Abstract
Previous studies demonstrated that transforming growth factor (TGT) β1 plays an immunosuppressive role in clinical tuberculosis. However, the contribution of TGF-β1 gene polymorphisms to human tuberculosis susceptibility remains undetermined. In this study, we showed that single-nucleotide polymorphisms (SNPs) in TGF-β1 gene were associated with increased susceptibility to tuberculosis in the discovery cohort (1533 case patients and 1445 controls) and the validation cohort (832 case patients and 1084 controls), and 2 SNPs located in the promoter region (rs2317130 and rs4803457) are in strong linkage disequilibrium. The SNP rs2317130 was associated with the severity of tuberculosis. Further investigation demonstrated that rs2317130 CC genotype is associated with higher TGF-β1 and interleukin 17A production. The mechanistic study showed that rs2317130 C allele affected TGF-β1 promoter activity by regulating binding activity to nuclear extracts. These findings provide insights into the pathogenic role of TGF-β1 in human tuberculosis and reveal a function for the TGF-β1 promoter SNPs in regulating immune responses during Mycobacterium tuberculosis infection.
Keywords: TGF-β1, single-nucleotide polymorphisms, Mycobacterium tuberculosis
Functional single-nucleotide polymorphism of rs2317130 CC genotype, up-regulating Mycobacterium tuberculosis–specific transforming growth factor β1 and interleukin 17A expression, is associated with increased tuberculosis susceptibility.
Tuberculosis, an infectious disease caused by Mycobacterium tuberculosis, remains a severe global health problem. According to the World Health Organization, in 2019, approximately 10 million new cases of tuberculosis were reported, and the latest global treatment success rate is 85%. China is one of 30 countries with a high tuberculosis burden [1]. M. tuberculosis is a highly successful pathogen; it has existed on earth for millions of years and has acquired various strategies to escape from host immune responses.
Although >2 billion people are infected with M. tuberculosis around the world, active tuberculosis develops in only 10% of them, while the majority remain latently infected without symptom [2], implying that host genetic factors play an important role in determining susceptibility to tuberculosis. In recent years, a number of studies have reported that genetic polymorphisms are associated with tuberculosis susceptibility [3–9]. In our previous studies, we also identified several genetic polymorphisms in interleukin 6, interleukin 1β, and Interferon alpha/beta receptor 1 genes associated with tuberculosis susceptibility in the Chinese population [10–12]. Thus, it is essential to determine the relationships between the genetic variations and susceptibility or resistance to tuberculosis, in order to comprehensively understand the pathogenesis of mycobacterial infection.
Transforming growth factor (TGF) β1 has been shown to regulate a broad spectrum of biological processes, including differentiation, chemotaxis, proliferation, and activation, in many immune cells [13, 14]. Numerous studies have showed that TGF-β1 gene polymorphisms are associated with susceptibility to many different kinds of diseases, including cancers [15–21]. TGF-β1 is produced in excess during tuberculosis and presented at the sites of active M. tuberculosis infection [22, 23]. Furthermore, it has been reported to inhibit T-cell responses and deactivate macrophages in patients with tuberculosis [24, 25]. In a tuberculosis granuloma model, it was shown that knockout of TGF-β1 benefits bacterial clearance by cytotoxic T cells [26]. Treatment with recombinant TGF-β1 significantly increased mycobacterial loads in the tissues of guinea pigs [27]. These observations suggest that TGF-β1 performs immunosuppressive activity and accelerates the progression of pulmonary tuberculosis. However, to date, there are extremely limited studies investigating the association of TGF-β1 gene polymorphisms with susceptibility to human tuberculosis.
In the current study, owing to the important role of TGF-β1 in the development of tuberculosis, we investigated the association between 10 single-nucleotide polymorphisms (SNPs) in the TGF-β1 gene and tuberculosis susceptibility in 2 case-control cohorts. After clear associations were found in some of these SNPs, we went on to clarify their function in regulating gene expression and elucidate their potential mechanisms.
MATERIALS AND METHODS
Study Participants and Samples
The discovery cohort including 1533 patients with active tuberculosis and 1445 healthy controls was recruited at The Third People’s Hospital of Shenzhen, as reported elsewhere [11]. Among the 1533 patients with active tuberculosis, 1432 had a diagnosis of pulmonary and 101, a diagnosis of extrapulmonary tuberculosis. Members of the validation tuberculosis study cohort—832 case patients with active tuberculosis (367 with pulmonary and 465 with extrapulmonary tuberculosis) and 1084 controls—were recruited at West China Hospital, Sichuan University, in Chengdu, Sichuan province. The study was approved by the ethics committee of The Third People’s Hospital of Shenzhen, and informed consent was obtained from each participant. For detailed information, see Supplementary Materials and Methods.
DNA Extraction, SNP Selection, and Genotyping
In total, 10 SNPs in TGF-β1 were selected according to methods described elsewhere [28], involving 6 SNPs in the promoter (rs2317130 C>T, rs11466313 DEL>AGG, rs17516265 A>C, rs3087453 C>G, rs4803457 T>C, and rs1800469 C>A) and 4 SNPs in the intron (rs2278422 C>G, rs8110090 A>G, rs4803455 C>A, rs11466334 C>T). SNP genotypes were determined by primer-extension mass spectrometry performed using the Sequenom MassARRAY system. For detailed information, see Supplementary Materials and Methods.
Statistical Analysis
The Hardy-Weinberg equilibrium for TGF-β1 polymorphism distribution was analyzed in healthy controls and case patients. The allele and genotype frequencies of SNPs in case patients and controls were compared using the Pearson χ 2 test. Unconditional logistic regression analyses adjusted by sex and age were performed to calculate odd ratios (ORs), and 95% confidence intervals (CIs) and corresponding corrected P values were calculated under 4 alternative models (multiplicative, additive, dominant, and recessive). One-way analysis of variance with the Newman-Keuls multiple comparison test was used for statistical analyses to compare differences among multiple groups. Haplotype block analysis and haplotype population frequency estimation were performed using Haploview 4.1 software (http://broad.mit.edu/mpg/haploview), and all statistical analyses were performed using GraphPad Prism software (version 5.0). Two-tailed statistical tests were conducted with a significance level of .05. For detailed functional assays, see Supplementary Materials and Methods. The data sets used and analyzed in the current study are available from the corresponding author on reasonable request.
RESULTS
Association Between TGF-β1 SNPs and Tuberculosis Susceptibility in Discovery Cohort
To determine whether TGF-β1 polymorphisms were associated with tuberculosis susceptibility, we selected 10 potential functional SNPs in the TGF-β1 gene, using the strategy described elsewhere [28]. The genotype distributions of the 10 SNPs in case patients and controls are coincident with Hardy-Weinberg equilibrium (P > .05). Among them, 5 of 10 SNPs, including 3 in the TGF-β1 promoter region (rs2317130, rs4803457, and rs11466313) and 2 in the TGF-β1 intron (rs4803455 and rs11466334), are significantly associated with tuberculosis susceptibility, while the remaining 5 SNPs (rs17516265, rs3087453, rs1800469, rs2278422, and rs8110090) have no association with tuberculosis susceptibility in the discovery cohort (Table 1). The rs2317130 C allele exhibits higher risk to tuberculosis susceptibility (odds ratio [OR], 1.14; 95% CI, 1.03–1.26; P = .01).
Table 1.
Association Between Transforming Growth Factor β1 Single-Nucleotide Polymorphisms and Tuberculosis Susceptibility in the Discovery Cohort
| No. (%) | Multiplicative Model | Additive Model | Dominant Model | Recessive Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP ID and Genotype | Control Group | Tuberculosis Group | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) |
| rs2317130 (5-near) | ||||||||||
| CC | 424 (29.4) | 515 (33.6) | .01 | 1.14 (1.03–1.26) | .01 | 1.29 (1.05–1.60) | .12 | 1.16 (.96–1.40) | .01 | 1.21 (1.04–1.42) |
| CT | 742 (51.3) | 756 (49.3) | .42 | 1.09 (.89–1.32) | ||||||
| TT | 279 (19.3) | 262 (17.1) | Reference | Reference | ||||||
| rs4803457(5-near) | ||||||||||
| TT | 405 (28.0) | 494 (32.2) | .01 | 1.14 (1.03–1.26) | .01 | 1.29 (1.05–1.59) | .13 | 1.15 (.96–1.38) | .01 | 1.22 (1.04–1.43) |
| CT | 747 (51.7) | 762 (49.7) | .44 | 1.08 (.89–1.31) | ||||||
| CC | 293 (20.3) | 277 (18.1) | Reference | Reference | ||||||
| rs11466313(5-near) | ||||||||||
| DEL.DEL | 427 (29.3) | 522 (34.0) | .002 | 1.17 (1.06–1.30) | .003 | 1.36 (1.11–1.68) | .02 | 1.23 (1.03–1.48) | .008 | 1.23 (1.05–1.44) |
| DEL.AGG | 712 (49.0) | 737 (48.1) | .14 | 1.16 (.95–1.40) | ||||||
| AGG.AGG | 306 (21.7) | 274 (17.9) | Reference | Reference | ||||||
| rs17516265(5-near) | ||||||||||
| AA | 1391 (96.3) | 1470 (95.9) | .55 | 1.12 (.78–1.61) | … | … | … | … | … | … |
| AC | 54 (3.3) | 62 (4.0) | … | … | ||||||
| CC | 0 (0) | 1 (.1) | … | … | ||||||
| rs8110090 (intron) | ||||||||||
| AA | 1427 (98.8) | 1515 (98.8) | .86 | 1.06 (.55–2.04) | … | … | … | … | … | … |
| AG | 18 (1.2) | 18(1.2) | … | … | ||||||
| GG | 0 (0) | 0 (0) | … | … | ||||||
| rs4803455 (intron) | ||||||||||
| CC | 532 (36.8) | 666 (43.4) | .001 | 1.20 (1.08–1.33) | .01 | 1.35 (1.07–1.70) | .17 | 1.16 (.94–1.44) | <.001 | 1.31 (1.14–1.53) |
| CA | 714 (49.4) | 682 (44.5) | .81 | 1.03 (.82–1.29) | ||||||
| AA | 199 (13.8) | 185 (12.1) | Reference | Reference | ||||||
| rs3087453 (5-near) | ||||||||||
| CC | 1444 (99.9) | 1532 (99.9) | .97 | 1.06 (.07–16.98) | … | … | … | … | … | … |
| CG | 1 (.1) | 1 (.1) | … | … | ||||||
| GG | 0 | 0 | … | … | ||||||
| rs2278422 (intron) | ||||||||||
| CC | 1376 (95.2) | 1452 (94.7) | .54 | 1.11 (.80–1.52) | .96 | 1.06 (.15–7.51) | .95 | 1.06 (.15–7.55) | .53 | 1.11 (.80–1.55) |
| CG | 67 (4.7) | 79 (5.2) | .87 | 1.18 (.16–8.60) | ||||||
| GG | 2 (.1) | 2 (.1) | Reference | Reference | ||||||
| rs1800469 (5-near) | ||||||||||
| CC | 403 (27.9) | 433 (28.2) | .90 | 1.01 (.91–1.11) | .91 | 1.01 (.83–1.24) | .98 | 1.00 (.85–1.19) | .83 | 1.02 (.87–1.19) |
| CA | 705 (48.8) | 742 (48.4) | .92 | 1.01 (.84–1.21) | ||||||
| AA | 337 (23.3) | 358 (23.4) | Reference | Reference | ||||||
| rs11466334 (intron) | ||||||||||
| CC | 1262 (87.3) | 1427 (93.1) | <.001 | 1.91 (1.50–2.43) | .11 | 2.54 (.79–8.28) | .13 | 2.40 (.74–7.80) | <.001 | 1.95 (1.52–2.51) |
| TC | 174 (12.0) | 102 (6.7) | .65 | 1.32 (.40–4.39) | ||||||
| TT | 9 (.7) | 4 (.2) | Reference | Reference | ||||||
Abbreviations: CI, confidence interval; ID, identifier; OR, odds ratio; SNP, single-nucleotide polymorphism.
At the genotype level, the patients with rs2317130 genotype CC have increased risk of tuberculosis in the additive and recessive model. Allele T of another SNP, rs4803457, is associated with increased tuberculosis susceptibility using the multiplicative, additive, and recessive model. In addition, allele DEL of rs11466313 is associated with an increased risk of tuberculosis in the multiplicative (OR, 1.17; 95% CI, 1.06–1.30; P = .002) and additive (1.36; 1.11–1.68; P = .003) models, and C allele of rs4803455 is susceptible to tuberculosis, with an adjusted OR of 1.20 (95% CI, 1.08–1.33, P = .001). Specifically, using the recessive model, the rs11466334 genotype CC was estimated to impart a 95% increase in risk (OR, 1.95; 95% CI, 1.52–2.51; P < .001).
Association Between TGF-β1 Haplotypes and Tuberculosis Susceptibility
Haplotype analysis of the 5 significant SNPs revealed strong linkage disequilibrium between rs2317130 and rs4803457 (Supplementary Figure 1). As shown in Table 2, further analyses of associations between TGF-β1 haplotypes and tuberculosis susceptibility revealed that haplotype H2 (TC, SNPs in the order of rs2317130 and rs4803457) reduces the risk to tuberculosis at P value of .03, with an OR of 0.85 (95% CI .74–.98), compared with haplotypes H1 (CT) and H3 (CC).
Table 2.
Association Between Transforming Growth Factor β1 Haplotypes and Tuberculosis Susceptibility
| Frequency | ||||||
|---|---|---|---|---|---|---|
| Haplotype ID | Codea | Haplotype | Tuberculosis Group | Control Group | P Value | OR (95% CI) |
| H1 | MM | CT | 0.57 | 0.53 | … | Reference |
| H2 | mm | TC | 0.41 | 0.45 | .03 | 0.85 (.74–.98) |
| H3 | mM | CC | 0.02 | 0.02 | .99 | 0.99 (.55–1.78) |
Abbreviations: CI, confidence interval; ID, identifier; OR, odds ratio.
aM represents major allele; m, minor allele. (Single-nucleotide polymorphisms are in the order of rs2317130 and rs4803457.)
Association of TGF-β1 Polymorphism rs2317130 With Severity of Tuberculosis but Not M. tuberculosis–Specific INF-γ Production
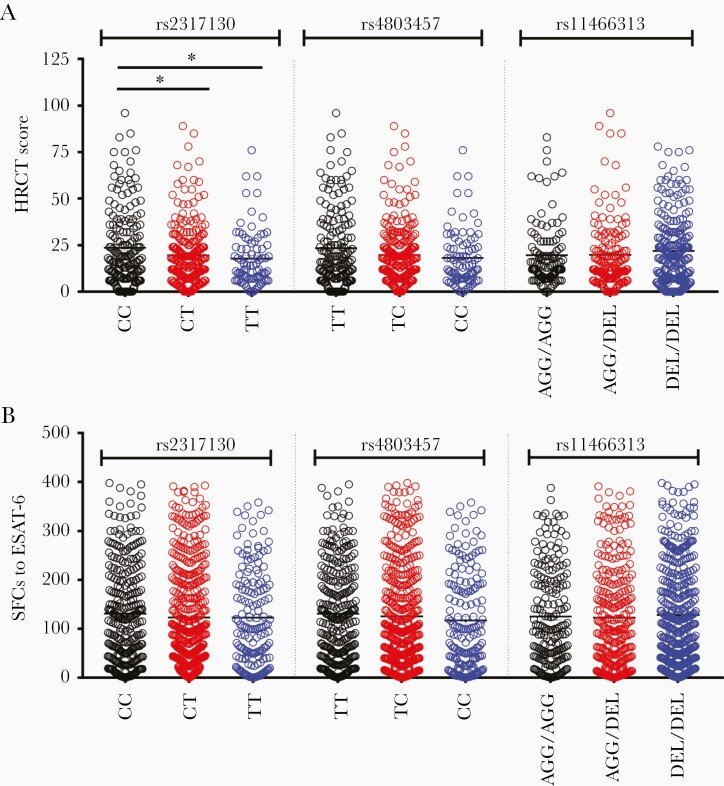
Next, we went on to investigate the 5 positive SNPs in their relationship with the severity of patients with tuberculosis. Therefore, high-resolution computed tomographic (HRCT) scores based on radiographic manifestations were used to directly quantify lung damage in patients with tuberculosis [29, 30]. Among 483 patients, the HRCT scores were significantly higher in carriers with rs2317130 genotype CC than in those with genotypes CT and TT (Figure 1A). In contrast, no significant differences in HRCT score were found in patients carrying other genotypes of rs4803457 and rs11466313 located in the promoter region (Figure 1A) and rs4803455 and rs11466334 located in the intron region (Supplementary Figure 2). However, when we tried to investigate the association between the above 5 SNPs and bacterial load, there were no differences in the allele frequency of these SNPs between patients with sputum culture positivity and those with culture negativity (Supplementary Table 3). In addition, no significant differences in the numbers of spot-forming cells producing interferon γ, erythrocyte sedimentation rates, and C-reactive protein levels were observed among patients with pulmonary tuberculosis carrying 3 TGF-β1 promoter variants (Figure 1B and Supplementary Figure 3). Taken together, these results indicated that the rs2317130 allele C was associated with increased severity of pulmonary tuberculosis.
Figure 1.
Transforming growth factor β1 rs2317130 single-nucleotide polymorphism is associated with the severity of pulmonary tuberculosis, but it has no effect on Mycobacterium tuberculosis–specific interferon γ production. A, High-resolution computed tomographic (HRCT) scores in 483 patients with tuberculosis carrying different genotypes of rs2317130, rs4803457, or rs11466313. B, Spot-forming cells (SFCs) to early secreted antigenic target 6 (ESAT-6) in 878 patients with tuberculosis carrying different genotypes of rs2317130 or rs4803457 or rs11466313. *P < .05.
Replication of Association Between TGF-β1 SNPs and Tuberculosis Susceptibility in Validation Cohort
Because the rs2317130, rs4803457, and rs11466313 SNPs located in the promoter region showed a pattern of linkage disequilibrium and association with clinical phenotypes, we further verified their association with tuberculosis susceptibility through an independent cohort collected from West China Hospital. According to Supplementary Table 4, under the multiplicative model, significantly higher frequencies of the C allele in rs2317130, T allele in rs4803457, and DEL allele in rs11466313 were observed among patients with active tuberculosis, compared with health controls. In the additive, dominant, or recessive model, the genotype frequency of these SNPs also showed significant differences between tuberculosis and healthy control groups (Supplementary Table 4). Thus, these results confirmed that these promoter SNPs are associated with susceptibility to active tuberculosis in the Chinese population.
Effect of SNP rs2317130 on M. tuberculosis–Specific TGF-β1 and Interleukin 17A Production
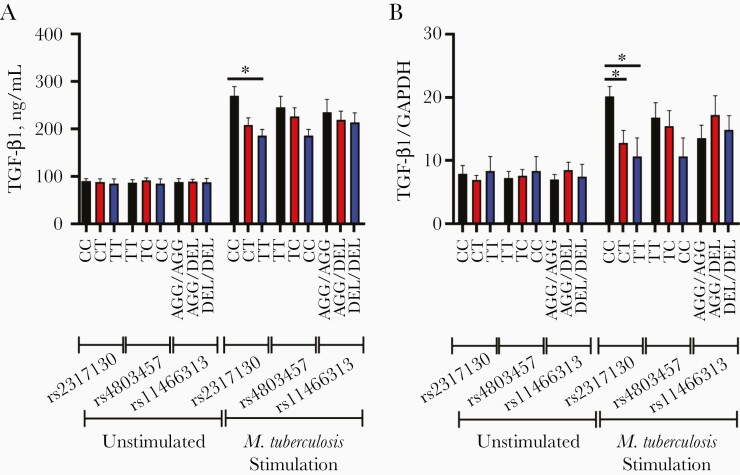
Next, we wonder whether these promoter SNPs could affect the production of TGF-β1 in response to M. tuberculosis stimulation. To test this, we assessed the expression of TGF-β1 in peripheral blood mononuclear cells (PBMCs) with or without M. tuberculosis stimulation, using quantitative polymerase chain reaction and enzyme-linked immunosorbent assays among various genotypes. As shown in Figure 2A and 2B, no significant difference was found in the TGF-β1 protein and messenger RNA (mRNA) levels of PBMCs from individuals carrying various genotypes without M. tuberculosis stimulation. After M. tuberculosis stimulation, the TGF-β1 protein and mRNA levels of PBMCs from individuals carrying various genotypes were all increased compared with those without M. tuberculosis stimulation (Figure 2A and 2B). Moreover, Figure 2A and 2B show that PBMCs from individuals carrying rs2317130 CC genotype produce significantly higher levels of TGF-β1 protein and mRNA than those carrying CT or TT genotype in response to M. tuberculosis stimulation. In contrast, PBMCs from individuals carrying different rs4803457 and rs11466313 genotypes with M. tuberculosis stimulation exhibit no significant differences in TGF-β1production at both protein and mRNA levels.
Figure 2.
The rs2317130 single-nucleotide polymorphism (SNP) affects Mycobacterium tuberculosis–specific transforming growth factor (TGF) β1 production. A, B, Peripheral blood mononuclear cells (n = 18) with various genotypes of rs2317130, rs4803457, and rs11466313 from healthy controls were unstimulated or stimulated (infected) with M. tuberculosis strain H37Rv at a multiplicity of infection of 10; 24 hours later, TGF-β1 production was compared using enzyme-linked immunosorbent assay (A) and quantitative polymerase chain reaction (B). Data represent means with standard errors of the mean. *P < .05. Abbreviation: GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
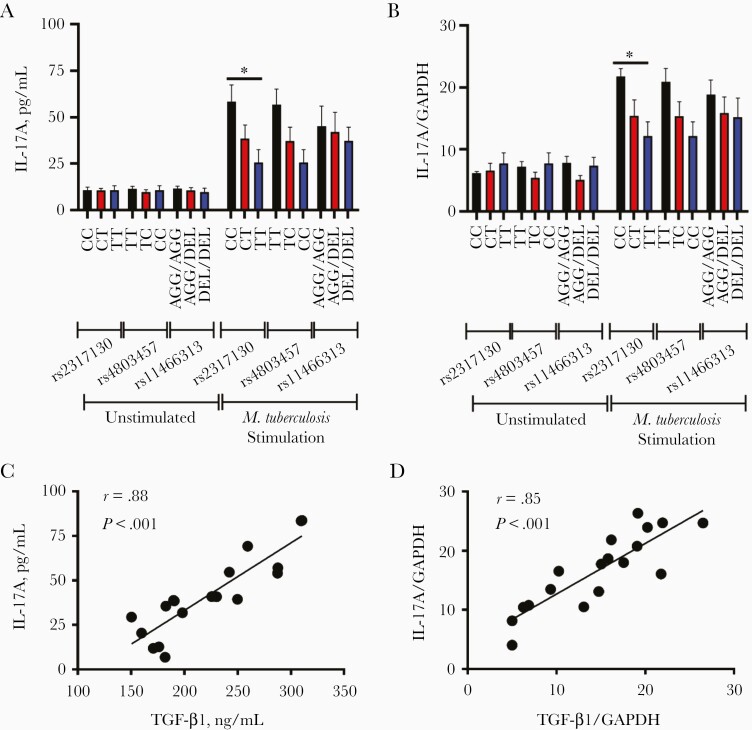
Interleukin 17A (IL-17A), the main effector cytokine of T-helper (Th) 17, promotes local inflammatory responses and may lead to the serious lung damage through recruitment of neutrophils, which play a pathogenic role and exacerbate tuberculosis disease [31, 32]. TGF-β1 is known to play a role in inducing the generation of IL-17A–producing cells [33]. We went on to test whether these SNPs were associated with the expression of IL-17A. As shown in Figure 3A and 3B, IL-17A expression showed a similar pattern like TGF-β1 at both protein and mRNA levels, and higher expression of IL-17A was observed in PBMCs carrying rs2317130 CC genotype than in those carrying genotypes TT in response to M. tuberculosis stimulation. In contrast, PBMCs carrying rs4803457 and rs11466313 genotypes exhibit no significant differences in IL-17A production (Figure 3A and 3B).
Figure 3.
The rs2317130 single-nucleotide polymorphism (SNP) affects Mycobacterium tuberculosis–specific interleukin 17A (IL-17A) production. A, B, Peripheral blood mononuclear cells (PBMCs) (n = 18) with various genotypes of rs2317130, rs4803457, and rs11466313 from healthy controls were unstimulated or stimulated (infected) with M. tuberculosis strain H37Rv at a multiplicity of infection of 10; after 24 hours, IL-17A production was compared using enzyme-linked immunosorbent assay (A) and quantitative polymerase chain reaction (B). C, D, Relationship between transforming growth factor (TGF) β1 and IL-17A expression at the protein (C) and messenger RNA (D) levels. Data represent means with standard errors of the mean. *P < .05. Abbreviation: GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Furthermore, the relationship between TGF-β1 and IL-17A expression was explored. The correlation coefficients between TGF-β1 and IL-17A expression both in protein and mRNA levels were 0.88 and 0.85, suggesting positive correlation existed in the expression between TGF-β1 and IL-17A (Figure 3C and 3D). In addition, the levels of TGF-β1 and IL-17A in serum samples from patients with active tuberculosis were also determined. The patients with tuberculosis carrying various genotypes showed no differences in the level of TGF-β1, although there was an increased trend for individuals with rs2317130 CC and rs4803457 TT genotype (Supplementary Figure 4A). However, the IL-17A level in serum was too low to be detected using available kits (Supplementary Figure 4B). Taken together, these results suggested that SNP rs2317130 affects M. tuberculosis–specific TGF-β1 and IL-17A production.
Influence of SNPs on TGF-β1 Gene Transcription Activity
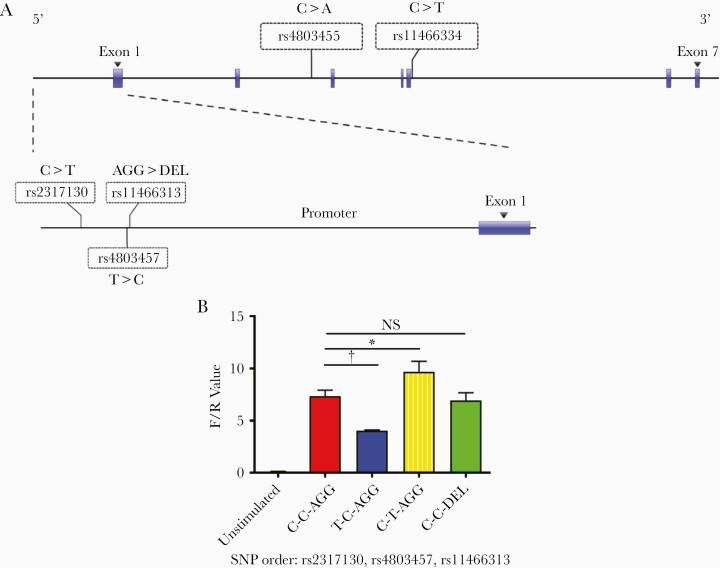
To evaluate the effect of promoter SNPs on TGF-β1 gene transcription activity, we constructed different haplotypes among 3 variants, as shown in Figure 4A and assessed these haplotypes using a dual-luciferase reporter assay. As shown in Figure 4B, we used haplotype C-C-AGG (SNPs in the order of rs2317130, rs4803457, and rs11466313) as a template; when the rs2317130 was mutated into protective allele T, the haplotype T-C-AGG showed decreased luciferase activity. In contrast, the site-directed mutation of rs4803457 to susceptible variants T, the haplotype C-T-AGG showed increased luciferase activity. However, after rs11466313 mutation from AGG to DEL, the haplotype C-C-DEL showed no difference in luciferase activity compared with haplotype C-C-AGG. In addition, haplotype C-T-AGG, which consisted of 2 susceptible variants, rs2317130 allele C and rs4803457 allele T, shows the highest transcriptional activity among C-T-AGG, T-C-AGG, C-C-AGG, and C-C-DEL (Figure 4B).
Figure 4.
Influence of single-nucleotide polymorphisms (SNPs) on transforming growth factor (TGF) β1 gene transcription activity. A, Schematic representation of the locations of 5 significant TGF-β1 gene SNPs. B, TGF-β1 promoter luciferase reporter plasmids carrying different haplotypes, with an SNP order of rs2317130, rs4803457, and rs11466313, were transfected into Hela cells. Firefly luciferase activities were determined and normalized to Renilla luciferase activities. TGF-β1 transcription activities in different haplotypes were compared. Data represent means with standard errors for 3 independent experiments. F/R Value is defined as the ratio of firefly luciferase activities and renilla luciferase activities. *P < .05; †P < .01.
Binding Activity of rs2317130 SNP to Nuclear Extracts
To determine whether the effects of SNPs rs2317130 and rs4803457 on TGF-β1 transcriptional activity were due to the alteration of binding efficiency to the nuclear extracts, electrophoretic mobility shift assay was performed. Biotin-labeled or unlabeled oligonucleotides containing specific alleles were incubated with nuclear extracts from human acute monocytic leukemia cell line-1 cells stimulated with or without M. tuberculosis. As shown in Figure 5A, both alleles of rs2317130 showed several shifted bands when incubated with nuclear protein extracts. Furthermore, these shifted bands could be blocked by unlabeled oligonucleotide competitors (Figure 5A, lanes 5, 6, 9, and 10). Interestingly, M. tuberculosis stimulation induced more complex 1 and additional complex 2 formation on the rs2317130 C oligonucleotide (Figure 5A, lanes 4 and 8) compared with T oligonucleotide (Figure 5A lanes 3 and 7, and Figure 5C), suggesting that SNP rs2317130 could alter nuclear extracts binding activity to TGF-β1 promoter region. On the other hand, distinct bands (complex 3) were shifted when biotin-labeled rs4803457 C probe were incubated with the nuclear extracts (Figure 5B, lanes 4 and 8), which was specifically blocked by unlabeled competitors (Figure 5B, lanes 5, 6, 9, and 10). However, no significant difference in complex 3 was observed between unstimulated and stimulated cells (Figure 5B, lanes 4 and 8, and Figure 5C).
Figure 5.
The rs2317130 single-nucleotide polymorphism (SNP) shows different binding activity to nuclear extracts. A, B, Nuclear extracts were extracted from phorbol-12-myristate-13-acetate-differentiated human acute monocytic leukemia cell line-1 cells stimulated (infected) with Mycobacterium tuberculosis strain H37Rv at a multiplicity of infection of 10 (24 hours after infection), or unstimulated cells, then incubated with C-probe or T-probe of rs2317130 (A) or rs4803457 (B) in the absence or presence of unlabeled competitors and subjected to electrophoretic mobility shift assay. C, Relative band intensity of each complex was analyzed using Image J software. Data represent means with standard errors of the mean for 3 independent experiments. *P < .05; †P < .01.
DISCUSSION
TGF-β1 has been reported to be up-regulated during tuberculosis and presented at sites of active M. tuberculosis infection [22, 23]. However, few studies have examined the relationships between TGF-β1 gene variations and tuberculosis susceptibility. Previous studies showed that the TGF-β1 gene polymorphism at −509C/T (rs1800469) and +869T/C (rs1800470) is correlated with the progression of several diseases [34–36]. So far, 3 TGF-β1 SNPs have been extensively investigated in tuberculosis among different populations,involving +869T/C+915G/C (rs1800471) and −509C/T. It was reported that the +869T/C genotype was significantly associated with increased tuberculosis susceptibility in India [37], but no association was observed in patient with tuberculosis from Japan [38]. In addition, the allele distribution of +915G/C shows no difference in Colombian patients with tuberculosis and healthy controls [39], and in Hong Kong Chinese adults, gene polymorphisms at −509C/T and +869T/C are not associated with tuberculosis susceptibility [40].
The ethnic differences in various populations may lead to these divergent findings, and another reason may be the lack of suitable functional SNP selection strategy. In the current study, SNP selection was as described previously [28], which is different from the usual way of tagging SNPs, in which SNPs were usually selected based only on the linkage disequilibrium in a genomic region. The SNP selection process focuses more on efficiency, which maximizes the coverage but minimizes the cost [41]. We focused on the SNPs located in putative transcription factor binding sites or microRNA target sites. Among these 10 selected potential functional SNPs in the TGF-β1 gene, 5 (50%) were efficiently identified to be significantly associated with tuberculosis susceptibility through this strategy.
In addition, TGF-β1 was required for development of Th17 cells that could produce IL-17A–regulating inflammatory responses [33, 42, 43]. In this study, we performed this correlation analysis between TGF-β1 polymorphisms and tuberculosis susceptibility. Our functional assay demonstrated that PBMCs from individuals carrying rs2317130 C allele in TGF-β1, which is associated with increased susceptibility to tuberculosis, produce significantly higher TGF-β1 in response to M. tuberculosis stimulation, which is consistent with a previous report that TGF-β1 is up-regulated during tuberculosis [22]. Moreover, similar to TGF-β1, PBMCs carrying rs2317130 C allele also produced higher levels of IL-17A in response to M. tuberculosis stimulation. Series of findings have suggested the involvement of IL-17A in tuberculosis pathology. In humans, IL-17 expression in lymphocytes from patients with active tuberculosis is correlated with disease severity [44]; in line with this, it was reported that enhanced Th17 responses were associated with high antigen load in drug-resistant tuberculosis [45]. From the viewpoint of human genetics, the high IL-17A–expressing rs2317130 C allele was associated with tuberculosis susceptibility and disease severity, indicating that persistent IL-17A production causes sustained lung damage and decreased protective immunity against M. tuberculosis infection.
The functional SNPs located in the promoter region may affect transcriptional activity, those in the exon region may result in variant translation, and those in the 3’ untranslated region may alter the miRNA-target interaction. The SNP rs2317130 is located in the promoter region of TGF-β1, and the luciferase reporter assay demonstrated that the rs2317130 C allele increased transcriptional activity of the TGF-β1 promoter in Hela cells. Moreover, electrophoretic mobility shift assay performance showed that the rs2317130 C allele affected recruitment of nuclear proteins to the promoter region of TGF-β1 in response to M. tuberculosis infection. Previous work has found that TGF-β1 expression could be regulated by various transcription factors, such as specificity protein 1, CCAAT-enhancer-binding protein β, activation protein-1, NF-κB, and Kruppel-like factor 4 (gut), in various experimental model systems [46–49]. However, we could not detect binding by these transcription factors of the TGF-β1 promoter region recruited by rs2317130 C allele (data not shown). Thus, binding proteins to the TGF-β1 promoter region recruited by the rs2317130 C allele need further investigation. In short, our data suggested that the rs2317130 C allele resulted in high levels of TGF-β1 and IL-17A in response to M. tuberculosis stimulation, by affecting TGF-β1 promoter transcriptional activity.
Given that TGF-β can inhibit immune cell proliferation and regulate CD4+ T cell differentiation into Foxp3+ regulatory T cells and Th17 cells that are thought to restrain the antituberculosis effector cell functions, TGF-β has been considered an attractive therapeutic target and shown to be an effective immunomodulatory strategy by absence of TGF-β expression or inhibition TGF-β effects with drugs or therapeutic antibodies in vitro and in vivo [50]. However, TGF-β, as a major fibroblast growth factor, is crucial to stimulating the necessary extracellular matrix proteins required for repairing tissue damaged by M. tuberculosis infection [50]. Therefore, reasonable therapeutic approaches are required to shift the balance in favor of a more protective immune response. Meanwhile, other nonimmune yet protective host responses will also be considered [50]. More research is needed to sufficiently balance the overall benefits, considering the full spectrum of host responses to M. tuberculosis infection.
To sum up, we have identified several SNPs of TGF-β1 gene that have significant association with tuberculosis susceptibility. We also found that the functional rs2317130 C allele up-regulating TGF-β1 and IL-17A expression was associated with disease severity. In addition, the rs2317130 C allele determines the promoter activity by affecting the binding activity with nuclear extract. However, our study also had some drawbacks. The cohort was limited to the Chinese populations, there was no retrospective follow-up study to assess variation in TGF-β1 after treatment, and allele-mediated transcription factors regulating TGF-β1 expression have not been identified. Even so, our findings provide critical genetic evidence of the pathogenic role of TGF-β1 in human tuberculosis in the Chinese population, suggesting that host-directed therapy targeting TGF-β1/IL-17A signaling could be an alternative therapeutic strategy, mitigating the severity of tuberculosis and improving clinical outcomes, particularly among individuals with increased TGF-β1–production genotypes.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We extend our appreciation to the physicians and nurses in the department of tuberculosis at The Third People’s Hospital of Shenzhen for recruiting and following up the donors in this study.
Author contributions. S. Z., J. B., Q. G., W. W., S. L., G. X., M. O., J. Z., X. H., and F. L. conducted the experiment, collected the data, and designed the figures; Guobao Li,, X. F., and Guanqiang Li collected and analyzed the data; and C. G. F., X. C., and G. Z. designed the study and wrote the manuscript.
Financial support. This work was supported by the Natural Science Foundation of China (grants 81873958 and 8192031), the Shenzhen Scientific and Technological Foundation (grants JCYJ20180228162321234 and JCYJ20180228162511084), the National Science and Technology Major Project for Control and Prevention of Major Infectious Diseases of China (grant 2017ZX10103004), the National Key Research and Development Plan (grant 2019YFC0840602), and the Sanming Project of Medicine in Shenzhen (grants SZSM201911009 and SZSM201612025).
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2019. 2019. [Google Scholar]
- 2. Murray CJ, Styblo K, Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis 1990; 65:6–24. [PubMed] [Google Scholar]
- 3. Zheng X, Li T, Chen Y, et al. Genetic polymorphisms of the P2X7 gene associated with susceptibility to and prognosis of pulmonary tuberculosis. Infect Genet Evol 2017; 53:24–9. [DOI] [PubMed] [Google Scholar]
- 4. Wang M, Xu G, Lü L, et al. Genetic polymorphisms of IL-17A, IL-17F, TLR4 and miR-146a in association with the risk of pulmonary tuberculosis. Sci Rep 2016; 6:28586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lü J, Pan H, Chen Y, et al. Genetic polymorphisms of IFNG and IFNGR1 in association with the risk of pulmonary tuberculosis. Gene 2014; 543:140–4. [DOI] [PubMed] [Google Scholar]
- 6. Wei Z, Wenhao S, Yuanyuan M, et al. A single nucleotide polymorphism in the interferon-γ gene (IFNG +874 T/A) is associated with susceptibility to tuberculosis. Oncotarget 2017; 8:50415–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ren G, You J, Gong X, et al. SP110 and PMP22 polymorphisms are associated with tuberculosis risk in a Chinese-Tibetan population. Oncotarget 2016; 7:66100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Zhang MM, Huang WW, et al. Polymorphisms in Toll-like receptor 10 and tuberculosis susceptibility: evidence from three independent series. Front Immunol 2018; 9:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qi H, Zhang YB, Sun L, et al. Discovery of susceptibility loci associated with tuberculosis in Han Chinese. Hum Mol Genet 2017; 26:4752–63. [DOI] [PubMed] [Google Scholar]
- 10. Zhang G, Zhou B, Wang W, et al. A functional single-nucleotide polymorphism in the promoter of the gene encoding interleukin 6 is associated with susceptibility to tuberculosis. J Infect Dis 2012; 205: 1694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang G, Boping, et al. Allele-specific induction of IL-1β expression by C/3β and PU.1 contributes to increased tuberculosis susceptibility. PLoS Pathog 2014; 10: e1004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang G, deWeerd NA, Stifter SA, et al. A proline deletion in IFNAR1 impairs IFN-signaling and underlies increased resistance to tuberculosis in humans. Nat Commun 2018; 9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol 2012; 13:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakao A, Afrakhte M, Morén A, et al. Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature 1997; 389:631–5. [DOI] [PubMed] [Google Scholar]
- 15. Hsieh YY, Chang CC, Tsai FJ, Peng CT, Yeh LS, Lin CC. Polymorphism for transforming growth factor beta 1-509 (TGF-B1-509): association with endometriosis. Biochem Genet 2005; 43:203–10. [DOI] [PubMed] [Google Scholar]
- 16. Achyut BR, Ghoshal UC, Moorchung N, Mittal B. Transforming growth factor-B1 and matrix metalloproteinase-7 promoter variants induce risk for Helicobacter pylori-associated gastric precancerous lesions. DNA Cell Biol 2009; 28:295–301. [DOI] [PubMed] [Google Scholar]
- 17. Hishida A, Iwata H, Hamajima N, et al. Transforming growth factor B1 T29C polymorphism and breast cancer risk in Japanese women. Breast Cancer 2003; 10:63–9. [DOI] [PubMed] [Google Scholar]
- 18. Woo SU, Park KH, Woo OH, et al. Association of a TGF-β1 gene -509 C/T polymorphism with breast cancer risk: a meta-analysis. Breast Cancer Res Treat 2010; 124:481–5. [DOI] [PubMed] [Google Scholar]
- 19. Wang HB, Song WG, Liu HQ, Fang F, Xiao Y. Role of TGFB1 polymorphism in the development of metastatic brain tumors in non-small cell lung cancer patients. Genet Mol Res 2015; 14:3545–50. [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Wu G, Herrle F, Niedergethmann M, Keese M. Single nucleotide polymorphisms of genes for EGF, TGF-β and TNF-α in patients with pancreatic carcinoma. Cancer Genomics Proteomics 2012; 9:287–95. [PubMed] [Google Scholar]
- 21. Cruz M, Fragoso JM, Alvarez-León E, et al. The TGF-B1 and IL-10 gene polymorphisms are associated with risk of developing silent myocardial ischemia in the diabetic patients. Immunol Lett 2013; 156:18–22. [DOI] [PubMed] [Google Scholar]
- 22. Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner JJ. Enhanced production of TGF-β by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculous granulomatous lung lesions. J Immunol 1995; 154:465–73. [PubMed] [Google Scholar]
- 23. Aung H, Sherman J, Tary-Lehman M, Toossi Z. Analysis of transforming growth factor-beta 1 (TGF-beta1) expression in human monocytes infected with Mycobacterium avium at a single cell level by ELISPOT assay. J Immunol Methods 2002; 259:25–32. [DOI] [PubMed] [Google Scholar]
- 24. Rajaram MV, Ni B, Dodd CE, Schlesinger LS. Macrophage immunoregulatory pathways in tuberculosis. Semin Immunol 2014; 26:471–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toossi Z, Ellner JJ. The role of TGFβ in the pathogenesis of human tuberculosis. Clin Immunol Immunopathol 1998; 87:107. [DOI] [PubMed] [Google Scholar]
- 26. Warsinske HC, Pienaar E, Linderman JJ, Mattila JT, Kirschner DE. Deletion of TGF-β1 increases bacterial clearance by cytotoxic T cells in a tuberculosis granuloma model. Front Immunol 2017; 8:1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dai G, McMurray DN. Effects of modulating TGF-beta 1 on immune responses to mycobacterial infection in guinea pigs. Tuber Lung Dis 1999; 79:207–14. [DOI] [PubMed] [Google Scholar]
- 28. Zhang G, Chen X, Chan L, et al. An SNP selection strategy identified IL-22 associating with susceptibility to tuberculosis in Chinese. Sci Rep 2011; 1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ors F, Deniz O, Bozlar U, et al. High-resolution CT findings in patients with pulmonary tuberculosis: correlation with the degree of smear positivity. J Thorac Imaging 2007; 22:154–9. [DOI] [PubMed] [Google Scholar]
- 30. Qiu Z, Zhang M, Zhu Y, et al. Multifunctional CD4 T cell responses in patients with active tuberculosis. Sci Rep 2012; 2:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoshino H, Laan M, Sjöstrand M, Lötvall J, Skoogh BE, Linden A. Increased elastase and myeloperoxidase activity associated with neutrophil recruitment by IL-17 in airways in vivo. J Allergy Clin Immunol 2000; 105:143–9. [DOI] [PubMed] [Google Scholar]
- 32. Eruslanov EB, Lyadova IV, Kondratieva TK, et al. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect Immun 2005; 73:1744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 2006; 441:231–4. [DOI] [PubMed] [Google Scholar]
- 34. Pulleyn LJ, Newton R, Adcock IM, Barnes PJ. TGFβ1 allele association with asthma severity. Hum Genet 2001; 109:623–7. [DOI] [PubMed] [Google Scholar]
- 35. Chou HT, Chen CH, Tsai CH, Tsai FJ. Association between transforming growth factor-β1 gene C-509T and T869C polymorphisms and rheumatic heart disease. Am Heart J 2004; 148:181–6. [DOI] [PubMed] [Google Scholar]
- 36. Sato F, Narita I, Goto S, et al. Transforming growth factor-β1 gene polymorphism modifies the histological and clinical manifestations in Japanese patients with IgA nephropathy. Tissue Antigens 2004; 64:35–42. [DOI] [PubMed] [Google Scholar]
- 37. Sivangala R, Ponnana M, Thada S, et al. Association of cytokine gene polymorphisms in patients with tuberculosis and their household contacts. Scand J Immunol 2014; 79:197–205. [DOI] [PubMed] [Google Scholar]
- 38. Niimi T, Sato S, Sugiura Y, et al. Transforming growth factor-beta gene polymorphism in sarcoidosis and tuberculosis patients. Int J Tuberc Lung Dis 2002; 6:510–5. [DOI] [PubMed] [Google Scholar]
- 39. Henao MI, Montes C, París SC, García LF. Cytokine gene polymorphisms in Colombian patients with different clinical presentations of tuberculosis. Tuberculosis (Edinb) 2006; 86:11–9. [DOI] [PubMed] [Google Scholar]
- 40. Mak JCW, Leung HCM, Sham ASK, et al. Genetic polymorphisms and plasma levels of transforming growth factor-β1 in Chinese patients with tuberculosis in Hong Kong. Cytokine 2007; 40:0–182. [DOI] [PubMed] [Google Scholar]
- 41. Sham PC, Ao SI, Kwan JS, et al. Combining functional and linkage disequilibrium information in the selection of tag SNPs. Bioinformatics 2007; 23:129–31. [DOI] [PubMed] [Google Scholar]
- 42. Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005; 6:1133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006; 24:179–89. [DOI] [PubMed] [Google Scholar]
- 44. Jurado JO, Pasquinelli V, Alvarez IB, et al. IL-17 and IFN-γ expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J Leukoc Biol 2012; 91:991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Basile JI, Geffner LJ, Romero MM, et al. Outbreaks of Mycobacterium tuberculosis MDR strains induce high IL-17 T-cell response in patients with MDR tuberculosis that is closely associated with high antigen load. J Infect Dis 2011; 204:1054–64. [DOI] [PubMed] [Google Scholar]
- 46. Presser LD, McRae S, Waris G. Activation of TGF-β1 promoter by hepatitis C virus-induced AP-1 and Sp1: role of TGF-β1 in hepatic stellate cell activation and invasion. PLoS One 2013; 8:e56367. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47. Abraham S, Sweet T, Khalili K, Sawaya BE, Amini S. Evidence for activation of the TGF-β1 promoter by C/EBPβ and its modulation by Smads. J Interferon Cytokine Res 2009; 29:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang Y, Wang Y, Liu Y, Wang N, Qi Y, Du J. Krüppel-like factor 4 transcriptionally regulates TGF-β1 and contributes to cardiac myofibroblast differentiation. PLoS One 2013; 8:e63424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lin W, Tsai WL, Shao RX, et al. Hepatitis C virus regulates transforming growth factor β1 production through the generation of reactive oxygen species in a nuclear factor κB-dependent manner. Gastroenterology 2010; 138:2509–18, 2518.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kiran D, Podell BK, Chambers M, Basaraba RJ. Host-directed therapy targeting the Mycobacterium tuberculosis granuloma: a review. Semin Immunopathol 2016; 38:167–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.