Abstract
Coenzyme Q10 (CoQ10), a lipid involved in ATP synthesis, exhibits very limited oral absorption, and its endogenous production decreases with ageing and with the occurrence of oxidative stress. Our group previously showed that monoglycerides omega-3 (MAG-OM3) increase OM3 plasma concentrations. Since CoQ10 is liposoluble, we hypothesised that its 48 h pharmacokinetics is higher when provided with MAG-OM3 compared to CoQ10 alone (in powder form) or added to rice oil (a neutral triacylglycerol oil). A randomised triple-blind crossover study was performed with fifteen men and fifteen women consuming the three supplements providing 200 mg of CoQ10 in a random order. Blood samples were collected before (t = 0) and 1, 3, 5, 6, 7, 8, 10, 11, 24 and 48 h after the supplement intake. Plasma total CoQ10 concentrations were analysed on ultrahigh-performance liquid chromatography coupled to a tandem mass spectrometer (UPLC-MS/MS). Participants were 26⋅1 ± 4⋅8 years old. When CoQ10 was provided with rice or MAG-OM3 oils, the 48 h area under the curve (AUC 0–48 h) was approximately two times higher compared to when provided without an oil. The delta max concentration (ΔCmax) of plasma CoQ10 was, respectively, 2 (MAG-OM3) and 2⋅5 (rice oil) times higher compared to CoQ10 alone. There was a significant sex by treatment interaction (P = 0⋅0250) for the AUC 0–6 h supporting that in postprandial, men and women do not respond the same way to the different supplement. Women had a higher CoQ10 concentration 48 h after the single-dose intake compared to men. We conclude that CoQ10 supplements must be provided with lipids, and their kinetics is different between men and women.
Key words: CoQ10, Crossover study, Monoglyceride omega-3, Pharmacokinetics
Abbreviations: AUC, area under the curve; C 0 h, plasma concentration at baseline; C 48 h, plasma concentration at 48 h post-single-dose intake; Cmax, maximum concentration; CoQ10, coenzyme Q10; CoQ9, coenzyme Q9; MAG, monoglyceride; OM3, omega-3 fatty acids; Tmax, time to reach maximum concentration
Introduction
Coenzyme Q10 (CoQ10), also known as ubiquinone, is an endogenously synthesised fat-soluble molecule. It is composed of a benzoquinone ring and 10 isoprene subunits(1). It has two main roles in the body. CoQ10 plays a major role in ATP synthesis by transporting electrons from complexes I and II to complex III via the Q cycle in the electron transport chain of the mitochondria and it also contributes to the proton gradient(2,3). CoQ10 is also a scavenger of free radicals, can regenerate other antioxidants’ pools like vitamin E(4) and can protect lipoproteins from lipid peroxidation at the initiation and propagation stages(2). A low level of blood CoQ10 has been reported in those with chronic diseases such as cardiovascular diseases, neurodegenerative diseases, diabetes and cancer(5,6). Moreover, during ageing, CoQ10's blood levels tend to decrease(7,8).
The total amount of CoQ10 in the body is approximately 2000 mg. Its daily nutritional intake is estimated at 3–6 mg/day but the body needs are estimated to be ~500 mg of CoQ10 every day(9). Therefore, the consumption of 30–100 mg of CoQ10 per day by healthy people and of 60–1200 mg of CoQ10 per day in those with a medical condition as described above is recommended(9). CoQ10 supplementation is considered safe and well tolerated without any serious adverse effects in human subjects(10). Its acceptable daily intake is 12 mg/kg/d which is about 720 mg/d for a person weighing 60 kg(10). Some studies even used CoQ10 doses up to 2400 mg/d, which was well tolerated with no safety concerns reported(11). Furthermore, it seems that dietary CoQ10 intake does not influence its endogenous synthesis and there is no long-term accumulation in the tissues(10). The main issue with CoQ10 supplementation is its low and incomplete absorption, and hence, its efficacy when provided as a supplement is doubted or limited(12). To overcome this issue, the most popular approaches focused on adding synthetic products such as liposomes, lipid-free nano-formulations, water-soluble CoQ10, CoQ10-loaded oleo gels and CoQ10 micellisation. Although these strategies have improved CoQ10 absorption and bioavailability, these synthetic products have no health benefits(12).
Our group previously conducted pharmacokinetic studies on omega-3 fatty acids (OM3) esterified in monoacylglycerols (MAG) and reported that plasma OM3 concentrations were two to three times higher 0–6 h post dose intake when OM3s were esterified in MAGs compared to when esterified in ethyl esters or triacylglycerols(13,14). The amphipathic nature of MAGs contributes to improving the emulsification of fats and producing micelles in the gastrointestinal tract(15–17). Therefore, we hypothesise that CoQ10 blood levels over a 48 h follow-up will be higher when provided with MAG-OM3 than CoQ10 alone (in powder form) or added to rice oil (a neutral triacylglycerol oil). The objective of the present study was to perform, in men and women, comparative pharmacokinetics of the three CoQ10 formulations and monitor concentrations of total CoQ10 in the plasma over 48 h after the single-dose intake.
Methods
Study participants
The present study was a randomised, triple-blind, crossover trial conducted at the Research Center on Aging, Centre Intégré Universitaire de Santé et des Services Sociaux de l'Estrie – Centre Hospitalier Universitaire de Sherbrooke (CIUSSSE–CHUS), in Sherbrooke (Quebec, Canada). This trial was revised and approved by the Research Ethics Committee of the CIUSSSE–CHUS (reference no. 2020-3280). The present study is registered at clinicaltrials.gov under no. NCT04035525.
Recruitment took place from June to December 2020. The main inclusion criteria were to be aged between 18 and 50 years old. Exclusion criteria included people with a particular diet such as being vegetarian or vegan, and/or with allergies to fish/seafood, people taking CoQ10 and/or omega-3 fatty acid supplements or who had taken these supplements daily in the previous 6 months. People with a body mass index of <18⋅5 kg/m2 or >34⋅9 kg/m2 were also excluded and those smoking tobacco or marijuana as well. Other exclusion criteria assessed during the phone call were: current or past performance athletes; alcohol and/or drug abuse; malnutrition; diabetes; presence of systemic, gastrointestinal, hepatic, renal, cardiac, thyroid or hormonal problems; or a diagnosis of schizophrenia, psychotic disorder, bipolarity, major depression (<5 years), panic disorder and/or obsessive-compulsive disorder. Also, women who were pregnant or lactating or going through menopause and people who had donated blood in the past month were excluded. Females of childbearing age were required to use a contraceptive method to avoid getting pregnant during the trial. After phone screening, participants were invited to the Research Center. At that time, participants were requested to read and sign the informed consent form. All participants provided written informed consent prior to the trial beginning. The fasted blood samples were then collected to assess blood biochemistry at baseline. These analyses were performed at the Centre Hospitalier Universitaire de Sherbrooke clinical laboratory and included HDL cholesterol, LDL cholesterol, triacylglycerols, fasting blood glucose concentration and glycated haemoglobin. Individuals with values outside the clinical reference range were excluded.
Randomisation and blinding
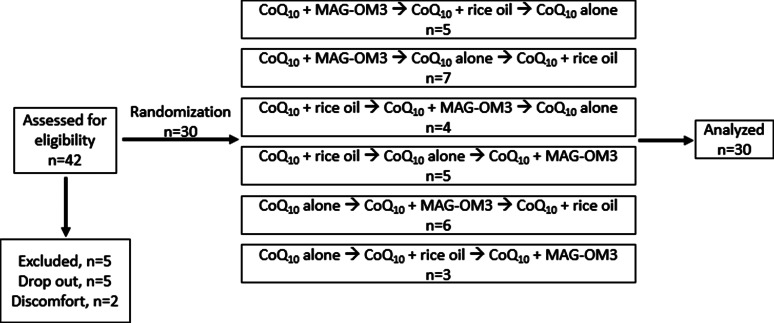
Randomisation of the treatment groups was performed using ‘https://www.randomizer.org/’. Treatment randomisation is described in a flow chart in Fig. 1. Participants, clinical staff and laboratory staff were blinded to treatment allocation. The CoQ10 + MAG-OM3 and CoQ10 + rice oil capsules were identical in size, shape and taste, but differed from the CoQ10 alone capsule. To maintain blinding, participants wore an eye mask when taking the supplements and a research staff not involved in the trial provided the capsules to participants to keep blinding. Also, each plasma sample was assigned a random number between 1 and 990 to avoid knowing the participant's number, treatment and the time at which the sample was analysed. These were unblinded only after performing all the CoQ10 analyses described below.
Fig. 1.
Clinical trial flow chart and randomisation of the treatments order to the CoQ10 + MAG-OM3, CoQ10 + rice oil or CoQ10 alone supplement. The n represents the number of participants allocated to the treatment sequence. CoQ10, coenzyme Q10; MAG-OM3, monoglycerides omega-3.
Study treatments
The study treatments consisted of (1) CoQ10 + MAG-OM3, (2) CoQ10 + rice oil (a neutral triacylglycerol oil) and (3) CoQ10 alone, in powder. Each capsule provided 100 mg of oxidised CoQ10, the most stable form of CoQ10, and 500 mg of oil, except for the CoQ10 alone which contained only 100 mg of CoQ10 in powder. The supplement content in CoQ10 was analysed by Labs Mart and it ranges from 108 to 117 mg/capsules (n 2 capsules/supplement type). The fatty acid profile of the capsules was determined using the modified AOCS Ce 1b-89 method and is detailed in Table 1.
Table 1:
Fatty acids content in the supplements
| CoQ10 + MAG-OM3 supplement (mg/capsule) |
CoQ10 + rice oil supplement (mg/capsule) |
|||
|---|---|---|---|---|
| Fatty acids | Mean | SD | Mean | SD |
| 8:0 | 0·0 | 0·0 | 0·4 | 0·5 |
| 10:0 | 0·9 | 0·4 | 3·4 | 3·7 |
| 14:0 | 1·5 | 0·0 | 1·5 | 0·1 |
| 16:0 | 6·1 | 0·7 | 89·6 | 2·8 |
| 16:1 n-7 | 0·5 | 0·0 | 0·9 | 0·0 |
| 17:1 | 0·4 | 0·0 | 0·1 | 0·3 |
| 18:0 | 6·6 | 0·0 | 9·5 | 0·3 |
| 18:1 n-9 | 11·3 | 0·8 | 190·4 | 5·8 |
| 18:1 n-7 | 3·3 | 0·0 | 4·0 | 0·1 |
| 18:2 n-6 | 3·6 | 0·7 | 144·1 | 4·4 |
| 18:3 n-6 | 0·7 | 0·0 | 1·6 | 0·1 |
| 18:3 n-3 | 1·2 | 0·0 | 4·3 | 0·1 |
| 20:0 | 5·2 | 0·1 | 3·6 | 0·1 |
| 20:1 | 14·3 | 0·2 | 2·4 | 0·1 |
| 20:2 | 2·4 | 0·1 | 0·0 | 0·0 |
| 20:3 n-6 | 2·9 | 0·0 | 0·0 | 0·0 |
| 20:4 n-6 | 12·9 | 0·2 | 0·0 | 0·0 |
| 20:3 n-3 | 1·3 | 0·1 | 0·0 | 0·0 |
| 20:5 n-3 | 224·3 | 3·5 | 0·0 | 0·0 |
| 22:0 | 0·0 | 0·0 | 1·3 | 0·1 |
| 22:1 | 1·7 | 0·1 | 0·0 | 0·0 |
| 22:5 n-6 | 3·4 | 0·1 | 0·0 | 0·0 |
| 22:5 n-3 | 14·3 | 0·1 | 0·0 | 0·0 |
| 22:6 n-3 | 95·3 | 1·6 | 0·0 | 0·0 |
| 24:0 | 0·0 | 0·0 | 2·5 | 0·1 |
| TOTAL Concentration | 414·1 | 8·9 | 459·8 | 18·7 |
n = 3 capsules/supplement form.
Participants consumed two capsules providing 200 mg of CoQ10 and 1 g of oil. This dose was selected because a study compared the pharmacokinetics of a single oral dose of 100 and 200 mg of CoQ10 and they reported an increase of 150 % of CoQ10 in the plasma with the 200 mg dose compared to an increase of 80 % with the 100 mg dose(18). Another study also obtained similar results(19). Therefore, the dose of 200 mg/d of CoQ10 was selected for the present study. Also, a dose of 1 g of oil was selected because it was similar to a previous study done by our group(13,14). A minimum of 1 week washout period was mandatory between treatments since CoQ10's half-life is 33 h(20). It is generally accepted for drugs that four to five times the half-life eliminate the active molecule at 94–97 %, which is considered to be below clinical significance(21). Therefore, in this trial, 6⋅9 d were required to eliminate CoQ10 at 97 %.
Study design
Participants were required to be fasted at arrival. A blood sample was then collected, and a standardised breakfast with the two capsules was provided. The breakfast provided 410 kcal (75 % carbohydrates, 22 % proteins, 3 % lipids) and was composed of two toasts with raspberry jam, a banana, a protein chocolate milk and a coffee or a tea. In the present study, meals were provided throughout the day to control the dietary fat intake. A low-fat menu was selected to limit bias in the CoQ10 metabolism. After consuming the capsules, the timer indicated the subsequent blood samples to be collected at 1, 3, 5, 6, 7, 8, 10, 11, 24 and 48 h post-single oral dose intake. Blood was collected in tubes containing citrate. Lunch and dinner were provided at 4 and 9 h post-single-dose intake, and they provided, respectively, 614 kcal (78 % carbohydrates, 17 % proteins, 5 % lipids) and 696 kcal (72 % carbohydrates, 23 % proteins, 5 % lipids). Lunch was composed of one serving of chow mein, baby carrots, an apple, a yogurt, a juice and a fig bar, and dinner was composed of one serving of General Tao, one slice of bread, one mini cucumber, apple sauce, protein chocolate milk, vegetable juice and a granola bar. Also, at 11 h post-dose intake, a snack (one granola bar and one package of gummy fruits) provided 210 kcal (87 % carbohydrates, 6 % proteins, 7 % lipids). The menu and food items consumed by a participant in the first pharmacokinetic study day were kept the same for the other two pharmacokinetic days to limit potential bias. Participants returned for a blood sample 24 and 48 h post-single-dose intake. During the study day, blood samples were kept on ice and centrifuged at 1700 g for 10 min at 4°C, and the plasma was aliquoted and stored at −80°C until further analysis.
CoQ10 extraction and analysis
Total CoQ10 was extracted from plasma samples, using the Hansen et al. method(22). Because reduced CoQ10 is unstable, plasma CoQ10 was oxidised with 1,4-benzoquinone to generate a stable CoQ10 analyte. To allow quantification, coenzyme Q9 (CoQ9) was added as an internal standard.
Briefly, samples were thawed at room temperature for 45 min. In total, 50 μl of plasma were transferred to a 1⋅5 ml tube along with 50 μl of 1,4-benzoquinone dissolved in methanol (0⋅4 mg/ml) and 50 μl of CoQ9 dissolved in 1-propanol (800 μg/l). After mixing for 15 min at room temperature, 1 ml of 1-propanol was added to the tube. Tubes were then mixed again for 1 min and centrifuged at 10 000 g for 3⋅5 min at 4°C. After centrifugation, 1 ml of the supernatant was transferred to an HPLC vial. In total, 300 μl of methanol was added to have the same solvent as the mobile phase of the liquid chromatography system. All vials were stabilised in the autosampler at 4°C for 30 min before being injected. One quality control sample was done per batch.
Analyses were performed on Waters Acquity UPLC H-Class system coupled to Waters Xevo TQ-S Micro tandem mass spectrometer. The column used was Waters Acquity UPLC BEH C18 2⋅1 × 50 mm, 1⋅7 μm and the pre-column was Waters Acquity UPLC BEH C18 Vanguard 2⋅1 × 5 mm, 1⋅7 μm and it was maintained at 25°C. The mobile phase was composed of methanol with 5 mm of ammonium formate, in isocratic mode. The injection volume was 1 μl, the flow was 0⋅4 ml/min and the total run time was 14 min per injection. The mass spectrometer was operated in the electrospray positive mode. The optimised detector parameters were set as follows: nitrogen desolvation was at 600°C with a flow rate of 1000 l/h, the nitrogen cone gas was set at 80 l/h, the argon collision gas was at 5 psi, the source temperature was as 150°C and the capillary voltage was set at 0⋅9 kV. The cone voltage was at 88 V for CoQ10 and 82 V for CoQ9. The collision energy was at 32 V for CoQ10 and at 30 V for CoQ9. Quantification was performed in multiple reaction monitoring mode with the following mass transitions: CoQ10 at m/z 863⋅6 → 197⋅1 and CoQ9 at 795⋅6 → 197⋅1. The dwell time for each transition was 0⋅037 s.
Calibration curves were freshly prepared daily using CoQ10 stock solution at the following concentrations: 0, 250, 500, 1500, 2000 and 2500 μg/l. An internal standard (CoQ9) at a concentration of 800 μg/l was also added in each of the calibration curve samples. Calibration curves were generated using MassLynx software by plotting the peak area ratio of CoQ10 to internal standard v. the CoQ10 concentration. Sample CoQ10 concentrations were calculated with a linear regression using the 1/x weighting factor.
Statistical analysis
Sample size was calculated with a website sample size calculator(23). The sample size was calculated based on the available data on the pharmacokinetics of CoQ10 that was the closest to our study design. López-Lluch et al. performed a pharmacokinetic study on seven different CoQ10 formulations and monitored CoQ10 plasma concentration over a 48 h period in fourteen healthy adults after a single oral dose intake of approximately 100 mg of CoQ10(24). Using the 0–48 h AUC of the olive oil/coconut oil + CoQ10 treatment (6⋅28 ± 3⋅07 mg/l*48 h) compared to the finely ground CoQ10 powder (8⋅94 ± 3⋅33 mg/l*48 h), we calculated, with a power of 80 % with an α = 0⋅05, a sample size of twenty-five participants was required. We, therefore, tested thirty participants to account for a potential 20 % attrition.
Area under the curves (AUC) were calculated using GraphPad Prism 7.03 software. The incremental AUC was calculated with the 48 h-curves, by ignoring the areas below the baseline. ΔCmax was defined as the CoQ10 maximum concentration minus the baseline CoQ10 plasma concentration. Tmax was defined as the time required to reach Cmax.
The primary outcome of the present study was the 0–48 h AUC of CoQ10. Secondary outcomes included 0–6 h AUC, ΔCmax, Tmax, C 0 h and C 48 h. Statistical analyses were performed using IBM SPSS Statistics 25 and GraphPad Prism 7.03 software. For the pharmacokinetic parameters, the Shapiro–Wilk normality test (α = 0⋅05) was performed, and data were not normally distributed. Therefore, Friedman's ANOVA statistical rank test for paired samples was used. Dunn's multiple comparison test was used for the comparison between treatments. For the comparison between sexes, a two-way ANOVA test was performed, with Bonferroni's multiple comparison test. However, for the ΔC 48 h, these data were analysed by fitting a mixed model, rather than by repeated-measures ANOVA, because there were missing values. Indeed, three participants refused to come back on one of their 48 h follow-up visits. The P interaction represents the combined effect of sex and supplement type, while P sex and P supplement represent the individual effects of sex or supplement.
Results
Participants baseline characteristics
Table 2 presents the baseline data of the participants. There was no significant difference between men and women. The participants’ mean age was 26⋅1 ± 4⋅8 years. Treatment randomisation is described in the flow chart (Fig. 1).
Table 2:
Baseline characteristics of the participants
| Total cohort (n = 30) |
Men (n = 15) |
Women (n = 15) |
|||||
|---|---|---|---|---|---|---|---|
| Baseline characteristics | Mean | SD | Mean | SD | Mean | SD | P-value |
| Age (years) | 26·1 | 4·8 | 26·7 | 4·3 | 25·5 | 5·2 | 0·4771 |
| BMI (kg/m2) | 25·3 | 3·6 | 25·6 | 3·5 | 25·1 | 3·8 | 0·7043 |
| Plasma TG* (mmol/L) | 0·9 | 0·6 | 0·9 | 0·4 | 1·0 | 0·7 | 0·9584 |
| Plasma HDL-C (mmol/L) | 1·4 | 0·3 | 1·3 | 0·3 | 1·4 | 0·2 | 0·1738 |
| Plasma LDL-C (mmol/L) | 2·5 | 0·8 | 2·5 | 0·8 | 2·4 | 0·8 | 0·7934 |
| Plasma glucose (mmol/L) | 4·5 | 0·4 | 4·4 | 0·4 | 4·5 | 0·4 | 0·5183 |
| HbA1c (%) | 5·0 | 0·3 | 5·1 | 0·3 | 5·0 | 0·3 | 0·6834 |
BMI = body mass index, TG = triglycerides, HDL-C = high-density lipoproteins cholesterol, LDL-C = low-density lipoproteins cholesterol, HbA1c = glycated hemoglobin. P values were evaluated by T test for unpaired measurements.
*For TG, the data was not normally distributed, and a nonparametric Mann-Whitney test was performed.
Primary outcome
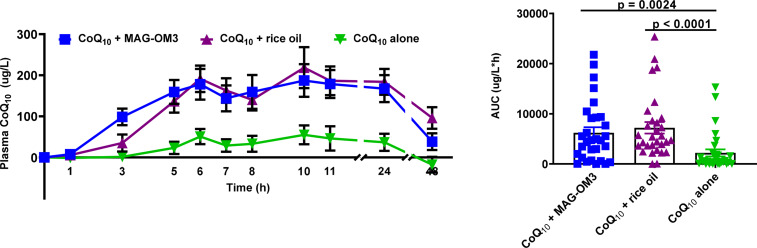
Fig. 2 and Table 3 present the 0–48 h pharmacokinetic curves and pharmacokinetic parameters of CoQ10. The 0–48 h AUC of CoQ10 combined with MAG-OM3 or rice oil was significantly higher than CoQ10 provided alone (Table 3).
Fig. 2.
Plasma CoQ10 concentrations over a 48 h period when combined with MAG-OM3, rice oil or alone. The results are expressed as the mean ± sem. P values were assessed by the Friedman nonparametric statistical analysis. CoQ10, coenzyme Q10; MAG-OM3, monoglycerides omega-3; AUC, area under the curve.
Table 3:
Pharmacokinetic parameters of CoQ10 in combination with MAG-OM3, rice oil or alone.
| CoQ10 + MAG-OM3 | CoQ10 + Rice oil | CoQ10 alone | |||||
|---|---|---|---|---|---|---|---|
| Pharmacokinetic parameters | Mean | SEM | Mean | SEM | Mean | SEM | P-value |
| Tmax (h) | 9·5 | 1·3 | 9·6 | 1·3 | 6·9 | 1·1 | 0·6128 |
| C0h (μg/L) | 611·6 | 42·0 | 617·9 | 43·4 | 581·8 | 40·2 | 0·0877 |
| ΔCmax (μg/L) | 281·1 A | 44·5 | 325·1 A | 50·1 | 127·8 B | 26·5 | 0·0015 |
| ΔC48h (μg/L) | 38·3 A | 20·5 | 96·1 B | 26·3 | −17·7 A | 19·2 | 0·0004 |
| AUC 0-6h (μg/L*h) | 576·7 A | 100·0 | 456·1 A | 73·1 | 161·7 B | 41·6 | <0·0001 |
| AUC 0-48h (μg/L*h) | 6211·0 A | 1097·0 | 7216·0 A | 1148·0 | 2199·0 B | 702·6 | <0·0001 |
Results are expressed as mean ± SEM. Tmax = time at which the maximum concentration is reached, C0h = plasma concentration at baseline, ΔCmax = maximum concentration, C48h = plasma concentration 48h post single dose intake, AUC 0-6 h = area under the curve over 6h, AUC 0-48h = area under the curve over 48h. P values were assessed by Friedman nonparametric statistical analysis, with Dunn's multiple comparison test. Data with different letters A and B are statistically different.
Secondary outcomes
The 0–6 h AUC of CoQ10 was significantly higher when CoQ10 was combined with MAG-OM3 or rice oil compared to CoQ10 alone (P < 0⋅0001). Plasma CoQ10 peaked 6 h and again 10 h post-single-dose intake (Fig. 2). Tmax and CoQ10 plasma concentrations at baseline were not different between treatments. CoQ10 concentrations still in the plasma 48 h after taking the supplements were 1⋅5–4⋅5 times higher for the CoQ10 + rice oil supplement compared to MAG-OM3 or CoQ10 alone (P = 0⋅0004) (Table 3). The ΔCmax of plasma CoQ10 was, respectively, 1 and 1⋅5 times higher when CoQ10 was provided with MAG-OM3 or rice oil compared to the powder supplement form (P = 0⋅0015) (Table 3).
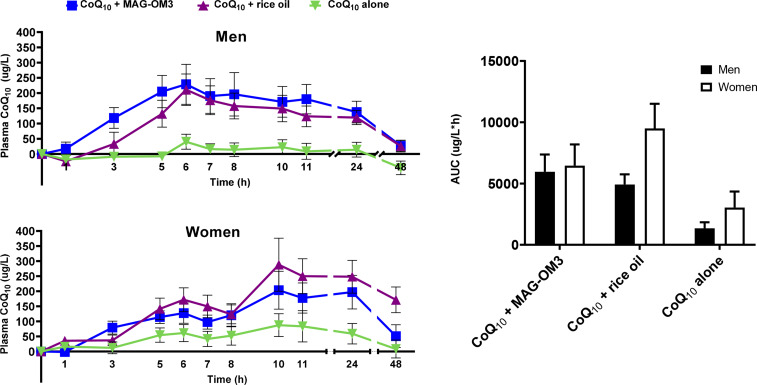
CoQ10 pharmacokinetic parameters were also assessed in relation to sexes (Fig. 3). There was no sex difference in the AUC 0–48 h, Tmax and ΔCmax. Forty-eight hours after the single-dose intake of CoQ10, women had, on average, eleven times higher CoQ10 plasma concentration than men (P = 0⋅0143). There was an interaction between sex and supplement form for the baseline concentration (C 0 h) and the AUC 0–6 h in the pharmacokinetics of CoQ10 between men and women (Fig. 3; Table 4). A significant sex by supplement interaction means that the effect of the supplements differs between men and women, even though the effect of sex alone is not significant. For the AUC 0–6 h, men had higher postprandial CoQ10 levels, whereas the highest concentrations in CoQ10 in women were reached 10 h after the single-dose intake.
Fig. 3.
Plasma CoQ10 concentration in men and women over a 48 h period when combined with MAG-OM3, rice oil or alone and AUC over a 48 h period. The results are expressed as the mean ± se. CoQ10, coenzyme Q10; MAG-OM3, monoglycerides omega-3; AUC, area under the curve.
Table 4:
Pharmacokinetic parameters of CoQ10 in combination with MAG-OM3, rice oil or alone in men and women
| Pharmacokinetic parameters | CoQ10 + MAG-OM3 | CoQ10 + Rice oil | CoQ10 Alone | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | P-value | |||||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | P interaction | P sex | P supplement | |
| Tmax (h) | 7·2 | 1·5 | 11·7 | 2·1 | 8·7 | 1·7 | 10·6 | 1·9 | 7·7 | 1·9 | 6·1 | 1·0 | 0·1772 | 0·3127 | 0·1795 |
| C0h (μg/L) | 589·6 | 54·6 | 633·6 | 65·3 | 658·7 | 58·4 | 577·1 | 64·4 | 614·8 | 64·5 | 548·9 | 48·9 | 0·0198 | 0·6691 | 0·2706 |
| ΔCmax (μg/L) | 279·8 | 67·6 | 282·4 | 60·2 | 373·1 | 52·1 | 373·2 | 85·8 | 87·0 | 18·4 | 168·7 | 48·3 | 0·5550 | 0·3588 | 0·0003 |
| ΔC48h* (μg/L) | 26·5 | 18·8 | 51·0 | 37·9 | 25·6 | 18·9 | 171·5 | 42·6 | -45·5 | 22·2 | 8·3 | 30·0 | 0·0696 | 0·0143 | 0·0002 |
| AUC 0-6h (μg/L*h) | 731·3 | 177·2 | 422·0 | 81·3 | 452·2 | 113·5 | 460·1 | 96·3 | 99·9 | 35·3 | 223·4 | 73·4 | 0·0250 | 0·6176 | <0·0001 |
| AUC 0-48h (μg/L*h) | 5957·2 | 1414·1 | 6464·9 | 1725·0 | 4927·9 | 824·8 | 9503·1 | 2007·1 | 1348·6 | 489·7 | 3049·5 | 1304·5 | 0·1707 | 0·1453 | <0·0001 |
Tmax = time at which the maximum concentration is reached, C0h = plasma concentration at baseline, ΔCmax = maximum concentration, ΔC48h = plasma concentration 48h post single dose intake, AUC 0-6 h = area under the curve over 6h, AUC 0-48h = area under the curve over 48h. P values were assessed by a 2-way ANOVA statistical analysis, with Bonferroni's multiple comparison test. The P interaction represents the combined effect of sex and type of supplement while P sex and P supplement represent the individual effect of sex or supplement.
*For the ΔC48h, these data were analyzed by fitting a mixed model, rather than by repeated measures ANOVA because there were missing values, i.e. 3 participants refused to come back on one of their 48h follow-up visit (n = 3).
Discussion
In the present study, we hypothesise that CoQ10 blood levels over a 48 h follow-up is higher when provided with MAG-OM3 than CoQ10 alone (in powder form) or added to rice oil (a neutral triacylglycerol oil). We report here that both CoQ10 + MAG-OM3 and CoQ10 + rice oil had approximately two times higher AUC 0–48 h than CoQ10 alone, and hence, our hypothesis is rejected. The rationale for using MAG-OM3 in the present study stem from a previous study done by our group supporting that when provided esterified in MAG, OM3 plasma concentrations were twice than when provided in a triacylglycerol or ethyl ester form and that concentration in the plasma 24 h after the single dose was also twice that of the other supplements(14). Moreover, our previous study also showed that there is an early effect of MAG-OM3 on fat absorption, and this would improve the AUC 0–6 h of CoQ10 compared to other formulations. However, our data showed that AUC 0–6 h was higher when CoQ10 was provided with MAG-OM3 and rice oil compared to when provided alone. Therefore, it seems that providing CoQ10 with MAG-OM3 did not reproduce the same postprandial benefit observed in Chevalier et al.(14) when investigating the metabolism of OM3. This suggests that liposoluble molecules like CoQ10 might only require to be solubilised in an oil to improve its absorption. However, its metabolism seems to be biphasic.
Indeed, we observed a biphasic shape of the CoQ10 pharmacokinetic curve with a CoQ10 peak at 6 h and again 10 h post-single-dose intake. This two-peak pattern has been reported in previous pharmacokinetic studies of CoQ10(9,25–27). For instance, Evans et al.(25) reported a biphasic distribution of CoQ10, with a first peak 6 h and a second peak between 12 and 24 h post-dose intake(25). One main explanation for this biphasic distribution is related to CoQ10 redistribution from tissues and its enterohepatic recycling after food intake 4 and 9 h post-single-dose intake(27,28). Lamber and Parks(28) hypothesised that ‘lipids secreted at the very onset of a meal are those that were consumed in an earlier meal, suggesting the presence of an enterocyte storage pool for triglycerides’(28). Thus, we hypothesise that CoQ10 might have been stocked in the liver or other tissues enriched in lipids and thereafter redistributed to the blood circulation after food intake(9,29). However, our results offer another explanation for this biphasic shape of CoQ10 metabolism. Indeed, our data support a difference in the COQ10 metabolism between men and women since we found that CoQ10 concentrations peaked at 10 h post-dose in women but at 6 h post-dose in men (Fig. 3). Other evidence is related to a sex by supplement interaction for AUC 0–6 h and a trend towards this interaction for ΔC 48 h (P = 0⋅0696). These results support that the CoQ10 metabolism in response to a CoQ10 supplement differs between men and women. Hence, men had a higher AUC 0–6 h than women and their concentration remained higher up to 24 h after the single-dose intake but returned close to baseline 48 h after the single-dose intake. On the other hand, 24 h after the single CoQ10 dose with MAG-OM3 or rice oil, women had ~30–60 % higher CoQ10 plasma concentrations than men and 48 h after the single-dose intake, women still had at least twice the CoQ10 plasma concentration level of men. Unlike the data of Wahlqvist et al.(30) suggesting that men have a better absorption and/or lower clearance of CoQ10 than women, here our data suggest that woman actually have lower clearance since their plasma CoQ10 concentrations are higher than men 24 and 48 h after the single-dose intake. This could be partially explained by a slower gastric motility(31) in women supporting why CoQ10 absorption peaked later in women, although Tmax was not statistically different between men and women. However, from our data, it seems that there are transiently higher concentrations of CoQ10 in women than men and there was a trend towards a sex by supplement interaction (P = 0⋅0696) in the ΔC 48 h concentration. Delta plasma CoQ10 concentrations in women receiving the CoQ10 + rice oil were about eleven times higher than the other supplements. Looking back at the data to see if this was the result of one or two outliers, we can confirm that it was not the case and that nine participants out of fifteen had a plasma concentration higher than 80 μg/l of CoQ10.
In the present study, we also found a sex by supplement interaction on the AUC 0–6 h. In the literature, there are discrepancies between studies in the AUC 0–6 h, and this result should therefore be interpreted with caution. For instance, one study reported a higher AUC(32), while another reported a higher concentration(30) in men than women after a single-dose intake of CoQ10. Conversely, one study reported higher CoQ10 bioavailability in women than in men, although this result was not significant(26). Therefore, it is highly recommended that future pharmacokinetics of CoQ10 should be evaluated in men and women since their absorption peak is different by sex together with their levels up to 48 h after the single-dose intake. This recommendation also needs to consider the baseline CoQ10 concentrations since previous studies reported higher levels in men than women(33–36). In the present study, there was an interaction between sex and supplement for C 0 h but our post hoc analysis was not able to determine where the differences were. We did not detect a significant P sex effect of C 0 h in the present study (Table 4).
The present study had strengths and limitations. In terms of strengths, the crossover design was robust enough to detect significant differences between treatments. Also, every participant was their own control, which aimed at limiting inter-individual variability. Indeed, CoQ10 supplementation is known to generate plasma response with high inter-individual variability(15,35,37,38) and although we had a strong research design, inter-individual variability remained important in the present study. The high inter-individual variability found in other studies could be explained by age, gender, redox state and health disorders(9,37). Another strength of the present study is the standardised procedures. For example, all the participant's dietary intake during the study days was controlled, limiting bias among the study days especially with respect to dietary fat intake. Treatment randomisation and the triple-blind design were also strengths since neither the participants nor the clinical staff were aware of which supplement order the participants were subjected to and all tubes were coded to avoid knowing the sampling time, participant number and supplement type the tube corresponded to. The present study also has limitations. Since this is a pharmacokinetics study of a single oral dose, it does not provide information regarding the efficacy nor the long-term effects of the CoQ10 supplements. Also, it does not represent the pharmacodynamics of the supplements. The study population was young men and women which perhaps does not represent the population that could benefit the most from this type of supplementation. It also would have been interesting to test the pharmacokinetics parameters of these supplements in older adults, people under statin therapy or people with fat malabsorption since these groups of people could benefit more from the CoQ10 supplementation(2,9) and their pharmacokinetics are likely to differ from the ones we obtained in the present study. Knowing that CoQ10 absorption is enhanced in the presence of lipids(15), the low-fat diet throughout the study day could have influenced the overall CoQ10's absorption downwards. However, the purpose of our control supplement (CoQ10 in powder form) was to evaluate CoQ10 absorption when it was administered alone, with limited oil consumption. It would not have been possible to evaluate this effect if provided with a high-fat diet. This is a limitation in the transfer of these results to the general population since most people eat a diet that has higher fat levels than the ones we provided here.
To conclude, the purpose of this pharmacokinetic study was to evaluate whether the 48 h pharmacokinetics of CoQ10 was enhanced when provided with MAG-OM3. Our results do not support this hypothesis since CoQ10 AUC 0–48 h was similar to when CoQ10 was provided with rice oil. We conclude that the administration of CoQ10 in combination with lipid carriers helps enhance its absorption and overall metabolism. However, one important finding of the present study is the different pharmacokinetics of CoQ10 between men and women, with higher level of CoQ10 in women than men when provided with MAG-OM3 or rice oil. The plus value of the MAG-OM3 supplements resides in its OM3 content and pharmacokinetics providing higher OM3 levels in the blood in postprandial. Specifically, these results are of potential interest for those with fat malabsorption issues but require to be tested in this population before a definite conclusion can be drawn.
Acknowledgments
This work was supported and reviewed by Biodroga Nutraceuticals. CoQ10 supplements were provided by Biodroga Nutraceuticals. The company did not generate the data or have access to the final graph until our team had completed all the analyses and requested unblinding of the study. They reviewed the manuscript but did not influence the interpretation or conclusions of the study.
The authors’ responsibilities were as follows: M.P. designed the present study with Biodroga Nutraceuticals; S.B. handled the samples in the laboratory, wrote the first draft of the manuscript, and analysed and interpreted the data with the help of A.V.; A.V. supervised the quality control of the CoQ10 analysis; A.V. and M.P. revised the manuscript and all authors read and approved the final manuscript.
M.P. held salary awards from the Canadian Institutes of Health Research. M.P. also holds the Research Chair on Lipid Metabolism in Aging funded by the Medical Research Center of the Université de Sherbrooke.
This trial was revised and approved by the Research Ethics Committee of the CIUSSSE–CHUS (reference no. 2020-3280). The present study is registered at clinicaltrials.gov under no. NCT04035525.
References
- 1.Freye ECK (2018) Coenzyme Q10 supplements which increase ATP synthesis within mitochondria and protect against toxic sodium azide. Coenzyme Q 2, 2–9. [Google Scholar]
- 2.Rodick TC, Seibels DR, Babu JR, et al. (2018) Potential role of coenzyme Q10 in health and disease conditions. Nutr Diet Suppl. doi: 10.2147/NDS.S112119. [DOI] [Google Scholar]
- 3.Cramer WA, Hasan SS & Yamashita E (2011) The Q cycle of cytochrome bc complexes: a structure perspective. Biochim Biophys Acta 1807, 788–802. doi: 10.1016/j.bbabio.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ernster L & Dallner G (1995) Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta BBA – Mol Basis Dis 1271, 195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 5.Arenas-Jal M, Suñé-Negre JM & García-Montoya E (2020) Coenzyme Q10 supplementation: efficacy, safety, and formulation challenges. Compr Rev Food Sci Food Saf 19, 574–594. doi: 10.1111/1541-4337.12539. [DOI] [PubMed] [Google Scholar]
- 6.Hernández-Camacho JD, Bernier M, López-Lluch G, et al. (2018) Coenzyme Q10 supplementation in aging and disease. Front Physiol 9. doi: 10.3389/fphys.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalén A, Appelkvist EL & Dallner G (1989) Age-related changes in the lipid compositions of rat and human tissues. Lipids 24, 579–584. doi: 10.1007/BF02535072. [DOI] [PubMed] [Google Scholar]
- 8.Knott A, Achterberg V, Smuda C, et al. (2015) Topical treatment with coenzyme Q10-containing formulas improves skin's Q10 level and provides antioxidative effects. Biofactors Oxf Engl 41, 383–390. doi: 10.1002/biof.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pravst I, Rodríguez Aguilera JC, Cortes Rodriguez AB, et al. (2020) Comparative bioavailability of different coenzyme Q10 formulations in healthy elderly individuals. Nutrients 12. doi: 10.3390/nu12030784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hidaka T, Fujii K, Funahashi I, et al. (2008) Safety assessment of coenzyme Q10 (CoQ10). BioFactors 32, 199–208. doi: 10.1002/biof.5520320124. [DOI] [PubMed] [Google Scholar]
- 11.Parkinson Study Group QE3 Investigators, Beal MF, Oakes D, et al. (2014) A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol 71, 543–552. doi: 10.1001/jamaneurol.2014.131. [DOI] [PubMed] [Google Scholar]
- 12.Pastor-Maldonado CJ, Suárez-Rivero JM, Povea-Cabello S, et al. (2020) Coenzyme Q10: novel formulations and medical trends. Int J Mol Sci 21, 8432. doi: 10.3390/ijms21228432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chevalier L & Plourde M (2020) Comparison of pharmacokinetics of omega-3 fatty acid supplements in monoacylglycerol or ethyl ester in humans: a randomized controlled trial. Eur J Clin Nutr 1–9. doi: 10.1038/s41430-020-00767-4. Published online October 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chevalier L, Vachon A & Plourde M (2021) Pharmacokinetics of supplemental omega-3 fatty acids esterified in monoglycerides, ethyl esters, or triglycerides in adults in a randomized crossover trial. J Nutr nxaa458. doi: 10.1093/jn/nxaa458. Published online February 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantle D & Dybring A (2020) Bioavailability of coenzyme Q10: An overview of the absorption process and subsequent metabolism. Antioxidants 9. doi: 10.3390/antiox9050386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao X, Bor G, Al-Hosayni S, et al. (2018) Structural characterization of self-assemblies of new omega-3 lipids: docosahexaenoic acid and docosapentaenoic acid monoglycerides. Phys Chem Chem Phys 20, 23928–23941. doi: 10.1039/C8CP04256J. [DOI] [PubMed] [Google Scholar]
- 17.Cuenoud B, Rochat I, Gosoniu ML, et al. (2020) Monoacylglycerol form of omega-3s improves Its bioavailability in humans compared to other forms. Nutrients 12, 1014. doi: 10.3390/nu12041014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohr D, Bowry VW & Stocker R (1992) Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta 1126, 247–254. [DOI] [PubMed] [Google Scholar]
- 19.Singh RB, Niaz MA, Kumar A, et al. (2005) Effect on absorption and oxidative stress of different oral coenzyme Q10 dosages and intake strategy in healthy men. BioFactors Oxf Engl 25, 219–224. doi: 10.1002/biof.5520250127. [DOI] [PubMed] [Google Scholar]
- 20.Bhagavan HN & Chopra RK (2006) Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res 40, 445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 21.Hallare J & Gerriets V (2020) Half life. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK554498/ (accessed October 2020). [PubMed] [Google Scholar]
- 22.Hansen G, Christensen P, Tüchsen E, et al. (2004) Sensitive and selective analysis of coenzyme Q in human serum by negative APCI LC-MS. Analyst 129, 45–50. doi: 10.1039/B308690A. [DOI] [PubMed] [Google Scholar]
- 23.Sample Size Calculator (2021) Sample Size Calculator. https://clincalc.com/stats/samplesize.aspx (accessed July).
- 24.López-Lluch G, del Pozo-Cruz J, Sánchez-Cuesta A, et al. (2019) Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition 57, 133–140. doi: 10.1016/j.nut.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Evans M, Baisley J, Barss S, et al. (2009) A randomized, double-blind trial on the bioavailability of two CoQ10 formulations. J Funct Foods 1, 65–73. doi: 10.1016/j.jff.2008.09.010. [DOI] [Google Scholar]
- 26.Constantinescu R, McDermott MP, DiCenzo R, et al. (2007) A randomized study of the bioavailability of different formulations of coenzyme Q10 (ubiquinone). J Clin Pharmacol 47, 1580–1586. doi: 10.1177/0091270007307571. [DOI] [PubMed] [Google Scholar]
- 27.Bhagavan HN & Chopra RK (2007) Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 7, S78–S88. doi: 10.1016/j.mito.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Lambert JE & Parks EJ (2012) Postprandial metabolism of meal triglyceride in humans. Biochim Biophys Acta 1821, 721–726. doi: 10.1016/j.bbalip.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasai T, Tokuda T, Ohmichi T, et al. (2016) Serum levels of coenzyme Q10 in patients with multiple system atrophy. PLoS ONE 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahlqvist M, BPharm NW, Savige G, et al. (1988) Bioavailability of two different formulations of coenzyme Q10 in healthy subjects. Asia Pac J Clin Nutr 4, 37–40. [PubMed] [Google Scholar]
- 31.Whitley HP & Lindsey W (2009) Sex-based differences in drug activity. Am Fam Physician 80, 1254–1258. [PubMed] [Google Scholar]
- 32.Weis M, Mortensen SA, Rassing MR, et al. (1994) Bioavailability of four oral coenzyme Q10 formulations in healthy volunteers. Mol Aspects Med 15, s273–s280. doi: 10.1016/0098-2997(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 33.Miles MV, Horn PS, Morrison JA, et al. (2003) Plasma coenzyme Q10 reference intervals, but not redox status, are affected by gender and race in self-reported healthy adults. Clin Chim Acta 332, 123–132. doi: 10.1016/S0009-8981(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 34.Niklowitz P, Onur S, Fischer A, et al. (2016) Coenzyme Q10 serum concentration and redox status in European adults: influence of age, sex, and lipoprotein concentration. J Clin Biochem Nutr 58, 240–245. doi: 10.3164/jcbn.15-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martucci A, Reurean-Pintilei D & Manole A (2019) Bioavailability and sustained plasma concentrations of CoQ10 in healthy volunteers by a novel oral timed-release preparation. Nutrients 11, 527. doi: 10.3390/nu11030527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molyneux SL, Florkowski CM, Lever M, et al. (2005) Biological variation of coenzyme Q10. Clin Chem 51, 455–457. doi: 10.1373/clinchem.2004.043653. [DOI] [PubMed] [Google Scholar]
- 37.Molyneux SL, Young JM, Florkowski CM, et al. (2008) Coenzyme Q10: is there a clinical role and a case for measurement? Clin Biochem Rev 29, 71–82. [PMC free article] [PubMed] [Google Scholar]
- 38.Miles MV (2007) The uptake and distribution of coenzyme Q(10). Mitochondrion 7, S72–S77. doi: 10.1016/j.mito.2007.02.012. [DOI] [PubMed] [Google Scholar]