Abstract
Respiratory syncytial virus (RSV) is the most important cause of viral lower respiratory tract illness (LRI) in infants and children worldwide and causes significant LRI in the elderly and in immunocompromised patients. The goal of RSV vaccination is to prevent serious RSV-associated LRI. There are several obstacles to the development of successful RSV vaccines, including the need to immunize very young infants, who may respond inadequately to vaccination; the existence of two antigenically distinct RSV groups, A and B; and the history of disease enhancement following administration of a formalin-inactivated vaccine. It is likely that more than one type of vaccine will be needed to prevent RSV LRI in the various populations at risk. Although vector delivery systems, synthetic peptide, and immune-stimulating complex vaccines have been evaluated in animal models, only the purified F protein (PFP) subunit vaccines and live attenuated vaccines have been evaluated in recent clinical trials. PFP-2 appears to be a promising vaccine for the elderly and for RSV-seropositive children with underlying pulmonary disease, whereas live cold-passaged (cp), temperature-sensitive (ts) RSV vaccines (denoted cpts vaccines) would most probably be useful in young infants. The availability of cDNA technology should allow further refinement of existing live attenuated cpts candidate vaccines to produce engineered vaccines that are satisfactorily attenuated, immunogenic, and phenotypically stable.
Respiratory syncytial virus (RSV) is the most important cause of viral lower respiratory tract illness (LRI) in infants and children worldwide (22, 55). Nearly 100,000 hospitalizations are attributable to RSV infection in the United States annually, resulting in costs approaching $300,000,000 per year (60). Infants who are premature (7, 35) or have chronic lung disease (52) or congenital heart disease (77) are at particular risk for severe RSV disease. Although traditionally regarded as a pediatric pathogen, RSV can also cause life-threatening pulmonary disease in bone marrow transplant recipients (44) and the elderly (36–38, 41, 84).
Although the importance of RSV as a respiratory pathogen has been recognized for over 30 years, a vaccine is not yet available because of several problems inherent in RSV vaccine development. The peak of severe disease and mortality associated with RSV infection occurs in infants younger than 3 months, who often have high titers of maternally derived RSV antibody. These young infants may not respond adequately to vaccination because of immunologic immaturity or suppression of the immune response by maternally derived antibody (18, 22, 71, 88). An RSV vaccine must also protect against the antigenically divergent groups A and B (see below). Most importantly, the vaccine must not potentiate naturally occurring RSV disease, as was observed with the formalin-inactivated RSV (FI-RSV) vaccine (45, 70, 74) (see below). Since serious RSV disease can occur in high-risk individuals who have experienced previous RSV infection, as well as in RSV-naive infants, it is also likely that more than one type of RSV vaccine will be needed to immunize all of those who would benefit from vaccination. The purpose of this review is to describe recent efforts to develop safe and effective RSV vaccines for populations at risk.
EPIDEMIOLOGY
By 2 years of age, almost all children will have been infected with RSV and approximately 50% will have been infected twice (62). Reinfection can occur throughout life and is usually symptomatic; however, RSV infection does not generally cause LRI in immunocompetent young adults and healthy older children (57).
RSV epidemics occur yearly during winter and early spring in temperate climates and during the rainy season in some tropical climates (86, 107). Humans are the only known reservoir for RSV. Spread of this highly contagious virus via contaminated nasal secretions requires close contact with an infected individual or contaminated environmental surface (56).
CLASSIFICATION AND STRUCTURE
RSV is a member of the genus Pneumovirus of the family Paramyxoviridae. This virus has a genome comprised of a single strand of negative-sense RNA, which is tightly associated with viral protein to form the nucleocapsid. The viral envelope is composed of a plasma membrane-derived lipid bilayer that contains virally encoded transmembrane proteins. A viral polymerase is packaged within the virion and transcribes genomic RNA into mRNA. The RSV genome (strain A2) is composed of 15,222 nucleotides, which encode three transmembrane surface proteins (F, G, and SH), two matrix proteins (M and M2), three nucleocapsid proteins (N, P, and L), and two nonstructural proteins (NS1 and NS2) (22). The surface fusion (F) and attachment (G) glycoproteins are the only viral components that induce RSV neutralizing antibody; they are therefore important targets of vaccine development. The F protein, in combination with G and SH, is responsible for fusion of the viral envelope with the host cell membranes and for the characteristic syncytium formation in cell culture. Its genome is highly conserved between RSV groups. The G protein mediates attachment to the host cell surface and is largely responsible for the antigenic diversity observed between RSV groups (see below).
Cross-neutralization studies have shown that RSV isolates can be classified into two groups, designated A and B (19). Although RSV A and B strains differ in all 10 viral proteins, the G glycoprotein shows the greatest divergence between groups, with only 53% amino acid homology between prototype RSV A and B viruses (68). Group A and group B viruses cocirculate during epidemics, although one may predominate (1, 63, 64, 85). Group A RSV infection may also cause more severe disease than group B RSV infection (79, 112, 120). RSV A and B viruses have been further classified into subgroups, and several investigators have described the regional and global epidemiology of these strains (2, 11, 12, 46, 108). The impact of antigenic diversity on RSV epidemiology is not completely understood, but it may partly explain the susceptibility to reinfection throughout life (5, 62) and the yearly variation in the severity of epidemics within communities (22).
IMMUNITY
Virus-specific immune responses are largely responsible for protection against RSV-associated LRI and recovery from RSV infection. Immunity to RSV is mediated via humoral and cellular effectors, including serum antibody (acquired as a result of infection or maternally derived in young infants), secretory antibody, and major histocompatibility complex class I-restricted cytotoxic T lymphocytes (3, 4, 15, 105, 125). Natural immunity to RSV is incomplete, and reinfection occurs throughout life, as demonstrated by epidemiologic studies (5, 62) and challenge studies in healthy young adults (58). Healthy older children and young adults, however, are usually protected against RSV-associated LRI. In general, humoral immune responses involving secretory antibodies and serum antibodies appear to protect against infection of the upper and lower respiratory tract, respectively, while cell-mediated responses directed against internal proteins appear to terminate infection.
The role of local immunity in the protection of the upper respiratory tract against RSV is suggested by the observation that passive transfer of neutralizing antibodies protects the lungs of cotton rats but does not significantly affect viral replication in the upper respiratory tract (99, 100, 121). However, cotton rats that were given live RSV via the respiratory tract were resistant to rechallenge for 6 to 12 months, suggesting that local immune responses inhibited reinfection (121). In adult volunteer studies, the presence of secretory neutralizing antibody but not serum antibody correlated with protection of the upper respiratory tract against RSV infection (83). In infants, the development of RSV-specific immunoglobulin A (IgA) in nasal secretions correlated temporally with disease clearance (81).
RSV replicates primarily in the respiratory epithelium. For this reason, serum neutralizing antibody does not prevent infection, as it does for pathogens that produce viremia, such as measles virus and varicella-zoster virus. However, high titers of serum neutralizing antibody against RSV do protect the lower respiratory tract against RSV infection, as has been demonstrated by animal studies (as noted above [99, 100, 121]), epidemiologic observations, and, most recently, clinical trials of RSV hyperimmune globulin (RSVIG) in high-risk infants (54, 98). High titers of maternally derived RSV antibody (as measured by IgG enzyme-linked immunosorbent assay [ELISA] or neutralization assay in maternal blood or cord blood) have been shown to correlate inversely with the incidence of RSV infection (47, 65) and the severity of RSV pneumonia in the first 6 months of life (76). In young children, the rate of reinfection with RSV and the rate of LRI at the time of reinfection also correlate inversely with the level of serum neutralizing antibody against RSV achieved after the primary infection (48). Finally, a randomized, prospective study of administration of RSVIG to high-risk infants showed that those who received monthly doses of 750 mg/kg had significant reductions in the rate and severity of LRI, as measured by the need for hospitalization, days spent in the hospital, and days spent in intensive care (54). Interestingly, the titer of RSV neutralizing antibody achieved in infants who received this dose of RSVIG was comparable to that demonstrated to protect the lungs of cotton rats against RSV infection in earlier studies (54, 61). The protective effect of RSVIG in these young infants has been confirmed by a subsequent placebo-controlled trial (98). Although the prophylactic efficacy of RSVIG has been established, RSVIG does not appear to ameliorate RSV disease when given therapeutically (106).
Primary infection with RSV does not always elicit an immune response that will protect the lower respiratory tract, since RSV-associated LRI can occur in young children experiencing their second episode of RSV (48, 62). The lack of protection may be explained in part by group specificity, since children with primary RSV infection develop a neutralizing antibody response in serum more frequently and of greater magnitude to the infecting strain than to the heterologous RSV strain (63). In addition, young infants develop levels of neutralizing antibody and F and G glycoprotein antibodies to RSV that are only 15 to 25% of those observed in older children (87). This latter study also demonstrated that the serum IgA response to the F glycoprotein is affected primarily by age, while the response to the G glycoprotein is affected primarily by the preexisting RSV IgG titer. The suboptimal response of young infants to primary infection with RSV has important implications for vaccine development, since it suggests that more than one dose of vaccine will probably be needed to induce adequate levels of RSV neutralizing antibody in this population.
Cell-mediated immunity appears to be important in the termination of RSV infection. Studies with mice suggest that antibodies are not essential to the clearance of virus (14). Children with defects in cell-mediated immunity and athymic or gamma-irradiated mice can shed virus indefinitely (14, 43). Although the adoptive transfer of primed T cells halts RSV replication in immunodeficient mice, the adoptive transfer of RSV-specific cytotoxic T lymphocytes may also potentiate disease (13), suggesting that there may be an immune component to RSV illness.
VACCINE DEVELOPMENT
General Considerations
A successful RSV vaccine should prevent serious RSV-associated LRI in those at risk. Several strategies have recently been used in RSV vaccine development, including the generation of peptide, subunit, and live virus vaccines. Of these, only inactivated, subunit, and live attenuated RSV vaccines have been evaluated in clinical trials to date. Although most efforts are directed toward immunizing at-risk populations, the possibility of immunizing pregnant women with an RSV subunit vaccine to enhance the level of serum neutralizing antibody passively transferred to infants is also being explored.
Experience with Formalin-Inactivated RSV Vaccine
In the early 1960s, an FI-RSV vaccine was prepared and tested in infants and children. The Bernett strain of RSV was initially propagated in human embryonic kidney cells and passaged in vervet monkey kidney cells. The infected cells were inactivated with formalin and concentrated by ultracentrifugation and alum precipitation. This preparation had a final concentration factor of 100 and was known as lot 100. Lot 100 was administered as two or three intramuscular doses separated by 1 to 3 months to infants and children between 2 months and 7 years of age (17, 45, 70, 74). Lot 100 not only failed to protect against wild-type (wt) RSV disease but also induced an exaggerated clinical response to wt RSV infection in infants who were RSV naive before vaccination. Many vaccinees were hospitalized with LRI; in one study, the hospitalization rate of vaccinees approached 80% compared to 5% in controls (74). Tragically, two infants who received lot 100 died following wt RSV infection, one at 14 months of age and the second at 16 months of age (74). RSV was readily isolated from the lower respiratory tracts of these infants, whose lungs also contained eosinophilic infiltrates (74).
The mechanisms responsible for the FI-RSV vaccine-associated disease enhancement are still not completely understood. Early studies with the limited amount of material available from these clinical trials suggested that FI-RSV induced humoral and cellular responses which were qualitatively and quantitatively different from those induced by wt RSV infection. Studies with banked sera from a subset of the subjects showed that recipients of FI-RSV developed high titers of serum antibodies to the RSV F glycoprotein as measured by ELISA but relatively low levels of RSV neutralizing activity as determined by the plaque reduction neutralization assay (89), suggesting that formalin inactivation selectively altered the protective epitopes located within the F and G surface glycoproteins of RSV (89). These infants also had poor F, G, and neutralizing antibody responses when subsequently infected with wt RSV (89). In addition, stored lymphocytes from FI-RSV vaccinees showed a greater proliferative response to RSV antigens than did those obtained from children naturally infected with wt RSV (75), and a peripheral eosinophilia was observed in some FI-RSV vaccinees (17).
The development of rodent models of enhanced pathologic changes following immunization with FI-RSV and challenge with wt RSV has allowed a more comprehensive analysis of the possible mechanisms of immunopotentiation of RSV disease. In mice, a priming immunization with wt RSV followed by challenge with wt RSV induced a Th-1-like response, with relatively increased levels of gamma interferon mRNA (51) whereas a priming immunization with FI-RSV followed by challenge with wt virus induced a Th-2-like response, with relative increases in the levels of interleukin-4 (IL-4) (51), IL-5, IL-10, and IL-13 cytokine mRNA (122). In addition, the depletion of CD4+ T cells (26) or of IL-4 or IL-10 (111) before RSV challenge abrogated the enhanced histopathologic changes in mice previously immunized with FI-RSV, further suggesting that the FI-RSV-induced pathologic changes in this animal model are Th-2 mediated.
Based upon these data, several investigators have developed a hypothesis to explain the enhanced disease observed in seronegative FI-RSV recipients. They postulated that FI-RSV vaccinees remained susceptible to infection with wt RSV because vaccination produced inadequate levels of neutralizing antibodies in serum and did not induce local immunity (32, 50, 88). Once the vaccinees were infected with wt RSV, the virus was not readily cleared because FI-RSV did not prime for CD8+ cytotoxic T-cell responses and the viral infection produced a direct cytopathic effect in the lower respiratory tracts of these infants. In addition, immunization with FI-RSV primed for a Th-2-like response, with increased local production of IL-4, IL-5, and IL-10, an influx of lymphocytes and eosinophils, the possible release of additional mediators, and resultant inflammation and bronchoconstriction (32, 92, 111, 122).
The clinical experience with FI-RSV and the information gleaned from animal models of disease enhancement suggest key features of an effective RSV vaccine for seronegative infants. The vaccine should induce protective levels of neutralizing antibody, as well as CD8+ RSV-specific cytotoxic T cells, and a pattern of CD4 response like that evoked by wt RSV. Although a live attenuated vaccine is most likely to exhibit these characteristics (32, 88), it is possible that novel immunization strategies that combine nonreplicating vaccines with cytokines or new adjuvants will also achieve these goals (92, 111).
Live Virus Vaccines
The failure of the parenterally administered, inactivated RSV vaccine and the subsequent recognition of the importance of local immunity in the prevention of RSV disease directed vaccine development toward live attenuated RSV mutants that could be delivered to the respiratory mucosa. Intranasal immunization with a live attenuated RSV vaccine should induce both systemic and local immunity and therefore protect against upper respiratory disease as well as LRI. Furthermore, the immune response to a live vaccine should closely resemble the response to natural infection and therefore should not produce enhanced disease upon exposure of the vaccinee to wt virus (88). In addition, it is likely that a live intranasal RSV vaccine will infect young infants, since other live attenuated intranasal respiratory virus vaccines have been shown to replicate in infants in the presence of maternally acquired antibody (18, 71). This feature will be critical for the success of a live attenuated RSV vaccine in young infants, since the target population (infants younger than 1 month) will invariably possess some maternally derived antibody. As noted above, it is unlikely that a single dose of vaccine would completely protect the lower respiratory tract against RSV infection, and so a successful RSV vaccine will probably have to be administered in multiple doses.
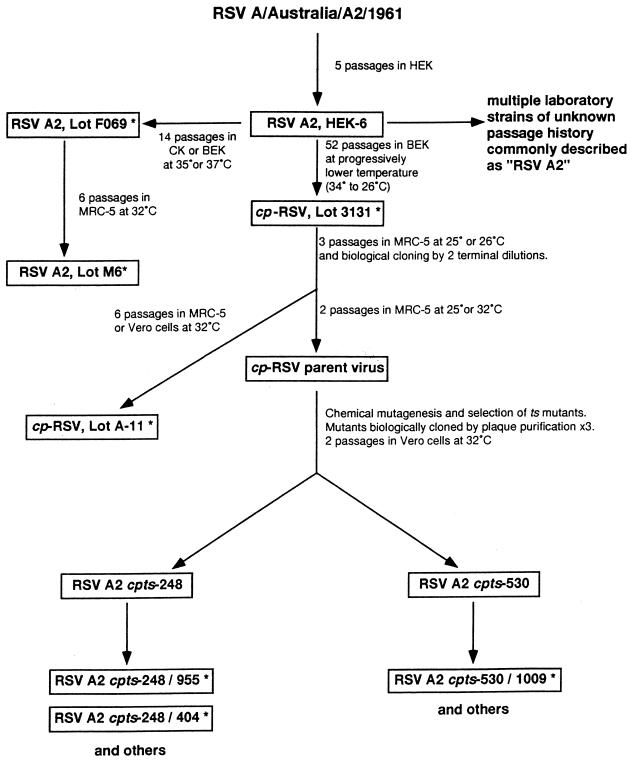
Several different strategies for the development of a live attenuated RSV vaccine were originally explored, including the creation of host range mutants, cold-passaged (cp) mutants, and temperature-sensitive (ts) mutants. The clinical evaluation of mutants developed between 1968 and 1976 that were either cp or ts (designated cpRSV [lot 3131], RSV ts-1, and ts-2) has been extensively summarized elsewhere (16, 32, 88). In brief, these vaccine candidates were either underattenuated (cpRSV and RSV ts-1) or overattenuated (RSV ts-2), and reversion to the wt (ts+) phenotype was observed in viral isolates obtained from infants and children who received the RSV ts-1 mutant. Transmission of the ts-1 mutant from vaccinated children to placebo recipients also occurred (73, 80). Importantly, enhanced disease was not observed when infants who received RSV ts-1 or cpRSV were naturally infected with wt RSV (80, 126). Although these early attempts to develop a live attenuated RSV vaccine were unsuccessful, they established the use of placebo-controlled, double-blind trials with postvaccination surveillance through RSV epidemics as the model for future evaluation of live attenuated RSV vaccines in children. In addition, cpRSV is the progenitor of promising cpts RSV vaccines currently being evaluated in children (Fig. 1) (see below).
FIG. 1.
Passage history of RSV A2 cpts vaccine candidates. Cell monolayer cultures were used to isolate or cold-passage RSV A2. HEK, human embryonic kidney cells; BEK, bovine embryonic kidney cells; MRC-5, a human diploid lung cell line; Vero, the well-characterized World Health Organization continuous cell line of African green monkey kidney cells; ∗, vaccines which have been evaluated in humans. Courtesy of James E. Crowe, Jr.
Investigators in the United Kingdom have also developed ts mutants of RSV group A, which were derived from the RSS-2 strain by chemical mutagenesis of virus grown in the MRC-5 human diploid cell line (82, 102). Two mutants, designated ts1A and ts19A, were derived by a single round of mutagenesis with 5-fluorouracil (5-FU); ts1B and ts19B were each derived from the respective A viruses by a second round of mutagenesis with ICR 340 or ICR 372; and the ts1C virus was derived from ts1B by a third round of mutagenesis with 5-FU. These mutant viruses display progressive levels of temperature-sensitive plaque formation in vitro, with ts1A being the least ts virus (no plaque formation at 39°C) and ts1C being the most ts virus (no plaque formation at 37°C). Each of these ts viruses has been evaluated in healthy adult volunteers. Although attenuated in comparison to wt RSV, the ts1A and ts1B viruses caused upper respiratory tract illnesses in adults and were therefore not sufficiently attenuated for further evaluation in children (82). Replication of the ts1C mutant in adults was not associated with illness (102), but evaluation in children is necessary to determine if this candidate vaccine is sufficiently attenuated. Recently, the complete nucleotide sequences of RSS-2 and ts1C and partial sequences of ts1A and ts1B were determined. A total of 37 nucleotide changes resulting in 13 amino acid substitutions occurred in the generation of ts1C; of these, 1 amino acid change occurred in P, 1 occurred in M1, 1 occurred in G, 2 occurred in F, and 8 occurred in L (113). The contribution of these individual mutations to the attenuation phenotype of ts1C in adults remains to be determined.
Bovine RSV has also been considered as a possible vaccine against human RSV despite significant differences in the predicted amino acid sequences of the F and G glycoproteins of bovine and human RSV (100, 101). Although rodents and owl monkeys infected with bovine RSV are protected against challenge with human RSV (96, 101), bovine RSV does not protect chimpanzees against human RSV challenge, despite high levels of virus replication in the upper respiratory tract (29). For this reason, bovine RSV is not a suitable candidate vaccine for clinical trials.
Investigators at Wyeth-Lederle Vaccines and Pediatrics have produced RSV A and B candidate cold-adapted and ts vaccines by serial passage of wt RSV A and B strains (RSV 3A and RSV 2B) in Vero cell culture at low temperature (103). Three RSV A mutants (p20E, p20F, and p28F) and four RSV B mutants (p33F, p24G, p20L, and p34L) were selected by biological cloning and displayed various levels of attenuation and immunogenicity in cotton rats. All of the RSV 3A mutants protected cotton rats against challenge with RSV A2 wt virus, and two of the RSV 2B mutants protected against challenge with RSV 18537 (B) wt virus. Some reversion to the ts+ phenotype was observed after growth of these mutants in vitro and in vivo (103). The RSV 2B33F mutant was also evaluated in seronegative chimpanzees and was found to be 10-fold restricted in replication in the upper respiratory tract and 1,000-fold restricted in replication in the lower respiratory tract compared with wt RSV 2B, although 2B33F was recovered from the lower respiratory tracts of some animals. Isolates of 2B33F recovered from seronegative chimpanzees also showed some loss of the ts phenotype (32). Further development of these mutants is required before they can be evaluated in clinical trials.
Finally, investigators at the Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Disease, National Institutes of Health, have produced a series of live attenuated RSV A candidate vaccines. These vaccines, which are all cpts, were derived from cpRSV by two rounds of random chemical mutagenesis with 5-FU. As shown in Fig. 1, this process generated candidate vaccines with a range of shutoff temperatures (35 to 37°C) that displayed a spectrum of attenuation in rodents and nonhuman primates (30, 31, 33, 34). Each of these candidate vaccines was shown to protect chimpanzees against challenge with wt RSV (30, 34, 72). Candidate vaccine viruses recovered from chimpanzees and nude mice showed greater stability of the ts phenotype than had previously been observed with the ts-1 virus (32). As a model for immunization of young infants who have maternally derived RSV antibody, chimpanzees were infused with RSVIG, given one of the cpts candidate vaccine viruses (248, 248/404, or 530/1009), and challenged 4 to 6 weeks later with wt RSV. Compared to control animals, chimpanzees that received one of these vaccines showed 1,000- to 10,000-fold restriction of challenge virus replication in the upper respiratory tract and 100,000-fold restriction of virus in the lower respiratory tract (34). The results of this study indicate that these cpts viruses can induce protective immune responses in the presence of passively acquired RSV antibody and suggest that these vaccines may therefore be immunogenic in young infants.
Three of these candidate intranasally administered vaccines have been evaluated in clinical trials. cpts 248/955 and 530/1009 vaccines were evaluated sequentially in adults, RSV-seropositive children, and RSV-seronegative children as young as 6 months. Although both of these vaccine candidates were attenuated in adults and seropositive children, neither vaccine was sufficiently attenuated in seronegative children to allow subsequent evaluation in very young infants (72). Importantly, the cpts 248/955 and 530/1009 vaccines retained the ts phenotype after prolonged replication in RSV-seronegative children (72). This was the first demonstration that an RSV mutant with a stable ts phenotype could be produced. In addition, disease enhancement was not observed when children who received either of these vaccines were observed through the subsequent RSV season. The cpts 248/404 vaccine is currently being evaluated in infants and young children. To date, this candidate vaccine appears to be safe, infectious, and immunogenic in RSV-seronegative infants as young as 6 months (72). Studies to evaluate this vaccine in younger infants are in progress.
The genetic basis of attenuation of these cpts candidate vaccines is being investigated. Sequence analysis of the cpts 248 virus and comparison with the parent cpRSV revealed that a single nucleotide substitution at position 10989 in the L (polymerase) gene, resulting in a predicted amino acid change of Gln to Leu, is responsible for acquisition of the ts phenotype (28). In comparison to cpts 248, cpts 248/404 virus has acquired an additional amino acid change (Asp to Glu) in the L gene and a noncoding nucleotide change in the M2 gene start sequence (42). In total, the nucleotide sequences of wt RSV A2 and cpts 248/404 differ at eight positions, leading to seven predicted amino acid changes (one in N, two in F, and four in the L gene). In the future, the contributions of these individual changes to the attenuation phenotypes of the cpts viruses can be evaluated by introducing single mutations into RSV cDNA and evaluating the mutants in rodents and primates (21, 28).
Genetically Engineered (cDNA-Derived) Vaccines
Recently, investigators have demonstrated the ability to recover infectious virus from cDNA clones of RSV (21). There are many potential applications of this technology to the field of RSV vaccine development. First, the attenuating mutations in a biologically derived candidate vaccine can be identified by introducing single mutations into RSV cDNA and evaluating the mutants in vitro and in vivo. By using this technique, the ts phenotype of the cpts 530 virus (Fig. 1) was mapped to a phenylalanine-to-leucine change at amino acid 521 (69). In addition, mutations present in current live attenuated RSV candidate vaccines could be combined to produce novel cDNA-derived vaccines that are sufficiently infectious and immunogenic yet attenuated and genetically stable (32). Deletion of a nonessential gene (such as the small hydrophobic protein SH gene) in combination with known attenuating cp and ts mutations might also produce a highly attenuated, genetically stable vaccine (10). Finally, it is possible to insert a single foreign gene into a recombinant RSV genome (9), so that a cDNA-derived bivalent RSV vaccine that contained the G genes from RSV A and B might be developed (9). Alternatively, immunomodulating genes (the IL-6 gene, for example) might be introduced in an effort to enhance immunogenicity in young infants (9).
Vector Delivery Systems
Recombinant vaccinia viruses expressing nine of the known RSV proteins were initially evaluated in BALB mice. Vaccinia virus M2 conferred short-lived protection in BALB/c (H-2d) mice, which was mediated by CD8+ cytotoxic T lymphocytes. In contrast, vaccinia virus F (vac-F) and vaccinia virus G (vac-G) each induced a neutralizing antibody response that provided long-term protection against wt RSV challenge in BALB mice of three major histocompatibility complex haplotypes (25). Sera from cotton rats immunized with vac-F or vac-G passively protected naive animals (25). Other investigators showed that vac-G derived from RSV B provided significant protection in cotton rats against RSV B but not RSV A challenge (109). However, intradermal immunization with vac-F or vac-G in seronegative chimpanzees produced low levels of neutralizing antibody and provided incomplete protection against challenge in the lower respiratory tract and no protection in the upper respiratory tract (23, 33). The lack of immunogenicity of these recombinants in chimpanzees, combined with concerns about the safety of vaccinia virus recombinants in young infants, make vaccinia virus-RSV recombinants unlikely candidate vaccines (32). Adenovirus-RSV recombinants expressing F (AdF), G, or F and G were shown to be immunogenic in dogs, but Ad7F, Ad4F, and Ad5F were poorly immunogenic when administered orally to seronegative chimpanzees (20, 66, 67). Although it is possible that other vector delivery systems for RSV will be developed, they will have to produce more immunogenic vaccines than those evaluated to date.
Subunit Vaccines
RSV F and G, the viral glycoproteins which induce neutralizing and protective antibodies (reviewed in reference 22), have been evaluated as potential candidate vaccines. Subunit RSV vaccines containing a novel chimeric FG glycoprotein or purified F protein have been developed and tested in a variety of rodent and primate models (8, 24, 29, 90, 91, 119, 124). In addition, two RSV F subunit vaccines (purified F protein 1 [PFP-1] and PFP-2) have been evaluated in clinical trials in the elderly (39, 40) and in children older than 1 year who are healthy (6, 94, 115) or who have cystic fibrosis (97) or bronchopulmonary dysplasia (53).
The chimeric RSV FG candidate vaccine consists of a immunoaffinity-purified, baculovirus-expressed fusion glycoprotein containing the ectodomains of RSV F and G (123). RSV FG delivered intramuscularly was shown to protect the lower respiratory tracts but not the upper respiratory tracts of rodents (8, 24, 124). When cotton rats that had previously received FG were challenged with wt RSV, enhanced pulmonary pathologic changes were observed by some investigators (24) but not by others (124). In African green monkeys, the FG candidate vaccine delivered intramuscularly induced low levels of RSV neutralizing antibody in serum and afforded minimal protection of the lower respiratory tract to challenge with wt virus; however, enhanced pulmonary pathologic changes were not observed in these animals. Intranasal immunization of mice with FG, with cholera toxin B (CTB) as an adjuvant, induced local IgG and IgA antibodies and protected the upper respiratory tract against challenge (93), but the utility of this approach requires further investigation.
Immunization with an immunoaffinity-purified preparation of the RSV F glycoprotein was also shown to protect the lower respiratory tracts but not the upper respiratory tracts of rodents against RSV challenge (119). Although initial studies did not demonstrate disease enhancement following challenge (91), some investigators later showed that cotton rats immunized with purified F had altered immune responses (high levels of F antibody relative to RSV neutralizing antibody) and enhanced histopathologic changes with challenged with wt RSV (90). Seronegative chimpanzees that received three doses of this vaccine developed low levels of RSV neutralizing antibody (29). As with the FG chimeric vaccine, intranasal immunization of mice with PFP-1 adsorbed to CTB protected the upper respiratory tract against challenge, and simultaneous intranasal immunization with F and CTB and parenteral immunization with F afforded upper and lower respiratory tract protection equivalent to that induced by live virus infection (118).
RSV F subunit vaccines adsorbed to alum have been evaluated in children older than 12 months with and without chronic underlying pulmonary disease, in healthy adults, and in institutionalized and ambulatory elderly subjects (Table 1). These vaccines, PFP-1 and PFP-2, contain the RSV F glycoprotein purified by immunoaffinity column chromatography or ion-exchange chromatography, respectively. Several observations can be made from these clinical trials. First, the vaccines were well tolerated in these populations: acute postvaccination reactions were minimal and enhanced disease was not observed (6, 39, 40, 53, 94, 97, 115). Second, 50 μg of vaccine was the most immunogenic of the doses tested, and fourfold or greater rises in RSV neutralizing-antibody titers were observed in approximately one-half to three-quarters of the vaccinees (Table 1). Finally, in some studies, the response to vaccination (defined as at least a fourfold rise in titer) correlated inversely with preimmunization neutralizing antibody titers in serum (39, 53), suggesting that these vaccines may not be able to increase neutralizing antibody responses beyond a certain level. Taken together, these studies suggest that RSV PFP vaccines are safe and moderately immunogenic in the populations (the elderly or children with chronic cardiac or pulmonary disease) for which they might be most useful. Recent studies with mice suggest that formulation of PFP-2 vaccine with the novel adjuvant Quillaja saponaria (QS-21) may augment neutralizing antibody and Th-1-type antibody responses (59). Clinical trials with PFP-2 adsorbed to QS-21 are in progress (95).
TABLE 1.
Evaluation of RSV F subunit vaccines in children and adults
| Study population | Vaccine | Dose (μg) | No. of subjects | % with ≥ 4 fold rise in RSV A2 neut Abg | % with RSV infectionh | % with RSV-associated LRI |
|---|---|---|---|---|---|---|
| Children | ||||||
| 18–36 moa | PFP-1 | 50 | 13 | 82 | 0 | 0 |
| Placebo | 0 | 13 | 8 | 31 | 15 | |
| 24–48 mob | PFP-1 | 20 | 13 | 42 | 10 | 0 |
| 50 | 10 | 70 | 12 | 0 | ||
| Placebo | 0 | 24 | 0 | 17 | 0 | |
| 12 mo–8 yr with CFc | PFP-2 | 50 | 17 | 53 | 23 | 18 |
| Placebo | 0 | 17 | 0 | 29 | 19 | |
| >12 mo with BPDd | PFP-2 | 50 | 10 | 90 | 10 | 10 |
| TIVi | 11 | 0 | 55 | 18 | ||
| Adults | ||||||
| Ambulatory elderlye | PFP-2 | 50 | 33 | 48 | 0 | 0 |
| Placebo | 0 | 31 | 0 | 0 | 0 | |
| Institutionalized elderlyf | PFP-2 | 50 | 37 | 25 | NTj | NT |
Data from reference 115.
Data from reference 94.
Data from reference 97. CF, cystic fibrosis.
Data from reference 53. BPD, bronchopulmonary dysplasia.
Data from reference 39.
Data from reference 40.
Measured within 4 weeks of the last vaccination received. neut Ab, neutralizing antibody.
As defined by viral detection in nasal secretions; repeat identification by antigen assay is counted as a single episode.
TIV, trivalent inactivated influenza vaccine.
NT, not tested.
Other Approaches
Synthetic peptide vaccines, F and G glycoprotein vaccines derived from bacterial expression in prokaryotes, and immune-stimulating complex-formulated vaccines have all been evaluated in mice (27, 78, 110, 116, 117). At present, it is unclear whether any of these vaccines will offer an advantage over the RSV PFP-2 subunit vaccine.
CONCLUSIONS
In the past decade, tremendous progress has been made in the development of RSV vaccines. Currently, two promising sets of candidate vaccines have been evaluated in clinical trials: the PFP subunit vaccines for immunization of the elderly and older RSV-seropositive children with chronic cardiac or pulmonary disease, and the cpts live attenuated vaccines, which would be used primarily for immunization of young infants. It is possible that combinations of different types of vaccines will be needed for certain populations; for example, optimal immune responses in the elderly might result from simultaneous administration of a live and a nonreplicating RSV vaccine, as has previously been demonstrated for influenza (49, 114). The availability of cDNA technology should allow further refinement of existing live attenuated candidate vaccines to produce engineered vaccines that are satisfactorily attenuated, immunogenic, and phenotypically stable. This technology might also be used to develop a bivalent (RSV A and B) chimeric vaccine, since an appropriate live attenuated RSV B candidate vaccine has not yet been identified.
The goal of RSV vaccination is not to prevent RSV infection but, rather, to prevent RSV-associated LRI. Efficacy studies of candidate vaccines will have to be designed with this goal in mind. If a monovalent RSV A vaccine is the first candidate to be assessed in an efficacy trial, it will also be important to determine whether heterotypic immunity is sufficient to protect against RSV B-associated LRI. Postvaccination surveillance for disease associated with wt RSV infection is a necessary component of all RSV vaccine trials in seronegative children: in phase I and II trials, the primary purpose is to monitor for enhanced disease, and in phase III trials, protection against RSV-associated LRI is also assessed.
Once efficacious vaccines are identified, issues of vaccine storage and delivery will also have to be addressed. This is particularly true for live attenuated vaccines, which must currently be stored at −70°C and are administered as nasal drops. Efforts are currently being made to increase the stability of these vaccines and to evaluate nasal spray delivery systems (104). It is to be hoped that delivery and storage systems will be developed that will make RSV vaccines accessible and affordable worldwide.
ACKNOWLEDGMENTS
We thank Brian Murphy, Peter Paradiso, and Peter Wright for their many helpful comments and suggestions. The editorial assistance of Martin Blair is gratefully acknowledged.
REFERENCES
- 1.Akerlind B, Norrby E. Occurrence of respiratory syncytial virus subtypes A and B strains in Sweden. J Med Virol. 1986;19:241–247. doi: 10.1002/jmv.1890190306. [DOI] [PubMed] [Google Scholar]
- 2.Anderson L J, Hendry R M, Pierik L T, Tsou C, McIntosh K. Multicenter study of strains of respiratory syncytial virus. J Infect Dis. 1991;163:687–692. doi: 10.1093/infdis/163.4.687. [DOI] [PubMed] [Google Scholar]
- 3.Bangham C R M, Cannon M J, Karzon D T, Askonas B A. Cytotoxic T-cell response to respiratory syncytial virus in mice. J Virol. 1985;56:55–59. doi: 10.1128/jvi.56.1.55-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangham C R M, Openshaw P J M, Ball L A, King A M Q, Wertz G W, Askonas B A. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986;137:3973–3977. [PubMed] [Google Scholar]
- 5.Beem M. Repeated infections with respiratory syncytial virus. J Immunol. 1967;98:1115–1122. [PubMed] [Google Scholar]
- 6.Belshe R B. Immunogenicity of purified F glycoprotein of respiratory syncytial virus: clinical and immune responses to subsequent natural infection in children. J Infect Dis. 1993;168:1024–1029. doi: 10.1093/infdis/168.4.1024. [DOI] [PubMed] [Google Scholar]
- 7.Berkovich S. Acute respiratory illness in the premature nursery associated with respiratory syncytial virus infections. Pediatrics. 1964;34:753–760. [PubMed] [Google Scholar]
- 8.Brideau R J, Wathen M W. A chimeric glycoprotein of human respiratory syncytial virus termed FG induces T-cell mediated immunity in mice. Vaccine. 1991;9:863–864. doi: 10.1016/0264-410x(91)90003-o. [DOI] [PubMed] [Google Scholar]
- 9.Bukreyev A, Camargo E, Collins P L. Recovery of infectious respiratory syncytial virus expressing an additional, foreign gene. J Virol. 1996;70:6634–6641. doi: 10.1128/jvi.70.10.6634-6641.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bukreyev A, Whitehead S S, Murphy B R, Collins P L. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J Virol. 1997;71:8973–8982. doi: 10.1128/jvi.71.12.8973-8982.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cane P A, Matthews D A, Pringle C R. Analysis of relatedness of subgroup A respiratory syncytial viruses isolated worldwide. Virus Res. 1992;25:15–22. doi: 10.1016/0168-1702(92)90096-r. [DOI] [PubMed] [Google Scholar]
- 12.Cane P A, Pringle C R. Evolution of subgroup A respiratory syncytial virus: evidence for progressive accumulation of amino acid changes in the attachment protein. J Virol. 1995;69:2918–2925. doi: 10.1128/jvi.69.5.2918-2925.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannon M J, Openshaw P J M, Askonas B A. T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannon M J, Stott E J, Taylor G, Aksonas B A. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunology. 1987;62:133–138. [PMC free article] [PubMed] [Google Scholar]
- 15.Chanock R M, Kim H W, Vargosko A J, et al. Respiratory syncytial virus. I. Virus recovery and other observations during 1960 outbreak of bronchiolitis, pneumonia, and minor respiratory diseases in children. JAMA. 1961;176:647–653. [PubMed] [Google Scholar]
- 16.Chanock R M, Murphy B M. Past efforts to develop safe and effective RSV vaccines. In: Meignier B, Murphy B, Ogra P, editors. Animal models of respiratory syncytial virus infections. Lyon, France: Merieux Foundation; 1991. pp. 35–42. [Google Scholar]
- 17.Chin J, Magoffin R L, Shearer L A, Schieble J H, Lennette E H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 18.Clements M L, Makhene M K, Karron R A, Murphy B R, Steinhoff M C, Subbarao K, Wilson M H, Wright P F Pediatric Care Center. Effective immunization with live attenuated influenza A virus can be achieved in early infancy. J Infect Dis. 1996;173:44–51. doi: 10.1093/infdis/173.1.44. [DOI] [PubMed] [Google Scholar]
- 19.Coates H V, Alling D W, Chanock R M. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol. 1966;83:299–313. doi: 10.1093/oxfordjournals.aje.a120586. [DOI] [PubMed] [Google Scholar]
- 20.Collins, P. L., A. R. Davis, M. D. Lubeck, et al. Evaluation of the protective efficacy of recombinant vaccinia viruses and adenoviruses that express respiratory syncytial virus glycoproteins, p. 79–84. In F. Brown, R. M. Chanock, H. S. Ginsberg, and R. A. Lerner (ed.), Vaccines 90: modern approaches to vaccines including the prevention of AIDS. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Collins P L, Hill M G, Camargo E, Grosfeld H, Chanock R M, Murphy B M. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci USA. 1995;92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins P L, McIntosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, editor. Fields virology. New York, N.Y: Raven Press; 1996. pp. 1313–1351. [Google Scholar]
- 23.Collins P L, Purcell R H, London W T, Lawrence L A, Chanock R M, Murphy B R. Evaluation in chimpanzees of vaccinia virus recombinants that express the surface glycoproteins of human respiratory syncytial virus. Vaccine. 1990;8:164–168. doi: 10.1016/0264-410x(90)90141-8. [DOI] [PubMed] [Google Scholar]
- 24.Connors M, Collins P L, Firestone C-Y, Sotnikov A V, Waitze A, Davis A R, Hung P P, Chanock R M, Murphy B R. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia-RSV recombinants or RSV. Vaccine. 1992;10:475–484. doi: 10.1016/0264-410x(92)90397-3. [DOI] [PubMed] [Google Scholar]
- 25.Connors M, Collins P L, Firestone C Y, Murphy B R. Respiratory syncytial virus (RSV) F, G M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991;65:1634–1637. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connors M, Kulkarni A B, Firestone C-Y, Holmes K L, Morse H C, III, Sotnikov A V, Murphy B R. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol. 1992;66:7444–7451. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corvaïa N, Tournier P, Nguyen T N, Haeuw J F, Power U F, Binz H, Andreoni C. Challenge of BALB/c mice with respiratory syncytial virus does not enhance the Th2 pathway induced after immunization with a recombinant G fusion protein, BBG2NA, in aluminum hydroxide. J Infect Dis. 1997;176:560–569. doi: 10.1086/514075. [DOI] [PubMed] [Google Scholar]
- 28.Crowe J, Jr, Firestone C Y, Whitehead S S, Collins P L, Murphy B R. Acquisition of the ts phenotype by a chemically mutagenized cold-passaged human respiratory syncytial virus vaccine candidate results from the acquisition of a single mutation in the polymerase (L) gene. Virus Genes. 1996;13:269–273. doi: 10.1007/BF00366988. [DOI] [PubMed] [Google Scholar]
- 29.Crowe J E., Jr Current approaches to the development of vaccines against disease caused by respiratory syncytial virus (RSV) and parainfluenza virus (PIV). A meeting report of the WHO Programme for Vaccine Development. Vaccine. 1995;13:415–421. doi: 10.1016/0264-410x(95)98266-d. [DOI] [PubMed] [Google Scholar]
- 30.Crowe J E, Jr, Phuong T B, Davis A R, Chanock R M, Murphy B R. A further attenuated derivative of a cold-passaged temperature-sensitive mutant of human respiratory syncytial virus (RSV cpts-248) retains immunogenicity and protective efficacy against wild-type challenge in seronegative chimpanzees. Vaccine. 1994;12:783–790. doi: 10.1016/0264-410x(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 31.Crowe J E, Jr, Bui P T, London W T, Davis A R, Hung P P, Chanock R M, Murphy B R. Satisfactorily attenuated and protective mutants derived from a partially attenuated cold-passaged respiratory syncytial virus mutant by introduction of additional attenuating mutations during chemical mutagenesis. Vaccine. 1994;12:691–709. doi: 10.1016/0264-410x(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 32.Crowe J E, Jr, Collins P L, Chanock R M, Murphy B R. Vaccines against respiratory syncytial virus and parainfluenza virus type 3. In: Levin M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 711–725. [Google Scholar]
- 33.Crowe J E, Jr, Collins P L, London W T, Chanock R M, Murphy B R. A comparison in chimpanzees of the immunogenicity and efficacy of live attenuated respiratory syncytial virus (RSV) temperature-sensitive mutant vaccines and vaccinia virus recombinants that express the surface glycoproteins of RSV. Vaccine. 1993;11:1395–1404. doi: 10.1016/0264-410x(93)90168-w. [DOI] [PubMed] [Google Scholar]
- 34.Crowe J E, Jr, Bui P T, Siber G R, Elkins W R, Chanock R M, Murphy B R. Cold-passaged, temperature-sensitive mutants of human respiratory syncytial virus (RSV) are highly attenuated, immunogenic, and protective in seronegative chimpanzees, even when RSV antibodies are infused shortly before immunization. Vaccine. 1995;13:847–855. doi: 10.1016/0264-410x(94)00074-w. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham C K, McMillan J A, Gross S J. Rehospitalization for respiratory illness in infants of less than 32 weeks’ gestation. Pediatrics. 1991;88:527–532. [PubMed] [Google Scholar]
- 36.Dowell S F, Anderson L J, Gary H E, Jr, Erdman D D, Plouffe J F, File T M, Jr, Marston B J, Breiman R F. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis. 1996;174:456–462. doi: 10.1093/infdis/174.3.456. [DOI] [PubMed] [Google Scholar]
- 37.Falsey A R, Cunningham C K, Barker W H, Kouides R W, Yuen J B, Menegus M, Weiner L B, Bonville C A, Betts R F. Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J Infect Dis. 1995;172:389–394. doi: 10.1093/infdis/172.2.389. [DOI] [PubMed] [Google Scholar]
- 38.Falsey A R, Treanor J J, Betts R F, Walsh E E. Viral respiratory infections in the institutionalized elderly: clinical and epidemiologic findings. J Am Geriatr Soc. 1992;40:115–119. doi: 10.1111/j.1532-5415.1992.tb01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falsey A R, Walsh E E. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in ambulatory adults over age 60. Vaccine. 1996;14:1214–1218. doi: 10.1016/s0264-410x(96)00030-8. [DOI] [PubMed] [Google Scholar]
- 40.Falsey A R, Walsh E E. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in the institutionalized elderly. Vaccine. 1997;15:1130–1132. doi: 10.1016/s0264-410x(97)00002-9. [DOI] [PubMed] [Google Scholar]
- 41.Falsey A R, Walsh E E. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis. 1998;177:463–466. doi: 10.1086/517376. [DOI] [PubMed] [Google Scholar]
- 42.Firestone C, Whitehead S S, Collins P L, Murphy B R, Crowe J E., Jr Nucleotide sequence analysis of the respiratory syncytial virus subgroup A cold-passaged (cp), temperature-sensitive (ts) cpts-248/404 live attenuated virus vaccine candidate. Virology. 1996;225:419–422. doi: 10.1006/viro.1996.0618. [DOI] [PubMed] [Google Scholar]
- 43.Fishaut M, Tubergen D, McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1980;96:179–186. doi: 10.1016/s0022-3476(80)80799-2. [DOI] [PubMed] [Google Scholar]
- 44.Fouillard L, Mouthon L, Laporte J P, Isnard F, Stachowiak J, Aoudjhane M, Lucet J C, Wolf M, Bricourt F, Douay L, Lopez M, Marche C, Najman A, Gorin N C. Severe respiratory syncytial virus pneumonia after autologous bone marrow transplantation: a report of three cases and review. Bone Marrow Transplant. 1992;9:97–100. [PubMed] [Google Scholar]
- 45.Fulginiti V A, Eller J J, Sieber O F, Joyner J W, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines: an aqueous trivalent parainfluenza virus vaccine and an alum-precipated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89:435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- 46.Garcia O, Martin M, Dopazo J, Arbiza J, Frabasile S, Russi J, Hortal M, Perez-Brena P, Martinez I, Garcia-Barreno B, et al. Evolutionary pattern of human respiratory syncytial virus (subgroup A): cocirculating lineages and correlation of genetic and antigenic changes in the G glycoprotein. J Virol. 1994;68:5448–5459. doi: 10.1128/jvi.68.9.5448-5459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glezen W P, Paredes A, Allison J E, Taber L H, Frank A L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98:708–715. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 48.Glezen W P, Taber L H, Frank A L, Kasel J A. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 49.Gorse G J, Otto E E, Powers D C, Chambers G W, Eickhoff C S, Newman F K. Induction of mucosal antibodies by live attenuated and inactivated influenza virus vaccines in the chronically ill elderly. J Infect Dis. 1996;173:285–290. doi: 10.1093/infdis/173.2.285. [DOI] [PubMed] [Google Scholar]
- 50.Graham B S. Pathogenesis of respiratory syncytial virus vaccine-augmented pathology. Am J Respir Crit Care Med. 1995;152:S63–S66. doi: 10.1164/ajrccm/152.4_Pt_2.S63. [DOI] [PubMed] [Google Scholar]
- 51.Graham B S, Henderson G S, Tang Y, Lu X, Neuzil K M, Colley D G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 52.Groothuis J R, Gutierrez K M, Lauer B A. Respiratory syncytial virus infection in children with bronchopulmonary dysplasia. Pediatrics. 1988;82:199–203. [PubMed] [Google Scholar]
- 53.Groothuis J R, King S J, Hogerman D A, Paradiso P R, Simoes E A. Safety and immunogenicity of a purified F protein respiratory syncytial virus (PFP-2) vaccine in seropositive children with bronchopulmonary dysplasia. J Infect Dis. 1998;177:467–469. doi: 10.1086/517377. [DOI] [PubMed] [Google Scholar]
- 54.Groothuis J R, Simoes E A F, Levin M J, Hall C B, Long C E, Rodriguez W J, Arrobio J, Meissner H C, Fulton D R, Welliver R C, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. N Engl J Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 55.Hall C B. Prospects for a respiratory syncytial virus vaccine. Science. 1994;265:1393–1394. doi: 10.1126/science.7915433. [DOI] [PubMed] [Google Scholar]
- 56.Hall C B, Douglas R G. Modes of transmission of respiratory syncytial virus. J Pediatr. 1981;99:100–103. doi: 10.1016/s0022-3476(81)80969-9. [DOI] [PubMed] [Google Scholar]
- 57.Hall C B, Geiman J M, Biggar R, Kotok D, Hogan P M, Douglas R G. Respiratory syncytial virus infections within families. N Engl J Med. 1976;294:414–419. doi: 10.1056/NEJM197602192940803. [DOI] [PubMed] [Google Scholar]
- 58.Hall C B, Walsh E E, Long C E, Schnabel K C. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 59.Hancock G E, Speelman D J, Frenchick P J, Mineo-Kuhn M M, Baggs R B, Hahn D J. Formulation of the purified fusion protein of respiratory syncytial virus with the saponin QS-21 induces protective immune responses in Balb/c mice that are similar to those generated by experimental infection. Vaccine. 1995;13:391–400. doi: 10.1016/0264-410x(95)98263-a. [DOI] [PubMed] [Google Scholar]
- 60.Heilman C A. Respiratory syncytial and parainfluenza viruses. J Infect Dis. 1990;161:402–406. doi: 10.1093/infdis/161.3.402. [DOI] [PubMed] [Google Scholar]
- 61.Hemming V G, Prince G A, Groothuis J R, Siber G R. Hyperimmune globulins in prevention and treatment of respiratory syncytial virus infections. Clin Microbiol Rev. 1995;8:22–33. doi: 10.1128/cmr.8.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henderson F W, Collier A M, Clyde W A, Jr, Denny F W. Respiratory-syncytial-virus infections, reinfections and immunity: a prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 63.Hendry R M, Burns J C, Walsh E E, Graham B S, Wright P F, Hemming V G, Rodriquez W J, Kim H W, Prince G A, McIntosh K, Chanock R M, Murphy B R. Strain-specific serum antibody responses in infants undergoing primary infection with respiratory syncytial virus. J Infect Dis. 1988;157:640–647. doi: 10.1093/infdis/157.4.640. [DOI] [PubMed] [Google Scholar]
- 64.Hendry R M, Pierik L T, McIntosh K. Prevalence of respiratory syncytial virus subgroups over six consecutive outbreaks: 1981–1987. J Infect Dis. 1989;160:185–190. doi: 10.1093/infdis/160.2.185. [DOI] [PubMed] [Google Scholar]
- 65.Holberg C J, Wright A L, Martinez F D, Ray C G, Taussig L M, Lebowitz M D. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol. 1991;133:1135–1151. doi: 10.1093/oxfordjournals.aje.a115826. [DOI] [PubMed] [Google Scholar]
- 66.Hsu K-H L, Lubeck M D, Davis A R, Bhat R A, Selling B H, Bhat B M, Mizutani S, Hung P P, Murphy B R, Collins P L, Chanock R M. Immunogenicity and protective efficacy of adenovirus vectored respiratory syncytial virus vaccine. In: Chanock R M, Brown F, Ginsberg H S, Lerner R A, editors. Vaccines 91: modern approaches to new vaccines including prevention of AIDS. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1991. pp. 293–297. [Google Scholar]
- 67.Hsu K-H L, Lubeck M D, Davis A R, Bhat R A, Selling B H, Bhat B M, Mizutani S, Murphy B R, Collins P L, Chanock R M. Immunogenicity of recombinant adenovirus-respiratory syncytial virus using Ad4, Ad5, and Ad7 vectors in dogs and a chimpanzee. J Infect Dis. 1992;166:769–775. doi: 10.1093/infdis/166.4.769. [DOI] [PubMed] [Google Scholar]
- 68.Johnson P R, Jr, Olmsted R A, Prince G A, Alling D W, Walsh E E, Collins P L. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J Virol. 1987;61:3163–3166. doi: 10.1128/jvi.61.10.3163-3166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Juhasz K, Whitehead S S, Bui P T, Biggs J M, Boulanger C A, Collins P L, Murphy B R. The temperature-sensitive (ts) phenotype of a cold-passaged (cp) live attenuated respiratory syncytial virus vaccine candidate, designated cpts530, results from a single amino acid substitution in the L protein. J Virol. 1997;71:5814–5819. doi: 10.1128/jvi.71.8.5814-5819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kapikian A Z, Mitchell R H, Chanock R M, Shvedoff R A, Stewart C E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 71.Karron R A, Steinhoff M C, Subbarao E K, Wilson M H, MacLeod K, Clements M L, Fries L F, Murphy B R Pediatric Care Center. Safety and immunogenicity of a cold-adapted influenza A (H1N1) reassortant virus vaccine administered to infants less than six months of age. Pediatr Infect Dis J. 1995;14:10–16. doi: 10.1097/00006454-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 72.Karron R A, Wright P F, Crowe J E, Jr, Clements M L, Thompson J, Makhene M, Casey R, Murphy B R. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus vaccines in chimpanzees and in human adults, infants and children. J Infect Dis. 1997;176:1428–1436. doi: 10.1086/514138. [DOI] [PubMed] [Google Scholar]
- 73.Kim H W, Arrobio J O, Brandt C D, Wright P, Hodes D, Chanock R M, Parrott R H. Safety and antigenicity of temperature-sensitive (ts) mutant respiratory syncytial virus (RSV) in infants and children. Pediatrics. 1973;52:56–63. [PubMed] [Google Scholar]
- 74.Kim H W, Canchola J G, Brandt C D, Pyles G, Chanock R M, Jensen K, Parrott R H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 75.Kim H W, Leikin S L, Arrobio J, Brandt C D, Chanock R M, Parrott R H. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr Res. 1976;10:75–78. doi: 10.1203/00006450-197601000-00015. [DOI] [PubMed] [Google Scholar]
- 76.Lamprecht C L, Krause H E, Mufson M A. Role of maternal antibody in pneumonia and bronchiolitis due to respiratory syncytial virus. J Infect Dis. 1976;134:211–217. doi: 10.1093/infdis/134.3.211. [DOI] [PubMed] [Google Scholar]
- 77.MacDonald N E, Hall C B, Suffin S C, Alexson C, Harris P J, Manning J A. Respiratory syncytial viral infection in infants with congenital heart disease. N Engl J Med. 1982;307:397–400. doi: 10.1056/NEJM198208123070702. [DOI] [PubMed] [Google Scholar]
- 78.Martin-Gallard A, Fleischer E, Doyle S A, Arumugham R, Collins P L, Hildreth S W, Paradiso P R. Expression of the G glycoprotein gene of human respiratory syncytial virus in Salmonella typhimurium. J Gen Virol. 1993;74:453–458. doi: 10.1099/0022-1317-74-3-453. [DOI] [PubMed] [Google Scholar]
- 79.McConnochie K M, Hall C B, Walsh E E, Roghmann K J. Variation in severity of respiratory syncytial virus infections with subtype. J Pediatr. 1990;117:52–62. doi: 10.1016/s0022-3476(05)82443-6. [DOI] [PubMed] [Google Scholar]
- 80.McIntosh K, Arbeter A M, Stahl M K, Orr I A, Hodes D S, Ellis E C. Attenuated respiratory syncytial virus vaccines in asthmatic children. Pediatr Res. 1974;8:689–696. doi: 10.1203/00006450-197407000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McIntosh K, Masters H B, Orr I, Chao R K, Barkin R M. The immunologic response to infection with respiratory syncytial virus in infants. J Infect Dis. 1978;138:24–32. doi: 10.1093/infdis/138.1.24. [DOI] [PubMed] [Google Scholar]
- 82.McKay E, Higgins P, Tyrrell D, Pringle C. Immunogenicity and pathogenicity of temperature-sensitive modified respiratory syncytial virus in adult volunteers. J Med Virol. 1988;25:411–421. doi: 10.1002/jmv.1890250405. [DOI] [PubMed] [Google Scholar]
- 83.Mills J, Van Kirk J E, Wright P F, Chanock R M. Experimental respiratory syncytial virus infection of adults. J Immunol. 1971;107:123–130. [PubMed] [Google Scholar]
- 84.Mlinaric-Galinovic G, Falsey A R, Walsh E E. Respiratory syncytial virus infection in the elderly. Eur J Clin Microbiol Infect Dis. 1996;15:777–781. doi: 10.1007/BF01701518. [DOI] [PubMed] [Google Scholar]
- 85.Mufson M A, Belshe R B, Orvell C, Norrby E. Respiratory syncytial virus epidemics: variable dominance of subgroups A and B strains among children, 1981–1986. J Infect Dis. 1988;157:143–148. doi: 10.1093/infdis/157.1.143. [DOI] [PubMed] [Google Scholar]
- 86.Mufson M A, Levin M J, Wash R E, Mocega-Gonzales H E, Krause H E. Epidemiology of respiratory syncytial virus infection among infants and children in Chicago. Am J Epidemiol. 1973;98:88–95. doi: 10.1093/oxfordjournals.aje.a121542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murphy B R, Alling D W, Snyder M H, Walsh E E, Prince G A, Chanock R M, Hemming V G, Rodriguez W J, Kim H W, Graham B S, Wright P F. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J Clin Microbiol. 1986;24:894–898. doi: 10.1128/jcm.24.5.894-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murphy B R, Hall S L, Kulkarni A B, Crowe J E, Collins P L, Connors M, Karron R A, Chanock R M. An update on approaches to the development of respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) vaccines. Virus Res. 1994;32:13–36. doi: 10.1016/0168-1702(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 89.Murphy B R, Prince G A, Walsh E E, Kim H W, Parrott R H, Hemming V G, Rodriguez W J, Chanock R M. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J Clin Microbiol. 1986;24:197–202. doi: 10.1128/jcm.24.2.197-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murphy B R, Sotnikov A V, Lawrence L A, Banks S M, Prince G A. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3–6 months after immunization. Vaccine. 1990;8:497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- 91.Murphy B R, Sotnikov A V, Paradiso P R, Hildreth S W, Jenson A B, Baggs R B, Lawrence L, Zubak J J, Chanock R M, Beeler J A, et al. Immunization of cotton rats with the fusion (F) and large (G) glycoproteins of respiratory syncytial virus (RSV) protects against RSV challenge without potentiating RSV disease. Vaccine. 1989;7:533–540. doi: 10.1016/0264-410x(89)90278-8. [DOI] [PubMed] [Google Scholar]
- 92.Neuzil K M, Johnson J E, Tang Y, Prieel J, Slaoui M, Gar N, Graham B S. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with respiratory syncytial virus FG subunit vaccine. Vaccine. 1997;15:525–532. doi: 10.1016/s0264-410x(97)00218-1. [DOI] [PubMed] [Google Scholar]
- 93.Oien N L, Brideau R J, Walsh E E, Wathen M W. Induction of local and systemic immunity against human respiratory syncytial virus using a chimeric FG glycoprotein and cholera toxin B subunit. Vaccine. 1994;12:731–735. doi: 10.1016/0264-410x(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 94.Paradiso P R, Hildreth S W, Hogerman D A, Speelman D J, Lewin E B, Oren J, Smith D H. Safety and immunogenicity of a subunit respiratory syncytial virus vaccine in children 24 to 48 months old. Pediatr Infect Dis J. 1994;13:792–798. doi: 10.1097/00006454-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 95.Paradiso, P. R. Personal communication.
- 96.Piazza R M, Johnson S A, Darnell M E R, Porter D D, Hemming V G, Prince G A. Bovine respiratory syncytial virus protects cotton rats against human respiratory syncytial virus infection. J Virol. 1993;67:1503–1510. doi: 10.1128/jvi.67.3.1503-1510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Piedra P A, Grace S, Jewell A, Spinelli S, Bunting D, Hogerman D A, Malinoski F, Hiatt P W. Purified fusion protein vaccine protects against lower respiratory tract illness during respiratory syncytial virus season in children with cystic fibrosis. Pediatr Infect Dis J. 1996;15:23–31. doi: 10.1097/00006454-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 98.Prevent Study Group. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics. 1997;99:93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 99.Prince G A, Horswood R L, Camargo E, Koenig D, Chanock R M. Mechanisms of immunity to respiratory syncytial virus in cotton rats. Infect Immun. 1983;42:81–87. doi: 10.1128/iai.42.1.81-87.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prince G A, Horswood R L, Chanock R M. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985;55:517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prince G A, Redington M, Piazza R M, Hemming V G. Bovine respiratory syncytial virus provides protection against human respiratory syncytial virus infection in cotton rats and primates. In: Meignier B, Murphy B, Ogra P, editors. Animal models of respiratory syncytial virus infections. Lyon, France: Merieux Foundation; 1991. pp. 133–136. [Google Scholar]
- 102.Pringle C R, Filipiuk A H, Robinson B S, Watt P J, Higgins P, Tyrrell D A J. Immunogenicity and pathogenicity of a triple temperature-sensitive modified respiratory syncytial virus in adult volunteers. Vaccine. 1993;11:473–478. doi: 10.1016/0264-410x(93)90290-e. [DOI] [PubMed] [Google Scholar]
- 103.Randolph V B, Kandis M, Stemler-Higgins P, Kennelly M S, McMullen Y M, Speelman D J, Weeks-Levy C. Attenuated temperature-sensitive respiratory syncytial virus mutants generated by cold adaptation. Virus Res. 1994;33:241–259. doi: 10.1016/0168-1702(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 104.Randolph, V. B. Personal communication.
- 105.Richardson L S, Yolken R H, Belshe R B, et al. Enzyme-linked immunosorbent assay for measurement of serological response to respiratory syncytial virus infection. Infect Immun. 1978;23:660–664. doi: 10.1128/iai.20.3.660-664.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodriguez W J, Gruber W C, Welliver R C, Groothuis J R, Simoes E A, Meissner H C, Hemming V G, Hall C B, Lepow M L, Rosas A J, Robertson C, Kramer A A. Respiratory syncytial virus (RSV) immune globulin intravenous therapy for RSV lower respiratory tract infection in infants and young children at high risk for severe RSV infections: Respiratory Syncytial Virus Immune Globulin Study Group. Pediatrics. 1997;99:454–461. doi: 10.1542/peds.99.3.454. [DOI] [PubMed] [Google Scholar]
- 107.Spence L, Barratt N. Respiratory syncytial virus associated with acute respiratory infections in Trinidadian patients. Am J Epidemiol. 1968;88:257–266. doi: 10.1093/oxfordjournals.aje.a120884. [DOI] [PubMed] [Google Scholar]
- 108.Storch G A, Anderson L J, Park C S, Tsou C, Dohner D E. Antigenic and genomic diversity within group A respiratory syncytial virus. J Infect Dis. 1991;163:858–861. doi: 10.1093/infdis/163.4.858. [DOI] [PubMed] [Google Scholar]
- 109.Sullender W M, Anderson K, Wertz G W. The respiratory syncytial virus subgroup B attachment glycoprotein: analysis of sequence, expression from a recombinant vector, and evaluation as an immunogen against homologous and heterologous subgroup virus challenge. Virology. 1990;178:195–203. doi: 10.1016/0042-6822(90)90394-7. [DOI] [PubMed] [Google Scholar]
- 110.Sullender W M, Britt W J. Antigenic and immunogenic analysis of group A and group B respiratory syncytial virus G proteins expressed from recombinant baculoviruses. J Gen Virol. 1996;77:641–648. doi: 10.1099/0022-1317-77-4-641. [DOI] [PubMed] [Google Scholar]
- 111.Tang Y W, Graham G S. Interleukin-12 treatment during immunization elicits a T helper cell type 1–like immune response in mice challenged with respiratory syncytial virus and improves vaccine immunogenicity. J Infect Dis. 1995;172:734–738. doi: 10.1093/infdis/172.3.734. [DOI] [PubMed] [Google Scholar]
- 112.Taylor C E, Morrow S, Scott M, Young B, Toms G L. Comparative virulence of respiratory syncytial virus subgroups A and B. Lancet. 1989;i:777–778. doi: 10.1016/s0140-6736(89)92592-0. [DOI] [PubMed] [Google Scholar]
- 113.Tolley K P, Marriott A C, Simpson A, Plows D J, Matthews D A, Longhurst S J, Evans J E, Johnson J R, Cane P A, Randolph V B, Easton A J, Pringle C R. Identification of mutations contributing to the reduced virulence of a modified strain of respiratory syncytial virus. Vaccine. 1996;14:1637–1646. doi: 10.1016/s0264-410x(96)00136-3. [DOI] [PubMed] [Google Scholar]
- 114.Treanor J J, Mattison H R, Dumyati G, Yinnon A, Erb S, O’Brien D, Dolin R, Betts R F. Protective efficacy of combined live intranasal and inactivated influenza A virus vaccines in the elderly. Ann Intern Med. 1992;117:625–633. doi: 10.7326/0003-4819-117-8-625. [DOI] [PubMed] [Google Scholar]
- 115.Tristram D A, Welliver R C, Mohar C K, Hogerman D A, Hildreth S W, Paradiso P. Immunogenicity and safety of respiratory syncytial virus subunit vaccine in seropositive children 18-36 months old. J Infect Dis. 1993;167:191–195. doi: 10.1093/infdis/167.1.191. [DOI] [PubMed] [Google Scholar]
- 116.Trudel M, Nadon F, Seguin C, Binz H. Protection of BALB/c mice from respiratory syncytial virus infection by immunization with a synthetic peptide derived from the G glycoprotein. Virology. 1991;185:749–757. doi: 10.1016/0042-6822(91)90546-n. [DOI] [PubMed] [Google Scholar]
- 117.Trudel M, Stott E J, Taylor G, Oth D, Mercier G, Nadon F, Seguin C, Simard C, Lacroix M. Synthetic peptides corresponding to the F protein of RSV stimulate murine B and T cells but fail to confer protection. Arch Virol. 1991;117:59–71. doi: 10.1007/BF01310492. [DOI] [PubMed] [Google Scholar]
- 118.Walsh E E. Humoral, mucosal, and cellular immune response to topical immunization with a subunit respiratory syncytial virus vaccine. J Infect Dis. 1994;170:345–350. doi: 10.1093/infdis/170.2.345. [DOI] [PubMed] [Google Scholar]
- 119.Walsh E E, Hall C B, Briselli M, Brandriss M W, Schlesinger J J. Immunization with glycoprotein subunits of respiratory syncytial virus to protect cotton rats against viral infection. J Infect Dis. 1987;155:1198–1204. doi: 10.1093/infdis/155.6.1198. [DOI] [PubMed] [Google Scholar]
- 120.Walsh E E, McConnochie K M, Long C E, Hall C B. Severity of respiratory syncytial virus infection is related to virus strain. J Infect Dis. 1997;175:814–820. doi: 10.1086/513976. [DOI] [PubMed] [Google Scholar]
- 121.Walsh E E, Schlesinger J J, Brandriss M W. Protection from respiratory syncytial virus infection in cotton rats by passive transfer of monoclonal antibodies. Infect Immun. 1984;43:756–758. doi: 10.1128/iai.43.2.756-758.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Waris M E, Tsou C, Erdman D D, Zaki S R, Anderson L J. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wathen M W, Brideau R J, Thomsen D R, Murphy B R. Characterization of a novel human respiratory syncytial virus chimeric FG glycoprotein expressed using a baculovirus vector. J Gen Virol. 1989;70:2625–2635. doi: 10.1099/0022-1317-70-10-2625. [DOI] [PubMed] [Google Scholar]
- 124.Wathen M W, Kakuk T J, Brideau R J, Hausknecht E C, Cole S L, Zaya R M. Vaccination of cotton rats with a chimeric FG glycoprotein of human respiratory syncytial virus induces minimal pulmonary pathology on challenge. J Infect Dis. 1991;163:477–482. doi: 10.1093/infdis/163.3.477. [DOI] [PubMed] [Google Scholar]
- 125.Watt P J, Zardis M, Lambden P R. Age related IgG subclass response to respiratory syncytial virus fusion protein in infected infants. Clin Exp Immunol. 1986;64:503–509. [PMC free article] [PubMed] [Google Scholar]
- 126.Wright P F, Shinozaki T, Fleet W, Sell S H, Thompson J, Karzon D T. Evaluation of a live, attenuated respiratory syncytial virus vaccine in infants. J Pediatr. 1976;88:931–936. doi: 10.1016/s0022-3476(76)81044-x. [DOI] [PubMed] [Google Scholar]