Highlights
-
•
This review focused on implemented HPV vaccine delivery strategies and their costs.
-
•
Insights on the costs and effectiveness of HPV vaccination strategies are provided.
-
•
Access was improved by increasing the availability and the uptake of HPV vaccines.
-
•
Costs varied by vaccine delivery strategy and target population.
-
•
Lessons learned can inform efficient and equitable allocation of vaccine resources.
Keywords: Human papillomavirus or HPV, Vaccine, Cost, Effectiveness or reach, Access, Delivery strategy
Abstract
Fifteen years following the approval of the first human papillomavirus (HPV) vaccine, cervical cancer continues to be a significant source of morbidity and mortality among women in low-resource settings. It is the second-leading cause of cancer-related deaths in women globally and the leading cause of cancer-related deaths in Sub-Saharan Africa. Vaccine delivery and programmatic costs may hinder the distribution of HPV vaccines in low-resource settings, and ultimately influence access to HPV vaccines. While reviews have been conducted on the cost-effectiveness of HPV vaccines, little is known about the cost and effectiveness of vaccination strategies. The purpose of this systematic review was to synthesize evidence on the cost and cost-effectiveness of vaccination strategies utilized to increase access to HPV vaccines. Search queries were created for CINAHL Plus, Embase, and PubMed. Our search strategy focused on articles that contained information on HPV vaccine uptake/reach, HPV vaccination costs, or the cost-effectiveness of HPV vaccination programs. We retrieved 773 articles from the databases, assessed 251 full-texts, and included 15 articles in our final synthesis. Countries without national HPV vaccination programs aimed to identify and adopt sustainable strategies to make HPV vaccines available to adolescents through demonstration programs. In contrast, countries with national vaccination programs focused on identifying cost-effective interventions to increase vaccination rates to meet nationally recommended standards. There is a dire need for HPV vaccination programs and intervention studies tailored to settings in low- and middle-income countries to increase access to HPV vaccines. Future studies should also evaluate the cost-effectiveness of implemented strategies.
1. Background
Human papillomavirus (HPV) is the most prevalent viral infection of the reproductive tract and the most common sexually transmitted infection (Centers for Disease Control and Prevention, 2019, World Health Organization, 2020). This is concerning because persistent infection with HPV can progress to precancerous and cancerous lesions. Malignancies caused by HPV occur in the anus, cervix, oropharynx, penis, rectum, vagina, and vulva (Centers for Disease Control and Prevention, 2020). Both sex-related and regional disparities exist in the burden and impact of HPV-associated malignancies.
The global estimate of cancers caused by HPV is approximately 640,000 annually, accounting for 29.5% of cancers caused by infections (Serrano et al., 2018). Of these, 570,000 are cervical cancer cases which result in 311,000 cervical cancer-related deaths (World Health Organization, 2020, Serrano et al., 2018). Cervical cancer is the leading cause of cancer-related deaths in Sub-Saharan Africa and the second-leading cause of cancer-related deaths in women globally (Ferlay et al., 2019). About 90% of cervical cancer deaths occur in low- and middle-income countries (LMICs) (World Health Organization, 2020). Cervical cancer incidence and mortality are significantly associated with poverty rates, urbanization, literacy rate, health expenditure per capita, human development index, and gender inequality index; with poverty rate and human development index explaining more than 52% of the variation in global mortality (Singh et al., 2012). The human development index combines factors such as educational attainment and gross national income to measure social and economic development, which highlights the role of economic and social inequalities in driving global trends in cervical cancer disparities (Klugman, 2011). Despite lower cervical cancer-related deaths in high-income countries (HICs), similar trends in disparities are associated with poverty and comparable socio-economic variables. For example, in the United States of America (USA), cancer disparities are associated with low income, low health literacy, and decreased access to medical facilities; rural Appalachia has higher rates of cervical cancer compared to urban regions (National Cancer Institute, 2020). In Australia, cervical cancer incidence is 52% higher in women in the lowest socio-economic status areas (Cancer Australia, 2021). Therefore, people with suboptimal access to health care and limited economic opportunities experience a higher disease burden from HPV and fewer prevention opportunities.
HPV vaccines prevent up to 90% of cancers caused by HPV infections (World Health Organization, 2020, St. Laurent et al., 2018, Nour, 2009). The vaccines are effective when administered before infection with HPV, ideally before sexual activity initiation (World Health Organization, 2020, Centers for Disease Control and Prevention, 2020). HPV vaccines are recommended for 9 to 26-year-olds as a 2-dose or 3-dose series (World Health Organization, 2020, Centers for Disease Control and Prevention, 2020).
The World Health Organization (WHO) recommends countries determine the cost-effectiveness of the HPV vaccine before implementing a national HPV vaccination program (World Health Organization, 2009). HPV vaccination may be cost-effective for LMICs if the total cost per vaccinated child is $10-to-25 United States dollars (US$) (World Health Organization, 2009). This estimated cost per child should include both the cost of the vaccines and programmatic costs. HPV vaccine delivery and programmatic costs could be negligible for HICs but constitute significant costs for LMICs; this warrants clarity on delivery costs (Fesenfeld et al., 2013). With support from Gavi, the Vaccine Alliance, more low-income countries have gained access to HPV vaccines for as little as US$ 4.50 per dose (Gavi. Human papillomavirus, 2020). Nevertheless, delivery and programmatic costs may hinder the distribution of HPV vaccines in low-resource settings, and ultimately influence the sustainability of HPV vaccination programs.
While reviews have been conducted on the cost-effectiveness of HPV vaccines, little is known about the cost and effectiveness of vaccination strategies that have been implemented to expand access to HPV vaccines. Studies have also reviewed the impact of HPV vaccination on gender equity (Portnoy et al., 2020) and country-specific HPV vaccination strategies (Holloway, 2019). The latter study focused on HPV vaccination strategies specific to the USA, which highlights the need to understand vaccination strategies across the world. To the best of our knowledge, no study has systematically reviewed studies on the cost and effectiveness of HPV vaccination strategies.
Our definition of access was guided by the concept definition by Gulliford and colleagues and includes four dimensions: (i) availability of health services, (ii) utilization of services, (iii) use of relevant services for satisfactory health outcomes, and (iv) equity of access (Gulliford et al., 2002). The utilization of services is impacted by acceptability, affordability, and physical accessibility of services. Once health services become readily available, the next challenge is vaccine uptake, which constitutes utilization of the available services. For the purpose of the paper, we will refer to the availability of HPV vaccines and the uptake of HPV vaccines to represent service availability and service utilization, respectively.
The purpose of our study was to review the cost and effectiveness of strategies that have been implemented to expand access to HPV vaccines. We conducted a systematic review of research on the cost and cost-effectiveness of HPV vaccination strategies according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Moher et al., 2009, Page et al., 2021).
2. Methods
2.1. Search strategy
We searched PubMed, Embase, and CINAHL Plus for articles about HPV vaccine uptake published through February 4th, 2020. Search terms included papillomavirus, vaccine, cost-effectiveness, and cost analysis. MeSH terminology, truncations, and Boolean operators were incorporated into the search strategy. Search terms and syntax were adapted to each database. The search queries used are provided (Supplementary data 1). All resulting articles were imported into Covidence for deduplication and screening (Covidence, 2020).
2.2. Inclusion/Exclusion criteria
We selected articles containing information on HPV vaccine uptake or reach, HPV vaccination strategy, and cost or cost-effectiveness. The scope included both intervention studies and evaluations of vaccination programs implemented to facilitate HPV vaccine uptake. We excluded articles that lacked cost data, did not include HPV vaccination as an outcome, did not implement a specific strategy to promote or increase vaccine uptake, literature reviews, and non-English articles.
2.3. Study selection
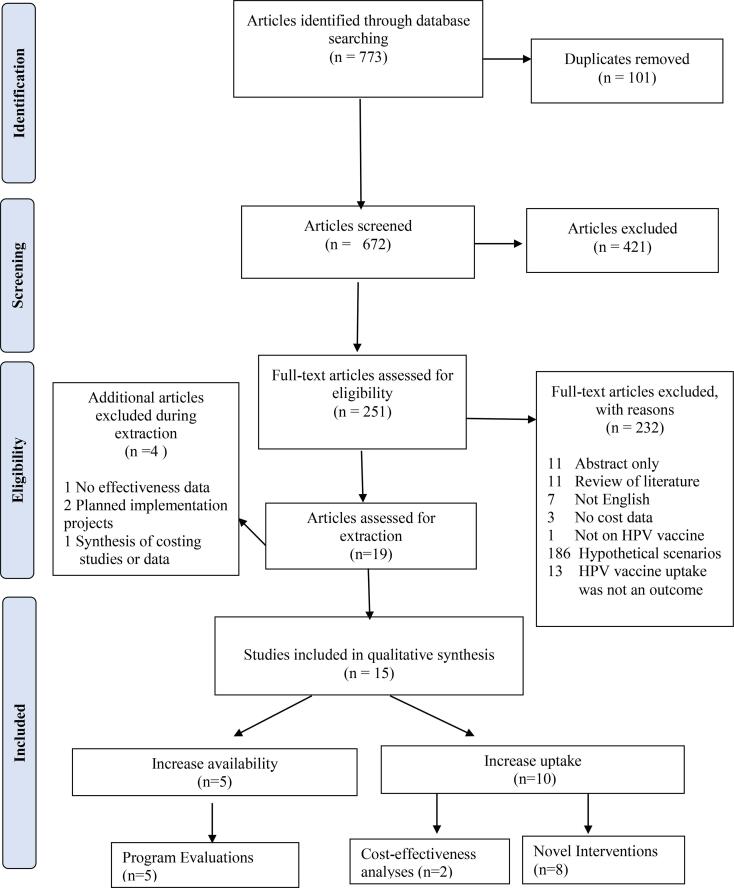
Three authors (AA, JL, and JW) screened articles in Covidence. All three authors independently completed the initial title and abstract screening to identify articles that would potentially be eligible for our review. Disagreement between authors was resolved by advancing the studies to full-text review. A.A., J.L, and J.W. completed the full-text review. All authors discussed inconsistencies, and agreement was reached by consensus. Additionally, articles identified from references during the full-text screening phase deemed eligible for our review were hand-searched. These articles were discussed as a team to determine eligibility for our study questions. The number of excluded studies and the rationale for exclusion are presented in the PRISMA flow diagram.
2.4. Data Extraction, Analysis, and synthesis
Data extraction and validation were discussed by three authors: AA, ES, and WT. All articles in our final selection were individually discussed during weekly team meetings by A.A., E.S., and W.T. Based on study design, the studies were categorized as program cost evaluations, cost-effectiveness analyses (CEAs), or novel interventions. The studies were synthesized qualitatively. There was notable heterogeneity with the results across studies, which impeded the possibility of any meaningful quantitative synthesis. We report summaries from studies in tables and synthesize the strategies across studies in a concept diagram.
2.5. Currency conversion
The summarized results in tables are presented in the currency year and values reported by the studies. We also report the effectiveness or reach associated with these cost values. Cost values in the manuscript are also in the currencies and years reported by the studies. To provide a better overall picture of delivery costs, we used the personal consumption expenditures price index (PCEPI) to calibrate program evaluation unit costs to May 2021 values for reference in Table 2. PCEPI is a measure of underlying inflation trends that are used to track changes in prices of goods and services, including healthcare expenditure (Bureau of Economic Analysis, 2021). It is prepared and released monthly by the Bureau of Economic Analysis (Bureau of Economic Analysis, 2021). We applied currency exchange values as needed (Organisation for Economic Co-operation and Development, 2000) to the World Bank values for per capita gross domestic product (GDP) (The World Bank, 2021). The per capita GDP for each of the countries was used to compare the incremental cost-effectiveness ratios (ICERs) as a percent of per capita GDP for the cost-effectiveness studies. These values are computed in the authors’ reporting year and are reported in Table 3.
Table 2.
Program evaluation unit costs
| First author | Country | Currency | Currency year | Total received HPV vaccine | Number of doses administered | Mean financial cost per dose | Mean financial cost per FIG | Mean economic cost per dose | Mean economic costs per FIG | §PCEPI December currency year | May 2021 financial cost per FIG | May 2021 economic cost per FIG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Levin | Peru Uganda Vietnam |
US$ | 2009 | 17,268 | 54,043 | 1.82 | 5.71 | 3.05 | 9.55 | 95.175 | 6.88 | 11.50 |
| Quentin | Tanzania | US$ | 2011 | 4,211 | 12,633 | 1.73 | 5.48 | 3.09 | 9.76 | 98.965 | 6.35 | 11.30 |
| Alonso | Mozambique | US $ | 2014 | 2,276 | 6,945 | 6.07 | 17.95 | 17.59 | 52.29 | 102.852 | 20.01 | 58.28 |
| Soi | Mozambique | US$ | 2014 | 9,669 | 29,007 | (y1: $30; y2: $19) | 36.9 | na | na | 102.852 | 41.13 | na |
| Hidle | Zimbabwe | US $ | 2016 | 5,724 | 11,599 | 19.76 | 40.03 | 45 | 91.19 | 105.005 | 43.70 | 99.55 |
HPV: human papillomavirus FIG: fully immunized girl. PCEPI: personal consumption expenditures price index. na: data not available.
The §PCEPI column represents the price index in December of the currency year that was used to estimate program costs. The PCEPI of the currency year reported and the PCEPI for May 2021 (US$ 114.631) were applied to inflate the costs per FIG to represent May 2021 cost values as presented in the last two columns.
Table 3.
Summary of cost effectiveness analyses
| Author (year) | Model | Perspective | Time Horizon | Currency Year | Discount Rate | Incremental Cost | Incremental Effect | Cost effectiveness Estimate | % GDP per capita | Uncertainty | Sensitivity Analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blakely et al. 2014 | Markov model BODE protocol |
Health system | 110 years | 2011 NZ$ | 3% | *NZ$ 4.65 million NZ$ 2.773 million NZ$ 3.784 million |
*266 QALYs 348 QALYs 382 QALYs |
$18,800 per QALY gained $34,700 per QALY gained $122,500 per QALY gained |
38.2% 71.4% 252.1% |
Yes | Yes |
| Wilson et al. 2020 | Markov model | Payer | 20 years | 2016 US$ | 3% | $158,048 | 1.80 LYS | US$$79,022 per LYS | 136.2% | no | Yes |
% GDP per capita represents the percentage of the per capita GDP of the ICER in the currency year for the study. World bank values for per capita GDP in reference years were used with rates obtained from OECD exchange rates database.
3. Results
3.1. Summary of included studies
We retrieved 773 articles. Of these, 672 articles were eligible for screening after removing 101 duplicates. After the title and abstract screening, 471 studies were excluded. We assessed 251 full-text studies. Nineteen were discussed for extraction. Four of the nineteen articles provided valuable information on the cost and effectiveness of HPV vaccination strategies but did not meet our inclusion criteria. These articles were excluded after the hand search did not yield additional information on the studies' costs and effectiveness results as needed for our inclusion criteria (Hutubessy et al., 2012, Karanth et al., 2017, Levin et al., 2014, Mahumud et al., 2020). Our final selection included 15 publications. The study selection process is presented in the flow diagram in Figure 1. The 15 studies included in our review were published between 2012 and 2020 and implemented across ten different countries (Mozambique, Zimbabwe, Peru, Uganda, Vietnam, Tanzania, New Zealand, USA, Belgium, and England). Five studies evaluated the cost of programs implemented in LMICs (Alonso et al., 2019, Hidle et al., 2018, Levin et al., 2013, Quentin et al., 2012, Soi et al., 2019). One study conducted a CEA of a comprehensive vaccination program that also delivered HPV vaccines, and another study conducted a CEA of an ongoing national HPV vaccination program (Blakely et al., 2014, Wilson et al., 2020). There were eight novel intervention studies, of which six were conducted in the USA (Morris et al., 2015, Szilagyi et al., 2013, Kempe et al., 2012, O'Leary et al., 2015, Fiks et al., 2013, Coley et al., 2018) and two in Europe (Lefevere et al., 2016, Mantzari et al., 2015). A summary of the study characteristics, including the costs and effectiveness of strategies utilized, is presented in Table 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram depicting the study selection process.
Table 1.
Summary of publications reviewed.
| Author & Year | Location | Gavi-supported | Study design | Target age cohort | Sample size | HPV vaccination outcomes assessed | Strategies Utilized | Main results of costs and effectiveness |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Alonso 2019 | Mozambique | ✓ | Demonstration program with retrospective micro costing | 10-year-old girls | 2 doses: 2,791 FIG3 doses: 2,276 FIG |
|
|
|
| Hidle 2018 | Zimbabwe | ✓ | Demonstration program with retrospective cost analysis | 10-year-old girls | 5,724 FIG |
|
|
|
| Levin 2013 | PeruUgandaVietnam | ✓ | Demonstration Program with retrospective micro costing | Adolescent girls | 17, 268 FIG |
|
|
|
| Quentin 2012 | Tanzania | --Sponsor-subsidized acquisition cost | Demonstration program with retrospective top-down cost analysis from project’s perspective | 10-to12-year-old girls (class 4 and class 6) | 4,211 FIG |
|
|
|
| Soi 2019 | Mozambique | ✓ | Demonstration program with retrospective micro costing | 10-year-old girls | Target population sizeYear 1: 8,556Year 2: 9,135 |
|
|
|
| ||||||||
| Blakely 2014 | New Zealand | – | Markov modelHealth system’s perspective | 12-year-ollds | National sample: 58,582 |
|
|
|
| Wilson 2020 | Texas, USA | – | Markov modelPayer’s perspective | Uninsured and low-income adults | 1,036 received HPV vaccines |
|
|
|
| ||||||||
| Coley 2018 | New York, USA | – | Randomized controlled trial | 11-to-13-year olds | Intervention: 81,558Control: 80,894 |
|
|
|
| Fiks 2013 | Philadelphia, USA | – | Randomized controlled trial; cluster and patient-level randomization | 11-to-17-year-old girls | Total: 22,486CDS: 5,557FFI: 5680CDS + FFI: 5,561No intervention: 5,68811 clinics |
|
|
|
| Kempe 2012 | Colorado, USA | – | Multi-method study: HPV vaccine demonstration project for girls only, and randomized controlled trial for boys | Sixth graders attending public schools; girls only for HPV vaccines | Total: 529Girls: 265 |
|
|
|
| Lefevere 2016 | Flanders, Belgium | – | Retrospective cohort study analyzing claims data | 12-to-18-year-old girls | Total: 6415Intervention: 850 |
|
|
|
| Mantzari 2015 | England, UK | – | Randomized controlled trial | 16-to-18-year-old girls | Total: 1000 |
|
|
|
| Morris 2015 | California, USA | – | Randomized controlled trial | 11-to-17-year-olds | Intervention groups: 1,797Phone call only: 3,253Unsampled controls: 116,356 |
|
|
|
| O’Leary 2015 | Colorado, USA | – | Randomized controlled trial | 11-to-17-year-olds | Intervention: 2,228Controls: 2,359 |
|
|
|
| Szilagyi 2013 | New York, USA | – | Random selection of participants. Participants select choice of intervention | 11-to-17-year-olds |
|
|
|
|
CDS - decision support for clinicians. FFI – family focused intervention/automated decision support to families. FIG - fully immunized girl.
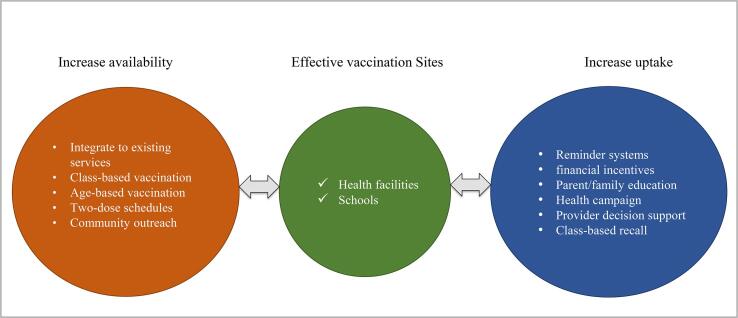
Two main strategies were observed in countries' efforts to increase access to HPV vaccines: (1) improving the availability of HPV vaccines and (2) increasing the number of people receiving HPV vaccines. Countries without national vaccination programs sought to identify sustainable strategies to make HPV vaccines available to adolescents via demonstration programs. Most studies accomplished this by conducting program cost evaluations using microcosting to determine which programs were most feasible for countries to adopt. In contrast, countries with existing national HPV vaccination programs identified cost-effective strategies to increase the uptake of the vaccines to meet nationally recommended HPV vaccination guidelines. Novel interventions were used to increase the uptake of HPV vaccines across study populations. We synthesized vaccine delivery strategies presented in the cost analyses into a concept map (Figure 2).
Figure 2.
Represents HPV vaccination strategies utilized across studies. Adolescents were effectively vaccinated in schools and health care facilities such as general practice clinics. Utilizing these existing infrastructures increased access to HPV vaccines by reaching eligible adolescents at accessible sites.
3.2. Effective vaccination sites
Effective vaccination sites utilized health facilities, clinics, and schools. A study that evaluated the cost-effectiveness of a national vaccination program in New Zealand found that the most cost-effective vaccination strategy was a mixed approach of vaccinating at both schools and primary care facilities (Blakely et al., 2014). Programs that took advantage of available resources at health care clinics and accessed target age groups through schools yielded a lower cost per vaccinated girl compared to programs that targeted hard-to-reach populations and had access to fewer resources.
3.3. Effective strategies
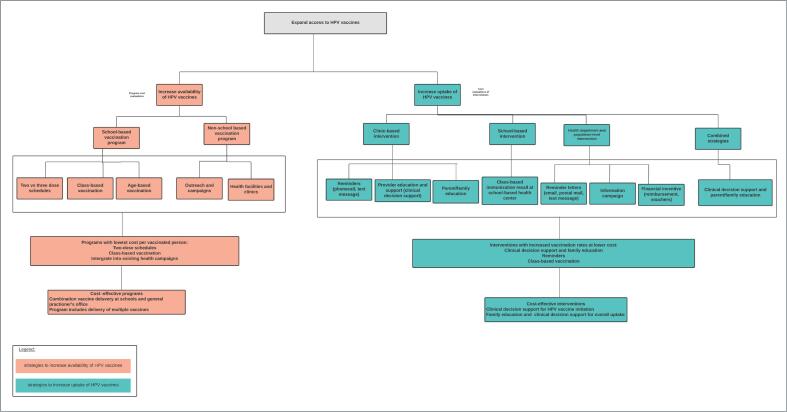
Effective strategies utilized to increase the availability of HPV vaccines included age-based vaccinations, class-based vaccinations, utilization of two-dose schedules, and integration of HPV vaccination into existing health infrastructure. Integrating school-based delivery into existing health systems was the most cost effective (Levin et al., 2013). Similarly, interventions that optimized existing school-based health center resources and primary care clinics yielded high vaccination rates at lower clinic costs and costs per vaccinated adolescent (Kempe et al., 2012, O'Leary et al., 2015, Fiks et al., 2013). Effective strategies to increase the number of vaccinated children include reminder systems, decision support for clinicians or providers, parent/family education, financial incentives, health campaigns, targeting uptake of multiple adolescent vaccines, and class-based immunization recall. A school-based immunization recall program had the highest percent increase (59%) in HPV vaccine initiation rates (Kempe et al., 2012). A detailed summary of vaccination strategies is provided in Supplementary figure 1.
3.4. Target population
All studies except one focused on the adolescent population to increase HPV vaccine rates (Table 1). Demonstration programs focused on vaccinating 10-to-12-year-old girls in a school-based setting and girls who received all recommended doses of HPV vaccines were referred as fully immunized girls (FIG). When a class-based strategy was utilized, the cost per FIG was lower compared to age-based vaccinations (Quentin et al., 2012). All study interventions were designed for 11-to-18-year-old adolescents to receive one or more doses of HPV vaccines and were conducted in HICs. Some interventions targeted girls only and others targeted all adolescents within the eligible age cohort.
3.5. Programmatic delivery costs
The implementation timeframe of program cost evaluation studies was one to two years and used a retrospective micro-costing approach from the health system or provider's perspective. Costs were reported in United States dollars (US$). Costs associated with HPV vaccine prices are disentangled from the qualitative synthesis of HPV vaccine delivery costs. However, it is noteworthy that all demonstration programs received HPV vaccines at subsidized costs. The Gavi negotiated price was estimated at US$ 5-to-5.70 per dose for studies that received HPV vaccines via Gavi-supported funding. The number of vaccinated girls, the total number of doses administered, and service delivery costs per FIG or dose administered are presented in Table 2.
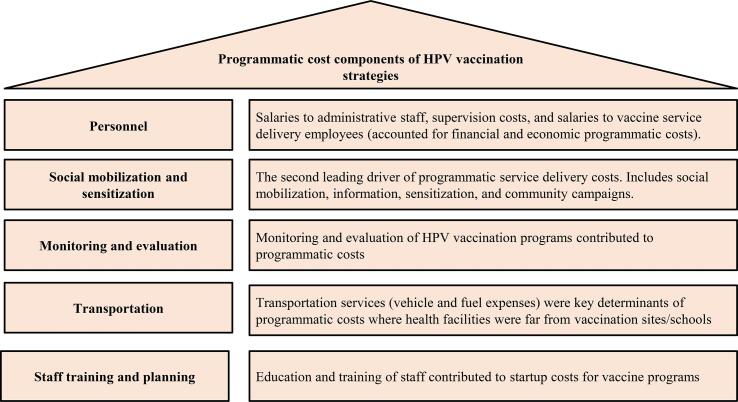
Programmatic cost evaluations reported both financial costs (paid monetary values) and economic costs (financial costs plus the opportunity costs associated with using resources for HPV vaccine delivery). Financial service delivery costs were largely payments to personnel (nurses, drivers, village health workers, school coordinators, etc.) and economic service delivery costs were related to personnel time dedicated to vaccination efforts. One study excluded salaries paid to existing staff in their financial and economic service delivery costs (Quentin et al., 2012). Fuel and transportation fees contributed considerably to both the total economic and financial delivery costs. The principal drivers of startup costs were expenditures related to personnel and running campaigns to raise community awareness. Programmatic service delivery costs are summarized in Figure 3.
Figure 3.
A description of the HPV vaccine delivery cost components itemized in the microcosting of the vaccination programs
Costs varied by vaccine delivery strategy and the country of program implementation. Costs for a school-based strategy in Uganda included transportation and personal costs due to the extensive distances between health facilities and schools (Levin et al., 2013). Introduction costs in Vietnam stemmed from resource-intensive micro-planning and training required by the Vietnamese government for school-based and health-center-based delivery strategies (Levin et al., 2013). Cost estimates to scale up a regional vaccination program in Tanzania were heavily impacted by salaries to international personnel and supervision, which accounted for more than 50% of vaccine delivery costs (Quentin et al., 2012). There was a marked decrease in average startup costs from year one to year two of an implementation of a demonstration program in Mozambique that aimed at estimating projected costs of a national scale-up of an HPV vaccination program (Soi et al., 2019). The greatest number of FIG 17,268 girls) resulted from the multi-regional study with an estimated financial and economic costs per FIG of US$ 5.71 and US$ 9.55 respectively, which were the lowest across all program cost evaluations (Levin et al., 2013).
3.6. Costs and effectiveness of novel interventions
Novel interventions were examined in eight studies with shared goals of increasing the uptake of HPV vaccines. The implementation time frame of these interventions ranged from six months to two years. All intervention studies were conducted in HIC which already had national HPV vaccination programs (Table 1).
Reminder systems. Reminder messages to parents and families were the most common strategy used to improve HPV uptake. Reminder systems targeting 11-to-17-year-olds in the USA yielded moderate increases in vaccine uptake with moderate financial costs per vaccinated child. The costs per vaccinated child using reminder interventions ranged from US$1.12 to US$30.95. The cost of a school-based immunization recall that targeted sixth grade girls ranged from $1.12 to $6.87 per child immunized (Kempe et al., 2012). Ninety five percent of girls needed the first dose of HPV vaccine and the recall system increased initiation rates by 59% (Kempe et al., 2012). A population-level mail reminder implemented by the state health department had the widest reach (81,558 adolescents) with the lowest incremental coverage rates (2.2% increase in the first dose compared to no intervention) for a total of US$30.95 per adolescent initiating vaccination (Coley et al., 2018). Another study evaluated an automated centralized recall system using mailed reminders and telephone calls for routine adolescent vaccines (Szilagyi et al., 2013). Both reminder methods had similar HPV vaccine initiation and completion rates and the cost of each adolescent was US$18.78 per year for mail reminders and US$16.68 for telephone reminders (Szilagyi et al., 2013). Meanwhile, the net cost of clinic-generated reminder messages ranged from US$ 855 to US$ 3394 per practice and US$ 2.64 to US$ 10.48 per child (O'Leary et al., 2015). Intervention implementation was less costly in clinics with existing electronic medical record systems (O'Leary et al., 2015). Additionally, the costs to the clinics more accurately represent estimates for practices in later stages of electronic health systems adoption than clinics in the early stages of adoption. Hence, reminders may not be suitable for hard-to-reach populations or in settings with technological limitations.
Financial incentives. Financial incentives were utilized to increase HPV vaccination rates in England and Belgium (Lefevere et al., 2016, Mantzari et al., 2015). Vaccination rates with financial incentives were higher for girls in their late teens than younger girls. While it is expected that financial incentives could be motivational to impact behavior change, removing out-of-pocket costs in settings where HPV vaccines are not free for adolescents could be more beneficial in increasing vaccination rates. Even though social deprivation did not have a significant moderating effect on HPV vaccine uptake (Mantzari et al., 2015), social equity analyses suggested that it is beneficial to target specific high-risk populations to optimize the use of scarce resources and reduce the burden of disease (Blakely et al., 2014). Many countries in Europe (Belgium and England included) now offer free gender-neutral HPV vaccines to target populations (European Parliamentary Forum for Sexual and Reproductive Rights, 2020).
Education and clinical decision support. Educating parents on the HPV vaccine’s role in cancer prevention and providing clinicians with tools to make strong recommendations to parents had substantial effect on both vaccine series initiation and completion rates. Among the intervention studies, only one study computed incremental costs for the interventions implemented (Fiks et al., 2013). This study focused on supporting clinicians and families in the clinic setting. An electronic medical record clinical decision support system for clinicians (CDS) was more effective at increasing HPV vaccine initiation rates with an incremental cost of US$6 compared to no intervention (Fiks et al., 2013). The family-focused interventions provided reminder phone calls, linked families with educational materials, and emphasized that the child's clinician recommended the vaccine (Fiks et al., 2013). This family-focused approach was most effective than CDS or no intervention at increasing HPV vaccine series completion rates with incremental costs of $10 for the second dose and $6 for the third dose. Combining both interventions most effectively accelerated timely receipt of vaccines with an incremental cost of $189 (compared to the family-focused approach) for receipt of the third dose.
3.7. Cost-effectiveness analysis
Two studies performed cost-effectiveness analyses using existing strategies and HPV uptake estimates from existing data as input parameters (Blakely et al., 2014, Wilson et al., 2020). Both studies used a Markov modeling approach and a discount rate of 3% (Table 3 below). Wilson and colleagues utilized data from a local health department to assess the cost-effectiveness of a comprehensive adult vaccination program for uninsured, low-income, high-risk adults (Wilson et al., 2020). Given a cost-effectiveness threshold of US$ 100,000 HPV vaccination was cost-effective with an incremental cost effectiveness ratio (ICER) of US$ 79,022 per life years saved (LYS) (Wilson et al., 2020). This ICER represented 136.2% of per capita GDP of the USA. In the study by Blakely and colleagues using data from New Zealand's (N.Z.) national girls' HPV vaccination program implemented in 2008, the ICER was NZ$18,800 per quality-adjusted life years (QALY) gained, compared to no vaccination (Blakely et al., 2014). This ICER represented 38.2% of New Zealand's per capita GDP. This study also assessed the equity impacts of HPV vaccination on Maori populations compared to non-Maori. The social equity analyses suggested that it is beneficial to target specific high-risk populations to optimize the use of scarce resources and reduce the burden of vaccine-preventable diseases. Other cost-effective strategies assessed were modification to a school-only program as per Australia, and a new mandatory law that requires active opt-out of vaccination as per some USA states
4. Discussion
HPV vaccines can prevent most cervical cancers and other HPV-attributable cancers. The WHO recommends HPV vaccines as critical to ending cervical cancer globally. England’s decline in cervical cancer rates and precancer rates provide country-level evidence on the benefits of investing in HPV vaccines for guaranteed protection against cervical cancer (Falcaro et al., 2021). Similarly, HPV vaccination was reported to be 100% effective in preventing cervical cancer in Nordic countries (Kjaer et al., 2020). While the vaccines are available at subsidized costs or for free to some countries, vaccine delivery costs could hamper the adoption of HPV vaccines into national immunization programs for low-income countries. Additionally, factors such as stigma associated with a vaccine that prevents sexually transmitted infections in adolescents have impacted acceptability and uptake of HPV vaccines across different regions (Escoffery et al., 2019;16. PMID:, Lim and Lim, 2019, Shah et al., 2021). As such, conscientious public health interventions are needed to promote HPV vaccination efforts. Behavioral interventions are necessary to promote the acceptability and uptake of HPV vaccines. Understanding the cost components associated with vaccine delivery strategies and their effectiveness will help identify opportunities for improvements to promote a wider reach. Not only are the financial costs critical for decision-making, but economic costs also frame a bigger picture on the total monetary and non-monetary implications of implementing HPV vaccination strategies.
Program evaluation studies reported both financial and economic costs while intervention studies focused on financial costs without considering economic costs. Opportunity costs such as the cost of diverting health professionals' time to effectively implement a program, or modifying existing workflow processes, are vital components in successful delivery programs. Similar to demonstration programs in developing countries, the study by Kempe and colleagues which delivered vaccines in urban schools, found that the time spent by staff to deliver the intervention significantly increased costs (Kempe et al., 2012). Implementing any of these approaches on a large scale will require reengineering of workflow. Optimizing processes remain critical to cost-effectively integrate these strategies into existing workflows and systems. Hence, given competing limited resources, reporting of costs and effectiveness of interventions and programs are crucial for optimal resource allocation.
None of the studies performed a CEA of the interventions. However, a study evaluating the CEA of interventions to increase HPV vaccine uptake was published while our review was ongoing (Spencer et al., 2020). This USA-based study indicated that quality improvement visits, centralized reminder systems, and school-based vaccination were cost-effective. Quality improvement visits were the most cost-effective intervention with a cost per QALY gained of US$ 1,538 (Spencer et al., 2020). The cost per QALY gained using the quality improvement visits represented 2.4% of the USA GDP per capita in 2020 (The World Bank, 2021). Hence, school-based vaccinations may not be the most cost-effective approach in certain countries as cost-effective delivery is highly influenced by national health infrastructure and immunization policies.
Globally, especially in LMICs, the focus of HPV vaccinations has been on adolescent girls. Toolkits exist for LMICs to estimate the cost of adding HPV vaccines to national immunization programs to prevent cervical cancer (Hutubessy et al., 2012). Portnoy and colleagues synthesized data from different studies to demonstrate the impact of HPV vaccination on gender equity (Portnoy et al., 2020). HPV vaccination decreases the incidence and mortality of cervical cancer, increases life expectancy for females, and subsequently increases labor force participation for women (Portnoy et al., 2020). Focusing on women and girls who are most vulnerable to HPV-associated cancers is an economically viable strategy in limited-resource settings. Gender-neutral vaccination is economically beneficial and it provides increased protection for everyone (Ng et al., 2018). Nonetheless, in limited resource settings, this approach may not be viable. Gavi subsidizes HPV vaccines for females only, focusing on cervical cancer prevention (Gavi, 2020). As such, even though it will be beneficial to vaccinate boys, the current evidence does not suggest this will be a sustainable approach for lower income countries. Balancing equitable access to HPV vaccines with cost constraints is a fundamental milestone that is yet to be achieved by many HPV vaccination programs.
4.1. Implications
The articles in this review provide evidence in support of vaccinating school-aged children in primary care settings and other accessible health facilities. The importance of clinical decision support is endorsed by evidence of study results. Empowering clinicians for collaborative decision making with parents increases vaccine initiation and completion considerably. Hence, healthcare provider-led education of parents and communities on the importance of HPV vaccination of children is vital to promote HPV vaccinations. Intentional and collaborative efforts are needed to increase HPV vaccination coverage.
Many HPV vaccination efforts were carried out in schools. School-based vaccination yielded dramatically lower costs per FIG than the outreach approach. The outreach settings were not specified; hence, it may be challenging to infer if all potential outreach sites would be significantly costlier. Catch-up vaccination options and interventions for eligible adolescents not vaccinated at schools or those not enrolled in school settings are critical to ensure a more equitable prevention approach for HPV-attributable cancers. Thus, raising questions on how to effectively vaccinate outside the school setting in resource-limited settings. HPV vaccination programs in these settings may benefit from the inclusion of community representatives in the early stages of planning. Public health officials must work collaboratively and creatively with communities. Ethical incentives that mobilize communities and parents to respond to vaccination efforts should be considered. Additionally, clear and consistent public health messaging that is culturally sensitive and safe are essential to effectively promote vaccination efforts.
There is a need for targeting interventions using a culturally relevant lens. Countries vary socio-culturally, politically, and economically. Thus, the lessons learned from this review may need to be modified and changed to best suit the needs of different countries and settings. Future studies on vaccine delivery programs must also be cognizant of cultural and livelihood factors (i.e., cropping season, skepticism of healthcare providers, HPV vaccine stigma) during the planning process to create vaccine programs that consider the cultural context of the setting in which the program is implemented.
5. Limitations
Our eligibility criteria restricted the inclusion of studies that did not provide cost data. Therefore, it is possible that we may have missed strategies that are effective at increasing HPV vaccination rates because they did not consider the costs of implementing their strategy. However, an integrative review on effective HPV vaccination strategies in the USA concluded that strong provider recommendations are essential for multi-component HPV vaccination strategies (Holloway, 2019). Therefore, it is likely that even after excluding studies without cost data, we were able to review studies that outlined approaches most effective at increasing the availability and uptake of HPV vaccinations. Another potential limitation is that we present our results in the currency year and values reported by the authors. However, we used the personal consumption expenditures price index to calibrate costs to May 2021 values and report these in Table 2 for reference.
6. Conclusion
The results from our review suggest that strategies used to expand access to HPV vaccines target two pathways: (i) increasing the availability and (ii) increasing the uptake of vaccines. All intervention studies were conducted in HICs. There is a dire need for intervention studies that are tailored to settings in LMICs to effectively increase uptake of HPV vaccines because rates of cervical cancer are continuously high in these regions. Given the current public sentiment towards vaccines, cultural and historical contexts must be considered when developing and implementing interventions for increasing the delivery of HPV vaccines. The inclusion of community representatives in the early phases of planning vaccine delivery programs will aid in tailoring creative, safe, and consistent public health messaging and interventions for HPV vaccines. Studies should also evaluate the cost-effectiveness of the implemented strategies and the cost-effectiveness of novel approaches. Future research on vaccine delivery must engage health systems, health professionals, communities, families, parents, and adolescents to develop culturally competent programs.
7. Availability of data and materials
Not applicable. All articles involved in the synthesis of our results are cited. Extracted data are reported in tables.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to thank Stella Seal at the Johns Hopkins Welch Library for support with developing the search protocol and retrieving articles from the databases.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2022.101734.
Contributor Information
Alvine M. Akumbom, Email: aakumbo1@jhu.edu.
Jennifer J. Lee, Email: jlee694@jh.edu.
Nancy R. Reynolds, Email: nancy.reynolds@jhu.edu.
Winter Thayer, Email: wthayer1@jhmi.edu.
Jinglu Wang, Email: jingluwang@stanfordhealthcare.org.
Eric Slade, Email: eslade@jhu.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- Centers for Disease Control and Prevention. STD facts - human papillomavirus (HPV). https://www.cdc.gov/std/hpv/stdfact-hpv.htm. Updated 2019. Accessed Feb 5, 2020.
- World Health Organization. Human papillomavirus (HPV) and cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. Updated 2020.
- Centers for Disease Control and Prevention. Cancers caused by HPV. https://www.cdc.gov/hpv/parents/cancer.html. Updated 2020. Accessed Feb 5, 2021.
- Serrano B., Brotons M., Bosch F.X., Bruni L. Epidemiology and burden of HPV-related disease. Best Practice & Research Clinical Obstetrics & Gynaecology. 2018;47:14–26. doi: 10.1016/j.bpobgyn.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer. 2019;144(8):1941–1953. doi: 10.1002/ijc.v144.8. [DOI] [PubMed] [Google Scholar]
- Singh GK, Azuine RE, Siahpush M. Global inequalities in cervical cancer incidence and mortality are linked to deprivation, low socioeconomic status, and human development. International journal of MCH and AIDS. 2012;1(1):17-30. https://www.ncbi.nlm.nih.gov/pubmed/27621956. 10.21106/ijma.12. [DOI] [PMC free article] [PubMed]
- Klugman J. Human development report 2011. sustainability and equity: A better future for all. United Nations Development Programme - HDRO Human Development Reports. 2011. https://papers.ssrn.com/abstract=2294671. Accessed Nov 1, 2021.
- National Cancer Institute. Cancer disparities. https://www.cancer.gov/about-cancer/understanding/disparities. Updated 2020.
- Cancer Australia. Cancer incidence. https://ncci.canceraustralia.gov.au/diagnosis/cancer-incidence/cancer-incidence. Updated 201Accessed Nov 1, 2021.
- St. Laurent J., Luckett R., Feldman S. HPV vaccination and the effects on rates of HPV-related cancers. Current problems in cancer. 2018;42(5):493–506. doi: 10.1016/j.currproblcancer.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Nour N.M. Cervical cancer: A preventable death. Rev Obstet Gynecol. 2009;2(4):240–244. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Weekly epidemiological record. 2009;84:117–132. [Google Scholar]
- Fesenfeld M., Hutubessy R., Jit M. Cost-effectiveness of human papillomavirus vaccination in low and middle income countries: A systematic review. Vaccine. 2013;31(37):3786–3804. doi: 10.1016/j.vaccine.2013.06.060. [DOI] [PubMed] [Google Scholar]
- Gavi. Human papillomavirus. https://www.Gavi.org/types-support/vaccine-support/human-papillomavirus. Updated 2020.
- Portnoy A., Clark S., Ozawa S., Jit M. The impact of vaccination on gender equity: Conceptual framework and human papillomavirus (HPV) vaccine case study. Int J Equity Health. 2020;19(1):10. doi: 10.1186/s12939-019-1090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway G.L. Effective HPV vaccination strategies: What does the evidence say? an integrated literature review. J Pediatr Nurs. 2019;44:31–41. doi: 10.1016/j.pedn.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Gulliford M., Figueroa-Munoz J., Morgan M., Hughes D., Gibson B., Beech R., Hudson M. What does 'access to health care' mean? J Health Serv Res Policy. 2002;7(3):186–188. doi: 10.1258/135581902760082517. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLOS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Economic Analysis. Personal consumption expenditures price index. https://www.bea.gov/data/personal-consumption-expenditures-price-index. Updated 2021.
- Organisation for Economic Co-operation and Development. Exchange rates: Total, national currency units/US dollar, 2000 – 2020. https://data.oecd.org/conversion/exchange-rates.htm.
- The World Bank. GDP per capita (current US$) - United States, New Zealand. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?end=2020&locations=US-NZ&start=1960&view=chart. Updated 2021.
- Hutubessy R., Levin A., Wang S., Morgan W., Ally M., John T., Broutet N. A case study using the united republic of tanzania: Costing nationwide HPV vaccine delivery using the WHO cervical cancer prevention and control costing tool. BMC medicine. 2012;10(1) doi: 10.1186/1741-7015-10-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanth S.S., Lairson D.R., Huang D., Savas L.S., Vernon S.W., Fernández M.E. The cost of implementing two small media interventions to promote HPV vaccination. Preventive Medicine. 2017;99:277–281. doi: 10.1016/j.ypmed.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Levin C.E., Van Minh H., Odaga J., Rout S.S., Ngoc D.N., Menezes L., Araujo M.A., LaMontagne D.S. Delivery cost of human papillomavirus vaccination of young adolescent girls in Peru, Uganda and Viet Nam. Bulletin of the World Health Organization. 2013;91(8):585–592. doi: 10.2471/BLT.12.113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A., Wang S.A., Levin C., Tsu V., Hutubessy R., Metcalfe J.Z. Costs of introducing and delivering HPV vaccines in low and lower middle income countries: Inputs for Gavi policy on introduction grant support to countries. PLOS ONE. 2014;9(6):e101114. doi: 10.1371/journal.pone.0101114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahumud R.A., Gow J., Alam K., Keramat S.A., Hossain M.G., Sultana M., Sarker A.R., Islam S.M.S. Cost-effectiveness of the introduction of two-dose bi-valent (cervarix) and quadrivalent (gardasil) HPV vaccination for adolescent girls in bangladesh. Vaccine. 2020;38(2):165–172. doi: 10.1016/j.vaccine.2019.10.037. [DOI] [PubMed] [Google Scholar]
- Alonso S., Cambaco O., Maússe Y., Matsinhe G., Macete E., Menéndez C., Sicuri E., Sevene E., Munguambe K. Costs associated with delivering HPV vaccination in the context of the first year demonstration programme in southern mozambique. BMC Public Health. 2019;19(1) doi: 10.1186/s12889-019-7338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidle A., Gwati G., Abimbola T., Pallas S.W., Hyde T., Petu A., McFarland D., Manangazira P. Cost of a human papillomavirus vaccination project, zimbabwe. Bulletin of the World Health Organization. 2018;96(12):834–842. doi: 10.2471/BLT.18.211904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentin W., Terris-Prestholt F., Changalucha J., Soteli S., Edmunds W.J., Hutubessy R., Ross D.A., Kapiga S., Hayes R., Watson-Jones D. Costs of delivering human papillomavirus vaccination to schoolgirls in Mwanza Region, Tanzania. BMC Med. 2012;10(1) doi: 10.1186/1741-7015-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soi C., Babigumira J.B., Chilundo B., Muchanga V., Matsinhe L., Gimbel S., Augusto O., Sherr K. Implementation strategy and cost of mozambique's HPV vaccine demonstration project. BMC Public Health. 2019;19(1) doi: 10.1186/s12889-019-7793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely T., Kvizhinadze G., Karvonen T., Pearson A.L., Smith M., Wilson N. Cost-effectiveness and equity impacts of three HPV vaccination programmes for school-aged girls in New Zealand. Vaccine. 2014;32(22):2645–2656. doi: 10.1016/j.vaccine.2014.02.071. [DOI] [PubMed] [Google Scholar]
- Wilson K.J., Brown H.S., Patel U., Tucker D., Becker K. Cost-effectiveness of a comprehensive immunization program serving high-risk, uninsured adults. Prev Med. 2020;130:105860. doi: 10.1016/j.ypmed.2019.105860. [DOI] [PubMed] [Google Scholar]
- Allison Kempe, Jennifer Barrow, Shannon Stokley, et al. Effectiveness and cost of immunization recall at school-based health centers. Pediatrics. 2012;129(6):e1446-e1452. https://doi.org/10.1542/peds.2011-2921. [DOI] [PubMed]
- O'Leary S, Lee M, Lockhart S, et al. Effectiveness and cost of bidirectional text messaging for adolescent vaccines and well care . Pediatrics. 2015;136(5):e1220-e1227. https://doi.org/10.1542/peds.2015-1089. [DOI] [PMC free article] [PubMed]
- Szilagyi P.G., Albertin C., Humiston S.G., Rand C.M., Schaffer S., Brill H., Stankaitis J., Yoo B.-K., Blumkin A., Stokley S. A randomized trial of the effect of centralized reminder/recall on immunizations and preventive care visits for adolescents. Acad Pediatr. 2013;13(3):204–213. doi: 10.1016/j.acap.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiks AG, Grundmeier RW, Mayne S, et al. Effectiveness of decision support for families, clinicians, or both on HPV vaccine receipt. Pediatrics. 2013;131(6):1114-1124. 10.1542/peds.2012-3122. [DOI] [PMC free article] [PubMed]
- Coley S, Hoefer D, Rausch-Phung E. A population-based reminder intervention to improve human papillomavirus vaccination rates among adolescents at routine vaccination age. Vaccine. 2018;36(32 Pt B):4904-4909. 10.1016/j.vaccine.2018.06.056. [DOI] [PubMed]
- Morris J., Wang W., Wang L., Peddecord K.M., Sawyer M.H. Comparison of reminder methods in selected adolescents with records in an immunization registry. Journal of Adolescent Health. 2015;56(5):S27–S32. doi: 10.1016/j.jadohealth.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Mantzari E., Vogt F., Marteau T.M. Financial incentives for increasing uptake of HPV vaccinations: A randomized controlled trial. Health Psychol. 2015;34(2):160–171. doi: 10.1037/hea0000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevere E., Hens N., De Smet F., Beutels P. The impact of non-financial and financial encouragements on participation in non school-based human papillomavirus vaccination: A retrospective cohort study. Eur J Health Econ. 2016;17(3):305–315. doi: 10.1007/s10198-015-0680-2. [DOI] [PubMed] [Google Scholar]
- European Parliamentary Forum for Sexual and Reproductive Rights. Cervical cancer prevention policy atlas 2020. https://www.epfweb.org/node/553. Updated 2020. Accessed Jan 20, 2022.
- Falcaro M., Castañon A., Ndlela B., Checchi M., Soldan K., Lopez-Bernal J., Elliss-Brookes L., Sasieni P. The effects of the national HPV vaccination programme in england, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: A register-based observational study. The Lancet. 2021;398(10316):2084–2092. doi: 10.1016/S0140-6736(21)02178-4. [DOI] [PubMed] [Google Scholar]
- Kjaer S.K., Nygård M., Sundström K., Dillner J., Tryggvadottir L., Munk C., Berger S., Enerly E., Hortlund M., Ágústsson Á.I., Bjelkenkrantz K., Fridrich K., Guðmundsdóttir I., Sørbye S.W., Bautista O., Group T., Luxembourg A., Marshall J.B., Radley D., Yang Y.S., Badshah C., Saah A. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four nordic countries. EClinicalMedicine. 2020;23:100401. doi: 10.1016/j.eclinm.2020.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoffery C., Riehman K., Watson L., Priess A.S., Borne M.F., Halpin S.N., Rhiness C., Wiggins E., Kegler M.C. Facilitators and barriers to the implementation of the HPV VACs (vaccinate adolescents against cancers) program: A consolidated framework for implementation research analysis. Prev Chronic Dis. 2019;16. PMID:;16 doi: 10.5888/pcd16.180406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P., Shetty V., Ganesh M., Shetty A.K. Challenges to human papillomavirus vaccine acceptability among women in south india: An Exploratory study. Am J Trop Med Hyg. 2021;105(4):966–973. doi: 10.4269/ajtmh.20-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A.S.E., Lim R.B.T. Facilitators and barriers of human papillomavirus vaccine uptake in young females 18–26 years old in singapore: A qualitative study. Vaccine. 2019;37(41):6030–6038. doi: 10.1016/j.vaccine.2019.08.053. [DOI] [PubMed] [Google Scholar]
- Spencer J.C., Brewer N.T., Trogdon J.G., Weinberger M., Coyne-Beasley T., Wheeler S.B. Cost-effectiveness of interventions to increase HPV vaccine uptake. Pediatrics. 2020;146(6) doi: 10.1542/peds.2020-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S.S., Hutubessy R., Chaiyakunapruk N. Systematic review of cost-effectiveness studies of human papillomavirus (HPV) vaccination: 9-valent vaccine, gender-neutral and multiple age cohort vaccination. Vaccine. 2018;36(19):2529–2544. doi: 10.1016/j.vaccine.2018.03.024. [DOI] [PubMed] [Google Scholar]
- Covidence, 2020. https://www.covidence.org. (Accessed 04 February 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable. All articles involved in the synthesis of our results are cited. Extracted data are reported in tables.