Abstract
Despite strong epidemiological evidence supporting an important role for Campylobacter upsaliensis as a human enteropathogen, it remains relatively unknown in the realm of clinical microbiology. Clinical studies indicate that infection with this organism usually is associated with benign self-limiting diarrhea. However, more serious illnesses, including spontaneous abortion and hemolytic-uremic syndrome, recently have been associated with human infections. Understanding of the virulence properties and molecular biology of C. upsaliensis is beginning to evolve. There is now a pressing need for controlled, prospective epidemiologic studies in addition to further in-depth investigation of the pathogenesis of this enteric campylobacter to more precisely define its role in human disease. Furthermore, since C. upsaliensis is sensitive to the antibiotics routinely used in Campylobacter selective media, widespread appreciation of the importance of this organism will rely on the development of widely applicable, effective techniques for its isolation.
The identification of novel pathogens is a perennial concern among microbiologists and others interested in the study of infectious diseases. In recent years, many novel gastrointestinal pathogens have come to attention. Some, such as enterohemorrhagic Escherichia coli (50), Cryptosporidium parvum (22), and Helicobacter pylori (8), have achieved widespread and fairly rapid recognition. The contribution of other microbes to human enteric diseases has been greeted with less certainty. Campylobacter upsaliensis is an organism which, despite consistently convincing epidemiological evidence supporting its role as a human enteric pathogen, languishes in the latter category. C. upsaliensis is rarely isolated in clinical laboratories and therefore is little known among clinicians. This underrecognition is due, at least in part, to the fact that C. upsaliensis is sensitive to the antibiotics routinely used in Campylobacter selective media. Heightened awareness and improved isolation techniques will undoubtedly yield higher C. upsaliensis isolation rates and help to bring this enteric pathogen to center stage in the realm of human enteric pathogens.
C. UPSALIENSIS AS A HUMAN PATHOGEN
In 1983, Sandstedt et al. (78) reported the presence of a novel catalase-negative Campylobacter sp. isolated frequently from the feces of dogs attending an animal clinic in Uppsala, Sweden. These organisms were originally referred to as the catalase-negative/catalase weak (CNW) group. In the year following their initial description, two reports described the isolation of CNW organisms from canine feces (24, 34). In 1985, Steele et al. (84) provided the first description of CNW organisms in human stools. On the basis of DNA homology studies, these organisms were shown to form a separate Campylobacter species, which was later named Campylobacter upsaliensis after the city in which it was first described (3). Since then, reports have emerged worldwide implicating C. upsaliensis as a human bacterial enteropathogen (23, 41, 55, 58, 61, 70, 88, 93). In fact, a number of investigators (41, 55, 58), have isolated C. upsaliensis from stools more frequently than C. coli, an acknowledged human enteropathogen.
C. upsaliensis is associated with acute self-limiting diarrhea but has also been isolated in the setting of chronic and recurrent diarrhea (39). Weight loss accompanying C. upsaliensis-related diarrhea also has been described (61). Moreover, C. upsaliensis can cause bacteremia in debilitated and immunocompromised patients (21) and has been associated with extraintestinal infections (33), spontaneous human abortion (44), hemolytic-uremic syndrome (17), and Guillain-Barré syndrome (37, 47).
Preliminary investigations into the virulence mechanisms of this organism have appeared only recently (detailed below). Koch’s postulates have not yet been fulfilled for this organism. Nevertheless, existing clinical and epidemiological data offer compelling evidence supporting the importance of C. upsaliensis as a human enteropathogen.
TAXONOMY
DNA hybridization studies performed by Sandstedt et al. (78) on the first reported isolates of C. upsaliensis indicate that they belong to a homogeneous group (80 to 96% intragroup relatedness and 40% relatedness to other thermotolerant Campylobacter spp.). Following this initial report, these organisms were referred to as the CNW group. The first human isolates of this organism (84) were confirmed as C. upsaliensis by using DNA probes which demonstrated their similarity to the CNW organisms originally isolated by Sandstedt et al. (78). In 1991 Sandstedt and Ursing (77) proposed the name Campylobacter upsaliensis for the CNW group; the name was validated in 1991 (3).
The G+C content of C. upsaliensis varies from 32.8 to 35.8 mol% (78, 91). This is somewhat greater than the G+C content of C. jejuni (30 to 32.6 mol%) and C. coli (30.8 to 32.5 mol%) but is similar to that described for other Campylobacter species including C. fetus (33.3 to 34.5 mol%) and C. hyointestinalis (33.6 to 35.2 mol%) (91). DNA-rRNA hybridization and immunotyping techniques (91) confirm the phylogenetic similarity of C. upsaliensis to other members of the Campylobacter genus.
A methyl-substituted menaquinone which appears unique to Campylobacter species is also present in C. upsaliensis (66). This menaquinone species, known as menaquinone-6, is a member of the isoprenoid quinone family present in the plasma membrane of bacteria and functions in electron transport (65).
LABORATORY DETECTION
C. upsaliensis is a microaerophilic, thermotolerant, motile, curved, gram-negative rod. The organism has a single polar or bipolar flagellum and exhibits the darting movements characteristic of Campylobacter spp. under phase-contrast microscopy. It forms smooth, pinpoint, greyish or translucent colonies on blood agar plates. Swarming may be observed when the organism is grown on moist media (77). Growth in broth requires supplementation with sheep blood (70) or fetal calf serum (9). The organisms are 0.3 to 0.4 μm wide and 1.2 to 3 μm long. On exposure to air, coccoid forms may appear.
This bacterium is oxidase positive, nitrate positive, and hippurate negative (71) (Table 1). C. upsaliensis is sensitive to nalidixic acid and, usually, to cephalothin. The presence of these antibiotics in the selective media generally used for the isolation of Campylobacter species (e.g., Skirrow’s medium) may well account for the suboptimal identification of C. upsaliensis in clinical specimens at most centers (4, 41, 70).
TABLE 1.
Phenotypic characteristics of Campylobacter speciesa
| Species | Characteristicb
|
G+C content (mol%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Catalase production | Nitrate reduction | Indoxyl Acetate | Hippurate hydrolysis | Tolerance to nalidixic acid | H2S production on TSI agar | Growth on potato starch | ||
| C. upsaliensis | −/w | + | + | − | − | − | + | 33–36 |
| C. jejuni | +/v | + | + | + | − | − | n | 30–32 |
| C. coli | + | + | + | − | − | v | n | 31–33 |
| C. lari | + | + | − | − | + | − | n | 31–33 |
| C. fetus | + | + | − | − | + | − | n | 33–34 |
| C. hyointestinalis | + | + | − | − | + | + | n | 35–36 |
| C. concisus | − | + | − | − | + | + | n | 38–39 |
| C. mucosalis | − | − | − | − | + | + | n | 38–39 |
| C. sputorum | − | + | − | − | + | + | n | 31–33 |
| C. helveticus | − | + | + | − | − | − | − | 34 |
| C. curvus | − | + | + | − | + | + | n | 43–47 |
| C. rectus | − | + | + | − | + | + | n | 42–46 |
| C. showae | + | + | + | − | − | + | n | 44–46 |
| C. hyoilei | + | + | n | − | − | + | n | 35 |
| C. gracilis | − | + | − | − | + | + | n | 44–46 |
Adapted from reference 10.
Test results: +, positive reaction; −, negative reaction; w, weak reaction; v, variable reaction; n, not known.
Successful isolation of C. upsaliensis from stool specimens currently relies on the use of a filtration method (41, 58, 64). This method enriches Campylobacter-infected fecal specimens and thereby helps increase the yield when grown on solid media (83). This is accomplished by using a filter with a pore size sufficiently large to permit passage of the small campylobacter organisms but small enough to exclude larger fecal contaminants. Goossens et al. (41) found that a filter system with a pore size of 0.45 μm resulted in less contamination than did one with 0.65-μm filters. However, the authors also pointed out that bacterial concentrations of less than 105 CFU per g of feces could not be detected by the filter method. Therefore, a more sensitive detection method (such as a specific genetic probe) might yield even higher isolation rates for C. upsaliensis in clinical specimens.
In addition to a lack of sensitivity, the use of the filtration method is more cumbersome than the use of selective agar media. Therefore, the development of selective media for the successful isolation of C. upsaliensis from clinical samples would be beneficial. Walmsley and Karmali (93) successfully used a selective medium containing cefoperazone (32 μg/ml), vancomycin (20 μg/ml), and cyclohexamide (100 μg/ml) without filtration to isolate C. upsaliensis from the stools of six pediatric patients. However, they did not directly compare the utility of this technique with that of the filtration method.
Aspinall et al. (4, 5) described the use of a new selective medium for the isolation of thermophilic campylobacters. A blood-free medium containing cefoperazone (8 μg/ml) amphotericin (10 μg/ml), and teicoplanin (4 μg/ml) (CAT) was compared with a commercially available Campylobacter selective medium containing cefperazone (32 μg/ml) and amphotericin (10 μg/ml) in a blood-free selective agar base (modified CCDA) and with a filtration method. CCDA and CAT demonstrated comparable isolation rates for campylobacters other than C. upsaliensis. Of significance, the CAT medium correctly isolated 84% of C. upsaliensis isolates from spiked fecal samples (comparable to 90% sensitivity for the filtration method in the same study), while the modified CCDA isolated only 29% of isolates. Further investigations comparing this technique with the filtration method are now required.
C. upsaliensis can be differentiated from C. jejuni by its lack of catalase activity and an inability to hydrolyze hippurate (77). It can be distinguished from C. coli and C. hyoilei by its lack of catalase activity and from C. lari, C. fetus and C. hyointestinalis by its lack of catalase activity and its sensitivity to nalidixic acid (2, 77) (Table 1). C. upsaliensis can be differentiated from the catalase-negative campylobacters (i.e., C. sputorum, C. concisus, C. curvus, C. rectus, and C. mucosalis) by its lack of hydrogen sulfide production on triple sugar iron (71, 77) and from C. gracilis by its positive oxidase test (86). The recently described C. helveticus (81) has many phenotypic and biochemical similarities to C. upsaliensis. Therefore, it may prove difficult to differentiate between these two organisms by conventional biochemical laboratory testing. However, the colony morphology of C. upsaliensis is distinctive (81). Pinpoint, grey colonies are typical of C. upsaliensis, whereas colonies of C. helveticus are flat and smooth with a watery, spreading appearance on blood agar. In addition, C. helveticus can be differentiated from C. upsaliensis by both its inability to reduce selenite and its lack of growth on potato starch medium (81). In conclusion, the most useful biochemical tests for the identification of C. upsaliensis in the clinical microbiology laboratory include those for catalase production, hippurate hydrolysis, nitrate reduction, oxidase activity, H2S production on triple sugar iron agar, and sensitivity to nalidixic acid (77).
A number of investigators have applied molecular techniques to directly identify enteric campylobacters from stools (27, 35, 68). Eyers et al. (27) identified regions of 23S rRNA genes specific for thermophilic Campylobacter strains, including C. upsaliensis. By designing oligonucleotide primers corresponding to these specific 23S rRNA regions, they were able to distinguish thermophilic Campylobacter strains from other fecal microorganisms and also to discriminate between individual Campylobacter species. Because isolation and accurate identification of Campylobacter species from fecal specimens by using standard phenotypic testing is problematic (68), PCR-based assays and other molecular methods soon may become standard identification techniques for these organisms.
Typing Methods
Since C. upsaliensis has only recently been recognized as a human pathogen, the development of applicable typing methods for this organism is still at an evolutionary stage. In two reports from South Africa (23, 56), few C. upsaliensis strains were typable by the lipopolysaccharide (heat-stable antigen) method of Penner et al. (72). A serotyping method based on the detection of heat-labile antigens was recently described by Lior and Woodward (59). This serotyping scheme recognizes seven serogroups, with groups 1, 2, 3, and 5 being the most common among human isolates of C. upsaliensis. No cross-reactivity is observed with C. jejuni, C. coli, or C. lari immune sera.
A number of typing methods have been compared in investigating a C. upsaliensis outbreak in four day care centres in Brussels in 1991 (36, 39). Thirty-four isolates were characterized by plasmid analysis, DNA restriction enzyme analysis, whole-cell protein analysis, restriction fragment length polymorphism (RFLP), and PCR typing. On the basis of the PCR and RFLP results, the outbreak was attributed to two closely related clonal variants of C. upsaliensis. However, no specific typing method examined was identified as ideal for widespread epidemiological typing studies of this organism.
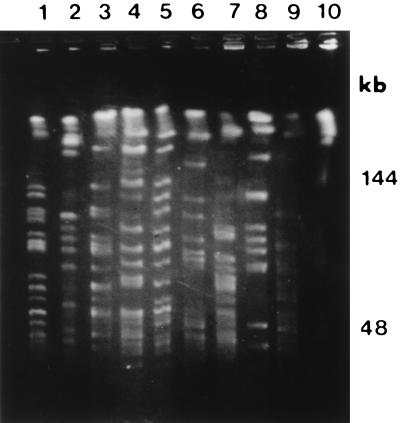
The observation of genotypic heterogeneity among strains of C. upsaliensis by RFLP (69) and pulsed-field gel electrophoresis (11) indicates the likely utility of molecular techniques for epidemiological purposes. Recently, we have shown little similarity across a range of C. upsaliensis strains (11) (Fig. 1). In that study, 19 C. upsaliensis strains obtained from the Laboratory Centre for Disease Control (Ottawa, Canada) and the type strain (ATCC 43954) were analyzed by pulsed-field gel electrophoresis. These C. upsaliensis strains were geographically diverse in origin and comprised both canine and human isolates. In contrast to similar studies on other campylobacters, genomic diversity was evident among C. upsaliensis isolates when a range of different rare-cutting restriction enzymes was used. These typing methods now warrant further evaluation in the setting of outbreaks of C. upsaliensis infection.
FIG. 1.
XhoI-generated macrorestriction patterns demonstrating genotypic heterogeneity among C. upsaliensis isolates. Reprinted from reference 11 with permission of the publisher.
EPIDEMIOLOGY
C. upsaliensis has been isolated from patients on four continents. However, it is sensitive to antibiotics, such as cephalothin, frequently used in selective media used for isolation of enteric campylobacters (41, 70, 93). Therefore, the contribution of C. upsaliensis to diarrheal disease and other human illnesses is difficult to determine from the present data.
Animal Reservoirs and Transmission
The observation that animals represent a reservoir for human infection with C. jejuni and C. coli (6, 7, 79) may also hold true for C. upsaliensis. Most of the isolates in the initial report by Sandstedt et al. (78) were from dogs with diarrhea. Davies et al. (24) described a C. upsaliensis isolate from a dog with chronic diarrhea. However, some isolates were from asymptomatic animals (28, 78), including all of the feline isolates identified in studies by Fox et al. (32) and Moreno et al. (64).
Most animal Campylobacter isolates were previously reported to be either C. jejuni or C. coli (6, 80). However, Moreno et al. (64), who isolated Campylobacter strains by a filtration method, documented extremely high rates of carriage of C. upsaliensis (66%) among domestic and laboratory cats. In contrast, only 3% of this animal population harbored C. jejuni and none carried C. coli. In a study of dogs, Sandstedt et al. (78) found that C. upsaliensis accounted for 63 (64%) of 98 strains of Campylobacter identified over 2 years. C. upsaliensis was also more commonly isolated than other campylobacters from canine feces in a study from Italy (28). Since the rate of Campylobacter carriage in dogs may be up to 75% (14, 45, 74), C. upsaliensis appears to be a common, albeit frequently unrecognized, environmental organism.
Evidence to support the transmission of C. upsaliensis infection from animals to humans comes from two reports implicating C. upsaliensis as a cause of both human enteritis and spontaneous abortion (40, 44). Goossens et al. (40) demonstrated C. upsaliensis in stools obtained from a 53-year-old man with acute onset of pyrexia and bloody diarrhea. A C. upsaliensis strain, apparently of some considerable similarity to that obtained from this patient, was also isolated from the patient’s asymptomatic dog. Gurgan and Diker (44) reported finding C. upsaliensis in cultures of blood and fetoplacental material from a woman suffering a spontaneous abortion at 18 weeks gestation. C. upsaliensis was also isolated from her asymptomatic household cat; analysis of protein profiles confirmed strong similarity between the human and feline bacterial isolates. Nonetheless, the transmission of C. upsaliensis from animals to humans remains to be conclusively proven. A recent study by Stanley et al. provides evidence against dog-to-human transmission of C. upsaliensis (82). In this study C. upsaliensis isolates from humans had a conserved 16S rRNA ribotype, which is not found among canine strains. Data from this study suggest that specific clones within C. upsaliensis are responsible for human disease.
Indirect evidence supporting the possibility of person-to-person spread of C. upsaliensis comes from two studies. Walmsley and Karmali (93) isolated C. upsaliensis from two asymptomatic patients in Toronto who had shared a hospital room (fecal cultures were performed because of contact with another child with a fecal Salmonella isolate). More recently, Goossens et al. (39) identified 34 children with C. upsaliensis in four day care centers in Brussels, Belgium. On the basis of multiple typing methods, it was demonstrated that the outbreaks of C. upsaliensis infection in three of the four centers were due to the same organism. Furthermore, the C. upsaliensis strain responsible for these outbreaks was closely related to the strain isolated from an out break in the fourth day care center.
Frequency of Isolation from Human Feces
C. upsaliensis contributes significantly to the total Campylobacter isolation rates in diarrhea. Over a period of 3 years, Goosens et al. (41) identified C. upsaliensis in 99 of a total of 15,185 stool specimens (0.65%); this represented 12% of all Campylobacter isolates in the study. Megraud and Bonnet (61) found C. upsaliensis in 9% of pediatric Campylobacter isolates. In Australia, Steele et al. (84) found a total of 104 Campylobacter isolates by a filter technique; 9 (8.7%) of these isolates were C. upsaliensis strains. Of note, C. upsaliensis was a major isolate in a subgroup of 217 stool specimens taken from children aged 3 years or younger, in which it accounted for 8 (26.7%) of 30 Campylobacter isolates. C. upsaliensis consistently accounts for over 20% of all Campylobacter isolates at The Red Cross War Memorial Children’s Hospital in South Africa (55). Lindblom et al. (58) recently showed that C. upsaliensis accounts for 18% of all Campylobacter isolates among children in Göteborg, Sweden. In this study, C. upsaliensis was observed six times more often in the stools of pediatric patients than was C. coli. Although it is difficult to extrapolate with confidence from these data to other populations, it appears that C. upsaliensis may account for over 10% of all fecal Campylobacter isolates. This figure may be closer to 20% among infants and young children.
Goossens et al. (41) reported that C. upsaliensis may also be a cause of traveller’s diarrhea. The population studied in this Belgian investigation included a large number of immigrants, mostly from Morocco. Many immigrants having travelled to their country of origin in the early summer to visit family would have been exposed to new members of the microbiological flora, often in unsanitary conditions. The authors suggested that higher isolation rates for C. upsaliensis infection noted in the late summer months might be explained by the return of these immigrants with newly acquired C. upsaliensis infections. Otherwise, there is a paucity of data concerning seasonal, demographic patterns and risk factors for acquisition of C. upsaliensis infection.
CLINICAL EXPERIENCE
Since the first description of human C. upsaliensis isolates from Australia (84), the organism has been found in human feces and blood cultures in France (61), South Africa (55, 56), Canada (88), the United States, (70), Belgium (38, 41), the United Kingdom (4), Austria (46), and Sweden (58). C. upsaliensis has also been identified in blood cultures obtained from febrile patients with and without immunodeficiency (21, 70).
Clinical Features Associated with Infection
Goossens et al. (41) evaluated stool specimens for the presence of C. upsaliensis in a large population in Belgium. By using a filtration method, C. upsaliensis was identified in 99 of a total of 15,185 specimens examined. Of the 77 patients (73 children and 4 adults) for whom clinical information was available, 92% had diarrhea. Typically the onset of illness was sudden and the symptoms were relatively mild, lasting for less than a week. Gross or occult blood was present in only 25% of samples, and leukocytes were detected in fecal smears in fewer than 20% of the patients.
A later study by the same group in Belgium documents person-to-person transmission of C. upsaliensis infection in day care centers in Brussels (39). Although few clinical details were provided in this paper, apparently the disease was mild and self-limiting in most cases. However, some children did experience chronic or recurrent diarrhea.
In a study in Toronto (93), C. upsaliensis was identified in stools from six children whose age ranged from 3.5 to 36 months. Three of the children had watery stools, vomiting, and anorexia at the time of the positive isolate. The illness was self-limiting in two of the children, but the third child had a prolonged illness, with diarrhea lasting for 3 weeks. One of the other children had fever of unknown origin (without diarrhea), and the remaining two children were completely asymptomatic. No other bacterial pathogens were isolated from the stools of these patients.
In another study in Canada, Taylor et al. (88) described seven C. upsaliensis isolates, five of which were from children less than 2 years old. All seven strains were isolated from patients with diarrhea. However, no other clinical description was provided. Megraud and Bonnet (61) identified C. upsaliensis in stool samples from seven French children, six of whom were younger than 10 months. All seven children had gastrointestinal disturbance, with the major features including diarrhea, vomiting, and fever. In three children, the illness lasted for more than 7 days. As in the majority of reports, no other stool pathogens were isolated from these children. The presence or absence of blood or pus cells in the stools of these patients was not discussed.
The clinical features of 11 patients (3 children and 8 adults) with C. upsaliensis in blood or stool specimens were described by Patton et al. (70). The stool specimens were isolated from three patients with vomiting, diarrhea, and fever. Two of these patients had abdominal pain, but only one had bloody diarrhea. One of the three patients with C. upsaliensis in the stool was leukopenic while on anticancer chemotherapy; the other two had been previously well. Eight patients ranging from 6 months to 83 years of age had C. upsaliensis in blood culture specimens. Six of these had an underlying medical condition which might have predisposed them to an opportunistic infection; no predisposing condition was identified in the other two patients. Three of the patients with positive blood culture results had diarrhea, and two others had undergone abdominal surgery. Otherwise, no specific source of infection with C. upsaliensis could be identified. This study offers strong supportive evidence that C. upsaliensis is a human pathogen causing enteritis and bacteremia in normal hosts and opportunistic infection in immunocompromised individuals.
C. upsaliensis bacteremia in the setting of immunodeficiency was also described in a case report by Chusid et al. (21). C. upsaliensis bacteremia was identified in a 16-year-old boy with acquired hypogammaglobulinemia secondary to nephrotic syndrome. A similar association between hypogammaglobulinemia and recurrent bacteremia due to C. jejuni infection has been noted (92).
Lastovica et al. (56) described the isolation of C. upsaliensis in cultures of 17 blood samples (from 16 pediatric patients) of a total of 28,576 blood cultures examined. The average age of the patients was 15.5 months, and all had an underlying illness (including eight with acute enteritis and six with kwashiorkor [protein-predominant malnutrition]). Although the presence of enteritis in these patients suggests an intestinal source of the C. upsaliensis bacteremia, the stools of these patients were not investigated for the presence of C. upsaliensis.
Recently, C. upsaliensis has been associated with hemolytic-uremic syndrome. Carter and Cimolai described a 14-year-old with abdominal pain and profuse watery diarrhea who developed microscopic hematuria, thrombocytopenia, and acute renal failure (17). A renal biopsy confirmed hemolytic-uremic syndrome, and C. upsaliensis was isolated from stools. No other pathogen, including sorbitol-negative Escherichia coli, was isolated, and PCR failed to amplify verotoxin genes from stools.
From these reports, it appears that C. upsaliensis is associated with a similar disease spectrum to that described previously for C. jejuni (71). An acute, self-limited diarrheal illness is the most usual presentation, while fever, vomiting, and abdominal pain are inconsistent features. In a minority of patients, blood or leukocytes are present in the stools. Asymptomatic infection may also occur. Young infants and children may be more frequently affected than adults. Infection in the pediatric age group may be associated with protracted diarrhea. Bacteremia occurs primarily in debilitated and immunocompromised individuals.
C. upsaliensis has also been isolated from other extraintestinal sites including a breast abscess (33) and fetoplacental material from a spontaneous human abortion (44). In addition, C. upsaliensis recently has been reported in association with postinfectious polyneuropathy (37, 47). Ho et al. documented an acute motor axonal neuropathy pattern of Guillain-Barré syndrome in a 64-year-old woman with antecedant C. upsaliensis-related diarrhea (47). Anti-C. upsaliensis lipopolysaccharide antibodies were present in her serum, including anti-ganglioside GM1 antibodies. Antibodies to GM1-like epitopes on lipopolysaccharides of C. jejuni strains associated with Guillain-Barré syndrome are thought to cross-react with the patient’s myelin sheath to produce nerve damage in this disorder. In another report, C. upsaliensis was isolated from a 4-year-old child in South Africa who was undergoing prolonged ventilation because of Guillain-Barré syndrome (37). To date, there has been no reported association of C. upsaliensis with Miller-Fischer syndrome, another polyneuropathy strongly associated with antecedent C. jejuni infection (75).
Immunological responses to C. upsaliensis following infection offer additional, indirect evidence for the pathogenicity of the organism in humans. Megraud and Bonnet (61) examined convalescent-phase serum samples from five patients who excreted C. upsaliensis in their stools. A complement fixation test showed that antibody titers against C. upsaliensis were significantly elevated in serum in three of the five patients. Goossens et al. (39) demonstrated specific immunoglobulin G (IgG), IgM, and IgA antibodies in serum in 21 of 26 affected children in a multicenter outbreak of C. upsaliensis infection involving four day care centers. Patton et al. (70) assayed C. upsaliensis isolates for their susceptibility to complement-mediated bactericidal activity and found that each of four stool isolates were susceptible to bactericidal activity present in normal human serum whereas seven of eight C. upsaliensis blood isolates displayed resistance. This indicates that the immunological responses of the host could play an important role in modulating the pathogenic effects of the organism; i.e., more invasive infections (e.g., bacteremia) occur only if host immune defenses mount a suboptimal response to an infecting strain.
Antimicrobial Therapy
Experience with antimicrobial treatment of C. upsaliensis infection is limited. In vitro testing reveals that the organism is typically sensitive to aminoglycosides, cephalosporins, tetracycline, and nalidixic acid. It is also usually sensitive to erythromycin, but resistant strains have been described (39, 70). C. upsaliensis strains are generally resistant to vancomycin, methicillin, piperacillin, and chloramphenicol (70).
Patients with C. upsaliensis bacteremia have been successfully treated with erythromycin (39, 70). Chusid et al. (21) documented the eradication of C. upsaliensis from blood cultures in a patient after 5 days of treatment with cefotaxime. In a study in Belgium (41), 11 patients with diarrhea and C. upsaliensis in stools received erythromycin and 2 received amoxicillin. The diarrheal symptoms disappeared and the organism was eradicated in all 13 patients receiving therapy. However, there have been no controlled trials of antibiotic treatment for C. upsaliensis-associated diarrhea. Therefore, the place of antibiotic therapy for use in the treatment of infection by C. upsaliensis has yet to be defined.
Although clinical studies provide evidence suggesting the importance of C. upsaliensis as a human enteropathogen, it has to be emphasized that each of the studies to date is uncontrolled. There is therefore a pressing need for controlled studies comparing C. upsaliensis isolation rates in patients with diarrhea with the rates in age-matched, asymptomatic controls. Experimental challenge of human volunteers and a variety of animal models are also required to fulfill each of Koch’s postulates and thereby confirm the enterovirulence of C. upsaliensis.
VIRULENCE PROPERTIES
Toxin production, adhesion to mucosal surfaces, and invasion and replication of organisms within epithelial cells are etiopathogenetic features of many bacterial infections (30). Colonization and infection by bacteria are initially dependent on the interaction of the bacteria with host surfaces. Bacterial adherence is therefore necessary before an organism can cause disease. Toxin production is used by many bacteria to cause damage to host cells, although the precise role of many toxins remains to be clarified. Invasion by organisms of host epithelial cells gives access to a rich nutrient environment and is also used to avoid host immune responses. Microbial pathogenicity is increasingly recognized as multifactorial, with many organisms using multiple complementary mechanisms to produce human disease (30).
The mechanisms by which enteric campylobacters cause human disease have not been clarified. In fact, of all the Campylobacter species, only C. jejuni has been studied in detail. Possible virulence factors in C. jejuni include flagellin (1), enterotoxin production (76), cytolethal distending toxin production (49), microbial adherence (57), and invasion of intestinal epithelial cells (26). In addition, some C. jejuni isolates demonstrate cytotoxic activity on HeLa and Chinese hamster ovary (CHO) cells (43). The relative importance, if any, of each of these mechanisms to the production of disease in humans remains to be determined. As illustrated in a recent comprehensive review of the field (51), the application of genetic techniques to the study of Campylobacter virulence mechanisms is required to more precisely determine the method by which these organisms cause disease. For instance, the generation of isogenic mutants lacking the gene(s) for putative virulence properties is the definitive way to elucidate the relative importance of individual virulence gene products.
The systematic study of virulence mechanisms in C. upsaliensis is in its infancy. Thus far, information on the pathophysiology of infection with this organism comes from only a handful of preliminary reports, which are considered in more detail below.
Motility
As with other Campylobacter species, C. upsaliensis is motile and has either a single flagellum or bipolar flagella. It is known that flagellar antigens show strong cross-reactivity among the Campylobacter species. A study in Toronto has shown that there is a flagellar antigen common to C. upsaliensis and other campylobacters of documented pathogenicity in humans (e.g., C. jejuni and C. coli (63). Therefore, the putative role of flagellin in the virulence of other Campylobacter species may also apply to C. upsaliensis. However, there have been no published investigations specifically evaluating the potential role of C. upsaliensis flagella as a virulence factor for the organism.
Adherence
Megraud et al. (62) reported that 16 CNW strains adhered to an endothelial cell monolayer in a similar fashion to other Campylobacter spp. An average of only 30% of the endothelial cells were infected in this study, and most of these infected cells had only a few adherent bacteria. This low level of adhesion may simply reflect the choice of cell line used in the study.
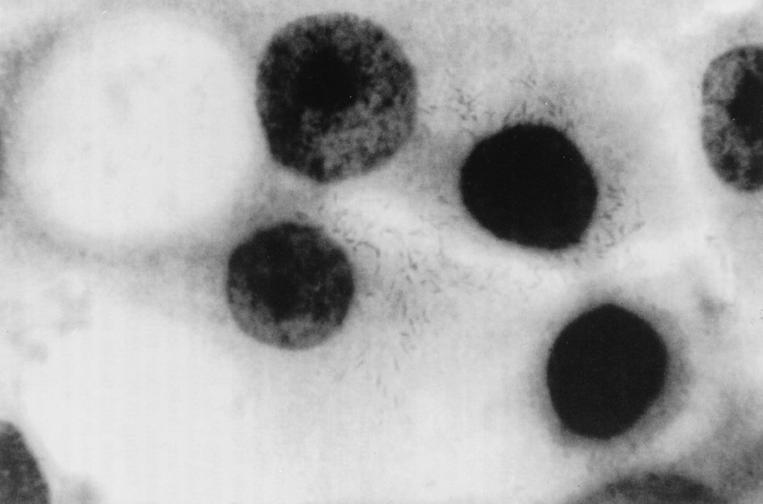
In the first in-depth study of the virulence of C. upsaliensis, Sylvester et al. (85) recently showed that C. upsaliensis binds to CHO and HEp-2 cells in tissue culture (Fig. 2). The manner in which C. upsaliensis bound to epithelial cells in vitro was comparable to the diffuse adherence pattern of C. jejuni. The authors also demonstrated the binding of C. upsaliensis to human small intestinal mucin and characterized the adherence of C. upsaliensis to intestinal lipids. In a thin-layer chromatography overlay binding assay, C. upsaliensis bound to phosphatidylethanolamine, phosphatidylserine, and gangliotetraosylceramide. Affinity was highest for phosphatidylethanolamine, and this lipid was detected in lipid extracts from three different cell lines (CHO, HEL, and HEp-2). Further work is needed to more exactly determine the nature and relevance in vivo of mucin and membrane lipid binding by this organism.
FIG. 2.
Electron micrograph (magnification, ca. ×800) demonstrating HEp-2 cells with adherent C. upsaliensis organisms. Reprinted from reference 85 with permission of the publisher.
Invasion
To date, there have been no reports on the potential role of bacterial internalization into the cytosol of host epithelial cells as a virulence mechanism for C. upsaliensis.
Toxin Production
Figura et al. (29) examined a number of atypical campylobacters for the production of cytotonic, cytotoxic, and cytolethal distending toxins. Each of the two C. upsaliensis strains tested in this study produced a cytolethal distending toxin. The presence or absence of cytotoxic and cytotonic toxins in these C. upsaliensis strains was not discussed. More recently, Pickett et al. (73) confirmed the likely presence of a cdtB homolog in the C. upsaliensis type strain. However, the putative C. upsaliensis cdt gene(s) has yet to be cloned.
Regardless of the identification of putative virulence factors for C. upsaliensis, proof of its role as a human enteropathogen will require the fulfillment of each of Koch’s postulates. Of these four postulates, only the second (i.e., the isolation of the organism from diseased subjects) has been demonstrated for C. upsaliensis. Therefore, there is a pressing need for controlled studies comparing isolation rates in symptomatic and asymptomatic controls and for animal (and human) challenge studies with subsequent reisolation of C. upsaliensis before this organism receives unequivocal recognition as a cause of human disease.
MOLECULAR BIOLOGY
Compared with many other gram-negative organisms such as E. coli and Salmonella spp., progress in Campylobacter molecular biology has been slow. For instance, natural transformation has only recently been described for campylobacters (94, 95). Moreover, the generation of E. coli-to-Campylobacter shuttle vectors (54) and suicide vectors (53) has occurred only relatively recently. In addition, a number of workers encountered difficulties in isolating and cloning genes in enteric campylobacters (87). These problems included failure of Campylobacter gene expression and instability of genes cloned in E. coli (16, 60). A number of possible reasons for these difficulties have been postulated, including differences in codon usage, methylation, and accessory gene requirements between Campylobacter species and E. coli (87). Many of these initial difficulties have now been overcome, and the genetic characterization of the campylobacters is proceeding more rapidly.
Until recently, the molecular biology of C. upsaliensis was largely unexplored. A number of investigators (23, 82) commented on the relative frequency of plasmids isolated from strains of C. upsaliensis (60 to 93%), which is considerably more frequent than the plasmid carriage of other campylobacters such as C. jejuni. Plasmid carriage appears to be more common among C. upsaliensis strains isolated from humans (82). However, there has been no systematic structural or functional study of these extrachromosomal genetic elements. Other isolated observations on the genetics of this organism include the presence of methylated chromosomal DNA (25) and internal transcribed spacers in 23S rRNA genes (27); both these features are found in other Campylobacter species also.
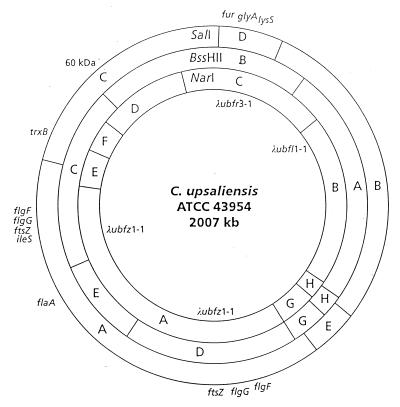
As an initial step toward understanding the molecular events involved in the pathogenesis of C. upsaliensis infection, we constructed a physical-genetic map of the chromosome of this organism (9) (Fig. 3). The genome of the C. upsaliensis type strain, ATCC 43954, is over 2 Mb and, therefore, considerably larger than the genomes of other enteric campylobacters such as C. jejuni (1.8 Mb) (52) and C. coli (1.7 Mb) (97). Additional studies indicate a range of genomic sizes (1.74 to 2.09 Mb) among clinical isolates of C. upsaliensis (11). Since the C. upsaliensis type strain appears to harbor an extensive duplication, it is possible that the increased size of some C. upsaliensis isolates is due to the presence of chromosomal duplications in these strains.
FIG. 3.
Physical genetic map of C. upsaliensis ATCC43954 generated with SalI, NarI, and BssHII. Reprinted (with a slight modification) from reference 9 with permission of the publisher.
At a macrorestriction level, C. upsaliensis demonstrates considerable genomic heterogeneity (11) (Fig. 1) reminiscent of the H. pylori genome (87). This finding is particularly intriguing in light of the relative evolutionary closeness of these two organisms and, conversely, the apparent dissimilarity of their respective ecological niches. However, preliminary evidence (12) does not suggest the presence of interstrain chromosomal rearrangement of genes in C. upsaliensis, which accounts for the remarkable variation observed among individual strains of Helicobacter pylori (48).
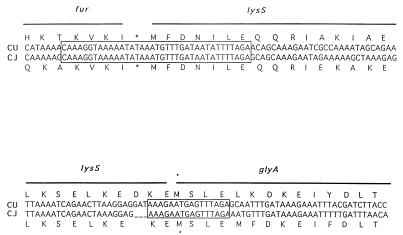
To identify de novo virulence-related genes in C. upsaliensis, we recently cloned and sequenced the iron uptake regulatory (fur) gene for this species (12). Fur acts as a transcriptional regulator repressing the expression of genes involved in iron homeostasis (42), the acid tolerance response (31), and general metabolism (90), as well as virulence genes (15, 67) and genes involved in protection of the organism from oxidative stress (89). Interestingly, the arrangement of genes downstream of C. upsaliensis fur is identical to that observed in C. jejuni (12, 18–20, 96). In fact, there is 100% identity at the nucleotide level across the regions spanning the junctions between fur and the two downstream open reading frames (Fig. 4). PCR experiments indicate that the fur-lysS-glyA arrangement of genes is highly conserved among the three human enteric campylobacters C. jejuni, C. upsaliensis, and C. coli. Northern analysis indicates the expression of polycistronic fur transcripts that probably encode Fur and LysS in both C. upsaliensis and C. jejuni (12, 20). The exact reason for the conservation of this close arrangement of apparently unrelated genes is unknown. However, it is noteworthy that a number of amino acyl-tRNA synthetases, including LysS, catalyze the synthesis of polyadenylated nucleotides whose levels are elevated when cells are exposed to heat or oxidative stress (13). It is conceivable that clustering of amino acyl-tRNA synthetase and fur genes in diverse bacterial species has a functional relevance and may be related to their complementary roles in cellular protection from oxidative stress.
FIG. 4.
Comparison of the gene arrangement downstream of fur in C. upsaliensis and C. jejuni. The upper diagram depicts the junction of the fur and lysS genes, and the lower diagram depicts the junction of the lysS and glyA genes. The C. upsaliensis (CU) DNA sequence is aligned above the respective C. jejuni (CJ) sequence. The deduced amino acid sequence for each organism is indicated above (C. upsaliensis) and below (C. jejuni) the respective nucleotide sequences. Asterisks indicate termination codons. Reprinted from reference 12 with permission of the publisher.
CONCLUSIONS
Failure to identify C. upsaliensis in the setting of many clinical microbiology laboratories is undoubtedly related to the sensitivity of this organism to the antibiotics routinely used in Campylobacter selective media. As a result, in the realm of clinical microbiology C. upsaliensis remains relatively obscure. Because it has received such scant attention, our understanding of this enteric campylobacter has lagged far behind that of related pathogens such as C. jejuni.
A considerable body of mainly epidemiological evidence now indicates the potential importance of C. upsaliensis as a cause of human enteric infection. With the development of improved isolation techniques, we anticipate renewed interest in this organism by those involved in the study of pathogenic bacteria. Future studies aimed at elucidation of the precise role of this organism in human disease and identification of its pathogenic mechanisms ultimately will allow the development of effective strategies aimed at treatment and prevention of C. upsaliensis-related infections.
ACKNOWLEDGMENTS
This work was supported by grants from the Medical Research Council of Canada. B.B. was the recipient of a Research Fellowship from the Medical Research Council of Canada and a grant from Janssen Pharmaceutica.
We are grateful to Brendan Drumm and Marguerite Clyne for their helpful comments during preparation of the manuscript.
REFERENCES
- 1.Aguero-Rosenfeld M E, Yang X, Nachamkin I. Infection of adult Syrian hamsters with flagellar variants of Campylobacter jejuni. Infect Immun. 1990;58:2214–2219. doi: 10.1128/iai.58.7.2214-2219.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderton M R, Korolik V, Coloe P J, Dewhirst F E, Paster B J. Campylobacter hyoilei sp. nov., associated with porcine proliferative eneteritis. Int J Syst Bacteriol. 1995;45:61–66. doi: 10.1099/00207713-45-1-61. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Validation of the publication of new names and new combinations previously effectively published outside the IJSB. Int J Syst Bacteriol. 1991;41:331. doi: 10.1099/00207713-41-4-580. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall S T, Wareing D R, Hayward P G, Hutchinson D N. Selective medium for thermophilic campylobacters including Campylobacter upsaliensis. J Clin Pathol. 1993;46:829–831. doi: 10.1136/jcp.46.9.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspinall S T, Wareing D R A, Hayward P G, Hutchinson D N. A comparison of a new selective medium (CAT) with membrane filtration for the isolation of thermophilic campylobacters including Campylobacter upsaliensis. J Appl Bacteriol. 1996;80:645–650. doi: 10.1111/j.1365-2672.1996.tb03269.x. [DOI] [PubMed] [Google Scholar]
- 6.Blaser M J, LaForce F M, Wilson N A, Wang W L L. Reservoirs for human campylobacteriosis. J Infect Dis. 1980;141:665–669. doi: 10.1093/infdis/141.5.665. [DOI] [PubMed] [Google Scholar]
- 7.Blaser M J, Berkowitz I D, LaForce F M, Cravens J, Reller L B, Wang W-L L. Campylobacter enteritis: clinical and epidemiological features. Ann Intern Med. 1979;91:179–185. doi: 10.7326/0003-4819-91-2-179. [DOI] [PubMed] [Google Scholar]
- 8.Bourke B, Jones N, Sherman P. Helicobacter pylori infection and peptic ulcer disease in children. Pediatr Infect Dis J. 1996;15:1–13. doi: 10.1097/00006454-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Bourke B, Sherman P, Louie H, Hani E, Islur P, Chan V L. Physical and genetic map of the genome of Campylobacter upsaliensis. Microbiology. 1995;141:2417–2424. doi: 10.1099/13500872-141-10-2417. [DOI] [PubMed] [Google Scholar]
- 10.Bourke B. M.D. thesis. Dublin, Ireland: University College Dublin; 1995. [Google Scholar]
- 11.Bourke B, Sherman P M, Woodward D, Lior H, Chan V L. Pulsed-field gel electrophoresis indicates genotypic heterogeneity among Campylobacter upsaliensis strains. FEMS Microbiol Lett. 1996;143:57–61. doi: 10.1111/j.1574-6968.1996.tb08461.x. [DOI] [PubMed] [Google Scholar]
- 12.Bourke B, Al Rashid S T, Bingham H L, Chan V L. Characterization of Campylobacter upsaliensis fur and its localization in a highly conserved region of the Campylobacter genome. Gene. 1996;183:219–224. doi: 10.1016/s0378-1119(96)00562-8. [DOI] [PubMed] [Google Scholar]
- 13.Brevet A, Chen J, Leveque F, Plateau P, Blanquet S. In vivo synthesis of adenylated bis(5′-nucleosidyl) tetraphosphates (Ap4N) by Escherichia coli aminoacyl-tRNA synthetases. Proc Natl Acad Sci USA. 1989;86:8275–8279. doi: 10.1073/pnas.86.21.8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruce D, Zochowski W, Fleming G A. Campylobacter infections in cats and dogs. Vet Rec. 1980;107:200–201. doi: 10.1136/vr.107.9.200. [DOI] [PubMed] [Google Scholar]
- 15.Calderwood S B, Mekalanos J J. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987;169:4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calva E, Torres J, Vazques M, Angeles V, de la Vega H, Ruiz-Palacios G M. Campylobacter jejuni chromosomal sequences that hybridize to Vibrio cholerae and Escherichia coli LT enterotoxin genes. Gene. 1989;75:243–251. doi: 10.1016/0378-1119(89)90270-9. [DOI] [PubMed] [Google Scholar]
- 17.Carter J E, Cimolai N. Hemolytic-uremic syndrome associated with acute Campylobacter upsaliensis gastroenteritis. Nephron. 1996;74:489. doi: 10.1159/000189403. [DOI] [PubMed] [Google Scholar]
- 18.Chan V L, Bingham H L. Lysyl-tRNA synthetase gene of Campylobacter jejuni. J Bacteriol. 1992;174:695–701. doi: 10.1128/jb.174.3.695-701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan V L, Bingham H, Kibue A, Nayndu P R V, Penner J L. Cloning and expression of the Campylobacter glyA gene in Escherichia coli. Gene. 1988;73:184–192. doi: 10.1016/0378-1119(88)90324-1. [DOI] [PubMed] [Google Scholar]
- 20.Chan V L, Louie H L, Bingham H L. Cloning and transcription regulation of the ferric uptake regulatory gene of Campylobacter jejuni TGH9011. Gene. 1995;164:25–31. doi: 10.1016/0378-1119(95)00477-n. [DOI] [PubMed] [Google Scholar]
- 21.Chusid M J, Wortmann D W, Dunne W M. “Campylobacter upsaliensis” sepsis in a boy with acquired hypogammaglobulinemia. Diagn Microbiol Infect Dis. 1990;13:367–369. doi: 10.1016/0732-8893(90)90003-e. [DOI] [PubMed] [Google Scholar]
- 22.Current W L, Garcia L S. Cryptosporidiosis. Clin Microbiol Rev. 1991;4:325–358. doi: 10.1128/cmr.4.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Da Silva-Tatley F M, Lastovica A J, Steyn L M. Plasmid profiles of Campylobacter upsaliensis isolated from blood cultures and stools of paediatric patients. J Med Microbiol. 1992;37:8–14. doi: 10.1099/00222615-37-1-8. [DOI] [PubMed] [Google Scholar]
- 24.Davies A P, Gebhart C J, Meri S A. Campylobacter-associated chronic diarrhoea in a dog. J Am Vet Med Assoc. 1984;184:469–471. [PubMed] [Google Scholar]
- 25.Edmonds P, Hall B M, Edwards W R, Hartline K M. Presence of methylated adenine in GATC sequences in chromosomal DNAs from Campylobacter species. J Bacteriol. 1992;174:8156–8157. doi: 10.1128/jb.174.24.8156-8157.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everest P H, Goosens H, Butzler J-P, Lloyd D, Knutton S, Ketley J M, Williams P H. Differentiation of Caco-2 cells as a model for enteric invasion by Campylobacter jejuni and C. coli. J Med Microbiol. 1992;37:319–325. doi: 10.1099/00222615-37-5-319. [DOI] [PubMed] [Google Scholar]
- 27.Eyers B, Chapelle S, Van Camp G, Goossens H, De Wachter R. Discrimination among thermophilic Campylobacter species by polymerase chain reaction amplification of 23S rRNA gene fragments. J Clin Microbiol. 1993;31:3340–3343. doi: 10.1128/jcm.31.12.3340-3343.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Figura N. Campylobacter spp. isolated from dog faeces. Lancet. 1991;338:1403. doi: 10.1016/0140-6736(91)92286-b. [DOI] [PubMed] [Google Scholar]
- 29.Figura, N., S. Zanchi, N. Partini, M. Bugnoli, P. Gugliemetti, R. Signori, and A. Rossolini. 1993. Campylobacters of unusual species in Siena, Italy. Acta Gastro-Enterol. Belg. 56(Suppl.):15.
- 30.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foster J W, Spector M P. How Salmonella survive against the odds. Annu Rev Microbiol. 1995;49:145–174. doi: 10.1146/annurev.mi.49.100195.001045. [DOI] [PubMed] [Google Scholar]
- 32.Fox J G, Maxwell K O, Taylor N S, Runsick C D, Edmonds P, Brenner D J. “Campylobacter upsaliensis” isolated from cats as identified by DNA relatedness and biochemical features. J Clin Microbiol. 1989;27:2376–2378. doi: 10.1128/jcm.27.10.2376-2378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaudreau C, Lamothe F. Campylobacter upsaliensis isolated from a breast abscess. J Clin Microbiol. 1992;30:1354–1356. doi: 10.1128/jcm.30.5.1354-1356.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gebhart C J, Ward G E, Finsmith L A. Abstracts of the 84th Annual Meeting of the American Society for Microbiology 1984. Washington, D.C: American Society for Microbiology; 1984. Occurrence of Campylobacter jejuni and a catalase-negative Campylobacter species in dogs and cats, abstr. C55; p. 245. [Google Scholar]
- 35.Giesendorf B A J, Quint W G V. Detection and identification of Campylobacter spp. using the polymerase chain reaction. Cell Mol Biol. 1995;41:625–638. [PubMed] [Google Scholar]
- 36.Giesendorf B A, Goossens H, Niesters H G, Van Belkum A, Koeken A, Endtz H P, Stegeman H, Quint W G. Polymerase chain reaction-mediated DNA fingerprinting for epidemiological studies on Campylobacter spp. J Med Microbiol. 1994;40:141–147. doi: 10.1099/00222615-40-2-141. [DOI] [PubMed] [Google Scholar]
- 37.Goddard E A, Lastovica A J, Argent A C. Campylobacter O:41 isolation in Guillain-Barre syndrome. Arch Dis Child. 1997;76:526–528. doi: 10.1136/adc.76.6.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goossens H, Pot B, Vlaes L, Van Den Borre C, Van Den Abbeele R, Van Naelten C, Levy J, Cogniau H, Marbehant P, Verhoef J, Kersters K, Butzler J-P, VanDamme P. Characterization and description of “Campylobacter upsaliensis” isolated from human faeces. J Clin Microbiol. 1990;28:1039–1046. doi: 10.1128/jcm.28.5.1039-1046.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goossens H, Giesendorf B A J, VanDamme P, Vlaes L, Van den Borre C, Koeken A, Quint W G V, Blomme W, Hanicq P, Koster D S, Hofstra H, Butzler J-P, Van der Plas J. Investigation of an outbreak of C. upsaliensis in day care centres in Brussels: analysis of relationships among isolates by phenotypic and genotypic typing methods. J Infect Dis. 1995;172:1298–1305. doi: 10.1093/infdis/172.5.1298. [DOI] [PubMed] [Google Scholar]
- 40.Goossens H, Vlaes L, Butzler J-P, Adnet A, Hanicq P, N’Jufom S, Massart D, De Schrijver G, Blomme W. Campylobacter upsaliensis enteritis associated with canine infections. Lancet. 1991;337:1486–1487. doi: 10.1016/0140-6736(91)93182-9. [DOI] [PubMed] [Google Scholar]
- 41.Goossens H, Vlaes L, DeBoeck M, Pot B, Kersters K, Levy J, De Mol P, Butzler J P, VanDamme P. Is “Campylobacter upsaliensis” an unrecognised cause of human disease? Lancet. 1990;335:584–586. doi: 10.1016/0140-6736(90)90359-d. [DOI] [PubMed] [Google Scholar]
- 42.Guerinot M L. Microbial iron transport. Annu Rev Microbiol. 1994;48:743–772. doi: 10.1146/annurev.mi.48.100194.003523. [DOI] [PubMed] [Google Scholar]
- 43.Guerrant R L, Wanke C A, Pennie R A, Barrett L J, Lima A A, O’Brien A D. Production of a unique cytotoxin by Campylobacter jejuni. Infect Immun. 1987;55:2526–2530. doi: 10.1128/iai.55.10.2526-2530.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gurgan T, Diker K S. Abortion associated with Campylobacter upsaliensis. J Clin Microbiol. 1994;32:3039–3094. doi: 10.1128/jcm.32.12.3093-3094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hastings D H. Campylobacter enteritis in pets. Lancet. 1978;ii:1249–50. doi: 10.1016/s0140-6736(78)92116-5. [DOI] [PubMed] [Google Scholar]
- 46.Hirschl A M, Pletschette M, Wolf D, Berger J, Diridl G, Rotter M L. Frequency of occurrence and characterization of catalase-negative campylobacters isolated from human faeces in Vienna. Int J Med Microbiol. 1990;272:547–553. doi: 10.1016/s0934-8840(11)80057-5. [DOI] [PubMed] [Google Scholar]
- 47.Ho T W, Hsieh S-T, Nachamkin I, Willison H J, Sheikh K, Kiehlbauch J, Flanigan K, McArthur J C, Cornblath D R, McKhann G M, Griffin J W. Motor nerve terminal degeneration provides a potential mechanism for rapid recovery in acute motor axonal neuropathy after Campylobacter infection. Neurology. 1997;48:717–724. doi: 10.1212/wnl.48.3.717. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Q, Taylor D E. Variability of gene order in different Helicobacter pylori strains contributes to genome diversity. Mol Microbiol. 1996;20:833–842. doi: 10.1111/j.1365-2958.1996.tb02521.x. [DOI] [PubMed] [Google Scholar]
- 49.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb Pathog. 1988;4:115–126. doi: 10.1016/0882-4010(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 50.Karmali M A. Infection by verotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:218–224. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ketley J M. Virulence of Campylobacter species: a molecular genetic approach. J Med Microbiol. 1995;42:312–327. doi: 10.1099/00222615-42-5-312. [DOI] [PubMed] [Google Scholar]
- 52.Kim N W, Bingham H, Khawaja R, Louie H, Hani E, Neote K, Chan V L. Physical map of Campylobacter jejuni TGH9011 and localization of 10 genetic markers by use of pulsed-field gel electrophoresis. J Bacteriol. 1992;174:3494–3498. doi: 10.1128/jb.174.11.3494-3498.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Labigne-Roussel A, Courcoux P, Tompkins L. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J Bacteriol. 1988;170:1704–1708. doi: 10.1128/jb.170.4.1704-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Labigne-Roussel A F, Harel J, Tompkins L. Gene transfer from Escherichia coli to Campylobacter species: development of shuttle vectors for the genetic analysis of Campylobacter jejuni. J Bacteriol. 1987;169:5320–5323. doi: 10.1128/jb.169.11.5320-5323.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lastovica, A. J., and E. LeRoux. 1993. Prevalence of Campylobacter spp. in the diarrhoeic stools and blood cultures of pediatric patients. Acta. Gastro-Enterol. Belg. 56(Suppl.):34.
- 56.Lastovica A J, Le Roux E, Penner J L. “Campylobacter upsaliensis” isolated from blood cultures of pediatric patients. J Clin Microbiol. 1989;27:657–659. doi: 10.1128/jcm.27.4.657-659.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee A, O’Rourke J L, Barrington P J, Trust T J. Mucus colonization as a determinant of pathogenicity in intestinal infection by Campylobacter jejuni—a mouse cecal model. Infect Immun. 1986;51:536–546. doi: 10.1128/iai.51.2.536-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindblom G-B, Sjogren E, Hansson-Westerberg J, Kaijser B. Campylobacter upsaliensis, C. sputorum sputorum and C. concisus as common causes of diarrhoea in children. Scand J Infect Dis. 1995;27:187–188. doi: 10.3109/00365549509019006. [DOI] [PubMed] [Google Scholar]
- 59.Lior, H., and D. L. Woodward. 1993. A serotyping scheme for Campylobacter upsaliensis. Acta Gastro-Enterol. Belg. 56(Suppl.):28.
- 60.Louie H, Chan V L. Cloning and characterization of the gamma-glutamyl phosphate reductase gene of Campylobacter jejuni. Mol Gen Genet. 1993;240:29–35. doi: 10.1007/BF00276880. [DOI] [PubMed] [Google Scholar]
- 61.Megraud F, Bonnet F. Unusual campylobacters in human faeces. J Infect. 1985;12:275–276. doi: 10.1016/s0163-4453(86)94398-7. [DOI] [PubMed] [Google Scholar]
- 62.Megraud F, Camborde F, Bonne F, Gavinet A M. Abstracts of the 14th International Congress of Microbiology 1986. 1986. Campylobacter CNW isolated from human faeces, abstr. P.B8-19. [Google Scholar]
- 63.Mills S D, Kurjanczyk L A, Penner J L. Identification of an antigen common to different species of the genus Campylobacter. J Clin Microbiol. 1988;26:1411–1413. doi: 10.1128/jcm.26.7.1411-1413.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moreno G S, Griffiths P L, Connerton I F, Park R W A. Occurrence of campylobacters in small domestic and laboratory animals. J Appl Bacteriol. 1993;75:49–54. doi: 10.1111/j.1365-2672.1993.tb03406.x. [DOI] [PubMed] [Google Scholar]
- 65.Moss C W, Kai A, Lambert M A, Patton C. Isoprenoid quinone content and cellular fatty acid composition of Campylobacter species. J Clin Microbiol. 1984;19:772–776. doi: 10.1128/jcm.19.6.772-776.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moss C W, Lambert-Fair M A, Nicholson M A, Guerrant G O. Isoprenoid quinones of Campylobacter cryoaerophilia, C. cinaedi, C. fennelliae, C. hyointestinalis, C. pylori, and “C. upsaliensis.”. J Clin Microbiol. 1990;28:395–397. doi: 10.1128/jcm.28.2.395-397.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ochsner U A, Vasil A I, Vasil M L. Role of the ferric uptake regulator of Pseudomonas aeruginosa in the regulation of siderophores and exotoxin A expression: purification and activity on iron-regulated promoters. J Bacteriol. 1995;177:7194–7201. doi: 10.1128/jb.177.24.7194-7201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.On S L W. Identification methods for campylobacters, helicobacters, and related organisms. Clin Microbiol Rev. 1996;9:405–422. doi: 10.1128/cmr.9.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Owen R J, Hernandez J. Genotypic variation in Campylobacter upsaliensis from blood and faeces of patients in different countries. FEMS Microbiol Lett. 1990;72:5–10. doi: 10.1016/0378-1097(90)90335-n. [DOI] [PubMed] [Google Scholar]
- 70.Patton C M, Shaffer N, Edmonds P, Barrett T J, Lambert M A, Baker C, Perlman D M, Brenner D J. Human disease associated with “Campylobacter upsaliensis” (catalase-negative or weakly positive Campylobacter species) in the United States. J Clin Microbiol. 1989;27:66–73. doi: 10.1128/jcm.27.1.66-73.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Penner J L. The genus Campylobacter: a decade of progress. Clin Microbiol Rev. 1988;1:157–172. doi: 10.1128/cmr.1.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Penner J L, Hennessy J N, Congi R V. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. Eur J Clin Microbiol. 1983;2:378–383. doi: 10.1007/BF02019474. [DOI] [PubMed] [Google Scholar]
- 73.Pickett C L, Pesci E C, Cottle D L, Russell G, Erdem A N, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB genes. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prescott J F, Bruin-Mosch C W. Carriage of Campylobacter jejuni in healthy and diarrheic animals. Am J Vet Res. 1981;42:164–165. [PubMed] [Google Scholar]
- 75.Rees J H, Soudain S E, Gregson N A, Hughes R A C. Campylobacter jejuni infection and Guillain-Barre syndrome. N Engl J Med. 1995;333:1374–1379. doi: 10.1056/NEJM199511233332102. [DOI] [PubMed] [Google Scholar]
- 76.Ruiz-Palacios G M, Torres J, Torres N I, Escamilla E, Ruiz-Palacios B R, Tamayo J. Cholera-like enterotoxin produced by Campylobacter jejuni. Lancet. 1983;ii:250–253. doi: 10.1016/s0140-6736(83)90234-9. [DOI] [PubMed] [Google Scholar]
- 77.Sandstedt K, Ursing J. Description of the Campylobacter upsaliensis sp. nov. previously known as the CNW group. Syst Appl Microbiol. 1991;14:39–45. [Google Scholar]
- 78.Sandstedt K, Ursing J, Walder M. Thermotolerant Campylobacter with no or weak catalase activity isolated from dogs. Curr Microbiol. 1983;8:209–213. [Google Scholar]
- 79.Skirrow M B. Campylobacter enteritis: a “new” disease. Br Med J. 1977;2:9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Skirrow M B. Campylobacter enteritis in dogs and cats: a “new” zoonosis. Vet Res Commun. 1981;5:13–19. doi: 10.1007/BF02214963. [DOI] [PubMed] [Google Scholar]
- 81.Stanley J, Burnens A P, Linton D, On S L W, Costas M, Owen R J. Campylobacter helveticus sp. nov., a new thermophilic species from domestic animals: characterization, and cloning of a species-specific DNA probe. J Gen Microbiol. 1992;138:2293–2303. doi: 10.1099/00221287-138-11-2293. [DOI] [PubMed] [Google Scholar]
- 82.Stanley J, Jones C, Burnens A, Owen R J. Distinct isolates of human and canine isolates of Campylobacter upsaliensis determined by 16S rRNA gene typing and plasmid profiling. J Clin Microbiol. 1994;32:1788–1794. doi: 10.1128/jcm.32.7.1788-1794.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steele T W, McDermott S N. The use of membrane filters applied directly to the surface of agar plates for the isolation of Campylobacter jejuni from feces. Pathology. 1984;16:263–265. doi: 10.3109/00313028409068535. [DOI] [PubMed] [Google Scholar]
- 84.Steele T W, Sangster N, Lanser J A. DNA relatedness and biochemical features of Campylobacter spp. isolated in Central and South Australia. J Clin Microbiol. 1985;22:71–74. doi: 10.1128/jcm.22.1.71-74.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sylvester F A, Philpott D, Gold B, Lastovica A, Forstner J F. Adherence to lipids and intestinal mucin by a recently recognized human pathogen, Campylobacter upsaliensis. Infect Immun. 1996;64:4060–4066. doi: 10.1128/iai.64.10.4060-4066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tanner A C R, Badger S, Lai C-H, Listgarten M A, Visconti R A, Socransky S S. Wolinella gen. nov., Wolinella succinogenes (Vibrio succinogenes Wolin et al.) comb. nov., and description of Bacteroides gracilis sp. nov., Wolinella recta sp. nov., Campylobacter concisus sp. nov., and Eikenella corrodens from humans with periodontal disease. Int J Syst Bacteriol. 1981;31:432–445. [Google Scholar]
- 87.Taylor D E. Genetics of Campylobacter and Helicobacter. Annu Rev Microbiol. 1992;46:35–64. doi: 10.1146/annurev.mi.46.100192.000343. [DOI] [PubMed] [Google Scholar]
- 88.Taylor D E, Hiratsuka K, Mueller L. Isolation and characterization of catalase-negative and catalase-weak strains of Campylobacter species, including “Campylobacter upsaliensis,” from humans with gastroenteritis. J Clin Microbiol. 1989;27:2042–2045. doi: 10.1128/jcm.27.9.2042-2045.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Touati D, Jacques M, Tardat B, Bouchard M, Despied S. Lethal oxidative damage and mutagenesis are generated by iron in Δfur mutants of Escherichia coli: protective role of superoxide dismutase. J Bacteriol. 1995;177:2305–2314. doi: 10.1128/jb.177.9.2305-2314.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsolis R M, Baumler A J, Stojiljkovic I, Heffron F. Fur regulon of Salmonella typhimurium: identification of new iron-regulated genes. J Bacteriol. 1995;177:4628–4637. doi: 10.1128/jb.177.16.4628-4637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vandamme P, Falsen E, Rossau R, Hoste B, Segers P, Tytgat R, De Lay J. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of genetic descriptions and proposal of Arcobacter gen. nov. Int J Syst Bacteriol. 1991;41:88–103. doi: 10.1099/00207713-41-1-88. [DOI] [PubMed] [Google Scholar]
- 92.Van der Meer J W M, Mouton R P, Daha M R, Schuurman R K B. Campylobacter jejuni bacteremia as a cause of recurrent fever in a patient with hypogammaglobulinemia. J Infect. 1986;12:235–239. doi: 10.1016/s0163-4453(86)94190-3. [DOI] [PubMed] [Google Scholar]
- 93.Walmsley S L, Karmali M A. Direct isolation of atypical thermophilic Campylobacter species from human feces on selective agar medium. J Clin Microbiol. 1989;27:668–670. doi: 10.1128/jcm.27.4.668-670.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y, Taylor D E. Natural transformation of Campylobacter species. J Bacteriol. 1990;172:949–955. doi: 10.1128/jb.172.2.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wassenaar T M, Bleumink-Pluym N M C, van der Zeijst B A M. Inactivation of the Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 1991;10:2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wooldridge K G, Williams P H, Ketley J M. Iron-repressive genetic regulation in Campylobacter jejuni: cloning and characterization of a fur homolog. J Bacteriol. 1994;176:5852–5856. doi: 10.1128/jb.176.18.5852-5856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan W, Taylor D E. Sizing and mapping of the genome of Campylobacter coli strain UA417R using pulsed-field gel electrophoresis. Gene. 1991;101:117–120. doi: 10.1016/0378-1119(91)90232-z. [DOI] [PubMed] [Google Scholar]