Abstract
Objectives
Up to 0.3% of Japanese have hypouricaemia. Most cases appear to result from a hereditary disease, renal hypouricaemia (RHUC), which causes exercise-induced acute kidney injury and urolithiasis. However, to what extent RHUC accounts for hypouricaemia is not known. We therefore investigated its frequency and evaluated its risks by genotyping a general Japanese population.
Methods
A cohort of 4993 Japanese was examined by genotyping the non-functional variants R90H (rs121907896) and W258X (rs121907892) of URAT1/SLC22A12, the two most common causative variants of RHUC in Japanese.
Results
Participants’ fractional excretion of uric acid and risk allele frequencies markedly increased at lower serum uric acid (SUA) levels. Ten participants (0.200%) had an SUA level ≤2.0 mg/dl and nine had R90H or W258X and were likely to have RHUC. Logistic regression analysis revealed these URAT1 variants to be significantly and independently associated with the risk of hypouricaemia and mild hypouricaemia (SUA ≤3.0 mg/dl) as well as sex, age and BMI, but these URAT1 variants were the only risks in the hypouricaemia population (SUA ≤2.0 mg/dl). W258X was only a risk in males with SUA ≤3.0 mg/dl.
Conclusion
Our study accurately reveals the prevalence of RHUC and provides genetic evidence for its definition (SUA ≤2.0 mg/dl). We also show that individuals with SUA ≤3.0 mg/dl, especially males, are prone to RHUC. Our findings will help to promote a better epidemiological understanding of RHUC as well as more accurate diagnosis, especially in males with mild hypouricaemia.
Keywords: renal hypouricaemia type 1 (RHUC1), urate transporter 1 (URAT1/SLC22A12), non-functional variants, fractional excretion of uric acid (FEUA), genetic epidemiology, mild hypouricaemia
Rheumatology key messages.
Genetic epidemiological analysis with a general Japanese population accurately showed the prevalence and risk of renal hypouricaemia.
Ninety percent of hypouricaemia (SUA ≤2.0 mg/dl) was caused by non-functional URAT1 variants.
Individuals with SUA ≤3.0 mg/dl, especially males, should be suspected of renal hypouricaemia.
Introduction
Urate, an end metabolite of purine bodies in humans [1], is excreted from the kidney and the intestine [2]. URAT1/SLC22A12 is a typical urate reabsorption transporter gene expressed in the renal proximal tubular cells in humans and is the molecular target of urate-lowering drugs such as benzbromarone, lesinurad and dotinurad. Non-functional variants of URAT1 cause the hereditary disorder renal hypouricaemia (RHUC) [3], which is characterized by low serum uric acid (SUA) levels and high fractional excretion of uric acid (FEUA; calculated as the uric acid clearance/creatinine clearance ratio; normal range 5.5–11.1% [4]). RHUC is relatively frequent in Japanese, non-Ashkenazi Jewish [4, 5] and European Roma populations [4, 6–8]. Non-functional variants of URAT1 [R90H (rs121907896) and W258X (rs121907892)] are known to be the two most frequent causative variants for RHUC in the Japanese population [3, 4, 9].
A clinical practice guideline (CPG) for RHUC has recently been developed [4] and, based on several epidemiological reports, strongly recommends testing for RHUC if patients have hypouricaemia (SUA ≤2.0 mg/dl). About 0.3% of Japanese reportedly have hypouricaemia [10]. However, the frequency of RHUC with genotyping among hypouricaemic individuals in the general Japanese population remains to be elucidated. Hence we investigated a cohort of 4993 Japanese participants and examined their SUA and FEUA while genotyping R90H and W258X of URAT1/SLC22A12. A risk evaluation was also conducted to reveal the factors that cause hypouricaemia in the general population.
Methods
Study participants
All 4993 Japanese individuals were recruited from the participants of the Shizuoka Area in the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study [11, 12]. Hypouricaemia was defined as an SUA of ≤2.0 mg/dl (≤120 µmol/l). Individuals with relatively low SUA [>2.0–≤3.0 mg/dl (>120–≤180 µmol/l)] were defined as having mild hypouricaemia [4]. Participants’ data on sex and age were obtained from their written questionnaires and their BMI data were obtained by physical measurements. FEUA {from the equation [urinary uric acid (mg/dl) × serum creatinine (mg/dl)]/[SUA (mg/dl) × urinary creatinine (mg/dl)]} was calculated from the results of blood and urine tests [4, 13].
Genetic and statistical analyses
Genomic DNA was extracted from whole peripheral blood cells [14]. Genotyping of p.R90H (c.269G>A) and p.W258X (c.774G>A) of URAT1 was performed by the TaqMan method (Life Technologies, Carlsbad, CA, USA) with a LightCycler 480 (Roche Diagnostics, Mannheim, Germany) [9]. Custom TaqMan assay probes were designed as follows [9]: for R90H, VIC-CCGCCACTTCCGC and FAM-CGCCGCTTCCGC; for W258X, VIC-CGGGACTGAACACTG and FAM-CGGGACTGGACACTG. The minor (i.e. risk) and major alleles were set to be A and G, respectively, for both R90H and W258X. All the heterozygotes and homozygotes of these variants were confirmed by direct sequencing using a 3130xl Genetic Analyzer (Life Technologies) [9] with the following primers: for R90H, forward 5′-GTTGGAGCCACCCCAAGTGAC-3′ and reverse 5′-GTCTGACCCACCGTGATCCATG-3′; for W258X, forward 5′-TGATGAACACGGGCACTCTC-3′ and reverse 5′-CTTTCCACTCGCTCCCCTAG-3′.
R software (version 3.4.0; http://www.r-project.org/) with the GenABEL package was used to calculate the linkage disequilibrium (r2). Logistic regression analysis using the backward elimination (likelihood ratio) method was conducted with SPSS version 22.0.0.0J (IBM Japan, Tokyo, Japan).
Results
The characteristics of the participants are shown in Supplementary Table S1, available at Rheumatology online. The call rates for R90H and W258X were >98% and both variants were in Hardy–Weinberg equilibrium (P > 0.05), suggesting no mistyping. Furthermore, no linkage disequilibrium was observed between these variants (r2 = 6.34 × 10−5), showing these variants to be mutually independent. The risk allele frequency (RAF) of R90H was 0.00276 (0.00333 in males and 0.00157 in females) and of W258X was 0.0225 (0.0236 in males and 0.0201 in females). These frequencies were relatively higher than in other populations, such as Africans and Europeans (Supplementary Table S2, available at Rheumatology online).
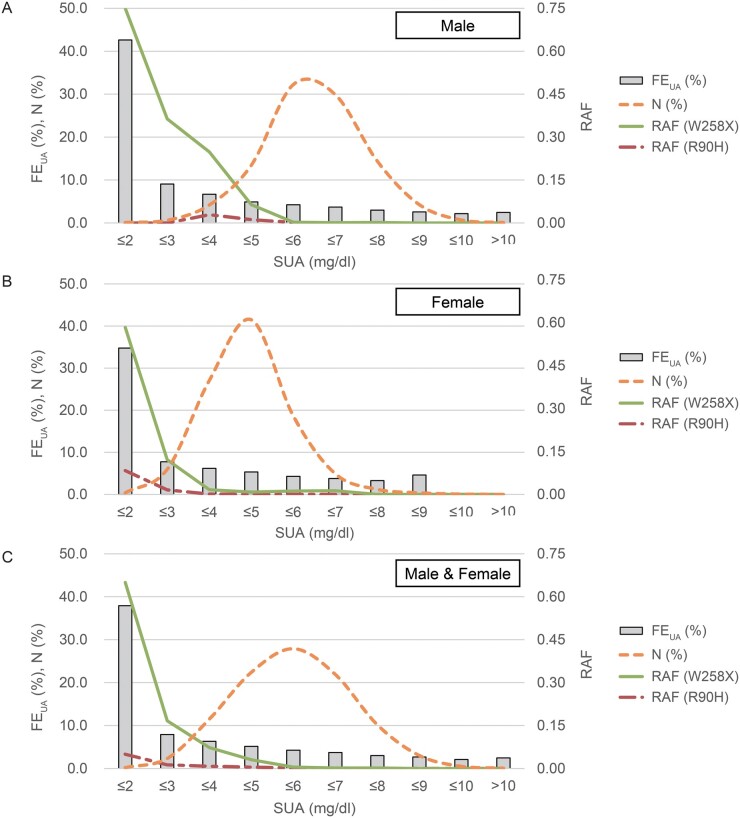
Fig. 1 shows the distribution of FEUA, participant numbers and RAFs of R90H and W258X along their SUA levels in male (Fig. 1A), female (Fig. 1B) and all participants (Fig. 1C). Whereas participants’ numbers seem to follow a normal distribution, their FEUA and RAFs of URAT1 variants were high when their SUA was low. The frequency of hypouricaemia (SUA ≤2.0 mg/dl) was 0.200% for all participants (10/4993), 0.118% in males (4/3399) and 0.376% in females (6/1594). Among 10 hypouricaemic subjects, all except for 1 female (65 years old, SUA = 2.0 mg/dl, FEUA = 12.4%) had non-functional variants of URAT1 and were estimated to have RHUC. The frequency of mild hypouricaemia was 2.34% for all (117/4993), 0.647% for males (22/3399) and 5.96% for females (95/1594). One 52-year-old male (SUA = 2.1 mg/dl) is likely to have had drug-induced mild hypouricaemia, since he was taking benzbromarone to treat gout. In the male population with hypouricaemia (SUA ≤2.0 mg/dl), the mean FEUA and RAF of W258X were 42.6% and reached 0.750, respectively, whereas they were 4.12% and 0.0236 in the overall male population. In the female population they were 34.8% and reached 0.583 for the hypouricaemic population, respectively, whereas they were 5.51% and 0.0201 in the entire female population. Although it was only a faint trend, FEUA was also higher when SUA was high in both sexes (Fig. 1), possibly due to the increased renal urate excretion in individuals with severe hyperuricaemia.
Fig. 1.
Distribution of participant numbers, FEUA and RAFs of URAT1 variants along SUA levels
As SUA levels decreased, FEUA and RAFs of non-functional URAT1 variants (R90H and W258X) increased in (A) males, (B) females and (C) all 4993 participants. This trend was more marked in the male population than in the female population. There were no female participants with SUA >9 mg/dl. FEUA: fractional excretion of uric acid; RAF: risk allele frequency; SUA: serum uric acid.
Together with the number of risk alleles of R90H and W258X of URAT1, logistic regression analysis was then conducted for the sex, age and BMI models, since all these factors typically affect SUA levels [15, 16]. As shown in Table 1, all of these factors, including URAT1 variants, were significantly and independently associated with risk in those with SUA ≤3.0 mg/dl (hypouricaemia and mild hypouricaemia). While both aging and gaining BMI are protective against hypouricaemia, URAT1 variants as well as sex (female) markedly increased its risk. However, sex, age and BMI were eliminated as covariates in the risk evaluation of hypouricaemia (SUA ≤2.0 mg/dl) and only URAT1 variants (R90H and W258X) significantly increased its risk (Table 1). In the same manner, logistic regression analyses were also performed for each sex (Supplementary Table S3, available at Rheumatology online). While all covariates retained their significance in females, only W258X was revealed to be a significant risk for the hypouricaemic and mild hypouricaemic population (SUA ≤3.0 mg/dl) in males.
Table 1.
Risk evaluation of hypouricaemia and mild hypouricaemia among 4993 Japanese participants
| Population | Factora | Odds ratio | 95% CI | P-value |
|---|---|---|---|---|
|
Hypouricaemia and mild hypouricaemia (SUA ≤3.0 mg/dl) |
Sexb | 9.52 | 5.74, 15.8 | 2.53 × 10–18 |
| Age | 0.968 | 0.946, 0.990 | 3.95 × 10–3 | |
| BMI | 0.832 | 0.772, 0.898 | 1.91 × 10–6 | |
| R90Hc | 22.7 | 6.34, 81.3 | 1.61 × 10–6 | |
| W258Xc | 29.4 | 18.1, 47.7 | 9.01 × 10–43 | |
|
| ||||
|
Hypouricaemia (SUA ≤2.0 mg/dl) |
R90Hc | 550.5 | 25.4, 11 930.5 | 5.79 × 10–5 |
| W258Xc | 302.8 | 38.2, 2401.6 | 6.40 × 10–8 | |
A logistic regression analysis using the backward elimination (likelihood ratio) method was conducted using models for sex, age, BMI and non-functional URAT1 variants (R90H and W258X). None of these covariates were eliminated in the risk evaluation of hypouricaemia and mild hypouricaemia (SUA ≤3.0 mg/dl), whereas sex, age and BMI were eliminated for hypouricaemia (SUA ≤2.0 mg/dl).
Calculation for sex was conducted for males as 1 and females as 2.
Calculations for the risk alleles of R90H and W258X were conducted for wild-type as 0, heterozygotes as 1 and homozygotes as 2.
Discussion
In the present study we showed, with 4993 Japanese, that FEUA and RAFs of non-functional URAT1 variants (R90H and W258X, the two most frequent causative variants of RHUC for Japanese) increased with lower SUA. Nine of the ten hypouricaemic participants had non-functional variants and were assessed as having RHUC. R90H and W258X as well as age, BMI and sex were revealed to be significantly and independently associated with the risk of hypouricaemia and mild hypouricaemia. Only R90H and W258X were demonstrated to be significant risks for hypouricaemia. The only risk for those with SUA ≤3.0 mg/dl (hypouricaemia and mild hypouricaemia) was W258X in the male population.
Depending on its causative genes, RHUC caused by the dysfunctional variants of URAT1/SLC22A12 and GLUT9/SLC2A9 is called RHUC type 1 (RHUC1) [3, 17] and type 2 (RHUC2) [14, 18], respectively, but the most common RHUC is RHUC1, by either W258X or R90H of URAT1 in Japanese individuals [4]. The present study revealed the frequency of hypouricaemia to be 0.118% in the male and 0.376% in the female participants, which is close to the reported levels of 0.2% and 0.4%, respectively, in the CPG for RHUC [4].
In the present study, the values of FEUA and RAFs of subjects harbouring non-functional URAT1 variants were clearly higher in the population with low SUA levels. Nine of ten individuals in the hypouricaemic population had non-functional variants of URAT1 and were estimated to have RHUC. In the male population with hypouricaemia, FEUA and RAF of W258X were >40% and reached 0.750, respectively, whereas they were 4.12% and 0.0236 in the overall male population. This trend was more marked in the male than in the female population, probably due to female hormones having a urate-lowering effect. Although 10 hypouricaemic participants in the present study were not clinically diagnosed with RHUC, 5 with homozygous variants in URAT1 must have had RHUC and the remaining 5 with heterozygous and/or high FEUA might have had RHUC with possibly other non-functional variants, because RHUC is a hereditary disorder (Mendelian Inheritance in Man: 220150 and 612076). The CPG for RHUC [4] strongly recommends RHUC be suspected if patients have an SUA ≤2.0 mg/dl, which is based only on past epidemiological reports. The present genetic epidemiological analysis also showed the characteristics of RHUC, low SUA (≤2.0 mg/dl) and high FEUA, and that most of the hypouricaemic population could be concluded to have RHUC.
Regression analyses were conducted to evaluate the risk of hypouricaemia and mild hypouricaemia: the results revealed that both of the URAT1 variants R90H and W258X as well as sex, age and BMI were significantly and independently associated with the risks of hypouricaemia and mild hypouricaemia (Table 1). Females were revealed to have close to a 10-fold higher risk of hypouricaemia and mild hypouricaemia than males (Table 1), but the actual risk to post-menopausal females might be lower, since female hormones have urate-lowering effects. Age and BMI were shown to be protective against hypouricaemia as expected, because they are well-known risks of hyperuricaemia [15, 16], which shows the opposite, that is, increased SUA levels. Also, as expected, having two non-functional variants of URAT1 displayed very strong risk (20- to 30-fold higher) on the progression of hypouricaemia and mild hypouricaemia (Table 1).
Another regression analysis was conducted to evaluate the risk of hypouricaemia alone using the same model as those for hypouricaemia and mild hypouricaemia (Table 1). Although R90H and W258X still showed a significantly strong effect, sex, age and BMI were eliminated as covariates. This result suggests that the URAT1 variants are the only risks or that they have much stronger effects than other factors in the hypouricaemic population.
When categorized by the participants’ sex, W258X was still revealed to be a significant risk in both sexes in the hypouricaemia and mild hypouricaemia population (Supplementary Table S3, available at Rheumatology online). Notably, in males, W258X was the only the risk to those with low SUA levels of ≤3.0 mg/dl. These results suggest that those with SUA ≤3.0 mg/dl, especially males, should be suspected of RHUC, in addition to the recommendation in the current CPG for RHUC [4] to suspect RHUC if patients have SUA ≤2.0 mg/dl. Although W258X no longer appeared to represent a risk of hypouricaemia in males, it might be due to the small number of hypouricaemic individuals (n = 4). R90H was also eliminated as a hypouricaemia risk in males due to its lower frequency than that of W258X; further analyses with larger populations are necessary to evaluate its risk.
RHUC is a hereditary disorder that clinically shows low SUA and high FEUA. Although RHUC itself is asymptomatic, patients often suffer from its severe complications, including exercise-induced acute kidney injury and urinary stones. Moreover, the pathophysiological elucidation of hypouricaemia or the effect of URAT1 variants will also promote the treatment of gout and hyperuricaemia by reducing states of high SUA [19, 20]. RHUC research would also clarify the physiological role of uric acid as well as help to answer clinical questions on gout, such as to what extent it is possible to lower patients’ SUA levels [19].
This is the first report to show the frequency and the risks of hypouricaemia using genotyping in an ordinary Japanese population. Our findings will help to provide genetic epidemiological evidence that almost all hypouricaemic individuals have RHUC. They will also assist accurate diagnosis of RHUC for those with SUA ≤3.0 mg/dl, especially in males.
Supplementary Material
Acknowledgements
The authors would like to thank all the participants involved in this study. We also wish to thank K. Morichika, M. Miyazawa, M. Seki, S. Suzuki, N. Yoshioka, Y. Miyoshi, M. Horie and R. Tanaka for the genetic analysis and technical assistance and N. Hamajima for sample collection. All procedures involved in this study were approved by the institutional ethical committees of the National Defense Medical College and Nagoya University and were performed in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Disclosure statement: The authors have declared no conflicts of interest.
Funding: This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, including JSPS KAKENHI grants (20H00566, 20K23152, 20K10522, 21H03350, 17H04128, 25293145 and 16H06279) and a Grant-in-Aid for Scientific Research on Innovative Areas (221S0002), the Ministry of Defense, the Kawano Masanori Memorial Foundation for the Promotion of Pediatrics and the Gout Research Foundation of Japan. The J-MICC Study was supported by Grants-in-Aid for Scientific Research from MEXT, including those for Priority Areas (17015018) and Innovative Areas (221S0001), as well as by a JSPS KAKENHI grant [16H06277 (CoBiA)].
Data availability statement
Data are available upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Wu XW, Lee CC, Muzny DM, Caskey CT.. Urate oxidase: primary structure and evolutionary implications. Proc Natl Acad Sci USA 1989;86:9412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dalbeth N, Choi HK, Joosten LAB. et al. Gout. Nat Rev Dis Primers 2019;5:69. [DOI] [PubMed] [Google Scholar]
- 3. Enomoto A, Kimura H, Chairoungdua A. et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002;417:447–52. [DOI] [PubMed] [Google Scholar]
- 4. Nakayama A, Matsuo H, Ohtahara A. et al. Clinical practice guideline for renal hypouricemia (1st edition). Hum Cell 2019;32:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suzuki T, Kidoguchi K, Hayashi A.. Genetic heterogeneity of familial hypouricemia due to isolated renal tubular defect. Jinrui Idengaku Zasshi 1981;26:243–8. [DOI] [PubMed] [Google Scholar]
- 6. Gabrikova D, Bernasovska J, Sokolova J, Stiburkova B.. High frequency of SLC22A12 variants causing renal hypouricemia 1 in the Czech and Slovak Roma population; simple and rapid detection method by allele-specific polymerase chain reaction. Urolithiasis 2015;43:441–5. [DOI] [PubMed] [Google Scholar]
- 7. Stiburkova B, Gabrikova D, Čepek P. et al. Prevalence of URAT1 allelic variants in the Roma population. Nucleosides Nucleotides Nucleic Acids 2016;35:529–35. [DOI] [PubMed] [Google Scholar]
- 8. Claverie-Martin F, Trujillo-Suarez J, Gonzalez-Acosta H. et al. URAT1 and GLUT9 mutations in Spanish patients with renal hypouricemia. Clin Chim Acta 2018;481:83–9. [DOI] [PubMed] [Google Scholar]
- 9. Sakiyama M, Matsuo H, Shimizu S. et al. The effects of URAT1/SLC22A12 nonfunctional variants, R90H and W258X, on serum uric acid levels and gout/hyperuricemia progression. Sci Rep 2016;6:20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wakasugi M, Kazama JJ, Narita I. et al. Association between hypouricemia and reduced kidney function: a cross-sectional population-based study in Japan. Am J Nephrol 2015;41:138–46. [DOI] [PubMed] [Google Scholar]
- 11. Hamajima N, J-MICC Study Group. The Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) to detect gene-environment interactions for cancer. Asian Pac J Cancer Prev 2007;8:317–23. [PubMed] [Google Scholar]
- 12. Asai Y, Naito M, Suzuki M. et al. Baseline data of Shizuoka area in the Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study). Nagoya J Med Sci 2009;71:137–44. [PMC free article] [PubMed] [Google Scholar]
- 13. Ichida K, Matsuo H, Takada T. et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun 2012;3:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsuo H, Chiba T, Nagamori S. et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet 2008;83:744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams PT. Effects of diet, physical activity and performance, and body weight on incident gout in ostensibly healthy, vigorously active men. Am J Clin Nutr 2008;87:1480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mikuls TR, Farrar JT, Bilker WB. et al. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis 2005;64:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawamura Y, Toyoda Y, Ohnishi T. et al. Identification of a dysfunctional splicing mutation in the SLC22A12/URAT1 gene causing renal hypouricaemia type 1: a report on two families. Rheumatology (Oxford) 2020;59:3988–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawamura Y, Matsuo H, Chiba T. et al. Pathogenic GLUT9 mutations causing renal hypouricemia type 2 (RHUC2). Nucleosides Nucleotides Nucleic Acids 2011;30:1105–11. [DOI] [PubMed] [Google Scholar]
- 19. Nakayama A, Matsuo H, Abhishek A, Ichida K, Shinomiya N, Guideline Development Committee of Clinical Practice Guideline for Renal Hypouricemia. First clinical practice guideline for renal hypouricemia: a rare disorder that aided the development of urate-lowering drugs for gout. Rheumatology (Oxford) 2021;60:3961–63. [DOI] [PubMed] [Google Scholar]
- 20. Toyoda Y, Kawamura Y, Nakayama A. et al. Substantial anti-gout effect conferred by common and rare dysfunctional variants of URAT1/SLC22A12. Rheumatology (Oxford) 2021;60:5224--32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.