Abstract
Objectives
Knee joint distraction (KJD) has been shown to result in long-term clinical improvement and short-term cartilage restoration in young OA patients. The objective of the current study was to evaluate MRI cartilage thickness up to 10 years after KJD treatment, using a 3D surface-based approach.
Methods
Twenty end-stage knee OA patients were treated with KJD. MRI scans (1.5 T) were performed before and at 1, 2, 5, 7, and 10 years after treatment. Tibia and femur cartilage segmentation and registration to a canonical surface were performed semi-automatically. Statistical parametric mapping with linear mixed models was used to analyse whole-joint changes. The influence of baseline patient characteristics was analysed with statistical parametric mapping using linear regression. Relevant weight-bearing parts of the femur were selected to obtain the average cartilage thickness in the femur and tibia of the most- (MAC) and least-affected compartment. These compartmental changes over time were analysed using repeated measures ANOVA; missing data was imputed. In all cases, P <0.05 was considered statistically significant.
Results
One and 2 years post-treatment, cartilage in the MAC weight-bearing region was significantly thicker than pre-treatment, gradually thinning after 5 years, but still increased at 10 years post-treatment. Long-term results showed that areas in the least-affected compartment were significantly thicker than pre-treatment. Male sex and more severe OA at baseline somewhat predicted shorter-term benefit (P >0.05). Compartmental analyses showed significant short- and long-term thickness increase in the tibia and femur MAC (all P <0.05).
Conclusion
KJD results in significant short- and long-term cartilage regeneration, up to 10 years post-treatment.
Trial registration
Netherlands Trial Register, https://www.trialregister.nl, NL419.
Keywords: OA, knee joint distraction, joint-preserving, MRI, cartilage thickness, long-term
Rheumatology key messages.
Knee joint distraction provides cartilage repair activity that after 10 years still shows structural benefit.
In the least-affected compartment, a delayed regenerative response seems to take place.
This could help find drivers of intrinsic cartilage repair, providing leads for cartilage repair strategies.
Introduction
End-stage knee OA is often treated with a total knee arthroplasty (TKA), which generally reduces knee pain and improves function [1]. However, in younger patients (<65 years), TKA treatment brings an increased risk of requiring complex and costly revision surgery later in life [2]. In these patients, a joint-preserving treatment could postpone a first TKA and possibly prevent future revision surgery. One such joint-preserving surgical treatment is knee joint distraction (KJD). In distraction surgery, the two bony ends of a joint are temporarily placed at a small distance from each other by an external frame, which is fixed to the bones with bone pins [3]. KJD has been evaluated in a limited number of clinical studies, including two randomized controlled trials, and the treatment has shown good results comparable with those of alternative surgical treatments (TKA and high tibial osteotomy) [4–10]. KJD has also been applied in regular care, where it has also been shown to result in clinical improvement [11]. In addition to clinical effects, cartilage restoration activity has been demonstrated on radiographs and MRI scans, especially in the first 2 years after treatment [12–16]. The first long-term clinical analyses showed beneficial results up to 9 years after treatment, and MRI scans up to 5 years after treatment showed better results in patients treated with KJD than in untreated OA patients from the OA initiative [14, 15]. However, despite the many studies that have been performed, MRI scans have not been evaluated long-term more than 5 years after KJD. The objective of this study was to evaluate MRI cartilage thickness up to 10 years after KJD treatment, looking not only at (sub)regional cartilage thickness measurements, but primarily at the whole articular area in 3 D using a surface-based approach[17].
Methods
Patients
Between 2006 and 2008, 20 patients with end-stage knee OA were included in an open prospective study. Inclusion criteria were age <60 years old, visual analogue scale of pain of ≥60 mm, radiographic signs of joint damage, and primarily tibiofemoral OA. Exclusion criteria were severe symptoms in both knees, history of inflammatory or septic arthritis, and severe malalignment (>10°). Patients were in regular care indicated for TKA surgery but treated with KJD instead because of their young age.
KJD treatment was performed using an external fixation frame consisting of two monotubes (Stryker), fixed to the femur and tibia on the lateral and medial side of the joint with four pairs of bone pins. The joint was distracted 2 mm at surgery, and gradually extended by 1 mm per day over the next 3 days until 5 mm distraction was reached, confirmed radiographically. After full distraction was completed, patients were discharged from the hospital, and encouraged to load the distracted joint, using crutches if necessary. After 2 months, the frame and pins were removed under anaesthesia, after which patients were discharged the same day, without further imposed rehabilitation protocol.
The study was approved by the medical ethical review committee of the University Medical Center Utrecht (04/086) and complied with the Declaration of Helsinki. All patients gave written informed consent.
MRI analyses
MRI scans (1.5 T) including a coronal 3 D spoiled gradient recalled echo sequence with fat suppression (SPGR-fs) were acquired shortly before and at 1, 2, 5, 7, and 10 years after surgical treatment. A slice thickness of 1.5 mm, repetition time of 20 ms, echo time of 9 ms, flip angle of 15 degrees, acquisition matrix of 512 × 512 pixels, and pixel size of 0.31 × 0.31 mm were used. Images were imported into Stradview v6.0 (University of Cambridge Department of Engineering, Cambridge, UK, in-house developed software freely available at https://mi.eng.cam.ac.uk/Main/StradView), which was used for semi-automatic cartilage segmentation. Initial contours were drawn manually for the tibia and femur every five slices, from which a 3 D isosurface was generated for the two bones separately. The inner and outer cartilage surfaces were measured automatically in every slice and checked manually. Data sampled along a vector at the normal to each vertex of the surface on the cartilage patches was used to calculate the distance between the inner and outer surface and with that obtain the cartilage thickness at each vertex via model-based deconvolution. This process was performed for every scan for patches of the femur, medial and lateral tibia separately, and has previously been described in more detail [17].
The outer surface of all obtained patches were registered to representative canonical surfaces using an initial similarity transformation and subsequent thin-plate spline registration, performed in wxRegSurf v18 (University of Cambridge Department of Engineering, Cambridge, UK, in-house developed software freely available at http://mi.eng.cam.ac.uk/∼ahg/wxRegSurf/) to allow the comparing of patches from multiple scans.
Initial analyses focused on the whole joint (patches). To analyse the average cartilage thickness on both sides of the joint separately, relevant medial and lateral weight-bearing parts of the femur were selected (cut out) from the canonical surface (and thus applied identically in all patients and time points) in wxRegSurf (Supplementary Fig. S1, available at Rheumatology online). An average cartilage thickness for both the femur and tibia on both sides of the joint could be generated by averaging the thickness values of all vertices in the four parts separately.
Statistical analyses
Whole-joint analyses
MATLAB R2020a and the SurfStat MATLAB package (https://www.math.mcgill.ca/keith/surfstat/, modified for this specific application by Graham Treece of the University of Cambridge) were used for whole-joint, vertex-wise data analysis and visualization. The average cartilage thickness was displayed for each time point separately by averaging data of all available patients at each specific time point. Statistical parametric mapping (SPM) was used for analysis of changes over time. SPM uses all subject values at each vertex for testing between time points and delivers P-values corrected for multiple comparisons [18]. For differences at each follow-up moment compared with baseline, SPM with linear mixed models was used. The influence of the baseline patient characteristics on the changes over time was also analysed with SPM, using a separate linear regression model for each different patient characteristic and its influence on short-term (2-year) and long-term (10-year) changes. In all cases, a threshold P-value of <0.05 was considered statistically significant. Since KJD has previously shown significant results mostly in the patients’ most affected compartment (MAC), patients were separated in two groups based on whether their MAC was the medial or lateral compartment.
Compartmental analyses
For each time point, the average cartilage thickness was calculated for the medial and lateral femur and tibia. Instead of analysing changes over time for the medial and lateral side areas, changes over time were analysed for the MAC (either medial or lateral) and least affected compartment (LAC; either lateral or medial). As such, the four different compartments analysed at each time point were the MAC and LAC femur and tibia. Compartmental statistical analyses were performed in IBM SPSS Statistics 25. In the case of data missing over the entire 10 years, for the statistical compartmental analyses (not for the whole-joint surface-based analyses) multiple imputation was performed for each compartment separately for all patients; missing data was replaced by the average of five imputations based on the data available before loss of follow-up data. This was considered valid, as previous data have shown that those patients who underwent arthroplasty after several years within the 10-year follow-up period had no significant change in clinical or structural radiographic outcome shortly before arthroplasty [15]. As a sensitivity analysis for imputation validity, patients with complete datasets were analysed separately. Changes over time were analysed using repeated measures ANOVA. Additionally, as patients filled out the WOMAC at the same time points MRI scans were performed, the influence of compartmental cartilage thickness changes over time on the change in total WOMAC over time was analysed using linear mixed models, with total WOMAC as outcome variable, a random intercept at patient level, and fixed effects of time and compartmental cartilage thickness. In case the cartilage thickness change in a compartment had a statistically significant influence, its influence on the change in WOMAC subscales (pain, function and stiffness) was analysed in separate models as well. In all cases, a P-value of <0.05 was considered statistically significant.
Results
Patients
All 20 patients were treated successfully; their characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Parameter | KJD patients (n = 20) |
|---|---|
| Age (years), mean (s.d.) | 48.5 (5.7) |
| BMI (kg/m2), mean (s.d.) | 29.6 (3.5) |
| Male sex, n (%) | 11 (55) |
| Kellgren–Lawrence grade, n (%) | |
| - Grade 0 | 0 (0) |
| - Grade 1 | 1 (5) |
| - Grade 2 | 3 (15) |
| - Grade 3 | 15 (75) |
| - Grade 4 | 1 (5) |
| Medial MAC, n (%) | 18 (90) |
KJD = knee joint distraction; MAC = most affected compartment.
No patients were lost to follow-up in the first 2 years. Between 2 and 5 years of follow-up, three patients were lost: one patient underwent a TKA; two patients underwent arthroscopy. Between 5 and 7 years, five patients were lost: four underwent TKA surgery; one refused further follow-up. Between 7 and 10 years, four patients were lost, all because of TKA surgery.
Whole-joint changes
The average cartilage thicknesses for the femur and the (medial and lateral) tibia of the 18 patients with a medial MAC are shown in Fig. 1. The cartilage on the medial side of both the femur and tibia was thinner than that on the lateral side, as indicated by the red vs green-blue colour. One and 2 years post-treatment, the cartilage in the medial weight-bearing region was on average thicker than pre-treatment (diminishing red intensity). Effects were clear at both the femur and tibia. After 2 years, the average medial cartilage thickness seemed to gradually decrease, though even at 10 years this did not yet seem lower than before treatment. On the lateral side, the cartilage thickness seemed to increase as well, especially long term (increasing blue intensity). The average cartilage results for the two patients with a lateral MAC are shown in Supplementary Fig. S2, available at Rheumatology online; these patients showed similar results, with the biggest changes seen on the lateral side of the joint.
Fig. 1.
Average whole-joint cartilage thickness
The average cartilage thickness of all patients whose medial compartment was the most affected, at baseline (n = 18) and 1 (n = 18), 2 (n = 18), 5 (n = 15), 7 (n = 11) and 10 (n = 7) years after treatment with knee joint distraction. Results are displayed on average right femur and tibia articular cartilage surfaces. The colour range is based on the minimum and maximum average values of the femur (0.78–3.00) and tibia (0.80–3.92) separately.
Changes in cartilage thickness compared with baseline for all patients with a medial MAC are shown in Fig. 2. As indicated by the dark blue areas, the initial increase in medial cartilage thickness was largely statistically significant after 1 year and, especially for the femur, at 2 years. The medial tibia showed some smaller significantly thicker areas up to 10 years after treatment. Long-term results showed that areas in the (lateral) LAC were significantly thicker than before treatment in both the femur and tibia. These statistical tests were not performed for patients with a lateral MAC, because of the small number of patients (n = 2).
Fig. 2.
Whole-joint changes in cartilage thickness
The change in cartilage thickness compared with baseline, for all patients whose medial compartment was the most affected, after 1 (n = 18), 2 (n = 18), 5 (n = 15), 7 (n = 11) and 10 (n = 7) years after treatment with knee joint distraction. Statistically significant changes are indicated by the darker colour map (P < 0.05), while non-significant areas are shown with faded colours (P > 0.05). Blue indicates an increase and red a decrease in cartilage thickness compared with baseline. Results are viewed on average right femur and tibia articular cartilage surfaces.
Compartmental changes
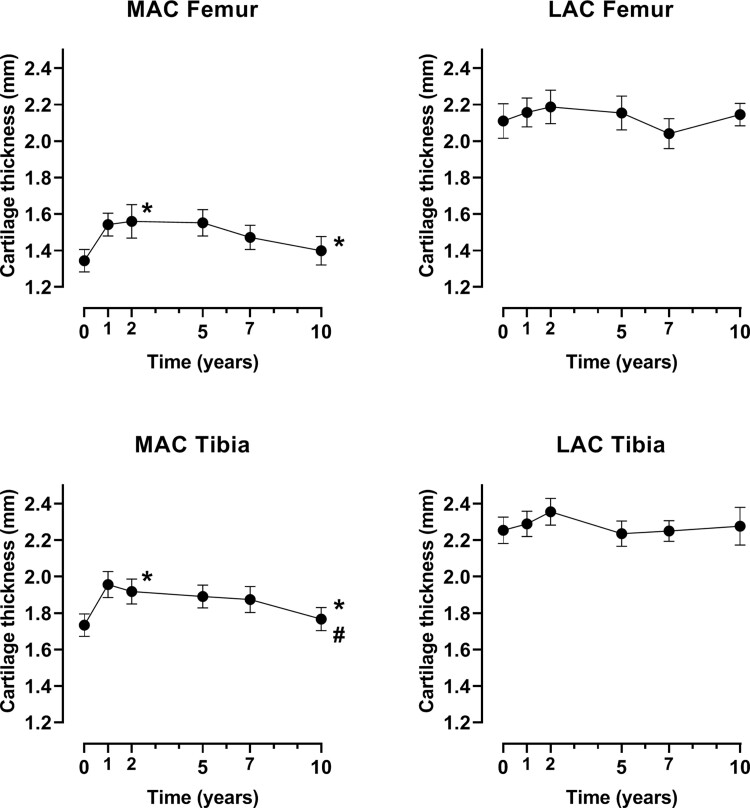
Figure 3 shows the results per compartment of the joint for all patients combined (for 18 of whom the MAC was the medial side and for 2 the lateral side). Both the MAC femur and tibia showed a significant increase over the 10-year period after treatment (both P <0.02), while the LAC femur and tibia did not (both P >0.2). As for the whole-joint analyses, the cartilage thickness of the four compartments showed a bi-phasic response after treatment: an initial cartilage prompt regeneration phase of up to 2 years, statistically significant for the MAC femur [baseline 1.3 (s.d. 0.3) – 2 years 1.6 (0.4); P = 0.010] and MAC tibia [1.7 (0.3) – 1.9 (0.3); P =0.016], and a gradual degeneration phase between 2 and 10 years, statistically significant for the MAC tibia [10 years 1.8 (0.3); P =0.044] but not the MAC femur [10 years 1.4 (0.3); P =0.072]. The LAC femur [2.1 (0.4) – 2.2 (0.4); P =0.343] and LAC tibia [2.2 (0.3) –2.4 (0.3); P =0.058] showed the same trend of an increase in the first 2 years, with some more variation in the years afterwards (both P >0.1). Since the MAC compartments clearly show lower cartilage thickness values even at baseline, Supplementary Fig. S3 (available at Rheumatology online) displays the compartmental cartilage thickness over time, using separate y-axis ranges for the subfigures, to better visualize the changes that occur in each compartment. The mean and 95% CI of all data points are shown in Supplementary Table S1, available at Rheumatology online.
Fig. 3.
Change over time for the four compartments
Missing data was imputed (n = 20 on all time points). * indicates significant (P <0.05) changes up until that time point from baseline: from baseline to 2 years and from baseline to 10 years. # indicates significant (P <0.05) changes from 2 years to 10 years. MAC = most affected compartment, LAC = least affected compartment. Mean and standard error are shown.
Because this analysis was performed with imputed data, a sensitivity analysis was performed including only the eight patients with full datasets. The results are shown in Supplementary Fig. S4, available at Rheumatology online, and show the same bi-phasic response.
Influence of baseline parameters
The influence of baseline parameters on the whole-joint 2- and 10-year changes are shown in Fig. 4 for all patients with a medial MAC. Over the short term (2 years), a higher age, lower BMI, male sex and a higher Kellgren–Lawrence grade seemed to result in a higher medial cartilage thickness increase. It should be noted, 75% of patients had Kellgren–Lawrence grade 3, however, so these results are based on only a very small number of patients. The long-term results (10 years) generally showed the opposite, although for sex and Kellgren–Lawrence grade it is important to note that at 10 years only one female patient was left with grade 2 and six male patients were left with grade 3. None of the results were statistically significant, although especially male sex and higher Kellgren–Lawrence grade seemed to have some positive influence on the 2-year change in the medial compartment.
Fig. 4.
Influence of baseline parameters on whole-joint changes
Influence of baseline parameters on the whole-joint 2-year (n = 18) and 10-year (n = 7) changes, for all patients with a medial most affected compartment. For continuous parameters (age and BMI), the colour map indicates the change per standard deviation increase; for sex, the colour map indicates male sex compared with female sex; for Kellgren–Lawrence (KL) grade, the colour map indicates the change per category increase.
Influence on clinical outcome
The influence of the compartmental cartilage thickness changes over time on the change in total WOMAC is shown in Table 2. As indicated, the 2-year cartilage thickness change did not have a significant influence on the 2-year change in total WOMAC for any of the compartments. However, the 10-year LAC tibia thickness change had a statistically significant influence on the 10-year total WOMAC change (P =0.031), with a relatively large effect estimate: 1 mm cartilage thickness increase could result in 24 points of total WOMAC increase. Looking at the WOMAC subscales separately, the 10-year LAC tibia thickness increase had a significant influence only on the WOMAC function scale [P =0.030; effect estimate 24.93 (95% CI 2.57–47.30)] but not on the other subscales (both P >0.05), although effect estimates were still relatively large (both >17.09).
Table 2.
Influence of cartilage changes on total WOMAC change
|
Change over 2 years |
Change over 10 years |
|||
|---|---|---|---|---|
| Effect estimate | P-value | Effect estimate | P-value | |
| MAC femur | –6.64 (–25.16 to 11.89) | 0.476 | 1.28 (–16.11 to 18.67) | 0.884 |
| LAC femur | –4.16 (–22.72 to 14.40) | 0.652 | 2.48 (–16.11 to 21.07) | 0.789 |
| MAC tibia | 5.50 (–13.32 to 24.26) | 0.562 | 4.79 (–13.76 to 23.35) | 0.609 |
| LAC tibia | 13.16 (–8.64 to 34.97) | 0.228 | 24.00 (2.29 to 45.69) | 0.031 |
LAC = least affected compartment; MAC = most affected compartment. Estimates and 95% CI are shown. Bold typeface other than for row and column headers indicates statistical significance.
Discussion
Ten years after treatment with KJD, in patients who did not convert to TKA, the beneficial effects of this treatment still appeared visible, even in this relatively small cohort. In these end-stage knee OA patients, KJD treatment resulted in significant short-term (1 to 2 years) cartilage regeneration in the MAC. While after 2 years this initial gain in cartilage thickness was gradually lost, 10 years after treatment the cartilage remained thicker than before treatment. This was seen in the whole-joint changes, as indicated in Fig. 1, but also compartmentally, as seen in Fig. 3 and Supplementary Figs S3 and S4, available at Rheumatology online. Even individually, all patients with data at 10 years showed an increase of at least 0.1 mm in one or more compartments, and six of eight patients showed a 10-year increase when averaging all compartments (data not shown). The gradual decrease after 2 years was likely the result of natural progression in loss occurring again after the 2-year regenerative response, potentially in combination with normal or even increased weight-bearing and movement, as a result of successful treatment and the experienced clinical improvement shown previously [15]. However, as we have no untreated control group, this cannot be verified. A good control group for these patients is difficult to find, since purposefully not treating patients with an indication for TKA, especially over multiple years, is impractical and ethically unsound.
In the LAC, a delayed cartilage response seems to take place, with significantly increased cartilage thickness in the long term in the whole-joint analyses. This is surprising, since thus far it has been thought that KJD does not have a clear effect on the cartilage in the LAC [16]. The compartmental analyses did not show a significant long-term increase in the LAC, but only a minimal increase between 5 and 10 years after treatment. For the analyses for all patients, these results could be affected by survivorship bias, but a similar effect was seen when looking only at patients with full 10-year datasets. Apparently, the LAC areas with a significant long-term increase are compensated for by a decrease in the remaining space of the LAC, resulting in the LAC barely changing in the compartmental analyses. This highlights the value of analytical approaches that fully reflect the spatial distribution of changes in the articular cartilage. Still, looking at Figs 1 and 3, the slight long-term increase in the LAC occurred in parallel with a decrease in the MAC, which for the MAC tibia was statistically significant. This may indicate increased loading on the MAC and decreased loading on the LAC over time, allowing regeneration in the LAC, either in a delayed response to the processes in the joint initiated by KJD treatment (described previously [19, 20]) or as a natural response that might occur even in untreated patients. It is also surprising that only the 10-year cartilage thickness change in the LAC tibia had a significant influence on the clinical outcome over 10 years. Previously, no association between clinical and structural changes had been found, and it is unexpected that changes in the LAC instead of the MAC could be related to better clinical response. Importantly, these analyses should be repeated in a larger group of patients to verify these results, especially since the effect was not significant over the first 2 years after treatment.
This is the first time that the cartilage thickness changes after KJD treatment have been shown topographically and over such a long time span, and it seems that the most significant cartilage regeneration moves from the exterior side of the MAC initially to more interiorly long term. Short-term (2-year) subregional analyses in a different cohort have been performed after KJD previously, and showed the most significant response on the exterior side of the MAC femur and tibia as well [16]. The exterior side of the MAC seems to be the most affected pre-treatment, meaning that perhaps the initial regenerative response takes place in the parts of the joint with thinner baseline cartilage, and a slower response takes place in the less affected parts, including the LAC. In fact, baseline MAC cartilage thickness has previously been shown to significantly predict a short-term (2-year) cartilage thickness increase, as has Kellgren–Lawrence grade [16]. In the current study, Kellgren–Lawrence grade did not have a statistically significant influence. Fifteen of the 20 patients had Kellgren–Lawrence grade 3, so there were only very small groups for grade 1 (n = 1), grade 2 (n = 3) and grade 4 (n = 1), hampering detection of statistically significant differences between the groups. Looking at the influence of Kellgren–Lawrence grade on the whole joint (Fig. 3) a higher grade does seem to result in a higher 2-year MAC cartilage increase, but no strong conclusions can be drawn here because of the small sample size. In general, the baseline parameters showed opposing results for the 2- and 10-year changes, indicating a distinction between a short- and long-term response, although in both cases the same beneficial effect. Performing short- and long-term MRI scans in a larger group of patients, ideally including for example biomarker analyses or MRI scans reflecting cartilage quality, could help in drawing stronger conclusions on different responses between (types of) patients.
This study had several limitations. First, the sample size of n = 20 was small, and there was no control group. Despite the small sample size, the results are clear and consistent with previously published short-term results in similar patients. In the current study, long-term MRI cartilage thickness after KJD treatment was evaluated for the first time, adding unique evaluations and conclusions not previously known. Another limitation is that only cartilage thickness was evaluated, not cartilage quality. It would be interesting to see whether the newly generated cartilage was of the same quality, and if the quality of the already present cartilage changed. While dGEMRIC and T2-mapping scans were performed in a different cohort, these were up to 2 years only (T2-mapping analyses being performed currently) [21]. Third, patients were lost over time, mostly due to the (delayed) placement of a joint prosthesis. The last data available before TKA have been included and represent the potential worsening of the joint, which remains the reference after data imputation. This may have resulted in underestimation of the cartilage thickness increase over time. On the other hand, imputation of data based on the data available for survivors may have led to overestimation of the repair activity over time, although none of the compartments showed a difference in cartilage thickness changes over the first 2 or 5 years between patients who did and did not complete 10 years of follow-up (data not shown; all P > 0.18). Also, the sensitivity analyses using the patients for whom all data were available demonstrated that the observed effects presented with imputed data for the whole group seem solid. Still, it remains important to remember that the long-term whole-group results may be an underestimation or, perhaps more likely, an overestimation of the actual cartilage regeneration effect, since patients were lost to follow-up because of additional surgery, making it likely that the remaining patients experienced greater treatment benefit. Last, as validation for the results of the current study, it could have been worthwhile to directly measure the cartilage thickness in the patients undergoing TKA. Unfortunately, in these patients the post-surgery material was not stored and no cartilage thickness was measured. Including this in the study protocol of future studies could give an opportunity for validation of the results. Future studies could also include registration of data that could possibly bias the measured cartilage thickness, such as activity monitoring, to further improve reliability of the data.
In conclusion, in these young end-stage knee OA patients, KJD treatment results in significant short-term cartilage regeneration in the MAC, of which the effects can still be seen after 10 years. Apparently, an initial boost of cartilaginous tissue repair provides a long-term tissue structure benefit. In the LAC, a delayed regenerative response seems to take place. Male sex and severity of joint damage may predict initial benefit, although this was lost over time. The observed intrinsic cartilage tissue repair activity upon KJD, specifically in the first 2 years, may be used to find the metabolic and mechanical drivers of intrinsic cartilage repair in general, providing novel leads for cartilage tissue repair strategies.
Funding: This study was funded by the ReumaNederland (Dutch Arthritis Society, project number LLP-9).
Disclosure statement: J.M. reports grants and personal fees from GlaxoSmithKline, personal fees from Moximed, and grants and personal fees from GE Healthcare, outside the submitted work. The other authors have declared no conflicts of interest.
Data availability statement
The data underlying this article cannot be shared publicly due to ethical restrictions related to participant consent. These restrictions are imposed by the institutional review board of the University Medical Center Utrecht, Utrecht, The Netherlands. The data will be shared on reasonable request by sending an email to the corresponding author or the Rheumatology department of the UMC Utrecht (urrci@umcutrecht.nl).
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Nilsdotter AK, Toksvig-Larsen S, Roos EM.. A 5 year prospective study of patient-relevant outcomes after total knee replacement. Osteoarthr Cartil 2009;17:601–6. [DOI] [PubMed] [Google Scholar]
- 2. Bayliss LE, Culliford D, Monk AP. et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet 2017;389:1424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mastbergen SC, Saris DBF, Lafeber FPJG.. Functional articular cartilage repair: here, near, or is the best approach not yet clear? Nat Rev Rheumatol 2013;9:277–90. [DOI] [PubMed] [Google Scholar]
- 4. Jansen MP, Boymans TAEJ, Custers RJH. et al. Knee joint distraction as treatment for osteoarthritis results in clinical and structural benefit: a systematic review and meta-analysis of the limited number of studies and patients available. Cartilage 2020; Advance Access published 22 July 2020, doi: 10.1177/1947603520942945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Der Woude JAD, Wiegant K, Van Heerwaarden RJ. et al. Knee joint distraction compared with total knee arthroplasty a randomised controlled trial. Bone Jt J 2017;99-B:51–8. [DOI] [PubMed] [Google Scholar]
- 6. van der Woude JAD, Wiegant K, van Heerwaarden RJ. et al. Knee joint distraction compared with high tibial osteotomy: a randomized controlled trial. Knee Surg Sport Traumatol Arthrosc 2017;25:876–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jansen MP, Besselink NJ, van Heerwaarden RJ. et al. Knee joint distraction compared with high tibial osteotomy and total knee arthroplasty: two-year clinical, radiographic, and biochemical marker outcomes of two randomized controlled trials. Cartilage 2019;12:181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoorntje A, Kuijer PPFM, Koenraadt KLM. et al. Return to sport and work after randomization for knee distraction versus high tibial osteotomy: is there a difference? J Knee Surg 2020; Advance Access published 23 November 2020, doi: 10.1055/s-0040-1721027 [Google Scholar]
- 9. Deie M, Ochi M, Adachi N. et al. A new articulated distraction arthroplasty device for treatment of the osteoarthritic knee joint: a preliminary report. Arthroscopy 2007;23:833–8. [DOI] [PubMed] [Google Scholar]
- 10. Aly TA, Hafez K, Amin O.. Arthrodiatasis for management of knee osteoarthritis. Orthopedics 2011;34:e3383–43. [DOI] [PubMed] [Google Scholar]
- 11. Jansen MP, Mastbergen SC, Heerwaarden R. V. et al. Knee joint distraction in regular care for treatment of knee osteoarthritis: a comparison with clinical trial data. PLoS One 2020;15:e0227975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Intema F, Van Roermund PM, Marijnissen ACA. et al. Tissue structure modification in knee osteoarthritis by use of joint distraction: an open 1-year pilot study. Ann Rheum Dis 2011;70:1441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wiegant K, van Roermund PM, Intema F. et al. Sustained clinical and structural benefit after joint distraction in the treatment of severe knee osteoarthritis. Osteoarthr Cartil 2013;21:1660–7. [DOI] [PubMed] [Google Scholar]
- 14. van der Woude JTAD, Wiegant K, van Roermund PM. et al. Five-year follow-up of knee joint distraction: clinical benefit and cartilaginous tissue repair in an open uncontrolled prospective study. Cartilage 2017;8:263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jansen MP, van der Weiden GS, Van Roermund PM. et al. Initial tissue repair predicts long-term clinical success of knee joint distraction as treatment for knee osteoarthritis. Osteoarthr Cartil 2018;26:1604–8. [DOI] [PubMed] [Google Scholar]
- 16. Jansen MP, Maschek S, van Heerwaarden RJ. et al. Changes in cartilage thickness and denuded bone area after knee joint distraction and high tibial osteotomy – post-hoc analyses of two randomized controlled trials. J Clin Med 2021;10:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. MacKay JW, Kaggie JD, Treece GM. et al. Three‐dimensional surface‐based analysis of cartilage MRI data in knee osteoarthritis: validation and initial clinical application. J Magn Reson Imaging 2020;52:1139–51. [DOI] [PubMed] [Google Scholar]
- 18. Turmezei TD, Treece GM, Gee AH. et al. Quantitative 3D analysis of bone in hip osteoarthritis using clinical computed tomography. Eur Radiol 2016;26:2047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watt FE, Hamid B, Garriga C. et al. The molecular profile of synovial fluid changes upon joint distraction and is associated with clinical response in knee osteoarthritis. Osteoarthr Cartil 2020;28:324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanjurjo-Rodriguez C, Altaie A, Mastbergen S. et al. Gene expression signatures of synovial fluid multipotent stromal cells in advanced knee osteoarthritis and following knee joint distraction. Front Bioeng Biotechnol 2020;8:1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Besselink NJ, Vincken KL, Bartels LW. et al. Cartilage quality (dGEMRIC index) following knee joint distraction or high tibial osteotomy. Cartilage 2020;11:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to ethical restrictions related to participant consent. These restrictions are imposed by the institutional review board of the University Medical Center Utrecht, Utrecht, The Netherlands. The data will be shared on reasonable request by sending an email to the corresponding author or the Rheumatology department of the UMC Utrecht (urrci@umcutrecht.nl).