Abstract
A man fully mRNA-vaccinated against COVID-19 presented to our hospital with an acute febrile illness, respiratory symptoms and a positive test for SARS-CoV-2. He was later found early into hospitalisation to have two morbid bacterial co-infections: Legionella pneumophila serogroup 1 and methicillin-resistant Staphylococcus aureus (MRSA). Although this patient was initially admitted for COVID-19 management, his initial presentation was remarkable for lobar pneumonia, hyponatraemia and rhabdomyolysis more compatible with Legionnaire’s disease than severe COVID-19. On discovery of MRSA pneumonia as a second bacterial infection, immunosuppressive COVID-19 therapies were discontinued and targeted antibiotics towards both bacterial co-infections were initiated. The patient’s successful recovery highlighted the need to have high suspicion for bacterial co-infections in patients presenting with community-acquired pneumonia and a positive SARS-CoV-2 test, as patients with serious bacterial co-infections may have worse outcomes with use of immunosuppressive COVID-19 therapies.
Keywords: pneumonia (infectious disease), medical management, COVID-19
Background
SARS-CoV-2 continues to be prevalent across the globe despite its emergence more than 2 years ago.1 Due to its wide prevalence, patients presenting to the emergency department with symptoms and signs of pneumonia are often found to test positive for SARS-CoV-2 in routine clinical workups for new pneumonia. While most patients with compatible signs of COVID-19 are solely infected by SARS-CoV-2, a minority of patients presenting to the hospital are also affected by bacterial superinfection, defined as a secondary bacterial pathogen causing pneumonia in patients with pre-existing viral infection.2 Superinfection is distinct from the concept of co-infections, defined as two or more pathogens causing concurrent infections, as superinfection specifically implies a preceding viral infection that predisposes to a bacterial infection that follows.2 Recognising bacterial co-infections in COVID-19 is crucial given they have been shown to increase mortality.3 In addition to requiring antibiotic therapy, patients with particularly morbid bacterial co-infections may also require a de-escalation of immunosuppressive therapies used for severe COVID-19. While studies of glucocorticoids, interleukin-6 inhibitors (eg, tocilizumab) and Janus kinase inhibitors (eg, baricitinib) have demonstrated improved survival in patients with COVID-19, the use of such immunomodulators carries a risk of infectious complications, and they should therefore be used cautiously in patients with confirmed, serious bacterial co-infections.4
Case presentation
A man in his 50s with no known medical history presented to the emergency department after a 1-day history of dyspnoea at rest and pleuritic left-sided chest pain. One week prior to admission, he had abrupt onset fevers, drenching night sweats, headache, productive cough, nasal congestion, sore throat and myalgias prompting SARS-CoV-2 testing, which was initially negative. Of note, he completed a COVID-19 vaccine series (mRNA-1273, Moderna) 4 months prior to presentation. On review of systems, he noted watery diarrhoea occurring four times per day over the past week and disorientation starting the day prior to presentation.
Further clinical history was significant only for a 35-pack-year smoking history. He reported no recent sick contacts at home or work. The patient reported frequent work-related travel within the northeastern USA where he designed cubicles for large commercial buildings with central air conditioning systems. During his travels, he would stay in hotels. He had been to several locations in Massachusetts during the 2 weeks prior to onset of symptoms and was travelling in New Hampshire when symptoms began. He also described a large water leak in his Connecticut apartment from sink plumbing in his bathroom ceiling 3 weeks prior to his presentation.
On arrival to the hospital, the patient was afebrile with a heart rate of 122 beats/min, blood pressure of 152/84 mm Hg, respiratory rate of 24 breaths/min and oxygen saturation of 89% on room air, requiring 4 L/min of supplemental oxygen to maintain saturation above 96%. His body mass index was noted to be 33.05 kg/m2. He was profusely diaphoretic and speaking slowly due to shortness of breath and fatigue. Rhonchi were auscultated in the left posterior lung fields with egophony in the left lower lung field. He exhibited diffuse muscle tenderness in the lower extremities but without discrete lesions, oedema or erythema. His neurological examination was normal.
Investigations
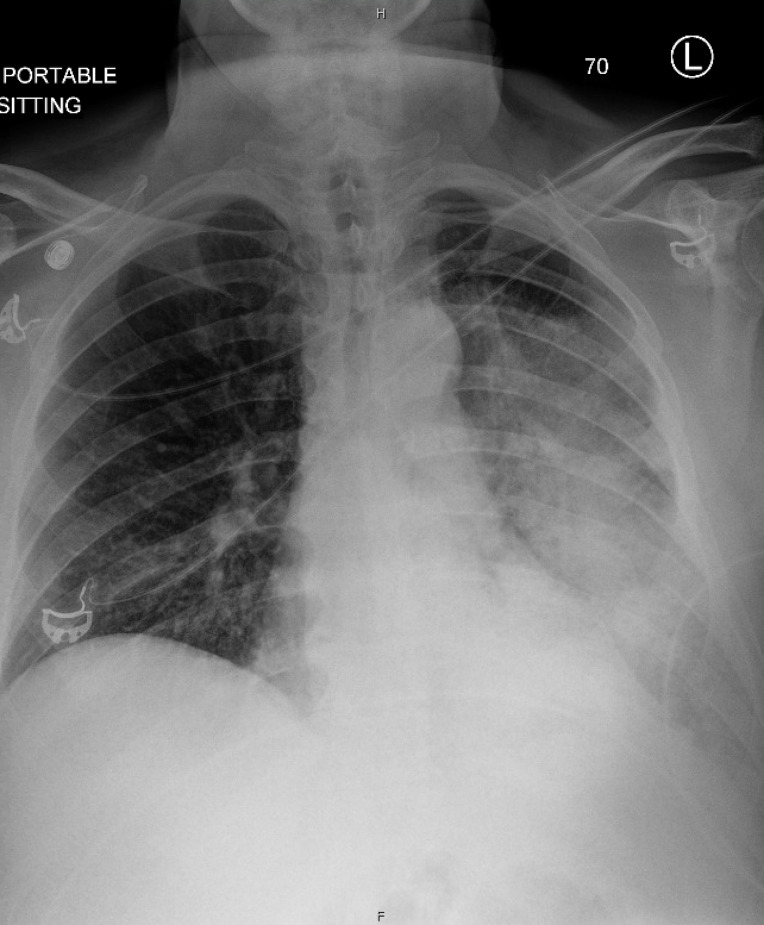
A SARS-CoV-2 PCR performed at the time of admission (1 week after the patient’s onset of symptoms and initial negative test) was positive with a high cycle threshold of 41.6. A chest X-ray demonstrated near complete opacification of the left hemithorax with air bronchograms (figure 1). He had a leucocytosis of 17 300 white cells/µL (reference range 4000–10 000/µL) with 96% neutrophils (reference range 37.0%–84.0%) and absolute lymphocyte count of 300 cells/µL (reference range 1000–4000/µL), elevated high-sensitivity C-reactive protein (hs-CRP) to >300.0 mg/L and elevated procalcitonin of 34.00 ng/mL (normal ≤0.25 ng/mL). Other blood tests were notable for hyponatraemia with a sodium of 123 mEq/L (reference range 136–144 mEq/L), acute kidney injury with a creatinine of 1.47 mg/dL (baseline 0.7 mg/dL), elevated total creatine kinase to 6343 U/L (reference range 11–204 U/L), and elevated transaminases with an aspartate aminotransferase (AST) of 354 U/L (reference range 10–35 U/L) and alanine aminotransferase (ALT) of 140 U/L (reference range 9–59 U/L, table 1). A urinalysis demonstrated 3+ blood with microscopy showing only 6–10 red blood cells per high-powered field concerning for rhabdomyolysis due to bacterial myositis or COVID-19-associated myositis (table 2).5 Additional infectious workup was notable for positive urine antigen testing for Legionella pneumophila serogroup 1, and routine sputum cultures grew methicillin-resistant Staphylococcus aureus (MRSA). A sputum culture eventually also grew L. pneumophila after 2 weeks (table 3). Urine antigen testing for Streptococcus pneumoniae was negative, and routine blood cultures were without growth. He was noted to have hyperglycaemia, obesity and lack of primary care engagement, so a haemoglobin A1c was checked and found to be 7.1%. Repeat SARS-CoV-2 PCR testing performed in the hospital 1 week after the patient’s admission test was negative.
Figure 1.
Portable chest X-ray demonstrating a large area of opacification with air bronchograms involving nearly the entire left hemithorax. There is sparing of the upper part of the left upper lobe.
Table 1.
Laboratory findings
| Investigations | Observed value | Reference range | Units |
| Sodium | 123 | 136–144 | mEq/L |
| Potassium | 3.1 | 3.3–5.1 | mEq/L |
| Chloride | 85 | 98–107 | mEq/L |
| CO2 | 20 | 20–30 | mEq/L |
| Glucose | 110 | 70–100 | mg/dL |
| Blood urea nitrogen | 35 | 6–20 | mg/dL |
| Creatinine | 1.47 | 0.40–1.30 | mg/dL |
| Calcium | 8.3 | 8.8–10.2 | mg/dL |
| White blood cells | 17.3 | 44.0-10.0 | 109/L |
| Haemoglobin | 148 | 120.0-180.0 | g/L |
| Platelets | 354 | 140.0-440.0 | 109/L |
| Differential count | Neutrophils: 96.0 | 37.0–84.0 | % |
| Lymphocytes: 2.0 | 8.0–49.0 | ||
| Monocytes: 1.0 | 4.0–15.0 | ||
| Bands: 1.0 | 0–10.0 | ||
| Alanine aminotransferase | 140 | 9–59 | U/L |
| Aspartate aminotransferase | 354 | 10–35 | U/L |
| Alkaline phosphatase | 60 | 9–122 | U/L |
| Total bilirubin | 0.7 | <1.2 | mg/dL |
| Albumin | 2.4 | 3.6–4.9 | g/dL |
| Procalcitonin | 34.0 | N/a | ng/mL |
| High-sensitivity C-reactive protein | >300.0 | N/a | mg/L |
| Total creatine kinase | 6343 | 11–204 | U/L |
| D-dimer | 4.92 | <0.56 | mg/L |
Table 2.
Urine tests
| Investigations | Observed value | Reference range | Units |
| Clarity | Cloudy | Clear | N/a |
| Colour | Yellow | Yellow | N/a |
| Specific gravity | 1.023 | 1.005–1.030 | N/a |
| pH | 5.5 | 5.5–7.5 | N/a |
| Protein | 2+ | Negative-trace | N/a |
| Glucose | Negative | Negative | N/a |
| Ketones | Negative | Negative | N/a |
| Blood | 3+ | Negative | N/a |
| Bilirubin | Negative | Negative | N/a |
| Leucocytes | Negative | Negative | N/a |
| Nitrites | Negative | Negative | N/a |
| Epithelial cells | Few | None-few | cells/low-powered field |
| White blood cells | 6–10 | 0–5 | cells/high-powered field |
| Red blood cells | 6–10 | 0–2 | cells/high-powered field |
| Bacteria | Moderate | None-few | cells/high-powered field |
| Hyaline casts | >10 | 0–3 | casts/low-powered field |
| Coarse granular casts | 4–10 | None | casts/low-powered field |
| Urine culture | <10 000 | <10 000 | colony-forming units/mL |
Table 3.
Microbiology
| Investigations | Observed value | Reference range | Units |
| SARS-CoV-2 (COVID-19) nasopharyngeal PCR | Positive | Negative | N/a |
| Blood cultures | Negative | Negative | N/a |
| Legionella pneumophila serogroup 1 urine antigen | Positive | Negative | N/a |
| Streptococcus pneumoniae urine antigen | Negative | Negative | N/a |
| Sputum culture | 2+ methicillin-resistant Staphylococcus aureus, 2+ normal flora | N/a | N/a |
| Legionella sputum culture | 3+ Legionella pneumophila | N/a | N/a |
Treatment
In the emergency department, the patient was initially started on empiric community-acquired pneumonia (CAP) treatment with ceftriaxone and doxycycline. He received one dose of each of these medications before being admitted to the medical floor, when the patient’s Legionella co-infection was recognised, and antibiotics narrowed to moxifloxacin for a 10-day course. In line with our hospital’s treatment guidelines for COVID-19, remdesivir and dexamethasone were initiated given the patient’s oxygen saturation was found to be 89% on room air. Baricitinib was also given in accordance with our guidelines, which recommend the addition of baricitinib for patients with both a hs-CRP level greater than 75 mg/L and supplemental oxygen requirement between 3 and 6 L/min. He was continued on remdesivir for 5 days of therapy; however, dexamethasone and baricitinib were held after only 2 days of therapy on identification of the patient’s second co-infection, MRSA. For the S. aureus pneumonia, he was initially started on cefazolin given a negative MRSA nasopharyngeal swab. However, cefazolin was switched to trimethoprim-sulfamethoxazole for a 7-day treatment course once susceptibility data became available. Intravenous normal saline was given for a total of 24 hours for acute kidney injury and rhabdomyolysis. Finally, the patient was maintained on prophylactic anticoagulation dosing of enoxaparin throughout the hospitalisation.
Outcome and follow-up
The patient’s acute kidney injury and rhabdomyolysis rapidly improved with intravenous fluids, resulting in normalisation of the serum creatinine and creatine kinase within 1 week. The patient’s heart rate and respiratory rate improved to normal after 24 hours of the initial therapies. However, after a 12-day hospital stay, he continued to require 2 L/min of supplemental oxygen to maintain a normal oxygen saturation, so the patient was discharged on 2 L/min of supplemental oxygen. He was off supplemental oxygen by his outpatient follow-up appointment 2 weeks later.
Discussion
This patient positive for SARS-CoV-2 presented with not one but two bacterial co-infections. In general, the rate of bacterial co-infection associated with COVID-19 is low (<4% at the time of presentation).6 This finding is surprising considering that the historical rate of bacterial co-infection associated with influenza is estimated to be 23%.7 S. aureus is the most common culprit of bacterial infection in patients with COVID-19 (representing 31% of respiratory superinfections and bloodstream co-infections), and co-infection by atypical bacteria like L. pneumophila is rare.6 Specifically, the incidence of Legionnaires’ disease in the USA, Europe and Australia is estimated to be 1.4–1.8 cases per 100 000 persons.8 Other groups have reported Legionnaire’s disease in patients with COVID-19, but our case is remarkable in that this patient did not have a pre-existing chronic pulmonary condition or recent history of immunosuppression.9 10
Although the guidelines of the American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) for the diagnosis of CAP recommend against routine Legionella and pneumococcal urine antigen testing, the guidelines do recommend testing patients with severe CAP or epidemiological risk factors for Legionnaire’s disease.11 Given that only 0.08% of patients with COVID-19 have a positive Legionella urine antigen test and the paucity of case reports of Legionella co-infection, applying the ATS/IDSA’s recommendation against routine Legionella urine antigen testing to patients presenting with COVID-19 appears reasonable.6 Our patient did not have severe pneumonia per the ATS/IDSA criteria; however, his history of frequent travel to worksites with central air conditioning system did qualify him for urine antigen testing. Of note, in addition to a history of recent travel, hospitalisation or residence in a long-term care facility, a history of returning to work in a recently reopened building may be considered as an epidemiological indication for urine antigen testing given the high number of building closures during the COVID-19 pandemic.12
The crux of this case lies in the clinical reasoning behind the diagnosis and treatment of this patient positive for SARS-CoV-2. First, this case highlights the importance of considering bacterial co-infection in patients who present with a positive SARS-CoV-2 test and CAP. Although the rate of bacterial co-infection in COVID-19 is low, the mortality rate of patients with COVID-19 with bacterial co-infection is higher than in patients with influenza with bacterial co-infection.3 Recognising bacterial co-infection in COVID-19 is guided by findings similar to those traditionally used in the recognition of superinfection in influenza. A rise in leucocyte counts, evidence of lobar consolidation or necrotising infection, and recurrence of fever are objective findings that should raise concern for bacterial co-infection.13 It should be noted that various guidelines provide different recommendations on when to begin empiric antibiotic therapy in patients with COVID-19, so there remains a role for clinician vigilance in assessing patients’ entire investigative workup to determine the need for antibiotic therapy.
Second, regarding the treatment of our patient, this case raised the question of how to balance the treatment of patients with positive SARS-CoV-2 testing and confirmed bacterial co-infections. While dexamethasone and adjunctive immunosuppressives like tocilizumab and baricitinib have been shown to decrease mortality in severe COVID-19, such therapies may predispose patients to serious infection and potentiate bacterial superinfection.14 15 Our patient with positive SARS-CoV-2 testing and confirmed Legionella and MRSA pneumonia raised concerns about whether patients with COVID-19 with confirmed, serious co-infections should be continued on immunosuppressive therapies directed towards COVID-19. Since there are no clear guideline-driven recommendations on how to approach this issue, we turned to the sum of the patient’s clinical, radiographic and microbiological data to assess which aetiology was the central driver of his illness. Our decision to hold dexamethasone and baricitinib was in part directed by the high cycle threshold result of the positive SARS-CoV-2 PCR test, which was consistent with prolonged SARS-CoV-2 shedding from a prior asymptomatic or mild COVID-19 infection. Additionally, the patient’s diarrhoea, hyponatraemia, acute kidney injury and rhabdomyolysis were highly consistent with Legionnaire’s disease. Given the patient had a negative SARS-CoV-2 PCR test 1 week after the patient’s positive admission PCR test, we felt our patient most likely presented with a prior COVID-19 infection that predisposed him to develop superinfection with co-infecting Legionella and MRSA, which were the likely central drivers of his illness.
Our patient’s successful recovery suggests not all patients with hypoxaemia and a positive SARS-CoV-2 test are obligated to receive immunosuppressive therapies for COVID-19. In patients with hypoxaemia, a positive SARS-CoV-2 test and findings more compatible with bacterial pneumonia than COVID-19 pneumonia, we recommend weighing risks and benefits of immunosuppressive therapies directed towards COVID-19, prioritising testing for bacterial pathogens and tailoring antibiotic therapy.
Learning points.
Bacterial co-infection in COVID-19 is uncommon but should be considered in patients with a constellation of symptoms and signs compatible with bacterial pneumonia, including atypical pneumonias such as Legionnaire’s disease.
Physicians should elicit a social history from patients presenting with COVID-19, as Legionella urine antigen testing should be performed in patients with epidemiological risk factors for Legionnaire’s disease in addition to patients with severe community-acquired pneumonia alone.
In patients presenting with positive SARS-CoV-2 testing and findings compatible with bacterial co-infections, the decision to withhold immunosuppressive therapies may be guided by the cycle threshold result of RT PCR testing and specific clinical features of the infectious syndrome.
Footnotes
Twitter: @ASanchez_PS
Contributors: This report was supervised by AS-M and written by AS, EIE and PW. Drs AS and PW were responsible for the initial diagnosis and management of this patient, and EIE and AS-M served as infectious disease consultants.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.WHO . Weekly epidemiological update on COVID-19 - 6 January 2022 [Internet]. Who.int. [cited 12 Jan 2022], 2022. Available: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-6-january-2022
- 2.Bengoechea JA, Bamford CG. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol Med 2020;12:e12560. 10.15252/emmm.202012560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shafran N, Shafran I, Ben-Zvi H, et al. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci Rep 2021;11:12703. 10.1038/s41598-021-92220-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 Treatment Guidelines [Internet] . Covid19treatmentguidelines.nih.gov. [cited 13 Jan 2022]. Available: https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/whats-new/
- 5.Saud A, Naveen R, Aggarwal R, et al. COVID-19 and myositis: what we know so far. Curr Rheumatol Rep 2021;23:63. 10.1007/s11926-021-01023-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westblade LF, Simon MS, Satlin MJ. Bacterial coinfections in coronavirus disease 2019. Trends Microbiol 2021;29:930–41. 10.1016/j.tim.2021.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein EY, Monteforte B, Gupta A, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta‐analysis. Influenza Other Respi Viruses 2016;10:394–403. 10.1111/irv.12398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dooling KL, Toews K-A, Hicks LA, et al. Active Bacterial Core Surveillance for Legionellosis - United States, 2011-2013. MMWR Morb Mortal Wkly Rep 2015;64:1190–3. 10.15585/mmwr.mm6442a2 [DOI] [PubMed] [Google Scholar]
- 9.Shimizu M, Chihara Y, Satake S, et al. Co-Infection with Legionella and SARS-CoV-2: a case report. JA Clin Rep 2021;7:62. 10.1186/s40981-021-00467-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verhasselt HL, Buer J, Dedy J, et al. COVID-19 Co-infection with Legionella pneumophila in 2 Tertiary-Care Hospitals, Germany. Emerg Infect Dis 2021;27:1535–7. 10.3201/eid2705.203388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic Society and infectious diseases Society of America. Am J Respir Crit Care Med 2019;200:e45–67. 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassell K, Davis JL, Berkelman R. Legionnaires' disease in the time of COVID-19. Pneumonia 2021;13:2. 10.1186/s41479-020-00080-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Co-infection and antimicrobial stewardship [Internet]. Idsociety.org. [cited 19 Oct 2021]. Available: https://www.idsociety.org/covid-19-real-time-learning-network/disease-manifestations--complications/co-infection-and-Antimicrobial-Stewardship/
- 14.Clarke B, Yates M, Adas M, et al. The safety of JAK-1 inhibitors. Rheumatology 2021;60:ii24–30. 10.1093/rheumatology/keaa895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favalli EG. Understanding the role of interleukin-6 (IL-6) in the joint and beyond: a comprehensive review of IL-6 inhibition for the management of rheumatoid arthritis. Rheumatol Ther 2020;7:473–516. 10.1007/s40744-020-00219-2 [DOI] [PMC free article] [PubMed] [Google Scholar]