Abstract
Functional cognitive disorder is common but underlying mechanisms remain poorly understood. Metacognition, an individual’s ability to reflect on and monitor cognitive processes, is likely to be relevant. Local metacognition refers to an ability to estimate confidence in cognitive performance on a moment-to-moment basis, whereas global metacognition refers to long-run self-evaluations of overall performance. Using a novel protocol comprising task-based measures and hierarchical Bayesian modelling, we compared local and global metacognitive performance in individuals with functional cognitive disorder. Eighteen participants with functional cognitive disorder (mean age = 49.2 years, 10 males) were recruited to this cross-sectional study. Participants completed computerized tasks that enabled local metacognitive efficiency for perception and memory to be measured using the hierarchical meta-d’ model within a signal detection theory framework. Participants also completed the Multifactorial Memory Questionnaire measuring global metacognition, and questionnaires measuring anxiety and depression. Estimates of local metacognitive efficiency were compared with those estimated from two control groups who had undergone comparable metacognitive tasks. Global metacognition scores were compared with the existing normative data. A hierarchical regression model was used to evaluate associations between global metacognition, depression and anxiety and local metacognitive efficiency, whilst simple linear regressions were used to evaluate whether affective symptomatology and local metacognitive confidence were associated with global metacognition. Participants with functional cognitive disorder had intact local metacognition for perception and memory when compared with controls, with the 95% highest density intervals for metacognitive efficiency overlapping with the two control groups in both cognitive domains. Functional cognitive disorder participants had significantly lower global metacognition scores compared with normative data; Multifactorial Memory Questionnaire-Ability subscale (t = 6.54, P < 0.0001) and Multifactorial Memory Questionnaire-Satisfaction subscale (t = 5.04, P < 0.0001). Mood scores, global metacognitive measures and metacognitive bias were not significantly associated with local metacognitive efficiency. Local metacognitive bias [β = −0.20 (SE = 0.09), q = 0.01] and higher depression scores as measured by the Patient Health Questionnaire-9 [β = −1.40 (SE = 2.56), q = 0.01] were associated with the lower global metacognition scores. We show that local metacognition is intact, whilst global metacognition is impaired, in functional cognitive disorder, suggesting a decoupling between the two metacognitive processes. In a Bayesian model, an aberrant prior (impaired global metacognition), may override bottom-up sensory input (intact local metacognition), giving rise to the subjective experience of abnormal cognitive processing. Future work should further investigate the interplay between local and global metacognition in functional cognitive disorder.
Keywords: functional cognitive disorder, metacognition, signal detection theory, depression, Bayesian model
Using a novel protocol comprising task-based measures and hierarchical Bayesian modelling, Bhome and McWilliams et al. report that local metacognition is intact but global metacognition is impaired in functional cognitive disorder. These findings, when incorporated within a Bayesian model of neuropsychiatric illness, may explain the pathogenesis of the condition.
Graphical Abstract
Graphical Abstract.
Introduction
Functional cognitive disorder (FCD) is an increasingly recognized condition and is seen in up to 25% of patients in cognitive assessment services.1 FCD is characterized by persistent and distressing cognitive symptoms that cause significant difficulty and cannot be explained by another disorder.1,2 Of note, patients with FCD may have co-morbid functional disorders and affective illnesses, but cognitive symptoms are in excess of those expected due to any co-morbidities. A canonical and often distinguishing diagnostic feature of FCD is cognitive internal inconsistency, which refers to a discrepancy between the individual’s subjective appraisal of their cognitive performance compared with objective evidence about that performance, as well as differences in cognitive performance under automatic and explicit control.2 Metacognition, the ability of an individual to reflect on and monitor their own cognitive processes,3,4 has been posited as a key mechanism contributing to cognitive internal inconsistency.1,5–8
The exact role of metacognitive processing in FCD remains poorly understood. Theoretical frameworks distinguish between two aspects of metacognition, global and local and evidence from behavioural experiments suggests that performance in one can modulate the other.9 Global metacognition refers to self-performance estimates (SPEs) about overall task performance, and in FCD these may be shaped by self-evaluation of cognitive performance, emotions associated with cognitive ability and perceived control over cognitive functioning.7,10 Global metacognition, measured using subjective rating tools, has been shown to be altered in people with FCD.7,11,12
Local metacognition—the ability to track changes in moment-to-moment cognitive performance—is focused on the appraisal of individual cognitive decisions as opposed to the evaluation of overall cognitive performance. Local metacognition can be characterized by two key parameters—metacognitive bias and metacognitive sensitivity. Metacognitive bias describes an individual’s average confidence level in their task performance, regardless of whether their judgments are objectively correct or incorrect—and therefore overlaps conceptually with notions of global metacognition. In contrast, metacognitive sensitivity refers to the ability to distinguish between correct and incorrect decisions using confidence ratings. This construct can be objectively quantified through a combination of task-based measures and Type 2 signal detection theory.13–15 However, metrics of metacognitive sensitivity are known to be confounded by variation in first-order cognitive performance and by an individual’s bias to give generally positive or negative confidence ratings.13 This has led to the development of model-based measures of metacognitive ‘efficiency’, such as meta-d’/d’, which characterize the level of metacognitive sensitivity relative to a particular level of task performance.15 Local metacognitive efficiency is likely to be relevant in FCD for two reasons. First, it is considered to play a role in directly modulating global metacognition which,9 as mentioned above, is itself distorted in FCD.7,11,12 Second, it likely regulates the expression of core symptoms of FCD, including increased but poorer quality monitoring for cognitive lapses,6,7 and misinterpretation of what may, in fact, be attentional lapses.16
In this study, we evaluated and compared global and local metacognition in FCD participants. Whilst global metacognition has previously been evaluated in people with FCD,7,11,12 ours is the first study to objectively measure local metacognition by using a novel protocol comprising task-based measures and hierarchical Bayesian modelling.13,17,18 We compared local metacognition in FCD with two healthy control groups constructed from previously collected data.19,20 In addition, we investigated whether depression and anxiety were associated with measures of global and local metacognition. Our primary hypothesis was that global and local metacognition are coupled and so both would be impaired in people with FCD. Secondarily, we hypothesized that symptoms of depression and anxiety may predict lower global self-confidence when giving metacognitive ratings, because the negative cognitions that arise in these conditions21 would affect individuals’ appraisal of their cognitive performance.
Materials and methods
Participants
Eighteen participants [aged 24–66 years, mean 49.22 (SD = 13.55); eight females] with an established diagnosis of FCD were recruited from two tertiary neuropsychiatric clinical services [National Hospital for Neurology and Neurosurgery (NHNN) (University College London Hospitals NHS Trust) and South West London and St George’s Mental Health Trust] between April 2019 and February 2020. Thirty potential participants were contacted in this period, and though not objectively evaluated, there were no noticeable differences in terms of clinical characteristics between those who participated and those who did not. Participants provided informed consent, which was obtained according to the Declaration of Helsinki principles for medical research.
Inclusion criteria
Inclusion criteria were age 18 or above, a diagnosis of FCD and provision of informed consent to participate in the study. The diagnosis of FCD was made in a tertiary specialist neuropsychiatry service (including assessment by a consultant neuropsychiatrist with expertise in this area) and based on the following criteria2: (i) the presence of one or more symptoms of impaired cognitive function causing clinically significant distress and/or impairment in functioning, (ii) evidence of cognitive internal inconsistency (see ‘Introduction’ section) and (iii) symptoms of impaired cognitive functioning that cannot be accounted for by an alternative medical condition. Patients with a diagnosis of probable or possible neurodegenerative disorder on the basis of neuroimaging, neuropsychological and clinical assessment were excluded, as were patients with a primary psychiatric disorder such as schizophrenia, bipolar affective disorder and personality disorder. Patients with co-morbid depression or anxiety were included if it was judged to be mild in severity and the cognitive symptoms were considered well in excess of those known to occur in those conditions.
Comparison groups
One group (Control Group 1)19 was drawn retrospectively from a large (n = 304) web study of healthy volunteers, recruited via the academic crowdsourcing site, Prolific (https://www.prolific.co/). Controls were matched by age alone, irrespective of gender, to the patient group, in the ratio of 3:1, respectively. The same tasks were undertaken as those completed by the FCD group, with an identical staircasing procedure used to control for first-order task performance, although there were some minor differences between task versions for the two groups, as the FCD group completed an earlier version of the tasks (see below).
A second control group (Control Group 2)20 was formed by re-analysing previously published behavioural data from a neuroimaging study of healthy volunteers at New York University (NYU), undertaking tasks of a similar psychophysical structure to our own.
Local metacognition
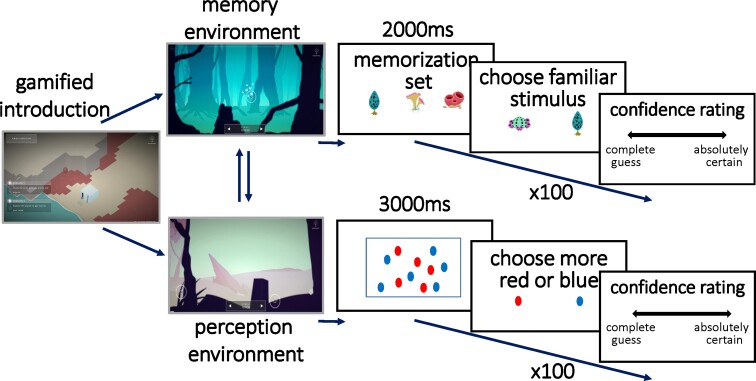
A task measuring metacognition for (working) memory and a task measuring metacognition for perception (vision) were administered. Each contained five mini-blocks of 20 trials, with each trial involving a two-alternative forced-choice task followed immediately by a metacognitive (confidence) rating, in line with standard task structures used in previous research.20 An optional set of five practice trials before each set of 20 trials allowed participants to familiarize themselves with the first-order task and the confidence rating sliding scale.
In each recognition memory trial, an array of stimuli is presented for 2 seconds for memorization. A novel stimulus is then presented together with a stimulus from the memorization set, and the participant is asked to indicate which stimulus they had memorized. The subject is then asked, ‘How confident are you?’ and must make a metacognitive judgement using a sliding scale, which is marked with the labels at either end of ‘complete guess’ or ‘absolutely certain’ (Fig. 1). Task difficulty is staircased to increase subject motivation and control first-order performance, by increasing or decreasing the number of items in the stimulus sets presented for memorization. The staircase aims to keep task accuracy for each subject around 70–72%, by adjusting difficulty in response to changes in first-order task performance in line with established protocols.22 When a subject gives an incorrect response, the task is made easier by one increment (decreasing by one the size of the memory stimulus set presented for memorization). When a subject gives two consecutive correct responses, the task is made more difficult by one increment (increasing by one the size of the memory stimulus set). The memory task undertaken by the FCD group employed stimuli, which were less visually appealing, with plain white, rather than coloured, stimuli. For each of the five memory task themes, there were 12 available stimuli for display to the FCD group, whereas the version used by the control group had 25 stimuli within each task theme.
Figure 1.
Task structure. Participants were tested on both recognition memory (left) and perceptual discrimination (right) tasks. In both tasks, participants are: (i) presented stimuli (for 2000 ms in the recognition memory task; 3000 ms in the perceptual discrimination task); (ii) asked to make an unspeeded two-alternative forced-choice based on presented stimuli and (iii) asked to make an unspeeded metacognitive judgement on a sliding scale.
In each perceptual discrimination trial, the subject is presented with an array of identical red and blue shapes for 3 seconds. Subjects are then asked whether there were more red or blue shapes. They then undertake the same metacognitive rating judgement as in the memory task, before moving onto the next perceptual trial (Fig. 1). The version given to the FCD group used less visually appealing stimuli and a first trial presenting 41 shapes of one colour and 25 of the other, whereas the control groups were shown 40 and 25. The difficulty was staircased by increasing or decreasing this difference, again aiming to converge the first-order task accuracy of each subject to 70–72%.22 When a subject gives an incorrect response, the task is made easier by one increment (increasing the difference by one, through increasing the larger number of coloured shapes presented). When a subject gives two consecutive correct responses, the task is made more difficult by one increment (decreasing the difference by one, through decreasing the larger number of coloured shapes presented).
The first 20 trials from each task (memory and perception) were removed prior to analysis to allow for learning effects and the difficulty staircase used to calibrate task difficulty to stabilize. Trials where subjects took longer than 30 seconds to respond to any instruction were also removed.
Self-reported measures
Global metacognition was measured using the Multifactorial Memory Questionnaire (MMQ).23 The MMQ has three subscales: MMQ-Ability measuring self-appraisal of memory ability, MMQ-Satisfaction measuring satisfaction and concern about memory and MMQ-Strategy which measures the use of compensatory strategies and cognitive aids in daily functioning. Lower scores in the first two subscales suggest lower global memory SPEs whilst higher scores in the MMQ-Strategy subscale indicate greater subjective use of memory strategies.
The Patient Health Questionnaire-9 (PHQ-9)24 and the Generalised Anxiety Disorder-7 (GAD-7)25 scale were used to measure symptoms of depression and anxiety, respectively.
Statistical analysis
The IBM SPSS Version 22 was used to calculate descriptive statistics for study variables including age, MMQ subscale scores, PHQ-9 and GAD-7. The MMQ subscale scores in our study for FCD patients were compared with normative data26 using independent t-tests.
To analyse local metacognitive data, the meta-d’ model was used to calculate metacognitive efficiency within a signal detection theoretic framework.15 Meta-d’ reflects an individual’s metacognitive sensitivity, namely, how well a subject discriminates correct from incorrect responses. The meta-d’/d’ ratio (M-ratio) is known as metacognitive efficiency, and quantifies metacognitive sensitivity (meta-d’) relative to task performance (d’). An optimal value for metacognitive efficiency is therefore 1. Local metacognitive efficiency was calculated using a hierarchical Bayesian model as implemented within the hierarchical meta-d’ (HMeta-d) toolbox18 in JAGS. This toolbox was developed with the aim of allowing robust estimates of local metacognitive efficiency in situations where limited per participant task data are available, such as in clinical populations. Hierarchical Bayesian modelling was used to generate group-level parameter estimates, thereby allowing direct inference on group comparisons (such as patients versus controls) whilst avoiding reliance on noisy point estimates of single-subject parameters.18 Certainty on these parameters (the group-level M-ratios), was determined by computing the 95% high-density interval (HDI) from the sampled estimates of posteriors.27 Metacognitive efficiency at the group level could thus be compared with existing data from healthy controls by computing the HDIs of the differences between the estimates of parameters obtained from each dataset.
An extended version of the HMeta-d model (the RHMeta-d model) has been developed to hierarchically estimate regression parameters relating metacognitive efficiency to covariates of interest.28 Whilst capitalizing on the power of hierarchical estimation, the extended model avoids the problems encountered by post hoc regressions on hierarchical model parameters such as unwanted shrinkage to the group mean. This model was used to regress MMQ, PHQ-9 and GAD-7 scores on memory M-ratio. Although the hierarchical regression model was able to return a robust characterization of influences on metacognitive efficiency at the group level, the small numbers of trials (N = 80) completed by each individual subject meant that it was not possible to reliably identify individual-level M-ratios.
Associations of demographic characteristics, affective symptomatology and other metacognitive measures with global metacognition, measured using MMQ-Ability, were evaluated using simple linear regression models. Covariates were not included because age and gender are known to not significantly affect MMQ subscale scores.26 Whilst measured and interpreted individually, for theoretical reasons there is likely to be a strong correlation between different MMQ subscales and so these were not included as covariates. A false discovery rate (FDR) correction was performed for the six dependent variables included in these analyses, using the Benjamini–Hochberg method.29
The M-ratio for memory was chosen rather than the M-ratio for perception in these analyses because the MMQ is most focussed on symptoms of memory impairment—rather than symptoms concerning other types of cognition—thereby enabling a domain-specific evaluation of the interplay between local and global metacognition.
Missing data
For particular metacognitive tasks or questionnaire measures, participants were excluded if they could not tolerate or complete testing and this is highlighted in the ‘Results’ section. Analyses were performed on available data without imputation.
Ethical approval
Ethical approval (ref: 18/LO/1056) from NHS Health Research Authority and Health and Care Research Wales (HCRW) and London-South East Research Ethics Committee (REC) was granted to carry out this study using FCD patients. Ethical approval for the collection of data from healthy volunteers was given by the University College London Ethics Committee (ref: 12/0006).
Data availability
Data used in this study will be shared upon reasonable request to the corresponding author. All data and statistics generated from this study are presented.
Results
Demographics, depression and anxiety scores
Of the 18 participants in this study, eight (44%) were female. The average age was 49.2 years (SD 13.6, range 24–62) (Table 1). Based on validated PHQ-9 cut-offs, 14 (82%) were in the depressed range, 3 (18%) with mild symptoms, 7 (41%) moderate, 3 (18%) moderately severe and 1 (6%) severe. Based on GAD-7 cut-offs, 10 (59%) of the participants were in the anxious range—7 (41%) with mild symptoms, 2 (12%) moderate and 1 (6%) severe symptoms of anxiety. Control Group 119 was matched for age and consisted of 54 individuals, with a mean age of 49.1 years (SD 13.3, range 24–62). Control Group 220 participants were significantly younger (n = 30, mean 24.97, SD = 4.44, range 18–33) in comparison to the FCD study population (t = 9.04, df = 46, P < 0.001).
Table 1.
Demographics, mood and global metacognitive characteristics of functional cognitive disorder participants compared with the normative sample
| FCD participants (n = 18) | Normative dataa (n = 401) | ||||
|---|---|---|---|---|---|
| Attribute | Mean (SD) | Range | Mean (SD) | Range | Statisticb |
| Age | 49.2 (13.6) | 24–66 | 71.4 (8.9) | 39–91 | t = 10.08, df = 417, P < 0.0001 |
| Gender, male, n (%) | 10 (56) | 116 (29) | χ 2 = 5.81, P = 0.02 | ||
| PHQ-9c | 11.5 (5.6) | 3–25 | |||
| GAD-7c | 7.0 (4.8) | 0–20 | |||
| MMQ-Satisfactionc | 26.9 (11.9) | 12–56 | 43.9 (13.7) | 7–72 | t = 5.04, df = 416, P < 0.0001 |
| MMQ-Abilityc | 30.4 (14.7) | 12–50 | 48.8 (11.2) | 0–80 | t = 6.54, df = 416, P < 0.0001 |
| MMQ-Strategyc | 36.8 (11.9) | 19–57 | 37.3 (10.4) | 1–64 | t = 0.19, df = 416, P = 0.85 |
GAD-7, Generalised anxiety disorder-7; PHQ-9, Patient Health Questionnaire-9; MMQ, Multifactorial Memory Questionnaire.
Normative MMQ data.23
Independent t-tests (MMQ data and age) and χ2 (gender) were used to compare FCD participants with normative MMQ data.
n = 17.
Local metacognition task
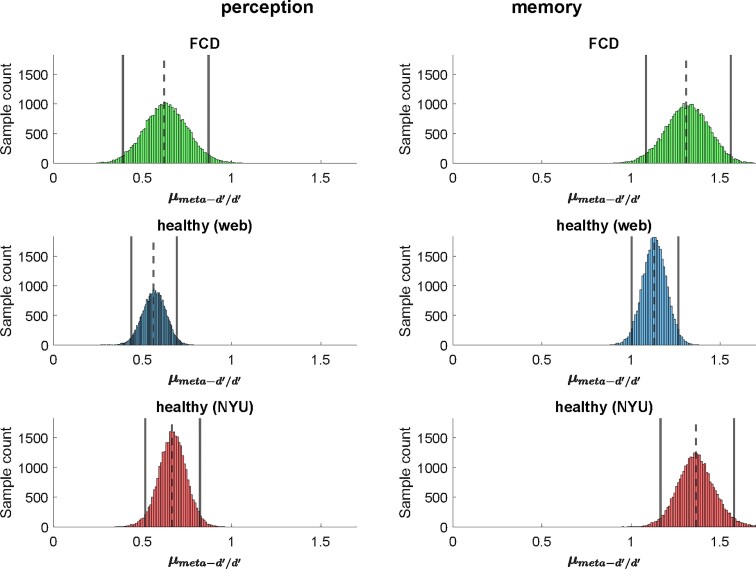
First-order task performance for each group is presented using the signal detection theory-derived metric d’ (Table 2). The minimal inter-subject variability in d’ indicates that task difficulty was appropriately titrated for each individual using staircases. Group-level parameters estimated for M-ratio were compared between FCD participants and the two control groups (Fig. 2). Analysis of perceptual metacognition data was undertaken for only 14 of the FCD participants: four were excluded for completing too few trials. The means of the posterior densities of group-level M-ratio estimates for FCD participants undertaking both the perceptual (0.62) and memory (1.31) tasks are very close to those found in the healthy Control Group 1 (0.56 and 1.13, respectively) and Control Group 2 (0.66 and 1.36). HDIs for the patient and control groups can be seen to be almost coincident, indicating no evidence of a difference in local metacognition between the groups (Table 2).
Table 2.
Measures of local metacognition
| Group | Perception | Memory | ||||
|---|---|---|---|---|---|---|
| n | d’a | M-ratiob (HDI) | n | d’a | M-ratiob (HDI) | |
| FCD | 14c | 1.26 (0.40) | 0.62 [0.39, 0.87] | 18 | 1.03 (0.19) | 1.31 (1.08, 1.56) |
| Controls 1 | 42 | 1.33 (0.23) | 0.56 [0.43, 0.69] | 54 | 1.14 (0.21) | 1.13 (1.00, 1.26) |
| Controls 2 | 24 | 1.13 (0.35) | 0.66 [0.51, 0.82] | 24 | 1.12 (0.40) | 1.36 (1.17, 1.58) |
FCD, functional cognitive disorder; HDI, 95% highest density interval of sampled M-ratios.
d’, a metric of objective task performance per participant: mean (SD).
Metacognitive efficiency, calculated as meta-d’/d’: mean (SD) of sampled group parameters.
n = 14 because four participants were unable to complete an adequate number of perception trials.
Figure 2.
Local metacognition is intact in FCD. Posterior densities of the estimates of group mean metacognitive efficiency (M-ratio) are shown for perception and memory, in FCD, Control Group 1 [healthy (web)] and Control Group 2 [healthy (NYU)]. Vertical bars show the 95% highest density interval for each M-ratio and dotted vertical bars the mean. Overlap between the HDIs across panels indicates that the FCD group has a similar level of local metacognitive efficiency compared with the controls in both domains.
All three groups had better metacognitive efficiency for memory than for perceptual tasks, consistent with previous findings. For each group of participants, in order to generate a Bayesian probability that M-ratios differed between the domains, differences were calculated between the posteriors from the analyses of the memory and of the perception task. This provided strong evidence for a difference, which was present in each of the three groups of participants (FCD: P = 0.9999; Control Group 1: P = 1; Control Group 2: P = 1).
Global metacognition
We compared global metacognition scores in our FCD participants with existing, validated normative data (Table 1).26 The FCD sample was significantly younger than the normative population (mean = 71.4, SD = 8.9, range = 39–91) (P < 0.001). Mean MMQ-Ability, a surrogate for global SPEs, was significantly lower in our study participants with FCD (n = 17, mean = 30.4, SD = 14.7, range = 12–50) compared with the normative sample (n = 401, mean = 48.8, SD = 11.2, range = 0–80) (t = 6.54, df = 416, P < 0.0001). MMQ-Satisfaction scores amongst FCD participants (n = 17, mean = 26.9, SD = 11.9, range = 12–56) were also significantly lower, indicating greater dissatisfaction with and concern about memory performance than the normative sample (n = 401, mean = 43.9, SD = 13.7, range = 7–72) (t = 5.04, df = 416, P < 0.0001).
Predictors of local and global metacognition
For the 17 FCD participants who completed questionnaire measures, two regressions were performed using the hierarchical model for memory M-ratio: one with metacognitive bias as the sole independent variable, and one with five covariates taken from the questionnaire measures (GAD-7, PHQ-9 and each MMQ subscale). The HDIs for our estimations of regression betas for each predictor all contained zero and therefore did not provide evidence for linear relationships (Table 3). However, given the small numbers of trials and subjects entering into this regression, we note a relatively strong underlying relationship would have been needed to override the hierarchical group prior to zero effect.
Table 3.
Hierarchical multiple regression predicting memory metacognitive efficiency
| Attribute | Mean sampled beta | HDIa |
|---|---|---|
| Regression 1 (five covariates) | ||
| GAD-7 | 0.018 | (−0.040, 0.074) |
| PHQ-9 | 0.011 | (−0.049, 0.074) |
| MMQ-Satisfaction | −0.008 | (−0.036, 0.020) |
| MMQ-Strategies | 0.003 | (−0.020, 0.024) |
| MMQ-Ability | 0.010 | (−0.011, 0.030) |
| Regression 2 (one covariate) | ||
| Metacognitive bias | −0.00014 | (−0.0057, 0.0057) |
PHQ-9, Patient Health Questionnaire-9; GAD-7, Generalised Anxiety Disorder-7; MMQ, Multifactorial Memory Questionnaire.
Sampled beta 95% Highest Density Interval. A 95% HDI, which did not span zero would provide evidence that the effect of that predictor on metacognitive efficiency differed significantly from zero.
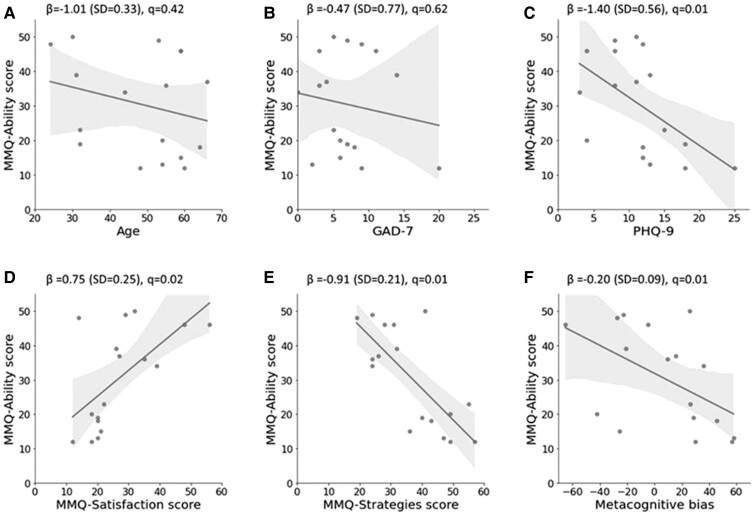
Simple linear regressions were used to evaluate predictors of global metacognition (Fig. 3). More severe depression as measured by PHQ-9 was significantly associated with lower global SPEs as defined by the MMQ-Ability scores [β = −1.40 (SE = 2.56), q = 0.01]. Greater satisfaction about memory performance was significantly associated with higher global SPEs [β = 0.75 (SE = 0.25), q = 0.02] whilst a greater use of strategies was significantly associated with lower global SPEs [β = −0.91 (SE = 0.21), q = 0.01] (Table 4).
Figure 3.
Relationship of age, affective symptomatology and metacognitive measures with global metacognition. Regression plots for FCD participants with MMQ-Ability score as the dependent variable (y-axis) and the following independent variables: (A) age; (B) GAD-7; (C) PHQ-9; (D) MMQ-Satisfaction score; (E) MMQ-Strategies score; (F) local metacognitive bias in the memory task. β coefficient values are presented along with q values that were calculated following FDR correction using the Benjamini–Hochberg method. GAD-7, Generalised Anxiety Disorder-7; PHQ-9, Patient Health Questionnaire-9; MMQ, Multifactorial Memory Questionnaire. n = 17 as one participant did not complete the MMQ questionnaire.
Table 4.
Association of demographic factors, mood and metacognitive measures with global metacognition (MMQ-Ability)
| Attribute | Adjusted beta (SE) | Beta confidence interval | P-valuea | q-valueb |
|---|---|---|---|---|
| Age | −1.01 (0.33) | −0.84 to 0.30 | 0.33 | 0.42 |
| Gender | 1.49 (7.36) | −14.20 to 17.17 | 0.84 | 0.840 |
| GAD-7 | −0.47 (0.77) | −2.11 to 1.17 | 0.55 | 0.62 |
| PHQ-9 | −1.40 (0.56) | −2.59 to −0.21 | 0.004 | 0.01 |
| MMQ-Satisfaction | 0.75 (0.25) | 0.23–1.28 | 0.008 | 0.02 |
| MMQ-Strategies | −0.91 (0.21) | −1.35 to 0.47 | 0.001 | 0.01 |
| Metacognitive bias (memory task) | −0.20 (0.09) | −0.40 to −0.01 | 0.004 | 0.01 |
GAD-7, Generalised Anxiety Disorder-7; PHQ-9, Patient Health Questionnaire-9; MMQ, Multifactorial Memory Questionnaire. In bold results showing FDR-corrected statistically significant associations.
P-values were analysed by a linear regression model using the ordinary least squares (OLS) method.
q-values were calculated following FDR correction using the Benjamini–Hochberg method.
Relationship between local and global metacognition
Local metacognitive bias in the memory task was significantly associated with MMQ-Ability [β = −0.20 (SE = 0.09), q = −0.01] suggesting that higher local confidence estimates in the memory task were unexpectedly associated with lower global SPEs about memory.
Discussion
Dysregulated metacognitive processing has been postulated as a unifying mechanism in FCD.1,5–8 A key finding in FCD is self-reports of low confidence in cognitive abilities in the absence of objective deficits, meaning that people with FCD explicitly report a metacognitive symptom. This study, therefore, sought to characterize local and global metacognitive processing in a sample of FCD patients. We found evidence for a dissociation between local and global metacognition: in FCD participants, local metacognitive efficiency was intact, whilst global metacognition was impaired. Additionally, in FCD, more strongly positive local metacognitive bias and higher depression scores were significantly associated with poorer global metacognition.
In our study, local metacognitive performance in FCD was similar to that in samples of healthy subjects.19,20 Metacognitive efficiency for memory tasks was higher than that in visual perception tasks as has been reported previously,20,30 which may be due to control processes playing a greater role on decision confidence in memory compared with visual perception tasks and/or differences in the structure of the underlying evidence (perceptual, mnemonic) entering the decision process.30
FCD participants had significantly lower self-appraisals of memory ability (lower SPEs) as well as less satisfaction about their memory compared with existing normative data. This is consistent with previous work,7,11 which also used self-reported questionnaires to measure global metacognition.
The dissociation between local and global metacognitive processing that we found in our study went against our primary hypothesis that both of these levels of metacognition would be impaired in FCD. However, it is possible that this dissociation is the very essence of cognitive internal inconsistency which is a canonical feature of FCD.2 An alternative explanation for our findings is that a person who is overly concerned about their cognitive performance might routinely attend more closely to and monitor that performance, so that they would ultimately develop effective local metacognitive discrimination that is indistinguishable from controls.
A dissociation between local and global metacognition can be explained using a Bayesian model of functional neurological disorder.31 Tailoring the model to FCD, this model posits that people with FCD have an abnormal set of expectations—or ‘priors’, in the Bayesian sense—which contribute to the decoupling of top-down predictions of cognitive performance and bottom-up sensory information about actual cognitive performance. Local metacognition describes the ability to dynamically monitor moment-to-moment cognitive performance, thereby playing a crucial role in evaluating performance on individual decisions.32 This would correspond to the bottom-up sensory component within such a Bayesian model.
Meanwhile, global metacognition is characterized by the construction of priors or expectations and if impaired, this prior may lead to predictions of poor cognitive performance. In other words, an abnormal prior (global metacognition) may override intact bottom-up sensory input (local computations of confidence in individual decisions), giving rise to the perceived experience of impaired cognitive performance. In such a computational model,31 as has been postulated previously,6 if higher-order brain functions responsible for local metacognition are uncoupled from the predictions of the prior (shaped by global metacognition), the resulting cognitive difficulties may be perceived as being outside the subject’s control.
The relationship between local and global metacognition remains unclear, even in healthy subjects. We found that, in FCD participants, greater local confidence (metacognitive bias) was significantly associated with lower global metacognitive estimates. This was unexpected, and is in contrast to work in healthy participants which shows that confidence for individual decisions at a local metacognitive level is positively coupled to global SPEs in tasks that require learning about performance over time.9 In FCD, it is possible that this usage of local confidence signals to update global SPEs becomes disrupted, reflecting a pathological decoupling and loss of interaction between local and global metacognitive processing. Recent work using brain imaging suggests that a midline network spanning the ventromedial prefrontal cortex and precuneus may simultaneously represent both local and global confidence signals—indicating a potential anatomical locus for an interaction between these levels.33 Such a hypothesis could be further tested in FCD by employing tasks that examine the learning of global SPEs over time.9 Global confidence estimates can also be incorporated as additional hierarchical levels within Bayesian models of metacognition. Mechanistically, such work could, therefore, also shed light on the mismatch between incorrect top-down predictions and bottom-up sensory input in the Bayesian model of FCD described above.
A potential therapeutic approach would be to target improving global metacognition directly in FCD.6 Metternich et al. randomized controlled study34 demonstrated that a group psychological intervention comprising cognitive restructuring and psychoeducation led to significant improvements in memory self-efficacy [derived from three subscales of the Metamemory in Adulthood Questionnaire (MIA)],35 a measure of global metacognition. Interestingly, global metacognition was significantly improved when measured at 6 months follow-up but not immediately at the end of the intervention, suggesting that it may take time for cognitive restructuring to take place. This delay may conceal the effectiveness of the intervention from clinicians and could contribute to the early termination of some psychological treatments because of lack of perceived efficacy.
Self-reported symptoms of depression but not anxiety were significantly associated with impaired global metacognition within the FCD group. The increased negative cognitions21 and neuroticism36 that people with depression experience may extend to their appraisal of their own cognitive performance. It might be expected that as an individual’s level of depression increases, their self-appraisal of cognitive performance will become more pessimistic and inaccurate, thereby leading to a reinforcing cycle. Clearly, not everyone with depression presents with global metacognitive deficits but when they arise co-morbidly there is likely to be a synergistic effect.37
Limitations
The two key variables of interest, local and global metacognition were compared with existing data sets of healthy controls, which were not matched for gender or psychiatric co-morbidities and only one of the control groups was matched for age. In terms of age, participants in one of the comparison groups for local metacognition were significantly younger than our study participants,20 although, if anything, there is some evidence that local metacognitive efficiency may worsen with age,38 thereby making our finding of no significant differences in local metacognitive processing between these groups more striking. Furthermore, this control group did not differ in local metacognitive parameters from the age-matched control group and FCD group. MMQ-Ability, which we used to measure global metacognition, has a negligible relationship with age and gender,26 and so not having a gender- and age-matched comparison group for global metacognition is unlikely to have influenced our results.
We did not measure objective cognitive performance in either the FCD or control groups. This is not a limitation when evaluating local metacognition because there is an inbuilt staircase to the computerized task, which adjusts difficulty to ensure all participants have the same accuracy on tasks thereby removing actual objective cognitive performance as a confounder. For global metacognition, objective cognitive performance may vary between the FCD group and normative sample, although reassuringly in neither group were participants with impaired cognitive performance suggestive of neurodegenerative disorders included.
Normative samples were also not matched for levels of depression or anxiety symptoms. This may be relevant in the analysis of local metacognition because previous work has demonstrated that symptom dimensions related to depression and anxiety are associated with slightly higher metacognitive efficiency scores.39 Future work is required to explore the relationship between depression, FCD and metacognition.
The measurement of global metacognition relied on the use of the MMQ which is a self-reported questionnaire raising the possibility of response bias. In this regard, the development of behavioural experiments and analysis pipelines that enable global metacognition to be quantified objectively would be timely.9
Finally, FCD is an aetiologically heterogeneous disorder.1,37,40,41 The small sample size in this study meant that it was not possible to classify participants with different subtypes of FCD,1,41 and it remains possible that there may be variability in metacognition between these subgroups. Additionally, there is a risk of sampling bias given that participants were recruited from tertiary neuropsychiatric services and may not be representative of the significantly wider population of FCD patients who are managed in primary and secondary care.1
Conclusions
Our findings suggest a decoupling of metacognitive processes in people with FCD wherein local metacognition is intact but global metacognition is impaired. Future work should incorporate recently developed behavioural experiments and analysis pipelines that evaluate how local metacognition influences global metacognition.9 A potential mechanistically plausible treatment may focus on re-establishing links between local and global metacognition.
In the meantime, given the lack of consensus on treatment in clinical practice, a psychological intervention focussed on improving global metacognition through cognitive restructuring and psychoeducation may be beneficial for FCD patients.
Acknowledgements
We thank Dr Jorge Morales for providing full and swift access to data re-analysed here, as well as to relevant analysis code.
Abbreviations
- FCD =
functional cognitive disorder
- GAD-7 =
Generalised Anxiety Disorder-7
- HDI =
high-density interval
- MMQ =
Multifactorial Memory Questionnaire
- M-ratio =
meta-d’/d’ ratio
- PHQ-9 =
Patient Health Questionnaire-9
- SPEs =
self-performance estimates
Funding
R.B. is supported by a Wolfson-Eisai Clinical Research Training Fellowship. R.J.H. is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre (NIHR UCLH BRC). A.M. is supported by the Mental Health and Justice Project funded by the Wellcome Trust (203376/2/16/Z). The Wellcome Centre for Human Neuroimaging is supported by core funding from the Wellcome Trust (203147/Z/16/Z). S.M.F. is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (206648/Z/17/Z). J.D.H. is funded by a Wellcome Clinical Research Career Development Fellowship (214547/Z/18/Z). For the purpose of Open Access, the author has applied a CC-BY public copyright licence to any author accepted manuscript version arising from this submission.
Competing interests
The authors report no competing interests.
References
- 1. McWhirter L, Ritchie C, Stone J, Carson A. Functional cognitive disorders: A systematic review. Lancet Psychiatry. 2020;7:191–207. [DOI] [PubMed] [Google Scholar]
- 2. Ball HA, McWhirter L, Ballard C, et al. Functional cognitive disorder: Dementia’s blind spot. Brain. 2020;143:2895–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fleming SM, Dolan RJ, Frith CD. Metacognition: Computation, biology and function. Philos Trans R Soc Lond B Biol Sci. 2012;367:1280–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nelson TO, Narens L. Metamemory: A theoretical framework and new findings. In Bower GH, ed. The psychology of learning and motivation. Academic Press; 1990; 125–173. [Google Scholar]
- 5. Bharambe V, Larner AJ. Functional cognitive disorders: Demographic and clinical features contribute to a positive diagnosis. Neurodegener Dis Manag. 2018;8:377–383. [DOI] [PubMed] [Google Scholar]
- 6. Bhome R, McWilliams A, Huntley JD, Fleming SM, Howard RJ. Metacognition in functional cognitive disorder —a potential mechanism and treatment target. Cogn Neuropsychiatry. 2019;24:311–321. [DOI] [PubMed] [Google Scholar]
- 7. Metternich B, Schmidtke K, Hull M. How are memory complaints in functional memory disorder related to measures of affect, metamemory and cognition? J Psychosom Res. 2009;66:435–444. [DOI] [PubMed] [Google Scholar]
- 8. Pennington C, Newson M, Hayre A, Coulthard E. Functional cognitive disorder: What is it and what to do about it? Pract Neurol. 2015;15:436–444. [DOI] [PubMed] [Google Scholar]
- 9. Rouault M, Dayan P, Fleming SM. Forming global estimates of self-performance from local confidence. Nat Commun. 2019;10:1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunlosky J, Thiede KW. Metamemory. In: Reisberg D, ed. The Oxford handbook of cognitive psychology. Oxford University Press; 2013:283–298. [Google Scholar]
- 11. Larner AJ. Metamemory: A construct with diagnostic utility in a cognitive disorders clinic? Int J Geriatr Psychiatry. 2018;33:553–554. [DOI] [PubMed] [Google Scholar]
- 12. Schmidtke K, Metternich B. Validation of two inventories for the diagnosis and monitoring of functional memory disorder. J Psychosom Res. 2009;67:245–251. [DOI] [PubMed] [Google Scholar]
- 13. Fleming SM, Lau HC. How to measure metacognition. Front Hum Neurosci. 2014;8:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galvin SJ, Podd JV, Drga V, Whitmore J. Type 2 tasks in the theory of signal detectability: Discrimination between correct and incorrect decisions. Psychon Bull Rev. 2003;10:843–876. [DOI] [PubMed] [Google Scholar]
- 15. Maniscalco B, Lau H. A signal detection theoretic approach for estimating metacognitive sensitivity from confidence ratings. Conscious Cogn. 2012;21:422–430. [DOI] [PubMed] [Google Scholar]
- 16. Teodoro T, Edwards MJ, Isaacs JD. A unifying theory for cognitive abnormalities in functional neurological disorders, fibromyalgia and chronic fatigue syndrome: Systematic review. J Neurol Neurosurg Psychiatry. 2018;89:1308–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carpenter J, Sherman MT, Kievit RA, Seth AK, Lau H, Fleming SM. Domain-general enhancements of metacognitive ability through adaptive training. J Exp Psychol Gen. 2019;148:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fleming SM. HMeta-d: Hierarchical Bayesian estimation of metacognitive efficiency from confidence ratings. Neurosci Conscious. 2017;2017:nix007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McWilliams A, Bibby H, Steinbeis N, David AS, Fleming SM. Age-related decreases in global metacognition are independent of local metacognition and task performance [Internet]. 2022. https://psyarxiv.com/nmhxv [DOI] [PMC free article] [PubMed]
- 20. Morales J, Lau H, Fleming SM. Domain-general and domain-specific patterns of activity supporting metacognition in human prefrontal cortex. J Neurosci. 2018;38:3534–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beevers CG, Miller IW. Unlinking negative cognition and symptoms of depression: Evidence of a specific treatment effect for cognitive therapy. J Consult Clin Psychol. 2005;73:68–77. [DOI] [PubMed] [Google Scholar]
- 22. Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49:467. [PubMed] [Google Scholar]
- 23. Troyer AK, Rich JB. Psychometric properties of a new metamemory questionnaire for older adults. J Gerontol B Psychol Sci Soc Sci. 2002;57:P19–P27. [DOI] [PubMed] [Google Scholar]
- 24. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spitzer RL, Kroenke K, Williams JBW, Lowe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006;166:1092–1097. [DOI] [PubMed] [Google Scholar]
- 26. Multifactorial Memory Questionnaire . Professional manual. https://www.baycrest.org/Baycrest_Centre/media/content/form_files/MMQ-Manual-2018_ebook.pdf. Accessed 31 March 2021.
- 27. Kruschke J. Doing Bayesian data analysis: A tutorial introduction with R and BUGS. Academic Press; 2010. [Google Scholar]
- 28. Harrison OK, Garfinkel SN, Marlow L, et al. The Filter Detection Task for measurement of breathing-related interoception and metacognition. Biol Psychol. 2021;165:108185. [DOI] [PubMed] [Google Scholar]
- 29. Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol. 1995;57:289–300. [Google Scholar]
- 30. Mazancieux A, Fleming SM, Souchay C, Moulin CJA. Is there a G factor for metacognition? Correlations in retrospective metacognitive sensitivity across tasks. J Exp Psychol Gen. 2020;149:1788–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edwards MJ, Adams RA, Brown H, Parees I, Friston KJ. A Bayesian account of ‘hysteria’. Brain. 2012;135:3495–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fernandez-Duque D, Baird JA, Posner MI. Awareness and metacognition. Conscious Cogn. 2000;9:324–326. [DOI] [PubMed] [Google Scholar]
- 33. Rouault M, Fleming SM. Formation of global self-beliefs in the human brain. Proc Natl Acad Sci U S A. 2020;117:27268–27276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Metternich B, Schmidtke K, Harter M, Dykierek P, Hull M. Development and evaluation of a group therapy for functional memory and attention disorder. Psychother Psychosom Med Psychol. 2010;60:202–210. [DOI] [PubMed] [Google Scholar]
- 35. Dixon RA, Hultsch DF, Hertzog C. The Metamemory in Adulthood (MIA) questionnaire. Psychopharmacol Bull. 1988;24:671–688. [PubMed] [Google Scholar]
- 36. McWilliams L. Neuroticism and depression. Br J Psychiatry. 2003;182:80. [DOI] [PubMed] [Google Scholar]
- 37. Bhome R, Huntley JD, Price G, Howard RJ. Clinical presentation and neuropsychological profiles of functional cognitive disorder patients with and without co-morbid depression. Cogn Neuropsychiatry. 2019;24:152–164. [DOI] [PubMed] [Google Scholar]
- 38. Palmer EC, David AS, Fleming SM. Effects of age on metacognitive efficiency. Conscious Cogn. 2014;28:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rouault M, McWilliams A, Allen MG, Fleming SM. Human metacognition across domains: Insights from individual differences and neuroimaging. Personal Neurosci. 2018;1:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pennington C, Hayre A, Newson M, Coulthard E. Functional cognitive disorder: A common cause of subjective cognitive symptoms. J Alzheimers Dis. 2015;48:S19–S24. [DOI] [PubMed] [Google Scholar]
- 41. Stone J, Pal S, Blackburn D, Reuber M, Thekkumpurath P, Carson A. Functional (psychogenic) cognitive disorders: A perspective from the neurology clinic. J Alzheimers Dis. 2015;48:S5–S17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this study will be shared upon reasonable request to the corresponding author. All data and statistics generated from this study are presented.