Abstract
Background
All-trans retinoic acid (ATRA) is a biologically active isomer of retinoic acid (RA). Topical ATRA (retin-a, retin-a micro, atralin, renova, and avita) is the active pharmaceutical ingredient for FDA-approved treatments for acne and skin wrinkles. Oral formulations (Vesanoid) treat acute promyelocytic leukemia, but oral dosing can induce severe side effects. Despite benefits in various rodent models of inflammatory bowel disease (IBD), toxicity and controversial clinical observations have diminished enthusiasm for ATRA IBD clinical trials. To circumvent these issues and to use ATRA’s key role in maintaining gut tolerance, we developed a poly(lactic-co-glycolic acid) (PLGA) microsphere (MS) encapsulated ATRA formulation aimed at directing ATRA delivery to immune structures of the gut, limiting systemic exposure. Initially, ATRA MS was developed as a component of a combinatorial product (TreXTAM) that also contained encapsulated transforming growth factor (TGF)-β and ATRA in a 1:2 w/w ratio. Although the combination was optimal, benefit was also observed when ATRA MS was given alone in the CD4+ CD25-T-cell adoptive transfer (ACT) colitis model.
Methods
We used the ACT and DSS-induced murine models of colitis to expand on the dose-dependent effects of oral ATRA MS when given alone. The DSS model was also used to compare the efficacy of ATRA MS and soluble ATRA, while healthy animals were used to compare the pharmacokinetics of the two drugs.
Results
In both the ACT and DSS-induced murine models of colitis, ATRA MS was observed to be effective in ameliorating disease. ATRA MS was also observed to be more effective than soluble ATRA in these models and displayed more favorable pharmacokinetics.
Conclusions
We suggest ATRA MS, as a standalone product, may attenuate IBD and perhaps limit fibrosis, while limiting systemic side effects.
Keywords: IBD, ATRA, Oral
Introduction
Crohn’s disease (CD), ulcerative colitis (UC), and the less common indeterminate colitis (IC) are chronic inflammatory processes of the gastrointestinal (GI) tract. Grouped together as inflammatory bowel disease (IBD), they cause significant morbidity for over one and a half million Americans.1 Diarrhea, nausea, abdominal pain, weight loss, and toxic mega colon (which can prove fatal)2 are additive to an increased risk for colorectal cancer.3 Anti-inflammatory agents and antibiotics4 are variably effective as first-line treatments; however, remissions remain all too fleeting and come with significant side effects.4 Gut fibrosis is a particularly dangerous complication often requiring surgery (required by 80% and 45% of CD and UC patients, respectively).4 The wide spread use of potent anti-inflammatory biological response modifiers or “biologics” (macromolecules that target lymphocytes or the cytokines they produce5,6) over the last few decades has achieved only modest impact on gut fibrosis.7 A postulated inflammation-independent fibrotic pathway may offer novel therapeutic targets aimed at gut fibrosis.8
Retinoic acid (RA), a metabolite of vitamin A (retinol), regulates a wide range of biological processes including embryonic development, reproduction, vision, cell growth and differentiation, apoptosis, and inflammation.9 All-trans retinoic acid (ATRA) is one of its biologically active isomers and the primary enzymatic product of retinaldehyde oxidation. All-trans retinoic acid is a key gut regulator in maintaining tolerance and driving immunity.10,11 Topical ATRA is marketed under several trade names (eg, Retin-A, Retin-A Micro, Atralin, Renova, and Avita) as FDA-approved treatments for acne and skin wrinkles. Oral formulations (Vesanoid) treat acute promyelocytic leukemia (APL) but can also come with black-box FDA warnings because of dose-limiting, severe, potentially life-threatening side effects, including retinoic acid syndrome in APL patients.12 Despite reports of ATRA benefits in rodent IBD models, including potential anti-fibrotic activity,13,14 it has never been tested in IBD clinical trials, perhaps due to dose-limiting toxicity and ambiguous clinical associations with IBD induction when given systemically.15 Efforts to circumvent these issues by tissue-targeted administration have included encapsulation16,17 and aerosolization18; to our knowledge, neither has progressed further into development or resulted in approved IBD products.
Our previous studies in this area reported that TreXTAM, an oral combinatorial product containing encapsulated transforming growth factor β (TGF-β) and ATRA in a 1:2 w/w ratio, could ameliorate disease in rodent colitis models19 via its ability to enhance regulatory T-cell activity. Although the combination was optimal, benefit was also observed when PLGA-encapsulated ATRA microspheres (ATRA MS) were given alone in the CD4+ CD25- adoptive cell transfer (ACT) colitis model. We herein confirm our previous report and expand on the dose-dependent effects of oral ATRA MS in the mouse ACT model. We also report for the first time on ATRA MS activity when given as a single agent in the mouse DSS-induced colitis model and on the activity, pharmacokinetics, toxicology, and physiochemical properties of scaled-up manufactured drug product. We suggest that oral ATRA MS may attenuate IBD and perhaps limit fibrosis with reduced systemic toxicity compared with other formulations of oral ATRA.
Materials and Methods
Preparation of ATRA MS
The ATRA MSs were prepared either by a benchtop process resulting in batch sizes of up to approximately 3 g or by a commercially applicable, scaled-up, spray-drying process allowing for kilogram-sized batches. Both the benchtop and spray-drying methods targeted 0.2% loading wt./wt. (2 mg of ATRA per gram of particles). However, extensive extraction studies, which make use of small samples of ATRA MS but do not analyze individual microspheres, suggest experimental loadings of approximately half. Thus, all measurements of experimental loading represent the aggregate loading of a large sample of microspheres, which is an appropriate approximation of the dosage quantity of ATRA MS, both in vitro and in vivo. Note that benchtop material is generally referred to as ATRA bMS, while spray-dried material is termed TPX7001 to indicate potential clinical utility.
Benchtop lots
All-trans retinoic acid (Sigma-Aldrich) was encapsulated into poly-lactic-co-glycolic acid microspheres (2 mg of ATRA per gram of particles) using a modification of the solvent evaporation technique.20 Briefly, 5.0 mg of ATRA was dissolved in 20-mL dichloromethane (DCM) to produce a 250-µg/mL solution in an amber vial. Eight mililiters of this solution was added to 1000 mg of 503H polymer (Resomer) dissolved in 32 mL of DCM to produce the oil phase. Twenty mililiters of Span 80 was also pipetted into this solution as a surfactant. To prepare the water phase, 600 mL of 1% poly(vinyl alcohol), MW25000 (PVA) was added to a glass beaker, which was placed on ice within a stainless-steel container. The water phase was then set under a Silverson mixer at a shear rate of 4000 rpm for 75 minutes as the oil phase was added in droplets via a glass pipette. The emulsion was then transferred to an overhead mixer with an additional liter of double-distilled water and spun for 225 minutes. The microspheres were washed, centrifuged, and lyophilized for 48 hours.
Spray drying
Alternatively, lots of ATRA MS were manufactured using a proprietary spray drying method developed by Therapyx in collaboration with Bend Scientific, Bend Oregon. Spray drying was conducted on a custom lab scale dryer with 35 kg/hr drying gas capacity equipped with a 2-fluid nozzle in Bend’s development facility, with an ingoing batch size of approximately 150 grams. Optimized sprays resulted in yields of approximately 90%. Exact manufacturing conditions remain proprietary. Secondary drying was performed by placing samples under vacuum for approximately 21 hours to reduce residual solvents (DCM). Physiochemical properties of ATRA MS are presented in Table 1. Microspheres produced by the spray-drying process as potential commercial product are referred to as TPX7001. Extraction studies on both benchtop and spray-dried material consistently confirmed an experimental loading of approximately half theoretical loading (data not shown).
Table 1.
Physiochemical properties of scale-up ATRA MS (TPX7001).
| Formulation | 97.8/2.0/0.2 PLGA/Span 80/ATRA |
|---|---|
| Residual Solvent (wt. %) | 2.9 ± 0.6 |
| Tg (°C) | 31.5 ± 0.2 |
| Physical State | Amorphous |
| Particle Morphology | Spheres |
Animal Models
Prophylactic model of DSS-induced colitis
Eight-week-old male and female C57BL/6 mice were kept at the Center for Advanced Preclinical In Vivo Research (CAPIR) at the University of Catania under standard laboratory conditions (nonspecific, pathogen-free) with free access to food and water and were allowed to adapt to their environment for at least 1 week before commencing the study.
Animals were housed within a limited-access rodent facility under controlled microbial conditions, which excluded murine pathogens, and were kept in groups with a maximum of 5 mice in polycarbonate cages (Tecniplast, Varese, Italy), according to the Italian legislation and as approved by the Italian Ministry of Health. This regimen does not exclude various species of Helicobacter. Cages were sterilized and filled with wood shavings as bedding material. Automatically controlled environmental conditions were set to maintain temperature at 20°C to 24°C with a relative humidity (RH) of 30% to 70%, 10 to 30 air changes per hour, and a natural dark:light cycle. Protection of animals used in the experiment is in accordance with Directive 2010/63/UE, enforced by the Italian D. Lgs 26/2014. Physical facilities and equipment for accommodation and care of animals are in accordance with the provisions of EEC Council Directive 86/609.
Severe combined immunodeficiency (SCID) mouse CD4+ T-cell transfer colitis model
Animals, method of CD4+ CD45- T-cell isolation, and method of induction of colitis were reported in our previous study.19
Treatment
Prophylactic administration of ATRA MS in DSS-induced colitis
The first treatment was given 24 hours before the induction of colitis (day 0) and continued every other day for 6 days (3 additional treatments on days 3, 5, and 7; total 4 treatments). As positive control group, 6 additional mice (3 male and 3 female) were treated per os with Sulfasalazine at the dose of 50 mg/Kg daily for 6 consecutive days starting from day 2. All test compounds were suspended in saline and always diluted in saline to obtain the required dosing. A stable milky suspension was obtained. Compounds were administered by gavage in a final volume of 0.2 mL per the schedule described previously. Treatment was identical in the study "Activity of TPX7001 compared with soluble ATRA in the murine model of DSS-induced colitis"; however, no positive control group was utilized.
Effect of dose on the efficacy of ATRA MS in the established ACT model of colitis
Treatment (3x per week via oral gavage) started when 10% of mice showed 5% or greater weight loss and/or soft or bloody stools. Doses (1, 5, or 25 mg/mouse) were suspended in 0.2 mL of saline immediately prior to administration. Treatment continued for 14 days, after which animals were humanely killed.
Pharmacokinetics of TPX7001 compared with soluble ATRA in mice
Animals (n = 18 per group) were treated with a single oral dose of either soluble ATRA (100 µg) or TPX7001 (50 mg). Blood was taken from animals at preselected time points (15, 30, 60, 120, 180, and 240 minutes).
Toxicology and pharmacokinetics of TPX7001 in a rat model
Animals were dosed by oral gavage at 2 mL/kg 3 times per week for 4 weeks (days 0, 2, 4, 7, 9, 11, 14, 16, 18, 21, 23, and 25). The concentrations of doses were dependent upon experimental group (30, 50, and 90 mg/kg), and dose volumes were calculated based upon most recent body weight. Although each dose suspension was mixed, the dose volume was loaded into a syringe for gavaging a single animal at a time.
Analytics
Disease score
Mice were monitored for disease development in a blinded fashion as was performed in our previous study.19
Histology
Proximal, mid, and distal colons (defined by equal division of the colon into 3 segments) from treated and control mice were fixed in 10% formalin, embedded in paraffin, sectioned (5 µm) and stained with hematoxylin and eosin (H&E). Five randomly selected sections from each mouse were scored for disease severity using the scoring method outlined in Table 2.
Table 2.
Histology scoring criteria.
| Score | Severity of Inflammation | Extent of Inflammation | Amount of Mucus | Degree of Proliferation |
|---|---|---|---|---|
| 0 | None | None | Normal | None |
| 1 | Mild lymphoid infiltration | Mucosal | Slight decrease | Mild increase in cell numbers and crypt length |
| 2 | Lymphoid infiltration/focal crypt degeneration | Submucosal | Severe depletion | Moderate increase. Focally marked increase |
| 3 | Multifocal crypt degeneration and/or erosions | Transmural | Severe depletion | Marked increase—entire section |
| 4 | NA | NA | Total absence | NA |
SAA levels
Serum levels of serum amyloid A (SAA) were measured using an ELISA kit (BioSource, Inc. Camarillo, CA) according to manufacturer’s instructions.
ATRA high performance liquid chromatography
The high performance liquid chromatography (HPLC) mobile phase was made of a 68:24:8 (v/v/v) ratio of acetonitrile:1% glacial acetic acid:ethanol.21 The mobile phase was adjusted to pH 7.0 using 1 N NaOH. High performance liquid chromatography analysis conditions used an isocratic HPLC system, which comprised a Waters Symmetry C18 column (3.5um, 3.0 × 150mm) at a flow rate of 0.6 mL/minute using pH 7.0 mobile phase, with a run time of 15 minutes per 10 μL injection. The HPLC column was kept in a column oven at 30ºC, and the samples were stored in the autosampler at 10ºC prior to injection. The retinoic acid was detected using a UV/Vis HPLC detector set at a wavelength of 356 nm. The standard all-trans retinoic acid eluted at 6.0 ± 0.5 minutes. No peak for all-trans retinoic acid was observed in injections of the HPLC mobile phase blank.
Statistical analysis
Significance (P ≤ .05) between experimental and control groups was determined using Student t test analysis. In experiments with multiple groups, homogeneity of intergroup variance was analyzed by ANOVA.
Results
Prophylactic Administration of ATRA MS Attenuates DSS-induced Colitis
To test the ability of various doses of ATRA MS to ameliorate IBD, the DSS-induced colitis model was initially employed, in which ATRA bMS served as the test compound. They were suspended in saline and diluted in saline to obtain the required dosing. A stable milky suspension was obtained. Compounds were administered by gavage in a final volume of 0.2 mL. We found that prophylactic (ie, beginning 24 hours before DSS challenge) treatment with ATRA bMS reduced weight loss up to 75% (Figure 1A) and improved stool scores up to approximately 25% (Figure 1B). Treatment also reduced serum amyloid A levels (Figure 1C) and improved colon weight to length ratios (Figure 1D). Although benefit, compared with animals treated with blank MS, was suggested across all doses, responses were nominally dose-dependent, in that significance (P < .05) was achieved only with the 20 mg per mouse dose. Similarly, significant histological benefit, in terms of reduced histological score and cellular infiltrates (Figure 1E, F), was achieved only with the 20-mg dose. Although Bai and colleagues13 had previously shown that soluble ATRA (0.3 mg), given as daily intraperitoneal injections beginning 3 days after DSS, reduced disease activity by approximately 50%, it is important to note that our most effective dose of ATRA was approximately 20 µg (assuming experimental loading of 0.1%); this is more than 10-fold lower than the effective dose of soluble ATRA reported by Bai and colleagues. In fact, in the DSS model, higher doses seemed less effective. Although the studies are not entirely comparable, taken together, they support the prediction that encapsulation will achieve therapeutic potency at much lower doses and with presumably reduced side effects.
Figure 1.
Effect of prophylactic ATRA bMS on disease in the DSS-induced colitis model. Body weights (A) and clinical observations (B) were taken daily. Serum was analyzed for SAA concentration (C). Colons were measured (D), then sectioned, prepared, and scored for all treatment groups, and histological scores compiled (E). Representative sections for each treatment group are presented (F). The histological sections obtained from blank-treated mice displayed a massive presence of inflammatory cells (such as neutrophils, macrophages, lymphocytes, and plasma cells), signs of edema, crypt loss, surface epithelial cell hyperplasia, and goblet cell reduction. Sections obtained from 20 mg ATRA bMS-treated mice displayed only a minor presence of inflammatory cells; edema was not observed, and both crypt spacing and goblet cell numbers appeared normal. Significance: ■, ♦, and ▲ denote P < .05, .01, and .001, respectively, compared with blank-treated mice.
Effect of Dose on the Efficacy of ATRA MS in the Established ACT Model of Colitis
In the next set of experiments, we utilized the same CD4+ CD25- T-cell transfer model of IBD executed in our previous studies.19 Dose response analysis was performed for ATRA bMS at 1, 5, and 25 mg/kg in order to expand upon the previously observed effective dose range. Mice with established disease were treated orally with increasing doses of ATRA bMS 3 times per week for 2 weeks. Selected disease markers were monitored. The differences between control (blank microspheres) and treatment groups in terms of cumulative percentage of body weight change (Figure 2A) and disease score (Figure 2B) were significant at all dose levels. Only the high-dose group showed significantly lower SAA levels (Figure 2C). Although the high-dose treatment improved colon weight to length ratios (data not shown), as in our previous studies, the effect did not reach statistical significance. Histological analysis confirmed benefit only for the high-dose group (Table 3). Thus, by all 3 measures, the 25-mg ATRA bMS dose appeared most effective as in our previous studies. These observations—in both the DSS and ACT models of colitis—incentivized the further development towards commercialization of ATRA MS alone for the treatment of IBD.
Figure 2.
Effect of therapeutic ATRA bMS on disease in the ACT colitis model. Body weights (A) and clinical observations (B) were taken daily. Serum was analyzed for SAA concentration (C). Colon weight to length ratios were calculated; differences between the treatment groups did not attain statistical significance (data not shown). *P < .05 and **P < .01.
Table 3.
histological analysis of colons in murine ACT model of colitis.
| Blank | 25 mg ATRA | |||
|---|---|---|---|---|
| Parameter | Average Score | Standard Dev. | Average Score | Standard Dev. |
| Inflammation Severity | 1.4 | 0.83 | 1.3 | 0.48 |
| Inflammation Extent | 1.6 | 0.99 | 1.5 | 0.53 |
| Mucus Amount | 1.6 | 1.18 | 1.6 | 1.07 |
| Proliferation Degree | 1.5 | 1.06 | 1.0 | 0.82 |
| Composite Score | 6.1 | 3.31 | 5.4 | 2.22 |
Characterization of Spray-dried ATRA MS (TPX7001)
Our benchtop process20 produces batches of up to 3 grams, which is insufficient for clinical studies and incompatible with potential commercialization. Therefore, we worked in collaboration with Bend Research (Bend, Oregon) on a fee-for-service basis to develop spray-drying manufacturing methods capable of producing kilogram-sized batches. We produced 2 large (>150 g) batches of drug product (TPX7001) using this method, which were remarkably consistent in terms of physiochemical properties, release kinetics, and long term (>1 year) stability (data not shown).
Three-day ATRA release patterns for TPX7001 vs those manufactured using the benchtop process are presented in Figure 3A. Both yielded approximately 6 to 7 ug/mL ATRA at 24 hours. This relatively large release of ATRA during the first 24-hour period from both forms of ATRA MS may represent the dissociation of ATRA that is loosely associated with the surface of the microspheres. For both products, approximately 50% reductions in released ATRA were observed after each successive 24-hour period. Particle diameters for TPX7001 were characterized using a particle size analyzer (LS 13 320 Laser Diffraction Particle Size Analyzer, Beckman Coulter, USA). Particle sizes ranged from 2 to about 60 µM, with a median size of about 18 µM (Figure 3B); a characteristic scanning electron micrograph is displayed in Figure 3C. Importantly, extraction studies on both benchtop and spray-dried material consistently confirmed an experimental loading of approximately half theoretical loading (data not shown). We next tested the in vivo activity of TPX7001.
Figure 3.
Three-day ATRA release patterns and physical appearance of spray-dried vs benchtop ATRA MS. All-trans retinoic acid levels in supernatants were measured by HPLC (A). Data are averages of duplicate runs from one to 3 separate release samples and are expressed as ug/mL, +/- SD. Particle diameters for spray-dried lots (B) were characterized using a laser diffraction particle size analyzer. An electron micrograph of the spray-dried material was used to determine particle morphology (C).
Activity of TPX7001 Compared With Soluble ATRA in the Murine Model of DSS-induced Colitis
To demonstrate in vivo activity of TPX7001 and to compare with soluble material, we tested both TPX7001 and soluble ATRA side by side in the prophylactic iteration of the murine model of DSS-induced colitis. We used TPX7001 at the optimal dose of 20 mg/mouse and soluble ATRA at 20 µg/mouse to give equivalent dose masses, assuming experimental loading. We found that pretreatment with either TPX7001 or soluble ATRA reduced disease parameters, including significantly attenuated weight loss (Figure 4A), stool score (Figure 4B), colon weight to length ratio (Figure 4C) and SAA concentration (Figure 4D). Importantly, TPX7001 appeared to perform better in all disease measures, indicating that the spray-dried encapsulated material performed at least as well as, if not better than, the soluble material. This conclusion was confirmed by histological analysis (Figure 4E, F) in that both TPX7001 and soluble ATRA reduced inflammatory cellular infiltrates and significantly improved histological score. In the histological analysis as well, TPX7001 appeared to perform better than soluble material.
Figure 4.
Effect of prophylactic TPX7001 vs soluble ATRA in the DSS-induced colitis model. Body weights (A) and clinical observations (B) were taken daily. Serum was analyzed for SAA concentration (D). Colons were measured (C), then sectioned, prepared with H&E, and scored for all treatment groups, and histological scores compiled (E). Representative sections for each treatment group are presented (F). Sections obtained from saline-treated mice show alteration to the goblet cell architecture within the mucosa. A diffuse infiltration of inflammatory cells into the stroma and submucosa was also observed, as well as significant edema and a consequent increase in colon wall thickness. Sections obtained from both the TPX7001- and soluble ATRA-treated mice show, relative to saline-treated mice, a reduction of inflammatory cell infiltration, edema, and epithelium hyperplasia. These sections displayed normal goblet cell architecture. *P < .05, **P < .01, and ***P < .001, respectively.
Pharmacokinetics of TPX7001 Compared With Soluble ATRA in Mice
To gain insight into the bioavailability and pharmacokinetics of ATRA following a single oral administration of soluble ATRA or TPX7001, we tested both compounds side by side in C57BL/6 mice. Animals were administered either soluble ATRA (100 ug) or TPX7001 (50 mg). In this study, we chose to assume theoretical loading, cognizant that effectively delivered doses of ATRA in the TPX7001-treated group might be half that of the soluble group. We chose to employ this higher dose of soluble ATRA relative to the experimental dose of ATRA in TPX7001 to afford the best opportunity to observe differences between groups. Serum was collected at predetermined timepoints, and animals were additionally evaluated for clinical signs and body weights prior to sacrifice. The obtained data show that TPX7001-treated animals reached a serum concentration peak 7 times lower and 30 minutes later than soluble ATRA-treated animals. In addition, the half-life of TPX7001 was observed to be approximately half that of soluble ATRA. The maximum serum concentration/dose and area-under-curve of TPX7001 were observed to be 7- and 10-fold less than those of soluble ATRA, respectively, showing significantly reduced systemic ATRA exposure in TPX7001-treated animals when compared with animals treated with matched-dose soluble ATRA (Figure 5).
Figure 5.
Serum levels of ATRA after a single oral dose of TPX7001 or soluble ATRA in Mice. Blood was taken via a terminal bleed at the indicated time points (n = 3 per time point), reduced to serum and levels of ATRA measured by HPLC. Data are expressed as ng/mL +/- standard deviation.
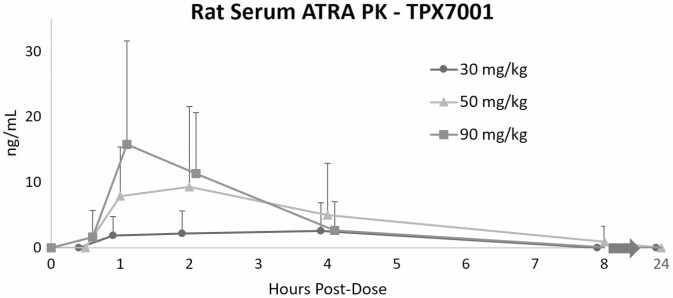
Toxicology and Pharmacokinetics of TPX7001 in a Rat Model
During development of our TGF-β and ATRA combination product (TreXTAM), we studied the pharmacokinetics of ATRA after oral administration of TPX7001 alone (as per FDA requirements) in 28-day thrice weekly GLP toxicology studies in rats. These included full industry-standard toxicology analyses, clinical observations, clinical pathology, necropsy, histopathology, and ophthalmology. There were no statistically significant differences in body weights or weekly food intake among groups and no significant organ weight changes. There were no test article-related histopathological or other findings, and no fibrosis was observed with even the highest doses at the end of treatment (day 28) or at the end of a 56-day recovery period (data not shown). The study showed that 3 times weekly oral administrations of TPX7001 at 30 mg/kg, 50 mg/kg, and 90 mg/kg for 4 weeks were well tolerated by the rats under the conditions of the study.
As part of this work, toxicokinetic studies were performed (Figure 6). We found dose-dependent transient increases in serum ATRA levels after TPX7001 dosing, with peaks between 1 and 2 hours. Levels were barely detectable after 8 hours and were not observed 24 hours after dosing, indicating no dose building with thrice weekly treatments. The Cmax, T1/2 and AUC in the rats were comparable with our previous studies.22
Figure 6.
Pharmacokinetics of TPX7001 in rats. Animals were bled at each indicated time point; blood was then processed to serum and levels of ATRA measured by HPLC. Data are expressed as ng/mL, +/- standard deviation.
Discussion
We report that TPX7001 is superior to soluble ATRA in the amelioration of multiple models of colitis, in that it displays more favorable systemic pharmacodynamics and equivalent efficacy when roughly equal-dose masses are orally administered. Oral delivery is the most convenient, cost-effective, and patient-compliant route for drug administration.23 However, systemic exposure to certain oral therapeutics, including ATRA, comes with significant toxicities; thus, limiting systemic exposure to ATRA while maintaining efficacy in the treatment of colitis is critical. Strategies to minimize systemic exposure while targeting disease signs include encapsulation in sustained-release microparticles. Such efforts have generally focused on siRNA,24 small molecules,25 peptides,26 and cytokines27 and involve polymer encapsulation accomplished via combinations of phase separation or precipitation, emulsion/solvent evaporation,28–35 and/or spraying methods.36–40 Poorly controlled release rates, loss of bioactivity during manufacturing, and difficulties with large-scale production of accurately sized microparticles remain formidable challenges. However, TPX7001 is a commercially viable PLGA-encapsulated ATRA formulation aimed at circumventing these issues and targeting delivery of ATRA to the immune structures of the gut.
Our initial efforts in this area were focused on encapsulated ATRA as part of an oral combinatorial product containing both encapsulated transforming growth factor β and ATRA in a 1:2 w/w ratio. Our previous studies showed this combination could ameliorate disease in rodent colitis models.19 Those studies also demonstrated activity with oral ATRA MS when given alone in the ACT model of murine IBD. We herein confirm those observations and report potentially clinically significant activity in prophylactic iterations of DSS-induced murine colitis. Doses used in the present studies for the DSS and ACT models were based on effective doses observed in our previous studies. We found oral dosing with 20 mg of ATRA MS provides benefit in both rodent models of colitis. Even assuming a 0.2% loading, this corresponds to a theoretical maximal ATRA dose of 40 µg ATRA. Using this conservative figure, simple allometric dosing would predict an effective human dose at about 100 mg/kg (about 7000 mg drug product) given every 3 days. This would correspond to a total dose of ATRA of 14 mg, or 0.2 mg/kg, which is 5-fold less than existing oral formulations of ATRA.41 However, it is important to remember that encapsulated drug substance is released slowly over days19 directly into the immune structures of the gut,27 with very different pharmacokinetics compared with soluble material. Thus, simple allometric scaling may yield an inflated estimate of the quantity of TPX7001 required for efficacy in humans. A linear extrapolation based on the ratio of small intestine length in mice and humans42 suggests that the effective dose of TPX7001 in humans may be as low as 400 mg, corresponding to a total dose of ATRA of 800 µg, or 12 mg/kg. However, it is important to note that the high dose of ATRA MS (60 mg) was not as effective as the low dose (20 mg) in the DSS colitis model. The reasons for this are unknown but may relate to differential effects on different gut cell types, including those with the ability to synthesize RA in response to inflammation and/or differentially express RARα, RARβ and RARγ receptors.43 How RA and RARs influence different immune cells,44 including B lymphocytes, T lymphocytes, and myeloid cells in the gut and how this modulation could vary depending on the cellular developmental stage and the milieu have recently been reviewed.45 Interestingly, this review discusses how ATRA may impact gut permeability via enhanced gut colonization of certain probiotics. All-trans retinoic acid may also directly impact gut permeability through altering the expression of tight junction proteins, as has been recently demonstrated in skin.46 Impact on leaky gut may suggest applications across other autoimmune diseases.47 We did not observe the phenomenon of diminishing effect at higher doses in the ACT model, where higher doses always appeared more efficacious. This may relate to more direct effects on aggressive autoreactive cells that drive disease in that model. Starting doses in clinical studies will be based on minimally effective doses and FDA-required toxicology studies.
Our preliminary pharmacokinetic studies in rodents suggest minimal systemic exposure of ATRA associated with oral dosing of TPX7001. In a preliminary side-by-side PK study in mice, even given that total doses of ATRA in TPX7001-treated group may have been half that of the soluble group, assuming experimental instead of theoretical loading, we still observed Cmax/D and AUC levels of TPX7001 at 7- and 10-fold less than those of soluble. This is consistent with our studies in rats where, importantly, we also found thrice-weekly administration of 90 mg/kg of TPX7001 over 28 days to be safe and generally well tolerated. Allometric dosing would suggest dosing of up to 7 g would be safe and well tolerated in humans. Given our estimates for efficacious doses in humans, the therapeutic window for TPX7001 may be as large as 10x the minimum effective dose. Of course, TPX7001 pharmacokinetics in humans will be determined in phase 1 studies.
An important aspect of the present studies was the demonstration that TPX7001 appears and behaves similarly to benchtop lots in terms of release pattern and physicochemical properties and remains active in IBD models. We are currently engaged in the synthesis of good manufacturing practice (GMP) lots suitable for use in IBD patients, where emergent issues including ultimate dose mass and the relationship between release pattern, loading efficiency, and lot consistency, will be resolved.
Our observations add to a wide body of literature reporting benefit of ATRA in rodent models of colitis, as we have reviewed.22,48 Observations included work in mouse DSS, 2,4,6-Trinitrobenzenesulfonic acid (TNBS),13,14 and TNFΔARE mutation models,49 along with our own previous work in rodent ACT and DSS IBD models.19 Observations in humans have been more equivocal, even controversial. Meta-analysis of available data sets did not support any increased risk of inflammatory bowel disease associated with even high doses of related products such as isotretinoin.15 On the contrary, all preclinical work in rodents and even one published epidemiological study50 suggest that ATRA might provide benefit to IBD patients. The clinical utility of exogenous ATRA as an IBD treatment remains to be tested. Treatment with TPX7001 is our clinical candidate aimed at that goal.
We are not the only group to attempt ATRA encapsulation in PLGA-based microspheres. A decade ago, Capurso and colleagues reported on a PLGA-based particulate drug delivery platform that released ATRA in vitro.16 Their method involved a single emulsion nanoparticle fabrication technique that produced very similar ATRA MS. Particles were submicron-sized (~ 0.25 µM) spherical PLGA MS with ATRA molecules entrapped in the polymer matrix. Loading was reported as 2 to 3 µg/ATRA per mg PLGA. The in vitro ATRA release curve was also quite similar, with an initial burst during the first 48 hours followed by slower, steady release over at least 5 days.
Importantly, this group performed in vitro mechanistic studies with these particles, alongside soluble ATRA, that confirmed ATRA abrogated interleukin (IL)-17 and IFN-γ production from T helper (Th)-17 cells, decreased intracellular expression of IL-17 (66%), and the key Th17 transcription factor RORγ (33%–50%). All-trans retinoic acid also enhanced regulatory T cells, both in terms of FOXp3 expression (about 4-fold) and IL-10 production (about 6-fold). Both soluble and particulate ATRA abrogated IL-17-dependent IL-6 production by cocultured fibroblasts. This work demonstrated that ATRA released from PLGA encapsulation, at least in vitro, performs as expected in terms of immunological bioactivity. More specifically, the data suggested a mechanism, in the context of IBD, whereby low doses of ATRA could provide a benefit, especially if it is delivered directly to the immune structures of the gut.
Finally, intestinal fibrosis is an all too common and potentially serious complication of IBD. Especially dangerous in the context of Crohn’s disease, it can often lead to bowel obstruction and stricture resection. Because of this alarming contribution to the morbidity and mortality of IBD, intestinal fibrosis has commanded significant attention. Driven by activated mesenchymal cells and marked by an excessive deposition of extracellular matrix (ECM), it was long thought to be an irreversible, inevitable consequence of chronic inflammation. Although potent anti-inflammatory biologic therapies have revolutionized IBD treatment, they have made little impact on fibrosis, calling into question the causal link between inflammation and fibrosis in IBD.
Even though ATRA has not been shown to treat IBD fibrosis specifically, and the present study does not address the potential impact of ATRA on gut fibrosis, there is a wide, suggestive literature based on the ability of ATRA to limit fibrosis in various other tissues.51–54 At the same time, a small number of reports also suggest that ATRA might enhance ECM production and exacerbate fibrosis both in vitro and in vivo. In 2013, this controversial point was reviewed in detail by Zhou and colleagues.55 Along with efforts to elucidate the mechanism, that body of work implies that exogenous ATRA treatments could be effective at reducing IBD fibrosis. This suggests a novel potential application for TPX7001 as a preventative treatment not only for IBD-associated flares/inflammation but also for gut fibrosis. This is the subject of forthcoming work from our laboratories.
To expand our understanding of the potential of TPX7001 to treat IBD outside of the context of acute flares, future studies will employ chronic colitis models, such as the IL-10-/- model in mice. This model is a well-established Th1 model of transmural colitis and cecal inflammation that has been observed to be ameliorated by various therapeutic agents, and its results tend to be reliable predictors of those agents’ effects in humans.56 Additionally, we plan to compare the efficacy of TPX7001 and existing therapeutics, such as sulfasalazine, budesonide, and anti-TNFα, in both acute and chronic models of colitis to facilitate direct comparison of the efficacy of TPX7001 to other products. In these experiments, we will also measure the response of pro- and anti-inflammatory cytokines to allow for a bolstering of the mechanism by which TPX7001 limits intestinal inflammation.
In summary, our studies confirm a wide body of evidence suggesting ATRA can provide benefit to IBD patients and specifically support the continued development of TPX7001 as a standalone product to treat IBD.
Funding
This work was supported by The National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award No 5R44AI080009. Research reported in this publication was supported by the National Institute of Allergy and Infectious Disease of the National Institutes of Health under award number 5R44AI080009-07.
Conflicts of Interest
D.A. holds shares in Therapyx, Inc. The authors declare that there are no other conflicts of interest.
References
- 1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9:405–410. [DOI] [PubMed] [Google Scholar]
- 3. Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. [DOI] [PubMed] [Google Scholar]
- 4. Podolsky DK. The current future understanding of inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2002;16:933–943. [DOI] [PubMed] [Google Scholar]
- 5. Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136:1182–1197. [DOI] [PubMed] [Google Scholar]
- 6. Neurath MF, Finotto S. Translating inflammatory bowel disease research into clinical medicine. Immunity. 2009;31:357–361. [DOI] [PubMed] [Google Scholar]
- 7. Cannom RR, Kaiser AM, Ault GT, et al. Inflammatory bowel disease in the United States from 1998 to 2005: has infliximab affected surgical rates? Am Surg. 2009;75:976–980. [PubMed] [Google Scholar]
- 8. Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:340–350.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stevison F, Jing J, Tripathy S, Isoherranen N. Role of retinoic acid-metabolizing cytochrome P450s, CYP26, in inflammation and cancer. Adv Pharmacol. 2015;74:373–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. von Boehmer H. Oral tolerance: is it all retinoic acid? J Exp Med. 2007;204:1737–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cassani B, Villablanca EJ, De Calisto J, et al. Vitamin A and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol Aspects Med. 2012;33:63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vahdat L, Maslak P, Miller WH Jr, et al. Early mortality and the retinoic acid syndrome in acute promyelocytic leukemia: impact of leukocytosis, low-dose chemotherapy, PMN/RAR-alpha isoform, and CD13 expression in patients treated with all-trans retinoic acid. Blood. 1994;84:3843–3849. [PubMed] [Google Scholar]
- 13. Bai A, Lu N, Zeng H, et al. All-trans retinoic acid ameliorates trinitrobenzene sulfonic acid-induced colitis by shifting Th1 to Th2 profile. J Interferon Cytokine Res. 2010;30:399–406. [DOI] [PubMed] [Google Scholar]
- 14. Hong K, Zhang Y, Guo Y, et al. All-trans retinoic acid attenuates experimental colitis through inhibition of NF-kappaB signaling. Immunol Lett 2014; 162: 34– 40. [DOI] [PubMed] [Google Scholar]
- 15. Coughlin SS. Clarifying the purported association between isotretinoin and inflammatory bowel disease. J Environ Health Sci 2015;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Capurso NA, Look M, Jeanbart L, et al. Development of a nanoparticulate formulation of retinoic acid that suppresses Th17 cells and upregulates regulatory T cells. Self Nonself. 2010;1:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ozpolat B, Lopez-Berestein G. Liposomal-all-trans-retinoic acid in treatment of acute promyelocytic leukemia. Leuk Lymphoma. 2002;43:933–941. [DOI] [PubMed] [Google Scholar]
- 18. Wang DL, Marko M, Dahl AR, et al. Topical delivery of 13-cis-retinoic acid by inhalation up-regulates expression of rodent lung but not liver retinoic acid receptors. Clin Cancer Res. 2000;6:3636–3645. [PubMed] [Google Scholar]
- 19. Conway TF, Hammer L, Furtado S, et al. Oral delivery of particulate transforming growth factor beta 1 and all-trans retinoic acid reduces gut inflammation in murine models of inflammatory bowel disease. J Crohns Colitis. 2015;9:647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeong YI, Song JG, Kang SS, et al. Preparation of poly(DL-lactide-co-glycolide) microspheres encapsulating all-trans retinoic acid. Int J Pharm. 2003;259:79–91. [DOI] [PubMed] [Google Scholar]
- 21. Hammer L, Furtado S, Mathiowitz E, Auci DL. Oral encapsulated transforming growth factor β1 reduces endogenous levels: Effect on inflammatory bowel disease. World J Gastrointest Pharmacol Ther. 2020;11:79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Auci D, Egilmez NK, Dryden, GW. Anti-fibrotic potential of all trans retinoic acid in inflammatory bowel disease. Gastroenterol Pancreatol Liver Disord. 2018;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamanaka YJ, Leong KW. Engineering strategies to enhance nanoparticle-mediated oral delivery. J Biomater Sci Polym Ed. 2008;19:1549–1570. [DOI] [PubMed] [Google Scholar]
- 24. Aouadi M, Tesz GJ, Nicoloro SM, et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pertuit D, Moulari B, Betz T, et al. 5-amino salicylic acid bound nanoparticles for the therapy of inflammatory bowel disease. J Control Release. 2007;123:211–218. [DOI] [PubMed] [Google Scholar]
- 26. Mathiowitz E, Jacob JS, Jong YS, et al. Biologically erodable microspheres as potential oral drug delivery systems. Nature. 1997;386:410–414. [DOI] [PubMed] [Google Scholar]
- 27. Chung AY, Li Q, Blair SJ, et al. Oral interleukin-10 alleviates polyposis via neutralization of pathogenic T-regulatory cells. Cancer Res. 2014;74:5377–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benoit MA, Baras B, Gillard J. Preparation and characterization of protein-loaded poly(epsilon-caprolactone) microparticles for oral vaccine delivery. Int J Pharm. 1999;184:73–84. [DOI] [PubMed] [Google Scholar]
- 29. Chu LY, Xie R, Zhu JH, et al. Study of SPG membrane emulsification processes for the preparation of monodisperse core-shell microcapsules. J Colloid Interface Sci. 2003;265:187–196. [DOI] [PubMed] [Google Scholar]
- 30. Lee SC, Oh JT, Jang MH, Chung SI. Quantitative analysis of polyvinyl alcohol on the surface of poly(D, L-lactide-co-glycolide) microparticles prepared by solvent evaporation method: effect of particle size and PVA concentration. J Control Release. 1999;59:123–132. [DOI] [PubMed] [Google Scholar]
- 31. Deng X, Zhou S, Li X, et al. In vitro degradation and release profiles for poly-dl-lactide-poly(ethylene glycol) microspheres containing human serum albumin. J Control Release. 2001;71:165–173. [DOI] [PubMed] [Google Scholar]
- 32. Ma GH, Su ZG, Omi S, et al. Microencapsulation of oil with poly(styrene-N,N-dimethylaminoethyl methacrylate) by SPG emulsification technique: effects of conversion and composition of oil phase. J Colloid Interface Sci. 2003;266:282–294. [DOI] [PubMed] [Google Scholar]
- 33. Supsakulchai A, Ma GH, Nagai M, Omi S. Preparation of uniform titanium dioxide (TiO2) polystyrene-based composite particles using the glass membrane emulsification process with a subsequent suspension polymerization. J Microencapsul. 2003;20:1–18. [PubMed] [Google Scholar]
- 34. Wei G, Pettway GJ, McCauley LK, Ma PX. The release profiles and bioactivity of parathyroid hormone from poly(lactic-co-glycolic acid) microspheres. Biomaterials. 2004;25:345–352. [DOI] [PubMed] [Google Scholar]
- 35. Yang YY, Chung TS, Ng NP. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials. 2001;22:231–241. [DOI] [PubMed] [Google Scholar]
- 36. Freitas S, Merkle HP, Gander B. Ultrasonic atomisation into reduced pressure atmosphere–envisaging aseptic spray-drying for microencapsulation. J Control Release. 2004;95:185–195. [DOI] [PubMed] [Google Scholar]
- 37. He P, Davis SS, Illum L. Chitosan microspheres prepared by spray drying. Int J Pharm. 1999;187:53–65. [DOI] [PubMed] [Google Scholar]
- 38. Mu L, Feng SS. Fabrication, characterization and in vitro release of paclitaxel (Taxol) loaded poly (lactic-co-glycolic acid) microspheres prepared by spray drying technique with lipid/cholesterol emulsifiers. J Control Release. 2001;76:239–254. [DOI] [PubMed] [Google Scholar]
- 39. Quaglia F, De Rosa G, Granata E, et al. Feeding liquid, non-ionic surfactant and cyclodextrin affect the properties of insulin-loaded poly(lactide-co-glycolide) microspheres prepared by spray-drying. J Control Release. 2003;86:267–278. [DOI] [PubMed] [Google Scholar]
- 40. Wang FJ, Wang CH. Sustained release of etanidazole from spray dried microspheres prepared by non-halogenated solvents. J Control Release. 2002;81:263–280. [DOI] [PubMed] [Google Scholar]
- 41. Roche Laboratories Inc. VESANOID (tretinoin) CAPSULES. 1998. https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/20438s004lbl.pdf. Accessed 04/01/2021.
- 42. Hugenholtz F, de Vos WM. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci. 2018;75:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Czarnewski P, Das S, Parigi SM, Villablanca EJ. Retinoic acid and its role in modulating intestinal innate immunity. Nutrients 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abdelhamid L, Luo XM. Retinoic acid, leaky gut, and autoimmune diseases. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li J, Li Q, Geng S. All-trans retinoic acid alters the expression of the tight junction proteins Claudin-1 and -4 and epidermal barrier function-associated genes in the epidermis. Int J Mol Med. 2019;43:1789–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yadav SK, Boppana S, Ito N, et al. Gut dysbiosis breaks immunological tolerance toward the central nervous system during young adulthood. Proc Natl Acad Sci U S A. 2017;114:E9318–E9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Auci DL, Egilmez NK. Synergy of transforming growth factor beta 1 and all trans retinoic acid in the treatment of inflammatory bowel disease: role of regulatory T cells. J Gastroenterol Pancreatol Liver Disord. 2016;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Collins CB, Aherne CM, Kominsky D, et al. Retinoic acid attenuates ileitis by restoring the balance between T-helper 17 and T regulatory cells. Gastroenterology. 2011;141:1821–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rashtak S, Khaleghi S, Pittelkow MR, et al. Isotretinoin exposure and risk of inflammatory bowel disease. JAMA Dermatol. 2014;150:1322–1326. [DOI] [PubMed] [Google Scholar]
- 51. Wang L, Potter JJ, Rennie-Tankersley L, et al. Effects of retinoic acid on the development of liver fibrosis produced by carbon tetrachloride in mice. Biochim Biophys Acta. 2007;1772:66–71. [DOI] [PubMed] [Google Scholar]
- 52. Tabata C, Kadokawa Y, Tabata R, et al. All-trans-retinoic acid prevents radiation- or bleomycin-induced pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:1352–1360. [DOI] [PubMed] [Google Scholar]
- 53. Choudhary R, Palm-Leis A, Scott RC 3rd, et al. All-trans retinoic acid prevents development of cardiac remodeling in aortic banded rats by inhibiting the renin-angiotensin system. Am J Physiol Heart Circ Physiol. 2008;294:H633–H644. [DOI] [PubMed] [Google Scholar]
- 54. Okoshi K, Kubo H, Nagayama S, et al. All-trans-retinoic acid attenuates radiation-induced intestinal fibrosis in mice. J Surg Res. 2008;150:53–59. [DOI] [PubMed] [Google Scholar]
- 55. Zhou TB, Drummen GP, Qin YH. The controversial role of retinoic acid in fibrotic diseases: analysis of involved signaling pathways. Int J Mol Sci. 2012;14:226–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang H, Vilches-Moure JG, Cherkaoui S, et al. Chronic model of inflammatory bowel disease in IL-10-/- transgenic mice: evaluation with ultrasound molecular imaging. Theranostics. 2019;9:6031–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]