Abstract
Local and regional habitat conditions associated with agricultural activity can fundamentally alter aquatic ecosystems. Increased nutrient inputs, channelization and reduced riparian habitat both upstream and locally contribute to the degradation of stream ecosystems and their function. Here, we examine stream food webs in watersheds that feed into Lake Erie to determine the effects of agricultural land cover on major food web energy pathways and trophic structure. Given that higher agricultural intensity can increase nutrient runoff and reduce the riparian zone and litter in-fall into streams, we predicted that generalist fish would derive less energy from the terrestrial pathway and become more omnivorous. Consistent with these predictions, we show that both mean terrestrial energy use and trophic position of the resident top consumer, creek chub (Semotilus atromaculatus), decrease with local agricultural intensity but not with watershed-level agriculture intensity. These findings suggest that local riparian buffers can maintain trophic structure even in the face of high whole-watershed agricultural intensity.
Keywords: habitat coupling, stable isotopes, agricultural development, trophic ecology
1. Introduction
The human population is expected to reach ca 9.7 billion by 2050 [1], accompanied by an increase in global agricultural footprint of 593 million ha [2]. This agriculture directly alters the land on which crops are grown but can also impact adjacent aquatic food webs at both local [3] and regional scales [4,5]. At the local scale, conventional farming and associated runoff can impact streams by increasing light regimes, siltation and nutrient inputs and by reducing riparian zones, all of which can have implications for the food webs of adjacent streams [6]. Local agriculture may therefore rewire stream food webs (sensu Bartley et al. [7]), altering processes that maintain the diversity and function of these important ecosystems.
While research on landscape-scale nutrient-driven impacts of agriculture on large lakes and oceans abound [8,9], the impacts of local terrestrial inputs on adjacent stream food webs are less studied. At the local stream scale, shifts in diversity in response to agriculture have been documented in a variety of taxa, ranging from diatoms to predatory fish [10,11]. More recently, efforts have been made to extend these results to food web structure and function (e.g. [12–14]). While studies have shown that increased nutrients and riparian removal can alter energy flows into ecosystems, it is not yet well understood how different forms of local agriculture mediate stream food web responses in agriculturally dominated watersheds [15].
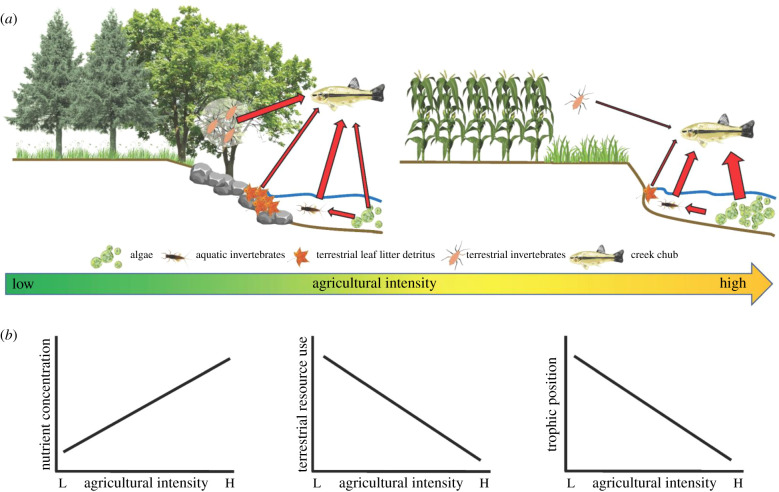
In a seminal contribution, Nakano & Murakami [16] established a seasonal perspective of how stream food webs respond to changes in aquatic and terrestrial production in relatively pristine temperate streams. Specifically, they documented asynchronous production in aquatic insects (spring pulse) and terrestrial riparian insects (summer pulse) as well as the consumption of these production events by both aquatic (fish) and terrestrial (bird and spider) consumers [17–20]. The generalist consumers in these studies ‘surfed' the seasonal waves of aquatic (spring) and terrestrial (summer) insect production. These results suggest a simple framework for predicting the impacts of agricultural intensification on the trophic dynamics of stream food webs (figure 1a). Since increasing agricultural intensity will result in higher nutrient runoff and reduce the extent of the riparian zone and in turn litter in-fall into adjacent streams, we predict that generalist top predators will derive less energy from the terrestrial pathway (Reduced Terrestrial Pathway Hypothesis; RTPH, figure 1b) and rely on more aquatic food sources such as invertebrates and algae. Given that increased nutrient runoff can lead to higher algal biomass, we also expect generalist fish will use this resource and occupy a reduced trophic position (figure 1b).
Figure 1.
A conceptual model of energy pathways in freshwater stream ecosystems located across a gradient of agricultural intensity, and subsequent predictions for the current study. (a) As agricultural land use increases, nutrient input and riparian habitat degradation increases, causing a reduction in terrestrial food sources and increase in autochthonous resources. (b) As a result, owing to the Reduced Terrestrial Pathway Hypothesis, we expect stream nutrient concentrations to increase with agricultural intensity, while terrestrial energy use and trophic position of generalist consumer fish decrease.
Here, we test the RTPH by examining stream food web responses across a natural gradient in local and regional level agricultural intensity, including conventional farms, farms using alternative land use practices (e.g. riparian buffers) and protected areas. We use stable isotope ratios of nitrogen (δ15N) and deuterium (δ2H) from white muscle to trace the time-integrated (weeks–months) energy sources and trophic position by the dominant resident omnivore in these streams (creek chub; Semotilus atromaculatus). Understanding the influence of local land use practices on the trophic ecology of stream fish in agriculture-dominated landscapes could inform conservation efforts and provide a test of theory on the impacts of global change on across-ecosystem coupling.
2. Methods
(a) . Study system
This study was carried out in streams along the north shore of Lake Erie, southwestern Ontario, Canada (electronic supplementary material, figure S1), a region with high cover of agricultural land. We collected stable isotope samples and supporting stream characteristic data from 20 sites across six major watersheds located in sand and till plains. Our focal species, the creek chub, effectively operates as the top predator in these systems (see electronic supplementary material, S1). Creek chub are highly generalized species capable of feeding on other fish, invertebrates and macrophytes and can act as top predator in streams when more typical predatory species are absent [21].
(b) . Field sampling
Sampling took place during July and August 2018. The sample sites spanned a gradient in agricultural pressure, from conservation areas to conventional farms, and highly and minimally impacted sites were included within each watershed where possible. The 20 sites had local per cent agriculture land uses ranging 0–92% (mean ± s.d. = 43% ± 27%), and average watershed-level agricultural land uses ranging 39–94% (mean ± s.d. = 77% ± 0.13%) (electronic supplementary material, table S1 and figure S1).
Stream characteristics were measured to categorize each site. We measured average buffer width (m), water turbidity (mg l−1), water temperature (°C), stream sinuosity, and July water nitrogen and phosphorus concentrations (mg l−1) (see electronic supplementary material, S1 for detailed sampling methods). For each site, we also calculated the proportion of agricultural land use in the watershed and within a 250 m radius of the site (i.e. proportion local agriculture). The local agriculture estimate differs from the watershed-level estimate in that it takes into consideration riparian buffer zones adjacent to study sites that are present in land cover maps that would not be included in coarser watershed-level estimates of land use. Watershed land use was determined using the Ontario Flow Assessment Tool and land cover raster maps (https://data.ontario.ca/dataset/ontario-land-cover-compilation-v20). See electronic supplementary material, S1 for details.
Creek chub sampling was conducted in 40 m reaches within the streams using a triple-pass electrofishing method [22] with the goal of obtaining 10 individuals per site to be analysed for stable isotopes. If fewer than 10 creek chub were captured, we set minnow traps overnight to attempt to achieve our target sample size. Fish were euthanized using an overdose of buffered tricaine methanesulfonate. Total length (mm) was measured, stomach contents were identified where possible and dorsal muscle was sampled for stable isotopes. Baseline samples, which included algae as the aquatic baseline and leaf litter detritus as the terrestrial baseline, were collected at each site to account for differences in basal stable isotope values. Samples were placed on ice and then frozen at −20°C until further processing (electronic supplementary material, table S2). See electronic supplementary material, S1 for details on analysis.
(c) . Stable isotopes
In the laboratory, baseline samples were thawed, rinsed and divided into three replicates of each baseline type with a minimum wet weight of 0.1 g. Samples were placed in a drying oven at 60°C for 48–72 h and then ground. Samples were split into two replicates and sent to accredited laboratories for quantification of δ15N and δ2H. Stable isotope values are conveyed in δ notation (units ‰), where δ2H or δ15N = ((Rsample/Rstandard) – 1) × 1000, where R is 2H/1H or 15N/14N. Values of δ15N generally increase from prey to consumer and provide information on trophic position [23], while values of δ2H provide discrimination between terrestrial and aquatic primary producers [24], with aquatic producers having lower δ2H values. Analytical information for stable isotope analysis is in electronic supplementary material, S2.
Environmental water contributes to the δ2H of aquatic organisms, so we had to account for the δ2H of water in our creek chub samples [24–27]. We adjusted the raw creek chub δ2H values for the contribution of environmental water using the following equations [27]:
and
where w1 and w2 are the proposed proportion of environmental water in aquatic primary producers and secondary consumers, respectively, with w1 assumed to be 0.17 [27]. Because we did not collect water samples at each site, we used the average δ2H water value (−62.7‰) from two streams located within our study region (electronic supplementary material, figure S1; [28,29]).
We used the δ2Hadjusted and δ15N values from individual creek chub and the mean aquatic and terrestrial baseline δ2H and δ15N values from each site to calculate the proportion of terrestrial energy use and trophic position for each individual creek chub. Proportion terrestrial energy use for each creek chub was calculated as [30]:
Estimates ≤ 0 or ≥ 1 were set to 0.001 or 0.999, respectively, to allow for logit transformation. Trophic position of each creek chub was calculated as [31]:
where 1 is the assumed trophic position of terrestrial baseline (detritus), and 3.4‰ is the assumed trophic enrichment factor [31]. We also used the two-baseline method to estimate trophic position to corroborate our results ([31]; see electronic supplementary material, S3).
(d) . Statistical analysis
We used a principal component analysis (PCA) and Pearson correlations to explore relationships between stream morphological, abiotic and biotic environmental variables from our sites. July phosphorus concentration was missing from one site, so to perform the PCA we used the average value from the rest of the sites to fill in this missing value. Turbidity, nitrogen, phosphorus, sinuosity and water temperature were log10-transformed to achieve normality.
We used linear regression to test for the potential influence of local and watershed-level percentage agriculture and mean fish total length on site-level mean proportion of terrestrial energy use (logit-transformed), and trophic position of creek chub and to test if the mean total length of creek chub across sites was influenced by proportion of local or watershed agriculture land use. While our study sites were spread across a range of local percentage agriculture values, most sites had a percentage watershed-level agriculture greater than 65%, with two sites having values of approximately 40%—this left a gap between approximately 40% and approximately 70% (electronic supplementary material, table S1). Thus, when testing for the relationships between watershed-level percentage agriculture on creek chub trophic metrics, we did so with and without the two sites with approximately 40% watershed agriculture.
3. Results
(a) . Agricultural gradient
The PCA indicated that a total of 68% of the variation within physiochemical variables is represented in the first (53%) and second (15%) axes (table 1, electronic supplementary material, figure S2). Parameters associated with the first axis were related to general agricultural activity, including proportion watershed agriculture, July nitrogen and phosphorus concentration, water turbidity and channel sinuosity. On the second axis, parameters including local proportion of agriculture, buffer width at the sampling site and water temperatures were represented (table 1, electronic supplementary material, figure S2). Of note, site buffer width was negatively correlated to both watershed (r = −0.80, p < 0.01) and local (−0.69, p < 0.01) proportion of agriculture (table 2). While moderate positive correlations of proportion of watershed agriculture with nitrogen (r = 0.56, p < 0.01) and phosphorous (r = 0.61, p < 0.01) concentrations were found, there were weak (r = 0.38, p = 0.09) and no apparent (r = 0.18, p = 0.49) correlations between local proportion of agriculture and nitrogen and phosphorous, respectively (table 2). Taken together, regional agriculture separated from local agriculture, allowing us to examine if local land modifications (e.g. riparian buffers) can alter stream food webs even in the face of regional agricultural signals.
Table 1.
(A) Eigenvalues and (B) loadings from a principal component analysis (PCA) that included stream characteristics and the proportion of local and watershed-level agriculture for each sampling site.
| (A) eigenvalues | ||||||||
|---|---|---|---|---|---|---|---|---|
| metric | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 |
| s.d. | 2.06 | 1.10 | 0.92 | 0.78 | 0.66 | 0.60 | 0.47 | 0.28 |
| proportion of variance | 0.53 | 0.15 | 0.11 | 0.08 | 0.05 | 0.05 | 0.03 | 0.01 |
| cumulative proportion | 0.53 | 0.68 | 0.79 | 0.86 | 0.92 | 0.96 | 0.99 | 1.00 |
| (B) loadings | ||||||||
| variable | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 |
| proportion watershed agriculture | −0.43 | 0.11 | −0.18 | 0.37 | −0.02 | −0.45 | −0.02 | −0.66 |
| buffer width | 0.39 | −0.44 | −0.01 | −0.22 | −0.18 | 0.08 | 0.56 | −0.51 |
| proportion local agriculture | −0.29 | 0.55 | 0.45 | 0.04 | −0.06 | 0.28 | 0.56 | −0.03 |
| log turbidity | −0.40 | −0.18 | 0.15 | −0.34 | −0.13 | 0.57 | −0.44 | −0.36 |
| log July nitrogen | −0.36 | 0.004 | −0.04 | −0.75 | −0.11 | −0.50 | 0.15 | 0.17 |
| log July phosphorus | −0.36 | −0.32 | −0.24 | 0.03 | 0.76 | 0.19 | 0.30 | 0.09 |
| log sinuosity | 0.36 | 0.24 | 0.42 | −0.27 | 0.60 | −0.20 | −0.23 | −0.33 |
| log water temperature | −0.19 | −0.55 | 0.71 | 0.27 | −0.05 | −0.26 | −0.02 | 0.16 |
Table 2.
Correlation matrix of biotic and abiotic characteristics measured at each sampling location. Correlation coefficients (r) and p-values were produced using the Pearson correlation coefficient method.
| proportion watershed agriculture | |||||||
|---|---|---|---|---|---|---|---|
| r = 0.49 | proportion local agriculture | ||||||
| p = 0.02 | |||||||
| r = −0.80 | r = −0.69 | buffer width | |||||
| p = <0.01 | p < 0.01 | ||||||
| r = 0.54 | r = 0.43 | r = −0.53 | turbidity | ||||
| p = 0.06 | p < 0.01 | ||||||
| p = 0.01 | |||||||
| r = 0.56 | r = 0.38 | r = −0.49 | r = 0.66 | nitrogen | |||
| p = 0.09 | p = 0.03 | p = <0.01 | |||||
| p = <0.01 | |||||||
| r = 0.61 | r = 0.18 | r = −0.45 | r = 0.61 | r = 0.49 | phosphorus | ||
| p = 0.49 | p = 0.05 | p = <0.01 | p = 0.03 | ||||
| p = <0.01 | |||||||
| r = −0.69 | r = −0.19 | r = 0.43 | r = −0.60 | r = −0.44 | r = −0.56 | sinuosity | |
| p = <0.01 | p = 0.43 | p = 0.06 | p < 0.01 | p = 0.05 | p = <0.01 | ||
| r = 0.25 | r = 0.12 | r = −0.07 | r = 0.42 | r = 0.19 | r = 0.32 | r = −0.24 | water temperature |
| p = 0.28 | p = 0.62 | p = 0.75 | p = 0.06 | p = 0.42 | p = 0.16 | p = 0.32 |
(b) . Trophic responses
Creek chub analysed for stable isotopes averaged 95 mm (n = 172, s.d. = 37, range = 27–226 mm) in length, with site-level means ranging from 54 to 135 mm (electronic supplementary material, table S2). Mean site creek chub total body length showed no relationship with the proportion of local (F1,18 = 0.77, p = 0.39) or watershed (F1,18 = 1.83, p = 0.19) agricultural land use. Although fish size can impact diet and trophic position, including total length of creek chub as a covariate did not improve the fit of any of the regressions presented below (see electronic supplementary material, S3).
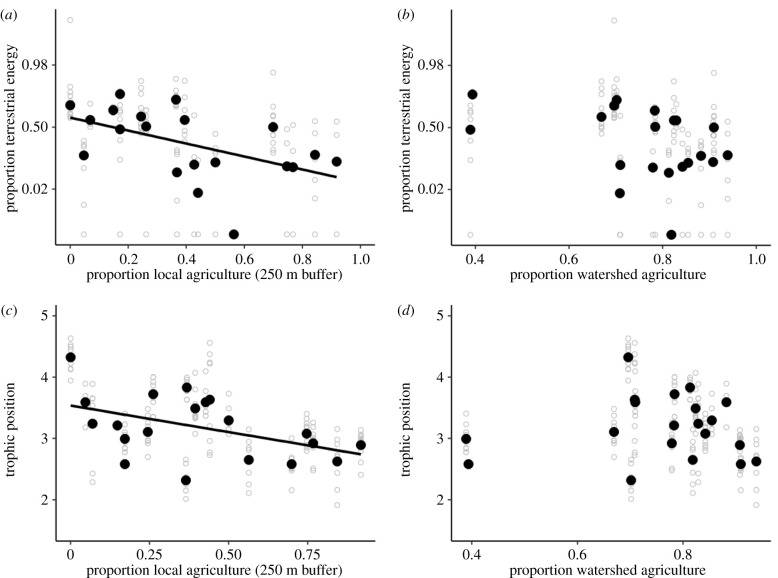
Mean logit proportion of terrestrial energy use and trophic position of creek chub each decreased with increasing local percentage agriculture within 250 m (logit terrestrial energy use: β = −4.19 ± 1.66, F1,18 = 6.34, p = 0.02, R2 = 0.26; trophic position: β = −0.87 ± 0.38, F1,18 = 5.10, p = 0.04, R2 = 0.22) (figure 2a,c). For example, at a site surrounded by no agriculture, creek chub on average accumulated 65% of their energy from terrestrial sources and occupied a trophic position of 3.5, whereas at a site surrounded by 92% agriculture (the maximum for our sites), creek chub used 4% terrestrial energy and occupied a trophic position of 2.7 (figure 2a,c). Comparable results were found by calculating trophic positions using the two-baseline method (see electronic supplementary material, S3; figure S3).
Figure 2.
Mean proportion of terrestrial energy use and trophic position of creek chub versus proportion of local (250 m buffer around study site) and watershed agricultural land use. Mean values per site are represented by solid black points, while individual fish estimates are depicted by hollow grey points. Estimates of terrestrial energy use and trophic position for each sampled fish were obtained using stable isotope ratios of deuterium (2H) and nitrogen, respectively (see electronic supplementary material, S1 and S2). Trend lines indicate a significant linear regression (p < 0.05).
We found no evidence that the mean logit proportion of terrestrial energy (F1,18 = 3.02, p = 0.10) nor mean trophic position (F1,18 = 0.06, p = 0.80) decreased as a function of whole watershed percentage agriculture (figure 2b,d). Similar results were found when removing the two sites with approximately 40% watershed agriculture (logit proportion terrestrial energy: F1,16 = 0.90, p = 0.36; trophic position: F1,16 = 2.10, p = 0.17; see electronic supplementary material, S3).
4. Discussion
Here, we hypothesized that agricultural land use would increase nutrient concentration in water and thus promote primary production and its use by aquatic food webs; simultaneously, the reduction of riparian zones should decouple stream webs from the terrestrial energy pathway (RTPH; figure 1). We found that stream food webs responded consistently with the RTPH, whereby the generalist consumer assimilated less terrestrial energy and occupied lower trophic positions, potentially owing to greater consumption of algae [27], as local agricultural increased. Intriguingly, this result was not influenced by regional agricultural land use, suggesting that even at sites with a high percentage of watershed agriculture, regional land use impacts could be mitigated with riparian buffers that increase leaf in-fall and terrestrial insects in stream food webs [16,32,33]. It is possible that greater light penetration at sites with higher agriculture could have led to higher algal production and contributed to our results. Nonetheless, our results suggest that local land use (e.g. riparian buffers) can influence the trophic structure of stream food webs in a heavily regionally impacted agricultural landscape [32–35].
Theory has generally argued that feeding across multiple food chains or ecosystems (i.e. coupling) by mobile predators can act as a potent stabilizer of food webs [36]. Specifically, using energy from two macrohabitats can reduce variability and/or maintain more stable equilibria [36–38]. Therefore, increasing feeding on a single energy pathway by consumers in highly impacted landscapes may alter the stability of these stream food webs. Our findings suggest that local riparian buffers and corridors can mitigate against potentially negative food web instability by balancing the relative use of terrestrial versus aquatic energy used by stream fish.
Our results are consistent with recent papers arguing that environmental change tends to asymmetrically impact macrohabiats and ecosystems, with generalist consumer behaviour tending to ‘rewire' ecosystems [7,39]. This study also highlights the mechanisms through which agricultural land use impacts the structure and function of stream ecosystems, and adds to the growing literature suggesting that riparian zones may play an important role in mitgating agricultural impacts on streams and rivers (e.g. [40,41]). We note that our study focused on a single year and season and does not consider seasonality or annual variation, both of which can impact the trophic structure of food webs [42,43] and warrant future research attention.
Acknowledgements
We thank Rob McLaughlin for comments, Christine Dulal-Whiteway and Neil Rooney for nutrient data, and reviewers for their comments.
Ethics
Research was conducted under an Animal User Protocol from the University of Guelph (AUP no. 3682) and Ontario Ministry of Natural Resources Forestry collection permit no. 1086855.
Data accessibility
Data and R code to reproduce the results of this paper have been deposited in the open-access repository Zenodo (https://doi.org/10.5281/zenodo.5838957) [44], with documentation provided in the Readme file. The data are provided in the electronic supplementary material.
Authors' contributions
E.J.C.: conceptualization, data curation, formal analysis, investigation, writing—original draft, writing—review and editing; M.M.G.: conceptualization, data curation, formal analysis, methodology, visualization, writing—original draft, writing—review and editing; M.K.G.: conceptualization, data curation, investigation, methodology, project administration, writing—review and editing; K.S.M.: conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, writing—original draft, writing—review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Competing interests
We declare we have no competing interests.
Funding
Research was funded by Food from Thought and an NSERC Discovery grant to K.S.M.
References
- 1. United Nations Department of Economic and Social Affairs Population Division. 2019 World Population Prospects 2019: highlights, ST/ESA/SER.A/423. New York, NY: United Nations. See https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf .
- 2.Searchinger T, Waite R, Hanson C, Ranganathan J. 2019. World Resources Report: Creating a Sustainable Food Future: A Menu of Solutions to Feed Nearly 10 Billion People by 2050. Washington, DC: World Resources Institute. [Google Scholar]
- 3.Hladyz S, Watkins SC, Whitworth KL, Baldwin DS. 2011. Flows and hypoxic blackwater events in managed ephemeral river channels. J. Hydrol. 401, 117-125. ( 10.1016/j.jhydrol.2011.02.014) [DOI] [Google Scholar]
- 4.Woodward A, Schreiner EG, Crain P, Brenkman SJ, Happe PJ, Acker SA, Hawkins-Hoffman C. 2008. Conceptual models for research and monitoring of Elwha dam removal—management perspective. Northwest Sci. 82, 59-71. ( 10.3955/0029-344X-82.S.I.59) [DOI] [Google Scholar]
- 5.Dodds WK, Bouska WW, Eitzmann JL, Pilger TJ, Pitts KL, Riley AJ, Schloesser JT, Thornbrugh DJ. 2009. Eutrophication of U. S. freshwaters: analysis of potential economic damages. Environ. Sci. Technol. 43, 12-19. ( 10.1021/es801217q) [DOI] [PubMed] [Google Scholar]
- 6.Hladyz S, et al. 2011. Stream ecosystem functioning in an agricultural landscape: the importance of terrestrial–aquatic linkages. Adv. Ecol. Res. 44, 211-276. ( 10.1016/B978-0-12-374794-5.00004-3) [DOI] [Google Scholar]
- 7.Bartley TJ, McCann KS, Bieg C, Cazelles K, Granados M, Guzzo MM, Macdougall AS, Tunney TD, Mcmeans BC. 2019. Food web rewiring in a changing world. Nat. Ecol. Evol. 3, 345-354. ( 10.1038/s41559-018-0772-3) [DOI] [PubMed] [Google Scholar]
- 8.Valiela I, Bartholomew M. 2015. Land–sea coupling and global-driven forcing: following some of Scott Nixon's challenges. Estuaries Coasts 38, 1189-1201. ( 10.1007/s12237-014-9808-3) [DOI] [Google Scholar]
- 9.Rabalais NN, Turner RE, Díaz RJ, Justić D. 2009. Global change and eutrophication of coastal waters. ICES J. Mar. Sci. 66, 1528-1537. ( 10.1093/icesjms/fsp047) [DOI] [Google Scholar]
- 10.Barrett KB, Haynes JM, Warton DI. 2017. Thirty years of change in a benthic macroinvertebrate community of southwestern Lake Ontario after invasion by four Ponto-Caspian species. Freshw. Sci. 36, 90-102. ( 10.1086/689576) [DOI] [Google Scholar]
- 11.Belore ML, Winter JG, Duthie HC. 2002. Use of diatoms and macroinvertebrates as bioindicators of water quality in Southern Ontario rivers. Can. Water Resour. J. 27, 457-484. ( 10.4296/cwrj2704457) [DOI] [Google Scholar]
- 12.Hladyz S, Cook RA, Petrie R, Nielsen DL. 2011. Influence of substratum on the variability of benthic biofilm stable isotope signatures: implications for energy flow to a primary consumer. Hydrobiologia 664, 135-146. ( 10.1007/s10750-010-0593-0) [DOI] [Google Scholar]
- 13.Lee KY, Graham L, Spooner DE, Xenopoulos MA. 2018. Tracing anthropogenic inputs in stream foods webs with stable carbon and nitrogen isotope systematics along an agricultural gradient. PLoS ONE 13, e0200312. ( 10.1371/journal.pone.0200312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price EL, Sertić Perić M, Romero GQ, Kratina P. 2019. Land use alters trophic redundancy and resource flow through stream food webs. J. Anim. Ecol. 88, 677-689. ( 10.1111/1365-2656.12955) [DOI] [PubMed] [Google Scholar]
- 15.Raitif J, Plantegenest M, Roussel JM. 2019. From stream to land: ecosystem services provided by stream insects to agriculture. Agric. Ecosyst. Environ. 270–271, 32-40. ( 10.1016/j.agee.2018.10.013) [DOI] [Google Scholar]
- 16.Nakano S, Murakami M. 2001. Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc. Natl Acad. Sci. USA 98, 166-170. ( 10.1073/pnas.98.1.166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakano S, Miyasaka H, Kuhara N. 1999. Terrestrial–aquatic linkages: riparian arthropod inputs alter trophic cascades in a stream food web. Ecology 80, 2435-2441. ( 10.1890/0012-9658(1999)080[2435:TALRAI]2.0.CO;2) [DOI] [Google Scholar]
- 18.Kato C, Iwata T, Nakano S, Kishi D. 2003. Dynamics of aquatic insect flux affects distribution of riparian web-building spiders. Oikos 103, 113-120. ( 10.1034/j.1600-0706.2003.12477.x) [DOI] [Google Scholar]
- 19.Murakami M, Nakano S. 2002. Indirect effect of aquatic insect emergence on a terrestrial insect population through predation by birds. Ecol. Lett. 5, 333-337. ( 10.1046/j.1461-0248.2002.00321.x) [DOI] [Google Scholar]
- 20.Kawaguchi Y, Nakano S. 2001. Contribution of terrestrial invertebrates to the annual resource budget for salmonids in forest and grassland reaches of a headwater stream. Freshw. Biol. 46, 303-316. ( 10.1046/j.1365-2427.2001.00667.x) [DOI] [Google Scholar]
- 21.Holm E, Mandrak NE, Burridge ME. 2009. The ROM field guide to freshwater fishes of Ontario, p. 146. Toronto, Canada: ROM. [Google Scholar]
- 22.Stanfield LW, Lester NP, Petreman IC. 2013. Optimal effort intensity in backpack electrofishing surveys. North Am. J. Fish Manag. 33, 277-286. ( 10.1080/02755947.2012.758200) [DOI] [Google Scholar]
- 23.Deniro MJ, Epstein S. 1981. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 45, 341-351. ( 10.1016/0016-7037(81)90244-1) [DOI] [Google Scholar]
- 24.Doucett RR, Marks JC, Blinn DW, Caron M, Hungate BA. 2007. Measuring terrestrial subsidies to aquatic food webs using stable isotopes of hydrogen. Ecology 88, 1587-1592. ( 10.1890/06-1184) [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson GM, Cole JJ, Pace ML. 2015. Deuterium as a food source tracer: sensitivity to environmental water, lipid content, and hydrogen exchange. Limnol. Oceanogr. Methods 13, 213-223. ( 10.1002/lom3.10019) [DOI] [Google Scholar]
- 26.Solomon CT, Cole JJ, Doucett RR, Pace ML, Preston ND, Smith LE, Weidel BC. 2009. The influence of environmental water on the hydrogen stable isotope ratio in aquatic consumers. Oecologia 161, 313-324. ( 10.1007/s00442-009-1370-5) [DOI] [PubMed] [Google Scholar]
- 27.Page HM, Cooper SD, Wiseman SW, Bennett D, Klose K, Sadro S, Nelson C, Even T. et al. 2017. Comparisons of stable isotope (C, H, N) signatures for revealing organic matter sources and trophic relationships in headwater streams. Can. J. Fish. Aquat. Sci. 74, 2110-2121. ( 10.1139/cjfas-2016-0322) [DOI] [Google Scholar]
- 28.Gibson JJ, Eby P, Stadnyk TA, Holmes T, Birks SJ, Pietroniro A. 2021. Dataset of 18O and 2H in streamflow across Canada: a national resource for tracing water sources, water balance and predictive modelling. Data Br. 34, 106723. ( 10.1016/j.dib.2021.106723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibson JJ, Holmes T, Stadnyk TA, Birks SJ, Eby P, Pietroniro A. 2020. 18O and 2H in streamflow across Canada. J. Hydrol. Reg. Stud. 32, 100754. ( 10.1016/j.ejrh.2020.100754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanden MJV, Vadeboncoeur Y. 2002. Fishes as integrators of benthic and pelagic food webs in lakes. Ecology 83, 2152-2161. ( 10.1890/0012-9658(2002)083[2152:FAIOBA]2.0.CO;2) [DOI] [Google Scholar]
- 31.Post DM. 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703-718. ( 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2) [DOI] [Google Scholar]
- 32.Babler AL, Pilati A, Vanni MJ. 2011. Terrestrial support of detritivorous fish populations decreases with watershed size. Ecosphere 2, 1-23. ( 10.1890/ES11-00043.1) [DOI] [Google Scholar]
- 33.Ward-Campbell B, Cottenie K, Mandrak N, McLaughlin R. 2017. Maintenance of agricultural drains alters physical habitat, but not macroinvertebrate assemblages exploited by fishes. J. Environ. Manage. 203, 29-39. ( 10.1016/j.jenvman.2017.07.032) [DOI] [PubMed] [Google Scholar]
- 34.Wilson HF, Xenopoulos MA. 2011. Nutrient recycling by fish in streams along a gradient of agricultural land use. Glob. Chang. Biol. 17, 130-139. ( 10.1111/j.1365-2486.2010.02284.x) [DOI] [Google Scholar]
- 35.Yates AG, Bailey RC. 2010. Covarying patterns of macroinvertebrate and fish assemblages along natural and human activity gradients: implications for bioassessment. Hydrobiologia 637, 87-100. ( 10.1007/s10750-009-9987-2) [DOI] [Google Scholar]
- 36.Rooney N, McCann K, Gellner G, Moore JC. 2006. Structural asymmetry and the stability of diverse food webs. Nature 442, 265-269. ( 10.1038/nature04887) [DOI] [PubMed] [Google Scholar]
- 37.Kondoh M. 2003. Foraging adaptation and the relationship between food-web complexity and stability. Science 299, 1388-1391. ( 10.1126/science.1079154) [DOI] [PubMed] [Google Scholar]
- 38.McCann KS, Rooney N. 2009. The more food webs change, the more they stay the same. Phil. Trans. R. Soc. B 364, 1789-1801. ( 10.1098/rstb.2008.0273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanchard JLA. 2015. A rewired food web. Nature 527, 7-8. ( 10.1038/nature16311) [DOI] [PubMed] [Google Scholar]
- 40.Carline RF, Walsh MC. 2007. Responses to riparian restoration in the Spring Creek watershed, Central Pennsylvania. Restor. Ecol. 15, 731-742. ( 10.1111/j.1526-100X.2007.00285.x) [DOI] [Google Scholar]
- 41.Dosskey MG, Vidon P, Gurwick NP, Allan CJ, Duval TP, Lowrance R. 2010. The role of riparian vegetation in protecting and improving chemical water quality in streams. J. Am. Water Resour. Assoc. 46, 261-277. ( 10.1111/j.1752-1688.2010.00419.x) [DOI] [Google Scholar]
- 42.McMeans BC, McCann KS, Humphries M, Rooney N, Fisk AT. 2015. Food web structure in temporally-forced ecosystems. Trends Ecol. Evol. 30, 662-672. ( 10.1016/j.tree.2015.09.001) [DOI] [PubMed] [Google Scholar]
- 43.Guzzo MM, Blanchfield PJ, Rennie MD. 2017. Behavioral responses to annual temperature variation alter the dominant energy pathway, growth, and condition of a cold-water predator. Proc. Natl Acad. Sci. USA 114, 9912-9917. ( 10.1073/pnas.1702584114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Champagne EJ, Guzzo MM, Gutgesell MK, McCann KS. 2022. Riparian buffers maintain aquatic trophic structure in agricultural landscapes. Zenodo. ( 10.5281/zenodo.5838957) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and R code to reproduce the results of this paper have been deposited in the open-access repository Zenodo (https://doi.org/10.5281/zenodo.5838957) [44], with documentation provided in the Readme file. The data are provided in the electronic supplementary material.