Abstract
Serum uric acid (SUA) is significantly elevated in obesity, gout, type 2 diabetes mellitus, and the metabolic syndrome and appears to contribute to the renal, cardiovascular and pulmonary comorbidities that are associated with these disorders. Most previous studies have focused on the pathophysiologic effects of high levels of uric acid (hyperuricemia). More recently, research has also shifted to the impact of hypouricemia, with multiple studies showing the potentially damaging effects that can be caused by abnormally low levels of SUA. Along with these observations, recent inconclusive data from human studies evaluating the treatment of hyperuricemia with xanthine oxidoreductase (XOR) inhibitors have added to the debate about the causal role of UA in human disease processes. SUA, which is largely derived from hepatic degradation of purines, appears to exert both systemic pro-inflammatory effects that contribute to disease and protective antioxidant properties. XOR, which catalyzes the terminal two steps of purine degradation, is the major source of both reactive oxygen species (O2.-, H2O2) and UA. This review will summarize the evidence that both elevated and low SUA may be risk factors for renal, cardiovascular and pulmonary comorbidities. It will also discuss the mechanisms through which modulation of either XOR activity or SUA may contribute to vascular redox hemostasis. We will address future research studies to better account for the differential effects of high versus low SUA in the hope that this will identify new evidence-based approaches for the management of hyperuricemia.
Keywords: U-shaped, Uric acid, Hyperuricemia, Hypouricemia, XOR inhibitors, H2O2
1. Introduction
Uric Acid (UA) is the end product of purine metabolism in all cells and is produced exclusively through the oxidation of xanthine and hypoxanthine by the enzyme xanthine oxidoreductase (XOR) [1]; a reaction that is necessary for the removal of nitrogenous waste products from the body. In humans, 3.0–6.8 mg/dL (178–400 μM) are considered normal levels of serum uric acid (SUA); however, many factors can influence these values such as age, gender and many disease processes. The pathophysiologic role of uric acid has been studied in a wide variety of disease processes and debated for decades, yet a complete understanding is still not at hand. Physiologically, UA plays a protective role as an antioxidant by scavenging singlet oxygen and preventing lipid peroxidation. It is believed that UA accounts for anywhere from 30 to 50% of the body's normal antioxidant capacity. We also have a good understanding of the role uric acid plays at levels of frank hyperuricemia (SUA >6.1 mg/dL) when uric acid crystal deposition can occur, resulting in the known pathologic process of gout [2]. Gout is highly prevalent worldwide (e.g., ∼3.9% of USA adults) [3] and has driven the development of medications aimed at lowering SUA through inhibition of the XOR enzyme, increasing urinary uric acid excretion (Uricosurics) or degrading soluble urate (Uricases) [2].
Since the early 2000s, research regarding UA has expanded to evaluate how UA impacts the body at both the high and low ends of what we have defined as physiologically normal (3.0–6.8 mg/dL) and how that definition of normal changes with different patient populations. For example, in patients with Type 2 Diabetes Mellitus (T2DM) [[4], [5], [6]] and metabolic syndrome (MetS) [[6], [7], [8]], hyperuricemia and excess XOR activity is common. Studies have shown that both T2DM and MetS are associated with increased cancer risk and chronic inflammation, and the possibility has been raised that hyperuricemia might be related to the increased risk of cancer incidence and metastasis [9,10]. Studies have also suggested that patients presenting with hyperuricemia are at a greater risk for cardiovascular (CV) and renal disease [[11], [12], [13], [14]], further suggesting a link between hyperuricemia and human cardiorenal and metabolic diseases [15]. More recently, research has also shifted to the impact of hypouricemia, with multiple studies showing the potentially detrimental effects that can be caused by abnormally low levels of SUA that are not merely explained by its antioxidant capacity [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]]. These studies indicate that uric acid plays a significant role in both physiology and pathology with the idea of a U-shaped association existing between SUA level and the prevalence and mortality in many diseases.
This review will highlight much of the current research regarding U-shaped associations with SUA as it relates to all-cause mortality, cardiovascular events and mortality, renal damage, neurologic disease, and other diseases including its potential impact on lung disorders. We will also discuss the results of past and ongoing clinical trials of mediations aimed at lowering SUA and how a U-shaped effect could be used to explain their findings. We will conclude with a discussion regarding the challenges to the current scope of research regarding UA and address ways in which we can develop future research studies to better account for U-shaped effects.
2. Pathophysiology of serum uric acid
Traditionally, the normal range of SUA has been defined as 3.0–6.8 mg/dL (178–400 μM). However, the idea of a one-size-fits-all mentality for UA is outdated, for it fails to account for the variability associated with age, gender, race, and comorbid diseases. Additionally, with many factors influencing purine metabolism, abnormalities in SUA are often multifactorial and variable among patients.
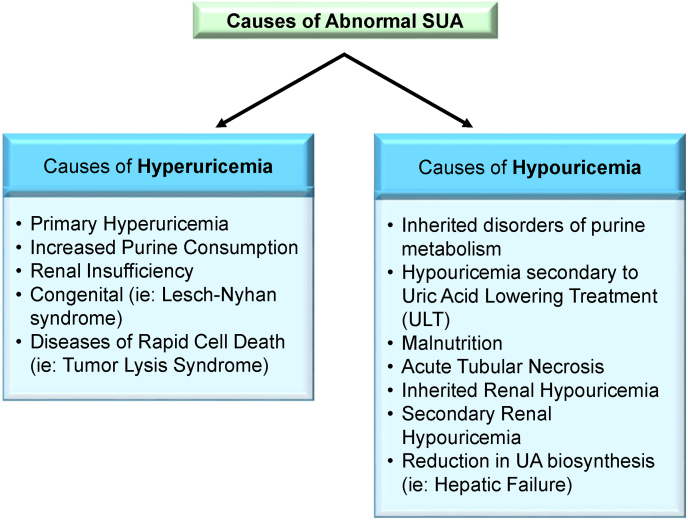
Hyperuricemia is a common disorder affecting ∼3.9% of USA adults [3]. It most commonly arises via decreased renal UA excretion, either as a result of primary genetic mechanisms or secondarily due to factors including functional renal impairment, hypertension, insulin resistance, obesity, and urate-elevating medications including thiazides and loop diuretics (Fig. 1). It can also be caused by UA overproduction, increased purine consumption, decreased small intestinal urate excretion, or a combination of all these mechanisms. Excessive SUA levels can result in precipitation of uric acid crystals in the connective tissue of joints leading to gouty arthritis and in the renal tubules leading to acute tubular necrosis.
Fig. 1.
Causes of Abnormal Serum Uric Acid (SUA). Both causes of abnormal high level of serum uric acid (hyperuricemia) and low level of serum uric acid level (hypouricemia) have primary and secondary etiologies as listed. The main cause of secondary hypouricemia is following uric acid lowering treatment (ULT).
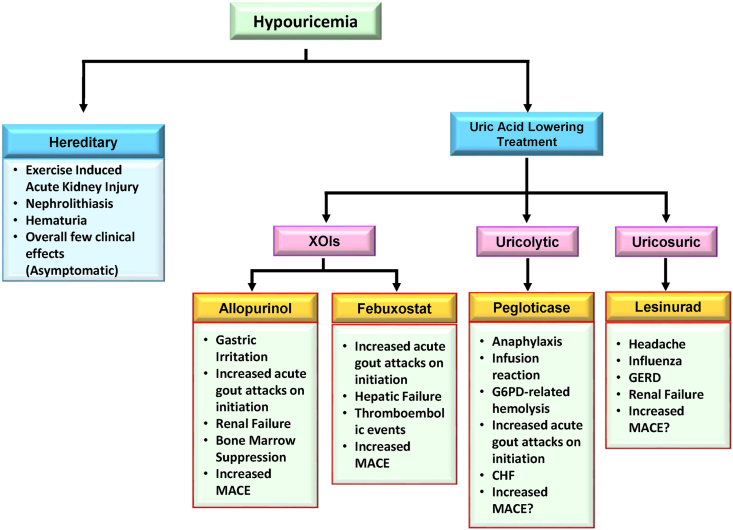
Conversely, hypouricemia is less common than hyperuricemia with studies estimating a prevalence of 0.51–1.39% [[35], [36], [37]]. Hypouricemia can be the result of either renal UA hyperexcretion or underproduction due to hereditary and iatrogenic causes (Fig. 2). Hereditary hypouricemia is often the result of genetic mutations in urate transporter 1 (URAT1/SLC22A12) or glucose transporter 9 (GLUT9/SLC2A9) [38,39]. Although generally asymptomatic, research has shown that hereditary hypouricemic patients are at increased risk for exercise-induced acute kidney injury potentially due to greater levels of filtered UA exceeding its reabsorption capacity [[40], [41], [42]]. This is in contrast to iatrogenic hypouricemia which is most commonly the result of urate-lowering therapies (ULT). The clinical significance of iatrogenic hypouricemia has not been well established. Additionally, complications resulting from hereditary hypouricemia have not been observed in iatrogenic cases. This raises the need for additional research to elucidate further the role of uric acid and the potential complications of hypouricemia secondary to uric acid lowering treatment (ULT).
Fig. 2.
Consequences of hypouricemia. Complications of both hereditary (primary) and ULT induced (secondary) hypouricemia have been listed. The most important of these is the ULT induced major adverse cardiovascular events (MACE).
3. U-shaped association of serum uric acid and overall cause mortality
Many studies have sought to evaluate the association between SUA and all-cause mortality, and whether SUA level can be used as an independent risk factor for all-cause mortality (Table 1). These studies have been conducted in various patient populations including healthy individuals [28,32], elderly patients [34], patients with chronic kidney disease (CKD) [43], and patients undergoing renal replacement therapy [16,18,20,23,44], all with varying results and study defined values for high and low SUA. Cho et al. conducted a retrospective analysis of 375,163 healthy participants in South Korea. The study revealed that SUA and all-cause mortality exhibited a U-shaped association in both men and women [32]. This association remained significant even after the authors adjusted for possible covariates such as body mass index (BMI), smoking status, alcohol intake, exercise, hypertension, diabetes, high cholesterol, and baseline glomerular filtration rate (GFR) [32]. This result is in slight contrast to a prospective study conducted in South Korea by Kang et al. in which they evaluated 27,490 healthy individuals(28). Primary results showed a U-shaped association between SUA and all-cause mortality in men and women, but only the association in men remained significant after adjusting for covariates [28]. Of particular interest in the Kang et al. study was that the lowest SUA group (<4 mg/dl) had a 35% increased mortality risk after covariate adjustment [28].
Table 1.
Epidemiologic studies evaluating U-Shaped effect of uric acid.
| Year | Reference | Study Population | Gender | Measurement | Low SUA | High SUA | Ref: |
|---|---|---|---|---|---|---|---|
| 2000 | Verdecchia et al. | Essential HTN | M/F | All-Cause /CV Events and Mortality | Men: <4.5 mg/dL Women: <3.2 mg/dL |
Men: >6.2 mg/dL Women: >4.6 mg/dL |
[45] |
| 2004 | Hsu SP et al. | ESRD | M/F | All-Cause Mortality | ≤6.0 mg/dL | ≥9.0 mg/dL | [16] |
| 2006 | Suliman ME et al. | ESRD | M/F | CV Mortality | <5.3 mg/dL | >8.9 mg/dL | [44] |
| 2007 | A. Mazza et al. | Elderly patients with NIDDM | M/F | CV Mortality | ≤0.29 mmol/L (4.9 mg/dL)a | ≥0.37 mmol/L (6.2 mg/dL)a | [17] |
| 2010 | Seet et al. | Asian patients with AIS | M/F | Functional outcomes | <280 μM/dL (4.7 mg/dL)a | >410 μM/dL (6.9 mg/dL)a | [46] |
| 2011 | Latif, W. et al. | ESRD | M/F | All-Cause /CV Mortality | <8.2 mg/dL | ≥8.2 mg/dL | [18] |
| 2012 | Lapsia et al. | Preoperative CV patients | M/F | AKI | <7.0 mg/dL | ≥7.0 mg/dL | [47] |
| 2013 | Feng et al. | Peritoneal dialysis | M/F | All-Cause Mortality | ≤7.0 mg/dL | ≥10.0 mg/dL | [20] |
| 2013 | Huang et al. | Japanese adult males | M | Muscle Strength | <5.4 mg/dL | ≥6.8 mg/dL | [48] |
| 2014 | Dahle et al. | Renal transplant patients | M/F | All-Cause /CV Mortality | 151–309 μM (2.6–5.2 mg/dL) a | 474–870 μM (8.0–14.7 mg/dL) a | [21] |
| 2015 | Kanda et al. | Healthy individuals | M/F | Loss of Kidney Function/AKI | <5.0 mg/dL | ≥6.5 mg/dL | [22] |
| 2016 | Bae et al. | ESRD | M/F | All-Cause Mortality | <5.5 mg/dL | ≥8.5 mg/dL | [23] |
| 2016 | Li et al.b | Healthy individuals with no history of DM. | M/F | Fasting plasma glucose (FPG) | – | – | [49] |
| 2016 | Oh et al. | Renal transplant patients | M/F | Allograft survival | ≤5.0 mg/dL | >8 mg/dL | [24] |
| 2016 | Zhang et al. | Healthy Japanese adults | M/F | CV Mortality | Men: <4.6 mg/dL Women: <3.3 mg/dL |
Men: >6.7 mg/dL Women: >5.1 mg/dL |
[50] |
| 2017 | Kamei et al. | Healthy Japanese adults | M/F | Incidence of nonfatal stroke | Men: ≤4.9 mg/dL Women: ≤3.7 mg/dL |
Men: ≥7.1 mg/dL Women: ≥5.5 mg/dL |
[51] |
| 2017 | Gwag et al. | Vasospastic Angina (VSA) | M/F | Major Adverse Cardiac Events (MACE) | ≤4.8 mg/dL | ≥6.0 mg/dL | [25] |
| 2017 | Hsieh et al. | CAPD patients | M/F | Technique failure in PD | ≤8.0 mg/dL | >8.0 mg/dL | [26] |
| 2017 | Hsieh et al. | CAPD patients | M/F | Residual Renal Function (RRF) | <0.372 mmol/L (6.3 mg/dL) a | ≥0.421 mmol/L (7.1 mg/dL) a | [27] |
| 2017 | Kang et al. | Healthy adults | M/F | All-Cause Mortality | ≤4.0 mg/dL | >8.0 mg/dL | [28] |
| 2017 | Lee et al. | Postmenopausal women | F | Arterial Stiffness | ≤3.8 mg/dL | ≥5.0 mg/dL | [29] |
| 2017 | Matsukuma et al. | Patients with IgAN | M/F | Development of ESRD | Men: <6.1 mg/dL Women: <4.4 mg/dL |
Men: >7.0 mg/dL Women: >5.3 mg/dL |
[30] |
| 2017 | Uedono et al. | Healthy individuals | M/F | Intrarenal hemodynamic parameters | <3.5 mg/dL | >6.0 mg/dL | [31] |
| 2017 | Wang et al.b | Chinese adults with a normal glucose tolerance test | M/F | Fasting Plasma Glucose (FPG) | – | – | [52] |
| 2018 | Cho et al. | General population | M/F | All-Cause, CV, and Cancer-related Mortality | Men <3.5 mg/dL Women <2.5 mg/dL |
Men ≥9.5 mg/dL Women ≥8.5 mg/dL |
[32] |
| 2018 | Srivastava et al. | CKD | M/F | Kidney Failure and All-Cause Mortality | 1.9–6.0 mg/dL | 8.7–15.2 mg/dL | [43] |
| 2018 | Tseng et al. | Elderly patients | M/F | All-Cause /CV Mortality | <3 mg/dL | ≥10.0 mg/dL | [34] |
| 2018 | Yang et al. | First-ever AIS | M/F | Functional outcomes | <190 μmol/L (3.2 mg/dL)a | >358 μmol/L (6.1 mg/dL) | [53] |
| 2018 | Latourte et al. | Healthy adults | M/F | Incidence of dementia | Men: <260 μmol/L (4.4 mg/dL) a Women: <209 μmol/L (3.5 mg/dL) a |
Men: ≥345 μmol/L (5.8 mg/dL) a Women: ≥292 μmol/L (4.9 mg/dL) a |
[54] |
| 2018 | Zhou et al. | Pregnancy | F | Maternal blood pressure | <0.140 mmol/L (2.4 mg/dL) a | >0.140 mmol/L (2.4 mg/dL) a | [55] |
| 2018 | Zhou et al. | Pregnancy | F | Fetal growth | <0.267 mmol/L (4.5 mg/dL) a | >0.267 mmol/L (4.5 mg/dL) a | [56] |
HTN: Hypertension; ESRD: End-Stage Renal Disease; NIDDM: Non-Insulin Dependent Diabetes Mellitus; AIS: Acute Ischemic Stroke; AKI; Acute Kidney injury; CAPD: Continuous Ambulatory Peritoneal Dialysis; IgAN: IgA Nephropathy; CKD: Chronic Kidney Disease.
Values in parentheses are a conversion to mg/dL for comparison.
Study did not primarily group patients based on SUA.
Another recent study published in 2018 by Tseng et al. evaluated the clinical impact of SUA in elderly patients. This retrospective analysis of 127,771 elderly patients initially showed a similar result to that of the Cho et al. [32] demonstrates that both low (<4 mg/dL) and high (>8 mg/dL) levels of SUA were independently associated with increased risk of all-cause mortality [34]. This association remained significant for the high SUA group but diminished for the low SUA group when adjustment was made for malnourishment [34].
In 2004, Hsu et al. conducted a retrospective analysis of patients with CKD on hemodialysis to evaluate the clinical impact of SUA on all-cause mortality [16]. The study was one of the first to show a U-shaped association in which the risk of all-cause mortality increased at both low (≤6.5 mg/dL) and high (≥9.0 mg/dL) levels of SUA [16]. In three prospective follow-up studies of patients with CKD (Stages 2–4) [43] and end-stage renal disease (ESRD) [23,44], similar results were obtained; all documenting a U-shaped relationship between SUA and the risk of all-cause mortality. Suliman et al. even noted that patients with ESRD and an SUA ≥9.0 mg/dL had an independent 2.0-fold increased risk of mortality [44].
These results differ from a later study by Latif et al. in which they evaluated the association of SUA and all-cause mortality in chronic hemodialysis patients [18]. This study concluded that mortality did increase in patients with a uric acid less than 8.2 mg/dL but decreased in patients with high SUA (≥8.2 mg/dL), suggesting a potentially protective effect of high UA in hemodialysis patients [18]. Specific to patients on continuous ambulatory peritoneal dialysis (CAPD), Feng et al. concluded that high SUA (≥10 mg/dL) was an independent risk factor for all-cause mortality [20]. This association did not remain significant for low SUA (≤7.0 mg/dL) after adjusting for covariates such as malnutrition and diabetes [20], suggesting that nutritional status may have an impact on the production of uric acid.
3.1. U-shaped association of serum uric acid and xanthine oxidoreductase activity with cardiac events and mortality
Just as with all-cause mortality, cardiac specific mortality and events are a major area of research as it relates to SUA and XOR activity. One of the reasons this area has become so highly researched is that multiple epidemiologic studies have reported a relationship between SUA and cardiovascular risk factors such as hypertension and metabolic syndrome [57]. One of the major populations at risk for hyperuricemia is patients with chronic kidney disease (CKD) due to their decreased ability to clear UA and dialysis patients that have a well-documented increase in the risk of cardiovascular disease (CVD) and sudden cardiac death (SCD) [58]. Evaluating the role of SUA as an independent risk factor and measuring changes in SUA in response to therapy as an assessment tool for the risk of cardiac events and mortality has proven difficult. One major challenge is that CVD is an enormous and broad category, encompassing a wide range of pathologic conditions and patient populations. Many studies have either evaluated narrowly specific types of CVD such as vasospastic angina (VSA) [25] or specific populations such as postmenopausal women [29] (Table 1). These results have made it difficult to extrapolate the data to a larger and more general population.
One of the earliest studies to propose a U-shaped association between SUA and cardiovascular events was the PIUMA study published in 2000 by Verdecchia et al. [45]. The study evaluated 1,720 individuals with essential HTN but no history of CVD, renal disease, cancer, or other significant medical problems. They showed increased risk at both high and low SUA leads to the study endpoint of CV events and all-cause mortality. In one of the few studies to evaluate a general population, Zhang et al. evaluated 36,313 Japanese individuals using data from the Evidence for Cardiovascular Prevention from Observational Cohorts in Japan study (EPOCH-JAPAN) [50]. Individuals were between the age of 35–89 and had no history of stroke, coronary heart disease, or cancer at baseline. They concluded that there was a U-shaped association between SUA and increased risk of CVD mortality in both men and women. No significant association was observed between SUA and mortality from non-CVD related mortality [50].
Two retrospective studies by A. Mazza et al. [17] and Tseng et al. [34] sought to evaluate the association between SUA and cardiovascular mortality in elderly patients (Age>65), with A. Mazza et al. specifically evaluating elderly patients with non-insulin dependent diabetes mellitus [17]. Both studies found a U-shaped association between SUA and cardiovascular mortality in both men and women [17,34]. Tseng et al. specifically noted an incremental increase in the risk of cardiovascular mortality at SUA>7 mg/dL and SUA<4 mg/dL [34]. The Tseng et al. study also evaluated the role of malnourishment in hypouricemia and found that the risk of cardiovascular mortality was strongly associated with the level of malnourishment as measured by the Geriatric Nutritional Risk Index (GNRI). This association was not statistically significant in patients without malnourishment [34].
A cross-sectional study by Dahle et al. evaluated patients who had undergone kidney transplantation and how SUA affected patient outcomes(21). They concluded that high SUA (474–870 μM; 8.0–14.7 mg/dL) was an independent risk factor for both all-cause and cardiovascular mortality and that low SUA (151–309 μM; 2.6–5.2 mg/dL) was associated with increased risk of mortality in patients with type 1, type 2, or new onset diabetes but not in nondiabetic patients [21].
Two unique population studies evaluated the risk of Major Adverse Cardiac Events (MACE) in patients with vasospastic angina (VSA) [25] and arterial stiffness in postmenopausal women [29]. The VSA study by Gwag et al. concluded that in patients with pure VSA, SUA less than or equal to 4.8 mg/dL was associated with a statistically significant increase in the risk of a MACE, while high SUA (≥6.0 mg/dL) did not have a statistically significant association with the risk of MACEs [25]. There was no association between SUA and risk of MACEs in patients with mixed VSA [25]. Following menopause, women are known to have increased SUA that advances with age [59]. This served as the basis for the study by Lee et al. that evaluated the relationship between SUA and arterial stiffness in postmenopausal women [29]. Arterial stiffness was determined by measuring the patient's brachial-ankle pulse wave velocity (ba-PWV). From this data, they concluded that there was a U-shaped association between SUA and arterial stiffness in postmenopausal Korean females [29]. A very recent retrospective study in 1,117 patients with infective endocarditis (IE) found a U-shaped relationship between the UA level and in-hospital death. The study concluded that both low and high levels of UA were predictive of increased short-term mortality in IE patients [60]. Also the same kind of study in 728 patients undergoing long-term peritdstoneal dialysis showed both SUA levels below 360 μmol/L and above 420 μmol/were found to be significant risk factors for developing CV events [61].
One of the most important research areas related to cardiac events and SUA is the activity of XOR, the enzyme responsible for the generation of uric acid [1]. Studies by both Fujimura et al. [62] and Otaki et al. [63] evaluated the association between the activity of the XOR enzyme and the risk of cardiac events. The study by Fujimura et al. retrospectively evaluated 408 patients admitted to the cardiac unit at Osaka Medical College in Japan. They concluded that there was a U-shaped association between XOR activity and the prevalence of a decreased left ventricular ejection fraction (LVEF) and increased brain natriuretic peptide (BNP) levels, both of which are used as markers of cardiac disease [62]. This association remained significant even after adjustment for characteristics such as age, sex and BMI, and patient-specific values such as hemoglobin A1C and diuretic use [62]. In a prospective evaluation of patients with a known history of congestive heart failure (CHF) by Otaki et al., patients were stratified based on their XOR activity, a U-shaped association between XOR activity and the risk of cardiac events was also shown [63].
3.2. U-shaped association of serum uric acid and renal disease
Renal function is one of the most important factors in the maintenance of UA homeostasis. The renal system is responsible for reabsorbing about 90% of the urate that is filtered, while also excreting 60–70% of the body's total UA [64]. This delicate interplay between SUA level, renal function, and associated renal disease makes for a frequent area of research. However, just as with CVD, the category of renal disease is broad and encompasses many different subcategories such as acute kidney injury (AKI) and chronic kidney disease (CKD) which complicates the ability to generalize conclusions regarding SUA and renal disease. Research regarding the association between SUA and renal damage has been evaluated in healthy individuals, AKI, CKD, kidney transplant, hemodialysis, and peritoneal dialysis patients (Table 1).
It is known that frank hyperuricemia due to increased UA production and decreased renal UA filtration can cause intratubular crystal precipitation resulting in acute tubular necrosis (ATN), a form of AKI [2]. Still, the association of SUA and AKI at levels below that of crystal precipitation is unclear. This was the focus of a 2012 study by Lapsia et al. in which they evaluated patients undergoing cardiovascular surgery and measured whether preoperative SUA was related to the development of AKI postoperatively [47]. Using a multivariate analysis, they concluded that SUA was an independent risk factor for the development of AKI and that both patients with low SUA and high SUA preoperatively (As defined by the study) had increased risk of developing AKI postoperatively [47]. A prospective study in 2015 by Kanda et al. evaluated the association between SUA and the risk of AKI in 9,847 healthy individuals in Japan with no medical history of CKD [22]. They showed a U-shaped association existed between SUA and the risk of diminishing kidney function in men, but not in women. In women, they found that only high SUA (≥6.5 mg/dL) was associated with an increased risk of kidney function loss. In both men and women, they concluded that low SUA (<5.0 mg/dL) was associated with decreased GFR [22].
As a follow up to that study, Uedono et al. sought to evaluate associations between SUA and the intrarenal hemodynamic parameters of renal plasma flow (RPF) and GFR in a small cohort of healthy individuals (n = 48). Using multivariate regression analysis, they concluded that SUA (<3.5 and >6.0 mg/dL) had an independent U-shaped association with decreased GFR and RPF [31]. They also concluded a significant and independent U-shaped association between SUA (<3.5 and >6.0 mg/dL) and increased afferent renal arteriolar resistance and decreased glomerular hydrostatic pressure. No association between SUA and efferent renal arteriolar resistance was noted [31].
Studies by Matsukuma et al. [30] and Srivastava et al. [43] evaluated the association between SUA and the development of renal failure in patients with CKD. The study by Matsukuma et al. evaluated patients with immunoglobulin A nephropathy (IgAN), which is the most common cause of glomerulonephritis worldwide [30]. They concluded that high SUA (Men >7.0 mg/dL and Women >5.3 mg/dL) was independently associated with an increased risk of developing ESRD. When they evaluated low SUA though (Men <6.1 mg/dL and Women <4.4 mg/dL), they found that only women had an increased risk of developing ESRD [30]. In the study by Srivastava et al., they evaluated patients with CKD (Stages 2–4) who were part of the Chronic Renal Insufficiency Cohort (CRIC) [43]. They concluded that in patients with CKD stage 3a or earlier (GFR≥45), high SUA (8.7–15.2 mg/dL) was associated with an increased risk of developing renal failure. This was in contrast to patients with CKD stage 4 (GFR<30) in which they found that for each one standard deviation greater UA concentration, patients had an independently associated 18% reduction in the risk of developing renal failure [43]. This result indicates a possible renal protective effect of hyperuricemia in patients with advanced CKD, which is similar to the result of a possible cardioprotective effect noted in the study by Latif et al. of chronic hemodialysis patients [18]. A 2016 study by Oh et al. evaluated SUA in living donor kidney transplant recipients. They found that SUA had an independent J-shaped association with the risk of loss of allograft kidney function [24].
Two retrospective studies, both by Hsieh et al., sought to evaluate how SUA is associated with peritonitis-related technique failure(26) and residual renal function (RRF) [27] in patients receiving continuous ambulatory peritoneal dialysis (CAPD). The first study evaluated the risk of technique failure and divided patients into two groups based on their baseline SUA (≤8.0 mg/dL and >8.0 mg/dL). They concluded that high SUA (>8.0 mg/dL) was independently associated with an increased risk of dialysis technique failure as compared to the normouricemic group (8.0 mg/dL) and that the elevated SUA group had a greater rate of peritonitis related failure [26]. The second study evaluated the association between SUA and RRF, an important prognostic indicator in CAPD. They concluded a U-shaped association between SUA and the rate of decline in RRF [27].
3.3. U-shaped association of serum uric and Neurological disease
One of the more recent areas of research regarding SUA has been in neurologic diseases and events (Table 1). A 2017 study by Kamei et al. [51] evaluated the association between SUA and nonfatal stroke. They used data from 155,322 Japanese individuals collected between 2008 and 2010 as part of the annual “Specific Health Check and Guidance in Japan.” They found that there was a strong independent association between elevated SUA and increased incidence of nonfatal stroke. They also noted that there was an increased risk of stroke seen in individuals with lesser SUA levels, suggesting a U-shaped association between SUA and the risk of nonfatal stroke [51].
Recently, the association between SUA and functional outcomes following an acute ischemic stroke (AIS) was evaluated in a recent 2018 study by Yang et al. [53]. They evaluated 710 Chinese adults following a first-time AIS to determine whether SUA was an independent risk factor of an unfavorable functional outcome three months following the AIS. When patients were stratified into deciles based on their SUA at the time of the AIS, there was a statistically significant U-shaped relationship between SUA and the risk of unfavorable outcomes in Chinese adults. This result was in agreement with a 2010 study by Seet et al. that found that both low (<280 μM; 4.7 mg/dL) and high SUA (>410 μM; 6.9 mg/dL) resulted in decreased functional outcomes in patients with AIS [46].
Research regarding a possible neuroprotective role of UA as it relates to dementia has been controversial. This was the focus of a study by Latourte et al. [54] in which they followed 1,598 study participants over a median follow-up of 10 years. The assessed the association between SUA and dementia, as well as brain aging markers noted on MRI. They found that the risk of dementia increased with increasing levels of SUA and that the association was strongest with vascular and mixed forms of dementia as compared to Alzheimer's. These results add more uncertainty as to the possible protective or degenerative role UA may play in dementia.
3.4. Potential role of U-Shape effect of uric acid in chronic obstructive pulmonary disease and pulmonary hypertension
With regard to lung diseases, the effect of UA on disease pathogenesis is not precisely apparent. UA has been found in respiratory secretions from the nose to the alveolus and is believed to play an important role in the protection of the respiratory epithelium from oxidants and inhaled toxins [65]. Many of the currently available studies have specifically examined the role of UA in chronic obstructive pulmonary disease (COPD), a disorder characterized by chronic inflammation [66] and associated with significant morbidity and mortality [[67], [68], [69]]. Also, an association of elevated serum uric acid (UA) in patients with pulmonary hypertension (PH) has been shown in 80% of adults and 60% of pediatric patients [[70], [71], [72], [73], [74]]. There are many potential mechanisms responsible for the elevated level of UA in cases with PH, some of which could be associated with disease pathogenesis (i.e. UA induced insulin resistance) [75]. Beside chronic renal disease which accounts for 10–15% of cases of PH with hyperuricemia [76], tissue hypoxia appears to be the most common reason for the high level of serum UA in patients with PH likely due to activation of xanthine oxidoreductase and increased purine metabolism with resultant uric acid production [77].
Studies have sought to evaluate SUA as a possible novel biomarker for respiratory disease and inflammation. Areas studied have included the relationship between SUA and acute exacerbations of COPD (AECOPD), global initiative for COPD (GOLD) criteria, and pulmonary function tests. Unfortunately, the results are limited and have been mainly inconclusive or contradictory.
In terms of an acute exacerbation of COPD (AECOPD), some studies have concluded that an elevated SUA at the time of admission to the hospital was associated with increased mortality at 30-days [78,79], 1-year [80], and overall [81]. However, other studies have concluded that there is no correlation between SUA and acute in-hospital [82] or 1-year mortality [78,81,83] following an AECOPD. In terms of morbidity, one study found no relationship between GOLD criteria and SUA but did find that patients with increased SUA had greater rates of hospitalization and antibiotic requirements [83]. Additionally, greater levels of SUA were associated with increased airflow limitations [84] and decreased FEV1 [85,86] on pulmonary function testing. Two studies evaluated the role of lower SUA in active smokers and concluded that lesser levels correlated with greater rates of COPD diagnosis and more severe disease [87,88].
These results indicate that both high and low levels of UA can be associated with worse morbidity and mortality in patients with COPD. While the idea of a U-shaped effect was not directly assessed in any of these studies, the results of the various study designs and inconsistencies between the results could likely be explained by this idea. We propose that future study designs focus on assessing the possible U-shaped effect of UA in pulmonary disease.
3.5. U-shaped association of serum uric acid and other disorders
While all-cause mortality, cardiovascular events and mortality, renal damage, and neurologic disease have been four of the most researched areas with regards to a U-shaped association of SUA, a handful of studies have evaluated this association in other areas as well (Table 1). A 2013 study by Huang et al. looked at SUA and sarcopenia in Japanese adult males. Their study evaluated how both low (<5.4 mg/dL) and high SUA (≥6.8 mg/dL) impacted the development of sarcopenia as measured by grip strength and leg extension power [48]. They concluded that there was an inverted J-shaped (U-shaped) association between SUA and both grip strength and leg extension power. Additionally, there was a statistically significant association between elevated SUA (≥6.8 mg/dL) and decreased grip strength even after adjustment for potential confounding factors such as age, BMI, diet, and comorbidities.
Two cross-sectional studies by Li et al. [49] and Wang et al. [52] sought to evaluate the relationship between SUA and fasting plasma glucose (FPG). Li et al. evaluated data from 100,348 adult individuals in China with no history of diabetes mellitus (DM) that were part of routine health screenings. Wang et al. evaluated 11,183 adult individuals in China. Both studies concluded a U-shaped relationship existed between FPG and SUA with Li et al. only looking at non-diabetic individuals and Wang et al. evaluating all individuals as a representation of the general population.
Lastly, two community-based cohort studies, both by Zhou et al., sought to assess the role of SUA on maternal blood pressure and fetal growth during pregnancy, respectively [55,56]. They noted that previous research had concluded a positive association between elevated SUA and the development of gestational hypertension, but no previous study has evaluated the impact of low SUA [55]. Data was collected from 1223 pregnant women who were part of the prospective Pregnancy Outcomes and Community Health (POUCH) study, which measured SUA during mid-pregnancy [89]. In regards to maternal blood pressure, they found that while there was a positive linear association between SUA and systolic blood pressure (SBP), both diastolic blood pressure (DBP) and mean arterial pressure (MAP) had a J-shaped relationship with SUA [55]. From these results, they concluded that a U-shaped association exists and that both high and low SUA are risk factors for the development of gestational hypertension [55]. When evaluating fetal growth during pregnancy, they used infant birth weight and gestational age to calculate gestation age-specific birthweight z-scores. They found that for infants who were small for gestational age (SGA), a U-shaped association existed and that both high and low SUA was associated with lower birth weight z-scores [56]. Among infants who were appropriate for gestational age (AGA), there was no significant association and for large for gestational age (LGA) infants, the relationship was linear [56].
4. Potential impact of U-Shaped association of serum uric acid on the outcome of uric acid lowering clinical trials
While the adverse clinical effects of frank hyperuricemia are well documented, newer research has begun supporting the idea of adverse effects occurring within the upper limits of the current “normal range.” This evidence has led to the development of an increasing number of pharmaceutical agents aimed at reducing SUA levels. In this section, we discuss many of the past clinical trials and their significance (Table 2, Table 3), as well as some of the ongoing clinical trials aimed at evaluating the safety and efficacy of these pharmaceutical agents (Table 4). Table 2, Table 3, Table 4 are arranged based on the date of publication and highlights many significant past and ongoing clinical trials.
Table 2.
Summary of clinical trials evaluating urate lowering therapies (ULTs).
| Year | Author | Trial | n | Population | Intervention (mg) | Duration | Measured Endpoints | Result | P-Value | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | Becker et al. | – | 153 | Gout | Febuxostat (40, 80, or 120) or Placebo | 28 days | SUA <6.0 mg/dL | Febuxostat was more effective than placebo | <0.001 | [106] |
| 2005 | Becker et al. | FACT | 762 | Gout | Febuxostat (80 or 120) or Allopurinol (300) | 52 weeks | SUA <6.0 mg/dL | Febuxostat was more effective than Allopurinol | <0.001 | [111] |
| 2006 | Siu et al. | – | 54 | CKD | Allopurinol 100–300 vs. usual therapy | 1 year | Reduced BP and slowing serum creatinine increase | No difference in systolic or diastolic blood pressure | N/S | [94] |
| Serum creatinine trended lesser with treatment. | N/S | |||||||||
| 2008 | Schumacher et al. | APEX | 1,072 | Gout | Febuxostat (80, 120, 240), Allopurinol (100 or 300), or Placebo | 28 weeks | SUA <6.0 mg/dL | At all doses, Febuxostat was more effective than Allopurinol or placebo | ≤0.05 | [112] |
| 2008 | Hare et al. | OPT-CHF | 405 | CHF | Oxypurinol 600 or Placebo | 24 weeks | Clinical Improvement of NYHA HF class | Oxypurinol did not produce clinical improvement. | N/S | [102] |
| 2009 | Schumacher et al. | FOCUS | 116 | Gout | Febuxostat [[40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], 115, [116], 117. (, [118], [119], [120]] | 5 years | SUA <6.0 mg/dL | Febuxostat resulted in the maintenance of SUA <6.0 mg/dL. | – | [107] |
| 2009 | Becker et al. | – | 1,086 | Gout | Febuxostat (80 or 120) or Allopurinol (300) | 31–40 months | SUA <6.0 mg/dL | Better goal SUA maintenance with Febuxostat than Allopurinol | – | [114] |
| 2010 | Becker et al. | CONFIRMS | 2,269 | Gout | Febuxostat (40 or 80) or Allopurinol (200–300) | 6 months | SUA <6.0 mg/dL | Greater dose (80 mg) Febuxostat was the most efficacious ULT. | <0.001 | [113] |
| 2010 | Momeni et al. | – | 40 | Diabetic Nephropathy | Allopurinol 100 vs. Placebo | 4 months | Reduction of proteinuria | 24-h protein was lesser in Allopurinol group | <0.049 | [97] |
| 2010 | Goicoechea et al. | – | 113 | CKD | Allopurinol 100 vs. continuation of usual therapy | 2 years | Renal disease progression, CV events, and hospitalizations. | Allopurinol decreased: | [91] | |
| Renal Disease Progression | 0.018 | |||||||||
| CV events | 0.039 | |||||||||
| Hospitalization risk | 0.033 | |||||||||
| 2011 | Kao et al. | – | 53 | CKD + LVH | Allopurinol 300 vs. Placebo | 9 months | Change in Left Ventricular Mass Index and Endothelial Function | Allopurinol reduced LVH | 0.036 | [100] |
| Allopurinol improved endothelial function | 0.009 | |||||||||
| 2011 | Kanbay et al. | – | 97 | Asymptomatic Hyperuricemia | Allopurinol 300 vs. No Treatment | 4 months | Endothelial Function and eGFR | Allopurinol Improved endothelial function | 0.003 | [95] |
| Allopurinol preserved renal function (eGFR) | 0.001 | |||||||||
| 2012 | European Medicines Agency | Studies C0405 and C0406 | 212 | Gout | Placebo x2wks Pegloticase 8 mg × 2wks Pegloticase 8 mg × 4wks |
6 months | SUA <6.0 mg/dL | Pegloticase has a dose-dependent response of lowering SUA | <0.001 | [133] |
| 2013 | Pai et al. | – | 183 | CKD | Allopurinol 100 vs. No Treatment | 2 years | CKD Progression | Allopurinol preserved renal function (eGFR) | 0.4* | [93] |
| 2014 | Hosoya et al. | – | 123 | CKD | Topiroxostat 160 vs. Placebo | 22 weeks | SUA and eGFR | Topiroxostat decreased SUA | <0.0001 | [134] |
| No change in eGFR | N/S | |||||||||
| 2015 | Goicoechea et al. | – | 107 | CKD | Allopurinol 100 vs. Placebo | 7 years | Renal and CV events | Reduction of Renal Events | 0.004 | [92] |
| Reduction of CV Events | 0.02 | |||||||||
| 2015 | Sircar et al. | – | 93 | CKD | Febuxostat 40 vs. Placebo | 6 months | eGFR | Febuxostat slowed eGFR decline | 0.05 | [108] |
| 2015 | Givertz et al. | EXACT-HF | 253 | Hyperuricemia + H.F. | Allopurinol 60 vs. Placebo | 24 weeks | Change in clinical status | No significant change in the composite clinical endpoints | N/S | [101] |
| 2016 | Xiao et al. | – | 125 | Normouricemic patients with chronic HF. | Allopurinol 300 vs. Control | 6 months | Cardiac Function | Allopurinol improved parameters of cardiac function | <0.01 | [99] |
| 2016 | Weng et al. | – | 2,460 | CKD | • Febuxostat “ab initio” (n = 40) • Other ULT to Febuxostat (n = 206) • Other ULT (n = 2,214) |
1 year | eGFR | Febuxostat preserved renal function (eGFR) | <0.001 | [109] |
| 2016 | Saag et al. | CLEAR 1 | 603 | Gout | Lesinurad 200/400 + Allopurinol vs. Placebo + Allopurinol | 12 months | SUA <6.0 mg/dL at 6 months | Combination of Lesinurad + Allopurinol was more efficacious | <0.0001 | [128] |
| 2016 | Bardin et al. | CLEAR 2 | 610 | Gout | Lesinurad 200/400 + Allopurinol vs. Placebo + Allopurinol | 12 months | SUA <6.0 mg/dL at 6 months | Combination of Lesinurad + Allopurinol was more efficacious | <0.001 | [129] |
| 2017 | Krishnamurthy et al. | – | 100 | Hyperuricemic Males | Allopurinol 200 vs. No Treatment | 3.4 years | eGFR | Allopurinol preserved renal function (eGFR) | <0.01 | [96] |
| 2017 | Jalal et al. | – | 80 | CKD | Allopurinol 300 vs. Placebo | 12 weeks | Endothelial Function | Allopurinol did not improve endothelial function | N/S | [98] |
| 2017 | Stamp et al. | – | 183 | Gout | Constant dose (269 mg/day)of Allopurinol (n = 93) or Allopurinol dose-escalation (n = 90) | 12 months | Reduction in SUA and TEAE | Dose-escalation was more efficacious in lowering SUA | <0.001 | [105] |
| 2017 | Tausche et al. | LIGHT | 214 | Gout | Lesinurad 400 vs. Placebo | 6 months | SUA <6.0 mg/dL | Lesinurad was more effective than placebo | <0.0001 | [130] |
| 2017 | Dalbeth et al. | CRYSTAL | 324 | Gout | Lesinurad 200/400 + Febuxostat vs. Placebo + Febuxostat | 12 months | SUA <5.0 mg/dL at month 6 | Combination of Lesinurad 400 + Febuxostat was most efficacious | <0.0001 | [131] |
| 2018 | Kimura et al. | FEATHER | 443 | CKD | Febuxostat dose escala tion to 40 mg/day vs. Placebo | 108 weeks | eGFR | No difference in eGFR decline | N/S | [110] |
| 2018 | Coburn et al. | – | 12,856 | Gout | Current dose of Allopurinol (n = 6,428) or Allopurinol dose-escalation (n = 6,428) | 10 years | All-Cause Mortality | Increase mortality associated with Allopurinol dose-escalation | HR 1.08+ | [104] |
| 2018 | Wada et al. | UPWARD | 65 | Hyperuricemia and Diabetic Nephropathy | Topiroxostat 160 vs. Placebo | 28 weeks | Change in urinary Albumin:Creatinine and eGFR. | Topiroxostat preserved renal function (eGFR). | 0.0303 | [135] |
| No change in urinary Albumin: Creatinine | N/S | |||||||||
| 2018 | White et al. | CARES | 6,190 | Gout + CVD | Allopurinol (200–600) vs. Febuxostat [[40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80]] | 32 months | First occurrence of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or urgent revascularization for unstable angina | Increase mortality and Cardiovascular events associated with Febuxostat | 0.047 | [116] |
| Nonfatal Myocardial Infarction | N/S | |||||||||
| Nonfatal Stroke | ||||||||||
| Urgent Revascularization for Unstable Angina | ||||||||||
| 2020 | MacDonald et al. | FAST | 6,142 | Hyperuricemia, with ≥1 additional CV risk factor | Febuxostat [[80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], 115, [116], 117. (, [118], [119], [120]] vs. Allopurinol (100–900) | 36 months | First occurrence of the Anti-Platelet Trialists’ Collaboration (APTC) cardiovascular endpoint of non-fatal myocardial infarction, non-fatal stroke or cardiovascular death | Febuxostat is non-inferior to allopurinol therapy with respect to the primary cardiovascular endpoint, and its long-term use is not associated with an increased risk of death or serious adverse events compared with allopurinol. | <0·0001 | [118] |
| 2019 | Kojima et al. | FREED | 1,070 | Hyperuricemia, with ≥1 additional CV risk factor | Febuxostat 10–40 vs. Allopurinol 100 (If SUA elevated) | 36 months | Development of cerebral or cardiorenovascular events and all deaths | Febuxostat lowers uric acid and delays the progression of renal dysfunction. | 0.041 | [122] |
*P-value indicates no significant change from baseline, indicating no disease progression.
+Study calculated hazard ratios (HR) instead of p-values.
TEAE: Treatment-Emergent Adverse Event; N/S: Non-Significant; NYHA: Ney York Heart Association; LVH: Left Ventricular Hypertrophy; ALT: Alanine Aminotransferase.
Table 3.
Treatment-Emergent Adverse Events (TEAE).
| Year | Study | Study Group (mg) | Serious TEAE | Cardiovascular Specific Events | Fatal Events | Ref |
|---|---|---|---|---|---|---|
| 2005 | Becker et al. | Placebo | 0% | – | 0% | [106] |
| Febuxostat | 3% (3/115) | – | 0% | |||
| 2005 | Becker et al. (FACT) | Allopurinol | 8% (19/253) | – | 0% | [111] |
| Febuxostat [80] | 4% (11/256) | – | <1% (2/256) | |||
| Febuxostat [120] | 8% (21/251) | – | <1% (2/251) | |||
| 2008 | Schumacher et al. | Placebo | 1% (2/134) | <1% (1/134) | 0% | [112] |
| Allopurinol | 4% (11/267) | 2% (5/267) | 0% | |||
| Febuxostat [80] | 3% (9/269) | 2% (5/269) | 0% | |||
| Febuxostat [120] | 4% (5/134) | <1% (1/134) | 0% | |||
| Febuxostat (240) | 3% (7/268) | <1% (1/268) | 0% | |||
| 2008 | Hare et al. (OPT-CHF) | Placebo | – | 2% (4/202)+ | 3% (6/202) | [102] |
| Oxypurinol (600) | – | 4% (8/203)+ | 5% (10/203) | |||
| 2009 | Schumacher et al. (FOCUS) | Febuxostat (40–120) | 18% (21/116) | 5% (6/116) | 0% | [107] |
| 2009 | Becker et al. | Allopurinol (300) | 21 events (12 per patient-year) | 5 events (3 per patient-year) | 0% | [114] |
| Febuxostat [80] | 165 events (11 per patient-year) | 46 events (3 per patient-year) | 7 (<1 per patient-year) | |||
| Febuxostat [120] | 73 events (9 per patient-year) | 17 events (2 per patient-year) | 3 (<1 per patient-year) | |||
| 2010 | Becker et al. (CONFIRMS) | Allopurinol (200/300) | 4.1% (31/756) | 0.4% (3/756) | 0.26% (2/756) | [113] |
| Febuxostat [40] | 2.5% (19/757) | 0% | 0% | |||
| Febuxostat [80] | 3.7% (28/756) | 0.4% (3/756) | 0% | |||
| 2010 | Goicoechea et al. | Allopurinol | 0% | 27% (15/56) | 4% (2/56) | [91] |
| Usual Treatment | 0% | 12% (7/57) | 0% | |||
| 2011 | Kao et al. | Placebo | 12% (3/26) | – | 0% | [100] |
| Allopurinol (300) | 11% (3/37) | – | 0% | |||
| 2012 | European Medicines Agency | Placebo | 11.6% (5/43) | 0% | 2.3% (1/43) | [133] |
| Pegloticase 8 mg every 4wks | 22.6% (19/84) | 3.6% (3/84) | 2.4% (2/84) | |||
| Pegloticase 8 mg every 2wks | 23.5% (20/85) | 4.7% (4/85) | 3.5% (3/85) | |||
| 2014 | Hosoya et al. | Placebo | 3% (2/60) | – | 0% | [134] |
| Topiroxostat [160] | 3% (2/62) | – | 0% | |||
| 2015 | Goicoechea et al. | Placebo | 43% (24/56)++ | 28% (16/57) | 0% | [92] |
| Allopurinol [100] | 16% (9/57)++ | 41% (23/56) | 0% | |||
| 2015 | Sircar et al. | Placebo | 0% | 0% | 0% | [108] |
| Febuxostat [40] | 0% | 0% | 0% | |||
| 2015 | Givertz et al. (EXACT-HF) | Placebo | 15% (19/125) | 2% (2/125) | 6% (7/125) | [101] |
| Allopurinol [60] | 20% (25/128) | 2% (2/128) | 6% (8/128) | |||
| 2016 | Xiao et al. | Control | 0% | 3.2% (2/63) | 1.6% (1/63) | [99] |
| Allopurinol (300) | 1.6% (1/62) | 8.1% (5/62) | 0% | |||
| 2016 | Saag et al. (CLEAR 1) | Placebo + Allopurinol | 5.5% (11/201) | 3.5% (7/201)+++ | 0% | [128] |
| Lesinurad (200) + Allopurinol | 4.5% (9/201) | 4.5% (9/201)+++ | 0.5% (1/201) | |||
| Lesinurad (400) + Allopurinol | 8% (16/201) | 4.0% (8/201)+++ | 0% | |||
| 2016 | Bardin et al. (CLEAR 2) | Placebo + Allopurinol | 3.9% (8/206) | 5.3% (11/206)+++ | 0% | [129] |
| Lesinurad (200) + Allopurinol | 4.4% (9/204) | 3.9% (8/204)+++ | 0% | |||
| Lesinurad (400) + Allopurinol | 9.5% (19/200) | 3.0% (6/200)+++ | 1% (2/200) | |||
| 2017 | Jalal et al. | Placebo | 0%^ | 0% | 0% | [98] |
| Allopurinol (300) | 0%^ | 2.6% (1/39) | 2.6% (1/39) | |||
| 2017 | Stamp et al. | Current Dose of Allopurinol | 27% (25/93) | 9% (8/93) | 5.4% (5/93) | [105] |
| Allopurinol Dose-Escalation | 24% (22/90) | 12% (11/90) | 5.6 (5/90) | |||
| 2017 | Tausche et al. (LIGHT) | Placebo | 3.7% (4/107) | 0.9% (1/107) | 0% | [130] |
| Lesinurad (400) | 8.4% (9/107) | 0.9% (1/107) | 0.9% (1/107) | |||
| 2017 | Dalbeth et al. (CRYSTAL) | Placebo + Febuxostat | 9.2% (10/109) | 1.8% (2/109) | 0% | [131] |
| Lesinurad (200) +Febuxostat | 5.7% (6/106) | 5.7% (6/106) | 0.9% (1/106) | |||
| Lesinurad (400) +Febuxostat | 8.3% (9/109) | 3.7% (4/109) | 0.9% (1/109) | |||
| 2018 | Kimura et al. (FEATHER) | Placebo | 16.7% (37/222) | 3.2% (7/222) | 0.5% (1/222) | [110] |
| Febuxostat | 21.9% (48/219) | 1.8% (4/219) | 0.5% (1/219) | |||
| 2018 | Coburn et al. | Current Dose of Allopurinol | – | 18.1 per 1,000 person-years+ | 38.7 per 1,000 person-years | [104] |
| Allopurinol Dose-Escalation | – | 19.8 per 1,000 person-years+ | 42.5 per 1,000 person-years | |||
| 2018 | Wada et al. (UPWARD) | Placebo | 9.1% (2/22) | – | 0% | [135] |
| Topiroxostat [160] | 7.0% (3/43) | 0% | ||||
| 2018 | White et al. (CARES) | Allopurinol (200–600) | 10.4% (321/3092) | 3.2% (100/3092)^^ | 6.4% (199/3092) | [116] |
| Febuxostat [[40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80]] | 10.8% (335/3098) | 4.3% (134/3098)^^ | 7.8% (243/3098) |
TEAE: Treatment-Emergent Adverse Event.
-Not reported by the study.
+Cardiovascular mortality reported.
++ Serious renal adverse events reported+++Includes both MACE and non-MACE (Major Adverse Cardiovascular Events).
^Total TEAEs (Study did not specify serious TEAEs).
^^Specifically Rate of Cardiovascular Death.
Table 4.
Summary of Ongoing Clinical Trials.
| Trial | Author | n | Population | Intervention (mg) | Duration | Measured Endpoints | Result | Ref |
|---|---|---|---|---|---|---|---|---|
| ALL-HEART | Mackenzie et al. | 5,215 | Ischemic Heart Disease | Allopurinol up to 600 mg daily vs. No treatment | 4 years | Composite of non-fatal myocardial infarction, non-fatal stroke or cardiovascular death. | Patient recruitment began in February 2014 with ongoing data collection and follow-up | [103] |
| LEAF-CHF | Yokota et al. | 200 | Chronic HF with Hyperuricemia | Febuxostat 60 mg vs. Control | 24 weeks | Change in plasma BNP levels. | Results Pending with ongoing data collection and follow-up | [123] |
Historically, the first-line medication used for the treatment of hyperuricemia has been Allopurinol, a purine-like analog that acts through inhibition of the enzyme xanthine oxidase (XO) [90]. It has been widely studied in many different patient populations and evaluated for safety and clinical efficacy. Multiple studies have sought to evaluate the use of Allopurinol in patients with CKD and CVD and whether it can be used to improve renal and cardiovascular parameters. In patients with CKD stages 3–4, some studies have shown a potential renal protective effect through reduced disease progression as measured by serum creatinine and eGFR [[91], [92], [93]]. On the other hand, another study failed to show any significant change in serum creatinine [94]. In two studies of patients with hyperuricemia but no history of CKD, Allopurinol treatment was shown to improve eGFR [95] and decrease serum creatinine levels [96]. In patients with diabetic nephropathy, treatment with Allopurinol was shown to reduce levels of 24-h urine protein [97].
In terms of CVD, Allopurinol has been hypothesized to have protective effects through improvement of vascular endothelial function. Multiple studies have attempted to evaluate this by looking at blood pressure, flow-mediated vasodilation, Ejection Fraction (EF), and other parameters, with mixed results. Some studies have shown improved blood pressure control in hyperuricemic patients both with [93] and without CKD [95]. Conversely, another study in patients with CKD concluded no significant change in systolic or diastolic blood pressure [94] and another found no change in endothelial function as measured by flow-mediated dilation [98]. In patients with Heart Failure (HF), two studies concluded that Allopurinol use improved cardiac function [99,100], while the results of the EXACT-HF and OPT-CHF studies found no statistically significant changes with Allopurinol use [101,102]. A large ongoing multicenter prospective study, known as the ALL-HEART study, is being conducted to evaluate Allopurinol use in patients over the age of 60 with a history of ischemic heart disease (IHD). The primary measured outcome is a composite of non-fatal myocardial infarction, non-fatal stroke, or cardiovascular death. Secondary outcomes include all-cause mortality, quality of life and cost-effectiveness of allopurinol [103].
A recently published large propensity-matched cohort study evaluated whether there is a dose-related protective effect with Allopurinol use [104]. The study used a 10-year observational, active-comparator design in which they evaluated patients who received care through the Veterans Health Administration (VHA) between 1999 and 2010. They reviewed the records of 12,856 individuals diagnosed with gout and who were receiving Allopurinol for treatment. They found that there was no association between dose escalation and improved survival, and that dose escalation was associated with a small (<10%) increase in all-cause mortality [104] (Table 3). The authors note that the results are limited due to the suboptimal dosing that was noted among all patients including dose-escalators. A much smaller prospective study (n = 183) also evaluated Allopurinol dose escalation and found that dose-escalation resulted in significantly more patients achieving a goal SUA of <6.0 mg/dL [105]. The study found no difference in mortality and only a slight difference in the rate of cardiovascular events, with 9% occurring in the control group and 12% in the Allopurinol group (Table 3).
While Allopurinol is still the most widely used medication for the management of hyperuricemia, several newer medications have been developed over the past decade. These additional pharmaceutical agents have allowed for the development of combination therapies resulting in greater and faster reductions in SUA. Approved for use by the Federal Drug Administration (FDA) in 2009, Febuxostat has become a widely prescribed xanthine-oxidase inhibitor (XOI), with the American College of Rheumatology (ACR) even initially recommending it as a first-line treatment for the management of gout along with Allopurinol [90]. In two placebo-controlled trials, Febuxostat was shown to decrease SUA more effectively than the placebo [106], a result maintained through the 5-year follow-up study [107]. Additional studies have evaluated Febuxostat's use in patients with CKD and whether it has any renal protective effects. Two studies, one 6 months in duration and the other one 12 months found that treatment with Febuxostat in patients with CKD slowed eGFR decline [108,109]. A third study of 108 weeks duration found no change in eGFR with Febuxostat treatment [110].
Since the development of non-purine analogs such as Febuxostat, there have been numerous studies comparing their efficacy to that of Allopurinol [[111], [112], [113], [114]]. The results of these studies have indicated that Febuxostat is equal to, if not more efficacious than, Allopurinol at lowering SUA at comparable doses, which is consistent even in patients with mild-moderate renal impairment [112,113]. Following these initial phase II and III clinical trials, it was noted that in the Febuxostat treatment groups, there was a greater rate of all-cause mortality, cardiovascular mortality, and cardiovascular thromboembolic events [90]. These results prompted the FDA to request additional clinically controlled safety data, which came in the form of the 2010 CONFIRMS trial, a 6-month study of 2,269 patients with gout and hyperuricemia [113]. CONFIRMS again demonstrated the clinical efficacy of Febuxostat at lowering SUA as compared to Allopurinol and also showed no statistically significant difference in all-cause mortality, cardiovascular events, or cardiovascular mortality between the treatment groups [113].
As a result of the previous clinical trials and pending its approval in 2009, the FDA required that Febuxostat's manufacturer conduct a post-marketing “randomized controlled trial of adequate size and duration to determine whether the use of Uloric (Febuxostat) is associated with a moderate increase in the risk of serious adverse cardiovascular outcomes as compared to Allopurinol” [115]. The resulting study became known as CARES (Cardiovascular Safety of Febuxostat and Allopurinol in Participants with Gout and Cardiovascular Comorbidities), a multicenter, double-blinded, noninferiority trial that evaluated 6,190 individuals with gout and known CVD. The study compared Febuxostat to Allopurinol with a primary composite endpoint of major adverse cardiac events (MACE). The CARES trial results were published in March 2018 and showed that while there was no significant difference in the primary end-point of MACEs, the rates of all-cause mortality and cardiovascular mortality were greater with Febuxostat than Allopurinol [116]. This result was significant because of its potential clinical implications and even prompted the FDA to issue a safety alert regarding Febuxostat [117]. As a result of the CARES trial, there was much anticipation for another prospective large RCT evaluating Febuxostat versus Allopurinol in patients with known CV risk factors (FAST trial) [118] and more clinical trials that were focused on the cardiovascular safety of XOIs [[119], [120], [121], [122], [123]]. However, the conclusions of these trials were not unanimous. Specifically, the conclusions of the FAST study differ from those of CARES with regard to secondary endpoints, despite similar trial sizes. While CARES showed an excess of cardiovascular death and all-cause mortality during the on-treatment [116]. This excess was not observed in FAST(118). The reasons for the differences between the two results may include: A) the baseline characteristics of the two trials were different, including the proportion of patients with established cardiovascular disease at baseline, the severity of cardiovascular disease, the severity of gout, B) the doses of study medication were different, C) the proportion of patients discontinued treatments, and the loss rate of follow-up of CARES was much greater than that of FAST, so the bias of CARES was greater than that of FAST. While the results of FAST study appear to be robust and reassuring in regards of cardiovascular tolerance, it does not allow firm conclusions to be drawn about patients with severe cardiovascular disease [124].
A recent systematic review and meta-analysis of randomized control trials of xanthine oxidase inhibitors was published in February 2018 [125]. The review evaluated randomized control trials (RCTs) of purine-like drugs such as Allopurinol and non-purine drugs such as Febuxostat. Outcomes evaluated were MACE, mortality, total cardiac events (TCE), and CV specific events. Reviewed RCTs were published in PubMed, EMBASE, Web of Science, Cochrane Central, and Lilacs prior to December 2016. In total, they included 81 articles which accounted for 10,684 patients and 6434 patient-years [125]. They concluded that treatment with Allopurinol protected myocardial infarction, hypertension, TCE, and serious TCE. On meta-regression, it was noted that increasing doses of Allopurinol were associated with an increased risk of TCE, especially at doses exceeding 300 mg/day, possibly indicating a loss of CV protection [125]. There was no significant increased or decreased risk of CV events noted with Febuxostat.
Lesinurad is a selective uric acid reuptake inhibitor that acts through inhibition of the uric acid transporter 1 (URAT1) [126]. It has been approved for use at a dose of 200 mg in combination with an XOI such as Allopurinol or Febuxostat [127]. It is not approved for use as a monotherapy or at doses greater than 200 mg due to increases in treatment-emergent adverse events (TEAE) and cardiovascular events (Table 3) [[128], [129], [130], [131]]. Pegloticase is a newer class of medication that acts as a recombinant uricase enzyme, allowing for the conversion of uric acid to the more soluble allantoin. Pegloticase is typically reserved for refractory cases of hyperuricemia due to a dose-dependent increase in Treatment-Emergent Adverse Events (TEAEs) including hemolytic anemia and cardiovascular events (Table 3) [132,133].
5. What are the mechanisms that might link the U-shape association of serum UA levels to cardiovascular comorbidities?
The mechanism underlying the U-shaped association between plasma UA levels and cardiovascular events remains unknown. The previously asked questions and uncertainties regarding the differential physiologic vs the pathogenic role of plasma UA and XOR in vascular function are still unanswered and valid [136]. In this section, we will only focus on the potential mechanisms that could link hyperuricemia and uric acid lowering treatment (ULT) to cardiovascular comorbidities. These mechanisms could also explain some of the injurious effects we see in other organ systems.
-
A)
Mechanisms of hyperuricemia induced vascular inflammation and remodeling.
UA has been found to activate inflammatory signaling pathways in vascular smooth muscle cells including the stimulation of MCP-1, COX-2, and the ERK and p38 MAP Kinases involved in their activation [137]. In hyperuricemic animal models, an elevated level of UA is associated with arteriolosclerotic vascular disease, characterized by vascular medial wall thickening, vascular smooth muscle cell proliferation, and luminal narrowing [138,139]. UA has been proposed to directly contribute to both essential hypertension and pulmonary hypertension [136,140]. The principal arguments surrounding UA as a risk factor related to the diverse effects of UA on oxidative stress, endothelial dysfunction, activation of the renin-angiotensin system, and restriction of NO bioavailability. Furthermore, modulating hyperuricemia with the XOR inhibitor allopurinol improves vascular parameters in hypertensive adolescents [141]. Mechanistically, UA stimulates endothelin-1 expression associated with NADPH oxidase, decreases endothelial NO production, increases arginase production, inhibits l-arginine transport, and can directly inactivate NO along with other pro-inflammatory process with potential vascular toxicity [142,143]. UA has also been found to decrease endothelial nitric oxide synthase (eNOS/NOS3) activity(144) (Fig. 3). This may be of great significance for the development of hypertension since genome-wide association studies (GWAS) have identified the eNOS down-regulating SNP rs3918226 as a highly significant (p = 2.58.10−13) risk factor for the development of hypertension(145).
-
B)
Potential mechanisms involved in Urate Lowering Treatment (ULT) induced cardiovascular injury.
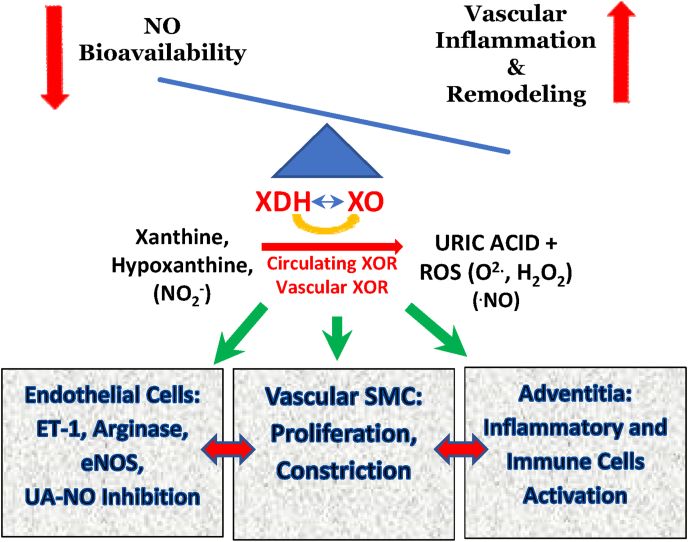
Fig. 3.
XOR is a major source of both uric acid and reactive oxygen species. Upregulation of circulating and vascular XOR result in increase in both systemic and locally derived ROS and UA which their imbalance has a role in perpetuating the inflammatory microenvironment seen in both systemic and pulmonary hypertension. ET-1, endothelin-1; eNOS, endothelial nitric oxide synthase; ROS, reactive oxygen species; SMC, smooth muscle cell; XDH, xanthine dehydrogenase; XO, xanthine oxidase.
Despite above observations regarding the detrimental effects of hyperuricemia on vascular inflammation, recent reports including the potential U-shaped association of plasma XOR activity with low LVEF and elevated BNP, independent of serum uric acid level [146] has only added more questions to our current concerns regarding the causality of uric acid in vascular pathology [[147], [148], [149], [150], [151], [152]]. Both Xanthine oxidoreductase and excess soluble urate (UA), the joint targets of XOR inhibitors (XOI) treatment, can exert noxious effects in the vasculature and other tissues [136,153,154]. Hence, the CARES study leaves us with more questions than answers concerning XOI effects on CV mortality [116,155]. One of these questions is how inhibition of XOR enzyme activity could affect the disease progression independent of UA level.
One potential mechanism is the differential effects of uric acid and XOR in the modulation of the redox status of the vascular system. UA is derived exclusively from the oxidation of xanthine and hypoxanthine by XOR [1]. XOR has wide tissue expression, can be released into the circulation, and binds the surface of endothelial cells [153,156]. XOR is up-regulated in animal models of inflammatory disease where it shows particularly high levels of activity and UA generation in inflammatory mononuclear phagocytes (MNP), including monocytes, macrophages, and dendritic cells(157,158). XOR, which catalyzes the terminal two steps of purine degradation, is the major source of both reactive oxygen species (ROS; O and H2O2) and UA ROS can have deleterious effects on biological processes and contribute to many pathological conditions such as inflammation, vascular injury, diabetes and hypertension. However, some ROS like H2O2 may act as a signaling molecule in many physiological processes. H2O2 is generated by mitochondria, some oxidases and also the metabolism of superoxide by superoxide dismutase (SOD). At low levels it contributes to signaling by oxidizing thiols such as glutathione (GSH) and thioredoxins and at greater levels it will develop toxic and highly reactive species including hydroxyl radicals(159). Although the exact role of H2O2 signaling in the cardiovascular system remains to be determined, there is evidence suggesting that H2O2 plays a significant role in vasoregulation under both physiological and pathological conditions(159). H2O2 is recognized as an endothelium derived hyperpolarizing factor (EDHF) with opposing vasoactive effects depending on its concentration, the vascular bed and redox status. At low concentrations (≤10 uM), endothelial cells are the primary cells involved in H2O2 induced arteriolar dilatation. The major signaling pathway involved in this responsdste is the COX1 – PGE2 axis and subsequent activation of smooth muscle cells (SMC). At greater concentrations of H2O2 (≥30 uM), the role of endothelial cells is diminished and replaced by direct effects of peroxide on SMCs through increase in K+ conductance(159). While H2O2 has its physiologic vasodilatory effects [160], this role will be more critical in pathologic conditions where nitrite-NO pathway is inefficient in maintaining the blood pressure (BP) (e.g. chronic hyperuricemia with vascular comorbidities) [161].
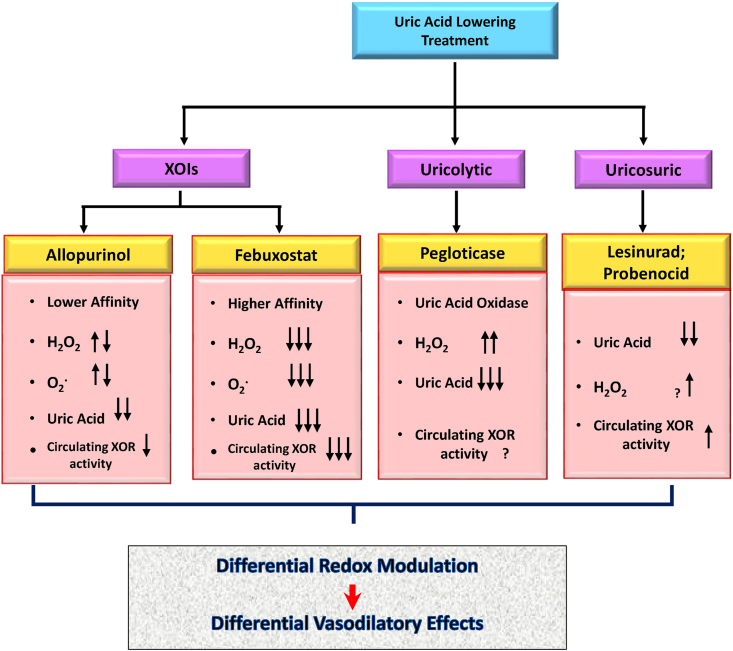
While XOIs can directly affect UA level, they can also differentially modify superoxide generation by the enzyme, and subsequently modulate oxidative stress and alter nitric oxide-redox balance [116,155]. This may explain the differential clinical outcome of using greater doeses of XOIs as well as different outcome when patients gout populations treated with XOIs has different baseline vascular comorbidities (CARES vs FAST trial). Uricosuric (e.g., Lesinurad) and uric acid oxidase (Pegloticase) can also modulate vascular redox status by potentially modulating XOR activity and changes in uric acid metabolism and its metabolic byproduct including H2O2 [162] (Fig. 3, Fig. 4).
Fig. 4.
Differential redox modulation in ULTs. Different uric acid lowering treatments (ULTs) will result in differential redox modulation of the systemic and pulmonary vasculature. Xanthine Oxidoreductase Inhibitors (XOIs) will have differential inhibitory effects on XOR activity mainly due to their differential affinity for the enzyme and mechanisms of inhibition (Add reference EE). Urocolytics and Uricosuric on the other hand exert their effects through changes in UA metabolism rather than directly inhibiting the enzyme. As a result, differential redox modulation in ULTs can have differential downstream physiologic effects on vascular response in different pathological condition (e.g. hypoxia, shear stress and inflammation).
We would propose that the missing component in most previous studies appears to be the lack of measurement of serum XOR activity which makes it difficult to draw a more definitive conclusion. Some of the presumed effects of serum SUA modulation could be explained through changes in XOR activity rather than changes in serum UA(163).
6. Next steps in understanding the role of U-shape effect of UA and XOR in vascular disease
The majority of previous animal studies on UA metabolism have been performed in preclinical models that do not recapitulate the observed pathology in humans. Hyperuricemia occurs mainly in higher primates, including humans, primarily due to the inactivation of the uricase gene during primate evolution [164,165]. There have been many challenges in generating animal models of hyperuricemia and current models do not yet reliably and consistently simulate the urate mediated hyperuricemia that occurs in humans [166]. Because pharmacological inhibitors of XOR decrease both UA and XOR activity, it has not been possible to determine their independent roles in inflammatory disease or to determine the potential therapeutic value of targeting them independently. Further, it has not been possible to critically measure the effects of newly discovered uricosurics (e.g., verinurad) in combination with other urate-lowering treatments in different clinical settings in vivo due to the low affinity of these drugs for rat and mouse URAT-1 [167] and due to lack of a reliable hyperuricemia model without functional uricase [166]. Future clinically relevant in vivo experiments should involve the use of humanized hyperuricemia models as well as conditional transgenic models of XOR which could help determine the differential role of UA and XOR activity in vascular biology.
7. Conclusion
Future guidelines should consider treating hyperuricemic patients based on their gender and associated comorbidities and to reduce the UA as a percentage of their baseline UA level rather than a fixed upper and lower threshold. As such, we agree with the previous EULAR recommendation of avoiding the maintenance of very low UA levels for chronic hyperuricemia management and further recommend an evidence-based and personalized approach for defining the correct therapeutic threshold with less potential cardiovascular toxicity(168,169).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgment
This work was supported by the National Institute of Health [5P01HL152961-02]; and the department of defense [DoD-W81XWH-14-1-0451, PR181125, PR191774].
Abbreviations
- ACR
American College of Rheumatology
- AECOPD, Ac
ute
- Exacerbation of COPD
- AIS
Acute Ischemic Stroke
- AK
, Acute Kidney Injury
- ATN
Acute Tubular Necrosis
- Ba-PWV
Brachial-ankle Pulse Wave Velocity
- BMI, Body
Mass Index
- CAPD
Continuous Ambulatory Peritoneal Dialysis
- CHF
, Congestive Heart Failure
- CKD
Chronic Kidney Disease
- COPD
, Chronic Pulmonary Chronic Disease
- CV
, Cardiovascular
- DBP
Diastolic Blood Pressure
- ESRD
End-Stage Renal Disease
- FDA
Federal Drug Administration GFR, Glomerular Filtration Rate
- FPG
Fasting Plasma Glucose
- GLUT9
, Glucose Transporter 9
- GNRI
, Geriatric Nutritional Risk Index
- IE
, Infective Endocarditis
- MACE
, Major Adverse Cardiac Events
- MetS
Metabolic Syndrome
- MPA
Mean Arterial Pressure
- LVEF
, Left Ventricular Ejection Fraction
- MNP
Mononuclear Phagocytes
- POUCH
Prospective Pregnancy Outcomes and Community Health
- RCTs
, Randomized Control Trials
- SBP
, Systolic Blood Pressure
- SGA
, Small for Gestational Age
- SUA
, Serum Uric Acid
- TCE
, Total Cardiac Events
- TEAE
, Treatment Emergent Adverse Events UA, Uric Acid
- ULT
, Urate L
- owering Treatments
- URAT1, Urate Transporter 1
- VHA
Veteran Health Administration
- VSA
, Vasospastic Angina
- XOR
, Xanthine Oxidoreductase
- XOIs
XOR inhibitors
References
- 1.Hille R. Molybdenum-containing hydroxylases. Arch. Biochem. Biophys. 2005;433:107–116. doi: 10.1016/j.abb.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat. Rev. Rheumatol. 2010;6:30–38. doi: 10.1038/nrrheum.2009.236. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y., Pandya B.J., Choi H.K. Prevalence of gout and hyperuricemia in the US general population: the national health and nutrition examination survey 2007-2008. Arthritis Rheum. 2011;63:3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 4.Deng Z., Gu Y., Hou X., Zhang L., Bao Y., Hu C., Jia W. Association between uric acid, cancer incidence and mortality in patients with type 2 diabetes: shanghai diabetes registry study. Diab. metab. res. rev. 2016;32:325–332. doi: 10.1002/dmrr.2724. [DOI] [PubMed] [Google Scholar]
- 5.Kodama S., Saito K., Yachi Y., Asumi M., Sugawara A., Totsuka K., Saito A., Sone H. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32:1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q., Yang Z., Lu B., Wen J., Ye Z., Chen L., He M., Tao X., Zhang W., Huang Y., Zhang Z., Qu S., Hu R. Serum uric acid level and its association with metabolic syndrome and carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc. Diabetol. 2011;10:72. doi: 10.1186/1475-2840-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee Y.J., Cho S., Kim S.R. A possible role of serum uric acid as a marker of metabolic syndrome. Intern. Med. J. 2014;44:1210–1216. doi: 10.1111/imj.12588. [DOI] [PubMed] [Google Scholar]
- 8.Lu W., Song K., Wang Y., Zhang Q., Li W., Jiao H., Wang G., Huang G. Relationship between serum uric acid and metabolic syndrome: an analysis by structural equation modeling. J. clinic. lipidol. 2012;6:159–167. doi: 10.1016/j.jacl.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Fini M.A., Elias A., Johnson R.J., Wright R.M. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin. Transl. Med. 2012;1:16. doi: 10.1186/2001-1326-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fini M.A., Lanaspa M.A., Gaucher E.A., Boutwell B., Nakagawa T., Wright R.M., Sanchez-Lozada L.G., Andrews P., Stenmark K.R., Johnson R.J. Brief report: the uricase mutation in humans increases our risk for cancer growth. Cancer Metabol. 2021;9:32. doi: 10.1186/s40170-021-00268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borghi C., Rosei E.A., Bardin T., Dawson J., Dominiczak A., Kielstein J.T., Manolis A.J., Perez-Ruiz F., Mancia G. Serum uric acid and the risk of cardiovascular and renal disease. J. Hypertens. 2015;33:1729–1741. doi: 10.1097/HJH.0000000000000701. discussion 1741. [DOI] [PubMed] [Google Scholar]
- 12.C. G.P., Young K.S., Michael L., K. C.H. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res. 2011;63:102–110. doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanbay M., Segal M., Afsar B., Kang D.H., Rodriguez-Iturbe B., Johnson R.J. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart. 2013;99:759–766. doi: 10.1136/heartjnl-2012-302535. [DOI] [PubMed] [Google Scholar]
- 14.Wang J., Qin T., Chen J., Li Y., Wang L., Huang H., Li J. Hyperuricemia and risk of incident hypertension: a systematic review and meta-analysis of observational studies. PLoS One. 2014;9:e114259. doi: 10.1371/journal.pone.0114259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson R.J. Why focus on uric acid? Curr. Med. Res. Opin. 2015;31(Suppl 2):3–7. doi: 10.1185/03007995.2015.1087979. [DOI] [PubMed] [Google Scholar]
- 16.Hsu S.P., Pai M.F., Peng Y.S., Chiang C.K., Ho T.I., Hung K.Y. Serum uric acid levels show a 'J-shaped' association with all-cause mortality in haemodialysis patients. Nephrol. Dial. Transplant. 2004;19:457–462. doi: 10.1093/ndt/gfg563. official publication of the European Dialysis and Transplant Association - European Renal Association. [DOI] [PubMed] [Google Scholar]
- 17.Mazza A., Zamboni S., Rizzato E., Pessina A.C., Tikhonoff V., Schiavon L., Casiglia E. Serum uric acid shows a J-shaped trend with coronary mortality in non-insulin-dependent diabetic elderly people. The CArdiovascular STudy in the ELderly (CASTEL) Acta Diabetol. 2007;44:99–105. doi: 10.1007/s00592-007-0249-3. [DOI] [PubMed] [Google Scholar]
- 18.Latif W., Karaboyas A., Tong L., Winchester J.F., Arrington C.J., Pisoni R.L., Marshall M.R., Kleophas W., Levin N.W., Sen A., Robinson B.M., Saran R. Uric acid levels and all-cause and cardiovascular mortality in the hemodialysis population. Clin. J. Am. Soc. Nephrol. : CJASN. 2011;6:2470–2477. doi: 10.2215/CJN.00670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lapsia V., Johnson R.J., Dass B., Shimada M., Kambhampati G., Ejaz N.I., Arif A.A., Ejaz A.A. Elevated uric acid increases the risk for acute kidney injury. Am. J. Med. 2012;125 doi: 10.1016/j.amjmed.2011.06.021. 302.e309-317. [DOI] [PubMed] [Google Scholar]
- 20.Feng S., Jiang L., Shi Y., Shen H., Shi X., Jin D., Zeng Y., Wang Z. Uric acid levels and all-cause mortality in peritoneal dialysis patients. Kidney Blood Pres. Res. 2013;37:181–189. doi: 10.1159/000350143. [DOI] [PubMed] [Google Scholar]
- 21.Dahle D.O., Jenssen T., Holdaas H., Leivestad T., Vardal M., Mjoen G., Reisaeter A.V., Toft I., Hartmann A. Uric acid has a J-shaped association with cardiovascular and all-cause mortality in kidney transplant recipients. Clin. Transplant. 2014;28:134–140. doi: 10.1111/ctr.12290. [DOI] [PubMed] [Google Scholar]
- 22.Kanda E., Muneyuki T., Kanno Y., Suwa K., Nakajima K. Uric acid level has a U-shaped association with loss of kidney function in healthy people: a prospective cohort study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bae E., Cho H.J., Shin N., Kim S.M., Yang S.H., Kim D.K., Kim Y.L., Kang S.W., Yang C.W., Kim N.H., Kim Y.S., Lee H. Lower serum uric acid level predicts mortality in dialysis patients. Medicine. 2016;95 doi: 10.1097/MD.0000000000003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh I.H., Kim J.E., Lee C.H., Kim G.H., Park J.S. A J-shaped association between serum uric acid level and allograft outcomes after living donor kidney transplantation. Artif. Organs. 2016;40:136–143. doi: 10.1111/aor.12519. [DOI] [PubMed] [Google Scholar]
- 25.Gwag H.B., Yang J.H., Park T.K., Song Y.B., Hahn J.Y., Choi J.H., Lee S.H., Gwon H.C., Choi S.H. Uric acid level has a U-shaped association with clinical outcomes in patients with vasospastic angina. J. Kor. Med. Sci. 2017;32:1275–1280. doi: 10.3346/jkms.2017.32.8.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh Y.-P., Chang C.-C., Kor C.-T., Yang Y., Wen Y.-K., Chiu P.-F., Lin C.-C. Relationship between uric acid and technique failure in patients on continuous ambulatory peritoneal dialysis: a long-term observational cohort study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2015-010816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh Y.P., Yang Y., Chang C.C., Kor C.T., Wen Y.K., Chiu P.F., Lin C.C. U-shaped relationship between uric acid and residual renal function decline in continuous ambulatory peritoneal dialysis patients. Nephrology. 2017;22:427–435. doi: 10.1111/nep.12613. [DOI] [PubMed] [Google Scholar]
- 28.Kang E., Hwang S.-s., Kim D.K., Oh K.-H., Joo K.W., Kim Y.S., Lee H. Sex-specific relationship of serum uric acid with all-cause mortality in adults with normal kidney function: an observational study. J. Rheumatol. 2017;44:380–387. doi: 10.3899/jrheum.160792. [DOI] [PubMed] [Google Scholar]
- 29.Lee H., Jung Y.H., Kwon Y.J., Park B. Uric acid level has a J-shaped association with arterial stiffness in Korean postmenopausal women. Kor. j. family med. 2017;38:333–337. doi: 10.4082/kjfm.2017.38.6.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsukuma Y., Masutani K., Tanaka S., Tsuchimoto A., Fujisaki K., Torisu K., Katafuchi R., Hirakata H., Tsuruya K., Kitazono T. A J-shaped association between serum uric acid levels and poor renal survival in female patients with IgA nephropathy. Hypertens. Res. : off. j. Jpn. Soci. Hyper. 2017;40:291–297. doi: 10.1038/hr.2016.134. [DOI] [PubMed] [Google Scholar]
- 31.Uedono H., Tsuda A., Ishimura E., Nakatani S., Kurajoh M., Mori K., Uchida J., Emoto M., Nakatani T., Inaba M. U-shaped relationship between serum uric acid levels and intrarenal hemodynamic parameters in healthy subjects. Am. J. Physiol. Ren. Physiol. 2017;312:F992–f997. doi: 10.1152/ajprenal.00645.2016. [DOI] [PubMed] [Google Scholar]
- 32.Cho S.K., Chang Y., Kim I., Ryu S., Hoboken N.J. Arthritis & rheumatology; 2018. U-Shaped Association between Serum Uric Acid Level and Risk of Mortality: A Cohort Study. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava A., Kaze A.D., McMullan C.J., Isakova T., Waikar S.S. Uric acid and the risks of kidney failure and death in individuals with CKD. Am. J. Kidney Dis. 2018;71:362–370. doi: 10.1053/j.ajkd.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tseng W.C., Chen Y.T., Ou S.M., Shih C.J., Tarng D.C., Taiwan geriatric kidney disease research, G. U-shaped association between serum uric acid levels with cardiovascular and all-cause mortality in the elderly: the role of malnourishment. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Son C.N., Kim J.M., Kim S.H., Cho S.K., Choi C.B., Sung Y.K., Kim T.H., Bae S.C., Yoo D.H., Jun J.B. Prevalence and possible causes of hypouricemia at a tertiary care hospital. Korean J. Intern. Med. (Engl. Ed.) 2016;31:971–976. doi: 10.3904/kjim.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichida K., Amaya Y., Okamoto K., Nishino T. Mutations associated with functional disorder of xanthine oxidoreductase and hereditary xanthinuria in humans. Int. J. Mol. Sci. 2012;13:15475–15495. doi: 10.3390/ijms131115475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bugdayci G., Balaban Y., Sahin O. Causes of hypouricemia among outpatients. Lab. Med. 2008;39:550–552. [Google Scholar]
- 38.Phay J.E., Hussain H.B., Moley J.F. Cloning and expression analysis of a novel member of the facilitative glucose transporter family, SLC2A9 (GLUT9) Genomics. 2000;66:217–220. doi: 10.1006/geno.2000.6195. [DOI] [PubMed] [Google Scholar]
- 39.Komoda F., Sekine T., Inatomi J., Enomoto A., Endou H., Ota T., Matsuyama T., Ogata T., Ikeda M., Awazu M., Muroya K., Kamimaki I., Igarashi T. The W258X mutation in SLC22A12 is the predominant cause of Japanese renal hypouricemia. Pediatr. Nephrol. 2004;19:728–733. doi: 10.1007/s00467-004-1424-1. [DOI] [PubMed] [Google Scholar]
- 40.Kuwabara M., Niwa K., Ohtahara A., Hamada T., Miyazaki S., Mizuta E., Ogino K., Hisatome I. Prevalence and complications of hypouricemia in a general population: a large-scale cross-sectional study in Japan. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furuhashi M., Mori K., Tanaka M., Maeda T., Matsumoto M., Murase T., Nakamura T., Koyama M., Moniwa N., Ohnishi H., Saitoh S., Shimamoto K., Miura T. Unexpected high plasma xanthine oxidoreductase activity in female subjects with low levels of uric acid. Endocr. J. 2018;65:1083–1092. doi: 10.1507/endocrj.EJ18-0127. [DOI] [PubMed] [Google Scholar]
- 42.Hershfield M.S. Reassessing serum urate targets in the management of refractory gout: can you go too low? Curr. Opin. Rheumatol. 2009;21:138–142. doi: 10.1097/BOR.0b013e3283257b83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srivastava A., Kaze A.D., McMullan C.J., Isakova T., Waikar S.S. Uric acid and the risks of kidney failure and death in individuals with CKD. Am. J. Kidney Dis. 2018;71:362–370. doi: 10.1053/j.ajkd.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suliman M.E., Johnson R.J., Garcia-Lopez E., Qureshi A.R., Molinaei H., Carrero J.J., Heimburger O., Barany P., Axelsson J., Lindholm B., Stenvinkel P. J-shaped mortality relationship for uric acid in CKD. Am. J. Kidney Dis. : the off. j. Nat. Kid. Found. 2006;48:761–771. doi: 10.1053/j.ajkd.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Verdecchia P., Schillaci G., Reboldi G., Santeusanio F., Porcellati C., Brunetti P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension The PIUMA study. Hypertension. 2000;36:1072–1078. doi: 10.1161/01.hyp.36.6.1072. [DOI] [PubMed] [Google Scholar]
- 46.Seet R.C., Kasiman K., Gruber J., Tang S.Y., Wong M.C., Chang H.M., Chan Y.H., Halliwell B., Chen C.P. Is uric acid protective or deleterious in acute ischemic stroke? A prospective cohort study. Atherosclerosis. 2010;209:215–219. doi: 10.1016/j.atherosclerosis.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Lapsia V., Johnson R.J., Dass B., Shimada M., Kambhampati G., Ejaz N.I., Arif A.A., Ejaz A.A. Elevated uric acid increases the risk for acute kidney injury. Am. J. Med. 2012;125:302. doi: 10.1016/j.amjmed.2011.06.021. e309-302.e317. [DOI] [PubMed] [Google Scholar]
- 48.Huang C., Niu K., Kobayashi Y., Guan L., Momma H., Cui Y., Chujo M., Otomo A., Guo H., Tadaura H., Nagatomi R. An inverted J-shaped association of serum uric acid with muscle strength among Japanese adult men: a cross-sectional study. BMC Muscoskel. Disord. 2013;14:258. doi: 10.1186/1471-2474-14-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H., Zha X., Zhu Y., Liu M., Guo R., Wen Y. An invert U-shaped curve: relationship between fasting plasma glucose and serum uric acid concentration in a large health check-up population in China. Medicine. 2016;95 doi: 10.1097/MD.0000000000003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang W., Iso H., Murakami Y., Miura K., Nagai M., Sugiyama D., Ueshima H., Okamura T. Serum uric acid and mortality form cardiovascular disease: EPOCH-Japan study. J. Atherosclerosis Thromb. 2016;23:692–703. doi: 10.5551/jat.31591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamei K., Konta T., Hirayama A., Ichikawa K., Kubota I., Fujimoto S., Iseki K., Moriyama T., Yamagata K., Tsuruya K., Narita I., Kondo M., Shibagaki Y., Kasahara M., Asahi K., Watanabe T. Associations between serum uric acid levels and the incidence of nonfatal stroke: a nationwide community-based cohort study. Clin. Exp. Nephrol. 2017;21:497–503. doi: 10.1007/s10157-016-1311-7. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y., Chi J., Che K., Chen Y., Sun X., Wang Y., Wang Z. Fasting plasma glucose and serum uric acid levels in a general Chinese population with normal glucose tolerance: a U-shaped curve. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y., Zhang Y., Li Y., Ding L., Sheng L., Xie Z., Wen C. U-shaped relationship between functional outcome and serum uric acid in ischemic stroke. Cell. Physiol. Biochem. 2018;47:2369–2379. doi: 10.1159/000491609. [DOI] [PubMed] [Google Scholar]
- 54.Latourte A., Bardin T., Richette P. Uric acid and cognitive decline: a double-edge sword? Curr. Opin. Rheumatol. 2018;30:183–187. doi: 10.1097/BOR.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 55.Zhou G., Holzman C., Luo Z., Margerison C. Maternal serum uric acid levels and blood pressure during pregnancy: a community-based cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018;222:64–69. doi: 10.1016/j.ejogrb.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Zhou G., Holzman C., Luo Z., Margerison C. Maternal serum uric acid levels in pregnancy and fetal growth. J. Matern. Fetal Neonatal Med. 2018:1–9. doi: 10.1080/14767058.2018.1484093. [DOI] [PubMed] [Google Scholar]
- 57.Feig D.I., Kang D.H., Johnson R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saravanan P., Davidson N.C. Risk assessment for sudden cardiac death in dialysis patients. Circ. Arrhythm. Electrophysiol. 2010;3:553–559. doi: 10.1161/CIRCEP.110.937888. [DOI] [PubMed] [Google Scholar]
- 59.Wingrove C.S., Walton C., Stevenson J.C. The effect of menopause on serum uric acid levels in non-obese healthy women. Metabolism. 1998;47:435–438. doi: 10.1016/s0026-0495(98)90056-7. [DOI] [PubMed] [Google Scholar]
- 60.Wei X., Fu B., Chen X., Chen W., Wang Z., Yu D., Jiang G., Chen J. U-shaped association between serum uric acid and short-term mortality in patients with infective endocarditis. Front. Endocrinol. 2021;12:750818. doi: 10.3389/fendo.2021.750818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Q., Wu C., Kuang W., Zhan X., Zhou J. Correlation analysis of low-level serum uric acid and cardiovascular events in patients on peritoneal dialysis. Int. Urol. Nephrol. 2021;53:2399–2408. doi: 10.1007/s11255-021-02902-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujimura Y., Yamauchi Y., Murase T., Nakamura T., Fujita S.-i., Fujisaka T., Ito T., Sohmiya K., Hoshiga M., Ishizaka N. Relationship between plasma xanthine oxidoreductase activity and left ventricular ejection fraction and hypertrophy among cardiac patients. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Otaki Y., Watanabe T., Kinoshita D., Yokoyama M., Takahashi T., Toshima T., Sugai T., Murase T., Nakamura T., Nishiyama S., Takahashi H., Arimoto T., Shishido T., Miyamoto T., Kubota I. Association of plasma xanthine oxidoreductase activity with severity and clinical outcome in patients with chronic heart failure. Int. J. Cardiol. 2017;228:151–157. doi: 10.1016/j.ijcard.2016.11.077. [DOI] [PubMed] [Google Scholar]
- 64.Bobulescu I.A., Moe O.W. Renal transport of uric acid: evolving concepts and uncertainties. Adv. Chron. Kidney Dis. 2012;19:358–371. doi: 10.1053/j.ackd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cross C.E., van der Vliet A., O'Neill C.A., Louie S., Halliwell B. Oxidants, antioxidants, and respiratory tract lining fluids. Environ. Health Perspect. 1994;102(Suppl 10):185–191. doi: 10.1289/ehp.94102s10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.The Global Initiative for Chronic Obstructive Lung Disease (GOLD).
- 67.Adeloye D., Chua S., Lee C., Basquill C., Papana A., Theodoratou E., Nair H., Gasevic D., Sridhar D., Campbell H., Chan K.Y., Sheikh A., Rudan I., Global Health Epidemiology Reference G. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J. Glob. Health. 2015;5 doi: 10.7189/jogh.05-020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Okutan O., Tas D., Demirer E., Kartaloglu Z. Evaluation of quality of life with the chronic obstructive pulmonary disease assessment test in chronic obstructive pulmonary disease and the effect of dyspnea on disease-specific quality of life in these patients. Yonsei Med. J. 2013;54:1214–1219. doi: 10.3349/ymj.2013.54.5.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flattet Y., Garin N., Serratrice J., Perrier A., Stirnemann J., Carballo S. Determining prognosis in acute exacerbation of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:467–475. doi: 10.2147/COPD.S122382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bendayan D., Shitrit D., Ygla M., Huerta M., Fink G., Kramer M.R. Hyperuricemia as a prognostic factor in pulmonary arterial hypertension. Respir. Med. 2003;97:130–133. doi: 10.1053/rmed.2003.1440. [DOI] [PubMed] [Google Scholar]
- 71.Hoeper M.M., Hohlfeld J.M., Fabel H. Hyperuricaemia in patients with right or left heart failure. Eur. Respir. J. 1999;13:682–685. doi: 10.1183/09031936.99.13368299. [DOI] [PubMed] [Google Scholar]
- 72.Van Albada M.E., Loot F.G., Fokkema R., Roofthooft M.T., Berger R.M. Biological serum markers in the management of pediatric pulmonary arterial hypertension. Pediatr. Res. 2008;63:321–327. doi: 10.1203/PDR.0b013e318163a2e7. [DOI] [PubMed] [Google Scholar]
- 73.Cerik I.B., Dindas F., Koyun E., Dereli S., Sahin A., Turgut O.O., Gul I. New prognostic markers in pulmonary arterial hypertension: CRP to albumin ratio and uric acid. Clin. Biochem. 2021 doi: 10.1016/j.clinbiochem.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 74.Uk Kang T., Park K.Y., Kim H.J., Ahn H.S., Yim S.Y., Jun J.B. Association of hyperuricemia and pulmonary hypertension: a systematic review and meta-analysis. Mod. Rheumatol. 2019;29:1031–1041. doi: 10.1080/14397595.2018.1537555. [DOI] [PubMed] [Google Scholar]
- 75.Zare E., Kafshbani P., Chenaghlou M., Noori M., Ghaemmaghami Z., Amin A., Taghavi S., Naderi N. ESC Heart Fail; 2021. Prognostic Significance of Insulin Resistance in Pulmonary Hypertension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peled N., Kassirer M., Shitrit D., Kogan Y., Shlomi D., Berliner A.S., Kramer M.R. The association of OSA with insulin resistance, inflammation and metabolic syndrome. Respir. Med. 2007;101:1696–1701. doi: 10.1016/j.rmed.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 77.Friedl H.P., Till G.O., Trentz O., Ward P.A. Role of oxygen radicals in tourniquet-related ischemia-reperfusion injury of human patients. Klin. Wochenschr. 1991;69:1109–1112. doi: 10.1007/BF01645168. [DOI] [PubMed] [Google Scholar]
- 78.Bartziokas K., Papaioannou A.I., Loukides S., Papadopoulos A., Haniotou A., Papiris S., Kostikas K. Serum uric acid as a predictor of mortality and future exacerbations of COPD. Eur. Respir. J. 2014;43:43–53. doi: 10.1183/09031936.00209212. [DOI] [PubMed] [Google Scholar]
- 79.Embarak S.S., A., Abdrabboh M., Mokhtar A. Serum uric acid as a biomarker for prediction of outcomes of patients hospitalized for acute exacerbation of chronic obstructive pulmonary disease. Egyp. J. Bronchol. 2014;8:115–120. [Google Scholar]
- 80.Ergün R., Ergün D. Prognostic value of serum uric acid level for short term survival in patients needing mechanical ventilation due to chronic obstructive pulmonary disease exacerbations. Int. J. Sci. Res. 2018;74 [Google Scholar]
- 81.Zhang X., Liu L., Liang R., Jin S. Hyperuricemia is a biomarker of early mortality in patients with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2015;10:2519–2523. doi: 10.2147/COPD.S87202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arslan Z.O.z., Ö., Karakoc F., Kara D., Naldan M., Uzlas E., Oral E., Aydın P. What are the factors affecting on the mortality of COPD patients in the ICU? W. Indian Med. J. 2017 [Google Scholar]
- 83.Vafaei A S.Z., Moghaddam SGh Mortazavi, Javid Arabshahi Z. The relationship between serum uric acid and severity of chronic obstructive pulmonary disease (COPD) J. Cardiothorac. Med. 2017;5:181–186. [Google Scholar]
- 84.Fukuhara A., Saito J., Sato S., Saito K., Fukuhara N., Tanino Y., Wang X., Rinno K., Suzuki H., Munakata M. The association between risk of airflow limitation and serum uric acid measured at medical health check-ups. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:1213–1219. doi: 10.2147/COPD.S126249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kahnert K., Alter P., Welte T., Huber R.M., Behr J., Biertz F., Watz H., Bals R., Vogelmeier C.F., Jorres R.A. Uric acid, lung function, physical capacity and exacerbation frequency in patients with COPD: a multi-dimensional approach. Respir. Res. 2018;19:110. doi: 10.1186/s12931-018-0815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aida Y., Shibata Y., Osaka D., Abe S., Inoue S., Fukuzaki K., Tokairin Y., Igarashi A., Yamauchi K., Nemoto T., Nunomiya K., Kishi H., Sato M., Watanabe T., Konta T., Kawata S., Kato T., Kubota I. The relationship between serum uric acid and spirometric values in participants in a health check: the Takahata study. Int. J. Med. Sci. 2011;8:470–478. doi: 10.7150/ijms.8.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Horsfall L.J., Nazareth I., Petersen I. Serum uric acid and the risk of respiratory disease: a population-based cohort study. Thorax. 2014;69:1021–1026. doi: 10.1136/thoraxjnl-2014-205271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nicks M.E., O'Brien M.M., Bowler R.P. Plasma antioxidants are associated with impaired lung function and COPD exacerbations in smokers. COPD. 2011;8:264–269. doi: 10.3109/15412555.2011.579202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holzman C., Bullen B., Fisher R., Paneth N., Reuss L., Prematurity Study G. Pregnancy outcomes and community health: the POUCH study of preterm delivery. Paediatr. Perinat. Epidemiol. 2001;15(Suppl 2):136–158. doi: 10.1046/j.1365-3016.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- 90.Robinson P.C., Dalbeth N. Febuxostat for the treatment of hyperuricaemia in gout. Expet Opin. Pharmacother. 2018;19:1289–1299. doi: 10.1080/14656566.2018.1498842. [DOI] [PubMed] [Google Scholar]
- 91.Goicoechea M., de Vinuesa S.G., Verdalles U., Ruiz-Caro C., Ampuero J., Rincon A., Arroyo D., Luno J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin. J. Am. Soc. Nephrol. : CJASN. 2010;5:1388–1393. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goicoechea M., Garcia de Vinuesa S., Verdalles U., Verde E., Macias N., Santos A., Perez de Jose A., Cedeno S., Linares T., Luno J. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am. J. Kidney Dis. : the off. j. Nat. Kid. Found. 2015;65:543–549. doi: 10.1053/j.ajkd.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 93.Pai B.H., Swarnalatha G., Ram R., Dakshinamurty K.V. Allopurinol for prevention of progression of kidney disease with hyperuricemia. Indian J. Nephrol. 2013;23:280–286. doi: 10.4103/0971-4065.114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siu Y.P., Leung K.T., Tong M.K., Kwan T.H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am. J. Kidney Dis. : the off. j. Nat. Kid. Found. 2006;47:51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 95.Kanbay M., Huddam B., Azak A., Solak Y., Kadioglu G.K., Kirbas I., Duranay M., Covic A., Johnson R.J. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin. J. Am. Soc. Nephrol. : CJASN. 2011;6:1887–1894. doi: 10.2215/CJN.11451210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krishnamurthy A., Lazaro D., Stefanov D.G., Blumenthal D., Gerber D., Patel S. The effect of allopurinol on renal function. J. Clin. Rheumatol. 2017;23:1–5. doi: 10.1097/RHU.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 97.Momeni A., Shahidi S., Seirafian S., Taheri S., Kheiri S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J. Kidney Dis. 2010;4:128–132. [PubMed] [Google Scholar]
- 98.Jalal D.I., Decker E., Perrenoud L., Nowak K.L., Bispham N., Mehta T., Smits G., You Z., Seals D., Chonchol M., Johnson R.J. Vascular function and uric acid-lowering in stage 3 CKD. J. Am. Soc. Nephrol. 2017;28:943–952. doi: 10.1681/ASN.2016050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao J., Deng S.B., She Q., Li J., Kao G.Y., Wang J.S., Ma Y. Allopurinol ameliorates cardiac function in non-hyperuricaemic patients with chronic heart failure. Eur. Rev. Med. Pharmacol. Sci. 2016;20:756–761. [PubMed] [Google Scholar]
- 100.Kao M.P., Ang D.S., Gandy S.J., Nadir M.A., Houston J.G., Lang C.C., Struthers A.D. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J. Am. Soc. Nephrol. 2011;22:1382–1389. doi: 10.1681/ASN.2010111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Givertz M.M., Anstrom K.J., Redfield M.M., Deswal A., Haddad H., Butler J., Tang W.H., Dunlap M.E., LeWinter M.M., Mann D.L., Felker G.M., O'Connor C.M., Goldsmith S.R., Ofili E.O., Saltzberg M.T., Margulies K.B., Cappola T.P., Konstam M.A., Semigran M.J., McNulty S.E., Lee K.L., Shah M.R., Hernandez A.F., Network N.H.F.C.R. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the xanthine oxidase inhibition for hyperuricemic heart failure patients (EXACT-HF) study. Circulation. 2015;131:1763–1771. doi: 10.1161/CIRCULATIONAHA.114.014536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hare J.M., Mangal B., Brown J., Fisher C., Jr., Freudenberger R., Colucci W.S., Mann D.L., Liu P., Givertz M.M., Schwarz R.P., Investigators O.-C. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J. Am. Coll. Cardiol. 2008;51:2301–2309. doi: 10.1016/j.jacc.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 103.Mackenzie I.S., Ford I., Walker A., Hawkey C., Begg A., Avery A., Taggar J., Wei L., Struthers A.D., MacDonald T.M., group A.-H. s. Multicentre, prospective, randomised, open-label, blinded end point trial of the efficacy of allopurinol therapy in improving cardiovascular outcomes in patients with ischaemic heart disease: protocol of the ALL-HEART study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-013774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coburn B.W., Michaud K., Bergman D.A., Mikuls T.R., Hoboken N.J. Arthritis & rheumatology; 2018. Allopurinol Dose Escalation and Mortality Among Patients with Gout: A National Propensity-Matched Cohort Study. [DOI] [PubMed] [Google Scholar]
- 105.Stamp L.K., Chapman P.T., Barclay M.L., Horne A., Frampton C., Tan P., Drake J., Dalbeth N. A randomised controlled trial of the efficacy and safety of allopurinol dose escalation to achieve target serum urate in people with gout. Ann. Rheum. Dis. 2017;76:1522–1528. doi: 10.1136/annrheumdis-2016-210872. [DOI] [PubMed] [Google Scholar]
- 106.Becker M.A., Schumacher H.R., Jr., Wortmann R.L., MacDonald P.A., Palo W.A., Eustace D., Vernillet L., Joseph-Ridge N. Febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase: a twenty-eight-day, multicenter, phase II, randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy in patients with gout. Arthritis Rheum. 2005;52:916–923. doi: 10.1002/art.20935. [DOI] [PubMed] [Google Scholar]
- 107.Schumacher H.R., Jr., Becker M.A., Lloyd E., MacDonald P.A., Lademacher C. Febuxostat in the treatment of gout: 5-yr findings of the FOCUS efficacy and safety study. Rheumatology. 2009;48:188–194. doi: 10.1093/rheumatology/ken457. [DOI] [PubMed] [Google Scholar]
- 108.Sircar D., Chatterjee S., Waikhom R., Golay V., Raychaudhury A., Chatterjee S., Pandey R. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized, placebo-controlled trial. Am. J. Kidney Dis. : the off. j. Nat. Kid. Found. 2015;66:945–950. doi: 10.1053/j.ajkd.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 109.Weng S.-C. Febuxostat is superior to traditional urate-lowering agents in reducing the progression of kidney function in chronic kidney disease patients. Cognet. Med. 2016;3 [Google Scholar]
- 110.Kimura K., Hosoya T., Uchida S., Inaba M., Makino H., Maruyama S., Ito S., Yamamoto T., Tomino Y., Ohno I., Shibagaki Y., Iimuro S., Imai N., Kuwabara M., Hayakawa H., Ohtsu H., Ohashi Y., Investigators F.S. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am. J. Kidney Dis: the off. J. of the Nat. Kid. Found. 2018 doi: 10.1053/j.ajkd.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 111.Becker M.A., Schumacher H.R., Jr., Wortmann R.L., MacDonald P.A., Eustace D., Palo W.A., Streit J., Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N. Engl. J. Med. 2005;353:2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 112.Schumacher H.R., Jr., Becker M.A., Wortmann R.L., Macdonald P.A., Hunt B., Streit J., Lademacher C., Joseph-Ridge N. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540–1548. doi: 10.1002/art.24209. [DOI] [PubMed] [Google Scholar]
- 113.Becker M.A., Schumacher H.R., Espinoza L.R., Wells A.F., MacDonald P., Lloyd E., Lademacher C. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res. Ther. 2010;12:R63. doi: 10.1186/ar2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Becker M.A., Schumacher H.R., MacDonald P.A., Lloyd E., Lademacher C. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. J. Rheumatol. 2009;36:1273–1282. doi: 10.3899/jrheum.080814. [DOI] [PubMed] [Google Scholar]
- 115.Uloric Summary Review. Division of Anesthesia, Analgesia, and Rheumatology. 2009. [Google Scholar]
- 116.White W.B., Saag K.G., Becker M.A., Borer J.S., Gorelick P.B., Whelton A., Hunt B., Castillo M., Gunawardhana L., Investigators C. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N. Engl. J. Med. 2018;378:1200–1210. doi: 10.1056/NEJMoa1710895. [DOI] [PubMed] [Google Scholar]
- FDA), F. a. D. A. FDA Adds Boxed Warning for Increased Risk of Death with Gout Medicine Uloric (Febuxostat).
- 118.Mackenzie I.S., Ford I., Nuki G., Hallas J., Hawkey C.J., Webster J., Ralston S.H., Walters M., Robertson M., De Caterina R., Findlay E., Perez-Ruiz F., McMurray J.J.V., MacDonald T.M. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet. 2020;396:1745–1757. doi: 10.1016/S0140-6736(20)32234-0. [DOI] [PubMed] [Google Scholar]
- 119.Su C.Y., Shen L.J., Hsieh S.C., Lin L.Y., Lin F.J. Comparing cardiovascular safety of febuxostat and allopurinol in the real world: a population-based cohort study. Mayo Clin. Proc. 2019;94:1147–1157. doi: 10.1016/j.mayocp.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 120.Singh J.A., Cleveland J.D. Comparative effectiveness of allopurinol and febuxostat for the risk of atrial fibrillation in the elderly: a propensity-matched analysis of Medicare claims data. Eur. Heart J. 2019;40:3046–3054. doi: 10.1093/eurheartj/ehz154. [DOI] [PubMed] [Google Scholar]
- 121.Kang E.H., Choi H.K., Shin A., Lee Y.J., Lee E.B., Song Y.W., Kim S.C. Comparative cardiovascular risk of allopurinol versus febuxostat in patients with gout: a nation-wide cohort study. Rheumatology. 2019;58:2122–2129. doi: 10.1093/rheumatology/kez189. [DOI] [PubMed] [Google Scholar]
- 122.Kojima S., Matsui K., Hiramitsu S., Hisatome I., Waki M., Uchiyama K., Yokota N., Tokutake E., Wakasa Y., Jinnouchi H., Kakuda H., Hayashi T., Kawai N., Mori H., Sugawara M., Ohya Y., Kimura K., Saito Y., Ogawa H. Febuxostat for cerebral and CaRdiorenovascular events PrEvEntion StuDy. Eur. Heart J. 2019;40:1778–1786. doi: 10.1093/eurheartj/ehz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yokota T., Fukushima A., Kinugawa S., Okumura T., Murohara T., Tsutsui H. Randomized trial of effect of urate-lowering agent febuxostat in chronic heart failure patients with hyperuricemia (LEAF-CHF) Int. Heart J. 2018;59:976–982. doi: 10.1536/ihj.17-560. [DOI] [PubMed] [Google Scholar]
- 124.Bardin T., Richette P. FAST: new look at the febuxostat safety profile. Lancet. 2020;396:1704–1705. doi: 10.1016/S0140-6736(20)32343-6. [DOI] [PubMed] [Google Scholar]
- 125.Bredemeier M., Lopes L.M., Eisenreich M.A., Hickmann S., Bongiorno G.K., d'Avila R., Morsch A.L.B., da Silva Stein F., Campos G.G.D. Xanthine oxidase inhibitors for prevention of cardiovascular events: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2018;18:24. doi: 10.1186/s12872-018-0757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miner J.N., Tan P.K., Hyndman D., Liu S., Iverson C., Nanavati P., Hagerty D.T., Manhard K., Shen Z., Girardet J.L., Yeh L.T., Terkeltaub R., Quart B. Lesinurad, a novel, oral compound for gout, acts to decrease serum uric acid through inhibition of urate transporters in the kidney. Arthritis Res. Ther. 2016;18:214. doi: 10.1186/s13075-016-1107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Terkeltaub R., Saag K.G., Goldfarb D.S., Baumgartner S., Schechter B.M., Valiyil R., Jalal D., Pillinger M., White W.B. Integrated safety studies of the urate reabsorption inhibitor lesinurad in treatment of gout. Rheumatology. 2019;58:61–69. doi: 10.1093/rheumatology/key245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saag K.G., Fitz-Patrick D., Kopicko J., Fung M., Bhakta N., Adler S., Storgard C., Baumgartner S., Becker M.A. Lesinurad combined with allopurinol: a randomized, double-blind, placebo-controlled study in gout patients with an inadequate response to standard-of-care allopurinol (a US-based study) Arthritis Rheumatol. 2017;69:203–212. doi: 10.1002/art.39840. [DOI] [PubMed] [Google Scholar]
- 129.Bardin T., Keenan R.T., Khanna P.P., Kopicko J., Fung M., Bhakta N., Adler S., Storgard C., Baumgartner S., So A. Lesinurad in combination with allopurinol: a randomised, double-blind, placebo-controlled study in patients with gout with inadequate response to standard of care (the multinational CLEAR 2 study) Ann. Rheum. Dis. 2017;76:811–820. doi: 10.1136/annrheumdis-2016-209213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tausche A.K., Alten R., Dalbeth N., Kopicko J., Fung M., Adler S., Bhakta N., Storgard C., Baumgartner S., Saag K. Lesinurad monotherapy in gout patients intolerant to a xanthine oxidase inhibitor: a 6 month phase 3 clinical trial and extension study. Rheumatology. 2017;56:2170–2178. doi: 10.1093/rheumatology/kex350. [DOI] [PubMed] [Google Scholar]
- 131.Dalbeth N., Jones G., Terkeltaub R., Khanna D., Kopicko J., Bhakta N., Adler S., Fung M., Storgard C., Baumgartner S., Perez-Ruiz F. Combination With Febuxostat in Patients With Tophaceous Gout: Findings of a Phase III Clinical Trial. Arthritis Rheumatol. Vol. 69. 2017. Lesinurad, a selective uric acid reabsorption inhibitor; pp. 1903–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Perez-Gomez M.V., Bartsch L.A., Castillo-Rodriguez E., Fernandez-Prado R., Kanbay M., Ortiz A. Potential dangers of serum urate-lowering therapy. Am. J. Med. 2019;132:457–467. doi: 10.1016/j.amjmed.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 133.CHMP Assessment Report - Krystexxa . European Medicines Agency; 2012. [Google Scholar]
- 134.Hosoya T., Ohno I., Nomura S., Hisatome I., Uchida S., Fujimori S., Yamamoto T., Hara S. Effects of topiroxostat on the serum urate levels and urinary albumin excretion in hyperuricemic stage 3 chronic kidney disease patients with or without gout. Clin. Exp. Nephrol. 2014;18:876–884. doi: 10.1007/s10157-014-0935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wada T., Hosoya T., Honda D., Sakamoto R., Narita K., Sasaki T., Okui D., Kimura K. Uric acid-lowering and renoprotective effects of topiroxostat, a selective xanthine oxidoreductase inhibitor, in patients with diabetic nephropathy and hyperuricemia: a randomized, double-blind, placebo-controlled, parallel-group study (UPWARD study) Clin. Exp. Nephrol. 2018;22:860–870. doi: 10.1007/s10157-018-1530-1. [DOI] [PubMed] [Google Scholar]
- 136.Neogi T., George J., Rekhraj S., Struthers A.D., Choi H., Terkeltaub R.A. Are either or both hyperuricemia and xanthine oxidase directly toxic to the vasculature? A critical appraisal. Arthritis Rheum. 2012;64:327–338. doi: 10.1002/art.33369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kanellis J., Watanabe S., Li J.H., Kang D.H., Li P., Nakagawa T., Wamsley A., Sheikh-Hamad D., Lan H.Y., Feng L., Johnson R.J. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 138.Kang D.H., Nakagawa T., Feng L., Watanabe S., Han L., Mazzali M., Truong L., Harris R., Johnson R.J. A role for uric acid in the progression of renal disease. J. Am. Soc. Nephrol. 2002;13:2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 139.Mazzali M., Kanellis J., Han L., Feng L., Xia Y.Y., Chen Q., Kang D.H., Gordon K.L., Watanabe S., Nakagawa T., Lan H.Y., Johnson R.J. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am. J. Physiol. Ren. Physiol. 2002;282:F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 140.Johnson R.J., Sanchez-Lozada L.G., Mazzali M., Feig D.I., Kanbay M., Sautin Y.Y. What are the key arguments against uric acid as a true risk factor for hypertension? Hypertension. 2013;61:948–951. doi: 10.1161/HYPERTENSIONAHA.111.00650. [DOI] [PubMed] [Google Scholar]
- 141.Johnson, R. J., Sanchez-Lozada, L. G., Mazzali, M., Feig, D. I., Kanbay, M., and Sautin, Y. Y. What are the key arguments against uric acid as a true risk factor for hypertension? Hypertension 61, 948-951. [DOI] [PubMed]
- 142.Neogi, T., George, J., Rekhraj, S., Struthers, A. D., Choi, H., and Terkeltaub, R. A. Are either or both hyperuricemia and xanthine oxidase directly toxic to the vasculature? A critical appraisal. Arthritis Rheum. [DOI] [PMC free article] [PubMed]
- 143.Puddu, P., Puddu, G. M., Cravero, E., Vizioli, L., and Muscari, A. Relationships among hyperuricemia, endothelial dysfunction and cardiovascular disease: molecular mechanisms and clinical implications. J. Cardiol. 59, 235-242. [DOI] [PubMed]
- 144.Park, J. H., Jin, Y. M., Hwang, S., Cho, D. H., Kang, D. H., and Jo, I. Uric acid attenuates nitric oxide production by decreasing the interaction between endothelial nitric oxide synthase and calmodulin in human umbilical vein endothelial cells: a mechanism for uric acid-induced cardiovascular disease development. Nitric Oxide 32, 36-42. [DOI] [PubMed]
- 145.Salvi, E., Kutalik, Z., Glorioso, N., Benaglio, P., Frau, F., Kuznetsova, T., Arima, H., Hoggart, C., Tichet, J., Nikitin, Y. P., Conti, C., Seidlerova, J., Tikhonoff, V., Stolarz-Skrzypek, K., Johnson, T., Devos, N., Zagato, L., Guarrera, S., Zaninello, R., Calabria, A., Stancanelli, B., Troffa, C., Thijs, L., Rizzi, F., Simonova, G., Lupoli, S., Argiolas, G., Braga, D., D'Alessio, M. C., Ortu, M. F., Ricceri, F., Mercurio, M., Descombes, P., Marconi, M., Chalmers, J., Harrap, S., Filipovsky, J., Bochud, M., Iacoviello, L., Ellis, J., Stanton, A. V., Laan, M., Padmanabhan, S., Dominiczak, A. F., Samani, N. J., Melander, O., Jeunemaitre, X., Manunta, P., Shabo, A., Vineis, P., Cappuccio, F. P., Caulfield, M. J., Matullo, G., Rivolta, C., Munroe, P. B., Barlassina, C., Staessen, J. A., Beckmann, J. S., and Cusi, D. Genomewide association study using a high-density single nucleotide polymorphism array and case-control design identifies a novel essential hypertension susceptibility locus in the promoter region of endothelial NO synthase. Hypertension 59, 248-255. [DOI] [PMC free article] [PubMed]
- 146.Fujimura Y., Yamauchi Y., Murase T., Nakamura T., Fujita S.I., Fujisaka T., Ito T., Sohmiya K., Hoshiga M., Ishizaka N. Relationship between plasma xanthine oxidoreductase activity and left ventricular ejection fraction and hypertrophy among cardiac patients. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cingolani H.E., Plastino J.A., Escudero E.M., Mangal B., Brown J., Perez N.G. The effect of xanthine oxidase inhibition upon ejection fraction in heart failure patients: La Plata Study. J. Card. Fail. 2006;12:491–498. doi: 10.1016/j.cardfail.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 148.Cardillo C., Kilcoyne C.M., Cannon R.O., 3rd, Quyyumi A.A., Panza J.A. Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients. Hypertension. 1997;30:57–63. doi: 10.1161/01.hyp.30.1.57. [DOI] [PubMed] [Google Scholar]
- 149.George J., Carr E., Davies J., Belch J.J., Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 150.Ogino K., Kato M., Furuse Y., Kinugasa Y., Ishida K., Osaki S., Kinugawa T., Igawa O., Hisatome I., Shigemasa C., Anker S.D., Doehner W. Uric acid-lowering treatment with benzbromarone in patients with heart failure: a double-blind placebo-controlled crossover preliminary study. Circ. Heart Fail. 2010;3:73–81. doi: 10.1161/CIRCHEARTFAILURE.109.868604. [DOI] [PubMed] [Google Scholar]
- 151.Cuspidi C., Facchetti R., Bombelli M., Sala C., Tadic M., Grassi G., Mancia G. Uric acid and new onset left ventricular hypertrophy: findings from the PAMELA population. Am. J. Hypertens. 2017;30:279–285. doi: 10.1093/ajh/hpw159. [DOI] [PubMed] [Google Scholar]
- 152.Kuwabara M., Sato Y., Kanbay M., Johnson R.J. Uric acid and left ventricular hypertrophy: a potentially new modifiable target? Am. J. Hypertens. 2017;30:229–231. doi: 10.1093/ajh/hpw195. [DOI] [PubMed] [Google Scholar]
- 153.Kelley E.E. A new paradigm for XOR-catalyzed reactive species generation in the endothelium. Pharmacol. Rep. : PR. 2015;67:669–674. doi: 10.1016/j.pharep.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Johnson R.J., Bakris G.L., Borghi C., Chonchol M.B., Feldman D., Lanaspa M.A., Merriman T.R., Moe O.W., Mount D.B., Sanchez Lozada L.G., Stahl E., Weiner D.E., Chertow G.M. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the national kidney foundation. Am. J. Kidney Dis. 2018;71:851–865. doi: 10.1053/j.ajkd.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Choi H., Neogi T., Stamp L., Dalbeth N., Terkeltaub R. New perspectives in Rheumatology: implications of the cardiovascular safety of febuxostat and allopurinol in patients with gout and cardiovascular morbidities trial and the associated food and drug administration public safety alert. Arthritis Rheumatol. 2018;70:1702–1709. doi: 10.1002/art.40583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fini M.A., Gaydos J., McNally A., Karoor V., Burnham E.L. Alcohol abuse is associated with enhanced pulmonary and systemic xanthine oxidoreductase activity. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;313:L1047–L1057. doi: 10.1152/ajplung.00570.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wright R.M., Ginger L.A., Kosila N., Elkins N.D., Essary B., McManaman J.L., Repine J.E. Mononuclear phagocyte xanthine oxidoreductase contributes to cytokine-induced acute lung injury. Am. J. Respir. Cell Mol. Biol. 2004;30:479–490. doi: 10.1165/rcmb.2003-0309OC. [DOI] [PubMed] [Google Scholar]
- 158.Chinnaiyan A.M., Huber-Lang M., Kumar-Sinha C., Barrette T.R., Shankar-Sinha S., Sarma V.J., Padgaonkar V.A., Ward P.A. Molecular signatures of sepsis: multiorgan gene expression profiles of systemic inflammation. Am. J. Pathol. 2001;159:1199–1209. doi: 10.1016/S0002-9440(10)62505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ratliff B.B., Abdulmahdi W., Pawar R., Wolin M.S. Oxidant mechanisms in renal injury and disease. Antioxidants Redox Signal. 2016;25:119–146. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wolin M.S., Alruwaili N., Kandhi S. Studies on hypoxic pulmonary vasoconstriction detect a novel role for the mitochondrial complex I subunit Ndufs2 in controlling peroxide generation for oxygen-sensing. Circ. Res. 2019;124:1683–1685. doi: 10.1161/CIRCRESAHA.119.315137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Feelisch M., Akaike T., Griffiths K., Ida T., Prysyahna O., Goodwin J.J., Gollop N.D., Fernandez B.O., Minnion M., Cortese-Krott M.M., Borgognone A., Hayes R.M., Eaton P., Frenneaux M.P., Madhani M. Long-lasting blood pressure lowering effects of nitrite are NO-independent and mediated by hydrogen peroxide, persulfides and oxidation of protein kinase G 1alpha redox signaling. Cardiovasc. Res. 2019 doi: 10.1093/cvr/cvz202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fini M.A., Stenmark K.R. Pegloticase and lowering blood pressure in refractory gout; is it uric acid or hydrogen peroxide? Eur. J. Intern. Med. 2019 doi: 10.1016/j.ejim.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 163.Polito L., Bortolotti M., Battelli M.G., Bolognesi A. Xanthine oxidoreductase: a leading actor in cardiovascular disease drama. Redox Biol. 2021;48:102195. doi: 10.1016/j.redox.2021.102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Alvarez-Lario B., Macarron-Vicente J. Uric acid and evolution. Rheumatology. 2010;49:2010–2015. doi: 10.1093/rheumatology/keq204. [DOI] [PubMed] [Google Scholar]
- 165.Oda M., Satta Y., Takenaka O., Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol. Biol. Evol. 2002;19:640–653. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- 166.Lu J., Dalbeth N., Yin H., Li C., Merriman T.R., Wei W.H. Mouse models for human hyperuricaemia: a critical review. Nat. Rev. Rheumatol. 2019 doi: 10.1038/s41584-019-0222-x. [DOI] [PubMed] [Google Scholar]
- 167.Tan P.K., Liu S., Gunic E., Miner J.N. Discovery and characterization of verinurad, a potent and specific inhibitor of URAT1 for the treatment of hyperuricemia and gout. Sci. Rep. 2017;7:665. doi: 10.1038/s41598-017-00706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Richette P., Doherty M., Pascual E., Bardin T. SUA levels should not be maintained <3 mg/dL for several years. Response to 'EULAR gout treatment guidelines by Richette et al: uric acid and neurocognition by Singh et al. Ann. Rheum. Dis. 2018;77:e21. doi: 10.1136/annrheumdis-2017-211423. [DOI] [PubMed] [Google Scholar]
- 169.Mouradjian M.T., Plazak M.E., Gale S.E., Noel Z.R., Watson K., Devabhakthuni S. Pharmacologic management of gout in patients with cardiovascular disease and heart failure. Am. J. Cardiovasc. Drugs. 2020;20:431–445. doi: 10.1007/s40256-020-00400-6. [DOI] [PubMed] [Google Scholar]