Introduction
Urticarial vasculitis (UV) can be associated with certain autoimmune diseases, malignancies, drugs, and infections but is most often idiopathic. In contrast to chronic spontaneous urticaria (CSU), UV’s association with parasitic infections is more scarcely reported in the literature. The following case involves a patient with urticarial eruptions clinically resembling CSU, but histologically compatible with UV, that completely resolved after the diagnosis and treatment of a chronic Strongyloides stercoralis infection.
Case report
A 61-year-old man who had immigrated to Canada from Cambodia over 30 years prior was referred to dermatology by his allergist for a 5-year history of severe recalcitrant urticaria. His past medical history included hypothyroidism and dyslipidemia, treated with levothyroxine and pravastatin, respectively. The patient had an extensive travel history, with trips to Hawaii, Costa Rica, the Caribbean, and Cambodia in the 6 years prior to the onset of urticaria. Lesions were initially present only during the winter months but had progressively worsened over the previous 2 years, despite the use of 40 mg of cetirizine daily and several courses of prednisone with doses of 25 to 40 mg. At the time of referral, the patient had also been on omalizumab for 3 months, with minimal improvement. He described his symptoms as itchy rashes lasting up to 24 hours before resolving and reappearing elsewhere (Fig 1). He denied ever having oropharyngeal swelling, fever, and arthralgia but indicated having intermittent upper portion of the right quadrant abdominal pain for over a year, without diarrhea. On physical examination, wheals were visible, with extensive involvement of the arms, torso, and thighs. No petechiae or significant hyperpigmentation was noted. A skin biopsy revealed leukocytoclastic vasculitis (LCV). Conjunctival injection was also noted and assessed by ophthalmology, which subsequently ruled out uveitis. Blood work revealed marked eosinophilia, with an absolute eosinophil count of 1.3 × 109 (18.9%). Complement levels, antinuclear antibodies, serum electrophoresis, and antibody testing for HIV, syphilis, human T-lymphotropic virus type I, and hepatitis B and C were unremarkable. A working diagnosis of UV was considered based on the histologic findings and the lack of improvement with conventional treatments for CSU. The patient started 100 mg of dapsone daily in addition to the omalizumab and antihistamines, with an improvement in his Urticaria Activity Score, which decreased from 3 to 1 or 2. A short course of low-dose oral prednisone was subsequently added, as the patient was experiencing mental distress from the lack of improvement of his pruritus. Given the suboptimal response to treatments, elevated eosinophilia, travel history, and gastrointestinal symptoms, he was referred to tropical medicine to rule out parasitosis. Stool microscopy revealed rhabditiform larvae, and serology testing was positive for S stercoralis. Omalizumab, dapsone, and all antihistamines were stopped prior to the initiation of treatment with oral ivermectin. All urticarial eruptions resolved promptly following the first cycle of ivermectin. The patient received a second course of ivermectin 2 weeks after the initial dose. One month after the initiation of treatment, a repeat stool sample was negative for Strongyloides and the eosinophilia had resolved. The patient remains off all treatments, and the lesions have not recurred after 6 months.
Fig 1.
Wheals on bilateral lower extremities.
Discussion
Strongyloidiasis is a widespread soil-transmitted infection caused by the human nematode S stercoralis. It is endemic to many tropical and subtropical regions, including the Southeastern United States. It may present with a variety of dermatologic manifestations, such as larva currens, generalized or localized urticarial eruptions, and thumbprint purpura. Other symptoms include digestive and, less commonly, respiratory complaints. Although most infections are asymptomatic, hyperinfection may occur in the context of immunosuppression, with mortality rates exceeding 85%. Even relatively short courses of corticosteroids in nonimmunocompromised patients have been associated with hyperinfection syndrome and death.1
Urticaria is significantly associated with strongyloidiasis and is present in over 25% of infected patients.2 The reported prevalence of parasitic infections among patients with CSU varies widely based on geographic location and ranges from 0% to 75%.3 Urticaria associated with symptomatic or occult Strongyloides infection in nonendemic locations has previously been reported. In a prospective Canadian study of 254 patients with chronic urticaria, only 1 patient was found to have an associated parasitic infection.4
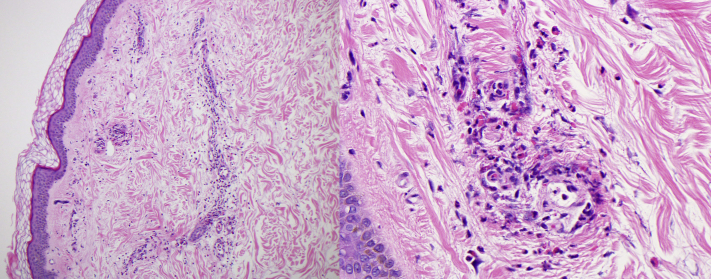
A clinical diagnosis of UV is usually made when both urticarial lesions and histologic findings of LCV are present.5 The patient’s biopsy demonstrated small-vessel neutrophilic infiltrates with karyorrhexis, lymphocytes, and eosinophils (Fig 2). These findings were consistent with LCV, and, in the clinical context, an initial diagnosis of UV was made. In our case, organisms were not observed on histology despite multiple step sections. However, it should be noted that biopsy specimens often fail to reveal larvae. Moreover, filariform larvae reach the skin from the bloodstream and migrate through the vessel walls of the papillary dermis. This may explain the inflammatory infiltrate noted around the small vessels on biopsy without any observable larvae.6
Fig 2.
Histologic examination showing leukocytoclastic vasculitis with numerous degranulating eosinophils in the blood vessel walls, a histologic image consistent with urticarial vasculitis (Hematoxylin and eosin stain; original magnification: 100× [left] and 400× [right].)
Parasitosis-induced UV is rare.7 Moreover, the diagnosis of a Strongyloides infection can be challenging, as symptoms can be vague, nonspecific, or absent. In this case, parasitosis was suspected in the absence of a clinical-pathologic correlation, the lack of response to conventional treatments for severe CSU, and the presence of eosinophilia. Eosinophil counts are often (but not always) elevated in Strongyloides infections. In chronic infections, eosinophilia is more intermittent but may be observed in up to two-thirds of cases.8 In CSU, serum eosinophil counts are usually normal. However, a recent study of 1259 patients with CSU identified eosinopenia in approximately 10% of cases.9 Lastly, eosinophilia is not an expected serologic finding in UV.10
With this in mind, we encourage clinicians to consider strongyloidiasis as a reversible cause of chronic urticaria, including in patients presenting in nonendemic countries and whose biopsy findings reveal leukocytoclasia. A higher index of suspicion is required when the response of CSU to conventional therapy is suboptimal, particularly in the presence of eosinophilia and a pertinent epidemiologic background. Finally, given the potentially devastating outcomes associated with hyperinfection syndrome, one should also consider strongyloidiasis screening prior to immunosuppressive therapy in any patient originating from an endemic country or having resided there for 6 months or more, regardless of symptoms or eosinophilia.1
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval: status: Not applicable.
References
- 1.Boggild A.K., Libman M., Greenaway C., McCarthy A.E. Committee to Advise on Tropical Medicine; Travel (CATMAT). CATMAT statement on disseminated strongyloidiasis: prevention, assessment and management guidelines. Can Commun Dis Rep. 2016;42(1):12–19. doi: 10.14745/ccdr.v42i01a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamarozzi F., Martello E., Giorli G., et al. Morbidity associated with chronic Strongyloides stercoralis infection: a systematic review and meta-analysis. Am J Trop Med Hyg. 2019;100(6):1305–1311. doi: 10.4269/ajtmh.18-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolkhir P., Balakirski G., Merk H.F., Olisova O., Maurer M. Chronic spontaneous urticaria and internal parasites--a systematic review. Allergy. 2016;71(3):308–322. doi: 10.1111/all.12818. [DOI] [PubMed] [Google Scholar]
- 4.Sibbald R.G., Cheema A.S., Lozinski A., Tarlo S. Chronic urticaria. Evaluation of the role of physical, immunologic, and other contributory factors. Int J Dermatol. 1991;30(6):381–386. doi: 10.1111/j.1365-4362.1991.tb03891.x. [DOI] [PubMed] [Google Scholar]
- 5.Brewer J.D., Davis M.D.P. Urticarial vasculitis. https://www.uptodate.com/contents/urticarial-vasculitis Accessed July 18, 2021.
- 6.Ly M.N., Bethel S.L., Usmani A.S., Lambert D.R. Cutaneous Strongyloides stercoralis infection: an unusual presentation. J Am Acad Dermatol. 2003;49(2 Suppl Case Reports):S157–S160. doi: 10.1067/mjd.2003.338. [DOI] [PubMed] [Google Scholar]
- 7.Shaigany S., Dabela E., Teich A.F., Husain S., Grossman M.E. Resolution of urticarial vasculitis after treatment of neurocysticercosis. J Am Acad Dermatol. 2015;72(1):e32–e33. doi: 10.1016/j.jaad.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell T., Lee D., Weinberg M., et al. Impact of enhanced health interventions for United States-bound refugees: evaluating best practices in migration health. Am J Trop Med Hyg. 2018;98(3):920–928. doi: 10.4269/ajtmh.17-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolkhir P., Church M.K., Altrichter S., et al. Eosinopenia, in chronic spontaneous urticaria, is associated with high disease activity, autoimmunity, and poor response to treatment. J Allergy Clin Immunol Pract. 2020;8(1):318–325.e5. doi: 10.1016/j.jaip.2019.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Venzor J., Lee W.L., Huston D.P. Urticarial vasculitis. Clin Rev Allergy Immunol. 2002;23(2):201–216. doi: 10.1385/CRIAI:23:2:201. [DOI] [PubMed] [Google Scholar]