Abstract
Background
Eliminating hepatitis C virus (HCV) will require effective treatment delivery to persons with substance use disorders (SUDs). We evaluated the relationship between ledipasvir/sofosbuvir treatment persistence (receiving 84 tablets), adherence, and sustained virologic response (SVR) in persons with human immunodeficiency virus (HIV)/HCV coinfection.
Methods
Of the 144 participants with HIV/HCV and SUDs, 110 initiated a 12-week treatment course under 1 of 3 conditions (usual care, peer mentors, and cash incentives). We used self-report, pharmacy pill counts, and expected date of refill to examine adherence. Persistent participants were categorized as high adherence (taking ≥90% of doses) or low adherence (taking <90% of doses).
Results
Most participants persisted on treatment after initiation (n = 105), with 95% (n = 100) achieving SVR. One third (34%) of participants had moderate/heavy alcohol use by the biomarker phosphatidylethanol ([Peth] ≥50 ng/mL), and 44% had urine toxicology positive for cocaine or heroin at enrollment. The proportion of persons with high adherence was 72% (n = 76), and the proportion of persons with low adherence was 28%. Although low adherence was associated with moderate/heavy alcohol use by PEth (relative risk = 2.77; 95% confidence interval, 1.50–5.12), SVR did not vary according to adherence (P = .702), and most participants (97%) with low adherence achieved SVR.
Conclusions
Treatment persistence led to high SVR rates among persons with HIV/HCV, despite imperfect adherence and SUDs.
Keywords: adherence, direct-acting antivirals, hepatitis C virus, substance use disorders, sustained virologic response
In this RCT of persons with HIV, HCV, and substance use disorders, 76% initiated a 12-week treatment course for HCV. Participants with high adherence (≥90% of doses) and low adherence (<90% of doses) had high rates of sustained virologic response.
Approximately 71 million people globally are living with hepatitis C virus (HCV) infection, 2.3 million of which are coinfected with human immunodeficiency virus (HIV) [1]. Among this coinfected population, more than half are persons who inject drugs, and many have active substance use disorders (SUDs) [2]. Persons with active HCV infection are at risk for life-threatening liver disease, hepatocellular carcinoma, and remain at risk for transmitting HCV infection [3, 4]. With the advent of highly effective direct-acting antivirals (DAAs), the World Health Organization established the goal of HCV elimination by 2030 with targets to diagnose and treat HCV in 90% of those infected and 80% of those eligible for treatment, respectively [1]. In this context, treatment of persons with active SUDs has been prioritized by HCV treatment guidelines to prevent progressive liver disease and stem onward transmission [5, 6].
Direct-acting antivirals are proving to be just as effective in populations of people who use drugs [7, 8], including those actively injecting and those on opioid-substitution therapy, compared to patients without a history of injection drug use [9]. However, persons with HCV and SUDs can face substantial barriers to curative treatment, including stigmatization within the healthcare system, lack of access to substance use treatment, and comorbid conditions such as depression [10–12]. Healthcare providers and systems may also obstruct treatment by requiring evidence of treatment readiness, which may include documentation of abstinence from drug or alcohol use and demonstration of compliance with scheduled visits [13, 14]. Obstacles related to treatment initiation and adherence could be addressed by research demonstrating successful strategies and outcomes.
In the SIMPLIFY study, the majority (97%) of persons who inject drugs completed a 12-week course of sofosbuvir/velpatasvir (SOF/VEL), and most (94%) achieved sustained virologic response (SVR) despite less than perfect adherence to once-daily pill taking [15]. Similarly, the CHAMPS study evaluated interventions to increase HCV treatment uptake and cure among persons with HIV, HCV, and SUDs. In this study, high rates of SVR were observed among persons who initiated a 12-week course of ledipasvir/sofosbuvir (LVD/SOF), and the most common reason for non-SVR was noninitiation of HCV treatment (24% of all persons who consented to treatment did not initiate) [16]. These studies suggest that the critical step in the HCV care continuum for many persons with active HCV infection is treatment initiation and introduces the possibility that HCV treatment with DAAs may be forgiving of missed doses. However, much of the research on HCV DAA efficacy in people who use drugs (1) only includes participants with high adherence, (2) has limited data on pill-taking adherence, or (3) requires participants to be in treatment for SUDs [17–21]. There are limited data related to HCV treatment outcomes for participants who complete treatment after the “expected” completion date. The aim of this secondary analysis is to examine “persistence” with the entire 84 tablet/12-week HCV treatment course and adherence to LDV/SOF (missed doses) and their relationship with SVR among persons with HIV, HCV, and SUDs.
METHODS
Study Population
The CHAMPS study (ClinicalTrials.gov Identifier NCT02402218) identified 194 people with HIV receiving HIV care at the Johns Hopkins HIV clinic who had not engaged in colocated HCV care within 8 months of entry. Eligible participants had chronic HCV genotype 1 infection and were HCV treatment naive. Other inclusion criteria included the following: age ≥18 years, CD4 count >100 mm3, estimated glomerular filtration rate ≥30 mL/min/1.73 m2, no evidence of hepatocellular carcinoma or decompensated liver disease, and life expectancy greater than 2 years [16]. After informed consent, 144 participants were provided access to 12 weeks of once-daily LDV/SOF at no cost (Harvoni; Gilead Sciences, Foster City, CA).
To evaluate strategies to facilitate treatment uptake and cure, participants were randomized to 1 of 3 conditions: (1) usual care (UC), (2) UC plus peer mentor support (peer), and (3) UC plus contingent cash incentive (cash). In brief, UC included linkage to an HCV provider and access to a nurse-led multidisciplinary team. In the peer group, trained peer mentors worked with participants before, during, and after treatment, and participants in the cash group received escalating incentives for visit attendance (maximum total $220). Incentives were not linked to pill counts or HCV response. Between August 2015 and October 2016, the CHAMPS study enrolled and randomized 144 participants to receive 12 weeks of LDV/SOF under 1 of 3 conditions: usual care, n = 36; peer mentor augmented care, n = 54; and cash incentive augmented care, n = 54. Of the 110 of 144 (76%) participants who initiated LDV/SOF, 100 (91%) achieved SVR; the SVR rate was similar in persons assigned to each condition (usual care, 92%; peer mentor, 91%; and cash incentive, 90%). Detailed methods and additional results are reported elsewhere [16].
Laboratory and Clinical Assessments
Laboratory monitoring included quantitative HCV ribonucleic acid (RNA) (COBAS TaqMan HCV Test v2.0; Roche Molecular Systems Inc., Branchburg, NJ) at treatment weeks 0, 4, 8, 12, and posttreatment weeks 6 and 12. At each visit, adverse effects and medication adherence were assessed by the study coordinators. CD4 cell count and HIV RNA level were measured at screening and as clinically indicated. Liver elastography (FibroScan 502 Touch; Echosens North America, Waltham, MA) was performed before HCV treatment and at posttreatment week 12. Drug and alcohol use was assessed at each study visit by questionnaires, including the 10-question Alcohol Use Disorders Identification Test (AUDIT) [22]. At study entry and treatment week 6, drug use was measured by urine toxicology, and alcohol use was measured by whole blood levels of phosphatidylethanol (PEth) (USDTL, Des Plaines, IL) [23].
Study Outcomes
The primary endpoint of the CHAMPS study was LDV/SOF initiation within 8 or 12 weeks of randomization (12 weeks if changes in HIV antiretroviral medications were required). Of the 144 participants randomized and provided access to HCV treatment, 34 participants did not initiate HCV treatment and were not considered in this adherence analysis. For the 110 participants who initiated HCV treatment, key secondary endpoints were SVR, HCV relapse, and HCV reinfection. Sustained virologic response was defined as an undetectable HCV RNA at 12 or more weeks after stopping LDV/SOF. Participants with undetectable HCV RNA at the end of treatment and detectable HCV RNA at posttreatment weeks 6 or 12 were assessed for HCV relapse versus reinfection using exposure history and virus characteristics.
Adherence Monitoring
Participants received bottles containing 28 tablets of LDV/SOF from the research pharmacists at the time of treatment initiation and at treatment weeks 4 and 8. Medication adherence was assessed using the dates the first and last pills were taken and via self-reported number of missed doses (weeks 4, 6, 8, and 12). Participants were instructed to return bottles to the pharmacy; however, distribution of the next 28-day supply of LDV/SOF was not contingent on the return of the previous bottle. At the time of refill, if the patient had pills remaining, the doses were returned to the participant to complete all 84 tablets of LDV/SOF. Participants who did not present for planned medication refill were contacted by the clinical (clinicians, nurses, or pharmacists) and study teams to facilitate treatment continuation, regardless of intervention arm. Participants randomized to the peer mentor condition had additional interactions with peers focused on treatment persistence and adherence.
Statistical Analysis
For this secondary analysis, treatment persistence was defined as the receipt of 84 tablets of LDV/SOF (all 3 bottles) regardless of how many days it took the participant to complete the treatment course. Treatment persistence was assessed among participants who initiated HCV treatment in the trial. Adherence was assessed among participants with treatment persistence using several methods including the following: (1) pharmacy-recorded first and last date of treatment; (2) pharmacy bottle (28 tablet) dispensing dates; and (3) patient self-report at week 6 and 12 study visits. The primary method for defining the adherence groups was based on missed doses, calculated based on the expected (84 days) versus the actual interval between the pharmacy-recorded first and last date of treatment. Any tablets returned to the pharmacy were also considered when calculating missed doses. For example, a participant with a pharmacy-reported end date on treatment day 88 instead of on treatment day 84 was considered to have missed tablets on 4 of the preceding 84 treatment days. A participant with a pharmacy-reported end date on treatment day 84 was considered to have complete (100%) adherence. For participants missing the treatment completion date, research staff contacted participants within 3 days of the expected completion date to obtain self-reported last dose, following up as needed.
Participants were categorized into high and low adherence groups based on missed doses over the 12-week, 84-tablet treatment course. High adherence was defined as missing 0–8 doses (≥90% adherence), and low adherence was defined as missing 9 or more doses of LDV/SOF (<90% adherence), consistent with the existing HIV and HCV adherence literature [24–27].
Participant characteristics were compared across adherence categories using χ 2 tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Relative risks (RRs) were estimated using Poisson regression with robust variance estimation. Variables such as “following a provider’s suggestion” were included in the bivariate analysis based on a priori hypotheses. Variables that were statistically significant (P < .05) in the bivariate models were included in a multivariable model. In addition, SVR was compared across adherence categories using a χ 2 test. The analysis was conducted using SAS software, version 9.4 (SAS Institute Inc., Cary, NC) and STATA, version 14.2 (StataCorp LLC, College Station, TX). The CHAMPS study was approved by the Johns Hopkins University School of Medicine Institutional Review Board and conducted in accordance with provisions of the Declaration of Helsinki and Good Clinical Practice guidelines.
RESULTS
Treatment Nonpersistence
Of the 110 participants who started HCV treatment, 5 (4.5%) participants discontinued treatment after receiving only 28 tablets of LDV/SOF (3 due to adverse events and 2 self-discontinued); none achieved SVR. The characteristics of participants with nonpersistence are reported in Supplemental Table 1.
Adherence Among Participants With Treatment Persistence
Among participants with treatment persistence (n = 105, 96%), most were male (59%), black (92%), and not employed (81%) with a median age of 55 years (Table 1). The median time to complete the 84-day treatment course was 85 days (range, 76 to 127 days) (Table 2).
Table 1.
Characteristics of Study Participants and Factors Associated With Nonadherence to 12-Weeks of LDV/SOF
| Characteristics | Total (n = 105)a | High Adherence (n = 76)b | Low Adherence (n = 29)b | Unadjusted Relative Risk (95% Confidence Interval) | Adjusted Relative Risk (95% Confidence Interval)c |
|---|---|---|---|---|---|
| Treatment Groups | |||||
| Usual Care | 23 (21.9) | 18 (78.3) | 5 (21.7) | 1 | |
| Peer | 43 (41.0) | 28 (65.1) | 15 (34.9) | 1.60 (0.67–3.87) | |
| Cash | 39 (37.1) | 30 (76.9) | 9 (23.1) | 1.06 (0.40–2.80) | |
| Age | |||||
| <55 | 54 (51.4) | 38 (70.4) | 16 (29.6) | 1 | |
| ≥55 | 51 (48.6) | 38 (74.5) | 13 (25.5) | 0.86 (0.46–1.61) | |
| Median (IQR) | 54.9 (51.1–59.3) | 55.1 (51.0–60.0) | 54.1 (51.9–57.1) | 0.98 (0.95–1.02) | |
| Sex | |||||
| Male | 62 (59.1) | 46 (74.2) | 16 (25.8) | 1 | |
| Female | 43 (40.9) | 30 (69.8) | 13 (30.2) | 1.17 (0.63–2.18) | |
| Race | |||||
| Not Black | 8 (7.6) | 5 (62.5) | 3 (37.5) | 1 | |
| Black | 97 (92.4) | 71 (73.2) | 26 (26.8) | 0.71 (0.27–1.86) | |
| Employment Status | |||||
| Unemployed | 85 (81.0) | 61 (71.8) | 24 (28.2) | 1 | |
| Employed | 20 (19.0) | 15 (75.0) | 5 (25.0) | 0.89 (0.38–2.04) | |
| Alcohol Used | |||||
| PEth <50 ng/mL | 65 (66.3) | 54 (83.1) | 11 (16.9) | 1 | 1 |
| PEth ≥50 ng/mL | 33 (33.7) | 16 (48.5) | 17 (15.5) | 3.04 (1.61–5.75) | 2.74 (1.48–5.08) |
| Self-Report Alcohol Use (AUDIT) | |||||
| Nonhazardous | 78 (74.3) | 59 (75.6) | 19 (24.4) | 1 | |
| Hazardous | 27 (25.7) | 17 (63.0) | 10 (37.0) | 1.52 (0.81–2.86) | |
| UTOX for Cocaine or Heroine | |||||
| Negative | 56 (56.0) | 42 (75.0) | 14 (25.0) | 1 | |
| Positive | 44 (44.0) | 29 (65.9) | 15 (34.1) | 1.36 (0.74–2.52) | |
| Self-Report Cocaine or Heroin | |||||
| No | 82 (78.1) | 61 (74.4) | 21 (25.6) | 1 | |
| Yes | 23 (21.9) | 15 (65.2) | 8 (34.8) | 1.36 (0.69–2.66) | |
| Depressionf | |||||
| CES-D <16 | 43 (41.0) | 33 (76.7) | 10 (23.3) | 1 | |
| CES-D ≥16 | 62 (59.0) | 43 (69.3) | 19 (30.7) | 1.32 (0.68–2.56) | |
| Emotional Supportg | |||||
| Low <33 | 41 (39.1) | 24 (58.5) | 17 (41.5) | 1 | 1 |
| High ≥33 | 64 (60.9) | 52 (81.2) | 12 (18.8) | 0.45 (0.24–0.85) | 0.56 (0.31–1.01) |
| Median (IQR) | 36 (24–40) | 38 (29–40) | 30 (20–40) | 0.96 (0.94–0.99) | |
| Follow Provider’s Suggestionsh | |||||
| Never/rarely | 11 (10.8) | 4 (36.4) | 7 (63.6) | 1 | 1 |
| Sometimes | 18 (17.7) | 13 (72.2) | 5 (27.8) | 0.44 (0.18–1.04) | 0.56 (0.23–1.35) |
| Usually/always | 73 (71.6) | 57 (78.1) | 16 (21.9) | 0.34 (0.18–0.64) | 0.46 (0.22–0.98) |
Abbreviations: AUDIT, alcohol use disorders identification test; CES-D, Centers for Depression Epidemiology Scale; IQR, interquartile range; LDV/SOF, ledipasvir/sofosbuvir; PEth, phosphatidylethanol.
aUnless otherwise specified, numbers reported are n (%). For the “Total” column, the percentages represent the proportion with the specified characteristic of the total number of participants (ie, column percentages).
bUnless otherwise specified, numbers reported are n (%). For the “High Adherence” and “Low Adherence” columns, the percentages represent the proportion with high/low adherence of the total number of participants with the specified characteristic (ie, row percentages).
cOnly characteristics with a relative risk specified were included in the multivariable model.
dPEth reported for n = 98; missing 7 participants (4, high; 2, moderate; 1 low).
eUTOX reported for n = 100; missing 5 participants (3, high and 2, moderate).
fCenters for Depression Epidemiology Scale; active depression defined as score ≥16.
gPatient-Reported Outcomes Measurement Information System Short Form v2.0, Emotional Support 8a; high support defined as short form score ≥33. Questions assess whether participants have someone they trust and someone who listens and understands their problems.
hAnswered “How often do you follow your provider’s suggestions exactly?” with usually or always, n = 102.
Table 2.
Pill Taking Behavior of Participants That Persisted on Treatment
| Pill Taking Behavior | Total N = 105 | High Adherence N = 76 | Low Adherence N = 29 | P Value |
|---|---|---|---|---|
| Median days to complete treatment (IQR) | 85 (84–93) | 84 (84–86) | 98 (95–101) | <.0001 |
| Range of days to complete treatment | 76–127 | 76–92 | 76–127 | |
| Pharmacy Report Days Overdue for 2nd Bottle | <.0001 | |||
| 0–2 | 95 (90.5) | 75 (98.7) | 20 (69.0) | |
| 3–5 | 2 (1.9) | 0 | 2 (6.9) | |
| 6–8 | 4 (3.8) | 1 (1.3) | 3 (10.3) | |
| ≥9 | 4 (3.8) | 0 | 4 (13.8) | |
| Pharmacy Report Days Overdue for 3rd Bottle | <.0001 | |||
| 0–2 | 87 (82.9) | 71 (93.4) | 16 (55.2) | |
| 3–5 | 2 (1.9) | 1 (1.3) | 1 (3.5) | |
| 6–8 | 6 (5.7) | 4 (5.3) | 2 (6.9) | |
| ≥9 | 10 (9.5) | 0 | 10 (34.5) | |
| Self-Report Missed Doses Week 6a | .02 | |||
| 0–2 | 101 (97.1) | 76 (100.0) | 25 (89.3) | |
| 3–5 | 2 (1.9) | 0 | 2 (7.1) | |
| 6–8 | 0 | 0 | 0 | |
| ≥9 | 1 (1.0) | 0 | 1 (3.6) | |
| Self-Report Missed Doses Week 12a | .003 | |||
| 0–2 | 92 (88.5) | 72 (94.7) | 20 (71.4) | |
| 3–5 | 6 (5.8) | 3 (3.9) | 3 (10.7) | |
| 6–8 | 4 (3.9) | 0 | 4 (14.3) | |
| ≥9 | 2 (1.9) | 1 (1.3) | 1 (3.6) |
Abbreviations: IQR, interquartile range.
aN = 104.
The SVR rate among participants who persisted on treatment with LDV/SOF was 95% (100 of 105). Of those with non-SVR, 3 participants had virologic failure, 1 had HCV reinfection (HCV genotype 1 subtype change after documentation of negative HCV RNA posttreatment), and 1 died of unknown causes before the SVR assessment.
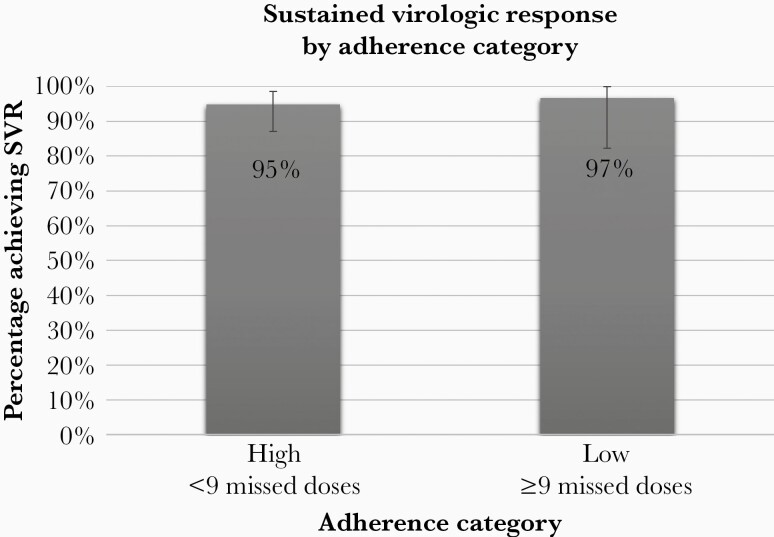
Among the 105 participants that persisted on HCV treatment, 76 (72%) had high adherence (median number of missed doses = 1; interquartile range [IQR] = 0–3; range = 0–8) and 29 (28%) had low adherence (median number of missed doses = 14; IQR = 12–17; range = 9–43). In addition, adherence to daily pill taking was not associated with SVR because 28 of the 29 participants (97%) in the low adherence category achieved SVR (P = .70) (Figure 1). The participant in the low adherence group that did not achieve SVR experienced HCV relapse after having taken 66 tablets over the 84-day period. He was retreated 18 months later and achieved SVR (participant No. 4, see Supplemental Table 1). The 2 participants in the high adherence group that did not achieve SVR due to virologic failure were not retreated as of >2 years poststudy (participant No. 2 [deceased] and No. 5, see Supplemental Table 1).
Figure 1.
Adherence to Daily Pill Taking was Not Associated with SVR.
Whereas participants with high adherence were consistent with their pill taking behavior over each treatment interval (weeks 4 and 8), those in the low adherence group demonstrated inconsistent pill taking behavior over time with the second and third bottles based on pharmacy bottle (28 tablet) dispensing dates (Table 2). Among participants with low adherence, 31% (9 of 29) were 3 or more days late for their second bottle of LDV/SOF, and 45% (13 of 29) were 3 or more days late for the third bottle. In contrast, among the adherent participants, only 1% (1 of 76) was 3 or more days late for the second bottle of LDV/SOF, and 7% (5 of 76) were 3 or more days late for their third bottle. All measures outlined in Table 2 were generally consistent in separating participants into low and high adherence groups.
Alcohol and Drug Use
Among the 105 persistent participants, 98 had blood alcohol exposure quantified at study entry; more than one third (n = 33) of these had PEth blood tests ≥50 ng/mL, consistent with moderate to heavy alcohol consumption over the preceding 14 days [28]. Moderate to heavy alcohol use was significantly associated with nonadherence to the treatment course and remained statistically significant in the multivariable model (crude RR = 3.04, confidence interval [CI] = 1.61 to 5.75; adjusted RR = 2.74, CI = 1.48 to 5.08). When measured by AUDIT, risky alcohol use over the past 12 months among participants was lower (27 of 105; 26% overall; 10 of 62; 16% among males; 17 of 43; 40% among females) and was not associated with adherence (RR = 1.52, CI = 0.81 to 2.86) (Table 1). Furthermore, 44% (44 of 100) of participants had a positive urine toxicology screening indicating recent cocaine or heroin use at study entry. Detection of heroin or cocaine by urine test was also not significantly associated with nonadherence (RR = 1.36, CI = 0.74 to 2.52). Similar results were obtained for self-reported cocaine or heroin use in the past 30 days (RR = 1.36, CI = 0.69 to 2.66) (Additional drug and alcohol use data reported in Supplemental Table 2).
Healthcare Provider Suggestions
Many participants with high adherence reported usually or always following a provider’s suggestion exactly (57 of 74, 77%) compared to participants with low adherence (16 of 28, 57%). The risk of nonadherence was 54% lower in participants who reported usually or always following a provider’s suggestions compared to those who indicated that they never/rarely follow a provider’s advice (adjusted RR = 0.46, CI = 0.22 to 0.98).
Depression and Emotional Support
By the Centers for Depression Epidemiology Scale (CES-D), 59% (62 of 105) of participants had evidence of depressive symptoms; this was not associated with adherence (P = .41). Relatedly, 61% (64 of 105) of participants reported high emotional support (having someone they trust and someone who listens and understands their problems) at study entry. The risk of nonadherence was 55% lower in participants who expressed high levels of emotional support from friends and family compared to those with low support (crude RR = 0.45, CI = 0.24 to 0.85). However, after adjusting for following a provider’s suggestions, a high level of emotional support was protective but no longer statistically significantly related to adherence (adjusted RR = 0.56, CI = 0.31 to 1.01), indicating that these measures of support and trust are related.
Discussion
In the CHAMPS study cohort, the primary reason for non-SVR was noninitiation of LDV/SOF (34 of 44 with non-SVR) [16]. Among the remaining 10 participants who initiated HCV treatment but did not achieve SVR, half (5 of 10) of the non-SVR were due to participants not completing the full 12-week course (nonpersistence). Among the 105 participants who persisted on treatment, 95% achieved SVR, and SVR rates were similar in patients with high and low adherence (95% vs 97%, respectively). Taken together, our data indicate that SVR can be achieved with 12 weeks of LDV/SOF despite imperfect adherence, indicating that the HCV treatment regimen may be forgiving of missed doses. These observations have important implications for HCV treatment strategies, especially among people who use drugs, and the concept of treatment readiness for these relatively short, finite courses of oral therapy. Consistent with the current literature, we found detection of heroin or cocaine by urine test was also not significantly associated with nonadherence, indicating the emphasis should be on linkage to HCV care and treatment initiation, despite perceived barriers to adherence such as substance use disorders [17, 29–31].
After initiation, ensuring treatment persistence, or the continuation of treatment in persons who report missed doses or are late in refilling medications, is key. Our data underscore the important role pharmacists can play in monitoring HCV treatment persistence as part of routine care [32]. Late or missing refills should prompt urgent intervention and collaboration among the patient’s care team to ensure that the treatment course continues until completion. Hepatitis C virus treatment prescribers should develop strategies along with the patient to facilitate treatment persistence, such as seamless prescription refills and continuation of treatment during hospitalization or incarceration [5, 6, 33, 34].
Our findings also support the treatment of persons actively using drugs and alcohol. Although moderate/heavy alcohol use was associated with a greater risk of nonadherence, this did not impact SVR. A related analysis by Irvin et al [35] found that heavy alcohol use (PEth ≥50 ng/mL) was not associated with failure to initiate HCV treatment or failure to achieve SVR among the CHAMPS study cohort. Therefore, if a participant’s alcohol use disorder can be identified, HCV treatment provides an opportunity for providers to educate patients about the effect of alcohol on advancing liver disease and fibrosis.
Similarly, we did not find an association between nonadherence and active cocaine or heroin use. This is consistent with findings from the primary manuscript that the proportion of participants initiating LDV/SOF and achieving SVR did not vary by active drug use [16]. In the ANCHOR study, people who inject drugs (PWID) and have HCV and an opioid use disorder were offered buprenorphine while initiating treatment for HCV [36]. The majority of PWID achieved SVR (82%) after apparent treatment persistence (84% completed all 3 bottles of SOF/VEL). Sustained virologic response was not associated with on-treatment drug use or imperfect daily adherence, defined as finishing treatment >7 days after the anticipated end date. Sustained virologic response was associated with completing ≥2 bottles of SOF/VEL and receiving opioid agonist treatment at week 24. Further research should develop the HCV care delivery model that coadministers HCV DAA therapy with medication-assisted therapy for opiate or alcohol use disorders. Integrated programs that concurrently treat HCV and substance use disorders may increase HCV treatment persistence and reduce the risk of HCV reinfection and liver damage in persons with SUDs [32, 37–40].
In our study, usually or always following a provider’s suggestion was significantly associated with a participant’s adherence. Following a provider’s suggestion can be interpreted as a sign of trust within the patient-provider relationship [41]. Trust and rapport have been shown to improve health outcomes across disease states and have been linked to improved medication initiation and adherence for HIV treatment [42–44]. In our study population of primarily black patients, this is important to understand in the context of the medical mistrust and racism that black communities experience when accessing healthcare [45], which has been shown to negatively influence medication adherence [46, 47]. Our findings indicate that when patients view their providers as a trusted source of information and advice, positive health behaviors may follow. Therefore, it is important to help providers continue to build trust with patients regardless of race or substance use disorder history while also addressing the mistrust their patients may have from past experiences. As related to HCV, providers must remove judgment from their practice by not withholding HCV medication prescriptions from their patients with SUDs. Instead, providing SUD treatment concurrently with HCV treatment as described above could give their patients a pathway to treatment success [36].
There are several important limitations to our study. First, this was a single-site study of persons engaged in comprehensive HIV care; therefore, results may not be generalizable to other settings with fewer resources or patients who are monoinfected with HCV. Second, although adherence was measured in multiple ways, including both subjective and objective measures, obtaining an accurate assessment of adherence is difficult; therefore, we report on adherence over the course of treatment and cannot report a specific number of consecutive days or weeks of missed doses that impacts SVR. Daily adherence is also difficult to assess even using more sophisticated technology, such as electronic blister packs, because these can be easily tampered with [48]. Wearable digital adherence monitoring technology could have been useful in this high-risk population to alert pharmacists and providers in real-time when a patient disengages from care [49]. Finally, we did not study HCV regimens with shorter durations, such as 8 weeks; however, a pooled analysis of adherence to 8 weeks of glecaprevir/pibrentasvir suggests that patients may achieve SVR despite missed doses [50]. Real-world studies are needed to confirm these observations with other HCV regimens, including SOF/VEL [36].
Conclusions
This study aimed to characterize adherence to HCV treatment with ledipasvir/sofosbuvir among persons with HIV who use drugs that had not been engaged in HCV care. Overall, HCV treatment adherence was not associated with SVR, because most of the participants in the low adherence category still achieved cure; this indicated that HCV treatment regimen may be forgiving of missed doses. As such, treatment should not be withheld from patients with substance use disorders despite its association with nonadherence. Instead, HCV treatment may be an ideal time to screen for alcohol use disorder and engage patients in SUD treatment. In this era of HCV treatment, patient persistence may be more attainable with help from a knowledgeable, trustworthy, and supportive treatment team of pharmacists and providers. Additional research should define the limitations of DAA persistence to create treatment courses that are highly effective and manageable for patient populations with competing sociomedical priorities.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was funded by the National Institutes of Health (NIH) (Grant Numbers R01DA16065, R37DA013806, U01DA036935, K24DA034621, and K23DA041294). This work was also made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR) and the Center for Clinical Data Analytics (funded in part by Grant Number UL1 TR001079), and the Johns Hopkins Center for AIDS Research (Grant Number P30AI094189). Study medication was provided by Gilead Sciences (Foster City, CA).
Potential conflicts of interest. S. B. reports consulting agreements with Gilead and AbbVie. M. S. S. reports consulting arrangements with Gilead and AbbVie and research grants to his institution from AbbVie, AssemblyBio, Janssen, and the NIH. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Global hepatitis report, 2017. Geneva: World Health Organization, 2017. [Google Scholar]
- 2. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16:797–808. [DOI] [PubMed] [Google Scholar]
- 3. Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis 2005; 9:383–98, vi. [DOI] [PubMed] [Google Scholar]
- 4. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol 2014; 61:S58–68. [DOI] [PubMed] [Google Scholar]
- 5. AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. Available at: http://www.hcvguidelines.org. Accessed 9 September 2020.
- 6. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018; 69:461–511. [DOI] [PubMed] [Google Scholar]
- 7. Eckhardt BJ, Scherer M, Winkelstein E, Marks K, Edlin BR. Hepatitis C treatment outcomes for people who inject drugs treated in an accessible care program located at a syringe service program. Open Forum Infect Dis 2018; 5: ofy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenthal ES, Silk R, Mathur P, et al. Concurrent initiation of hepatitis C and opioid use disorder treatment in people who inject drugs. Clin Infect Dis 2020; 71:1715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graf C, Mücke MM, Dultz G, et al. Efficacy of direct-acting antivirals for chronic hepatitis C virus infection in people who inject drugs or receive opioid substitution therapy: a systematic review and meta-analysis. Clin Infect Dis 2020; 70:2355–65. [DOI] [PubMed] [Google Scholar]
- 10. Cachay ER, Hill L, Wyles D, et al. The hepatitis C cascade of care among HIV infected patients: a call to address ongoing barriers to care. PLoS One 2014; 9:e102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris M, Rhodes T. Hepatitis C treatment access and uptake for people who inject drugs: a review mapping the role of social factors. Harm Reduct J 2013; 10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Biancarelli DL, Biello KB, Childs E, et al. Strategies used by people who inject drugs to avoid stigma in healthcare settings. Drug Alcohol Depend 2019; 198:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allyn P, O’Malley S, Ferguson J, Tseng C, Chew K, Bhattacharya D. Attitudes and potential barriers towards hepatitis C treatment in patients with and without HIV coinfection. Int J STD AIDS 2018; 29:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Asher AK, Portillo CJ, Cooper BA, Dawson-Rose C, Vlahov D, Page KA. Clinicians’ views of hepatitis C virus treatment candidacy with direct-acting antiviral regimens for people who inject drugs. Subst Use Misuse 2016; 51:1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cunningham EB, Amin J, Feld JJ, et al. ; SIMPLIFY study group . Adherence to sofosbuvir and velpatasvir among people with chronic HCV infection and recent injection drug use: the SIMPLIFY study. Int J Drug Policy 2018; 62:14–23. [DOI] [PubMed] [Google Scholar]
- 16. Ward K, Falade-Nwulia O, Moon J, et al. A randomized controlled trial of cash incentives or peer support to increase HCV treatment for persons with HIV who use drugs: the CHAMPS study. Open Forum Infect Dis 2019; 6:ofz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dore GJ, Altice F, Litwin AH, et al. ; C-EDGE CO-STAR Study Group . Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med 2016; 165:625–34. [DOI] [PubMed] [Google Scholar]
- 18. Mason K, Dodd Z, Guyton M, et al. Understanding real-world adherence in the directly acting antiviral era: a prospective evaluation of adherence among people with a history of drug use at a community-based program in Toronto, Canada. Int J Drug Policy 2017; 47:202–8. [DOI] [PubMed] [Google Scholar]
- 19. Min JE, Pearce LA, Janjua NZ, Ti L, Nosyk B. The causal effect of opioid agonist treatment on adherence to direct-acting antiviral treatment for hepatitis C virus. Open Forum Infect Dis 2020; 7: ofaa418. [Google Scholar]
- 20. Norton BL, Akiyama MJ, Arnsten JH, Agyemang L, Heo M, Litwin AH. High HCV cure rates among people who inject drugs and have suboptimal adherence: a patient-centered approach to HCV models of care. Int J Drug Policy 2021; 93:103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cunningham EB, Hajarizadeh B, Amin J, et al. ; SIMPLIFY and D3FEAT study groups . Adherence to once-daily and twice-daily direct-acting antiviral therapy for hepatitis C infection among people with recent injection drug use or current opioid agonist therapy. Clin Infect Dis 2020; 71:e115–24. [DOI] [PubMed] [Google Scholar]
- 22. Volk RJ, Steinbauer JR, Cantor SB, Holzer CE 3rd. The Alcohol Use Disorders Identification Test (AUDIT) as a screen for at-risk drinking in primary care patients of different racial/ethnic backgrounds. Addiction 1997; 92:197–206. [PubMed] [Google Scholar]
- 23. Eyawo O, McGinnis KA, Justice AC, et al. ; VACS Project team . Alcohol and mortality: combining self-reported (AUDIT-C) and biomarker detected (PEth) alcohol measures among HIV infected and uninfected. J Acquir Immune Defic Syndr 2018; 77:135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fogarty L, Roter D, Larson S, Burke J, Gillespie J, Levy R. Patient adherence to HIV medication regimens: a review of published and abstract reports. Patient Educ Couns 2002; 46:93–108. [DOI] [PubMed] [Google Scholar]
- 25. Weiss JJ, Bräu N, Stivala A, Swan T, Fishbein D. Review article: adherence to medication for chronic hepatitis C - building on the model of human immunodeficiency virus antiretroviral adherence research. Aliment Pharmacol Ther 2009; 30:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mathes T, Antoine SL, Pieper D. Factors influencing adherence in hepatitis-C infected patients: a systematic review. BMC Infect Dis 2014; 14:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ortego C, Huedo-Medina TB, Llorca J, et al. Adherence to highly active antiretroviral therapy (HAART): a meta-analysis. AIDS Behav 2011; 15:1381–96. [DOI] [PubMed] [Google Scholar]
- 28. Hahn JA, Emenyonu NI, Fatch R, et al. Declining and rebounding unhealthy alcohol consumption during the first year of HIV care in rural Uganda, using phosphatidylethanol to augment self-report. Addiction 2016; 111:272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grebely J, Hajarizadeh B, Dore GJ. Direct-acting antiviral agents for HCV infection affecting people who inject drugs. Nat Rev Gastroenterol Hepatol 2017; 14:641–51. [DOI] [PubMed] [Google Scholar]
- 30. Norton BL, Fleming J, Bachhuber MA, et al. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic. Int J Drug Policy 2017; 47:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Litwin AH, Soloway IJ, Cockerham-Colas L, et al. Successful treatment of chronic hepatitis C with triple therapy in an opioid agonist treatment program. Int J Drug Policy 2015; 26:1014–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Radley A, de Bruin M, Inglis SK, et al. Clinical effectiveness of pharmacist-led versus conventionally delivered antiviral treatment for hepatitis C virus in patients receiving opioid substitution therapy: a pragmatic, cluster-randomised trial. Lancet Gastroenterol Hepatol 2020; 5:809–18. [DOI] [PubMed] [Google Scholar]
- 33. Swan D, Long J, Carr O, et al. Barriers to and facilitators of hepatitis C testing, management, and treatment among current and former injecting drug users: a qualitative exploration. AIDS Patient Care STDS 2010; 24:753–62. [DOI] [PubMed] [Google Scholar]
- 34. Robaeys G, Grebely J, Mauss S, et al. ; International Network on Hepatitis in Substance Users . Recommendations for the management of hepatitis C virus infection among people who inject drugs. Clin Infect Dis 2013; 57(Suppl 2):S129–37. [DOI] [PubMed] [Google Scholar]
- 35. Irvin R, Chander G, Ward KM, et al. Unreported alcohol use was common but did not impact hepatitis C cure in HIV-infected persons who use drugs. J Viral Hepat 2020; 27:476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosenthal ES, Silk R, Mathur P, et al. Concurrent initiation of hepatitis C and opioid use disorder treatment in people who inject drugs. Clin Infect Dis 2020; 71:1715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dimova RB, Zeremski M, Jacobson IM, Hagan H, Des Jarlais DC, Talal AH. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin Infect Dis 2013; 56:806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Read P, Gilliver R, Kearley J, et al. Treatment adherence and support for people who inject drugs taking direct-acting antiviral therapy for hepatitis C infection. J Viral Hepat 2019; 26:1301–10. [DOI] [PubMed] [Google Scholar]
- 39. Norton BL, Beitin A, Glenn M, DeLuca J, Litwin AH, Cunningham CO. Retention in buprenorphine treatment is associated with improved HCV care outcomes. J Subst Abuse Treat 2017; 75:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Radley A, Robinson E, Aspinall EJ, Angus K, Tan L, Dillon JF. A systematic review and meta-analysis of community and primary-care-based hepatitis C testing and treatment services that employ direct acting antiviral drug treatments. BMC Health Serv Res 2019; 19:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Glanz K, Rimer BK, Viswanath K.. Health Behavior and Health Education Theory, Research, and Practice. 4th ed. San Francisco, CA: Jossey-Bass, 2008. [Google Scholar]
- 42. Saha S, Jacobs EA, Moore RD, Beach MC. Trust in physicians and racial disparities in HIV care. AIDS Patient Care STDS 2010; 24:415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Armstrong K, Rose A, Peters N, Long JA, McMurphy S, Shea JA. Distrust of the health care system and self-reported health in the United States. J Gen Intern Med 2006; 21:292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schneider J, Kaplan SH, Greenfield S, Li W, Wilson IB. Better physician-patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection. J Gen Intern Med 2004; 19:1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dale SK, Bogart LM, Wagner GJ, Galvan FH, Klein DJ. Medical mistrust is related to lower longitudinal medication adherence among African-American males with HIV. J Health Psychol 2016; 21:1311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pellowski JA, Price DM, Allen AM, Eaton LA, Kalichman SC. The differences between medical trust and mistrust and their respective influences on medication beliefs and ART adherence among African-Americans living with HIV. Psychol Health 2017; 32:1127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blackstock OJ, Addison DN, Brennan JS, Alao OA. Trust in primary care providers and antiretroviral adherence in an urban HIV clinic. J Health Care Poor Underserved 2012; 23:88–98. [DOI] [PubMed] [Google Scholar]
- 48. Samet JH, Sullivan LM, Traphagen ET, Ickovics JR. Measuring adherence among HIV-infected persons: is MEMS consummate technology? AIDS Behav 2001; 5:21–30. [Google Scholar]
- 49. Sulkowski M, Luetkemeyer AF, Wyles DL, et al. Impact of a digital medicine programme on hepatitis C treatment adherence and efficacy in adults at high risk for non-adherence. Aliment Pharmacol Ther 2020; 51:1384–96. [DOI] [PubMed] [Google Scholar]
- 50. Brown A, Welzel TM, Conway B, et al. Adherence to pan-genotypic glecaprevir/pibrentasvir and efficacy in HCV-infected patients: a pooled analysis of clinical trials. Liver Int 2020; 40:778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.