Abstract
Objective
We investigated the autoantibody (autoAb) profiles in ANA+ individuals lacking systemic autoimmune rheumatic disease (SARD) and early SARD patients to determine the key differences between these groups and identify factors that are associated with an increased risk of symptomatic progression within the next 2 years in ANA+ individuals.
Methods
Using custom antigen (Ag) microarrays, 144 IgM and IgG autoAbs were surveyed in 84 asymptomatic and 123 symptomatic (48 UCTD and 75 SARD patients) ANA+ individuals. AutoAbs were compared in ANA+ individuals lacking a SARD diagnosis with ≥2 years follow-up (n = 52), including all those who demonstrated progression (n = 14) during this period, with changes over time assessed in a representative subset.
Results
We show that ANA+ individuals have autoAb to many self-Ags that are not being captured by current screening techniques and very high levels of these autoAbs are predominantly restricted to early SARD patients, with SLE patients displaying reactivity to many more autoAgs than the other groups. In general, the symptoms that developed in progressors mirrored those seen in SARD patients with similar patterns of autoAbs. Only anti-Ro52 Abs were found to predict progression (positive predictive value 46%, negative predictive value 89%). Surprisingly, over 2 years of follow-up the levels of autoAbs remained remarkably stable regardless of whether individuals progressed or not.
Conclusion
Our findings strongly argue that development of assays with an expanded set of auto-Ags and enhanced dynamic range would improve the diagnostic and prognostic ability of autoAb testing.
Keywords: antinuclear antibodies, microarray analysis, SLE, rheumatic diseases, Ro52 antigen
Rheumatology key messages.
Ag microarrays have an improved ability to discriminate between ANA+ individuals with and without SARDs.
Very high levels of autoAbs against autoAg subcomponents are restricted to SARD patients.
Anti-Ro52 Ab is a predictor of progression in ANA+ individuals lacking a SARD diagnosis.
Introduction
Although ANAs are a hallmark of systemic autoimmune rheumatic disease (SARD) and predate the onset of clinical symptoms, they are also found in ∼20% of healthy women, the majority of whom will never progress to SARD [1, 2]. Currently the serological changes that distinguish ANA+ individuals who will progress to SARD from those who will not remain largely unknown. While studies suggest that the number of autoantibody (autoAb) specificities increases as individuals progress to SARD [3–8] and that some autoAbs, such as anti-dsDNA or anti-Sm Abs in SLE, tend to develop just prior to symptom onset [6], it is unclear whether these can be used as markers of impending disease onset.
We examined this question by assessing the type and abundance of autoAbs in a large cohort of ANA+ individuals with no SARD symptoms (ANA+NS) and patients with UCTD, some of whom demonstrated symptomatic progression over the subsequent 2 years. Since conventional commercially available autoAb testing methods examine only a small number of autoAb specificities (<15), a custom antigen (Ag) microarray was used to survey autoAbs to a large number of autoAgs (>140). The type and abundance of autoAbs were contrasted between ANA+ individuals lacking a SARD diagnosis and patients with untreated early SARD and between progressors and non-progressors to identify autoAbs associated with an increased risk of progression.
Patients and methods
Subjects and data collection
From July 2013 to October 2018, individuals referred for a rheumatology assessment because of a recently discovered positive ANA test were serially recruited at the Toronto Western and Mount Sinai Hospitals. At the first visit the subjects underwent an extensive history and physical exam seeking symptoms or signs of SARD, with all demographic and clinical data being recorded on a standardized form [9]. Individuals with an ANA ≥1:160 were stratified into three groups: ANA+NS individuals lacking SARD criteria as defined by the 1997 ACR criteria for SLE [10], 2013 ACR-EULAR criteria for SSc [11] or 2016 ACR–EULAR criteria for SS [12]; UCTD patients with one or more SARD criteria but with insufficient criteria for a diagnosis; or early (within 2 years of diagnosis) untreated (except antimalarials) SARD patients. Healthy controls (HCs) were also recruited and were confirmed to be ANA negative (ANA−HC), with positive HCs being reclassified into the ANA+NS group (20%). For ANA+NS and UCTD individuals recruited after July 2015 (n = 81), yearly follow-up was offered. Individuals who developed new ANA-associated SARD criteria during this time were considered clinical progressors and those with an increase in the levels or in the number of ANA specificities were termed serological progressors. The study was approved by the Research Ethics Boards of both recruiting hospitals and all participants provided written informed consent.
Measurement of autoAbs
ANAs were quantified by indirect immunofluorescence using the Kallestad HEp-2 kit (BioRad Laboratories, Hercules, CA, USA) through the University Health Network. The Bioplex 2200 ANA Screening System (BioRad Laboratories) was used to measure the serum levels of anti-dsDNA, -chromatin, -Ro, -La, -Sm, -SmRNP, -RNP, -Jo-1, -Scl-70, -centromere and -ribosomal P Abs.
Ag microarrays containing 144 autoAgs (Supplementary Table S1, available at Rheumatology online) were generated and probed using a previously published protocol [13–15] (full details are outlined in Supplementary data S1, available at Rheumatology online).
Measurement of the IFN signature
Total RNA was isolated from whole peripheral blood archived in Tempus tubes (Applied Biosystems, Waltham, MA, USA) and gene expression was quantified by using a custom array (NanoString Technologies, Seattle, WA, USA). Log2 normalized abundances of five IFN-induced genes (EPSTI1, IFI44L, LY6E, OAS3, RSAD2) were summed to generate IFN5 scores, as previously described [16].
Statistical analysis
Full details of statistical analysis are given in Supplementary data S1, available at Rheumatology online. Briefly, the net fluorescent intensities (NFI) were log2 transformed and linear modelling was performed using the R package limma (version 3.32.10; R Foundation for Statistical Computing, Vienna, Austria), with batch, sex and age incorporated as covariates. A dual threshold of false discovery rate (FDR) <0.05 and |coefficient| >1 was used to identify statistically significantly differentially abundant probes as compared with ANA−HCs.
To identify differences among progressors and non-progressors within ANA+NS and/or UCTD patients, non-parametric Wilcoxon rank sum tests were used, with a median fold change to assess effect size.
For all other statistical analyses, GraphPad Prism version 8.3.1 (GraphPad Software, San Diego, CA, USA) was used. When two groups were compared, a Mann–Whitney U test was performed for continuous variables and a Fisher’s exact test for discrete variables. Differences between three or more groups were determined using the Kruskal–Wallis test followed by Dunn’s post-test for multiple comparisons.
Results
Demographic and serologic characteristics of the study populations are presented in Table 1, with the SARD criteria seen in the UCTD group provided in Supplementary Table S2, available at Rheumatology online. With the exception of Jo-1 autoAbs, which were seen infrequently and only in SLE patients, the same autoAb specificities and levels were seen in these ANA+ groups as were seen in early SARD patients, at least at the level of the statistical power available in this study. Thus, using conventional commercially available autoAb testing methods, there is considerable overlap in the titre and patterns of autoAbs observed in ANA+NS and UCTD patients with early SARD, suggesting that these assays cannot be used to discriminate between these patient groups.
Table 1.
Study participant characteristics
| Characteristics | ANA−HC (n = 38) | ANA+NS (n = 84) | UCTD (n = 48) | SARD (n = 75) | SSc (n = 16) | SS (n = 33) | SLE (n = 26) | Followed ≥2 years |
|
|---|---|---|---|---|---|---|---|---|---|
| Non-progressors (n = 38a) | Clinical progressors (n = 12) | ||||||||
| Female, n (%) | 27 (71) | 79 (94) | 45 (93.7) | 67 (89.3) | 14 (87.5) | 26 (78.8) | 26 (100) | 36 (95) | 10 (83) |
| Age, years, mean (s.d.) | 30.9 (11.5) | 43.6 (13.6) | 44.0 (15.2) | 47.7 (15.3) | 55.9 (12.6) | 51 (13) | 38.1 (15.1) | 42.9 (14.7) | 44.7 (11.6) |
| Caucasian, n (%) | 19 (47.5) | 49 (58.3) | 34 (70.8) | 47 (62.6) | 11 (69) | 25 (75) | 11 (42) | 29 (76) | 7 (58) |
| Anti-Ro+ mother, n (%)b | 0 (0) | 7 (8.3) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ANA titre, n (%) | |||||||||
| 1:160 | 0 (0) | 19 (22.6)c | 11 (22.9)c | 5 (6.6) | 0 (0) | 2 (6) | 1 (3.8) | 7 (18) | 3 (25) |
| 1:320 | 0 (0) | 11 (13) | 9 (18.7) | 10 (13.3) | 0 (0) | 5 (15) | 5 (31.9) | 8 (21) | 0 (0) |
| 1:640 | 0 (0) | 27 (32.1) | 16 (33.3) | 23 (30.6) | 3 (18.7) | 13 (39) | 7 (26.9) | 13 (34) | 4 (33) |
| >1:640 | 0 (0) | 26 (30.9)c | 13 (27)c | 38 (50.6) | 13 (81.2) | 13 (39) | 12 (46.1) | 10 (26) | 5 (42) |
| Specific Abs, mean (s.d.) | 0 | 0.8 (1)c | 0.9 (1.1)c | 2.2 (1.5) | 1.3 (0.9) | 1.9 (0.7) | 3.1 (2.2) | 0.7 (0.8) | 1 (1.4) |
| Specific Abs, n (%) | |||||||||
| 0 | 38 (100) | 42 (50)c | 18 (37.5)c | 4 (5.3) | 1 (6.2) | 0 (0) | 3 (11.5) | 19 (50) | 3 (25) |
| 1 | 0 (0) | 25 (29.7) | 22 (45.8) | 25 (33.3) | 11 (68.7) | 9 (27) | 5 (19.2) | 14 (36) | 3 (25) |
| 2 | 0 (0) | 12 (14.3)c | 5 (10.4)c | 27 (36) | 3 (18.7) | 20 (60) | 3 (11.5) | 3 (7.8) | 4 (33) |
| 3 | 0 (0) | 1 (1.2)c | 1 (2) | 7 (9.3) | 1 (6.2) | 3 (9) | 4 (15.3) | 2 (10) | 0 (0) |
| 4 | 0 (0) | 1 (1.2)c | 2 (4) | 7 (9.3) | 0 (0) | 1 (3) | 5 (19.2) | 0 (0) | 0 (0) |
| ≥5 | 0 (0) | 2 (2.4) | 1 (2) | 6 (8) | 0 (0) | 0 (0) | 6 (23) | 0 (0) | 2 (16) |
| IFN5 score, median (IQR) | 49 (48–51)c | 53 (50–63)c | 55 (49–64)c | 66 (56–69) | 55 (50–66) | 67 (61–68) | 67 (61–67) | 53 (48–61) | 59 (47–65) |
24 ANA+NS and 14 UCTD.
Identified as ANA+ following birth of a child born with congenital heart block or other manifestations of neonatal lupus. Other indications for ordering the ANA test by the referring doctors in the study population were arthralgia, skin rash and fatigue. cSignificantly (P < 0.05) different from SARD. Values significantly (P < 0.05) different from ANA−HCs are in bold.
Among the 81 ANA+NS and UCTD individuals recruited after July 2015, 52 had completed at least 2 years of follow-up, 17 had <2 years and 12 were lost to follow-up (15%). No serological differences were noted between those who completed or were lost to follow-up. So far, 12 of the 52 individuals followed ≥2 years (23%) have demonstrated symptom progression with the development of new SARD criteria and 2 showed serological progression. Within this group, 10 individuals received antimalarials (19%), 6 of which were taking it at study initiation and 4 of whom started during follow-up. Details of the criteria that defined progression are provided in Supplementary Table S3, available at Rheumatology online.
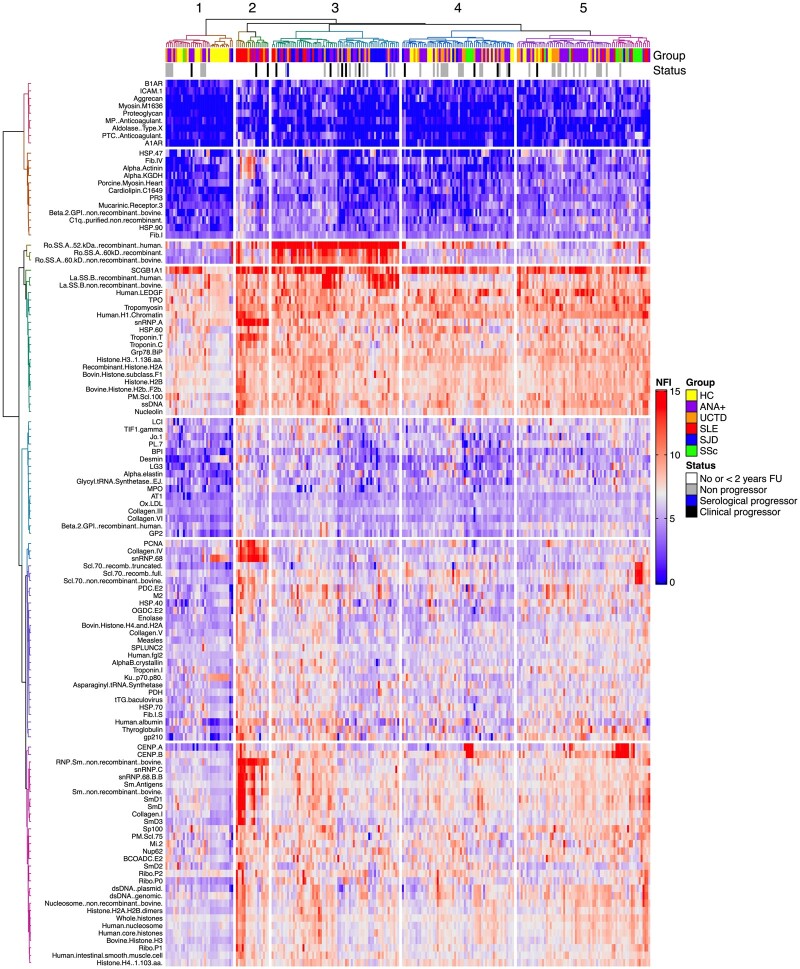
Early SARD patients cluster by diagnosis and a subset of ANA+NS and UCTD patients are admixed with them
Using linear modelling, IgG autoAb abundance differed significantly from ANA−HCs in at least one of the ANA+ groups for 117 of the Ags tested (Supplementary Table S4, available at Rheumatology online). The results of unsupervised hierarchical clustering for these Ags are shown in Fig. 1. Five distinct clusters of subjects were identified based on the pattern of autoAbs. Within these clusters, patients with the same SARD diagnosis tended to cluster together (SLE and SS, P < 0.00001; SSc, P = 0.002; Fisher’s exact test). However, there was substantial admixture of ANA+NS and UCTD patients, with ANA−HCs (see clusters 1 and 4 and the left side of cluster 5) or early SARD patients (clusters 2 and 3 and the right side of cluster 5). In general, the symptoms seen in the UCTD patients, or the new criteria that developed in either ANA+NS or UCTD patients, paralleled those seen in the early SARD patients with whom they were clustered (Supplementary Table S5, available at Rheumatology online).
Fig. 1.
Heat map showing the results of unsupervised hierarchical clustering analysis
IgG autoAb NFI levels shown in red are high and those in blue are low. The status annotations indicate the diagnostic groups (HC, ANA− healthy controls; ANA+, asymptomatic ANA+) and progression (black bar clinical progression, blue bar serological progression, grey bar no progression in the subsequent 2 years). ANA−HC, ANA+ individuals lacking a SARD diagnosis with no follow-up (FU) or <2 years of FU and early SARD patients (SS=SJD) are denoted as No or <2 years FU. Only autoAbs that were significantly different at a dual threshold of FDR <0.05 and |coefficient| >1 on linear modelling for at least one of the ANA+ groups as compared with ANA−HCs are included in the heat map.
In contrast to the IgG autoAb results, IgM autoAb abundance differed significantly from ANA−HCs for only 71 of the Ags tested and no clustering by diagnosis was seen (Supplementary Fig. S1, available at Rheumatology online).
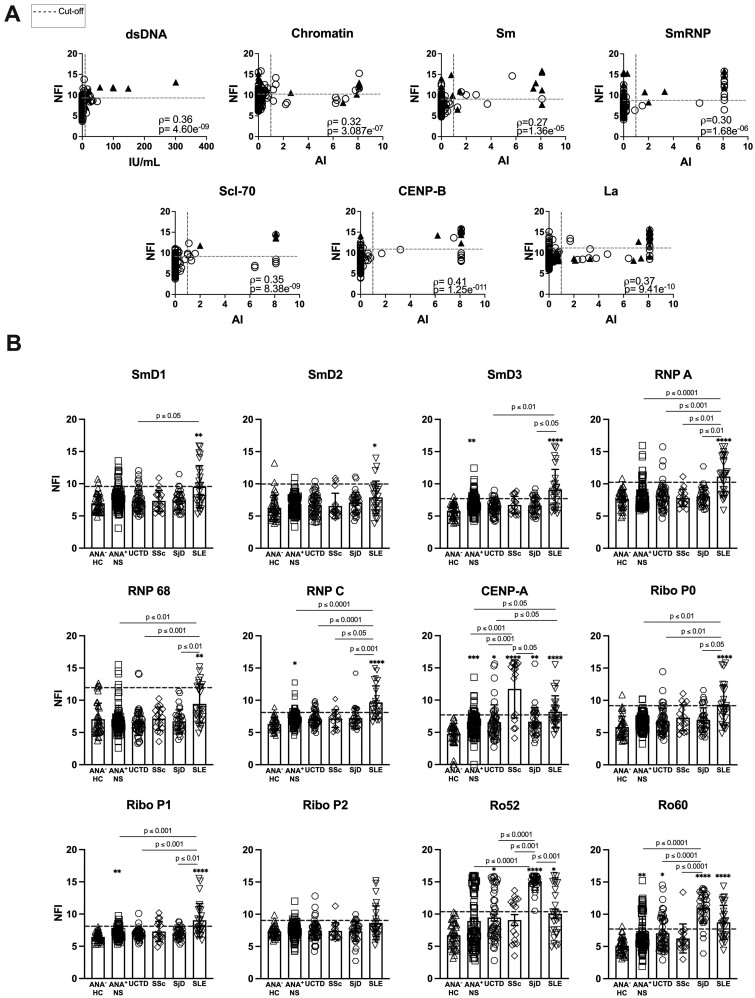
Very high levels of IgG autoAbs against certain nuclear antigen subcomponents may help to classify early SARD patients
In general, there was only a weak positive correlation between the levels of the autoAbs detected by the Bioplex system and the corresponding autoAbs in the microarray (Fig. 2A). This appeared to result from an increased sensitivity of the microarray to detect elevated levels of autoAbs as compared with the Bioplex system. This was particularly apparent for lupus-related autoAgs, such as H1 chromatin, Sm and SmRNP. However, occasionally high levels of autoAbs were detected by the Bioplex system in the absence of corresponding elevations in the microarray.
Fig. 2.
Very high levels of IgG autoAbs as measured by microarray may help to classify early SARD patients
(A) Correlations between the levels of the IgG autoAbs detected by microarray and the corresponding autoAbs in the Bioplex system. Filled triangles indicate patients with the most relevant SARD diagnosis: SLE for dsDNA, chromatin, Sm, and SmRNP; SS (SjD) for La; and SSc for Scl-70 and CENP-B. Open circles represent all the other study participants. The dashed lines indicate the cut-off for elevated levels (horizontal for microarray and vertical for the Bioplex system). Statistical significance was determined using Spearman’s correlation coefficient. (B) Levels of some IgG autoAb subcomponents included on the microarray that are not on the Bioplex system. Bars represent the mean (s.d.). The dashed line indicates the cut-off for elevated levels (ANA−HC mean + 2 s.d.). Statistical significance was determined using the Kruskal–Wallis test with Dunn’s post-test for multiple comparisons. Significant differences relative to ANA−HC are indicated by asterisks (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001) and differences for other groups by P-values. Non-significant differences are not displayed.
For the majority of Ags, the microarray appeared to have a larger dynamic range than the Bioplex system (Fig. 2A) and thus it could detect differences in autoAb levels between individuals above the upper limit of detection of the Bioplex system. Examination of individuals with the highest levels of autoAbs, as detected by microarray, revealed that for many autoAbs the majority of these individuals were early SARD patients, suggesting that very high levels of these autoAbs have increased specificity for SARD (Supplementary Fig. S2 and Supplementary Table S6, available at Rheumatology online).
Very high levels of autoAbs to autoAg subcomponents that were assessed only by microarray (SmD, RNP and ribosomal P subcomponents in SLE and CENP-A in SSc) were also predominantly restricted to early SARD patients (Fig. 2B) and have similarly increased specificity for SARD (Supplementary Table S6, available at Rheumatology online).
Due to a lack of follow-up data, it is unknown whether ANA+ individuals lacking a SARD diagnosis with very high levels of these autoAbs are at increased risk of progression.
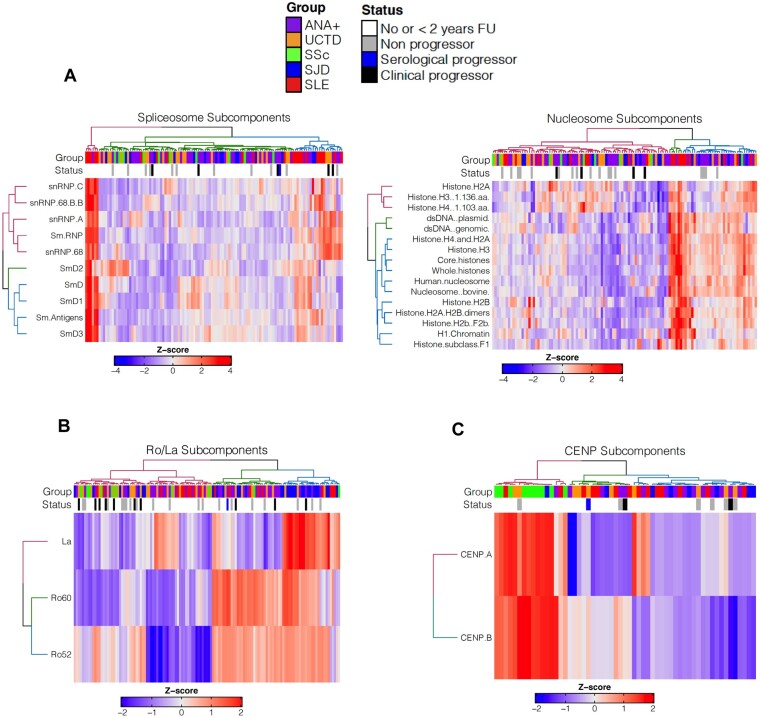
Early SARD patients demonstrate more epitope spreading
Simultaneous reactivity against many of the Sm and/or RNP subcomponents of the spliceosome was a characteristic of SLE patients (Fig. 3A) and was also seen in a small proportion of ANA+NS and UCTD patients. Although follow-up data on these patients are limited, two-thirds of patients with follow-up progressed over the next 2 years. Broad reactivity to the various subcomponents of the nucleosome was also seen in SLE, as well as occasionally in patients with SS and a significant number of ANA+NS and UCTD patients (Fig. 3B far right), none of whom has demonstrated progression thus far.
Fig. 3.
Heat maps showing IgG reactivity to clusters of autoAgs that are seen at high prevalence in SARD
(A) Spliceosome and nucleosome subcomponents, (B) Ro52, Ro60 and La and (C) centromeric protein A and B autoAb levels in ANA+ individuals with reactivity to at least one of the autoAgs being surveyed in the plot. The status annotation indicates progression (black bar is clinical and blue bar is serological) and the ANA+ group classification. ANA−HC, ANA+ individuals lacking a SARD diagnosis with no follow-up (FU) or <2 years of FU; early SARD patients (SS=SJD) are denoted as No or <2 years FU. AutoAb panels for each heat map were selected based on their high prevalence in SLE, SS and SSc, respectively.
Similar results were observed for SS, where concurrent reactivity to Ro60, La and often Ro52 was observed, but was also seen in a subset of other SARD patients, ANA+NS and UCTD patients (Fig. 3B). In contrast, the presence of both anti-CENP-A and anti-CENP-B was a distinctive characteristic of early SSc patients [17–19], being rarely detected in the other ANA+ groups (Fig. 3C).
Taken together, these findings indicate that the extent of epitope spreading appears to be increased in early SARD patients as compared with most ANA+ individuals who lack a SARD diagnosis. It is unclear at present whether those individuals with a pattern of reactivity similar to early SARD are at increased risk of imminent progression.
Most ANA+ individuals with a negative Bioplex assay demonstrated reactivity to nuclear autoAgs on the microarray
Since the microarray surveyed many more nuclear Ags than measured by the Bioplex system, we examined whether ANA+ individuals who lacked autoAbs on the Bioplex system were reactive to nuclear Ags contained in the microarray. The majority of these individuals had Abs to ssDNA and/or nucleosome components on the microarray (Supplementary Fig. S3, available at Rheumatology online). In addition, anti-Ro60, -Ro52, -La and -spliceosome component Abs were detected in a number of individuals who were not detected by the Bioplex system. These observations suggest that there is considerable reactivity to nuclear Ags in ANA+ individuals by immunofluorescence that is being missed by traditional screening techniques.
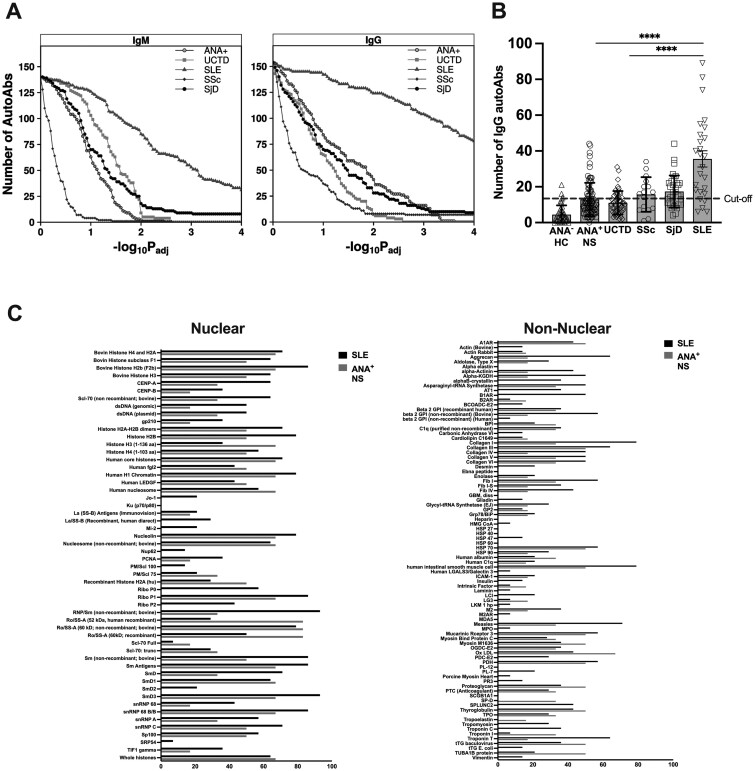
Many more IgG autoAbs were elevated in SLE than in the other ANA+ groups
Many more IgM and IgG autoAbs were elevated in SLE than in the other ANA+ groups (Fig. 4A). To better understand whether these differences represented a subset of individuals who had many elevated autoAbs or whether in general the autoAb levels were higher in these patients, we calculated the number of autoAbs that were elevated in each individual (>2 s.d. above the mean for ANA−HCs). Once again, the number of autoAgs recognized was considerably higher in SLE than in any other group (Fig. 4B). Many SLE patients recognized >30 autoAgs [n = 14 (54%)] and a subgroup recognized >50 autoAgs [n = 6 (23%)]. Age, ethnicity, clinical disease activity (determined by the SLEDAI-2K without the complement or dsDNA components), IFN5 scores and clinical manifestations did not differ among these groups. Interestingly, a subset of ANA+NS individuals also had elevations of >30 different ANA specificities [n = 6 (7%)]. However, as none have completed follow-up, it is not known whether they will be at an increased risk of progression. Fig. 4C depicts the proportion of ANA+NS individuals and SLE patients who showed reactivity against the indicated nuclear and non-nuclear autoAgs for those subjects who had autoAbs to >30 of the autoAgs on the microarray. In general, there was considerable overlap between the nuclear autoAgs that were recognized in ANA+NS and SLE.
Fig. 4.
Many more IgG autoAbs were elevated in SLE than in the other ANA+ groups
(A) P-value sensitivity plot indicating the number of autoAbs determined to be significantly associated with each group at various significance thresholds relative to ANA−HCs. For downstream analyses, a threshold of 0.05 was used. (B) Number of autoAbs recognized by each ANA+ group (SS=SjD). Reactivity was considered present if the autoAb levels exceeded 2 s.d. above the mean for ANA−HCs. Bars represent the mean (s.d.) for each group. Statistical significance was determined using the Kruskal–Wallis test with Dunn’s post-test for multiple comparisons. Non-significant differences are not displayed; **P ≤ 0.01, ****P ≤ 0.0001. (C) Proportion of SLE patients and ANA+NS individuals with >30 autoAbs that recognized each nuclear and non-nuclear Ag in the microarray. Only autoAgs recognized by one of the groups are shown.
These findings suggest that tolerance is more disrupted in SLE than other SARDs and indicate that a subset of ANA+NS share this characteristic.
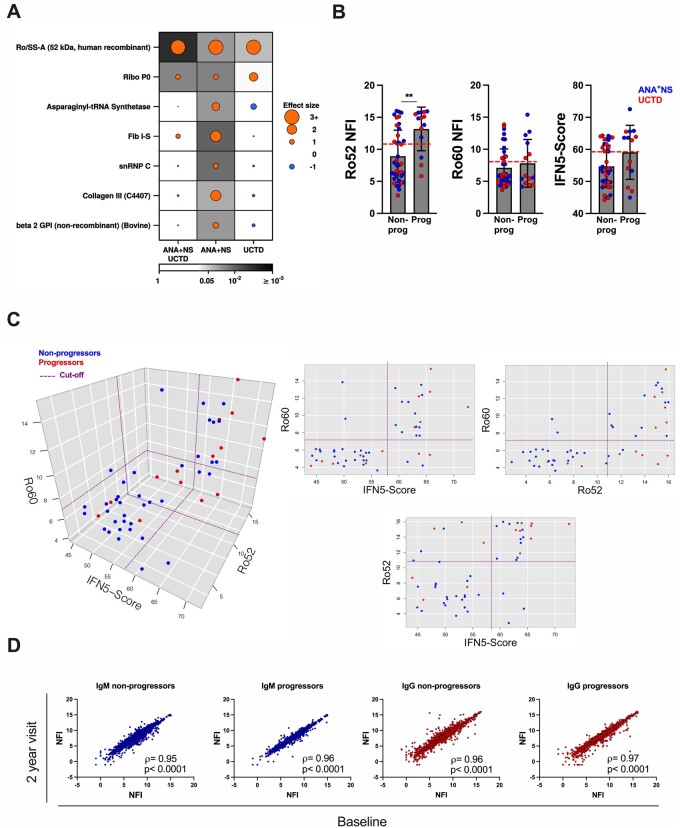
The presence of IgG anti-Ro52 Abs is associated with progression in both ANA+NS and UCTD individuals
To determine whether there are autoAb specificities that predict disease progression in ANA+ individuals lacking a SARD diagnosis, the levels of autoAbs were contrasted between progressors (n = 14) and non-progressors (n = 36) with at least 2 years of follow-up. As shown in Fig. 5A, only the levels of anti-Ro52 Abs were significantly associated with disease progression in ANA+NS, UCTD and the combined group of ANA+NS and UCTD progressors. Of the 24 patients with high anti-Ro52 Abs, 11 progressed over the 2 year period whereas only 3 of 28 individuals lacking these Abs progressed (P = 0.027). Notably, measurement of anti-Ro52 Abs by microarray outperformed measurement of anti-Ro Abs by the Bioplex system for the prediction of imminent progression, with a greater positive predictive value (46% vs 40%), negative predictive value (89% vs 81%) and sensitivity (79% vs 57%) and comparable specificity (66% vs 68%). In contrast, the number of autoAb specificities did not differ significantly between progressors and non-progressors [mean progressors 11 (s.d. 1.63), non-progressors 9.7 (4.7)].
Fig. 5.
AutoAbs associated with progression in ANA+NS and UCTD individuals
(A) Dotmap showing autoAbs with significant differences between progressors and non-progressors (P < 0.05 and |median fold change| >1). Dot size indicates effect size, colour shows direction (orange = higher abundance in progressors, blue = lower abundance in progressors) and background shading shows unadjusted P-value. (B) Levels of IgG anti-Ro52 Abs, IgG anti-Ro60 Abs and IFN5 scores in progressors and non-progressors that completed 2 years of follow-up. ANA+NS are indicated in blue [n = 31 (7 progressors)] and UCTD in red [n = 21 (7 progressors)]. Bars represent the mean (s.d.). Statistical significance was determined using the Mann–Whitney test. Non-significant differences are not displayed; **P ≤ 0.01. Red horizontal dashed lines indicate the cut-off for elevated levels, determined as >2 s.d. above the ANA−HC mean. (C) Three-dimensional plot and corresponding orthographic views depicting the interrelationship between IFN5 score and the levels of anti-Ro52 and anti-Ro60 Abs. Red dots represent progressors and blue non-progressors. The dashed purple lines correspond to the cut-off for elevation for each component. (D) Log2-log2 plot contrasting the levels of IgM and IgG autoAbs at baseline and at 2 years of follow-up (visit 2) in progressors and non-progressors. The statistical significance of the correlation between the two visits was determined using Spearman’s correlation coefficient.
Consistent with previous studies, the IFN5 score significantly correlated with the levels of IgG anti-Ro60 and -Ro52 Abs (ρ = 0.579, q = 1.42 × 10−16 and ρ = 0.467, q = 1.02 × 10−9, respectively) [16, 20, 21]. Furthermore, anti-Ro60 and -Ro52 Abs often cosegregate [22], which was confirmed in the current study (ρ = 0.53, q = 0.05). Given the close association between anti-Ro52 Abs, anti-Ro60 Abs and the IFN5 score [16, 20, 21], we examined the interrelationship between them and progression. In contrast to anti-Ro52 Ab, neither anti-Ro60 Ab nor IFN5 score levels were significantly increased in progressors as compared with non-progressors (Fig. 5B), and progression appeared to be most closely associated with elevated levels of anti-Ro52 (Fig. 5C). Although ANA+ individuals lacking a SARD diagnosis with very high IFN5 scores (>65) appeared to be enriched for progressors, there was a high degree of overlap with individuals with high levels of anti-Ro52 Abs. Similarly, individuals with high anti-Ro52 Abs who lacked IFN5 score elevations also demonstrated an increased rate of progression, suggesting that elevation of anti-Ro52 Abs is the more important factor.
Although anti-lens epithelium-derived growth factor (anti-LEDGF) Abs have been proposed to be associated with a lack of progression in ANA+ individuals [23–26], we were unable to reproduce these findings. Of 52 individuals with ≥2 years of follow-up, 13 had anti-LEDGF Abs, 2 of which have evolved, suggesting that this autoAb cannot be used to discriminate non-progressors from progressors. Similarly, despite previous reports suggesting that a positive Sjö test might indicate progression to SS, elevated levels of some components of this test were not seen in ANA+ individuals who progressed to SS nor were they seen in early SS (Supplementary Fig. S4, available at Rheumatology online).
To explore whether changes over time were associated with imminent clinical progression, changes in the autoAb profile from baseline to 2 years follow-up were compared between progressors (n = 10 clinical, n = 2 serological; the remaining 2 clinical progressors evolved after this part of the study was concluded) and a subset of age- and ethnicity-matched ANA+NS individuals and UCTD patients who had not progressed. There were no statistically significant differences over time between progressors and non-progressors, with both demonstrating minimal changes over the 2 year period (Figure 5D), nor were there significant differences over time between individuals with high (≥1:640) or low (≤1:320) ANA titres.
Discussion
Previous studies have contrasted the performance of different immunoassay techniques to detect autoAbs in individuals with SARD [20, 27–29]. However, in general these studies have investigated their diagnostic utility in comparison with HCs, unaffected relatives and/or individuals with non-SARD musculoskeletal conditions [2, 30–33]. To our knowledge, this is the first study to contrast the effectiveness of current commercially available techniques with autoAg microarrays to discriminate between early SARD patients and a large number of ANA+ individuals who lack a SARD diagnosis, representing the typical types of ANA+ individuals who are referred to a rheumatologist’s office for evaluation.
We show that the subset of individuals with very high levels of nuclear autoAbs on microarray is significantly enriched for early SARD patients, as opposed to ANA+NS or UCTD patients, indicating that measurement of autoAbs above currently established upper limits of detection would result in improved diagnostic specificity. Furthermore, early SARD patients often had very high levels of Abs to multiple subcomponents of the same nuclear Ags or particles, suggesting substantial epitope spreading. This finding is consistent with previous literature from retrospective studies of banked serum prior to SARD diagnosis showing that the number of different autoAb specificities increases over time [5, 6, 34, 35]. However, our study shows that this not only applies to different autoAgs, but also to different subcomponents within the same autoAgs (or particles) and suggests that the presence of Abs to multiple components of the same Ags might result in improved diagnostic accuracy for early SARD. Although we did not measure IgG subclasses, we do not think this would improve the diagnostic accuracy of the microarray, since analysis of IgG subclasses by ELISA for selected autoAgs (Ro52, Ro60 and RNP) revealed no differences between ANA+NS, UCTD and SARD patients (C. Munoz-Grajales, unpublished observations).
In the Bioplex system, anti-Ro52 Abs are measured together with -Ro60 Abs and reported as a single value. Consistent with a previous study comparing relatives of SLE patients who progressed to SLE with those who did not [36], anti-Ro Ab levels, as measured by the Bioplex system, were higher in progressors than non-progressors. Here we show that discriminating between Ro52 and Ro60 autoAbs provides an enhanced ability to predict disease progression in ANA+ individuals lacking a SARD diagnosis, with anti-Ro52 but not anti-Ro60 Abs being predictive in both ANA+NS and UCTD. Why anti-Ro52 Abs are uniquely predictive is currently unclear. Ro52, also known as TRIM21 [37], acts as an intracellular Fc receptor, binding to microorganisms containing immune complexes and, through its E3 ubiquitin ligase function, targets them to the proteasome for degradation [38]. It has been proposed that autoAbs are produced against TRIM21 when bacteria- or virus-containing TRIM21 complexes from dead cells are presented to the immune system through linked recognition [39], much in the way that rheumatoid factors are produced. Thus the presence of this autoAb may reflect the presence of bacterial or viral triggers that promote disease development. Alternatively, anti-Ro52 Abs may play an active role in promoting the progressive immune dysregulation that leads to disease, as they have been shown to target regions of the molecule that bind to Fc and sterically inhibit its E3 ligase activity [40]. Since TRIM21 plays an important role in negatively regulating several members of the interferon regulatory factor family, impairment of its function could lead to increased responses following toll-like receptor activation, resulting in increased elaboration of type I IFNs and cytokines involved in the Th17 pathway [41].
Previous work examining progression to SARD (SLE and SS) in symptomatic ANA+ individuals with insufficient criteria for a SARD diagnosis found that the best predictors of progression over the subsequent year were elevated levels of IFN-induced gene expression in the peripheral blood and a family history of autoimmunity [42]. Although previously published data from our laboratory confirmed that a subset of ANA+ individuals lacking a SARD diagnosis have high IFN5 scores and that ANA+NS progressors have higher IFN5 levels than non-progressors, a significant association with imminent progression was not found in UCTD patients or when ANA+ individuals lacking a SARD diagnosis were considered as a whole [43]. Here we confirm these findings in a larger group of patients followed for a longer period of time. There was also no association between a family history of autoimmunity and progression. Given the association between anti-Ro60 and anti-Ro52 Abs with elevated IFN5 scores, it was important to discriminate whether progression was predominantly driven by the elevated levels of IFN or whether this was due to the association between IFN levels and these autoAbs. Our findings suggest that it is the presence of anti-Ro52 Abs, rather than the elevated IFN5 score, that is most closely associated with progression.
Surprisingly, in the prospective component of this study, the type and levels of the IgM and IgG autoAbs remained relatively stable over time regardless of whether individuals progressed. This lack of change was not due to treatment with antimalarials, which have been shown to delay the accumulation of autoAb specificities in pre-SLE [44], as only a small proportion of the longitudinally followed study participants received this treatment. Instead, differences in study design may be responsible for the discrepancy between our findings and previous studies [4–7, 34, 35]. One important difference is that previous studies examined homogeneous groups of patients that developed a specific SARD, whereas our study examined a more heterogeneous group of ANA+ individuals with diverse outcomes. The short duration of follow-up in our study may also have precluded identification of changes in autoAb levels, either because autoAb levels rise slowly or, alternatively, because imminent progressors may have already undergone substantial serologic progression prior to clinical progression. The observation that many of the individuals who progressed within the subsequent 2 years co-clustered with early SARD patients in heat maps examining the extent of epitope spreading (Fig. 3) supports this later possibility. Furthermore, it is notable that the two ANA+ individuals who lacked a SARD diagnosis and who demonstrated serologic progression on the Bioplex system have not progressed. These findings suggest that spreading of the immune response may occur well in advance of clinical progression and that other immunologic events are required for conversion of asymptomatic to symptomatic autoimmunity, a concept that we are currently exploring.
While the presence of autoAbs to a diverse array of nuclear and non-nuclear autoAgs has been previously noted in SLE [20, 27–29], here we show that this can also be seen in some ANA+ individuals lacking a SARD diagnosis. This finding indicates that a broad breach of B cell tolerance can be seen in the absence of clinical disease activity and raises the possibility that it results from the genetic polymorphisms that promote disease. Whether this broad breach of tolerance indicates that these individuals are irrevocably committed to eventually develop SLE is currently unknown.
In summary, our findings strongly argue that development of assays with an expanded set of autoAgs and enhanced dynamic range would improve the diagnostic and prognostic ability of autoAb testing.
Supplementary Material
Acknowledgements
J.W. receives salary support from a Pfizer Chair Research Award, the Arthritis Centre of Excellence and the Schroeder Arthritis Institute. L.H. receives salary support from the Arthritis Society Stars Career Development Award. P.B. receives salary support and sits on the scientific advisory boards of Intersect Diagnostics and BioSymetrics. C.M.-G. is supported by a PhD salary award from the Arthritis Society.
All authors were involved in drafting the article or revising it critically for important intellectual content and all authors approved the final version to be published. J.W. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. C.M.-G., A.C. and J.W. were responsible for study conception and design. C.M.-G., S.R.J., Z.T., Z.A., D.B., L.H., A.B., A.C. and J.W. were responsible for the acquisition of data. C.M.-G., S.D.P., P.C.B. and J.W. were responsible for the analysis and interpretation of data.
Funding: This study was funded by a grant from the Canadian Institutes of Health Research (FRN 159563) to J.W. and A.C. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
All data underlying the article are available in the article or online supplementary material.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Slight-Webb S, Lu R, Ritterhouse LL. et al. Autoantibody-positive healthy individuals display unique immune profiles that may regulate autoimmunity. Arthritis Rheumatol 2016;68:2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li QZ, Karp DR, Quan J. et al. Risk factors for ANA positivity in healthy persons. Arthritis Res Ther 2011;13:R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eriksson C, Kokkonen H, Johansson M. et al. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther 2011;13:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bourn R, James JA.. Preclinical lupus. Curr Opin Rheumatol 2015;27:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McClain MT, Arbuckle MR, Heinlen LD. et al. The prevalence, onset, and clinical significance of antiphospholipid antibodies prior to diagnosis of systemic lupus erythematosus. Arthritis Rheum 2004;50:1226–32. [DOI] [PubMed] [Google Scholar]
- 6. Arbuckle MR, McClain MT, Rubertone MV. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003;349:1526–33. [DOI] [PubMed] [Google Scholar]
- 7. Olsen NJ, Karp DR.. Autoantibodies and SLE—the threshold for disease. Nat Rev Rheumatol 2014;10:181–6. [DOI] [PubMed] [Google Scholar]
- 8. Zhi-De H, Deng AM.. Autoantibodies in pre-clinical autoimmune disease. Clin Chim Acta 2014;437:14–8. [DOI] [PubMed] [Google Scholar]
- 9. Armstrong SM, Wither JE, Borowoy AM. et al. Development, sensibility, and validity of a systemic autoimmune rheumatic disease case ascertainment tool. J Rheumatol 2017;44:18–23. [DOI] [PubMed] [Google Scholar]
- 10. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1997. [DOI] [PubMed] [Google Scholar]
- 11. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. [DOI] [PubMed] [Google Scholar]
- 12. Shiboski CH, Shiboski SC, Seror R. et al. 2016 ACR-EULAR classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol 2017;69:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balboni I, Limb C, Tenenbaum JD, Utz PJ.. Evaluation of microarray surfaces and arraying parameters for autoantibody profiling. Proteomics 2008;8:3443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chruscinski A, Huang FYY, Nguyen A. et al. Generation of antigen microarrays to screen for autoantibodies in heart failure and heart transplantation. PLoS One 2016;11:e0151224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh H, Henry KA, Wu SST. et al. Reactivity profiles of broadly neutralizing anti-HIV-1 antibodies are distinct from those of pathogenic autoantibodies. AIDS 2011;25:1247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wither J, Johnson SR, Liu T. et al. Presence of an interferon signature in individuals who are anti-nuclear antibody positive lacking a systemic autoimmune rheumatic disease diagnosis. Arthritis Res Ther 2017;19:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehra S, Walker J, Patterson K, Fritzler MJ.. Autoantibodies in systemic sclerosis. Autoimmun Rev 2013;12:340–54. [DOI] [PubMed] [Google Scholar]
- 18. Hudson M, Mahler M, Pope J. et al. Clinical correlates of CENP-A and CENP-B antibodies in a large cohort of patients with systemic sclerosis. J Rheumatol 2012;39:787–94. [DOI] [PubMed] [Google Scholar]
- 19. Hanke K, Becker MO, Brueckner CS. et al. Anticentromere-A and anticentromere-B antibodies show high concordance and similar clinical associations in patients with systemic sclerosis. J Rheumatol 2010;37:2548–52. [DOI] [PubMed] [Google Scholar]
- 20. Li QZ, Zhou J, Wandstrat AE. et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol 2007;147:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kennedy WP, Maciuca R, Wolslegel K. et al. Association of the interferon signature metric with serological disease manifestations but not global activity scores in multiple cohorts of patients with SLE. Lupus Sci Med 2015;2:e000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schulte-Pelkum J, Fritzler M, Mahler M.. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev 2009;8:632–7. [DOI] [PubMed] [Google Scholar]
- 23. Ortiz-Hernandez GL, Sanchez-Hernandez ES, Casiano CA.. Twenty years of research on the DFS70/LEDGF autoantibody-autoantigen system: many lessons learned but still many questions. Autoimmun Highlights 2020;11: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Infantino M, Pregnolato F, Bentow C. et al. Only monospecific anti-DFS70 antibodies aid in the exclusion of antinuclear antibody associated rheumatic diseases: an Italian experience. Clin Chem Lab Med 2019;57:1764–9. [DOI] [PubMed] [Google Scholar]
- 25. Mahler M, Parker T, Peebles CL. et al. Anti-DFS70/LEDGF antibodies are more prevalent in healthy individuals compared to patients with systemic autoimmune rheumatic diseases. J Rheumatol 2012;39:2104–10. [DOI] [PubMed] [Google Scholar]
- 26. Shovman O, Gilburd B, Chayat C. et al. Prevalence of anti-DFS70 antibodies in patients with and without systemic autoimmune rheumatic diseases. Clin Exp Rheumatol 2018;36:121–6. [PubMed] [Google Scholar]
- 27. Olsen NJ, Li QZ, Quan J. et al. Autoantibody profiling to follow evolution of lupus syndromes. Arthritis Res Ther 2012;14:R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guthridge CJ, Gross T, Quintero M. et al. Expanded autoantibody profiles for subsetting of native American, African American, and European American patients with systemic lupus erythematosus. ACR Open Rheumatol 2020;2:415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quan J, Lakhanpal A, Reddy MM. et al. Discovery of biomarkers for systemic lupus erythematosus using a library of synthetic autoantigen surrogates. J Immunol Methods 2014;402:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jeong S, Hwang H, Roh J. et al. Evaluation of an automated screening assay, compared to indirect immunofluorescence, an extractable nuclear antigen assay, and a line immunoassay in a large cohort of Asian patients with antinuclear antibody-associated rheumatoid diseases: a multicenter retrospective study. J Immunol Res 2018;2018:9094217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Op De Beéck K, Vermeersch P, Verschueren P. et al. Antinuclear antibody detection by automated multiplex immunoassay in untreated patients at the time of diagnosis. Autoimmun Rev 2012;12:137–43. [DOI] [PubMed] [Google Scholar]
- 32. Lewis MJ, McAndrew MB, Wheeler C. et al. Autoantibodies targeting TLR and SMAD pathways define new subgroups in systemic lupus erythematosus. J Autoimmun 2018;91:1–12. [DOI] [PubMed] [Google Scholar]
- 33. Leuchten N, Hoyer A, Brinks R. et al. Performance of antinuclear antibodies for classifying systemic lupus erythematosus: a systematic literature review and meta-regression of diagnostic data. Arthritis Care Res 2018;70:428–38. [DOI] [PubMed] [Google Scholar]
- 34. Theander E, Jonsson R, Sjöström B. et al. Prediction of Sjögren’s syndrome years before diagnosis and identification of patients with early onset and severe disease course by autoantibody profiling. Arthritis Rheumatol 2015;67:2427–36. [DOI] [PubMed] [Google Scholar]
- 35. Heinlen LD, McClain MT, Merrill J. et al. Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Arthritis Rheum 2007;56:2344–51. [DOI] [PubMed] [Google Scholar]
- 36. Munroe ME, Young KA, Kamen DL. et al. Discerning risk of disease transition in relatives of systemic lupus erythematosus patients utilizing soluble mediators and clinical features. Arthritis Rheumatol 2017;69:630–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee AYS. A review of the role and clinical utility of anti-Ro52/TRIM21 in systemic autoimmunity. Rheumatol Int 2017;37:1323–33. [DOI] [PubMed] [Google Scholar]
- 38. Rhodes DA, Isenberg DA.. TRIM21 and the function of antibodies inside cells. Trends Immunol 2017;38:916–26. [DOI] [PubMed] [Google Scholar]
- 39. Burbelo PD, Teos LY, Herche JL, Iadarola MJ, Alevizos I.. Autoantibodies against the Immunoglobulin-binding region of Ro52 link its autoantigenicity with pathogen neutralization. Sci Rep 2018;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Espinosa A, Hennig J, Ambrosi A. et al. Anti-Ro52 autoantibodies from patients with Sjögren’s syndrome inhibit the Ro52 E3 ligase activity by blocking the E3/E2 interface. J Biol Chem 2011;286:36478–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Espinosa A, Dardalhon V, Brauner S. et al. Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23-Th17 pathway. J Exp Med 2009;206:1661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Md Yusof MY, Psarras A, El-Sherbiny YM. et al. Prediction of autoimmune connective tissue disease in an at-risk cohort: prognostic value of a novel two-score system for interferon status. Ann Rheum Dis 2018;77:1432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hafiz W, Nori R, Bregasi A. et al. Fatigue severity in anti-nuclear antibody-positive individuals does not correlate with pro-inflammatory cytokine levels or predict imminent progression to symptomatic disease. Arthritis Res Ther 2019;21:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. James JA, Kim-Howard XR, Bruner BF. et al. Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus 2007;16:401–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data underlying the article are available in the article or online supplementary material.