Summary
The antiretroviral treatment (ART) developed to control HIV infection led to a revolution in the prognosis of people living with HIV (PLWH). PLWH underwent from suffering severe disease and often fatal complications at young ages to having a chronic condition and a life expectancy close to the general population. Nevertheless, chronic age-related diseases increase as PLWH age. The harmful effect of HIV infection on the individual's immune system adds to its deterioration during ageing, exacerbating comorbidities. In addition, PLWH are more exposed to risk factors affecting ageing, such as coinfections or harmful lifestyles. The ART initiation reverses the biological ageing process but only partially, and additionally can have some toxicities that influence ageing. Observational studies suggest premature ageing in PLWH. Therefore, there is considerable interest in the early prediction of unhealthy ageing through validated biomarkers, easy to implement in HIV-clinical settings. The most promising biomarkers are second-generation epigenetic clocks and integrative algorithms.
Keywords: HIV, Ageing, Epigenetic clocks, Biomarkers
Introduction
The PLWH are ageing because of reduced mortality due to ART effectiveness.1 This has led to a global increase in their life expectancy by more than 50 years,2 mainly in Western countries where the proportion of PLWH over 50 approaches 40%.3 Projections in the Dutch cohort ATHENA estimate that by 2030 this population will increase to 73%.4 This is a significant epidemiological and demographic change from the early years of the epidemic, which brings new public health and clinical challenges.
Prolonged survival of PLWH translates into an increase of non-AIDS-defining illnesses associated with age. These include frailty and metabolic, cardiovascular, cancer, bone, and neurodegenerative disorders.5,6 Even with successful ART, the difference in comorbidity-free years between PLWH and the general population persists.2 This excess of morbidity suggests premature ageing in PLWH, both caused by the HIV infection itself and complex interaction of ART effects, chronic viral co-infections and lifestyle/behavioural factors. Nonetheless, ageing is highly variable and heterogeneous among individuals, and its underlying mechanisms are not completely clear yet, constituting a challenge for its characterization.7 A critical question arises on whether HIV infection accelerates ageing (events occur earlier than in the general population) by mechanisms common to the ageing process, or accentuates ageing (events occur at the same age but more frequently than in the general population) by becoming an additional risk factor for other chronic conditions.8
Understanding how PLWH age and how to measure it has become a priority for improving their medical management. In this review, we explore the present knowledge of ageing with HIV infection and future challenges. Additionally, we analyze available biomarkers that could be transferred into the clinic to evaluate the health status of PLWH and monitor interventions design to achieve healthy ageing.
HIV infection and ageing
The biological deterioration observed during ageing is attributed to a failure in homeostatic maintenance and repair mechanisms at the cellular and molecular level, which entails malfunctions in different physiological systems, including the immune system. Indeed, a functional decline in both the innate and adaptive immune responses leads to the phenomenon of immunosenescence.9 Senescent cells accumulating in different tissues and organs induce metabolic failures that accelerate the process of ageing,10 although the extent to which the immune system regulates this age-related accumulation is unknown.10,11 This progressive dysfunction of the immune system characterizes by thymus involution, naïve-memory cell imbalance, low CD4/CD8 ratio, TCR repertoire reduction, T-cell senescence, immunodeficiency and a chronic low-grade inflammation.12
As untreated PLWH show a progressive dysfunction of the immune system similar to the elderly general population, HIV infection has been considered a model of accelerated immunosenescence.8,13 Indeed, the frequencies of naïve cells and the innate immune response in young HIV-infected individuals resembles that observed in the uninfected elderly population.14 HIV infection leads to elevated immune activation markers such as CD38 and HLA-DR molecules on T-cells,15 and the release of soluble inflammatory factors such as IFN, IL-6, TNF, sCD14, sCD163.16 Other cell types, such as B-cells, monocytes and natural killers (NK) also show signs of activation and dysfunction,17,18 In this regard, a recent study showed a high-inflammatory profile in B-cells of HIV-infected individuals compared to non-HIV.19 Likewise, an excessive accumulation of senescent cells in other tissues may also be a source of pro-inflammatory cytokine secretion in HIV positive individuals.20 The levels of inflammatory cytokines during the whole course of infection in PLWH are like those observed in healthy controls 4–12 years older, which generate a state of low-grade chronic inflammation that contributes to the development of comorbidities.21
Another characteristic of HIV infection that parallels ageing is the replicative senescence of CD4 and CD8 T cells, identified by the increase in CD57+, CD28−CD27− phenotypes.22 During untreated infection, the CD28−CD8+ T cell subpopulation represents approximately 65% of all CD8 cells,23 similar to the 60% observed in non-infected individuals above 80 years of age.24 Likewise, an increase in the exhaustion of HIV-specific and non-specific T-cells has been observed, which affects the homeostasis of the different cell subpopulations.25
HIV per se may also have a direct effect on other mechanisms of ageing such as loss of proteostasis, mitochondrial dysfunction, stem cell exhaustion or epigenetic alterations. Some viral proteins like Tat, Nef or Vpr can induce T-cell apoptosis, interfere with autophagy and promote cellular senescence.26 HIV infection itself causes depletion of mitochondrial DNA (mtDNA) levels, induces ROS production and deregulates the methylome at multiple sites27,28 Lower methylation levels in HLA locus in PLWH have been associated with lower CD4/CD8 ratios.
Therefore, the effect of HIV on the different hallmarks of ageing and its contribution to the onset of age-related comorbidities seems clear. However, how much of the ageing process in PLWH is directly attributable to HIV infection remains somewhat elusive. We should keep in mind that many other factors (e.g. coinfections, lifestyle, and concomitant treatments) contribute to premature ageing and act as confounders. For instance, CMV infection, which has a pivotal role in driving immune ageing in the general population,29 is more frequent in PLWH. Likewise, socioeconomic or harmful lifestyle factors that contribute to ageing (e.g. smoking, adiposity, alcohol, poverty) are more prevalent in PLWH than in the general population.30 In addition, the influence of ART regimen and time on treatment further complicates data interpretation, as ART-related toxicities may contribute to age-related illnesses.
The effect of ART in ageing
The control of HIV viremia by ART has a remarkable positive effect on the ageing of PLWH: it significantly prolongs their life expectancy and prevents AIDS and non-AIDS related events, transforming HIV infection into a chronic and manageable condition.31 Furthermore, the START study showed the benefits of early ART initiation, regardless of the immunological status, to prevent serious AIDS-related and non-AIDS-related events.32 However, despite virological suppression during effective ART, compared to the HIV negative population, PLWH show in general persistent inflammation and immune dysregulation33 which can negatively impact healthspan and ageing dynamics. For example, the persistent elevation of immune biomarkers such as IL-6 and D-dimer has been independently associated with age-related comorbidities and mortality in PLWH on suppressive ART.34
Additionally, lifelong use of antiretroviral drugs can induce harmful effects on some tissues and organs that may limit the benefit of ART on ageing. Most antiretroviral clinical trials assess viral load as the primary surrogate endpoint for infection control but lack long-term follow-up to assess hard clinical endpoints related to ageing and mortality.
Since the approval of AZT in 1987, antiretrovirals have evolved toward less toxic and better tolerated regimens. The cumulative toxicity in patients who start newer regimens differs from those starting treatment decades ago. Especially with non-thymidine nucleoside reverse transcriptase inhibitors or older generation protease inhibitors that induced antiviral therapy-induced senescence (AVTIS) and were associated with significant toxicities, especially lipodystrophy, dyslipidemia, and insulin resistance.35,36 These effects may persist irreversibly years after switching to new regimens.37
New antiretrovirals are not completely free from short- and long-term toxicities. For instance, mitochondrial dysfunction is a known effect of long-term exposure to ART and occurs independently of that caused by HIV infection itself.38 Current antiretrovirals are considered to have significantly less mitochondrial toxicity,39 but still produce some toxicity in specific cell types, potentially contributing to neurocognitive disorders and cardiovascular risk.40,41
The mechanisms by which antiretrovirals can contribute to ageing are probably multifactorial, but some toxicities have been well described for certain classes of antiretrovirals. Tenofovir disoproxil difumarate is associated with bone and kidney toxicities,42,43 and abacavir with cardiovascular risk.44 Boosted protease inhibitors cause metabolic toxicities, such as insulin resistance or dyslipidemia and produce senescence in endothelial arterial cells.36 Integrase inhibitors have been linked to weight gain and neuropsyquiatric symptoms.45,46
At present, treatment strategies focus on achieving viral suppression with fewer antiretroviral drugs, i.e., dual antiretroviral therapies to avoid side effects for this lifelong use. But there is controversy on whether these alternative ART regimens may also have a detrimental effect on immune activation.47
Although the global benefit of ART in PLWH is beyond doubt, the appearance of these drug-related toxicities can affect their health, ageing, and survival. The real contribution of current antiretrovirals to cellular injury is yet to be defined. Moreover, the use of multiple medications due to the increase in comorbidities brings extra drug interactions that need control. Balancing the beneficial effect on the virus and immune system and the deleterious effect of drug-induced toxicities is essential for preventing premature ageing in this population.
How do we measure ageing?
A good measure of the ageing process must assess the individual's capacities beyond its deficits to understand its healthy or unhealthy status and the consequences of it. To date, there is a lack of quantitative and qualitative attributes to define this process objectively. Since individuals age at different rates,7 it is important to differentiate between chronological age and biological age. By definition, biological age refers to the general health condition of an individual at a specific time of its chronological age. Since chronological age is just the passage of time, it has limitations to measure the physiological functions and accurately reflect the health status of an individual.48 Thus, biological age has become a more reliable parameter to capture differences in morbidity and mortality risks among populations.
The multi-factorial nature of ageing in PLWH makes it difficult to find a single measurement useful to determine biological age, so a combination of different parameters would be appropriate. Moreover, the disparity of studies in different populations, the small number of patients in many of them and the lack of longitudinal studies make unclear the exact weight of HIV infection and confounding factors. This situation further complicates by concerns that ART toxicities may contribute to ageing. Therefore, the study of ageing in PLWH requires extensive data on the longitudinal evolution of multiple biomarkers over a long-time span before deterioration and compare them with well matched-controls.
Cohort and observational longitudinal studies
Cohorts provide an ideal tool to study ageing and demographic changes occurring in PLWH. HIV cohorts established years ago revealed ageing characteristics of individuals who have become old. However, HIV cohorts on average have younger individuals than general population cohorts; which would be conditional on the age at the time of comorbidities onset. Comparisons between cohorts need to be restricted to those with similar age distribution.49 But age is not the only confounding factor when comparing different cohorts: ethnicity, sex, lifestyle, and coinfections should also be controlled. It would be useful to have specific cohorts designed to study ageing and its consequences, similarly to ageing cohorts in the general population (i.e. InCHIANTI http://inchiantistudy.net/). For this matter, new cohorts like the COBRA, AGEhiV, POPPY, GEPPO and FUNCFRAIL have been designed to uncover aspects of ageing in PLWH but are still not representative of the entire HIV population.50 Besides, they lack prospective data collection on age-related biomarkers and clinical outcomes related to ageing on a routine basis. It is essential to include these data for better describing ageing trajectories. In this respect, the AGEhiV cohort with PLWH and well-matched negative controls found that prevalence of frailty, as well as transition to frailty overtime was higher in PLWH than HIV-negative controls, and a long cumulative exposure to ddC increased the odds to become frail.6
One of the oldest cohorts established to study ageing is the VACS (Veterans Ageing Cohort Study) (https://medicine.yale.edu/intmed/vacs/). This cohort is one of the most extensively studied and has produced the VACS index to assess disease severity and mortality risk.51 An advantage of this cohort is the fact that HIV-negative controls are not only well matched for age, ethnicity, and clinical site, but are also drawn from the same underlying population. However, like other ageing cohorts it does not completely captures the whole HIV ageing population characteristics. Other cohort studies have served to develop and validate several tools to predict age-related events in PLWH. Among them, the risk of new AIDS or death derived from the EuroSIDA cohort, or the risk of cardiovascular events or progression to chronic kidney disease derived from the D: A: D cohort (both accessible online: http://www.chip.dk/Tools-Standards/Clinical-risk-scores). Some clinical scores to assess ageing conditions used in the general population have also been tested in PLWH but not validated, such as the Fracture Risk Assessment Tool (FRAX) (accessible online https://www.sheffield.ac.uk/FRAX/). Conditions like frailty are measured preferentially with the Fried Frailty Phenotype but other scores, such as Clinical Frailty Scale, 400-m walk pace, SPPB or the chair rise pace could be considered. Several biomarkers have been proposed to assess frailty in the general population (levels of DHEA, testosterone, vitamin D, PTH, IGF1, myokines, HbA1c, amino acids).52 However, there is currently no reliable biomarker or combination of biomarkers to diagnose frailty, as they are not specific and are affected by many confounding factors. Moreover, the assessment of frailty in PLWH gives still heterogeneous results; therefore, further longitudinal studies are required on how best to diagnose this phenotype in PLWH, and what factors are associated. This is one of the objectives of the FUNCFRAIL cohort.
These clinical risk scores include routine clinical and biochemical parameters and are relatively simple to apply in the clinical practice for individual risk assessment, although they can be time consuming.
Need for useful ageing biomarkers
There is a growing interest in the early prediction of age-related diseases utilizing biomarkers in PLWH, and through these respond whether the ageing process is accelerated, accentuated or both.
According to the National Institute of Health Biomarkers Definitions Working Group, a biomarker is “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention”.53 To study ageing, we need biomarkers that can capture the inter-individual variability of biological age before becomes clinically relevant. A good biomarker has to be useful for prognosis but also for monitoring treatment interventions, easy to implement in clinical settings, and fulfil several criteria: (1) mark the individual stage of ageing and predict mortality better than chronological age; (2) monitor systemic ageing and not the effects of a disease, and (3) allow longitudinal non-invasive tracking.54
A broad range of biomarkers has been proposed for the study of ageing (Table 1). Among them, blood-based biomarkers are the most widely used due to their non-invasive nature and their feasibility to be performed in population studies and routine clinical settings.55
Table 1.
Examples of available molecular and cellular biomarkers of healthy ageing.
| Type | Biomarker | Hallmarks of cell ageing covered | reference |
|---|---|---|---|
|
Nucleic acid-based |
Telomere lenght | Telomere attrition | 54, 56 |
| Epigenetic clocks | Epigenetic alterations | 54, 55,71 | |
| Mitocondrial DNA | Mitochondrial dysfunction | 87 | |
|
Protein-based |
Apolipoprotein J/Clusterin (ApoJ/CLU) | Cellular senescence | 55, 90 |
| Proteasome subunits | Loss of proteostasis | 90 | |
|
Metabolic-based |
NAD/NADH ratio | Mitochondrial dysfunction | 11, 55 |
| Lipid alterations in plasma | Deregulated nutrient sensing | 55, 91 | |
| Plasma levels of essential amino-acids | Deregulated nutrient sensing | 55, 93 | |
|
Immunologic |
Soluble inflammatory markers (sCD14, sCD163, IL-6, hsCRP, D-dimer) | Inflammageing | 13 |
| Cell surface molecules (CD28, CD96) | Cellular senescence and exhaustion | 13 | |
| Integrative | MARK-AGE | Epigenetic alterations Mitochondrial dysfunction Deregulated nutrient sensing Inflammageing |
90 |
Molecular and cellular biomarkers in HIV ageing
Telomere length
Telomere length (TL) is inversely correlated with age and has long been recognized as a biomarker of ageing,56 as well as a predictor of mortality in the general population.57 Telomere attrition can be measured in any tissue and cell type but most studies have been performed in blood. Leucocyte TL varies according to the level of telomerase activity, which depends on cell type and the number of divisions. For instance, there are important differences in TL between CD4 and CD8 T cells. Additionally, B lymphocytes show longer TL than T lymphocytes.58 Within the T lymphocyte maturation cascade, naïve cells have longer TL than memory T cells. On the contrary, memory B lymphocytes have telomeres longer than naïve B lymphocytes.58 Understanding these differences is important when blood TL is used to measure ageing in PLWH with different stages of disease.
Compared to uninfected adults, PLWH have shorter leucocyte TL; which negatively correlates with viral load in untreated patients (Table 2).59,60 This TL attrition is pronounced and occurs rapidly after seroconversion,61,62 Early introduction of ART partially recovers leucocyte TL because of the immune reconstitution process that shifts towards less differentiated cells with longer TL.63 This positive effect of ART on TL, diminishes when ART initiation is delayed.64 Likely, this deceleration in TL recovery despite successful ART is hampered by the extension of initial damage. The ART benefit on TL is maintained in long-term treated aviremic HIV-infected adults, although more longitudinal studies are needed to confirm it.65 What seems evident is that leucocyte TL depends on the equilibrium between viral replication and immune activation, and it can be reverted. How the dynamics between leucocyte TL and immune reconstitution in PLWH can lead to premature ageing remains unsettled.
Table 2.
Studies of ageing in PLWH using different biomarkers.
| Biomarker | Study | Main finding | Reference |
|---|---|---|---|
|
Telomere length |
231 HIV-infected compared with 691 uninfected individuals | HIV-infected individuals had shorter telomere | 59 |
| Longitudinal study on 31 HIV-infected IV drug users | Telomere length decreased rapidly after seroconversion | 62 | |
| Longitudinal study in 201 individuals from the NEAT001/ANRS143 clinical trial | Improvement of mean leucocyte telomere length after initiation of ART | 63 | |
| 333 HIV-infected individuals from the SWISS cohort with CAD compared with 745 controls without CAD | Leucocyte TL measured 9 years before, associated with coronary artery events | 67 | |
|
Horvath's clock |
374 ART-naïve HIV-infected individuals compared with 34 uninfected individuals | HIV-infected individuals had higher epigenetic age acceleration than uninfected controls | 77 |
| Meta-analysis using 6 data sets from HIV-infected patients | HIV infection increases Epigenetic age by 5·2 years in blood and 7·4 years in brain | 79 | |
| Longitudinal study in 63 aviremic HIV patients under ART | Epigenetic age does not accelerate in well-controlled individuals | 81 | |
| NEAT001/143 Longitudinal substudy in 168 HIV patients before and after ART | ART partially reverses Epigenetic age acceleration | 78 | |
|
GrimAge and PhenoAge cloks |
Longitudinal study in 63 aviremic HIV patients under ART | Higher EAA may predict clinical events in PLWH | 81 |
| 69 PLWH and 38 HIV-seronegative controls | PLWH had higher PhenoAge than controls | 85 | |
| mtDNA | cross-sectional analysis 156 female HIV-infected patients and 133 HIV-negative controls, enrolled in CARMA cohort | Lower mitochondrial DNA content was associated with metabolic syndrome | 68 |
| Metabolomics | Cross-sectional analysis in 80 HIV+ men above 50 years of age | Abnormal lipidomic profiles associates with frailty in HIV+ men on ART | 93 |
| Immunologic | Cross-sectional study in 106 treated PLWH and 103 HIV-negative controls | Age advancement increased by 5·6 years in well-controlled PLWH. | 100 |
| MARK-AGE | Cross-sectional analysis of 134 PLWH on suppressive antiretroviral therapy (COBRA) cohort) and 35 matched-controls | Age advancement increased by 3·5 in PLWH with low CD4 T cells | 70 |
A recent study observed that shorter TL was associated with age-related comorbidities including cardiovascular diseases (CVD) and neurocognitive impairment, supporting the used of TL as a biomarker to estimate the impact of HIV on long-term health.66 Furthermore, in participants from the Swiss HIV Cohort Study (SHCS) TL -measured 9 years before the event- was independently associated with CVD after adjusting for traditional risk factors.67 Nevertheless, no association between TL and cardiovascular risk was observed in women from the CARMA cohort.68
Although these data suggest leucocyte TL could be a biomarker to measure ageing in PLWH, there are some technical and sample challenges. The lack of a gold standard and the different techniques for TL measurement hinder results comparison. There is also the inter-individual TL variability and differences across tissues. Another point is the heterogeneous blood cell population and transient changes reflecting the state of activation of the immune system in HIV infection, which in the short-term may have nothing to do with ageing. Moreover, many studies are cross-sectional and their results to predict mortality or age-related diseases need confirmation in large longitudinal studies.
Epigenetic clocks
Epigenetic ageing biomarkers based on changes in DNA methylation levels (DNAm) have been used in recent years to clarify whether HIV infection is associated with a premature biological ageing process. Most of the research in PLWH has been performed on blood samples using different DNAm-based algorithms on a wide range of CpG positions.28,69,70 Among the first-generation clocks, the most extensively used is the multi-tissue Horvath's clock, based on 353 CpG sites.71 This clock was initially trained on chronological age and although can capture biological age, new clocks have been developed to better predict disease and mortality. PhenoAge is a surrogate measure of phenotypic age based on 513 CpG sites, and is considered an accurate predictor of mortality, healthspan, cardiovascular disease, and other morbidities.72 The GrimAge estimator, which is calculated based on chronological age, sex, and DNA methylation-based surrogates for seven plasma proteins and smoking pack-years, also predicts time to death and comorbidities.73 The accuracy of epigenetic clocks has been broadly validated, and they have become attractive biomarkers because allow estimating physiological ageing through the calculation of epigenetic age acceleration (EAA), defined as an epigenetic age greater than that predicted based on chronological age. EAA is a good predictor for the occurrence of age-related comorbidities and mortality.74
In the absence of ART, PLWH experience -compared to uninfected individuals- an acceleration of epigenetic ageing measured in blood of between 2 and 15 years depending on the study.75, 76, 77, 78 Furthermore, a study carried out in a cohort of 31 individuals who inject drugs determined that this epigenetic ageing begins rapidly after seroconversion.62 On the other hand, an acceleration of epigenetic ageing of 2–5 years has been observed in HIV patients on ART and virologically suppressed.79,28 The disparity in age advancement among studies is most likely due to different characteristics of HIV population and control groups they were compared with. This age advancement in PLWH is lower when the control group are HIV-negative individuals with similar lifestyles than when the comparison is with blood donors.70 Therefore, it is important to compare epigenetic ageing with well-matched controls to evaluate the levels of premature ageing, or whether is accelerated or accentuated.
Although most studies favour the hypothesis of premature biological ageing, certain aspects of the dynamics of epigenetic ageing during HIV infection remain poorly understood. Firstly, the effect that ART initiation has on the epigenetic age is little known. A sub-study on 168 ART-naïve HIV-infected individuals from the NEAT001/ANRS143 clinical trial found that EAA reduced by 1·1–3·6 years two years after starting ART in four epigenetic age estimators (Horvath's and Hannum's clocks, PhenoAge and GrimAge).78 A similar improvement in epigenetic age was observed in two other studies: in 19 HIV patients from the VACS cohort after 7–11 years from the start of treatment76; and in 15 HIV-infected individuals followed during 24 months where epigenetic age was measured at three time points.80 Secondly, the lack of longitudinal studies in patients with stable ART does not allow us to determine whether epigenetic ageing is only accentuated or continues to accelerate during virologically controlled HIV infection. A recent longitudinal study with 63 long-term aviremic PLWH on stable ART found no differences on EAA after 4 years of follow-up,81 suggesting that epigenetic age does not accelerate over time and most likely there is a phenomenon of accentuation due to the initial damage caused by HIV infection. Data from the same study suggest the risk of suffering a serious non-AIDS event increased by 10–14% for each year of EAA increase. Finally, there are no data on the possible impact of different antiretroviral regimens on epigenetic ageing. In the aforementioned NEAT study, there were no differences in epigenetic age acceleration by treatment groups.78 Likewise, it is not entirely clear what aspects of cellular ageing are being represented in the epigenetic clocks. There is evidence that inflammation-related and antiviral response genes associate with this epigenetic ageing.82,83
Besides predicting biological age, the study of ageing should address the longitudinal behaviour of DNAm biomarkers in predicting health deterioration, age-related phenotypes and diseases in the context of HIV. It is important to identify what clinical end-points for PLWH (mortality risk, CVD, chronic kidney disease, dementia, etc.) are best predicted by each biomarker. In the general population, Horvath's clock performs better to predict all-cause mortality risk in men; while PhenoAge and GrimAge show higher performance in mortality risk prediction in women.84 The same study found that the combination of DNAm biomarkers and frailty index improved this predictive value. Some epigenetic metrics have been associated with frailty showing an increase of epigenetic age in frail PLWH,69 although a recent report associated EAA to a decrease in cognitive performance but not with frailty.85 Other DNAm-based biomarkers have also been related to frailty, prognosis and mortality in PLWH.83,86 All these data suggest epigenetic clocks could become an accurate biomarker in ageing. In our opinion, second generation epigenetic clocks such as PhenoAge should be the ones to choose for survival analysis and risk of developing serious non-AIDS events because show better prediction of the functional decline and morbidity and mortality risks in the general population. However, there is a need for more extensive data and validation in PLWH before using them in specific applications in both public health and clinical practice. DNA methylation is dynamic and longitudinal studies will help understand how it influences within individual's changes of ageing. One important point to mention is that they have been trained in a small number of sample sets and need to be tested in different populations, ethnicities, genders, lifestyles and diseases. Another limitation is the cost and available technology, which needs highly trained personnel. Finally, DNA methylation data needs validation as a predictive biomarker for survival time and non-AIDS clinical events.
Mitochondrial DNA
Progressive mitochondrial dysfunction and oxidative stress are also hallmarks of ageing.9 Reactive oxygen species (ROS) cause mtDNA mutations or deletions, leading to impairment in mitochondrial functions and ultimately to senescence and ageing.87 HIV infection directly induces mitochondrial ROS production reducing mtDNA level.27 It is important to note that historically mitochondrial damage in PLWH was associated with Nucleoside/Nucleotide Reverse Transcriptase Inhibitors exposure such as stavudine or zidovudine,88 so again there is the interplay between ART and the ageing process. Moreover, some protease inhibitors can also increase oxidative stress.88
The use of mtDNA as a biomarker of ageing in PLWH needs further exploration. Lower mtDNA has been associated with metabolic syndrome in the CARMA cohort, but no association was observed with another cellular marker of ageing such as leucocyte telomere length.68 In another study, chronological age, HIV and ethnicity were associated with lower mtDNA.89 In this same study, a subgroup of participants was followed longitudinally and observed that the rate of change in leucocyte TL and mtDNA levels/year were inversely correlated. There was also an increase in TL attrition and mtDNA loss when viral control was not achieved.
Proteomic and metabolomic markers
The accumulation of secondary metabolites of biochemical pathways altered during the ageing process and linked to longevity could have a relevance in the evaluation of this process. Their analyses through metabolomics and proteomics techniques could be useful since changes in metabolism are rapid and reveal the individual's actual physiological status. Some integrative algorithms designed to measure ageing have incorporated markers based on proteins and their modifications, as well as lipid and amino acid profiles associated with different metabolic dysfunctions. Among them, serum levels detection of ApoJ/CLU protein (which associates with premature senescence) or the presence of protein damage in blood, such as proteasome subunits that indicate proteasome function impairment associated with age.90
Metabolomics analyses in PLWH on ART revealed that lipid alterations were linked to markers of inflammation, microbial translocation, and hepatic dysfunction; and associated with frailty.91,92 Another study on individuals on successful long-term ART for more than five years matched with HIV-negative healthy individuals showed differences on 250 plasma metabolites. Plasma levels of essential amino acids were lower in PLWH than healthy controls and machine-learning predictions indicate that PLWH had higher risks of inflammatory and neurological diseases.93
Systemic inflammation parameters and immunosenescence markers
In HIV infection, persistent inflammation and systemic immune activation cause premature immunosenescence. It is important to determine which markers could be useful to follow ageing and age-related comorbidities in well-controlled PLWH. A recent study found that sCD14 molecule was higher in ART-naïve PLWH than in healthy controls, and continue to be elevated despite years of successful treatment. The sCD163 was also higher in these ART-naïve patients and reduced after ART initiation but did not normalize. Moreover, inflammatory molecules, including CCL23, MMP1, TRAIL, and TRANCE, were also elevated in PLWH and associated with different age-related comorbidities such as cardiovascular disease or neurocognitive decline.94 Additionally, in a study performed in participants from the COBRA cohort compared with blood donors, the age advancement was associated with a specific monocyte profile related to inflammation and a T cell phenotype associated with immune senescence and exhaustion.95 However, another study found some residual T-cell dysfunction in 780 aviremic PLWH but no evidence of age acceleration.96 A recent study suggests that the loss of CD96 molecule results in a suboptimal response to HIV antigens and could be a useful biomarker for CD8 T cell senescence and dysfunction in PLWH.97
Classical biomarkers of systemic inflammation such as IL-6, C-reactive protein (CRP) and D-dimers, as well as a low CD4/CD8 ratio and high markers of immune activation (CD8+/CD38+, CD8+/HLA-DR+) have been associated with frailty in the HIV population.98 Some SNPs in IL-6 and IL-10 have been correlated with higher epigenetic age and the IL-6 C allele carriers had an increased risk of suffering non-AIDS conditions.99 IL-6 and IL-10 SNPs may help to identify PLWH who are at high risk for accelerated ageing. A recent study integrated many of these biomarkers of systemic inflammation and immune activation (IL-6, D-dimer, CD38-HLA-DR, TNF, etc.) in a 25-parameter model called IMPA-25 (Immunological Age Prediction) as an indicator of “immunological age” in PLWH. Application of IMAP-25 revealed 5·6 years of age advancement in PLWH.100
Other markers
All aforementioned studies have been performed in blood, but other tissues can manifest ageing differently. In this regard, neuroimaging has been used as a biomarker for age-related brain atrophy. A large sample from the COBRA cohort (267 individuals) was analyzed using a machine-learning model of healthy brain; which had been trained in an independent cohort of 2,100 individuals from the general population. PLWH had greater brain-PAD score (brain-predicted age difference = brain-predicted brain age - chronological age), and this increase was associated with a decrease in cognitive performance.101 In a latter longitudinal study, the same group found no evidence of age acceleration after successful ART treatment.102
Outstanding questions
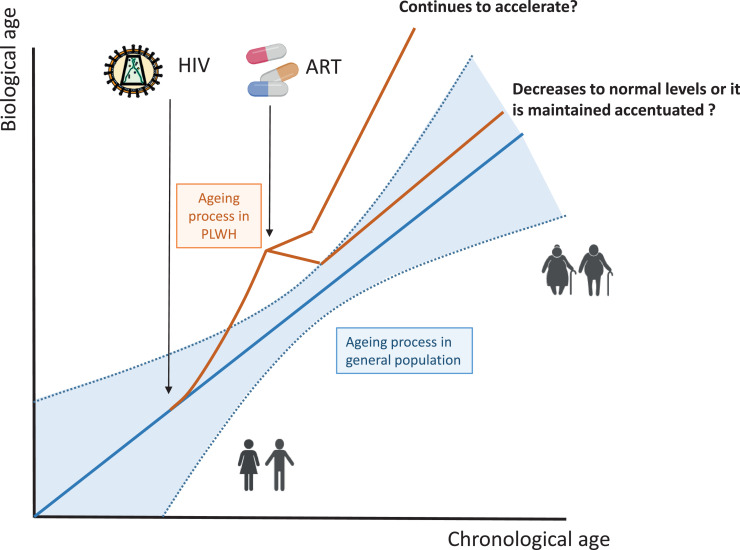
The importance of ageing and its impact on PLWH requires new strategies to address these changes in demographics. HIV infection inflicts – especially under detectable viremia- significant damage on the organism, particularly upon the immune system, which in the long term can cause ageing. But the dynamics of this ageing process need further exploration in longitudinal studies. It is essential to clarify under which circumstances it accelerates or becomes accentuated; and whether its partial or complete reversion is feasible (Figure 1).
Figure 1.
Interplay between HIV, ART and ageing. Hypothetical dynamics of the biological ageing process after HIV infection based on the studies performed in PLWH (red line) up to date. In untreated infection, the process seems more accelerated than non-HIV infection (blue line). With the introduction of ART, the biological ageing reverts. In this stage, the biological ageing is probably more accentuated than accelerated but the extent of this reversion remains unsettled.
As ageing is multifactorial, the combination of biomarkers summarized into a score may be more accurate at measuring the residual healthspan than a single biomarker. An example of this combined approach is the MARK-AGE algorithm, an integrative biomarker composed of a panel of 10 biomarkers, developed for measuring age advancement.95 But in order to be useful, a biomarker needs to be optimized for the use in clinical practice. Therefore, it is important to have large cohorts of PLWH including well-matched HIV negative controls, and with a longitudinal follow-up. Unmeasured confounders such as lifestyle habits, diet, socioeconomic background, smoking, alcohol, etc. contribute to age advancement and since the prevalence of them is higher in PLWH, it is important to control for them.
Future studies would focus in three areas:
-
a.
Establishment of ageing cohorts of naïve and treated PLWH and well-matched controls to account for different lifestyles and sociodemographic factors.
-
b.
Large longitudinal studies with integrative biomarkers (epigenetic markers in combination with few immunological markers or clinical scores) to accurately estimate biological ageing for different clinical endpoints (mortality or serious non-AIDS events).
-
c.
Optimization of biomarkers for the use in routine clinical practice.
In our opinion, the best strategy to deal with the complexity of the ageing process in PLWH would require a multi-marker approach, incorporating clinical scores, integrative biomarkers and patient self-reports related to lifestyles.
Search strategies and selection criteria
A literature search was electronically carried out in academic databases PubMed, MEDLINE and Google scholar by the authors using the search terms “ageing”, “ageing and HIV”, “ageing and ART and HIV”, “toxic effects of ART in HIV”, “biomarker of ageing”, “biomarker of ageing and HIV”, “HIV and age-related diseases”, “HIV and comorbidities”. After screening the abstracts, relevant publications from appropriate papers added as additional literature sources. Latest search date: 30th of October 2021. Most articles published within the last 5 years have been used.
Contributors
B.R. – Conceptualization and design, Writing – original draft. J.C., A.E-C., J.R-C. - Writing – review & editing. J.R.A. – Conceptualization, Writing – review & editing
All authors read and approved the final version of the manuscript.
Declaration of interests
B.R. declares personal fees from GILEAD and non-financial support from ViiV Healthcare, outside the submitted work. J.C. declares personal fees from GILEAD outside the submitted work. A.E.C. declares no conflict of interest. J.R.C. declares personal fees from GILEAD and ViiV Healthcare, outside the submitted work. J.R.A declares personal fees from GILEAD, Janssen, ViiV Healthcare, MSD, Aelix and Theranos, outside the submitted work.
Acknowledgements
The authors acknowledge the financial support from Instituto de Salud Carlos III-Fondo Social Europeo (Grants FI17/00194, CM17/00064 and PI18/000569). The funders were not involved in the study in any way.
Contributor Information
Berta Rodés, Email: berta.rodes@salud.madrid.org.
José Ramón Arribas, Email: joser.arribas@salud.madrid.org.
References
- 1.Fontela C., Aguinaga A., Moreno-Iribas C., et al. Trends and causes of mortality in a population-based cohort of HIV-infected adults in Spain: comparison with the general population. Sci Rep. 2020;10:8922. doi: 10.1038/s41598-020-65841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcus J.L., Leyden W.A., Alexeeff S.E., et al. Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000–2016. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autenrieth C.S., Beck E.J., Stelzle D., Mallouris C., Mahy M., Ghys P. Global and regional trends of people living with HIV aged 50 and over: estimates and projections for 2000–2020. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smit M., Brinkman K., Geerlings S., et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15:810–818. doi: 10.1016/S1473-3099(15)00056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schouten J., Wit F.W., Stolte I.G., et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59:1787–1797. doi: 10.1093/cid/ciu701. [DOI] [PubMed] [Google Scholar]
- 6.Verheij E., Wit F.W., Verboeket S.O., et al. Frequency, risk factors, and mediators of frailty transitions during long-term follow-up among people with HIV and HIV-negative AGEhIV cohort participants. J Acquir Immune Defic Syndr. 2021;86:110–118. doi: 10.1097/QAI.0000000000002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahadi S., Zhou W., Schüssler-Fiorenza Rose S.M., et al. Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nat Med. 2020;26:83–90. doi: 10.1038/s41591-019-0719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pathai S., Bajillan H., Landay A.L., High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014;69:833–842. doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ovadya Y., Landsberger T., Leins H., et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun. 2018;9:5435. doi: 10.1038/s41467-018-07825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desdín-Micó G., Soto-Heredero G., Aranda J.F., et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science. 2020;368:1371–1376. doi: 10.1126/science.aax0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittelbrunn M., Kroemer G. Hallmarks of T cell aging. Nat Immunol. 2021;22:687–698. doi: 10.1038/s41590-021-00927-z. [DOI] [PubMed] [Google Scholar]
- 13.Sokoya T., Steel H.C., Nieuwoudt M., Rossouw TM. HIV as a Cause of Immune Activation and Immunosenescence. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/6825493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appay V., Fastenackels S., Katlama C., et al. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS. 2011;25:1813–1822. doi: 10.1097/QAD.0b013e32834640e6. [DOI] [PubMed] [Google Scholar]
- 15.Lu L., Wang J., Yang Q., Xie X., Huang Y. The role of CD38 in HIV infection. AIDS Res Ther. 2021;18:11. doi: 10.1186/s12981-021-00330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauce D., Pourcher V., Ferry T., Boddaert J., Slama L., Allavena C. Immune activation and chronic inflammation: is there an additional effect of HIV in a geriatric population? Medicine (Baltimore) 2021;100:e25678. doi: 10.1097/MD.0000000000025678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moir S., Fauci AS. B-cell exhaustion in HIV infection: the role of immune activation. Curr Opin HIV AIDS. 2014;9:472–477. doi: 10.1097/COH.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 18.Prabhu V.M., Singh A.K., Padwal V., Nagar V., Patil P., Patel V. Monocyte based correlates of immune activation and viremia in HIV-infected long-term non-progressors. Front Immunol. 2019;10:2849. doi: 10.3389/fimmu.2019.02849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frasca D., Pallikkuth S., Pahwa S. Metabolic phenotype of B cells from young and elderly HIV individuals. Immun Ageing. 2021;18:35. doi: 10.1186/s12979-021-00245-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohli J., Veenstra I., Demaria M. The struggle of a good friend getting old: cellular senescence in viral responses and therapy. EMBO Rep. 2021;22:e52243. doi: 10.15252/embr.202052243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angelovich T.A., Hearps A.C., Maisa A., et al. Viremic and virologically suppressed HIV infection increases age-related changes to monocyte activation equivalent to 12 and 4 years of aging, respectively. J Acquir Immune Defic Syndr. 2015;69:11–17. doi: 10.1097/QAI.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 22.Bellon M., Nicot C. Telomere dynamics in immune senescence and exhaustion triggered by chronic viral infection. Viruses. 2017;9:289. doi: 10.3390/v9100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tassiopoulos K., Landay A., Collier A.C., et al. CD28-negative CD4+ and CD8+ T cells in antiretroviral therapy-naive HIV-infected adults enrolled in adult clinical trials group studies. J Infect Dis. 2012;205:1730–1738. doi: 10.1093/infdis/jis260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fagnoni F.F., Vescovini R., Mazzola M., et al. Expansion of cytotoxic CD8+ CD28- T cells in healthy ageing people, including centenarians. Immunology. 1996;88:501–507. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breton G., Chomont N., Takata H., et al. Programmed death-1 is a marker for abnormal distribution of naive/memory T cell subsets in HIV-1 infection. J Immunol. 2013;191:2194–2204. doi: 10.4049/jimmunol.1200646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagathu C., Cossarizza A., Béréziat V., Nasi M., Capeau J., Pinti M. Basic science and pathogenesis of ageing with HIV: potential mechanisms and biomarkers. AIDS. 2017;31(Suppl 2):S105–S119. doi: 10.1097/QAD.0000000000001441. [DOI] [PubMed] [Google Scholar]
- 27.Miura T., Goto M., Hosoya N., et al. Depletion of mitochondrial DNA in HIV-1-infected patients and its amelioration by antiretroviral therapy. J Med Virol. 2003;70:497–505. doi: 10.1002/jmv.10423. [DOI] [PubMed] [Google Scholar]
- 28.Gross A.M., Jaeger P.A., Kreisberg J.F., et al. Methylome-wide analysis of Chronic HIV infection reveals five-year increase in biological age and epigenetic targeting of HLA. Mol Cell. 2016;62:157–168. doi: 10.1016/j.molcel.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolich-Žugich J., Čicin-Šain L., Collins-McMillen D., et al. Advances in cytomegalovirus (CMV) biology and its relationship to health, diseases, and aging. Geroscience. 2020;42:495–504. doi: 10.1007/s11357-020-00170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atallah N., Adjibade M., Lelong H., et al. How healthy lifestyle factors at midlife relate to healthy aging. Nutrients. 2018;10:854. doi: 10.3390/nu10070854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wada N.I., Jacobson L.P., Margolick J.B., et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.INSIGHT START Study Group. Lundgren J.D., Babiker A.G., et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cobos Jiménez V., Wit F., Joerink M., Maurer I., et al. T-cell activation independently associates with immune senescence in HIV-infected recipients of long-term antiretroviral treatment. J Infect Dis. 2016;214:216–225. doi: 10.1093/infdis/jiw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grund B., Baker J.V., Deeks S.G., et al. Relevance of interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen J., D'Agostino L., Tuzer F., Torres C. HIV antiretroviral therapy drugs induce premature senescence and altered physiology in HUVECs. Mech Ageing Dev. 2018;175:74–82. doi: 10.1016/j.mad.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Afonso P., Auclair M., Caron-Debarle M., Capeau J. Impact of CCR5, integrase and protease inhibitors on human endothelial cell function, stress, inflammation and senescence. Antivir Ther. 2017;22:645–657. doi: 10.3851/IMP3160. [DOI] [PubMed] [Google Scholar]
- 37.Gelpi M., Afzal S., Fuchs A., et al. Prior exposure to thymidine analogs and didanosine is associated with long-lasting alterations in adipose tissue distribution and cardiovascular risk factors. AIDS. 2019;33:675–683. doi: 10.1097/QAD.0000000000002119. [DOI] [PubMed] [Google Scholar]
- 38.Schank M., Zhao J., Moorman J.P., Yao ZQ. The impact of HIV- and ART-induced mitochondrial dysfunction in cellular senescence and aging. Cells. 2021;10:174. doi: 10.3390/cells10010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birkus G., Hitchcock M.J.M., Cihlar T. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2002;46:716–723. doi: 10.1128/AAC.46.3.716-723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velichkovska M., Surnar B., Nair M., Dhar S., Toborek M. Targeted mitochondrial COQ10 delivery attenuates antiretroviral-drug-induced senescence of neural progenitor cells. Mol Pharm. 2019;16:724–736. doi: 10.1021/acs.molpharmaceut.8b01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y.F., Dugas TR. Endothelial mitochondrial senescence accelerates cardiovascular disease in antiretroviral-receiving HIV patients. Toxicol Lett. 2019;317:13–23. doi: 10.1016/j.toxlet.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Warriner A.H., Mugavero M., Overton ET. Bone alterations associated with HIV. Curr HIV/AIDS Rep. 2014;11:233–240. doi: 10.1007/s11904-014-0216-x. [DOI] [PubMed] [Google Scholar]
- 43.Heron J.E., Bagnis C.I., Gracey DM. Contemporary issues and new challenges in chronic kidney disease amongst people living with HIV. AIDS Res Ther. 2020;17:11. doi: 10.1186/s12981-020-00266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorjee K., Baxi S.M., Reingold A.L., Hubbard A. Risk of cardiovascular events from current, recent, and cumulative exposure to abacavir among persons living with HIV who were receiving antiretroviral therapy in the United States: a cohort study. BMC Infect Dis. 2017;17:708. doi: 10.1186/s12879-017-2808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sax P.E., Erlandson K.M., Lake J.E., et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71:1379–1389. doi: 10.1093/cid/ciz999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kheloufi F., Boucherie Q., Blin O., Micallef J. Neuropsychiatric events and dolutegravir in HIV patients: a worldwide issue involving a class effect. AIDS. 2017;31:1775–1777. doi: 10.1097/QAD.0000000000001557. [DOI] [PubMed] [Google Scholar]
- 47.van Welzen B.J., Oomen P.G.A., Hoepelman AIM. Dual antiretroviral therapy-all quiet beneath the surface? Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.637910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamczyk M.R., Nevado R.M., Barettino A., Fuster V., Andrés V. Biological versus chronological aging: JACC focus seminar. J Am Coll Cardiol. 2020;75:919–930. doi: 10.1016/j.jacc.2019.11.062. [DOI] [PubMed] [Google Scholar]
- 49.Sabin C.A., Reiss P. Epidemiology of ageing with HIV: what can we learn from cohorts? AIDS. 2017;31(Suppl 2):S121–S128. doi: 10.1097/QAD.0000000000001374. [DOI] [PubMed] [Google Scholar]
- 50.Milic J., Russwurm M., Cerezales Calvino A., Brañas F., Sánchez-Conde M., Guaraldi G. European cohorts of older HIV adults: POPPY, AGEhIV, GEPPO, COBRA and FUNCFRAIL. Eur Geriatr Med. 2019;10:247–257. doi: 10.1007/s41999-019-00170-8. [DOI] [PubMed] [Google Scholar]
- 51.Tate J.P., Sterne J.A.C., Justice A.C. Veterans Aging Cohort Study (VACS) and the Antiretroviral Therapy Cohort Collaboration (ART-CC). Albumin, white blood cell count, and body mass index improve discrimination of mortality in HIV-positive individuals. AIDS. 2019;33:903–912. doi: 10.1097/QAD.0000000000002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al Saedi A., Feehan J., Phu S., et al. Current and emerging biomarkers of frailty in the elderly. Clin Interv Aging. 2019;14:389–398. doi: 10.2147/CIA.S168687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 54.Guerville F., De S., Barreto P., Ader I., et al. Revisiting the hallmarks of aging to identify markers of biological age. J Prev Alzheimers Dis. 2020;7:56–64. doi: 10.14283/jpad.2019.50. [DOI] [PubMed] [Google Scholar]
- 55.Rivero-Segura N.A., Bello-Chavolla O.Y., Barrera-Vázquez O.S., Gutierrez-Robledo L.M., Gomez-Verjan JC. Promising biomarkers of human aging: in search of a multi-omics panel to understand the aging process from a multidimensional perspective. Ageing Res Rev. 2020;64 doi: 10.1016/j.arr.2020.101164. [DOI] [PubMed] [Google Scholar]
- 56.Blackburn E.H., Epel E.S., Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 57.Fazzini F., Lamina C., Raschenberger J., et al. Results from the German Chronic Kidney Disease (GCKD) study support association of relative telomere length with mortality in a large cohort of patients with moderate chronic kidney disease. Kidney Int. 2020;98:488–497. doi: 10.1016/j.kint.2020.02.034. [DOI] [PubMed] [Google Scholar]
- 58.Lin J., Epel E., Cheon J., et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J.C.Y., Leung J.M., Ngan D.A., et al. Absolute leukocyte telomere length in HIV-infected and uninfected individuals: evidence of accelerated cell senescence in HIV-associated chronic obstructive pulmonary disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alejos B., Stella-Ascariz N., Montejano R., et al. Determinants of blood telomere length in antiretroviral treatment-naïve HIV-positive participants enrolled in the NEAT 001/ANRS 143 clinical trial. HIV Med. 2019;20:691–698. doi: 10.1111/hiv.12791. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez-Serna A., Ajaykumar A., Gadawski I., et al. Rapid decrease in peripheral blood mononucleated cell telomere length after HIV seroconversion, but not HCV seroconversion. J Acquir Immune Defic Syndr. 2017;76:e29–e32. doi: 10.1097/QAI.0000000000001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leung J.M., Fishbane N., Jones M., et al. Longitudinal study of surrogate aging measures during human immunodeficiency virus seroconversion. Aging (Albany NY) 2017;9:687–705. doi: 10.18632/aging.101184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stella-Ascariz N., Montejano R., Rodriguez-Centeno J., et al. Blood telomere length changes after ritonavir-boosted darunavir combined with raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults infected with HIV-1. J Infect Dis. 2018;218:1523–1530. doi: 10.1093/infdis/jiy399. [DOI] [PubMed] [Google Scholar]
- 64.Raffenberg M., Engel T., Schoepf I.C., et al. Impact of delaying antiretroviral treatment during primary HIV infection on telomere length. J Infect Dis. 2021;10:1775–1784. doi: 10.1093/infdis/jiab186. [DOI] [PubMed] [Google Scholar]
- 65.Montejano R., Stella-Ascariz N., et al. Impact of Nucleos(t)ide reverse transcriptase inhibitors on blood telomere length changes in a prospective cohort of aviremic HIV-infected adults. J Infect Dis. 2018;218:1531–1540. doi: 10.1093/infdis/jiy364. [DOI] [PubMed] [Google Scholar]
- 66.Mehta S.R., Iudicello J.E., Lin J., et al. Telomere length is associated with HIV infection, methamphetamine use, inflammation, and comorbid disease risk. Drug Alcohol Depend. 2021;221 doi: 10.1016/j.drugalcdep.2021.108639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Engel T., Raffenberg M., Schoepf I.C., et al. Telomere length, traditional risk factors, HIV-related factors and coronary artery disease events in swiss persons living with HIV. Clin Infect Dis. 2021;73:e2070–e2076. doi: 10.1093/cid/ciaa1034. [DOI] [PubMed] [Google Scholar]
- 68.Russell E., Albert A., Côté H., et al. Rate of dyslipidemia higher among women living with HIV: a comparison of metabolic and cardiovascular health in a cohort to study aging in HIV. HIV Med. 2020;21:418–428. doi: 10.1111/hiv.12843. [DOI] [PubMed] [Google Scholar]
- 69.Sánchez-Conde M., Rodriguez-Centeno J., Dronda F., et al. Frailty phenotype: a clinical marker of age acceleration in the older HIV-infected population. Epigenomics. 2019;11:501–509. doi: 10.2217/epi-2018-0130. [DOI] [PubMed] [Google Scholar]
- 70.De Francesco D., Wit F.W., Bürkle A., et al. Do people living with HIV experience greater age advancement than their HIV-negative counterparts? AIDS. 2019;33:259–268. doi: 10.1097/QAD.0000000000002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levine M.E., Lu A.T., Quach A., et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018;10:573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu A.T., Quach A., Wilson J.G., et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY) 2019;11:303–327. doi: 10.18632/aging.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marioni R.E., Shah S., McRae A.F., et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25. doi: 10.1186/s13059-015-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rickabaugh T.M., Baxter R.M., Sehl M., et al. Acceleration of age-associated methylation patterns in HIV-1-infected adults. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nelson K.N., Hui Q., Rimland D., et al. Identification of HIV infection-related DNA methylation sites and advanced epigenetic aging in HIV-positive, treatment-naive U.S. veterans. AIDS. 2017;31:571–575. doi: 10.1097/QAD.0000000000001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang C.X., Schon E., Obeidat M., et al. Occurrence of accelerated epigenetic aging and methylation disruptions in human immunodeficiency virus infection before antiretroviral therapy. J Infect Dis. 2021;223:1681–1689. doi: 10.1093/infdis/jiaa599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Esteban-Cantos A., Rodríguez-Centeno J., Barruz P., et al. Epigenetic age acceleration changes 2 years after antiretroviral therapy initiation in adults with HIV: a substudy of the NEAT001/ANRS143 randomised trial. Lancet HIV. 2021;8:e197–e205. doi: 10.1016/S2352-3018(21)00006-0. [DOI] [PubMed] [Google Scholar]
- 79.Horvath S., Levine AJ. HIV-1 infection accelerates age according to the epigenetic clock. J Infect Dis. 2015;212:1563–1573. doi: 10.1093/infdis/jiv277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sehl M.E., Rickabaugh T.M., Shih R., et al. The effects of anti-retroviral therapy on epigenetic age acceleration observed in HIV-1-infected adults. Pathog Immun. 2020;5:291–311. doi: 10.20411/pai.v5i1.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Esteban-Cantos A., Montejano R., Rodríguez-Centeno J., et al. Longitudinal changes in epigenetic age acceleration in aviremic HIV-infected recipients of long-term antiretroviral treatment. J Infect Dis. 2021:jiab338. doi: 10.1093/infdis/jiab338. [DOI] [PubMed] [Google Scholar]
- 82.Sundermann E.E., Hussain M.A., Moore D.J., et al. Inflammation-related genes are associated with epigenetic aging in HIV. J Neurovirol. 2019;25:853–865. doi: 10.1007/s13365-019-00777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shu C., Justice A.C., Zhang X., et al. DNA methylation biomarker selected by an ensemble machine learning approach predicts mortality risk in an HIV-positive veteran population. Epigenetics. 2021;16:741–753. doi: 10.1080/15592294.2020.1824097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li X., Ploner A., Wang Y., et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife. 2020;9:e51507. doi: 10.7554/eLife.51507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shiau S., Arpadi S.M., Shen Y., et al. Epigenetic aging biomarkers associated with cognitive impairment in older African American adults with HIV. Clin Infect Dis. 2021;73:1982–1991. doi: 10.1093/cid/ciab563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X., Hu Y., Aouizerat B.E., et al. Machine learning selected smoking-associated DNA methylation signatures that predict HIV prognosis and mortality. Clin Epigenetics. 2018;10:155. doi: 10.1186/s13148-018-0591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pinto M., Moraes CT. Mechanisms linking mtDNA damage and aging. Free Radic Biol Med. 2015;85:250–258. doi: 10.1016/j.freeradbiomed.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hunt M., Payne BAI. Mitochondria and ageing with HIV. Curr Opin HIV AIDS. 2020;15:101–109. doi: 10.1097/COH.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 89.Hsieh A.Y.Y., Kimmel E., Pick N., et al. Inverse relationship between leukocyte telomere length attrition and blood mitochondrial DNA content loss over time. Aging (Albany NY) 2020;12:15196–15221. doi: 10.18632/aging.103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bürkle A., Moreno-Villanueva M., Bernhard J., et al. MARK-AGE biomarkers of ageing. Mech Ageing Dev. 2015;151:2–12. doi: 10.1016/j.mad.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 91.Cassol E., Misra V., Holman A., Kamat A., Morgello S., Gabuzda D. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect Dis. 2013;13:203. doi: 10.1186/1471-2334-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yeoh H.L., Cheng A.C., Cherry C.L., et al. Immunometabolic and lipidomic markers associated with the frailty index and quality of life in aging HIV+ men on antiretroviral therapy. EBioMedicine. 2017;22:112–121. doi: 10.1016/j.ebiom.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Babu H., Sperk M., Ambikan A.T., et al. Plasma metabolic signature and abnormalities in HIV-infected individuals on long-term successful antiretroviral therapy. Metabolites. 2019;9:E210. doi: 10.3390/metabo9100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Babu H., Ambikan A.T., Gabriel E.E., et al. Systemic inflammation and the increased risk of inflamm-aging and age-associated diseases in people living with HIV on long term suppressive antiretroviral therapy. Front Immunol. 2019;10:1965. doi: 10.3389/fimmu.2019.01965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Francesco D., Sabin C.A., Reiss P., Kootstra NA. Monocyte and T cell immune phenotypic profiles associated with age advancement differ between people with HIV, lifestyle-comparable controls and blood donors. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.581616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hove-Skovsgaard M., Zhao Y., Tingstedt J.L., et al. Impact of age and HIV status on immune activation, senescence and apoptosis. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.583569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bunet R., Nayrac M., Ramani H., et al. Loss of CD96 expression as a marker of HIV-specific CD8+ T-cell differentiation and dysfunction. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.673061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Falutz J. Frailty in people living with HIV. Curr HIV/AIDS Rep. 2020;17:226–236. doi: 10.1007/s11904-020-00494-2. [DOI] [PubMed] [Google Scholar]
- 99.Sundermann E.E., Hussain M.A., Moore D.J., Horvath S., Lin D.T.S., Kobor M.S., et al. Inflammation-related genes are associated with epigenetic aging in HIV. J Neurovirol. 2019;25:853–865. doi: 10.1007/s13365-019-00777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Armas L.R., Pallikkuth S., Pan L., Rinaldi S., Pahwa R., Pahwa S. Immunological age prediction in HIV-infected, ART-treated individuals. Aging (Albany NY) 2021;13:22772–22791. doi: 10.18632/aging.203625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cole J.H., Underwood J., Caan M.W.A., et al. Increased brain-predicted aging in treated HIV disease. Neurology. 2017;88:1349–1357. doi: 10.1212/WNL.0000000000003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cole J.H., Caan M.W.A., Underwood J., et al. No evidence for accelerated aging-related brain pathology in treated human immunodeficiency virus: longitudinal neuroimaging results from the comorbidity in relation to AIDS (COBRA) project. Clin Infect Dis. 2018;66:1899–1909. doi: 10.1093/cid/cix1124. [DOI] [PubMed] [Google Scholar]