Abstract
STUDY QUESTION
What are patients’ and fertility staff views of talking about possible IVF/ICSI failure and need for multiple cycles in treatment planning?
SUMMARY ANSWER
Healthcare professionals (HCPs) typically plan treatment on a cycle-by-cycle basis but HCPs and patients see benefits in talking about possible IVF/ICSI failure and the consequent need for multiple cycles to better prepare patients for this possibility, to support them through treatment challenges and to foster a sense of collaboration with the clinic in achieving the shared goal of treatment success.
WHAT IS KNOWN ALREADY
Many patients need more than one round of IVF/ICSI stimulation to achieve their parenthood goals. About 60% of patients are willing to plan for multiple cycles of treatment in advance of treatment engagement. However, it is not clear how patients are informed about the high possibility of failure and the subsequent need for multiple cycles during their treatment planning consultations, and how approaches could be optimized.
STUDY DESIGN, SIZE, DURATION
Qualitative focus groups with HCPs working at fertility clinics, patient advocates employed by patient charities (April 2020) and patients (July and August 2020). Patients were eligible if they had had a consultation to start a first/repeat stimulated IVF/ICSI cycle in the 8 weeks prior to participation, were aged 18 or older (upper age limit of 42 years for women), in heterosexual relationships and fluent in English. Eligible HCPs and patient advocates were those employed at a fertility clinic or charity, respectively.
PARTICIPANTS/MATERIALS, SETTINGS, METHOD
Focus group topic guides progressed from general questions about fertility consultations to if and how the possibility of treatment failure and need for multiple cycles was introduced and discussed in (attended/own) clinics. After, preferences regarding planning IVF/ICSI on a multi-cycle or cycle-by-cycle basis were explored. Focus groups were recorded, and recordings transcribed and analysed using framework analysis to identify shared, unique and incongruent themes across participant groups.
MAIN RESULTS AND THE ROLE OF CHANCE
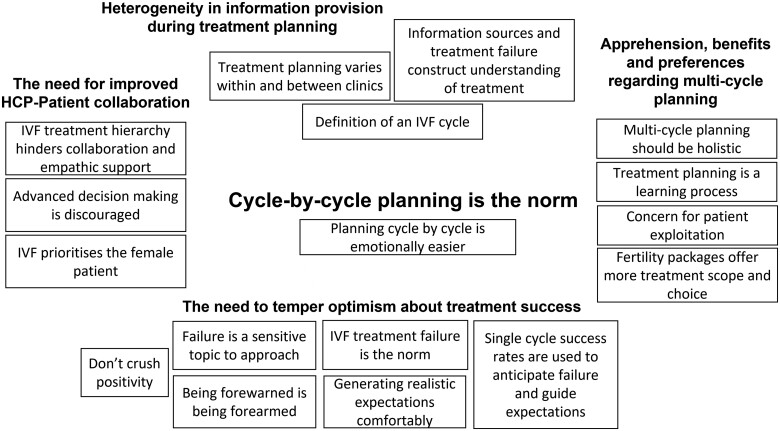
Twelve HCPs, 2 patient advocates and 10 patients participated in six semi-structured online focus group discussions. All patients were childless and had been trying to conceive for ∼3 years. Framework analysis generated four themes and one meta-theme across participant groups. The meta-theme showed planning IVF on a cycle-by-cycle basis is the norm at clinics and that this affects how treatment is planned and the acceptability of a shift towards planning for multiple cycles, which was perceived as beneficial despite some apprehension. The four themes were: (i) heterogeneity in information provision during treatment planning; (ii) the need for improved HCP-patient collaboration; (iii) the need to temper optimism about treatment success; and (iv) apprehension, benefits and preferences regarding multi-cycle planning.
LIMITATIONS, REASONS FOR CAUTION
Most patients were women from private fertility clinics with no previous treatment experience recruited from social media websites, mainly associated with patient support groups. Similarly, most HCPs were women from private fertility clinics.
WIDER IMPLICATIONS OF THE FINDINGS
The findings suggest that shifting from cycle-by-cycle to multi-cycle approaches in IVF planning is possible. Achieving this shift, like other shifts in IVF (e.g. single embryo transfer), is likely to require collaboration among all stakeholders (e.g. users, staff, policymakers, regulators) to ensure that costs and benefits are balanced through using appropriate benchmarks, avoiding deflating optimism, fostering a sense of collaboration and supporting patients through challenges of multi-cycle IVF.
STUDY FUNDING/COMPETING INTEREST(S)
This research is funded by an Investigator-Sponsor Noninterventional Study from Merck Serono Ltd (MS200059_0010), an affiliate of Merck KGaA, Darmstadt, Germany. ‘Merck KGaA, Darmstadt, Germany reviewed the manuscript for medical accuracy only before journal submission. The Authors are fully responsible for the content of this manuscript, and the views and opinions described in the publication reflect solely those of the authors’. Prof. J.B. reports personal fees from Merck KGaA, Darmstadt, Germany, Merck AB an affiliate of Merck KGaA, Darmstadt Germany, Theramex, Ferring Pharmaceuticals A/S, grant from Merck Serono Ltd, outside the submitted work and that she is co-developer of Fertility Quality of Life (FertiQoL) and MediEmo app. Dr S.G. reports consultancy fees from Ferring Pharmaceuticals A/S, Access Fertility and SONA-Pharm LLC, and grants from Merck Serono Ltd, an affiliate of Merck KGaA, Darmstadt, Germany. Dr C.H. declares no conflicts of interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: infertility, psychology, IVF/ICSI planning, healthcare professional, patient, qualitative
Introduction
According to the Human Fertilisation and Embryology Authority (HFEA), for patients undergoing IVF using their own eggs, one in every four embryos transferred resulted in a live birth in 2018 (Human Fertilisation and Embryology Authority (HFEA), 2020). Consequently, many patients need more than one round of IVF/ICSI stimulation to achieve their parenthood goals and the National Institute for Health and Care Excellence (NICE) guidelines recommend patients are offered up to three complete cycles (National Institute for Health and Care Excellence (NICE), 2013). Patients start treatment with strong intentions to do the cycles they need to achieve pregnancy and around 50% do three complete cycles (Gameiro et al., 2013; McLernon, 2016). Despite this, current clinical practice tends to employ single cycle success rates to plan a course of treatment with patients on a cycle-by-cycle basis (McLernon, 2016). By cycle-by-cycle is meant planning with patients one stimulated cycle of IVF/ICSI at a time, with decisions about doing more cycles only happening at the end of each failed cycle. By multi-cycle is meant acknowledging the possibility of cycle failure and benefits of undergoing multiple cycles to maximize chances of success, and planning from the start to do multiple cycles. Multi-cycle treatment planning implies anticipating decisions that may need to be made across the course of treatment and preparing for typical challenges patients may face when undergoing multiple cycles and which may undermine their initial intentions, the most difficult of these being the experience of an unsuccessful cycle and associated negative emotional reactions (e.g. Smeenk et al., 2004; Gameiro et al., 2012; Domar et al., 2018).
Previous research based on the Theory of Planned Behaviour (Ajzen 1991) and the health belief model (Rosenstock, 1974) showed that most patients are willing to plan for multiple cycles prior to treatment engagement and that they perceive this would allow them to better plan for the resources they need to invest (e.g. financial, time), to learn from the cycles they do and would make them more resilient to challenges faced during treatment (Harrison et al., 2021). This is supported by audit studies showing that about 50% of patients continue with treatment after an unsuccessful cycle (Gameiro et al., 2013; McLernon, 2016). Together, this research highlights that reframing treatment to patients as a multi-cycle journey, i.e. changing the way IVF/ICSI treatment is planned with patients to acknowledge the possible need of undergoing multiple cycles and plan for it from the start, is likely aligned to patients’ preferences and may therefore be a requirement for patient-centred care. Psychological theories also support the value of generating realistic intentions about treatment via planning and anticipating how to behave in the face of possible negative outcomes or challenges (e.g. cycle failure, physical side-effects of treatment, anticipatory anxiety), as means to strengthen the link between initial intentions and actual behaviour (Gollwitzer, 1999) and therefore prevent discontinuation. However, pervasiveness of cycle-by-cycle planning suggests there might be challenges in doing so. For instance, patients who feel less able to cope with the challenges of treatment, namely emotional distress and loss of control, physical effects of procedures and affordability, may resist conversations about the possibility of failure (Harrison et al., 2021). Healthcare professionals (HCPs) may also do cycle-by-cycle planning due to fear of being perceived as taking advantage of desperate patients (Thompson, 2005), which is congruent with many patients expressing dissatisfaction about being given false hopes (Peddie et al., 2005). Cycle-by-cycle planning may also reflect weaknesses in fertility care provision, for instance, a neglect of the longer-term implications of treatment (especially emotional ones) and lack of continuity in care (Coulter and Cleary, 2001; van Empel et al., 2010). The aims of the present study were to examine HCPs’ and patients’ perspectives on approaches to treatment planning, to explore the acceptability of a practice shift towards multi-cycle planning and consider how such planning may best be achieved.
Materials and methods
Participants
Eligible HCPs were those employed at a fertility clinic at the time of the study. Eligible patient advocates were individuals employed by patient focused fertility charities with a role to provide access to support and information to people affected by fertility issues. Patients were eligible to participate if they had had a consultation to start a first or repeat stimulated cycle of IVF/ICSI within 8 weeks prior to participating in the focus group, were aged 18 or older, and able to respond in English. The upper age limit of 42 years for women was applied due to the age limit for funded fertility treatment in the UK (Human Fertilisation and Embryology Authority (HFEA), 2019). Patients were excluded if they had been advised to stop IVF/ICSI, had had more than two complete cycles or if their most recent consultation (i.e. within the previous 8 weeks) was for a frozen embryo transfer. Complete cycles were defined as all embryo transfers (including frozen) resulting from one episode of ovarian stimulation. Participants were also excluded if they had undergone IVF/ICSI for pre-implementation genetic diagnosis because of a genetic disorder, fertility preservation, surrogacy or were using donated gametes (egg or sperm). Participating patients (but not HCPs) were offered £20 as a reimbursement of their time.
Materials
HCPs and patient focus groups (3–6 participants, 60–95 min duration) were carried out separately to facilitate the suggested safe environment participants need to share their views beliefs and attitudes (Morgan and Krueger, 1993; Krueger and Casey, 2000; Madriz, 2000). However, for each of the HCP focus groups, where possible due to availability, we invited a patient advocate to attend (see Table I) to represent patient perspectives and stimulate discussions. C.H. (female) facilitated the virtual (Zoom) focus groups with S.G. or J.B. (both female) and video recorded the discussion to aid the transcription process.
Table I.
Focus group composition and participant characteristics.
| Group | Focus group composition | Type of clinic (NHS/Private) | Duration of focus group (mins) | Gender | Age range (M) a | Years trying range (M) b | Number of previous cycles |
|---|---|---|---|---|---|---|---|
| 1 | 4 × HCP (2 × Consultant, 1 × Urologist, 1 × Trainee Consultant) | 2 × Private (Consultants) 1 × NHS (Urologist) 1 × NHS and Private (Trainee Consultant) | 55 | 3 × Female 1 × Male | |||
| 2 | 2 × HCP (2 × Nurse) 1 × Patient Advocate | 1 × Private 1 × NHS | 70 | 3 × Female | |||
| 3 | 3 × HCP (all Counsellors) | 1 × Private (Male) 1 × NHS 1 × NHS and Private | 76 | 2 × Female 1 × Male | |||
| 4 | 3 × HCP (2 × Consultant, 1 × Embryologist) 1 × Patient Advocate | 1 × Private (Consultant) 2 × NHS and Private (Consultant & Embryologist) | 66 | Female | |||
| 5 | 5 × Patient | 4 × Private 1 × NHS | 90 | 5 × Female | 29–36 (33) | 2.20–3.30 (2.86) | 4 × 0 cycles, 1 × 1 cycle |
| 6 | 5 × Patient | 3 × Private 2 × NHS | 95 | 4 × Female 1 × Male | 32–37 (33) | 2.00–4.00 (3.16) | 4 × 0 cycles, 1 × 1 cycle |
HCP, healthcare professional; NHS, National Health Service.
Median age.
Mean time trying in years.
Focus groups employed topic guides consisting of 11–15 questions with informal clarification prompts (Krueger and Casey, 2000). Topic guides were amended as focus groups were carried out with new questions being added to ensure the consolidation of any information obtained that was deemed important but not initially included in the topic guide. The questions for HCPs and patients were the same but wording was adapted for participant group. Topics covered (in order) approach to conducting consultations (e.g. who took the consultation, format as individual or group session, duration, what was said), understanding of complete cycle and multiple cycles of treatment, approach to talking about treatment success and possible failure, possible need for multiple cycles, treatment intentions after the experience of an unsuccessful cycle and perceptions and preferences for planning consultations if planning IVF/ICSI as a multi-cycle treatment endeavour were adopted.
Procedure
Professional societies in the UK (British Fertility Society (BFS), British Infertility Counselling Association (BICA)) were contacted (via chairs) with information about the study and members asked to contact the research team if interested. Patients were recruited via Facebook and Instagram with the assistance of patient charities (e.g. Fertility Network UK) and social influencers, with adverts asking people to e-mail the researchers if they were interested. C.H. screened people for eligibility, contacted them via email with more information about the study and a consent form, with those returning the consent allocated to a focus group based on their availability. Each focus group started with more information about the aims of study, procedure for the focus group, a set of ground rules (e.g. confidentiality, feeling free to express opinions even if they differed from others, no right or wrong answers) and reminded of video recording, as per consent. The procedure was the same for HCPs and patients. The ethics committee at the School of Psychology, Cardiff University provided ethical review and approval for the study (EC.20.03.10.5992).
Data management and analysis
Each focus group video was transcribed verbatim and imported into NVivo version 12 (QSR International, 1999) software for analysis of qualitative research. Data were analysed using framework analysis (Ritchie and Lewis, 2003). Framework analysis is a matrix-based technique that allows for the organization of data in meaningful themes and categories while additionally allowing for the examination of shared, unique and incongruent thematic content across participant groups using five interconnected stages: familiarization, coding, indexing, charting, mapping and interpretation (Ritchie and Lewis, 2003; Gale et al., 2013). Briefly, C.H. transcribed focus group discussions, thoroughly read and re-read each transcript and listened back to the audio-recorded interviews (familiarization). Using inductive coding C.H. coded meaningful segments of the text (coding) and researchers (C.H., S.G. and J.B.) met on several occasions to discuss the codes and achieve agreement on interpretation. The final set of codes was applied to all the transcripts (indexing). A data sheet was then used to generate a matrix and the data were ‘charted’ into the matrix and summarized by code (row), and participant group (e.g. consultants, nurses; column) using illustrative quotations (charting). Finally, textual data analysis was presented as a summary accompanied by a thematic map and illustrative verbatim quotations (mapping and interpretation). Within illustrative quotations, the use of […] indicated part of the quotation was not presented because it was not relevant, whereas (text) indicated additional text added for clarity (i.e. reliability, comprehensibility). Grammatical errors were corrected. Participant number was indicated with P.
Results
Recruitment outcome
Six focus groups were conducted. Four focus groups with consultants, nurses, patient advocates and counsellors were held during COVID-19 lockdown (April 2020) and two with patients after the clinics had re-opened during July and August 2020. Table I shows the total number of HCPs and patients participating in the six focus groups, the composition of the groups and participant characteristics. All patients were childless and in heterosexual relationships.
Themes
Framework analysis produced a matrix consisting of 52 codes, abstracted into 17 categories, synthesized into four themes and one meta-theme as shown in Fig. 1. Framework analysis showed all themes and categories of codes were present among HCPs and patients apart from the categories ‘IVF prioritises the female patient’ and ‘IVF treatment failure is the norm’, which were unique to patients. Although most coding applied to both participant groups, perceptions and nuance of planning consultations differed between HCPs and patients as highlighted in the textual description below.
Figure 1.
Thematic map showing the meta-theme, main themes and categories of codes generated from framework analysis.
Meta-theme
Cycle-by-cycle planning is the norm
The meta-theme identified that planning treatment on a cycle-by-cycle basis was the norm for planning treatment with patients. Using single cycle success rates to guide consultations was perceived to be easier than using cumulative rates. Cycle-by-cycle planning was perceived by HCPs to circumvent the discussion of the financial implications of treatment and the high likelihood of treatment failure:
‘Some people just want it to work the first time because they only have one funded cycle.’ P9, Counsellor
‘It feels too uncomfortable to start those discussions.’ P13, Trainee consultant
According to patients, one reason cycle-by-cycle planning could be preferred is to stay in the present:
‘I prefer the focus to be on that one cycle […] the cycle we are currently undergoing.’ P24, Patient
The cycle-by-cycle norm was apparent in every theme identified from the focus groups as presented next.
Main themes
Heterogeneity in information provision during treatment planning
This theme identified that the phase (prior to or after engagement with clinic) and stage (e.g. initial or repeat consultation) of treatment and sources of information (e.g. staff, clinic, media, social network) were seen to determine how treatment was communicated to and understood by patients, who wanted comprehensive, trustworthy and timely information.
Prior to engagement with specialized fertility care, patients reported their understanding of IVF to be as per what they viewed to be the societal misconception of IVF, namely that undergoing IVF would result in having a baby:
‘Outside of IVF world, there is an assumption that IVF is a ticket to having a baby and if you do it, it happens.’ P22, Patient
Once patients had direct involvement in the clinic process, their understanding of IVF was shaped by each individual clinic’s approach to information provision (e.g. what information, using what source). This resulted in heterogeneity in both HCPs and patients’ reports of what, how and when information was provided when planning IVF/ICSI treatment:
‘We discuss their individual treatment […]. What scans or needs come in, what other appointments, all the medication, the consents, all the other information around their screening, their bloods, etc.’ P5, Nurse
Although there was heterogeneity between clinics in terms of what information was provided, HCPs appeared to have designated roles in information provision. As expected, consultants reported informing on medical aspects of treatment, but counsellors provided information and support about both medical and the emotional aspects of treatment, with nurses reporting tailoring information to circumstance and patient needs:
‘Making sure that they (patients) get given the right information at the right time.’ P5, Nurse.
HCPs and patients’ views on amount and quality of information provision were incongruent. For example, while HCPs reported providing comprehensive and thorough information about treatment to patients, patients perceived gaps in information provision. Patients reported not being fully informed about all aspects of treatment (e.g. financial implications, support available) and needing to depend on other information sources (e.g. social media) to fill knowledge gaps prior to treatment. Patients reported this independent research to be very difficult because the reliability of the information is unknown. Consequently, patients indicated that the level of information provided by the clinics and individual HCPs could be improved to deter them from having to do their own independent research. However, there was concern that too much information from the clinic or HCPs could be overwhelming, particularly if provided during their initial 1-h planning consultation:
‘Any more information and guidance I think would always be appreciated because you don't really know how much of what's on the internet is factual.’ P19, Patient
‘If I’d had too much at the start, I wouldn’t have really known what to do with it.’ P22, Patient
The need for improved HCP-patient collaboration
This theme identified that collaboration between HCPs and patients could be strengthened. Patients reported they did not feel a sense of collaboration with HCPs, particularly consultants, when it came to treatment planning and continuation. First, patients described a sense of disappointment with their consultations reporting them to feel ‘depersonalised’ (P23, Patient) and ‘transactional’ (P23, 24, 22, Patients) with too little focus given on the psychological impact of infertility and previous treatment experiences:
‘They didn't acknowledge the first (cycle) had failed when we went for our second one. He was very much okay, your kind of the train off you go and there wasn't any, ah, you know, are you guys, okay? How are you finding it? It must be difficult coming back a second time. It wasn’t like that, it was very much like carry on like nothing had happened.’ P22, Patient
Second, the bulk of conversations, documentation and appointments were seen to be directed towards the woman. This resulted in patients perceiving HCPs to see partners as providing a supportive role rather than being considered as patients going through the same treatment journey. This orientation was reported to make partners (in these examples men) feel ‘helpless’ (P24, Patient) and side-lined from the treatment process. This perceived lack of partner integration was reported to be exacerbated by the COVID-19 pandemic due to the restrictions it placed on the partner:
‘My husband definitely felt […] a bit like a spare part because all the documentation is patient and partner.’ P22, Patient
Third, counsellors suggested that consultations could be perceived as paternalistic by patients. HCPs responsible for planning consultations were reported to exercise their professional status to give patients little room for involvement in the treatment decision-making process or options at different points in treatment (including after failure). Patient advocates concurred, reporting patients too often feel the HCP-patient power imbalance and intimidated when starting treatment for the first time. While this power imbalance was also experienced by patients, patients reported subscribing and placing trust in their consultants’ expertise:
‘It's pretty much what I was told. This is the best option for us and just kind of went with it. I've just counted on their medical expertise […] For me it was pretty much believing what they had said and going with it.’ P19, Patient
Finally, consultants and nurses reported providing patients with the information necessary for informed treatment decision-making about the different treatment outcomes that could be experienced (e.g. failed fertilization, poor response to medication), but patients often reported not being informed about these outcomes until they occurred and that discussion of these prior to treatment was discouraged:
‘I mentioned […] are we looking at that (donor eggs)? And the response was no, no, you're really young, […] (we don’t) need to look at that (option) yet.’ P15, Patient
The need to temper optimism about treatment success
This theme indicated participants to concur that tempering optimism about treatment success should be a priority in planning consultations. Participants perceived tempering could be achieved by discussing the possibility of treatment failure and the subsequent possible need for multiple cycles prior to treatment engagement. However, participants perceived this to be a delicate and complex matter requiring balancing realities, hopes and resources (mental, financial).
All participants were aware of the high possibility of treatment failure and HCPs reported there was much strategizing in bringing up the topic directly with patients. HCPs reported setting treatment expectations with patients using single cycle success rates and reference data (e.g. HFEA data, clinic data), but in addition, trying to anticipate how each patient will react to the possibility of failure in deciding about how to communicate this:
‘To gauge how they're going to respond to the information that you're giving them because we have limited access to their personas and where they have been, the journey they have been on.’ P5, Nurse
HCPs reported being more likely to comprehensively discuss the possibility of failure and the need for multiple cycles when patients had a poor prognosis (i.e. based on age, anti-Mullerian hormone, history of treatment failure) or had financial means (i.e. due to having sufficient funds or National Health Service (NHS) funding or financial package). The reasoning in these cases was that such patients were mentally and financially prepared for, or able to undergo, multiple cycles. In contrast, HCPs and patients reported that for good prognosis patients with no previous treatment experience these topics tended to be discussed superficially or indirectly, in terms of treatment funding, or discussed only if initiated by the patient. The reasoning (among HCPs) for these cases was that patients would have a limited capacity to receive negative or realistic information in combination with good chances of success:
‘Psychologically I think they're just prepared differently if their Clinical Commissioning Groups offers them two (cycles) […] there's a message that the first one might not work, (otherwise) why would they offer two if the first one's going to work.’ P9, Counsellor
When failure-related information was provided it was reported to be provided in small doses towards the end of the appointment, so that patients could consider it in their own time. For example, nurses described information provision to be a process of ‘sowing the seeds’ (P4, Nurse) and ‘drip feed(ing)’ (P5, Nurse). The hesitancy in discussing topics of failure and multiple cycles was very much linked to HCPs not wanting to be perceived as unsupportive or discouraging of treatment engagement:
‘I don't want to use that initial consultation to become very negative saying […] there's a higher probability that you're not going to get pregnant in the first cycle […] you're doomed before you start […].’ P1, Consultant
Patients, in contrast, were found to have mixed views on maintaining optimism. On the one hand, patients reported a desire to be proactive in constructing their own personal treatment plans and preparing for the realities of treatment including failure. On the other hand, most patients perceived hope and optimism to be important, and that too much negativity would not be appropriate during an initial consultation:
‘You want to be realistic, and you want to be told transparently, this are […] the risks and these are the benefits […] if this doesn't work, this is what's going to happen, but you don't want to be negative either.’ P15, Patient
Apprehension, benefits and preferences regarding multi-cycle planning
This theme indicated a general apprehension among HCPs and patients towards clinics adopting a multi-cycle approach to planning IVF. However, participants also reported benefits of shifting to multi-cycle planning and could readily provide descriptions of what they would like if multi-cycle planning were to become the norm.
First, HCPs expressed apprehension concerning their ability to plan for multiple cycles prior to the patient having engaged in treatment because they rely on the knowledge gained from an unsuccessful cycle to plan future cycles. Consequently, HCPs referred to the first cycle of treatment as ‘a test cycle’ (P4, Nurse), a ‘learning cycle’ (P10, Consultant), a ‘trial and error thing’ (P9, Counsellor):
‘You don’t know how (the patient is) going to respond until they’ve been through that first cycle of treatment.’ P4, Nurse
Secondly, participants were concerned that planning for the possibility of multiple cycles meant that patients would have to commit financially to engage in at least three cycles of treatment at a given clinic, like buying fertility treatment packages. This in turn elicited a concern that multi-cycle planning could exploit patients, for example taking away their freedom to choose to move clinics after an unsuccessful cycle, or just be inappropriate for some patients (low prognosis, no ability to fund multiple cycles):
‘If you've got a success rate of 5% or less, we don't feel that it's appropriate to […] (take) people's money […] (when) it's not going to work for them.’ P1, Consultant
‘It’s very hard to talk to some people about maybe needing three or four (cycles), because there's no way they're going to be able to afford them.’ P7, Counsellor
Thirdly, planning for multiple cycles prior to treatment engagement was perceived to deflate patient optimism:
‘Those patients come to us with an element of hope, and you can't squash that at the first appointment.’ P5, Nurse
Despite these misgivings, consultants, nurses and patients did see benefits in fertility financial packages. HCPs reported multi-cycle packages to reduce patient anxiety, help manage treatment expectations and enable patients to relax because they have paid for multiple cycles of treatment. These benefits were reported to be the result of having been forewarned about treatment, the likelihood that success would involve more than one cycle and having financially planned for multiple cycles of treatment prior to treatment engagement:
‘People who actually pay for a multiple cycle package from the beginning, start their whole journey far less anxious.’ P10, Consultant
HCPs, patient advocates and patients were able to describe what they would like included in multi-cycle consultations, if they were to become the norm, and to make suggestions of how to deliver such consultations. Table II amalgamates these (explicit and implicit) suggestions, with illustrative quotes, into three sections: forewarning, collaboration and support, and perseverance with personal plans. Patients and HCPs expressed a preference for a more comprehensive approach to treatment planning and saw benefits in discussing the likelihood of success and failure with patients. They also made suggestions about how to approach this discussion, for example introducing the possibility of needing multiple cycles earlier on in the treatment journey, specifically, patient advocates suggested that general practitioners (GPs) could initiate these conversations. This early preparation was suggested by both patients and HCPs to help patients prepare for the realities of treatment while also maintaining a sense of treatment optimism:
Table II.
Suggestions of information to include in multi-cycle planning consultations with illustrative quotes.
| Components of consultation | What information to include and how to deliver it | Illustrative quotes |
|---|---|---|
| Forewarning | ||
| Expectations to be managed from the very start |
|
‘Success rates based on their own circumstances. So their age her AMH BMIs etc’. P5, Nurse |
| Ways to discuss failure with patients |
|
|
| Ways to improve information provision |
|
‘More information and guidance would always be appreciated because you don't really know how much of what's on the internet is factual’. P19, Patient |
| Collaboration and support | ||
| Ways to boost collaboration and emotional support |
|
|
| Perseverance with personal treatment plans | ||
| Ways to boost perseverance with treatment plan |
|
|
AMH, anti-Mullerian hormone; BICA, British Infertility Counselling Association; HCP, healthcare professional.
‘You need to be realistic and truthful and transparent. But you also need to be using that element of hope that they have.’ P4, Nurse
Patients also reported that more discussions about the possible outcomes of treatment that could lead to treatment failure would help stimulate a sense of support and collaboration from the clinic in addition to reducing the self-blame patients can experience when this happens. Patients with prior experience of treatment failure reported that knowing failure was the norm was reassuring and should therefore be made more evident to patients prior to treatment engagement. Consultants also reported that multi-cycle consultations should include tools and resources (e.g. flowchart, coping skills) to prepare and equip patients for all the different elements of treatment (e.g. medical financial, emotional):
‘When I had the call, my consultation, with my consultant and the nurse, I felt instantly better and positive about it because I was told, you know, it's okay and this happens sometimes, and this is what we will do to try and rectify it. So that was beneficial […] One of the things she said that really reassured us was that, you know, this is the norm. You are the norm. You know, some people are very, very lucky, um, but not everyone is that lucky.’ P15, Patient
Discussion
The central finding of this study is that planning treatment cycle-by-cycle is regarded as the known, clinically and emotionally safe way to plan IVF treatment but there is potential to move to a multi-cycle approach. Participants indicated that HCPs implementing multi-cycle planning consultations should aim to provide comprehensive and trustworthy information and foster HCP-patient collaboration to help patients form realistic treatment expectations. This should, however, be provided while also being considerate of individual patient treatment journeys. We propose that if these suggestions were adopted then multi-cycle planning would be more acceptable and feasible to implement, potentially eventually becoming the norm.
Ambivalence surrounded the idea of moving from a cycle-by-cycle to multi-cycle norm. HCPs expressed apprehension about shifting the cycle-by-cycle norm, because of the risks of doing so, e.g. deflating optimism, confronting financial realities. These appear to be key barriers for staff but not for patients or patient advocates. Despite HCPs apprehension, all participants perceived multi-cycle planning as beneficial and could readily provide suggestions about desirable content for a multi-cycle consultation. Namely, to prepare for the worst but expect the best, and to engender a sense of collaboration and support throughout the subsequent treatment journey. Results suggest that a shift towards multi-cycle planning is possible and desirable, but the content of consultations needs to be crafted to fully target patients’ needs and HCPs should be supported in managing its implementation, much as has been the case with other shifts in practice (e.g. single embryo transfers). Future research needs to develop and evaluate single versus multi-cycle planning approaches and their impacts on valued outcomes (e.g. live birth rates, patient quality of life).
Our results suggest that to make the transition from cycle-by-cycle to multi-cycle planning three components seem to be especially valued by HCPs and patients: forewarning and expectation management, collaboration between patient and clinic and support through challenges. These concepts sit well with psychological theories concerned with forming intentions, implementing intentions to achieve goals and coping with stress (Rosenstock, 1974; Lazarus and Folkman, 1984; Ajzen, 1991; Gollwitzer, 1999). These concepts suggest that what patients look for in creating a ‘personalized treatment plan’ is to form a realistic understanding of what treatment truly entails and then be provided with assurance of support to manage the demands they will encounter as they carry out their plan. Research does suggest that all these concepts are important and that supporting patients in this way is likely to help sustain quality of life during treatment (Gameiro et al., 2015), as well as perseverance with personal plans (Gameiro et al., 2012; Huppelschoten et al., 2013; Pedro et al., 2013). The implication for clinical practice is that patients need time to discuss treatment plans with their HCPs and comprehensive and trustworthy resources to plan in advance about how to cope with events in treatment (e.g. cycle failure), instead of being offered support after events have occurred, as has traditionally been the focus of patient support. We collated suggestions from patients and HCPs on how to approach such discussions, including what information to include in a planning consultation and how to deliver it. Clinics can consider how they can integrate these suggestions within their own culture of patient communication to ensure patients do not need to search for external information sources. Future research needs to consider what associated resources should be, but from the present focus groups, the requirements seem to be that these should be accessible before the start of treatment, tailored and with a positive but realistic outlook.
The theme, the need to temper optimism about treatment success, indicates setting up expectations and the discussion of possible failure to be effortful for HCPs as it potentially involves tailoring (e.g. to patient prognosis, emotional capacity, finances), intuitive psychological profiling, the judicious use of the nuanced language of hope and sensitive forewarning. However, results suggest that doctors need not be as apprehensive because patients express a desire to be more informed about the possibility of failure prior to treatment engagement which suggests patients may be much more resilient and open to discussions than previously assumed. This willingness is consistent with recent quantitative research showing patients are willing to plan for the possibility of doing multiple cycles of treatment and that perceived benefits are mostly related with increased perceptions of being able to do what it takes to achieve their goal of parenthood (Harrison et al., 2021). This is in line with psychological conceptualizations of hope as not only believing that a desired outcome will happen but also perceiving one knows what to do to make that desired outcome happen and is able to motivate oneself to do so (Snyder, 2002). Overall, these results suggest that the language of hope used during planning consultations does not need to focus exclusively on the chances of treatment success but on reassuring patients they can undergo treatment and the team is there to support them through it. While approaching treatment planning in this way may be challenging for HCPs, psychological theories could help to address the complexity of expectation and uncertainty management exchange (Han et al., 2019).
Given the apprehension that surrounded the shift to multi-cycle planning and its association with patient exploitation, it could be argued that multi-cycle planning is not suitable for patients who cannot or do not want to do multiple cycles. However, as we have previously argued, considering the need for multiple cycles is essential for informed consent (Gameiro et al., 2013). Nonetheless, to be patient-centred, the personal circumstances of each patient should be considered in how consultations are structured so that patients’ reproductive autonomy is not compromised. For patients planning to do one cycle, due to financial constraints or personal preferences, this could mean reassuring them from the start that, even when treatment is unsuccessful, most patients go on to live hopeful and fulfilling lives, and signposting them to adequate support resources in case of failure (e.g. www.myjourney.pt). Ultimately, even doing all planned treatment, around 40% of people will not achieve pregnancy (McLernon, 2016). Research suggests that having acted consistently with plans can be protective when deciding to end treatment or when facing unsuccessful treatment, as it can provide patients with a sense of having done all they could to achieve parenthood. This is proposed to reduce the possibility of experiencing decisional conflict and regret (Gameiro and Finnigan, 2017). Overall, all conversations about planning, regardless of the approach taken, need to be carefully and sensitively handled so that discussions about treatment engagement are not interpreted as exploitation or interfering with patients’ autonomy.
The theme heterogeneity in information provision during treatment planning also highlights that patients and HCPs do not always communicate effectively. There were several instances of HCPs perceiving that they had provided required information or support but that patients had not; or vice versa. While these findings were specific to how a course of fertility treatment is planned and communicated to patients, they could reflect a broader patient–staff communication gap in fertility clinics. Dissatisfying communications have been reported previously, as have been the strain they can cause on staff (Boivin et al., 2017) and patients, even affecting their motivation to comply with treatment recommendations (Pedro et al., 2013). Future communication research needs to unpack what are the communication issues and how best to address them. There is a fair amount of bad news to be delivered in clinics, and indeed preparing patients for the possibility of failure could be considered as such. Multiple research studies show HCPs feel less stressed in communicating bad news when they are trained to do so according to a pre-defined protocol (Simpson and Bor, 2001). If a sense of collaboration is to be established between clinics and patients, adequate communication skills from all staff members that have direct contact with patients need to be ensured, as negative interactions occur even with office personnel (Dancet et al., 2010).
Strengths and limitations
Most patients were childless women from private fertility clinics with no previous treatment experience recruited from social media websites, associated with patient support groups. These results could mean people attracted to the study may be more motivated to do multiple cycles of treatment, have fewer economic stresses and thus the financial means to engage in multiple cycles of treatment. However, it should be noted that the inclusion criteria and recruitment methods were similar to those used in a preceding study (Harrison et al., 2021), where 44% of participants expressed some form of ambivalence about multi-cycle planning. Similarly, most HCPs were women from private fertility clinics. Informative comparisons across treatment stage, gender and funding source were not possible and it may be that the views expressed may reflect the profile of participants. Moreover, the views expressed may reflect beliefs rather than experiences of what happens during planning consultations. For example, counsellors and embryologists are not typically involved in planning treatment with patients and therefore their views in the present study represent their perceptions of what might be occurring when consultants and nurses consult with their patients and not what actually occurs. Overall, it is reassuring to note that a plurality of views was identified during the focus groups and many participants expressed concerns and reservations. Indeed, the qualitative nature of the study and the fact that HCPs and patients participated in focus groups separately, provided a safe environment and opportunity for them to voice their experiences and opinions in their own words and without regard to the views of other groups. Taken together these results offer reassurance that the data provide a comprehensive picture of the main views of patients and HCPs towards multi-cycle planning.
However, the results do suggest that having both patient and HCPs together would add value because often they did not appear to have consensus or detailed understanding of the issues that each faced. Framework analysis was appropriate for the aims of the focus groups because it allowed the pre-determined themes in the focus group guide to be explored while also providing the opportunity for new themes to emerge. Framework analysis additionally allowed examination of shared, unique and incongruent thematic content across participant groups.
Conclusion
Treatment for IVF/ICSI is dominated by a reliance on planning treatment cycle-by-cycle but patients and HCPs positively view planning treatment on a multi-cycle basis provided misgivings could be addressed. This could be achieved by providing HCPs and patients with information, resources and/or training to: (i) support HCPs to deliver multi-cycle consultations forewarning patients about the possibility of needing more than one complete cycle of treatment and inviting them to formulate a treatment plan that acknowledges this; (ii) provide patients with a sense of collaboration and support during treatment; and (iii) support patients to persevere with their treatment plan even when they face treatment challenges. We present result-informed suggestions on how to approach these three components of multiple-cycle planning. A cultural shift in IVF/ICSI planning would allow for the positives of multi-cycle planning to be adopted by all those involved in the fertility sector (regardless of funding eligibility or status) and would better mirror the reality of the IVF treatment process for many patients.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Acknowledgements
Thank you to Fertility Network UK, Fair Treatment for the Women of Wales and all the fertility clinics and social influencers for helping advertise the study.
Authors’ roles
C.H., S.G. and J.B. contributed to the conception and design of the study, the acquisition of data and the analysis and interpretation of data. They drafted all versions of the article and approved the final version for publication.
Funding
This research was financially supported by Merck Serono Ltd, an affiliate of Merck KGaA, Darmstadt, Germany. ‘Merck KGaA, Darmstadt, Germany reviewed the manuscript for medical accuracy only before journal submission. The Authors are fully responsible for the content of this manuscript, and the views and opinions described in the publication reflect solely those of the authors’.
Conflict of interest
Prof. J.B. reports personal fees from Merck KGaA, Darmstadt, Germany, Merck AB an affiliate of Merck KGaA, Darmstadt Germany, Theramex, Ferring Pharmaceuticals A/S, grant from Merck Serono Ltd, outside the submitted work and that she is co-developer of Fertility Quality of Life (FertiQoL) and MediEmo app. Dr S.G. reports consultancy fees from Ferring Pharmaceuticals A/S, Access Fertility and SONA-Pharm LLC, and grants from Merck Serono Ltd, an affiliate of Merck KGaA, Darmstadt, Germany. Dr C.H. declares no conflicts of interest.
References
- Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Processes 1991;50:179–211. [Google Scholar]
- Boivin J, Bunting L, Koert E, Ieng U C, Verhaak C.. Perceived challenges of working in a fertility clinic: a qualitative analysis of work stressors and difficulties working with patients. Hum Reprod 2017;32:403–408. [DOI] [PubMed] [Google Scholar]
- Coulter A, Cleary PD.. Patients’ experiences with hospital care in five countries. Health Aff (Millwood) 2001;20:244–252. [DOI] [PubMed] [Google Scholar]
- Dancet EA, Nelen WL, Sermeus W, De Leeuw L, Kremer JA, D'Hooghe TM.. The patients’ perspective on fertility care: a systematic review. Hum Reprod Update 2010;16:467–487. [DOI] [PubMed] [Google Scholar]
- Domar AD, Rooney K, Hacker MR, Sakkas D, Dodge LE.. Burden of care is the primary reason why insured women terminate in vitro fertilization treatment. Fertil Steril 2018;109:1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S.. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gameiro S, Boivin J, Dancet E, De Klerk C, Emery M, Lewis-Jones C, Thorn P, Van Den Broeck U, Venetis C, Verhaak CM. et al. ESHRE guideline: routine psychosocial care in infertility and medically assisted reproduction-a guide for fertility staff. Hum Reprod 2015;30:2476–2485. [DOI] [PubMed] [Google Scholar]
- Gameiro S, Boivin J, Peronace L, Verhaak C.. Why do patients discontinue fertility treatment? A systematic review of reasons and predictors of discontinuation in fertility treatment. Hum Reprod Update 2012;18:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gameiro S, Finnigan A.. Long-term adjustment to unmet parenthood goals following ART: a systematic review and meta-analysis. Hum Reprod Update 2017;23:322–337. [DOI] [PubMed] [Google Scholar]
- Gameiro S, Verhaak CM, Kremer JA, Boivin J.. Why we should talk about compliance with assisted reproductive technologies (ART): a systematic review and meta-analysis of ART compliance rates. Hum Reprod Update 2013;19:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollwitzer PM. Implementation intentions: strong effects of simple plans. Am Psychol 1999;54:493–503. [Google Scholar]
- Han PK, Babrow A, Hillen MA, Gulbrandsen P, Smets EM, Ofstad EH.. Uncertainty in health care: towards a more systematic program of research. Patient Educ Couns 2019;102:1756–1766. [DOI] [PubMed] [Google Scholar]
- Harrison C, Gameiro S, Boivin B.. Patient willingness, preferences and decision-making about planning for three complete cycles of IVF/ICSI treatment. Hum Reprod 2021;36:1339–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Fertilisation and Embryology Authority (HFEA). Commissioning Guidance for Fertility Treatment (2019). London: Human Fertilisation and Embryology Authority, 2019.
- Human Fertilisation and Embryology Authority (HFEA). Fertility Treatment 2018: Trends and Figures. London: Human Fertilisation and Embryology Authority, 2020.
- Huppelschoten AG, Van Dongen AJCM, Philipse ICP, Hamilton CJCM, Verhaak CM, Nelen WLDM, Kremer JAM.. Predicting dropout in fertility care: a longitudinal study on patient-centredness. Hum Reprod 2013;28:2177–2186. [DOI] [PubMed] [Google Scholar]
- Krueger R, Casey M.. Focus Groups: A Practical Guide for Applied Research, 3rd edn. Newbury Park, CA: Sage, 2000. [Google Scholar]
- Lazarus RS, Folkman S.. Stress, Appraisal, and Coping. New York, NY: Springer Publishing Company, 1984. [Google Scholar]
- Madriz E. Focus groups in feminist research. In: Denzin NK, Lincoln YS (eds). Handbook of Qualitative Research. Thousand Oaks, CA: Sage, (3rd ed). 2000, 835–850. [Google Scholar]
- McLernon DJ. Predicting the chances of live birth after one or more complete cycles of in vitro fertilisation: population based study of linked cycle data from 113873 women. BMJ 2016;355:i5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL, Krueger RA.. When to use focus groups and why. In: Successful Focus Groups: Advancing the State of the Art. Newsbury Park, CA: Sage, Vol 1. 1993, 3–19. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Fertility Problems: Assessment and 554 Treatment. UK: NICE Clinical Guideline CG156, 2013. [PubMed]
- Peddie VL, van Teijlingen E, Bhattacharya S.. A qualitative study of women's decision-making at the end of IVF treatment. Hum Reprod 2005;20:1944–1951. [DOI] [PubMed] [Google Scholar]
- Pedro J, Canavarro MC, Boivin J, Gameiro S.. Positive experiences of patient-centred care are associated with intentions to comply with fertility treatment: findings from the validation of the Portuguese version of the PCQ-infertility tool. Hum Reprod 2013;28:2462–2472. [DOI] [PubMed] [Google Scholar]
- QSR International. NVivo Qualitative Data Analysis Software [Software]. 1999. https://qsrinternational.com/nvivo/nvivo-products/ (22 September 2021, date last accessed).
- Ritchie J, Lewis J.. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage, 2003. [Google Scholar]
- Rosenstock IM. The health belief model and preventive health behavior. Health Educ Monogr 1974;2:354–386. [Google Scholar]
- Simpson R, Bor R.. ‘I’m not picking up a heart-beat’: experiences of sonographers giving bad news to men during ultrasound scans. Brit J Med Psycho 2001;74:255–272. [PubMed] [Google Scholar]
- Smeenk J, Verhaak C, Stolwijk A, Kremer J, Braat D.. Reasons for dropout in an in vitro fertilization/intracytoplasmic sperm injection program. Fertil Steril 2004;81:262–268. [DOI] [PubMed] [Google Scholar]
- Snyder CR. Hope theory: rainbows in the mind. Psychol Inq 2002;13:249–275. [Google Scholar]
- Thompson C. Making Parents: The Ontological Choreography of Reproductive Technologies. Cambridge, MA: MIT Press, 2005. [Google Scholar]
- van Empel IWH, Nelen WLDM, Tepe ET, van Laarhoven EAP, Verhaak CM, Kremer JAM.. Weaknesses, strengths and needs in fertility care according to patients. Hum Reprod 2010;25:142–149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.