Abstract
The cardiovascular performance of salmonids in aquaculture can be impaired by acute climate warming, posing risks for fish survival. Exercise training and functional feeds have been shown to be cardioprotective in mammals but their action on the fish heart and its upper thermal performance has not been studied. To investigate this, rainbow trout were trained at a moderate water velocity of 1 body length per second (bl s−1) for 6 h per day, either alone or in combination with one of two functional feed-supplements, allicin and fucoidan. After 6 weeks of exercise training and feeding, maximum heart rate and the temperature coefficient of heart rate were significantly higher in the trained fish as compared to untrained ones. There was a slight increase in hematocrit in trained control fish reared on a normal diet (TC group) compared to untrained fish fed with the same diet (CC). This implies that exercise training enhanced oxygen delivery to trout tissues via an increase of cardiac blood flow in warm water. However, cardiac thermal tolerance was not affected by exercise training or feeding, except from the temperature of peak heart rate which was higher in the trained group fed with fucoidan supplement (TF) as compared to the untrained group fed with same diet (CF). Allicin supplement caused a significant reduction in the maximum heart rate and the temperature coefficient of heart rate, especially in trained fish, while fucoidan supplement did not cause any effect on heart rate. No differences were observed in growth performance among groups. However, fish fed with fucoidan-supplemented diet had a slight reduction in feed conversion efficiency. We suggest further investigations to understand the antagonistic effect of allicin supplemental feeding and exercise training on cardiovascular performance. More studies are also required to investigate if other exercise training intensities could increase cardiac thermal tolerance.
Keywords: Allicin, Fucoidan, Maximum heart rate, Rainbow trout, Exercise training, Thermal tolerance
Highlights
-
•
Exercise training at 1 bl s−1 increased the hematocrit values of rainbow trout.
-
•
Exercise training at 1 bl s−1 increased the maximum heart rate and temperature coefficient of rainbow trout.
-
•
Exercise training at 1bl s−1 did not enhance the cardiac thermal tolerance of rainbow trout.
-
•
Functional feeds, allicin and fucoidan, did not improve the cardiovascular system of rainbow trout at high temperatures.
Abbreviations
- BMG
body mass gain
- bl s−1
body length per second
- CC
untrained control group fed with normal diet
- CA
untrained group fed with allicin-supplemented diet
- CF
untrained group fed with fucoidan-supplemented diet
- ECG
electrocardiogram
- FCE
feed conversion efficiency
- fHmax
maximum heart rate
- Hct
hematocrit
- Hb
hemoglobin
- HSI
hepatosomatic index
- K
condition factor
- Q10
temperature coefficient
- RVM
relative ventricle mass
- SGR
specific growth rate
- Tarr
arrhythmia temperature
- Tpeak
temperature of peak heart rate
- Tabt
Arrhenius break point temperature
- TC
trained group fed with normal diet
- TA
trained group fed with allicin-supplemented diet
- TF
trained group fed with fucoidan-supplemented diet
1. Introduction
The warming climate is affecting the biological functions of many, if not all, marine and freshwater fish living in natural habitats and aquaculture systems, posing risks for their survival (Ficke et al., 2007; Pörtner and Knust, 2007; FAO et al., 2016; Handisyde et al., 2017). In natural settings when temperature increases, wild fish populations might have the opportunity to migrate to cooler areas (McKenzie et al., 2021a). However, barriers such as dams and canals as well as drought can negatively impact the movement of wild fish to cooler habitats (NRDC et al., 2008). The warming climate can also affect fish in aquaculture that may not be able to change their habitat. Especially in shallow ponds and tanks, farmed fish rely on their phenotypic plasticity in order to acclimate to heat waves. However, these species may not be able to react swiftly enough to survive, since mass mortalities of fish and shellfish have been reported by the farmers after heat waves (FAO et al., 2018; Green et al., 2019).
One reason for mortalities could be that temperature sensitive physiological processes, such as cardiac function, become impaired during extreme temperature challenges (Haverinen and Vornanen, 2020). Acute temperature changes challenge fish hearts to maintain regular function while also increasing circulation to match a rising metabolic rate (Clark et al., 2008; Farrell et al., 2009). When temperature reaches the fish's upper thermal limit, cardiac output collapses due to bradycardia and there is an increase in the occurrence of cardiac arrhythmias (Haverinen and Vornanen, 2020). Limitations in heart performance and its plasticity during heat waves could, therefore, be influencing the health and survival of fish. This is a special concern for fish in the aquaculture industry since domestication and current rearing practices have caused severe cardiac abnormalities (Poppe et al., 2003; Mercier et al., 2000; Brijs et al., 2020; Frisk et al., 2020) which can limit their cardiovascular capacity to respond warming. There is, thus, urgent need to find ways to improve the cardiovascular performance of fish at warming temperatures.
Exercise training is a promising strategy for increasing cardiac performance of aquaculture fish (Palstra and Planas, 2011). Also in nature, the habitat characteristics like temperature and flow velocity could affect the fish performance and cause “natural training” (Eliason et al., 2017). Swimming exercise-training at moderate speeds has been shown to have beneficial effects on maximum cardiac performance but also on growth, swimming and muscular capacity, brain plasticity, stress tolerance, and disease resistance in salmonids (Walker and Emerson, 1978; Houlihan and Laurent, 1987; Farrell et al., 1990, 1991; Claireaux et al., 2005; Anttila et al., 2011; Castro, 2012; Davison and Herbert, 2013; Grisdale-Helland et al., 2013; Mes et al., 2020; McKenzie et al., 2021b). For example, training intensity, equal to 60% of maximal swimming capacity, has shown to improve the cardiovascular performance of rainbow trout (Oncorhynchus mykiss) in in situ perfused heart analysis (Farrell et al., 1991). However, the question remains whether exercise training could enhance maximum cardiac performance during warming and the cardiac thermal tolerance. Here, we explored a novel idea that exercise training at 1 body length per second (bl s−1) could improve the maximum cardiovascular performance and its thermal limits of farmed rainbow trout.
Another option to enhance the cardiovascular performance of fish at high temperatures could be changing the composition of their feed, as recently shown by Hardison et al. (2021). Functional feeds are enriched diets with natural components derived generally from terrestrial plants and seaweeds with potential benefits in the prevention and treatment of cardiovascular diseases (Hasler et al., 2000; Marriott, 2000). Two prevalent functional feeds, the allicin and fucoidan, have been shown to act against cardiovascular disorders in humans and mammals. Allicin, an organosulfur substance isolated from the terrestrial plant garlic (Allium sativum), has been shown to have antiplatelet, anti-atherosclerotic and antiarrhythmic properties in mammals (Prasad et al., 1995; Banerjee and Maulik, 2002; Huang et al., 2013; Cui et al., 2020). Allicin is an inhibitor that caused a reduction in hepatic lipid synthesis in mice fed with a high-fat diet and reduced heart oxidative stress in a rat model with chronic kidney disease (García-Trejo et al., 2016; Shi et al., 2019). The molecular mechanism by which allicin acts against cardiovascular disorders is partly the reduction of oxidative stress, which allows the restoration of mitochondrial function (Marón et al., 2020). Fucoidan, on the other hand, is a sulfated polysaccharide isolated from marine brown algae (Fucus vesiculosus), that has also been shown to act against cardiovascular disorders in mammals. The molecular mechanism of fucoidan relies on the activation of enzymatic antioxidants, suppression of inflammatory cytokines and nitric oxide-mediated disorders in cardiomyocytes (Thomes et al., 2010; Zaporozhets and Besednova, 2016). The mechanisms underlying those protective effects of fucoidan are associated with significant improvements in oxidative stress, cardiac systolic and diastolic function, ventricular rhythm, and vascular density (Manzo-Silberman et al., 2011; Tsai et al., 2017; Chang et al., 2019).
In aquaculture, dietary supplementations of allicin increase growth performance and feed conversion efficiency, stimulate appetite, promote intestinal development, improve immunity and control infectious diseases in rainbow trout and other commercial fish species (Nya and Austin, 2009; Nya et al., 2010; Militz et al., 2013; Lee et al., 2014; Huang et al., 2020). Fucoidan has also been reported to enhance the growth response of barramundi (Lates calcarifer) (Tuller et al., 2014; Purcell-Meyerink et al., 2021). Yet, the effects of allicin and fucoidan on teleost cardiovascular performance and its thermal tolerance have not been experimentally evaluated even though there have been discussions that allicin might be beneficial in this respect (Crumlish and Austin, 2020).
The objective of this study was to test whether exercise training at moderate intensity alone or together with allicin- or fucoidan-supplement feeding could have an effect on the maximum cardiovascular performance and its thermal tolerance in juvenile farmed rainbow trout. We compared the maximum heart rates (fHmax), induced by atropine and isoproterenol, during acute warming, and measured the temperatures where trout showed signs for cardiac arrhythmias (Tarr) between control and treated groups after 6 weeks. To our knowledge, this is the first study to test the effects of exercise training together with functional feeds on the cardiac performance and its thermal tolerance in teleosts. Besides cardiovascular measurements, we also evaluated whether the treatments could influence the growth performance of fish – important knowledge for aquaculture. The combined the effect of exercise training and dietary modifications has important applications in aquaculture and ecology.
2. Materials and methods
2.1. Ethical approval
All the experimental procedures followed the EU legal framework (Directive 2010/63/EU) and the Finnish legislation (Decree no. 812/2010, Helsinki 2013) for the protection of animals used for scientific purposes. The specific ethics license number for current study was ESAVI/11880/2019. The experiments were carried out at the Natural Resources Institute Finland (Luke) in Enonkoski during the spring-summer of 2019.
2.2. Experimental fish and design
Experiments were conducted on juvenile rainbow trout (age 1+, n = 216; body mass = 66.3 ± 1.3 g; fork-length = 17.2 ± 0.3 cm), which were randomly assorted into six experimental groups in duplicate tanks (n = 18 per group). The tanks were randomly distributed across the aquaculture facility to prevent tank/human disturbance effects. At the beginning of the experimental trial, fish size was approximately similar and the density of the fish in the tanks followed aquaculture recommendations.
The untrained groups were divided into control group CC, which was untrained trout fed with normal (control) diet, group CA, which was untrained trout fed with allicin-supplemented diet, and group CF, which was untrained trout fed with fucoidan-supplemented diet. The exercise trained groups were divided into group TC, which was trained trout fed with a normal diet, group TA, which was trained trout fed with allicin-supplemented diet, and group TF, which was trained trout fed with fucoidan-supplemented diet. Each experimental group was divided in two identical duplicate tanks. The fish were reared in 230 L circular flow-through plastic tanks (0.51 m height × bottom area 0.45 m2) with freshwater coming from the nearby Lake Ylä-Enonvesi (62°04′01″N, 29°00′00″E). The water temperature, oxygen level and daily light cycle were following the ambient natural conditions. At the first week of the exercise training and feeding, the average water temperature was 5.5 ± 0.3 °C, and at the last week, it was 13.9 ± 0.7 °C (Supplementary Figure 1). Dissolved water oxygen level was measured daily using an optical oxygen meter (HACH HQ30d flexi, HACH Company, USA) and it was around 86 ± 7.1% O2 saturation throughout the experimental period. Trout were fed to satiation once a day for five days per week. The amount of food was recorded daily for each tank to calculate feed intake, as the total average dry food consumed (g) per number of fish in each tank.
2.3. Diet preparations
During the 6-weeks experimental period, trout were fed with a commercial diet (3.5-mm Hercules pellets with 43% crude protein, 28% crude fat, 1.5% crude fiber, 4.8% ash, 0.7% calcium, 0.9% phosphorus, 0.5% sodium, vitamin A, vitamin D3, vitamin E, vitamin C3 and astaxanthin with the energy level of 24 MJ kg−1) produced at Raisio Aqua Oy in Turku (Finland).
The CC and TC groups were fed only with the commercial diet. Groups CA and TA were fed with the commercial diet, supplemented with allicin as Allimed® liquid (1000 mg L−1, Allicin International Company, East Sussex, UK), while the CF and TF groups diet were supplemented with fucoidan as MariVet® (powder from Fucus vesiculosus and Undaria pinnatifida, Marinova Pty Ltd, Cambridge TAS, Australia). The concentration of fucoidan in MariVet® powder was 398 mg g−1. MariVet® also contained marine polyphenols (131 mg g−1) and mannitol (125 mg g−1). The concentrations of Allimed® and MariVet® for the current study were selected according to Nya et al. (2010) and Tuller et al. (2014). Allimed® was sprayed into pelleted food at a 1 mL 100 g−1 food and MariVet® was manually mixed at 10 g kg−1 food for fucoidan. After mixing the supplements to food pellets, the pellets were spray-coated with fish oil (1%, Möller Orkla Care Oy, Finland) to ensure the binding of supplements and reduced their dissolution to water when feeding the fish. After overnight air-drying the food was vacuum-coated and stored in plastic bags at 4 °C and used within one month. Control groups' diets were prepared in the same way but without the addition of the supplements. The feeds were prepared in 300 g batches.
2.4. Exercise training
During the 6-week experimental period, the untrained trout were swimming against an average water flow velocity of 0.3 bl s−1 in circular plastic tanks (24 h). The trained fish were exposed to average water flow velocity of 1.0 bl s−1 for 6 h per day (from 8 a.m. to 2 p.m.), and rest for 18 h at 0.3 bl s−1. All the fish were fed 1 h after the exercise training ended. The exercise training was conducted 5 days per week. During the weekends fish were swimming with a velocity of 0.3 bl s−1. The water flow velocity was calculated as an average velocity from three different spots from the tank. The flow velocity was measured with OTT C small current meter with a propeller (Lindell, Germany).
2.5. Growth performance and feed utilization
One month before the experiment started trout were passive integrated transponder (PIT, #AB10350; HS code: 85235290, Loligo Systems, Viborg, Denmark) tagged under anesthesia (100 ppm buffered MS-222). At the same time, the initial mass and length of the fish were measured. These parameters were also measured at the end of the experiment. The growth performance parameters such as gain in body mass, specific growth rate, feed conversion efficiency, and condition factor were calculated according to the following formulas:
| Body mass gain (BMG) % = (Wt - W0) × 100 / W0, where W0 = initial trout mass in grams and Wt = final mass in grams |
| Specific growth rate (SGR) % = ((ln Wt) - ln(W0))/T x 100, where ln = natural logarithm and T = time interval (days) |
| Feed conversion efficiency (FCE) = mass gained (g) / feed intake (g), where feed intake was the total average dry food consumed (g) per number of fish in tank |
| Condition factor (K) = (mass (g) / length (cm) 3) × 100 |
2.6. Hematological and tissue analysis
At the end of the experiment, the blood, liver, and ventricle samples were collected from the rainbow trout groups. Blood samples were taken with heparinized syringes from the caudal vein of the fish. The hematocrit (Hct) of blood was measured in micro-hematocrit capillary tubes spun for 5 min at 15000 g (StatSpin MP Centrifuge) by the Wintrobe method, and Hct was reported as a percentage of packed red cell volume (%). In order to measure hemoglobin concentration (Hb), 10 μl of blood was diluted to 1 ml of Drabkin's solution (50 mg K3Fe(CN)6 (Merck, Espoo, Finland), 12.5 mg KCN (Pharmakon Inc, NJ, USA), 40 mg KH2PO4 (MilliporeSigma, Darmstadt, Germany), in 175 ml dH2O) and the absorbance of the solutions was measured as triplicates with PerkinElmer EnSpine™ 2300 Multilabel plate reader at 540 nm. The Hb was calculated as described by Wells et al. (2005). The heart ventricle was weighed after removal atrium, bulbus arteriosus, and blood. Liver was also removed and weighed. The relative ventricle mass (RVM) and the hepatosomatic index (HSI) were calculated using the following formulas:
| Relative ventricle mass (RVM) % = ventricle mass (g) / body mass (g) × 100 |
| Hepatosomatic index (HSI) % = liver mass (g) / body mass (g) × 100 |
2.7. Assessment of cardiovascular function in vivo
To study the cardiac performance of trout during acute warming, maximum heart rate (fHmax), temperature of peak heart rate (Tpeak), and the two transition temperatures, the Arrhenius break point temperature (Tabt) and the arrhythmia temperature (Tarr) of fHmax were measured in vivo using electrocardiograms (ECGs) as previously described by Casselman et al. (2012) and Anttila et al. (2017). The cardiovascular measurements of the fish were done within 7 days at the end of the experiment. In order to avoid bias and other unwanted effects such as temperature fluctuations within those 7 days, each day fish from each group were analyzed in random order.
The ECGs of the fish were detected with custom made silver electrodes (two detecting electrodes and the ground electrode immersed in chambers), which were placed underneath trout and below the heart. ECG signal was amplified with Grass P122 AC/DC Strain Gage Amplifier (Grass Technologies, Warwick, USA) and detected with BIOPAC Data Acquisition Unit MP100 (BIOPAC® Systems, Inc. Goleta, CA, USA) and Acknowledge software (ver. 3.9.1.).
For the heart measures, trout (n = 9 per group) were anesthetized in buffered tricaine methanesulfonate (100 ppm MS-222, Western Chemical, Inc. WA, USA) and their weight was recorded. Thereafter the fish were immersed in oxygen and temperature regulated chambers. The chambers were PVC pipes cut lengthwise, water circulation and temperature controlling of chambers were performed with Lauda RC6 (Lauda- Königshofen, Germany). Trout were maintained anesthetized (60 ppm buffered MS-222) and a nozzle was inserted in the mouth of the fish providing oxygen-rich water to gills during measurements. The temperature in the system was kept at 10 °C at the beginning of measurements. The anesthetized trout were stationary and fully submerged during the measurements to reduce skeletal muscle activity and minimize electrical interference. After 30 min of equilibration period at 10 °C, atropine sulfate solution was injected to fish intraperitoneally (1 μl g−1; concentration of 2.5 μg g−1; Alfa Aesar by ThermoFisher Scietific, Karlsruhe, Germany), and followed with isoproterenol injection (1 μl g−1; concentration of 8 ng g−1; Sigma-Aldrich Chemie GmbH, Munich, Germany) 15 min later. Both reagents were dissolved into 0.9% NaCl (Sigma-Aldrich, Helsinki, Finland) and prepared daily. Fifteen minutes after the isoproterenol injection, the temperature was started to increase stepwise: 1 °C per 6 min according to Casselman et al. (2012). The heart rate was allowed to stabilize before each temperature step. The warming continued until cardiac arrhythmias were observed i.e. QRS complex or P wave was missing, indicating arrhythmia temperature Tarr. After that, fish were immediately removed and killed with cranial percussion and the length of the fish was recorded. Fish that had noisy signal (no QRS complexes were able to be observed), or complications after the injections (fish developed cardiac arrhythmias shortly after injection) were omitted from analyses. In total, four fish were removed based on these criteria (final n-numbers per groups are presented in Table 2).
Table 2.
Cardiac performance and its thermal tolerance parameters of trained and untrained rainbow trout fed with different diets for 6 weeks. The cardiac responses were measured during an acute temperature challenge (1 °C per 6 min). The table shows data of peak heart rate (fHpeak) and temperature where it was achieved (TPeak) during the thermal challenge as well as Arrhenius break point temperature (Tabt) of fHmax and temperature where cardiac arrhythmias appeared (Tarr) for each trout group. All values are presented as means ± SEM.
| Trout groups | CC |
CA |
CF |
TC |
TA |
TF |
|---|---|---|---|---|---|---|
| (n = 8) | (n = 9) | (n = 9) | (n = 8) | (n = 8) | (n = 8) | |
| Water velocity | 0.3 bl s−1 (control) | 1.0 bl s−1 (training) | ||||
| Cardiac parameters | ||||||
| fHpeak | 142.3 ± 8.54 | 145.1 ± 2.84 | 141.8 ± 5.53 | 158.4 ± 4.70 | 143.6 ± 8.45 | 158.9 ± 7.56 |
| Tabt (°C) | 15.8 ± 0.46 | 16.5 ± 0.50 | 16.5 ± 0.38 | 16.9 ± 0.29 | 15.4 ± 0.63 | 16.5 ± 0.38 |
| TPeak (°C) | 20.1 ± 0.74AB | 20.5 ± 0.44AB | 19.4 ± 0.50A | 20.9 ± 0.55AB | 19.1 ± 0.85AB | 21.3 ± 0.67B |
| Tarr (°C) | 21.9 ± 0.59 | 22.8 ± 0.60 | 22.9 ± 0.71 | 22.3 ± 0.52 | 22.2 ± 1.20 | 23.5 ± 0.92 |
Abbreviations of trout groups have been previously mentioned in Table 1. Significant differences between groups are indicated with different letters.
The fHmax in each temperature step was calculated by counting R-R intervals for ten final heartbeats per temperature step. The peak heart (fHpeak) indicated the highest heart rate value recorded during temperature increase and the Tpeak temperature where fHpeak occurred. The Tabt indicated the temperature where rate functions first fail to follow an exponential increase with temperature and was calculated according to Yeager and Ultsch (1989). This was done by plotting the natural logarithm of fHmax (ln fHmax) against the inverse of Kelvin temperature (1/T). The two linear regression lines were fitted into plots of the ln fHmax versus 1/T. The intersection of these two regression lines indicated that the Tabt at which temperature-induced increase in fHmax shifts to a lower exponent. Temperature dependency of the fHmax was expressed as a temperature coefficient (Q10) calculated with the formula: Q10 = (R2/R1) (10/(T2 -T1)), where T1 and T2 are the temperatures that produce the fHmax values for R1 and R2 respectively.
2.8. Statistical analysis
All data in the text and figures, except the original recordings of ECG in vivo, are presented as mean ± SEM (standard error of the mean). The Shapiro-Wilk normality test and the Brown-Forsythe equal variance test were used to examine the distribution and variance of variables. The effect of the diet and exercise training on growth, hematological, organ, and cardiovascular variables (BMG, SGR, FCE, K, Hct, Hb, RVM, HSI, fHpeak, Tabt, Tpeak and and arrTarr) were assessed using a two-way ANOVA, while the fHmax and Q10 at different temperatures were analyzed with three-way ANOVA. Pairwise multiple comparisons between groups were identified using Holm-Sidak's post hoc analysis. All the statistical analyses were performed with SigmaPlot 14 (Systat Software Inc., San Jose, CA, USA, www.sigmaplot.com). Statistical significance was accepted at P ≤ 0.05.
3. Results
No mortalities were observed in any of the groups throughout the 6-week training program.
3.1. Growth and morphological parameters
The results of body parameters such as mean growth, relative ventricle mass and hepatosomatic index as well as feed conversion efficiency and hematological parameters are presented in Table 1. The Supplementary Table 1 also contains the average growth parameters of each duplicate tank per group. Overall, no differences were found among groups on body mass gain (F = 1.95, P = 0.15 diet; F = 0.56, P = 0.45 exercise training; F = 0.30, P = 0.74 interaction between diet x exercise training), specific growth rate (F = 1.42, P = 0.24 diet; F = 0.47, P = 0.49 exercise training; F = 0.49, P = 0.61 diet x exercise training), condition factor (F = 0.54, P = 0.58 diet; F = 1.26, P = 0.26 exercise training; F = 0.09, P = 0.91 diet x exercise training), relative ventricle mass (F = 1.06, P = 0.35 diet; F = 1.11, P = 0.30 exercise training; F = 0.18, P = 0.83 diet x exercise training) and hepatosomatic index (F = 1.98, P = 0.15 diet; F = 0.12, P = 0.73 exercise training; F = 0.99, P = 0.38 diet x exercise training). Diet had a significant effect on feed conversion efficiency (F = 4.71, P = 0.01), while exercise training did not influence feed conversion efficiency (F = 0.06, P = 0.81) and there was not an interaction between diet and exercise training (F = 0.63, P = 0.53). The post-hoc test revealed a significant 38%-decrease of FCE in trained fish fed fucoidan-supplemented diet (TF) compared to trained fish fed with allicin-supplemented diet (TA) (t = 2.66; P = 0.02). Exercise training and diet did not have effect on a hemoglobin (F = 0.40, P = 0.67 diet; F = 0.12, P = 0.74 exercise training). However, for hematocrit there was significant interaction between diet and exercise training (F = 3.08, P = 0.05). The post-hoc test revealed a significant increase of Hct by exercise training in fish fed with control diet (t = 2.05, P = 0.05), while in other diet groups exercise training did not have any effect (Table 1). A similar trend was also seen with hemoglobin (F = 2.47, P = 0.09 interaction between diet x exercise training).
Table 1.
Growth, hematological, and tissue parameters of rainbow trout fed with normal diet (control group), allicin-supplemented diet and fucoidan-supplemented diet and raised under two different water velocities, control (0.3 bl s−1) and moderate swimming exercise training (1.0 bl s−1). Measurements were taken after 6 weeks of feeding and exercise training. All values are presented as means ± SEM with number of samples for each group in parentheses.
| Trout groups | CC | CA | CF | TC | TA | TF | ||
|---|---|---|---|---|---|---|---|---|
| Water velocity | 0.3 bl s−1 (control) | 1.0 bl s−1 (training) | ||||||
| Growth parameters | ||||||||
| W0 | 65.36 ± 3.32 (19) | 68.23 ± 3.15 (17) | 70.22 ± 2.65 (18) | 65.35 ± 2.62 (17) | 60.66 ± 3.21 (15) | 62.94 ± 2.58 (18) | ||
| Wt | 103.45 ± 4.69 (20) | 109.90 ± 5.64 (17) | 110.23 ± 4.64 (19) | 107.96 ± 5.16 (19) | 102.95 ± 5.54 (16) | 98.53 ± 5.05 (20) | ||
| BMG % | 62.05 ± 3.99 (19) | 63.49 ± 7.62 (17) | 57.13 ± 4.46 (18) | 66.37 ± 5.99 (17) | 70.78 ± 5.86 (15) | 55.81 ± 5.19 (18) | ||
| SGR % | 1.70 ± 0.41 (19) | 1.68 ± 0.18 (17) | 1.59 ± 0.10 (18) | 1.78 ± 0.13 (17) | 1.88 ± 0.12 (15) | 1.54 ± 0.13 (18) | ||
| FCE | 1.12 ± 0.07 AB (19) | 1.08 ± 0.11 AB (17) | 1.03 ± 0.08 AB (18) | 1.15 ± 0.10 AB (17) | 1.31 ± 0.11 A (15) | 0.81 ± 0.08 B (18) | ||
| K | 1.74 ± 0.41 (20) | 1.36 ± 0.02 (17) | 1.38 ± 0.02 (19) | 1.36 ± 0.02 (19) | 1.33 ± 0.03 (16) | 1.32 ± 0.02 (20) | ||
| Hematological parameters | ||||||||
| Hct % | 34.5 ± 2.45A (16) | 41.3 ± 1.21AB (13) | 39.7 ± 2.44AB (10) | 44.2 ± 4.13B (15) | 38.8 ± 2.58AB (14) | 41.6 ± 1.42AB (14) | ||
| Hb g/l | 57.22 ± 6.29 (9) | 68.75 ± 6.2 (8) | 72.42 ± 3.72 (7) | 73.0 ± 4.01 (9) | 63.0 ± 8.66 (7) | 67.2 ± 4.07 (9) | ||
| Tissue parameters | ||||||||
| RVM % | 0.08 ± 0.00 (10) | 0.09 ± 0.00 (8) | 0.08 ± 0.00 (10) | 0.09 ± 0.00 (10) | 0.09 ± 0.00 (8) | 0.08 ± 0.00 (10) | ||
| HSI % | 0.77 ± 0.04 (9) | 0.88 ± 0.05 (8) | 0.80 ± 0.03 (9) | 0.84 ± 0.07 (9) | 0.85 ± 0.04 (8) | 0.74 ± 0.03 (9) | ||
Abbreviations CC: untrained trout fed with normal diet; CA: untrained trout fed with allicin-supplemented diet; CF: untrained trout fed with fucoidan-supplemented diet; TC: trained trout fed with normal diet; TA: trained trout fed with allicin-supplemented diet; TF: trained trout fed with fucoidan-supplemented diet; W0 = initial trout mass in grams; Wt = final mass in grams; BMG: body mass gain; SGR: specific growth rate; FCE = Feed conversion efficiency; CF: K; Hct: hematocrit; Hb: hemoglobin; RVM: relative ventricle mass; HSI: hepatosomatic index. Significant differences between groups are indicated with different letters.
3.2. Cardiac and thermal tolerance parameters
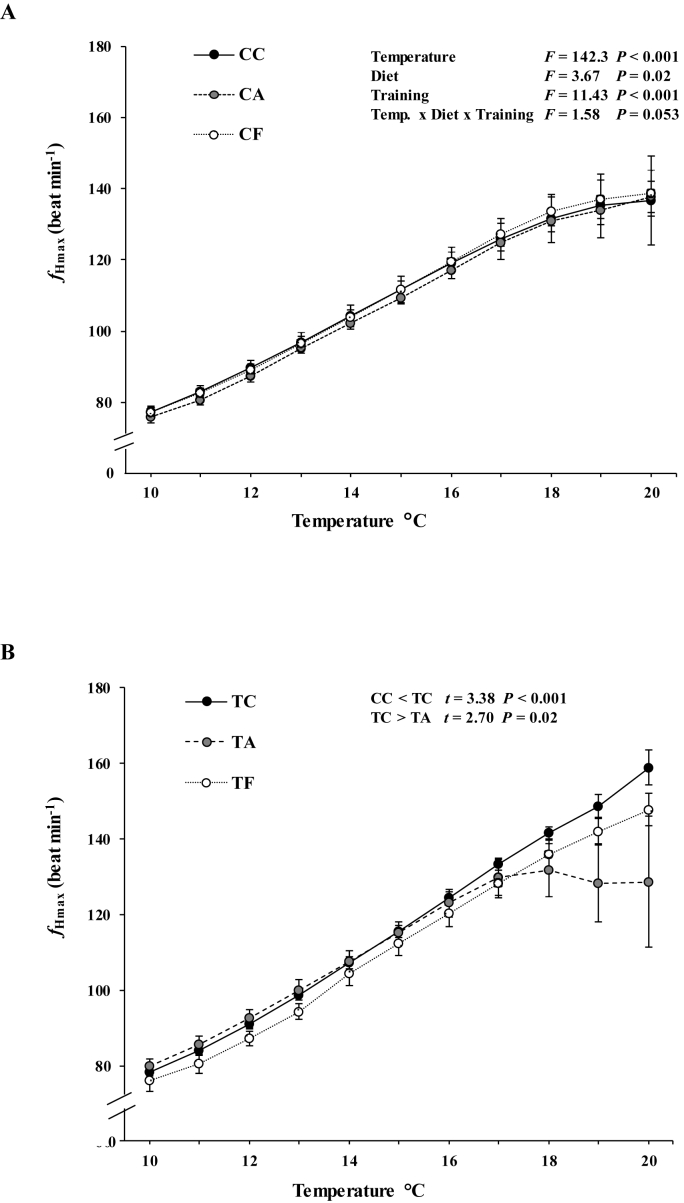
The fHmax increased significantly during the acute warming in all groups (F = 142.3, P < 0.001). Both diet (F = 3.67; P = 0.02) and exercise training (F = 11.43; P < 0.001) affected the fHmax during the acute temperature change from 10 °C to 20 °C, and there was almost a significant interaction between diet, training and temperature (F = 1.58, P = 0.053) (Fig. 1A). Post-hoc tests revealed that exercise training increased the average fHmax during the warming challenge of trout (t = 3.38, P < 0.001). The difference between trained and untrained groups was 11.3 beats per minute at 20 °C. The post-hoc tests also revealed that trout fed with allicin-supplemented diet had lower heart rate (fHmax) during warming as compared to group fed with control diet (t = 2.70; P = 0.02) and the difference between allicin and control groups was 18.4 beats per minute at 20 °C. The reduction of fHmax was especially evident in trained fish where the allicin fed had the lowest fHmax during the thermal challenge (Fig. 1B) and the difference between TC and TA groups was 37.8 beats per minute at 20 °C.
Fig. 1.
Maximum heart rate (fHmax; in beats per minute, bpm) of (A) untrained and (B) trained rainbow trout fed with different diets during an acute warming from 10 to 20 °C. Values are presented as mean ± SEM. CC: untrained trout fed with normal diet; CA: untrained trout fed with allicin-supplemented diet; CF: untrained trout fed with fucoidan-supplemented diet; TC: trained trout fed with normal diet; TA: trained trout fed with allicin-supplemented diet; TF: trained trout fed with fucoidan-supplemented diet.
With regards to the thermal tolerance parameters of cardiac function, neither diet nor the exercise training affected the Tabt (F = 0.74, P = 0.48 diet; F = 0.00, P = 0.98 exercise training; F = 2.80, P = 0.07 diet x exercise training) or Tarr (F = 1.00, P = 0.37 diet; F = 0.01, P = 0.91 exercise training; F = 0.47, P = 0.62 diet x exercise training) within any of the groups (Table 2). The fHpeak values did not differ significantly among groups (F = 0.45, P = 0.63 diet; F = 3.44, P = 0.07 exercise training; F = 0.52, P = 0.47 diet x exercise training). Even though diet (F = 0.57, P = 0.55) and exercise training (F = 0.52; P = 0.47) did not have an effect on the temperature of fHpeak, the Tpeak, there was significant interaction between diet and exercise trainings (F = 3.45, P = 0.04). According to post-hoc test the trained group fed with fucoidan-supplemented diet (TF) had 1.9 °C higher Tpeak than the untrained trout fed a similar diet (CF) (t = 2.03, P = 0.04) (Table 2). There were no significant differences in Tpeak between trained and untrained groups in the control and allicin fed fish (CC vs TC t = 0.82, P = 0.41; CA vs TA t = 1.61, P = 0.11).
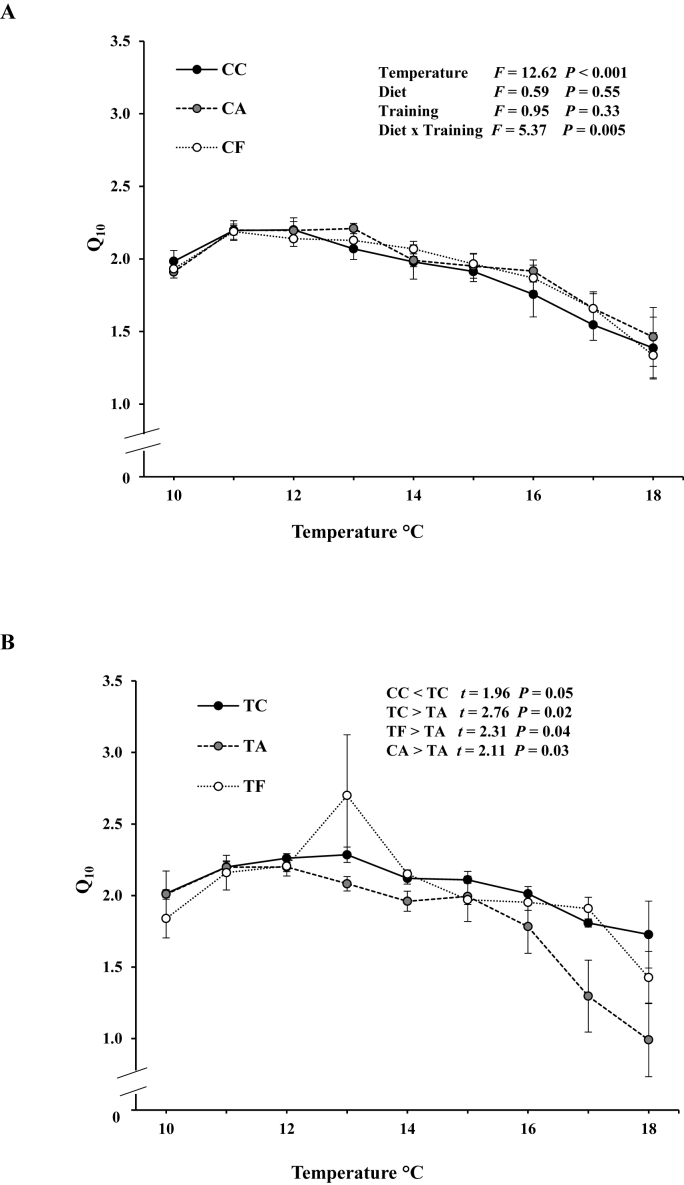
In general, the temperature had a significant main effect on Q10, as Q10 reduced when temperature increased (F = 12.62, P < 0.001) (Fig. 2). The diet and exercise training did not affect the Q10 values during warming (F = 0.59, P = 0.55 diet; F = 0.95, P = 0.33 exercise training). However, there was an interaction between diet and exercise training (F = 5.37, P = 0.005) (Fig. 2A), and post-hoc comparisons revealed (Fig. 2B) that the trained group fed with a normal diet had significantly higher Q10 (t = 1.96, P = 0.050) values at the high temperatures compared to untrained group fed with the same diet. Furthermore, the trained group fed with a normal diet (TC) had also higher Q10 compared to trained group fed with allicin-supplement (TA) (t = 2.76, P = 0.02) but not with fucoidan-supplement (TF) (t = 0.45, P = 0.64). Between allicin and fucoidan supplements, trained groups fed with the fucoidan had higher Q10 values during warming (t = 2.31, P = 0.04). The trained group fed with allicin-supplement had lower Q10 than untrained group fed with allicin (t = 2.11, P = 0.03).
Fig. 2.
The Q10 of maximum heart rate changes between each 1 °C temperature step during acute warming of (A) untrained and (B) trained rainbow trout. Values are presented as mean ± SEM. CC: untrained trout fed with normal diet; CA: untrained trout fed with allicin-supplemented diet; CF: untrained trout fed with fucoidan-supplemented diet; TC: trained trout fed with normal diet; TA: trained trout fed with allicin-supplemented diet; TF: trained trout fed with fucoidan-supplemented diet.
4. Discussion
In this study we hypothesized that exercise training at a moderate speed of 1 bl s−1 and dietary supplementation of the functional feeds, allicin and fucoidan, would positively influence cardiac performance, and cardiac thermal tolerance of farmed rainbow trout as well as their growth. Our results indicate that the supplementation of allicin (CA) or fucoidan (CF) in the diet did not improve the cardiac performance or growth in untrained groups. However, the exercise training increased the trout's fHmax and Q10 of heart rate at high temperatures. When the two-factors, exercise training and supplement feeding, were combined they did not work synergistically. Allicin significantly reduced the fHmax of the fish. This was especially evident in trained fish. Changes in cardiac performance in response to exercise training did not cause similar positive changes when trout were simultaneously fed with allicin. In other words, allicin supplementation eliminated the positive effects of training. Further research at a molecular level is required to understand the unwanted antagonistic effects of exercise training and supplement feeds in trout.
4.1. Cardiac function and thermal tolerance
One way to evaluate the performance of fish under environmental changes, e.g. warming climate, is to measure their heart rate and thermal tolerance parameters of cardiac function (Casselman et al., 2012). For instance, Tabt estimates the temperature when fish achieves its optimum performance, Tpeak is a temperature indicating the limit for maximum performance, and Tarr indicates the critical temperature when fish heart's performance starts to collapse (arrhythmias appear) (Casselman et al., 2012). Fish can, however, modulate their cardiac performance and its thermal tolerance to survive from changing temperatures (see review in Eliason and Anttila, 2017). Recently, it has been shown that diet has significant effect on cardiac performance of fish during warming. The supplementation of Ulva spp. to diet of the omnivorous fish opaleye (Girella nigricans) reduced their maximum heart rate (fHmax) when compared to fish fed with carnivorous diet without changing their thermal tolerance parameters (Hardison et al., 2021). Here, the aim was to discover if supplement feeding could improve the cardiac performance of the fish during warming. The allicin and fucoidan supplements, however, did not produce any beneficial effects on the cardiac performance parameters (fHpeak and fHmax during acute warming) nor cardiac thermal tolerance (Tabt, TPeak, and Tarr). Combining the fucoidan feeding to exercise training, however, had a positive effect on TPeak. Previous studies have shown that fucoidan could influence the cardiac function in mammals. In particular, the treatment with fucoidan enhanced the ventricular rhythm through reduction of QT intervals and improved the swimming endurance of aging mice (Chang et al., 2019). Also, enhanced myocardial contractility and reduced risk of ischemic heart disease have been reported after administration with dietary fucoidan in a rat model with diabetes (Li et al., 2011; Yu et al., 2014). Similarly, cardioprotective effects have been observed with allicin, through mechanisms that prevented ventricular arrhythmias and reduced myocardial infarctions in rats (Huang et al., 2013; Ma et al., 2017). However, in the current study, the only positive effect was the improvement of TPeak with fucoidan feeding when the fish were training while allicin reduced the maximum heart rate (fHmax) and its temperature coefficient (Q10). To date, there are no previous experiments on whether allicin or fucoidan could influence the cardiac function of any fish species. It is possible, though less likely, that other concentrations of allicin and fucoidan than those used in this study, might be more effective.
Exercise training, on the other hand, increased the fHmax of trout during the acute thermal challenge. Similarly, the trained trout had significantly higher Q10 at high temperatures compared to untrained ones. It could be that high fHmax enables high blood flow to fish tissues in trained trout when temperature rises. Even though fHmax was increased by a modest swimming training intensity, there was no statistically significant increase in cardiac thermal tolerance parameters (Tabt, TPeak and Tarr) of trained trout. Previous work in rainbow trout has shown that aerobic swimming exercise training at water velocities ≥1 bl s−1 positively affected stroke volume, cardiac output, and maximum power output in situ (Farrell et al., 1991). Here, in addition to the elevated fHmax values, we observed that trained trout fed with control diet had high Hct levels which indicate increased oxygen delivery to tissues. Thorarensen et al. (1993) found a similar increase of Hct with swimming in chinook salmon (Oncorhynchus tshawytscha). Both Hct and fHmax results in current study suggest that exercise training intensity at 1 bl s−1 was at an aerobic level, due to a potential higher oxygen delivery to tissues. However, despite the promising results, it could be that higher training intensity is needed to enhance the thermal tolerance of the fish, or induce changes in cardiac size since there were no significant differences in relative ventricle mass among groups.
Surprisingly, fHmax values were lower at high temperatures in the trained group fed with the allicin-supplement (TA) when compared to the normal diet (TC) treatment. Since swimming exercise training alone (TC vs. CC) increased fHmax, it seems that there is a possible antagonistic interaction with allicin feeding and exercise training. A similar result was seen in Q10 values which suggests that the combination of allicin and exercise training has a suppressing effect on the capacity of physiological processes, such as the heart rate, to response to environmental warming. The current study cannot reveal the reason for the observed reduction. However, we can speculate that a reason might be the regulation of blood pressure, and therefore cardiac function during warming. In mammals, garlic has been shown to reduce both systolic and diastolic blood pressure via stimulation of nitric oxide (NO) production and inhibition of the angiotensin-converting enzyme (Batiha et al., 2020). Similarly, swimming training has been shown to increase NO production and its bioactivity (Green et al., 2004). In fish, though, venous pressure needs to be kept consistent or even increase at high temperatures to enable cardiac filling (Sandblom and Gräns, 2017). The trained trout fed with allicin may have needed a low fHmax at high temperatures, to enable enough time for cardiac filling.
4.2. Growth performance
Besides evaluating the cardiac function and its thermal tolerance, we also measured the different growth parameters of the fish. Here, allicin supplement did not influence growth performance (BMG, SGR), nutrient utilization (FCE), or the general condition (K) of trout after 6 weeks. Although the same trend in somatic parameters was seen for trout fed fucoidan supplement, the trained group (TF) showed relatively low levels of FCE and BMG. In the current study, the FCE was calculated with assumption that all the fish in the same tank were feeding the same way. This could induce some bias into calculations since some fish might not eat same amount as other fish (e.g. larger fish bullying smaller fish and preventing them from feeding). Therefore, strong conclusions cannot be made about possible negative effects of fucoidan on FCE. In general, the condition factors of the fish in all groups were relatively high (1.3–1.8). Therefore, it could be that trout were already at their maximum growth performance. Furthermore, it could be that the growth parameters might be influenced if the experimental trial had lasted longer than 6 weeks. Another reason why allicin and fucoidan supplements did not affect untrained trout could be that there was no need for extra energy, protection against pathogenic microorganisms, or atherosclerosis that these supplements provide.
In the current study, trout were exposed to an exercise training challenge that often increases metabolism in fish, at least during exercise (Soofiani and Priede, 1985; Grisdale-Helland et al., 2013). However, in the current study, no differences were found in growth nor morphological parameters after exercise training irrespective of diet. This suggests that moderate swimming exercise training intensity for trout (1 bl s−1) might not have been high enough to induce a significant demand for more energy. In previous reviews, exercise-training at moderate intensities at the range of 0.75–1.5 bl s−1 has been shown to have positive effects on growth, feed intake, and swimming efficiency of juvenile salmonids (Davison, 1989, 1997; Jobling et al., 1993), with a few exceptions (Kiessling et al., 1994; Skov et al., 2011). Therefore, in terms of growth and feeding, we believe that swimming exercise training at velocity of 1 bl s−1 is at the range of intermediate velocities, at which juvenile trout are expected to swim relatively economically (Webb, 1971) without high energy demand. Moreover, in the present study we have shown that the HSI was unaffected within trained groups fed with functional feeds or normal diet. This also indicates that exercise intensity at 1 bl s−1 did not cause energy depletion, and therefore juvenile trout during exercise were able to adjust their energy stores (e.g. hepatic glucose) to maintain growth and swimming. Our results seem to be in agreement with a previous study where trout exposed to 1.5 bl s−1 showed a steady energy budget by balancing glucose production and glucose utilization (Shanghavi and Weber, 1999). Future studies could test if supplement feeding would have more beneficial effects if the fish were trained with higher intensity or if the duration of program would be longer.
In conclusion, we have shown that trout exposed to aerobic exercise training were able to maintain a robust fHmax at high temperatures and possibly increase the oxygen delivery to tissues via increased Hct. A higher fHmax did not, however, increase cardiac thermal tolerance of trained fish except when training was combined with fucoidan food supplement that increased the TPeak. as compared to untrained fucoidan fed fish. Allicin, on the other hand, reduced the fHmax and Q10 of heart rate, especially the ones of trained fish. However, on whole, dietary allicin and fucoidan do not improve cardiac and upper thermal tolerance performance of trout suggesting that these supplements, with used concentrations, are not providing any beneficial effects on cardiac performance. Our finding may be significant in fish hatcheries in terms of establishing 'swimming training regimes' and understanding the ability of fish to survive acute thermal conditions. Our results also show that cardiac function is plastic and different environmental conditions, for example water flow rate, can modulate the thermal performance of heart which could have implementations in nature as well when fish are e.g. migrating. More studies are, however, required for testing the effects different training intensities on cardiac thermal tolerance as well as revealing, at a molecular level, the combined effects of feeding and exercise training.
Funding
This work was supported by the Kone Foundation, Finland.
CRediT authorship contribution statement
Anna Papadopoulou: Conceptualization, Investigation, Methodology, Formal analysis, Visualization, Data curation, Writing – original draft. Luca Pettinau: Conceptualization, Investigation, Methodology, Data curation, Writing – review & editing, Visualization. Eila Seppänen: Investigation, Resources, Writing – review & editing, Project administration, Funding acquisition. Asko Sikanen: Investigation, Resources, Writing – review & editing. Katja Anttila: Conceptualization, Investigation, Writing – original draft, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank all technical staff of the Natural Resources Institute Finland (Luke) in Enonkoski for taking care of fish at facilities and practical help during the experiment. We are grateful to Raisio Aqua Oy which kindly provided the feed used in the experiment.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crphys.2022.02.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Anttila K., Jokikokko E., Erkinaro J., Järvilehto M., Mänttäri S. Effects of training on functional variables of muscles in reared Atlantic salmon Salmo salar smolts: connection to downstream migration pattern. J. Fish. Biol. 2011;78:552–566. doi: 10.1111/j.1095-8649.2010.02871.x. [DOI] [PubMed] [Google Scholar]
- Anttila K., Mauduit F., Le Floch S., Claireaux G., Nikinmaa M. Influence of crude oil exposure on cardiac function and thermal tolerance of juvenile rainbow trout and European sea bass. Environ. Sci. Pollut. Res. Int. 2017;24:19624–19634. doi: 10.1007/s11356-017-9609-x. [DOI] [PubMed] [Google Scholar]
- Batiha G.E.-S., Beshbishy A.M., Wasef L.G., Elewa Y.H.A., Al-Sagan A.A., Abd El-Hack M.E., Taha A.E., Abd-Elhakim Y.M., Devkota H.P. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): a review. Nutrients. 2020;12:1–21. doi: 10.3390/nu12030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S.K., Maulik S.K. Effect of garlic on cardiovascular disorders: a review. Nutr. J. 2002;1:1–14. doi: 10.1186/1475-2891-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brijs J., Hjelmstedt P., Berg C., Johansen I.B., Sundh H., Roques J.A.C., Ekström A., Sandblom E., Sundell K., Olsson C., Axelsson M., Gräns A. Prevalence and severity of cardiac abnormalities and arteriosclerosis in farmed rainbow trout (Oncorhynchus mykiss) Aquaculture. 2020;526:735417. doi: 10.1016/j.aquaculture.2020.735417. [DOI] [Google Scholar]
- Castro V. PhD Thesis. Norwegian University of Life Sciences, Ås, Norway. 2012. Aerobic exercise training for improving robustness of Atlantic salmon (Salmo salar) 978-82-575-1065-7. [Google Scholar]
- Casselman M.T., Anttila K., Farrell A.P. Using maximum heart rate as a rapid screening tool to determine optimum temperature for aerobic scope in Pacific salmon Oncorhynchus spp. J. Fish. Biol. 2012;80:358–377. doi: 10.1111/j.1095-8649.2011.03182.x. [DOI] [PubMed] [Google Scholar]
- Chang P.-M., Li K.-L., Lin Y.-C. Fucoidan–fucoxanthin ameliorated cardiac function via IRS1/GRB2/SOS1, GSK3β/CREB pathways and metabolic pathways in senescent mice. Mar. Drugs. 2019;17:69. doi: 10.3390/md17010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claireaux G., McKenzie D.J., Genge A.G., Chatelier A., Aubin J., Farrell A.P. Linking swimming performance, cardiac pumping ability and cardiac anatomy in rainbow trout. J. Exp. Biol. 2005;208:1775–1784. doi: 10.1242/jeb.01587. [DOI] [PubMed] [Google Scholar]
- Clark T.D., Sandblom E., Cox G.K., Hinch S.G., Farrell A.P. Circulatory limits to oxygen supply during an acute temperature increase in the Chinook salmon (Oncorhynchus tshawytscha) Am. J. Physiol. Cell Physiol. 2008;295:1631–1639. doi: 10.1152/ajpregu.90461.2008. [DOI] [PubMed] [Google Scholar]
- Crumlish M., Austin B. In: Climate Change and Infectious Fish Diseases. Wallingford. Woo P.T.K., Leong J.A., Buchmann K., editors. Oxfordshire; Boston: 2020. Aeromoniosis (Aeromonas salmonicida) p. 211. [DOI] [Google Scholar]
- Cui T., Liua W., Chenb S., Yua C., Lia Y., Zhang J.Y. Antihypertensive effects of allicin on spontaneously hypertensive rats via vasorelaxation and hydrogen sulfide mechanisms. Biomed. Pharmacother. 2020;128:1–12. doi: 10.1016/j.biopha.2020.110240. [DOI] [PubMed] [Google Scholar]
- Davison W. Training and its effects on teleost fish. Comp. Biochem. Physiol. Physiol. 1989;94:1–10. doi: 10.1016/0300-9629(89)90775-5. [DOI] [Google Scholar]
- Davison W. The effects of exercise training on teleost fish, a review of recent literature. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1997;117:67–75. doi: 10.1016/S0300-9629(96)00284-8. [DOI] [Google Scholar]
- Davison W., Herbert N.A. In: Swimming Physiology of Fish. Palstra A.P., Planas J.V., editors. Springer-Verlag Berlin Heidelberg; 2013. Swimming-enhanced growth; pp. 177–202. [DOI] [Google Scholar]
- Eliason E.J., Anttila K. In: The Cardiovascular System. Gamperl A.K., Gillis T.E., Farrell A.P., Brauner C.J., editors. Academic Press; 2017. Temperature and cardiovascular system; pp. 235–297. [Google Scholar]
- Eliason E.J., Gale M.K., Whitney C.K., Lotto A., Hinch S.G. Intraspecific differences in endurance swim performance and cardiac size in sockeye salmon (Oncorhynchus nerka) parr tested at three temperatures. Can. J. Zool. 2017;95:425–432. doi: 10.1139/cjz-2016-0248. [DOI] [Google Scholar]
- FAO . In: Summary of the Findings of the Intergovernmental Panel on Climate Change Fifth Assessment Report. Rome, Italy. Seggel A., DeYoung C., editors. 2016. Climate change implications for fisheries and aquaculture. 978-92-5-109260-6. [Google Scholar]
- FAO . In: Impacts of Climate Change on Fisheries and Aquaculture. Synthesis of Current Knowledge, Adaptation and Mitigation Options. Rome, Italy. Barange M., Bahri T., Beveridge M.C.M., Cochrane K.L., Funge-Smith S., Poulain F., editors. 2018. Climate change and aquaculture: interactions with fisheries and agriculture. 978-92-5-130607-9. [Google Scholar]
- Farrell A.P., Eliason E.J., Sandblom E., Clark T.D. Fish cardiorespiratory physiology in an era of climate change. Can. J. Zool. 2009;87:835–851. doi: 10.1139/Z09-092. [DOI] [Google Scholar]
- Farrell A.P., Johansen J.A., Steffensen J.F., Moyes C.D., West T.G., Suarez R.K. Effects of exercise training and coronary ablation on swimming performance, heart size, and cardiac enzymes in rainbow trout, Oncorhynchus mykiss. Can. J. Zool. 1990;68:1174–1179. doi: 10.1139/z90-174. [DOI] [Google Scholar]
- Farrell A.P., Johansen J.A., Suarez R.K. Effects of exercise-training on cardiac performance and muscle enzymes in rainbow trout, Oncorhynchus mykiss. Fish Physiol. Biochem. 1991;9:303–312. doi: 10.1007/BF02265151. [DOI] [PubMed] [Google Scholar]
- Ficke A.D., Myrick C.A., Hansen L.J. Potential impacts of global climate change on freshwater fisheries. Rev. Fish Biol. Fish. 2007;17:581–613. doi: 10.1007/s11160-007-9059-5. [DOI] [Google Scholar]
- Frisk M., Høyland M., Zhang L., Vindas M.A., Øverli Ø., Johansen I.B. Intensive smolt production is associated with deviating cardiac morphology in Atlantic salmon (Salmo salar L.) Aquaculture. 2020;529:735615. doi: 10.1016/j.aquaculture.2020.735615. [DOI] [Google Scholar]
- García-Trejo E.M.A., Arellano-Buendía A.S., Argüello-García R., Loredo-Mendoza M.L., García-Arroyo F.E., Arellano-Mendoza M.G., Castillo-Hernández M.C., Guevara-Balcázar G., Tapia E., Sánchez-Lozada L.G., Osorio-Alonso H. Effects of allicin on hypertension and cardiac function in chronic kidney disease. Oxid. Med. Cell. Longev. 2016;2016:1–13. doi: 10.1155/2016/3850402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.J., Maiorana A., O'Driscoll G., Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J. Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T.J., Siboni N., King W.L., Labbate M., Seymour J.R., Raftos D. Simulated marine heat wave alters abundance and structure of Vibrio populations associated with the Pacific Oyster resulting in a mass mortality event. Microb. Ecol. 2019;77:736–747. doi: 10.1007/s00248-018-1242-9. [DOI] [PubMed] [Google Scholar]
- Grisdale-Helland B., Takle H., Helland S.J. Aerobic exercise increases the utilization efficiency of energy and protein for growth in Atlantic salmon post-smolts. Aquaculture. 2013;406–407:43–51. doi: 10.1016/j.aquaculture.2013.05.002. [DOI] [Google Scholar]
- Handisyde N., Telfer T.C., Ross L.G. Vulnerability of aquaculture-related livelihoods to changing climate at the global scale. Fish Fish. 2017;18:466–488. doi: 10.1111/faf.12186. [DOI] [Google Scholar]
- Hasler C.M., Kundrat S., Wool D. Functional foods and cardiovascular disease. Curr. Atherosclerosis Rep. 2000;2:467–475. doi: 10.1007/s11883-000-0045-9. [DOI] [PubMed] [Google Scholar]
- Haverinen J., Vornanen M. Reduced ventricular excitability causes atrioventricular block and depression of heart rate in fish at critically high temperatures. J. Exp. Biol. 2020;223:1–10. doi: 10.1242/jeb.225227. [DOI] [PubMed] [Google Scholar]
- Hardison E.A., Kraskura K., Van Wert J., Nguyen T., Eliason E.J. Diet mediates thermal performance traits: implications for marine ectotherms. J. Exp. Biol. 2021;224:jeb242846. doi: 10.1242/jeb.242846. [DOI] [PubMed] [Google Scholar]
- Houlihan D.F., Laurent P. Effects of exercise training on the performance, growth and protein turnover of rainbow trout (Salmo gairdneri) Can. J. Fish. Aquat. Sci. 1987;44:1614–1621. doi: 10.1139/f87-195. [DOI] [Google Scholar]
- Huang W., Yao C., Liu Y., Xu N., Yin Z., Xu W., Miao Y., Mai K., Ai Q. Dietary allicin improved the survival and growth of large yellow croaker (Larimichthys crocea) larvae via promoting intestinal development, alleviating inflammation and enhancing appetite. Front. Physiol. 2020;11:587–674. doi: 10.3389/fphys.2020.587674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Wang Y., Cao Y.G., Qi H.P., Li L., Bai B., Liu Y., Sun H.L. Antiarrhythmic effects and ionic mechanisms of allicin on myocardial injury of diabetic rats induced by streptozotocin. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013;386:697–704. doi: 10.1007/s00210-013-0872-1. [DOI] [PubMed] [Google Scholar]
- Jobling M., Baardvik B.M., Christiansen J.S., Jørgensen E.H. The effects of prolonged exercise training on growth performance and production parameters in fish. Aquacult. Int. 1993;1:95–111. doi: 10.1007/BF00692614. [DOI] [Google Scholar]
- Kiessling A., Higgs D.A., Dosanjh B.S., Eales J.G. Influence of sustained exercise at two ration levels on growth and thyroid function of all-female chinook salmon (Oncorhynchus tshawytscha) in seawater. Can. J. Fish. Aquat. Sci. 1994;51:1975–1984. doi: 10.1139/f94-200. [DOI] [Google Scholar]
- Lee D.-H., Lim S.-R., Han J.-J., Lee S.-W., Ra C.-S., Kim J.-D. Effects of dietary garlic powder on growth, feed utilization and whole body composition changes in fingerling sterlet sturgeon, Acipenser ruthenus. Asian-Australas. J. Anim. Sci. 2014;27:1303–1310. doi: 10.5713/ajas.2014.14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Gao Y., Xing Y., Zhu H., Shen J., Tian J. Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia-reperfusion injury in rats via regulating the inflammation response. Food Chem. Toxicol. 2011;49:2090–2095. doi: 10.1016/j.fct.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Ma L.-N., L D., Li, Li S.-C., Hao X.-M., Zhang J.-Y., He P., Li Y.-K. Allicin improves cardiac function by protecting against apoptosis in rat model of myocardial infarction. Chin. J. Integr. Med. 2017;23:589–597. doi: 10.1007/s11655-016-2523-0. [DOI] [PubMed] [Google Scholar]
- Manzo-Silberman S., Louedec L., Meilhac O., Letourneur D., Michel J.B., Elmadbouh I. Therapeutic potential of fucoidan in myocardial ischemia. J. Cardiovasc. Pharmacol. 2011;58:626–632. doi: 10.1097/FJC.0b013e3182308c64. [DOI] [PubMed] [Google Scholar]
- Marón F.J.M., Camargo A.B., Manucha W. Allicin pharmacology: common molecular mechanisms against neuroinflammation and cardiovascular diseases. Life Sci. 2020;249:117513. doi: 10.1016/j.lfs.2020.117513. [DOI] [PubMed] [Google Scholar]
- Marriott B.M. Functional foods: an ecologic perspective. Am. J. Clin. Nutr. 2000;71:1728–1734. doi: 10.1093/ajcn/71.6.1728S. [DOI] [PubMed] [Google Scholar]
- McKenzie D.J., Zhang Y., Eliason E.J., Schulte P.M., Claireaux G., Blasco F.R., Nati J.J.H., Farrell A.P. Intraspecific variation in tolerance of warming in fishes. J. Fish. Biol. 2021;98:1536–1555. doi: 10.1111/jfb.14620. [DOI] [PubMed] [Google Scholar]
- McKenzie D.J., Palstra A.P., Planas J., MacKenzie S., Bégout M.-L., Thorarensen H., Vandeputte M., Mes D., Rey S., De Boeck G., Domenici P., Skov P.V. Aerobic swimming in intensive finfish aquaculture: applications for production, mitigation and selection. Rev. Aquacult. 2021;13:138–155. doi: 10.1111/raq.12467. [DOI] [Google Scholar]
- Mercier C., Aubin J., Lefrançois C., Claireaux G. Cardiac disorders in farmed adult brown trout, Salmo trutta L. J. Fish. Dis. 2000;23:243–249. doi: 10.1046/j.1365-2761.2000.00237.x. [DOI] [Google Scholar]
- Mes D., Palstra A.P., Henkel C.V., Mayer I., Vindas M.A. Swimming exercise enhances brain plasticity in fish. R. Soc. Open Sci. 2020;7:1–17. doi: 10.1098/rsos.191640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Militz T.A., Southgate P.C., Carton A.G., Hutson K.S. Efficacy of garlic (Allium sativum) extract applied as a therapeutic immersion treatment for Neobenedenia sp. management in aquaculture. J. Fish. Dis. 2013;37:451–461. doi: 10.1111/raq.12485. [DOI] [PubMed] [Google Scholar]
- Nya E.J., Austin B. Use of garlic, Allium sativum, to control Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum) J. Fish. Dis. 2009;32:963–970. doi: 10.1111/j.1365-2761.2009.01100.x. [DOI] [PubMed] [Google Scholar]
- Nya E.J., Dawood Z., Austin B. The garlic component, allicin, prevents disease caused by Aeromonas hydrophila in rainbow trout, Oncorhynchus mykiss (Walbaum) J. Fish. Dis. 2010;33:293–300. doi: 10.1111/j.1365-2761.2009.01121.x. [DOI] [PubMed] [Google Scholar]
- NRDC . In: Trout in Trouble. New York, USA. Kinsella S., Spencer T., Farling B., editors. 2008. The Impacts of global warming on trout in the interior West. [Google Scholar]
- Palstra A.P., Planas J.V. Fish under exercise. Fish Physiol. Biochem. 2011;37:259–272. doi: 10.1007/s10695-011-9505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe T.T., Johansen R., Gunnes G., Tørud B. Heart morphology in wild and farmed Atlantic salmon Salmo salar and rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 2003;57:103–108. doi: 10.3354/dao057103. [DOI] [PubMed] [Google Scholar]
- Prasad K., Laxdal V.A., Yu M., Raney B.L. Antioxidant activity of allicin, an active principle in garlic. Mol. Cell. Biochem. 1995;148:183–189. doi: 10.1007/BF00928155. [DOI] [PubMed] [Google Scholar]
- Purcell-Meyerink D., Packer M.A., Wheeler T.T., Hayes M. Aquaculture production of the brown seaweeds Laminaria digitata and Macrocystis pyrifera: applications in food and pharmaceuticals. Molecules. 2021;26:1–41. doi: 10.3390/molecules26051306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pörtner H.O., Knust R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science. 2007;315:95–97. doi: 10.1126/science.1135471. [DOI] [PubMed] [Google Scholar]
- Sandblom E., Gräns A. In: The Cardiovascular System. Gamperl A.K., Gillis T.E., Farrell A.P., Brauner C.J., editors. Academic Press; 2017. Form, function and control of the vasculature; pp. 369–433. [DOI] [Google Scholar]
- Shanghavi D.S., Weber J.M. Effects of sustained swimming on hepatic glucose production of rainbow trout. J. Exp. Biol. 1999;202:2161–2166. doi: 10.1242/jeb.202.16.2161. [DOI] [PubMed] [Google Scholar]
- Shi X., Zhou X., Chu X., Wang J., Xie B., Ge J., Guo Y., Li X., Yang G. Allicin improves metabolism in high-fat diet-induced obese mice by modulating the gut microbiota. Nutrients. 2019;11:1–14. doi: 10.3390/nu11122909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov P.V., Larsen B.K., Frisk M., Jokumsen A. Effects of rearing density and water current on the respiratory physiology and haematology in rainbow trout, Oncorhynchus mykiss at high temperature. Aquaculture. 2011;319:446–452. doi: 10.1016/j.aquaculture.2011.07.008. [DOI] [Google Scholar]
- Soofiani N.M., Priede I.G. Aerobic metabolic scope and swimming performance in juvenile cod, Gadus morhua L. J. Fish. Biol. 1985;26:127–138. doi: 10.1111/j.1095-8649.1985.tb04249.x. [DOI] [Google Scholar]
- Thomes P., Rajendran M., Pasanban B., Rengasamy R. Cardioprotective activity of Cladosiphon okamuranus fucoidan against isoproterenol induced myocardial infarction in rats. Phytomedicine. 2010;18:52–57. doi: 10.1016/j.phymed.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Thorarensen H., Gallaugher P.E., Kiessling A.K., Farrell A.P. Intestinal blood flow in swimming chinook salmon Oncorhynchus tshawyntscha and the effects of hematocrit on blood flow distribution. J. Exp. Biol. 1993;179:115–129. doi: 10.1242/jeb.179.1.115. [DOI] [Google Scholar]
- Tsai C.-H., Nam P., Lin Y.-C. Fucoidan and fucoxanthin ameliorate cardiac function of aging canine. Asian J. Anim. Vet. Adv. 2017;12:294–301. doi: 10.3923/ajava.2017.294.301. [DOI] [Google Scholar]
- Tuller J., De Santis C., Jerry D.R. Dietary influence of fucoidan supplementation on growth of Lates calcarifer (Bloch) Aquacult. Res. 2014;45:749–754. doi: 10.1111/are.12029. [DOI] [Google Scholar]
- Walker G.M., Emerson L. Sustained swimming speeds and myotomal muscle function in the trout, Salmo gairdneri. J. Fish. Biol. 1978;13:475–481. doi: 10.1111/j.1095-8649.1978.tb03457.x. [DOI] [Google Scholar]
- Webb P.W. The swimming energetics of trout: I. Thrust and power output at cruising speeds. J. Exp. Biol. 1971;55:489–520. doi: 10.1242/jeb.55.2.489. [DOI] [PubMed] [Google Scholar]
- Wells R.M.G., Baldwin J., Seymour R.S., Christian K., Brittain T. Red blood cell function and haematology in two tropical freshwater fishes from Australia. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005;141:87–93. doi: 10.1016/j.cbpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Yeager D.P., Ultsch G.R. Physiological regulation and conformation: a BASIC program for the determination of critical points. Physiol. Zool. 1989;62:888–907. doi: 10.1086/physzool.62.4.30157935. [DOI] [Google Scholar]
- Yu X., Zhang Q., Cui W., Zeng Z., Yang W., Zhang C., Zhao H., Gao W., Wang X., Luo D. Low molecular weight fucoidan alleviates cardiac dysfunction in diabetic goto-kakizaki rats by reducing oxidative stress and cardiomyocyte apoptosis. J. Diabetes Res. 2014;2014:1–14. doi: 10.1155/2014/420929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaporozhets T., Besednova N. Prospects for the therapeutic application of sulfated polysaccharides of brown algae in diseases of the cardiovascular system: review. Pharm. Biol. 2016;54:3126–3135. doi: 10.1080/13880209.2016.1185444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.