Abstract
Objective
Treatment with CTLA-4Ig blocks T-cell activation and is clinically effective in RA. However, it is unknown if specific CD4+ T-cell subsets in blood at baseline predict remission after CTLA-4Ig, or other biological treatments with different modes of action, and how treatment affects CD4+ T cells in patients with untreated early RA (eRA).
Methods
This study included 60 patients with untreated eRA from a larger randomized trial. They were treated with methotrexate combined with CTLA-4Ig (abatacept, n = 17), anti-IL6 receptor (tocilizumab, n = 21) or anti-TNF (certolizumab-pegol, n = 22). Disease activity was assessed by clinical disease activity index (CDAI), DAS28, swollen joint counts, tender joint counts, CRP and ESR. The primary outcome was CDAI remission (CDAI ≤ 2.8) at week 24. Proportions of 12 CD4+ T-cell subsets were measured by flow cytometry at baseline and after 4, 12 and 24 weeks of treatment.
Results
In patients treated with CTLA-4Ig, the proportions of PD-1+TFh and CTLA-4+ conventional CD4+ T cells at baseline predicted CDAI remission at week 24. CD4+ T-cell subset proportions could not predict remission after treatment with anti-IL6R or anti-TNF. The percentage of regulatory T cells (Tregs) expressing CTLA-4 decreased in all treatment arms by 24 weeks, but only CTLA-4Ig treatment significantly reduced the proportions of Tregs and PD-1+T follicular helper (TFh) cells.
Conclusion
These findings indicate that circulating proportions PD-1+TFh and CTLA-4+ conventional CD4+ T cells at baseline may serve as predictive biomarkers for remission in early RA after CTLA-4Ig treatment.
Keywords: Rheumatoid arthritis, CD4+, T cells, remission, CTLA-4Ig, anti-IL6R, anti-TNF
Rheumatology key messages.
Proportions of PD-1+TFh and CTLA-4+CD4+ T cells predict remission after CTLA-4Ig treatment in eRA.
CTLA-4Ig, anti-IL6R or anti-TNF treatment has different effects on circulating CD4+ T-cell subsets.
Introduction
RA is a chronic autoimmune disease that is characterized by synovial inflammation and subsequent joint destruction if not treated early and effectively [1]. In RA, CD4+ T cells have been identified as key promotors of disease pathology through genetic association studies as well as functional in vivo and in vitro studies (reviewed in [2, 3]). However, the contribution of specific CD4+ T-cell subsets in RA inflammation is not fully understood. In patients with untreated early RA (eRA) (a subset of patients included in the present study), we have previously shown that circulating proportions of T helper (Th) 2 and Th17 cells as well as CTLA-4+CD4+ T cells are higher in patients compared with healthy controls [4]. We also showed that baseline proportions of Th2, Th17 and Th1Th17 as well as CTLA-4+CD4+ T cells correlate with disease activity in male but not female patients [5].
The introduction of biological DMARD (bDMARD) has resulted in improved clinical outcomes for individuals diagnosed with RA. In patients with established RA, successful treatment with bDMARDs has been shown to alter the proportions of specific T-cell subsets in the circulation. Treatment with CTLA-4Ig (abatacept), an inhibitor of T-cell activation that blocks co-stimulatory receptors CD80/86 on antigen presenting cells [6], has been shown to reduce the proportions of both activated TFh, Th17 and regulatory T cells (Treg) [7, 8]. Anti-IL6 receptor (tocilizumab) or soluble TNF receptor (etanercept) treatment were found to increase the proportion of Tregs and reduce the proportion of Th17 cells [9, 10]. However, the findings are not consistent as others have shown that anti-IL6R does not affect proportions of Th17 cells [11], and that TNF blocking therapy does not alter proportions of Tregs [12]. The effects of bDMARDs on CD4+ T-cell subset proportions in patients with established RA are, however, difficult to compare due to differences in disease duration and treatment history, as well as inconsistent markers used to define the T-cell subsets. Moreover, most studies have not compared the effects of different bDMARDs head-to-head. In untreated eRA patients, the comparative effects of different bDMARDs on circulating proportions of specific CD4+ T-cell subsets have never been studied.
Despite the introduction of bDMARDs, <50% of eRA patients achieve strict remission (CDAI ≤ 2.8) within one year of treatment [13, 14]. Thus far, no useful biomarkers for selection of targeted bDMARDs or prediction of remission have been discovered [15]. Because bDMARDs have distinct modes of action, different biomarkers may be required to predict treatment outcome for each type of bDMARD. As CTLA-4Ig functions as an inhibitor of T-cell activation, circulating proportions of certain CD4+ T-cell subsets might serve as biomarkers for remission after CTLA-4Ig treatment.
In a cohort of 60 untreated eRA patients treated with CTLA-4Ig, anti-IL6R or anti-TNF, we examined whether CD4+ T-cell subsets at baseline could predict remission after 24 weeks of treatment, and we also compared the effects of the three therapies head-to-head on proportions of circulating CD4+ T-cell subsets.
Methods
Patients
This study was done on a subset of patients from three of the four randomized arms in the NORD-STAR trial, a phase four investigator-initiated, randomized, observer-blinded clinical trial [16], and followed 60 individuals with untreated eRA during their first 24 weeks of treatment. Patients’ baseline characteristics are shown in Table 1. Patients were diagnosed according to the ACR/EULAR 2010 criteria. No patients had received prior DMARD or corticosteroid treatment. The inclusion criteria were: ≥18 years of age, ≥2 swollen joints (66 joint count), ≥2 tender joints (68 joint count), DAS28-CRP of ≥3.2 and a symptom duration <24 months (retrospective patient-reported symptoms). Patients also had to be RF-positive or ACPA-positive or have a CRP level ≥10 mg/l. All patients were recruited at the rheumatology clinics at Sahlgrenska University Hospital (n = 46) or at Skåne University Hospital (n = 14), Sweden. All study participants gave informed consent and the regional ethics committees of Stockholm (Dnr. 2011/2069–31/4) and Gothenburg (Dnr. 691–12 and amendment T270-13) approved the study protocol. The study was conducted in compliance with the Helsinki Declaration and all patients gave written informed consent.
Table 1.
Baseline characteristics of untreated early RA patients in each respective treatment arm
| MTX + CTLA-4Ig (n = 17) | MTX + anti-IL6R (n = 21) | MTX + anti-TNF (n = 22) | P-value | |
|---|---|---|---|---|
| Age, yearsa | 61 (21-77) | 51 (25-72) | 60.5 (21-71) | 0.22c |
| Symptom duration, monthsa,b | 6 (2-23) | 6 (1.5-21) | 4.5 (1-18) | 0.62c |
| Female sex, n (%) | 12 (71) | 14 (67) | 13 (59) | 0.79d |
| Smoker (%)e | 2 (12) | 4 (19) | 4 (18) | 0.83d |
| CRP, mg/La | 10 (2-92) | 6 (0.3-25) | 18.5 (2-180) | 0.04c |
| ESR, mm/ha | 28 (8-101) | 19 (5-37) | 32.5 (7-98) | 0.03c |
| SJC66a | 8 (3-19) | 12 (3-17) | 11.5 (3-28) | 0.23c |
| TJC68a | 13 (3-25) | 12 (2-47) | 14.5 (2-35) | 0.60c |
| SJC28a | 6 (3-14) | 9 (2-13) | 7.5 (3-24) | 0.51c |
| TJC28a | 9 (0-13) | 8 (0-24) | 7.5 (1-27) | 0.86c |
| DAS28-CRPa | 4.9 (3.8-6.5) | 4.7 (2.7-6.9) | 5.4 (3.2-8.3) | 0.14c |
| DAS28-ESRa | 5.2 (4.2-7.2) | 5.2 (2.6-7.1) | 5.8 (3.6-8.7) | 0.17c |
| CDAIa | 26.0 (14.3-41.7) | 27.8 (10.5-62.1) | 28.1 (10.1-68.7) | 0.77c |
| ACPA+, n (%) | 15 (88) | 18 (86) | 17 (77) | 0.67d |
| RF+, n (%) | 12 (71) | 18 (86) | 13 (59) | 0.17d |
| ACPA+ and RF+, n (%) | 11 (65) | 15 (71) | 13 (59) | 0.70d |
| ACPAneg and RFneg, n (%) | 1 (6) | 0 0 | 5 (23) | 0.03d |
Abatacept (CTLA-4Ig), tocilizumab (anti-IL6R), certolizumab-pegol (anti-TNF). aMedian and range. bRetrospective patient-reported pain in the joints before RA diagnosis. cDifference between treatment arms, Kruskal–Wallis test followed by Dunn’s multiple comparisons. dDifference between treatment arms, Fishers exact test. eCurrent daily smoker.
Study format and clinical evaluation
The complete treatment protocol and results from the primary clinical outcome at week 24 in the full NORD-STAR cohort have been published elsewhere [16]. In brief, patients who fulfilled the inclusion criteria were randomized to four different treatment arms. As the focus of this study was to investigate the effect of targeted treatments, only patients treated with biological DMARDs were analysed. All patients received MTX escalated to 25 mg/week within the first 4 weeks. In addition to MTX, patients received CTLA-4Ig (abatacept; Bristol Myers Squibb, New York City, NY, USA), anti-IL6R (tocilizumab; Hoffmann-La Roche, Basel, Switzerland) or anti-TNF (certolizumab-pegol; UCB, Brussels, Belgium). Oral steroids were not allowed. The primary clinical outcome was the achievement of remission according to the Clinical Disease Activity Index (CDAI) at week 24 [17]. Disease activity was assessed at baseline (before the start of treatment), and after 4, 12 and 24 weeks of treatment by the following parameters: swollen joint counts in 28 and 66 joints (SJC28 and SJC66, respectively), tender joint counts in 28 and 68 joints (TJC28 and TJC68, respectively), CRP, ESR, CDAI and DAS28 [18]. ACPA and RF positivity was determined at the start of the study by multiplexed anti-CCP test (BioPlex from BioRad, Hercules, CA, USA) and nephelometry (Beckman Coulter, Brea, CA, USA), respectively, and positivity was determined according to cut-off levels in the local clinical immunology laboratories. Blood samples were drawn at baseline (within 1–2 weeks of RA diagnosis) and after 4, 12 and 24 weeks of treatment. T-cell subset proportions were analysed by flow cytometry.
Flow cytometry
To reduce variability, all flow cytometry analyses were performed by the same staff at the Clinical Immunology Laboratory and Transfusion Medicine at the Sahlgrenska University Hospital in Gothenburg. Peripheral blood mononuclear cells (PBMCs) were separated from whole blood by the use of Lymphoprep (Axis-Shield, Oslo, Norway). In fresh PBMC, T-cell subsets were defined and characterized by the use of flow cytometry, as previously described [4, 5]. In brief, PBMCs were stained with fluorochrome-conjugated antibodies against CD4, CD45RA, CCR4, CCR6, CXCR3, CXCR5, CD25, CD127, PD-1 and CTLA-4 (full list of antibodies is found in Supplementary Table S1, available at Rheumatology online). For CTLA-4 measurements, cells were fixed and permeabilised to detect both surface and intracellular CTLA-4. Antibodies were mixed and prepared in batches for 20–30 samples at a time to reduce between-sample variability caused by differences in antibody concentration. The stained samples were acquired on a FACS Canto II (BD Biosciences) equipped with FACS Diva software (BD Biosciences). BD FACSDiva™ CS&T IVD beads (BD Biosciences) were used for daily quality control and to ensure consistent performance of the flow cytometer. We found no significant batch effects in the flow cytometry data when the samples were grouped in batches based on the time of analysis (6 month intervals, data not shown). Flow cytometry data were analysed blindly in FlowJo software (Tree Star, Ashland, OR, USA) by K.A. The following T-cell subsets were analysed: Th1, Th1Th17, Th2, CXCR3+Th2, Th17 and CXCR3+Th17 were reported as a proportion of CD45RAneg memory cells (Supplementary Fig. S1A, available at Rheumatology online), regulatory T cells (Tregs), follicular regulatory T cells (TFregs), conventional CD4+ T cells (NonTregs) and T follicular helper cells (TFh) reported as a proportion of CD4+ cells (Supplementary Fig. S1B, available at Rheumatology online), and the fraction of CTLA-4 positive cells of Tregs and NonTregs, respectively (Supplementary Fig. S1C, available at Rheumatology online). A detailed gating strategy is presented in Supplementary Fig. S2, available at Rheumatology online [4, 5]. Only fresh PBMC were analysed, because freezing and thawing of PBMC stored in fetal calf serum with 7.5% dimethyl sulfoxide affected the expression of several markers used to categorize T-cell subsets (Supplementary Fig. S3, available at Rheumatology online).
Statistical analysis
Multivariate factor analysis (SIMCA-P+ software; Umetrics, Umeå, Sweden) by orthogonal projection to latent structures-discriminant analysis (OPLS-DA) was used to investigate the association between circulating proportions of CD4+ T-cells subsets and whether patients did, or did not, achieve remission (CDAI ≤ 2.8) at week 24. Log transformation was applied to all variables in multivariate factor analysis to normalize the data, and each variable was scaled by dividing it by one standard deviation (unit variance scaling) to ensure that all variables were given equal weight in the models. The quality of each of the OPLS-DA models was measured by the parameters R2 (i.e. how well the variance of the data is explained by the model) and Q2 (i.e. how well the model predicts the variable data). To avoid the problem of multiple testing, univariate statistical analyses were only applied to the variables that contributed most to each of the respective OPLS models. The univariate analyses performed were Mann–Whitney U test, paired Wilcoxon matched-pairs signed rank test, Spearman rank correlation test, Kruskal–Walls test followed by Dunn’s multiple comparison test (GraphPad Prism Software, La Jolla, CA, USA) and Fisher’s exact test (IBM SPSS Statistics, Armonk, NY, USA) as described in the respective figure legends. Logistic regression, presented as area under the curve (AUC) in receiver operating characteristic curves (ROC curves), was used to test whether specific T-cell subset proportions at baseline could predict remission at week 24 (Graphpad Prism Software). Cut-off values were chosen for the T-cell subsets that had ROC curves with a significant AUC based on the sensitivity/specificity that generated a positive predictive value (PPV) for the largest number of patients. The PPV and negative predictive value (NPV) were calculated based on the remission rate for each respective treatment arm. For all univariate analyses, a P-value ≤0.05 was regarded as statistically significant (*P ≤0.05, ** P ≤0.01, *** P ≤0.001 and **** P ≤0.0001).
Results
Biological treatments display distinct effects on circulating proportions of T-cell subsets in patients with untreated eRA
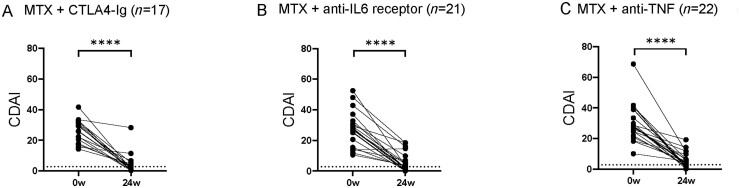
Baseline characteristics for untreated eRA patients in each treatment arm are shown in Table 1. There were no significant differences in median age or in symptom duration between treatment arms. Composite measures of disease activity DAS28 and CDAI, as well as TJC, SJC and number of ACPA+ and/or RF+ patients did not differ significantly between treatment arms. However, baseline CRP, ESR and number of ACPAneg and RFneg patients were higher in the anti-TNF arm than in the anti-IL6R arm. CDAI decreased significantly from baseline to week 24 in all treatment arms (Fig. 1A–C).
Fig. 1.
Difference in disease activity at baseline and after 24 weeks
RA disease activity as measured by the Clinical Disease Activity Index (CDAI) at baseline (0w) and at 24 weeks (24w) in patients treated with (A) methotrexate (MTX) + CTLA-4Ig (abatacept, n=17), (B) MTX + anti-IL6 receptor (tocilizumab, n=21) and (C) MTX + anti-TNF (certolizumab-pegol, n=22). Dotted line indicates limit for remission (CDAI≤2.8). Paired Wilcoxon matched-pairs signed rank test *P ≤0.05, **P ≤0.01, ***P ≤0.001 and ****P ≤0.0001.
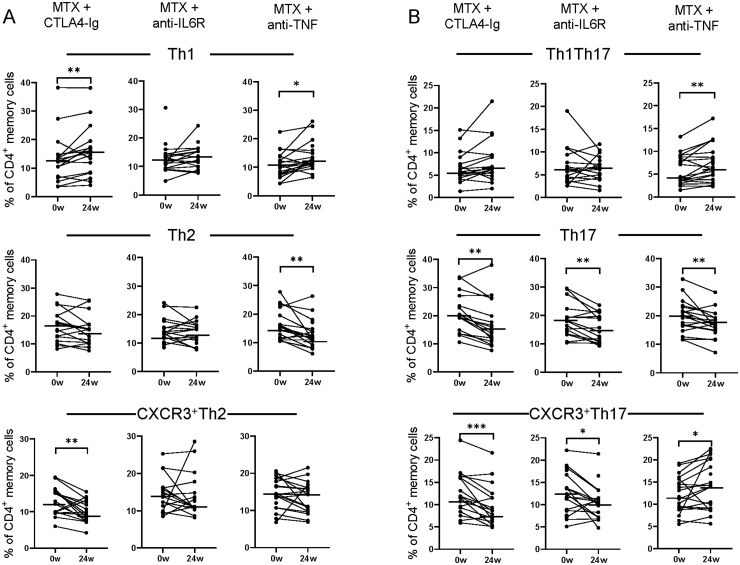
To investigate the effect of different bDMARDs on circulating proportions of T-cell subsets, we first examined the proportions of T-cell subsets at baseline and after 24 weeks of treatment in each arm (data from all time points, including baseline, week 4, 12 and 24 are shown in Supplementary Fig. S4, available at Rheumatology online). There were no significant differences in baseline T-cell subset proportions between treatment arms (Supplementary Table S2, available at Rheumatology online). Patients treated with CTLA-4Ig had lower proportions of memory CD4+ T cells at week 24 compared with baseline (P=0.0004, data not shown). With regard to classical and non-classical Th subsets, CTLA-4Ig decreased proportions of CXCR3+Th2, Th17 and CXCR3+Th17 cells at week 24 compared with baseline, while the proportions of Th1 cells increased (Fig. 2A–B). The decrease in CXCR3+Th2, Th17 and CXCR3+Th17 proportions was not related to the reduction in CDAI (Spearman correlation, r = 0.056 P=0.83, r=–0.30 P=0.25 and r = 0.22 P=0.39, respectively). Anti-IL6R treatment had no effect on the proportion of memory CD4+ T cells (P=0.8, data not shown), but resulted in lower proportions of Th17 and CXCR3+Th17 cells at week 24 compared with baseline (Fig. 2B). Treatment with anti-TNF resulted in decreased proportions of Th2 and Th17 cells at week 24 compared with baseline (Fig. 2A–B). The proportions of memory CD4+ T cells increased after anti-TNF treatment (P=0.02, data not shown), as well as proportions of Th1, Th1Th17 and CXCR3+Th17 cells (Fig. 2A–B). Thus, despite a significant reduction in disease activity in all treatment arms, the change in circulating proportions of memory CD4+ T cells and several subpopulations thereof differed between treatments.
Fig. 2.
Difference in T helper cell subset proportions at baseline and after 24 weeks of treatment
Comparison of the proportions of (A–B) Th1, Th2, CXCR3+Th2, ThTh17, Th17 and CXCR3+Th17 of CD45RAneg memory cells in circulation at baseline (0w) and at 24 weeks (24w) in patients treated with methotrexate (MTX) + CTLA-4Ig (abatacept, n=17), MTX + anti-IL6 receptor (tocilizumab, n=21) and MTX + anti-TNF (certolizumab-pegol, n=22), respectively. Paired Wilcoxon matched-pairs signed rank test *P ≤0.05, **P ≤0.01, ***P ≤0.001 and ****P ≤0.0001. Horizontal bars indicate median.
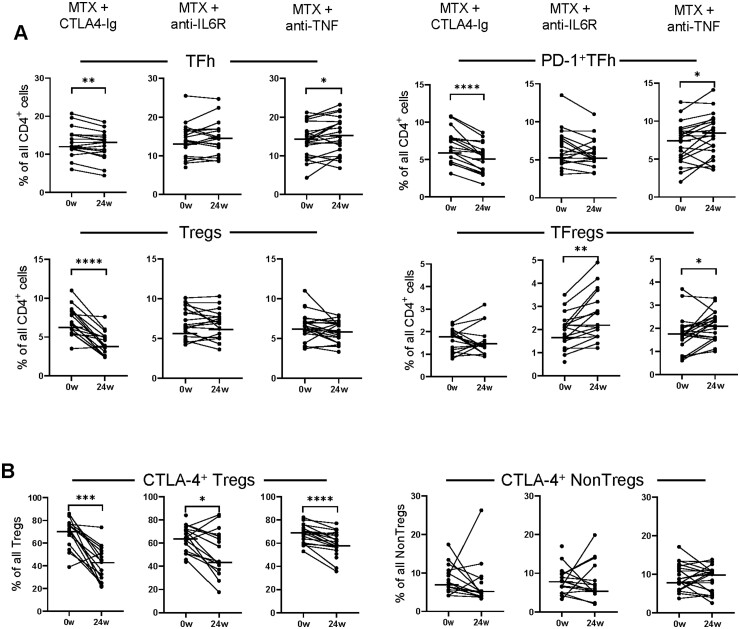
With regard to follicular T helper and regulatory T cells, CTLA-4Ig treatment resulted in reduced proportions of PD1+TFh, Tregs and Tregs expressing CTLA-4 at week 24 compared with baseline, while proportions of TFh increased (Fig. 3A–B). Anti-IL6R treatment decreased the proportions of Tregs expressing CTLA-4 but increased the proportions of TFregs at week 24 compared with baseline (Fig. 3A–B). Treatment with anti-TNF resulted in decreased proportions of Tregs expressing CTLA-4 (Fig. 3B) at week 24 compared with baseline, while proportions of TFh, PD-1+TFh and TFregs increased (Fig. 3A). Thus, the change in circulating T-cell subset proportions markedly differed between treatment arms, although the fraction of Tregs that express CTLA-4 decreased from baseline to week 24 in all treatment arms.
Fig. 3.
Difference in CD4+ T-cell subset proportions at baseline and after 24 weeks of treatment
Comparison of the proportions of (A) TFh, PD-1+TFh, regulatory T cells (Tregs) and follicular regulatory T cell (TFregs) of all CD4+ cells, as well as (B) the proportion of Tregs and conventional CD4+ T cells (NonTregs) that express CTLA-4 in circulation at baseline (0w) and at 24 weeks (24w) in patients treated with methotrexate (MTX) + CTLA-4Ig (abatacept, n=17), MTX + anti-IL6 receptor (tocilizumab, n=21) and MTX + anti-TNF (certolizumab-pegol, n=22), respectively. Paired Wilcoxon matched-pairs signed rank test *P ≤0.05, **P ≤0.01, ***P ≤0.001 and ****P ≤0.0001. Horizontal bars indicate median.
Circulating proportions of specific CD4+ T-cell subsets at baseline predict remission in patients treated with CTLA-4Ig
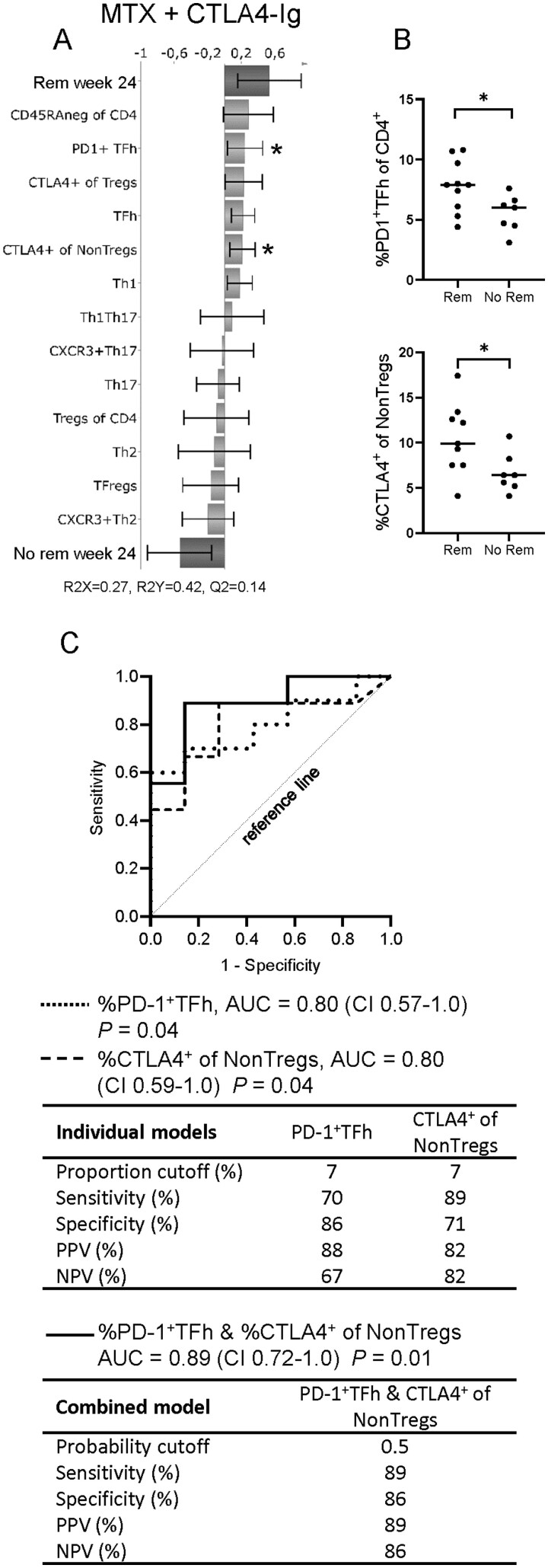
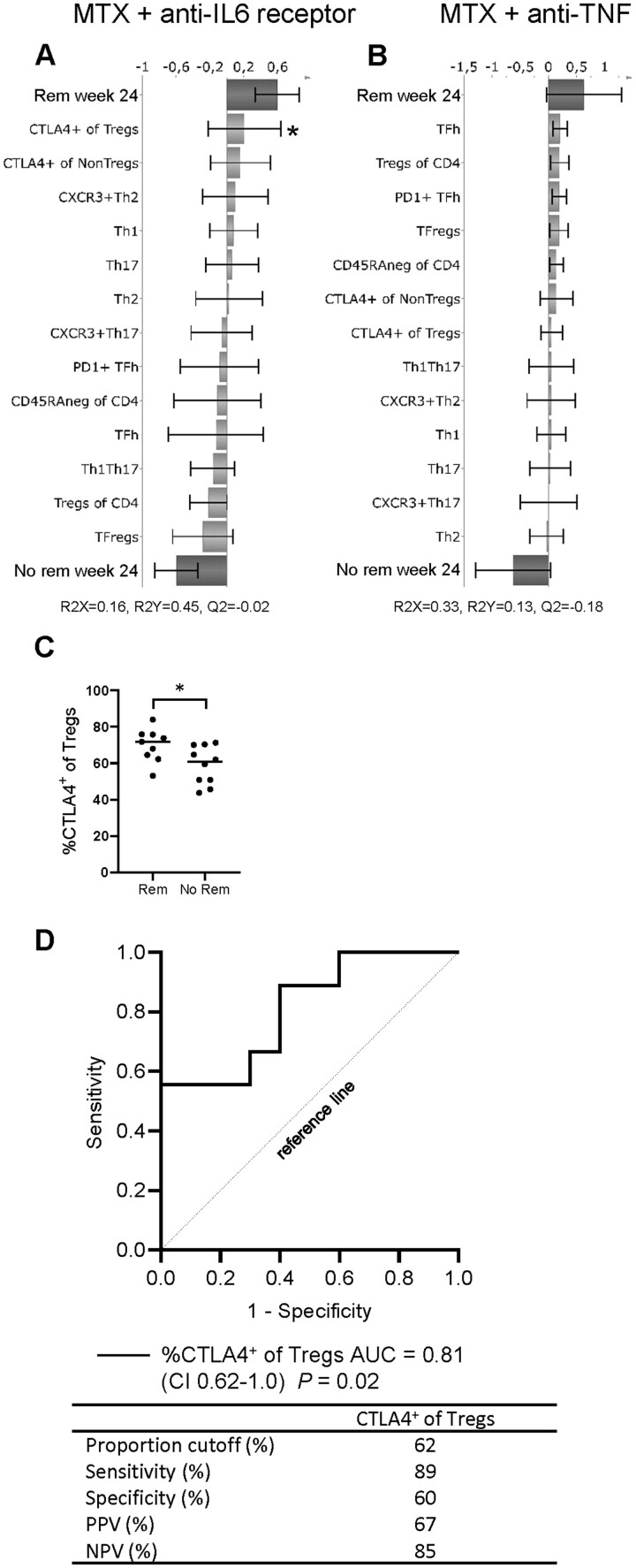
We subsequently examined whether proportions of CD4+ T-cell subsets at baseline could predict remission (CDAI ≤ 2.8) at week 24 in each treatment arm, respectively. OPLS-DA was used to identify T-cell subset proportions that associated with remission at week 24. T-cell subsets that showed the strongest relation to remission status in OPLS-DA were also evaluated by univariate analysis and logistic regression analysis, presented as ROC curves, to assess their diagnostic value. OPLS-DA showed that multiple T-cell subsets associated with remission in the CTLA-4Ig treatment arm (Fig. 4A). In an OPLS-DA loading plot, the X-variables (T-cell subset proportions at baseline) extend in either a positive or negative direction to indicate their association with remission (binary Y-variable) at week 24. Univariate analyses showed that proportions of PD-1+TFh and NonTregs that express CTLA-4 were significantly higher at baseline in patients who achieved CDAI remission at week 24 than those who did not (Fig. 4B). In ROC analysis, the AUC for the proportions of PD-1+TFh and NonTregs that express CTLA-4 at baseline were in both analyses 0.8 (CI 0.57–1.0 and 0.59–1.0, respectively) (Fig. 4C). When both subsets PD-1+TFh and NonTregs expressing CTLA-4 were combined into a single model, AUC increased to 0.89 (CI 0.72–1.0) and sensitivity increased to 89% with no reduction in specificity (Fig. 4C). Therefore, higher proportions of these subsets in combination at baseline would indicate a higher probability of remission in patients who are treated with CTLA-4Ig. OPLS-DA models for the anti-IL6R and anti-TNF arms showed poor association to CDAI remission (Fig. 5A–B). However, in the anti-IL6R treatment arm the proportion of Tregs that express CTLA-4 was significantly higher in patients who achieved remission than in those who did not (Fig. 5C). The ROC curve for the proportion of Tregs expressing CTLA-4 at baseline had an AUC of 0.81 (CI 0.62–1.0), but the specificity was only 60% (Fig. 5D). The anti-TNF arm contained the majority of the double seronegative (ACPAneg and RFneg) patients. When these patients were removed from the OPLS-DA model, a Q2 value of –0.29 was obtained, and when seronegativity was added as a parameter in the original model, Q2 decreased to –0.23 (data not shown). A negative Q2 value indicates that a model is not predictive and, therefore, unreliable. Thus, the anti-TNF OPLS-DA model was not improved by either removing or adding seronegativity as a component in the analysis. In conclusion, our findings show that circulating proportions of specific T-cell subsets may predict remission in untreated eRA patients treated with MTX combined with CTLA-4Ig, but not with anti-IL6R or anti-TNF.
Fig. 4.
Predicting remission in patients treated with CTLA-4Ig by circulating proportions of CD4+ T-cell subsets
(A) OPLS-DA column loading plot showing the association between remission (binary Y-variable) and T-cell subset proportions (X-variables) in patients treated with methotrexate (MTX) + CTLA-4Ig. (B) Comparison of circulating proportions of PD-1+TFh and conventional CD4+ T cells (NonTregs) expressing CTLA-4 at baseline in patients who did or did not achieve remission (CDAI≤2.8) at week 24. Mann–Whitney U-test, *P ≤0.05. Bars indicate median. (C) ROC curves of the proportions of PD-1+TFh and NonTregs expressing CTLA-4 at baseline, in individual regression models (dotted and dashed lines, respectively) or combined in one multiple regression model (black line). AUC: area under the curve; CI: 95 percent confidence interval; PPV: positive predictive value (PPV); NPV: negative predictive value.
Fig. 5.
The relationship between circulating proportions of T-cell subsets at baseline and remission at week 24
OPLS-DA column loading plots showing the association between remission (binary Y-variable) and T-cell subset proportions (X-variables) in patients treated with (A) MTX + anti-IL6 receptor (tocilizumab) or (B) MTX + anti-TNF (certolizumab-pegol). (C) Comparison of circulating proportions of regulatory T cells (Tregs) expressing CTLA-4 in patients who did or did not achieve remission by week 24 (anti-IL6 receptor arm). Two tailed Mann–Whitney U-test, *P ≤0.05. Bars indicate median. (D) ROC curve of the proportion of regulatory T cells (Tregs) expressing CTLA-4 at baseline for prediction of remission by week 24. AUC: area under the curve; CI: 95 percent confidence interval; PPV: positive predictive value (PPV); NPV: negative predictive value.
Discussion
The use of bDMARDs has improved clinical outcomes for individuals diagnosed with RA, but less than half of eRA patients achieve remission within one year of treatment [13, 14]. Quantitative biomarkers for the optimal selection of targeted treatment are needed to further improve outcomes. Circulating proportions of specific CD4+ T-cell subsets may represent usable biomarkers for CTLA-4Ig selection in untreated eRA. For the first time, we here investigated the effect of three different bDMARDs head-to-head on circulating proportions of CD4+ T-cell subsets in patients with untreated eRA and if specific CD4+ T-cell subsets could predict remission in patients treated with CTLA-4Ig and compared with two other biological treatment modalities. We report that CTLA-4Ig, anti-IL6R and anti-TNF treatment display distinct effects on multiple CD4+ T-cell subsets but all decreased the proportions of CTLA-4+ Tregs. We also demonstrate that the proportions of PD-1+TFh cells and NonTregs expressing CTLA-4 at baseline may predict remission in untreated eRA patients treated with CTLA-4Ig, but not anti-IL6R or anti-TNF.
In the present study, the disease activity decreased in all three treatment arms. In line with the reduced level of inflammation in the patients, we found significantly reduced proportions of CTLA-4+ Tregs across all treatment arms. This is likely a result of a less need for T-cell regulation when the inflammatory activity is lower. CTLA-4 is expressed by activated Tregs and it is a crucial part of Treg suppressor function [19], and animal studies have shown that Tregs require IL-2 to maintain suppressor function [20]. Indeed, patients with eRA have increased plasma concentration of IL-2 compared with healthy controls [21]. Therefore, decreased inflammation and plasma levels of IL-2 may contribute to the reduced proportions of CTLA-4+ Tregs after treatment with CTLA-4Ig, anti-IL6R and anti-TNF.
CTLA-4Ig treatment resulted in reduced proportions of total memory CD4+ T cells and several of the analysed T helper subsets. Indeed, CTLA-4Ig inhibits T-cell activation and subsequent differentiation into effector phenotypes by blocking the interaction between CD28 on T cells and co-stimulatory receptors CD80/86 on antigen presenting cells [6]. Specifically, we found that proportions of Th17, CXCR3+Th2, CXCR3+Th17 and PD-1+TFh cells decreased significantly, while the proportions of TFh increased. Note that PD-1 expression by TFh indicate recent antigen exposure and that PD-1+TFh is the activated population of the TFh subset [22]. In line with our findings, CTLA-4Ig treatment reduced the proportions of Th17 cells and activated TFh cells in patients with established RA [7, 23], and decreased the proportions of PD-1+TFh cells in patients with primary Sjögren’s syndrome [24]. The proportions of ICOS+PD-1+TFh and CCR7negPD-1+TFh also decreased after 1 year of CTLA-4Ig treatment in patients with recent onset type 1 diabetes [25]. However, these studies did not measure proportions of Th2, CXCR3+Th2 or CXCR3+Th17 cells. We found that CTLA-4Ig treatment also reduced the proportions of Tregs. Similarly, CTLA-4Ig treatment in patients with established RA results in a decrease in proportions of Tregs [26].
The only T helper subset proportions that were significantly reduced by IL-6 blocking therapy were the Th17 and CXCR3+Th17 subsets. Indeed, IL-6 is a primary inducer of Th17 differentiation [27], and eRA patients have increased proportions of Th17 cells in circulation and elevated plasma concentration of IL-6 compared with healthy controls [4, 21]. Thus, decreased proportions of Th17 and CXCR3+Th17 cells in eRA patients treated with anti-IL6R is likely a result of suppressed Th17 differentiation. Treatment with anti-TNF resulted in increased proportions of CD4+ memory T cells and several T helper subsets. In concordance with our findings, one study showed increased proportions of CD4+ memory T cells and TFh cells after anti-TNF treatment, but opposite to our study they also found increased Th17 proportions [7]. The latter may be due to differences in response to anti-TNF therapy, as proportions of Th17 cells decrease in patients who respond to anti-TNF therapy, while Th17 proportions increase in non-responders [28]. Reduced migration into inflamed joints may contribute to the increased proportions of CD4+ memory T cells after anti-TNF therapy, as transendothelial migration is mediated in part by endothelial expression of the adhesion molecule VCAM-1 that is induced by TNF [29].
We hypothesized that circulating proportions of CD4+ T-cell subsets might be used as biomarkers for selection of CTLA-4Ig treatment. We here show that baseline proportions of PD-1+TFh cells and NonTregs expressing CTLA-4 predicted remission in untreated eRA patients who were treated with CTLA-4Ig. Furthermore, we found that the PPV and NPV improved when proportions of PD-1+TFh cells and NonTregs expressing CTLA-4 were combined into a single prediction model. In contrast, baseline CD4+ T-cell subset proportions could not predict remission after anti-IL6R or anti-TNF treatment. In established RA, a previous study that compared the effect of CTLA-4Ig, anti-IL6R and anti-TNF on T-cell proportions showed that baseline proportions of TFh correlate with the reduction in disease activity only in patients treated with CTLA-4Ig [7]. CTLA-4Ig also appears more effective in seropositive RA patients [30, 31], which can be explained by the fact that TFh are specialized B-cell supporting cells that mediate B-cell activation and antibody production. Indeed, CTLA-4Ig treatment reduces the proportion of switched memory B cells as well as autoantibody levels in RA patients [32].
The use of a clearly defined cohort of treatment-naïve eRA patients should be considered the first strength of this study. Secondly, for the first time we compare the effects of three different bDMARDs on circulating proportions of CD4+ T-cell subsets head-to-head in a randomized clinical trial. Lastly, by analysing fresh PBMC, we avoid the effect of freezing and/or ex vivo stimulation that may have contributed to inconsistent T-cell data in prior studies. However, analysis of peripheral blood may not be fully representative of immunological changes in the joints. The prediction analyses also did not account for other possibly predictive factors, such as age, sex, autoantibodies or smoking status. Finally, the use of a binary outcome variable, i.e. remission, prevents measurement of a possible dose response relation between the predictor and the outcome.
Conclusion
While CTLA-4Ig, anti-IL6R and anti-TNF therapy all result in a significant reduction in disease activity in untreated eRA patients, they have distinct effects on circulating proportions of CD4+ T-cell subsets. Proportions of PD-1+TFh cells and of NonTregs expressing CTLA-4 combined may serve as prognostic markers in untreated eRA patients treated with CTLA-4Ig. If these findings can be replicated in a larger cohort, patients more likely to benefit from CTLA-4Ig treatment could be identified earlier and more accurately, potentially reducing treatment costs and improving quality of life.
Supplementary Material
Acknowledgements
We thank Elke Theander at Lund University for participating in the recruitment of patients and we thank the staff at the Clinical Immunology Laboratory of the Sahlgrenska University Hospital for their technical assistance in collecting the flow cytometry data.
Funding: This study was supported by grants from Swedish Research Council, the IngaBritt and Arne Lundberg’s foundation and the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement.
Disclosure statement: A-K.H.E. declares that she has received consulting fees from AbbVie and Pfizer unrelated to this work. R.vV. reports that he has received institutional grants from BMS, GSK, Lilly, Pfizer, Roche and UCB for research and educational programs, consulting fees from AbbVie, AstraZeneca, Biogen, Biotest, Celgene, Galapagos, Gilead, Janssen, Pfizer, Servier and UCB as well as speaking fees from AbbVie, Galapagos, Janssen, Pfizer and UCB, all non-related to this work. The remaining authors report no conflict of interest.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author J.A. or A.R.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Smolen JS, Aletaha D, McInnes IB.. Rheumatoid arthritis. Lancet 2016;388:2023–38. [DOI] [PubMed] [Google Scholar]
- 2. Kim K, Bang SY, Lee HS, Bae SC.. Update on the genetic architecture of rheumatoid arthritis. Nat Rev Rheumatol 2017;13:13–24. [DOI] [PubMed] [Google Scholar]
- 3. Firestein GS, McInnes IB.. Immunopathogenesis of rheumatoid arthritis. Immunity 2017;46:183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pandya JM, Lundell AC, Hallstrom M. et al. Circulating t helper and t regulatory subsets in untreated early rheumatoid arthritis and healthy control subjects. J Leukoc Biol 2016;100:823–33. [DOI] [PubMed] [Google Scholar]
- 5. Aldridge J, Pandya JM, Meurs L. et al. Sex-based differences in association between circulating t cell subsets and disease activity in untreated early rheumatoid arthritis patients. Arthritis Res Ther 2018;20:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peach RJ, Linsley PS.. Ctla4ig: a novel immunoglobulin chimera with immunosuppressive properties. Methods 1995;8:116–23. [Google Scholar]
- 7. Nakayamada S, Kubo S, Yoshikawa M. et al. Differential effects of biological dmards on peripheral immune cell phenotypes in patients with rheumatoid arthritis. Rheumatology 2018;57:164–74. [DOI] [PubMed] [Google Scholar]
- 8. Piantoni S, Regola F, Scarsi M, Tincani A, Airò P.. Circulating follicular helper t cells (cd4+cxcr5+icos+) decrease in patients with rheumatoid arthritis treated with abatacept. Clin Exp Rheumatol 2018;36:685. [PubMed] [Google Scholar]
- 9. Samson M, Audia S, Janikashvili N. et al. Brief report: inhibition of interleukin-6 function corrects th17/treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum 2012;64:2499–503. [DOI] [PubMed] [Google Scholar]
- 10. Lina C, Conghua W, Nan L, Ping Z.. Combined treatment of etanercept and mtx reverses th1/th2, th17/treg imbalance in patients with rheumatoid arthritis. J Clin Immunol 2011;31:596–605. [DOI] [PubMed] [Google Scholar]
- 11. Kikuchi J, Hashizume M, Kaneko Y. et al. Peripheral blood cd4+cd25+cd127low regulatory t cells are significantly increased by tocilizumab treatment in patients with rheumatoid arthritis: increase in regulatory t cells correlates with clinical response. Arthritis Res Ther 2015;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blache C, Lequerré T, Roucheux A. et al. Number and phenotype of rheumatoid arthritis patients' cd4+cd25hi regulatory t cells are not affected by adalimumab or etanercept. Rheumatology 2011;50:1814–22. [DOI] [PubMed] [Google Scholar]
- 13. Emery P, Bingham CO, Burmester GR. et al. Certolizumab pegol in combination with dose-optimised methotrexate in DMARD-naïve patients with early, active rheumatoid arthritis with poor prognostic factors: 1-year results from c-early, a randomised, double-blind, placebo-controlled phase iii study. Ann Rheum Dis 2017;76:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bijlsma JWJ, Welsing PMJ, Woodworth TG. et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (u-act-early): a multicentre, randomised, double-blind, double-dummy, strategy trial. The Lancet 2016;388:343–55. [DOI] [PubMed] [Google Scholar]
- 15. Smolen JS, Aletaha D, Barton A. et al. Rheumatoid arthritis. Nat Rev Dis Primers 2018;4:18001. [DOI] [PubMed] [Google Scholar]
- 16. Hetland ML, Haavardsholm EA, Rudin A. et al. Active conventional treatment and three different biological treatments in early rheumatoid arthritis: phase iv investigator initiated, randomised, observer blinded clinical trial. BMJ 2020;371:m4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson J, Caplan L, Yazdany J. et al. Rheumatoid arthritis disease activity measures: american college of rheumatology recommendations for use in clinical practice. Arthritis Care Res 2012;64:640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prevoo ML, van 't Hof MA, Kuper HH. et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 19. Qureshi OS, Zheng Y, Nakamura K. et al. Trans-endocytosis of cd80 and cd86: a molecular basis for the cell-extrinsic function of ctla-4. Science 2011;332:600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan MY, Low JS, Tanimine N. et al. Differential roles of il-2 signaling in developing versus mature tregs. Cell Rep 2018;25:1204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kokkonen H, Söderström I, Rocklöv J. et al. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum 2010;62:383–91. [DOI] [PubMed] [Google Scholar]
- 22. He J, Tsai LM, Leong Y. et al. Circulating precursor ccr7lopd-1hi cxcr5+ cd4+ t cells indicate tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 2013;39:770–81. [DOI] [PubMed] [Google Scholar]
- 23. Fukuyo S, Nakayamada S, Iwata S. et al. Abatacept therapy reduces cd28+cxcr5+ follicular helper-like t cells in patients with rheumatoid arthritis. Clin Exp Rheumatol 2017;35:562–70. [PubMed] [Google Scholar]
- 24. Verstappen GM, Meiners PM, Corneth OBJ. et al. Attenuation of follicular helper t cell–dependent b cell hyperactivity by abatacept treatment in primary sjögren's syndrome. Arthritis Rheum 2017;69:1850–61. [DOI] [PubMed] [Google Scholar]
- 25. Edner NM, Heuts F, Thomas N. et al. Follicular helper t cell profiles predict response to costimulation blockade in type 1 diabetes. Nat Immunol 2020;21: 1244–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pieper J, Herrath J, Raghavan S. et al. Ctla4-ig (abatacept) therapy modulates t cell effector functions in autoantibody-positive rheumatoid arthritis patients. BMC Immunol 2013;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou L, Ivanov II, Spolski R. et al. Il-6 programs th-17 cell differentiation by promoting sequential engagement of the il-21 and il-23 pathways. Nat Immunol 2007;8:967–74. [DOI] [PubMed] [Google Scholar]
- 28. Chen DY, Chen YM, Chen HH, Hsieh CW. et al. Increasing levels of circulating th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-tnf-α therapy. Arthritis Res Ther 2011;13:R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bryant J, Ahern DJ, Brennan FM.. Cxcr4 and vascular cell adhesion molecule 1 are key chemokine/adhesion receptors in the migration of cytokine-activated t cells. Arthritis Rheum 2012;64:2137–46. [DOI] [PubMed] [Google Scholar]
- 30. Alemao E, Postema R, Elbez Y, Mamane C, Finckh A.. Presence of anti-cyclic citrullinated peptide antibodies is associated with better treatment response to abatacept but not to TNF inhibitors in patients with rheumatoid arthritis: a meta-analysis. Clin Exp Rheumatol 2020;38:455–66. [PubMed] [Google Scholar]
- 31. Gottenberg JE, Ravaud P, Cantagrel A. et al. Positivity for anti-cyclic citrullinated peptide is associated with a better response to abatacept: data from the ‘orencia and rheumatoid arthritis’ registry. Ann Rheum Dis 2012;71:1815–9. [DOI] [PubMed] [Google Scholar]
- 32. Scarsi M, Paolini L, Ricotta D. et al. Abatacept reduces levels of switched memory b cells, autoantibodies, and immunoglobulins in patients with rheumatoid arthritis. J Rheumatol 2014;41:666–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author J.A. or A.R.