Abstract
Since its emergence 25 years ago, group B streptococcus has become recognized as a cause of serious illness in newborns, pregnant women, and adults with chronic medical conditions. Heavy colonization of the genital tract with group B streptococcus also increases the risk that a woman will deliver a preterm low-birthweight infant. Early-onset infections (occurring at <7 days of age) are associated with much lower fatality than when they were first described, and their incidence is finally decreasing as the use of preventive antibiotics during childbirth increases among women at risk. New serotypes of group B streptococcus have emerged as important pathogens in adults and newborns. Clinical and laboratory practices—in obstetrics, pediatrics, and clinical microbiology—have an impact on disease and/or its prevention, and protocols established at the institutional level appear to be critical tools for the reduction of perinatal disease due to group B streptococcus. Since intrapartum antibiotics will prevent at best only a portion of the full burden of group B streptococcal disease, critical developments in vaccine evaluation, including study of polysaccharide-protein conjugate vaccines, offer the potential for enhanced prevention in the relatively near future.
A quarter of a century ago, a series of articles in the Journal of Pediatrics heralded the emergence of group B streptococcus (GBS) as the leading cause of sepsis and meningitis in newborn nurseries around the country (21, 28, 64, 108). Last year, the Centers for Disease Control and Prevention (CDC) (153), American College of Obstetricians and Gynecologists (ACOG) (10), and American Academy of Pediatrics (AAP) (8) issued consensus guidelines on the prevention of perinatal GBS disease. New and reemerging infectious diseases continue to attract attention (101), but scientific interest in emerging infections often follows a tortuous path from exploration of a novel agent or syndrome to the eventual discovery and implementation of control strategies. Consideration of GBS as an emerging infection—which now can be considered substantially preventable—provides particular lessons about the struggle to control infectious disease threats, present and future.
This review will consider shifting paradigms in the epidemiology of GBS disease in the United States, where transformations in population profile, medical practices, and society’s approach to health promotion are moving GBS disease concerns from newborn nurseries to nursing homes and from intensive care units to outpatient health maintenance settings. Developments in GBS vaccines (89, 142, 152), methods for rapid detection of the organism (18, 185), prevention strategies (152, 153), and global perspectives on the disease burden (148, 178) have been recently reviewed.
CLINICAL SYNDROMES AND SEVERITY OF DISEASE
Group B Streptococcal Disease in Infants
Early- and late-onset syndromes.
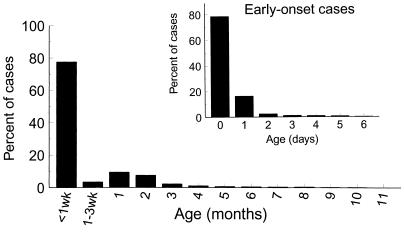
GBS infections in infants are restricted to very early infancy. Approximately 80% of infant infections occur in the first days of life, so-called early-onset disease. Late-onset infections occur in infants between 1 week and 2 to 3 months of age (Fig. 1).
FIG. 1.
Age distribution of invasive GBS disease in infants, by age in months, weeks, or (for early-onset cases only) days. The data are based on active surveillance in four geographic areas of the United States in 1993 and were obtained from the CDC.
Newborns with early-onset GBS disease acquire the organism intrapartum from their mothers, who are colonized with GBS in the genital tract. Most early-onset disease results from ascending spread of the organism into the amniotic fluid, where aspiration of contaminated amniotic fluid leads to invasive disease in some infants. Perinatal transmission can occur across intact membranes (92). The pathogenesis of late-onset disease is less well understood, although some cases probably reflect acquisition of the organism during passage through the birth canal. Although about 50% of mothers of infants with late-onset disease were found to carry the same GBS serotype as that causing infection in their infants, the source of infection in other infants is unclear (15, 53). Nosocomial and community sources are probably involved in some cases of late-onset disease (173), but the risk factors are not well understood. Even for infants with late-onset infections whose mothers have the same serotype, the precise mechanism of apparent mother-infant transmission has not been identified.
Clinical syndromes of GBS disease in newborns include sepsis, meningitis, pneumonia, cellulitis, osteomyelitis, and septic arthritis. Other syndromes which have been reported among older children include endocarditis (6, 161) and epiglottitis (190). Bloodstream infections, with or without pneumonia, are the main manifestation of GBS disease in infancy and are evident in 89% of cases (42). Meningitis is less common, occurring in approximately 10% of neonatal infections (42). Other manifestations are rare. GBS disease is diagnosed by isolation of the organism from a usually sterile site, primarily blood or cerebrospinal fluid. The contribution of GBS to clinical sepsis and pneumonia for which cultures are negative is not known.
The incidence of early- and late-onset GBS disease has varied in studies conducted in the United States (Table 1). Estimates of disease occurrence in single hospitals may not represent rates in more heterogeneous populations, and samples of hospitals organized around research protocols may have high-risk patient profiles. Even when population-based surveillance has been used, a method which identifies all cases in a geographic area instead of only those presenting to selected health care facilities, the incidence has varied between geographic areas (42, 150, 191).
TABLE 1.
Incidence of neonatal GBS disease during the last 10 years in the United States
| Setting | Date of study | No. of births | Incidence (cases per 1,000 births) of:
|
Reference(s) | |
|---|---|---|---|---|---|
| Early-onset disease | Late-onset disease | ||||
| Single hospital | 1986–1994 | 119,931 | 1.95 | 0.24 | 158 |
| Nine hospitals | 1987–1989 | 61,809 | 3.21 | 179 | |
| Multistate population based | 1990 | 180,000 | 1.4 (whites, 1.1; blacks, 2.0) | 0.3 (whites, 0.19; blacks, 0.67) | 191 |
| Multistate population based | 1991–1992 | 124,464 | 1.8 (2.3 in Ga., 2.3 in Tenn., 2.2 in Md., 1.6 in SF,a 1.0 in Mo.) | 150 | |
| Twelve hospitals | 1991–1993 | 7,606b | 5.9b | 7.7b | 166, 167 |
| Multistate population based | 1993–1995 | 190,000 | 1.7 (1993), 1.4 (1994), 1.3 (1995) | 0.5 | 42 |
SF, San Francisco.
Very low birth weight (<1,500 g) only; number of births for late-onset rate was 6,911.
GBS disease occurs substantially more often in African American infants than in other racial groups (191). Although part of this difference may relate to the higher prevalence among African Americans of risk conditions such as low birth weight and prematurity, maternal colonization with GBS is significantly more common among African American women than others (121, 140).
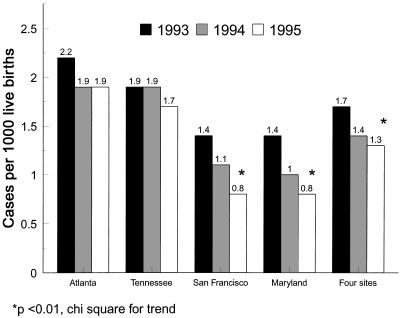
Recent reports of geographic variation in early-onset GBS disease probably reflect differences in the intensity of prevention activities (42). In areas under continued CDC surveillance from 1993 to 1995, early-onset GBS disease declined 43% in two areas but not significantly in the other sites (Fig. 2). Declines in the incidence of early-onset disease occurred while late-onset disease and disease in adults remained constant, supporting the interpretation that the decline was due to practices which interrupt intrapartum transmission. Further declines appear evident in 1996 and 1997 (42a).
FIG. 2.
Incidence (cases per 1,000 births) of invasive early-onset GBS disease by year and area. The data are based on active surveillance in four geographic areas of the United States in 1993 to 1995. Reprinted from reference 42.
Outcomes of infection.
In addition to acute illness due to GBS, which is itself costly, GBS infections in newborns can result in death, disability, and, in rare instances, recurrence of infection. GBS disease reported during the 1970s was fatal in 55% of cases (13). Fatality in the 1980s was in the range of 10 to 15% (123, 151, 179, 184). More recent estimates of death among newborns suggest even lower fatality ratios (4 to 6%) for cases identified by population-based surveillance during the 1990s (42, 191). Prompt recognition and early antibiotic therapy are probably responsible for the improved survival, since even birthweight-specific mortality has declined since the 1970s (99).
Chronic sequelae of acute illness tend to be the most expensive component of infectious diseases, including GBS disease (116). Most estimates of long-term morbidity due to GBS infection in newborns date from cases treated during the 1970s and 1980s (21, 43, 57, 176), where evidence of neurologic sequelae after meningitis was impressive. Neurologic sequelae associated with GBS infection are most profound among premature infants (126). The extent and nature of any disabilities which may occur as a result of extracorporeal membrane oxygenation, a technology now used for treatment of certain cases of GBS sepsis and other life-threatening conditions, is unclear at present (72).
Although GBS infections are usually responsive to therapy with penicillin or related antimicrobial agents, episodes of recurrent GBS infection have been reported (38, 80, 146, 151). Population-based retrospective surveillance in Atlanta during 1982 and 1983 identified 1 of 106 (0.9%) infants with a second episode of invasive GBS disease (151), and prospective active surveillance in Maryland from November 1991 through September 1993 identified only one recurrent infection among the 241 infants that survived a first episode of GBS disease (0.4% incidence) (80). In contrast to the low recurrence rate identified in infants, the Maryland investigators reported that 4.3% of first episodes of invasive GBS disease in adults were followed by at least one more episode (80).
Risk factors for infection.
Between 15 and 35% of pregnant women are colonized by GBS in the vagina and/or rectum, yet the incidence of neonatal GBS disease is 1 to 2 infants per 1,000 births. Several factors increase the chances that an infant will develop early-onset GBS disease (Table 2). Certain apparent risk factors, such as the risk of neonatal GBS disease complicating subsequent pregnancies, were derived from anecdotal reports (39, 60); the sample sizes of most epidemiologic studies on GBS disease have been insufficient to detect true increased risks associated with very rare exposures.
TABLE 2.
Conditions associated with increased risk of early-onset group B streptococcal disease
| Risk factor for early-onset GBS infections | Representative reference(s) |
|---|---|
| Premature delivery | 21, 53, 60, 150, 151, 184 |
| Low birth weight | 1, 19, 34, 44, 124, 151, 165, 179, 184 |
| Increased interval between membrane rupture and delivery | 1, 19, 21, 34, 53, 60, 151, 165, 184 |
| Rupture of membranes before labor onset | 1, 150 |
| Amnionitis, intrapartum fever | 1, 21, 34, 53, 150 |
| Maternal vaginorectal colonization with GBS | 5, 34, 53 |
| Heavy (dense) colonization with GBS | 11, 53, 68, 120, 124, 174 |
| African American race | 42, 151, 191 |
| Maternal age, <20 years | 150, 151 |
| GBS bacteriuria during current pregnancy | 118, 128, 183 |
| Low level of antibody to type-specific capsular polysaccharide | 22, 24 |
| Previous stillbirth or spontaneous abortion | 1, 151 |
| Multiple gestation/sibling of affected twin | 56, 125 |
| Previous delivery of infant with GBS disease | 39, 60 |
| Cesarean section | 34, 60 |
| Urinary tract infection in pregnancy | 150 |
| Prolonged duration of intrauterine monitoring | 1 |
Major factors increasing the risk that an infant will develop invasive GBS disease can be conceptualized as those related to increased host susceptibility and those associated with a large inoculum of the organism. Although the virulence of type III organisms is probably related to their disproportionate occurrence in meningitis and late-onset disease, strain virulence has not been clearly linked with early-onset cases, which are caused by all serotypes identified in genital carriers. The proportion of early-onset disease due to each serotype appears to reflect the distribution of serotypes identified from maternal carriers (20).
An infant’s susceptibility to GBS is increased when the level of anticapsular antibody to the infecting serotype is low, as is the case when the maternal antibody level is low (24, 27), as well as when infants born to mothers with adequate antibody levels are born before 34 weeks gestation, since transplacental transport of immunoglobulin G is reduced early in gestation. The inoculum of GBS to which the baby is exposed can be large either because maternal colonization is particularly dense or because obstetric complications develop which permit multiplication of the bacteria at the time of labor (11, 14, 124). Some obstetric manipulations, such as intrauterine monitoring (1, 49, 188), chorionic villus sampling (61), and numerous vaginal examinations (187), may facilitate the ascending spread of GBS or enhance the GBS inoculum to which the infant is exposed. Maternal bacteriuria due to GBS is probably indicative of a large inoculum present in the genital tract as well (183). Heavy colonization with GBS has been identified more frequently among African American women, a finding which may explain the higher risk of both early- and late-onset GBS disease among African Americans (93, 121, 140). Inoculum will also increase in deliveries following prolonged duration of membrane rupture.
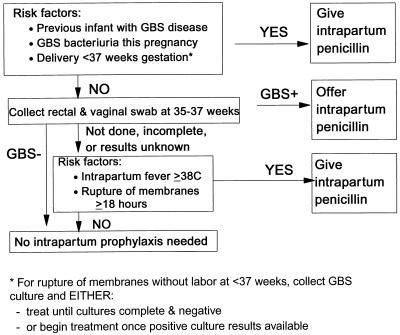
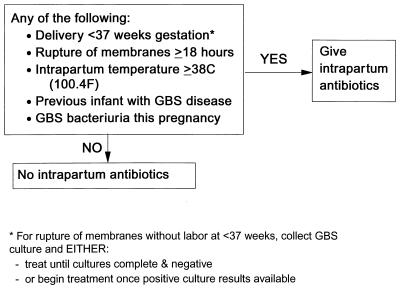
Among the various risk factors, six clinical and microbiologic characteristics of at-risk mothers have been targeted in one or both of the two prevention strategies recently promoted by the CDC, ACOG, and AAP (8, 10, 153) (Fig. 3 and 4). These factors are previous delivery of an infant with GBS disease, maternal GBS bacteriuria during the pregnancy, maternal GBS colonization late in pregnancy, onset of labor or membrane rupture before 37 weeks, prolonged rupture of ruptures for at least 18 h, and maternal amnionitis evidenced by intrapartum fever.
FIG. 3.
Screening-based approach to prevention of perinatal GBS disease. Reprinted from reference 153.
FIG. 4.
Risk-based approach to prevention of perinatal GBS disease. Reprinted from reference 153.
Group B Streptococcus-Related Morbidity in Pregnant Women
Invasive and noninvasive syndromes.
Among pregnant women, GBS causes clinical illness ranging from mild urinary tract infection to life-threatening sepsis and meningitis. Invasive disease among pregnant and postpartum women is defined as occurring when GBS is isolated from a normally sterile site, primarily blood or cerebrospinal fluid. Most invasive maternal infections are bloodstream infections, but osteomyelitis (103), endocarditis (157), and meningitis (2, 37, 63) have also been described.
In a retrospective population-based study conducted in Atlanta during 1982 and 1983, the incidence of invasive GBS disease among pregnant women was estimated as 22 cases per 100,000 births, and pregnancy-associated infections comprised 8% of all invasive disease (14 of 177 total invasive cases, 107 of which occurred in newborns) (155). More recent surveillance (1989 to 1990) in Atlanta showed that 15% of all invasive GBS disease occurred in pregnant women (65 of 424 total) (59). Multistate surveillance conducted in 1990 identified a total of 600 invasive GBS cases, 67 (11%) of which occurred in pregnant women (191). The outcome of pregnancy was known for 43 women with invasive GBS disease; 21 (49%) delivered infants with no apparent illness, 15 (35%) delivered infants who survived neonatal GBS disease, 4 (9%) women had infants who died of neonatal GBS disease, and 3 (7%) had pregnancies that resulted in stillbirth (191). Surveillance for invasive GBS disease among pregnant women may underestimate the burden of perinatal GBS infections in areas where collection of blood cultures from women with amnionitis or intrapartum fever is not conducted routinely.
Noninvasive syndromes in pregnancy and the postpartum period purported to be due to GBS, in addition to urinary tract disease (118, 170, 183), include amnionitis, endometritis, wound infections (postcesarean and postepisiotomy), cellulitis, and fasciitis (115, 168, 187). GBS is isolated from the genital tract in approximately one-fourth of women, so the organism can frequently be isolated from specimens such as placental surfaces without its playing a causative role in concurrent pathologic findings. GBS is clearly a cause of stillbirth in some instances (47). However, many stillbirths for which the pathogen is isolated from either the placenta or the products of conception, without careful attention to specimen handling and pathologic review of tissue samples, are unlikely to be the result of GBS infection. Careful bacteriologic study of amnionitis reveals that GBS is a major contributor to intra-amniotic infection. Gibbs et al. (69) isolated GBS from ∼15% of women with intrapartum infection; the organism was often present in association with other bacteria. Determining the role of specific members of the vaginal flora in adverse fetal outcomes and amnionitis has been the focus of substantial research in recent years (84, 96, 143). Increasing evidence points to a causative role in amnionitis and preterm low birth weight for genital microbes such as GBS and organisms associated with bacterial vaginosis (85, 139).
Relation between GBS colonization and prematurity.
The importance of infection as a cause of preterm delivery is gaining increasing recognition (84, 85, 114). A recent review of the epidemiology of preterm birth (30) suggested that distinct etiologic pathways lead to spontaneous preterm labor and preterm premature rupture of the membranes. Infection may play a role in both of these pathways, since intra-amniotic infection rates are elevated both in women with preterm labor with intact membranes and in those with preterm premature rupture of membranes (30). Studies which assessed the role of GBS colonization in prematurity have identified diverse results (4, 107, 109, 138).
Regan et al. studied cervical colonization with GBS in 6,706 parturients in New York (138). Among the 123 women who delivered at ≤32 weeks gestation, 38% were GBS carriers, compared with 13% of women who delivered after 32 weeks. The incidence of gestation at ≤32 weeks was 5.4% among colonized women compared with 1.8% among the general population (138). Smaller studies conducted by Alger et al. (4) in Maryland and McDonald et al. (109) in Adelaide, Australia, identified strong associations between GBS colonization and gestation of <37 weeks. A study conducted by Matorras among women in Madrid found no relation between GBS colonization and prematurity (107). A later report by Regan et al. of results of the Vaginal Infections and Prematurity Study (139) found a significant association between heavy colonization with GBS and risk of preterm low birth weight. This risk was not evident among the portion of heavily colonized women who received third-trimester antibiotics in an uncontrolled manner.
Although increasing evidence points to an important role of GBS colonization in some instances of preterm low birth weight, the effectiveness of antenatal antibiotics in GBS-colonized women in prevention of preterm or low-birth-weight deliveries has not been consistently demonstrated. One study which randomized women with GBS bacteriuria during pregnancy to received antenatal penicillin or placebo tablets did document a reduction in primary rupture of the membranes and preterm labor (170). However, a randomized controlled clinical trial of erythromycin treatment for GBS-colonized women which was conducted as part of the Vaginal Infection and Prematurity Study failed to identify any reduction in adverse pregnancy outcomes among treated women (94); the absence of a protective effect of antenatal antibiotics persisted even when only subjects with the highest compliance with either the antibiotic or placebo regimen were compared.
The effect of GBS colonization on the latency period between preterm premature rupture of membranes and delivery was evaluated by Towers et al. (172). Parturients with and without GBS colonization had a similar latency period between preterm premature rupture of the membranes and delivery.
These studies suggest that GBS is an important contributor to preterm low birth weight but that additional studies are needed to find effective approaches to preventing this serious outcome.
Risk factors for maternal infectious outcomes.
Because GBS colonizes a substantial proportion of parturients, many women with infectious complications of pregnancy can be expected to carry GBS without the organism playing a causative role in disease. To determine the proportion of peripartum infection attributable to group B streptococci, Yancey et al. (187) prospectively investigated risk factors for peripartum infection in a population in which vaginal cultures for GBS colonization were being routinely collected 2 weeks before delivery in conjunction with studies of rapid detection methods. Among the cohort of 823 women completing the study follow-up, 216 (26%) were colonized with GBS. Chorioamnionitis or endometritis occurred in 21% (45 of 216) of the colonized women compared with 12% (72 of 607) of the noncolonized women. Multivariate analysis, adjusted for confounders, revealed that GBS colonization was an independent risk factor for chorioamnionitis (odds ratio, 1.9; 95% confidence interval, 1.0 to 3.7) but not for endometritis. The risk of chorioamnionitis was highest among women with the heaviest degree of colonization. Other exposures which were independently associated with a risk of chorioamnionitis were duration of membrane rupture for more than 6 h, duration of internal monitoring for more than 12 h, and undergoing more than six vaginal examinations prior to delivery.
Group B Streptococcal Disease in Nonpregnant Adults
Clinical syndromes of GBS infection: incidence and case fatality ratio.
Invasive GBS disease occurring outside the perinatal period has been recognized practically since the advent of the serologic classification system (136, 137). However, recent efforts to define the magnitude of all invasive disease due to GBS demonstrated that a substantial burden of GBS-related illness occurs outside the better recognized risk periods of pregnancy and early infancy (59, 123, 155, 191).
Population-based surveillance for GBS disease conducted during 1982 and 1983 in metropolitan Atlanta identified an incidence of invasive GBS disease among men and nonpregnant women of 2.4 cases per 100,000 persons 20 years of age or older (155). The mean age of patients was 59 years, and the case fatality rate was 32%. Prospective population-based surveillance conducted in Atlanta during 1989 to 1990 revealed an incidence of 4.4 per 100,000 nonpregnant adults 18 years or older, an increase of 83% over the earlier estimate (59). The mean age of the patients was 62 years, and 21% of patients died in hospital.
A population-based study of invasive GBS disease during 1991 and 1992 conducted in three metropolitan areas showed that 21% of GBS patients died in hospital (87). The subjects died a median of 5 days after collection of the specimen from which GBS was isolated. In-hospital mortality was more common among persons 65 years of age or older (28 and 13%, respectively [P = 0.008) and was higher among those with hypothermia (42%) on admission than in those presenting with fever (12%) or intermediate temperatures (21%). Apparent differences in mortality related to the presenting clinical syndrome (pneumonia-associated mortality, 41%; nonpneumonic presentations, 18%) were not significant after adjustment to take account of age (87).
The clinical presentations of invasive GBS disease among nonpregnant adults most often take the form of primary bacteremia. In a population-based series of 219 cases, Jackson et al. described bacteremia without evident focus among 42% of patients, with skin or soft tissue infection being the next most common presentation (21%) (87). Pneumonia (12%), urosepsis (9%), endocarditis (6%), peritonitis (4%), meningitis (4%), and empyema (1%) occurred much less frequently. Skin or soft tissue infections included cellulitis (9%), infected peripheral ulcers (6%), osteomyelitis (5%), septic arthritis (3%), and decubiti or wound infections (87). Similar distributions of clinical syndromes were identified in earlier population-based series (59, 155). The sizable contribution of polymicrobial bacteremia to invasive GBS disease presentations was noted by Farley et al. (59), who reported that 42 (32%) of 132 patients with GBS bacteremia had additional organisms isolated from blood. The most common of these organisms were staphylococci and enterococci (59). Jackson et al. also noted polymicrobial bacteremia in a large percentage of patients; 53 (26%) of 201 adults with GBS bacteremia had other organisms isolated from blood cultures collected on the same day (87). The age distribution, mortality rate, and proportion of patients with nosocomial infections were similar between patients with polymicrobial bacteremia and other subjects (87).
Risk factors for community-acquired GBS infection.
Reports on series of adult patients with invasive GBS disease from individual hospitals provided the impression that this pathogen predominantly affects debilitated hosts (29, 54, 65, 66, 102, 175), supporting Lancefield’s initial view that serogroup B streptococci were saprophytic organisms (98). Although underlying chronic diseases such as diabetes mellitus and cancer were often present in patients reported in these case series (152), the increased risk of GBS disease associated with these conditions has been quantitated in only a few recent investigations.
Using estimates of the prevalence of cancer and diabetes mellitus in the general population and clinical information on cases of GBS disease identified in metropolitan Atlanta during 1982 to 1983 (155) and 1989 to 1990 (59), investigators found a significant increase in the risk of invasive GBS disease in persons with diabetes mellitus and cancer. Schwartz et al. estimated that persons with diabetes mellitus had nearly 14 times the risk of invasive GBS disease compared with other adults (155). Farley et al. (59) also identified diabetes as an important risk factor for invasive GBS disease among nonpregnant adults but found that the increased risk was highest among younger adults compared with persons of the same age without diabetes mellitus. The risk of GBS was increased 11- to 30-fold in persons with diabetes who were 20 to 64 years old but was increased 3.7- to 5.7-fold in persons with diabetes who were older than 64 years, compared with the same age population. Both studies also identified an increased risk of GBS among persons with cancer. Schwartz et al. estimated that such persons had a 16-fold increase in risk of GBS disease, and Farley et al. estimated that the risk was 28-fold in cancer patients between the ages of 20 and 49 years but only 4-fold in cancer patients between 50 and 69 years. Among persons with cancer who were 70 years or older, the risk of GBS disease was similar to that among persons the same age without cancer.
A different method was used by Jackson et al. to identify risk factors for invasive GBS disease (87). GBS cases were identified by population-based surveillance in three metropolitan areas, and up to three controls for each case were matched based on the admitting hospital. Unlike the cohort analyses described above, in this study medical records for both cases and controls were reviewed, providing data on multiple underlying conditions within a subject. These data permitted analysis of risk factors with adjustment for potential confounders by multivariate analysis. Several risk factors for community-acquired GBS disease among nonpregnant adults emerged from the investigation. Among hospitalized subjects, older age was an independent risk factor for invasive GBS disease. After adjustment for age, the following conditions each were independent risk factors: diabetes mellitus, previous stroke, decubitus ulcer, cirrhosis, breast cancer, and neurogenic bladder. Adjusted odds ratios ranged from 3.0 (diabetes mellitus) to 9.7 (cirrhosis). Of patients with community-acquired GBS disease, 63% (108 of 171) had at least one of these underlying conditions. This study failed to identify increased risk associated with cancers other than breast cancer or with persons infected with the human immunodeficiency virus (HIV). By selecting hospitalized persons as controls, the study may have been biased away from finding some real risk factors, since the control population was likely to have a high prevalence of serious medical conditions. It is also possible that the general category of malignancies contains heterogeneous conditions, some of which do increase the risk of invasive bacterial infections while others do not. For example, the association between breast cancer and invasive GBS disease may be related to surgical disruption of lymphatic drainage, rather than to a general immunologic predisposition to bacteremia. Several case patients with breast cancer presented with cellulitis; cellulitis involved the arm or chest wall on the side of a mastectomy (n = 5), the chest wall opposite to the side of the mastectomy (n = 1), and the arm after axillary node dissection (n = 1).
Risk factors for nosocomial GBS infection.
Multistate population-based surveillance suggests a substantial proportion of invasive GBS disease among adults is acquired nosocomially. Nosocomial GBS disease, defined as occurring when GBS is isolated only from cultures collected more than 2 days after admission, was identified in 22% of nonpregnant adults with invasive GBS disease (87). Compared with controls matched for hospital and admitting service, subjects with nosocomial GBS disease were 30 times more likely (adjusted odds ratio, 30.9; 95% confidence interval, 5.2 to 184.1) to have had central venous lines placed preceding the onset date. Other independent risk factors for nosocomial GBS disease were diabetes mellitus, congestive heart failure, and seizure disorders (87).
Residents of nursing homes are likely to be elderly and to have one or more serious chronic illness, which put them at high risk for invasive GBS disease. Hall et al. (78) used population-based data on GBS disease from Maryland collected during 1991 to 1995 and data collected in licensed nursing homes to determine the risk of GBS disease among nursing-home residents compared with the general population. The incidence of invasive GBS among nursing-home residents was estimated as 68.6/100,000, compared with 16.5/100,000 in community residents aged ≥60 years (relative risk, 4.2). No direct evidence of patient-to-patient transmission was determined in that evaluation, and the contribution of secondary disease among nursing-home residents appears to be small. Cases occurred in 59 different nursing homes; in 14 (24%) of those homes, two or more cases were identified over the 5-year study period. Only four instances (5% of 82 total cases among nursing-home residents) were identified in which illness onset occurred within 1 month of another case in the same nursing home.
HOST FACTORS
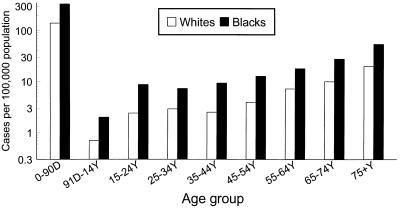
The most prominent feature of the epidemiology of GBS infections is the pronounced effect of age on incidence (Fig. 5). Among pediatric infections, the restriction of most GBS infections to the newborn period is striking. Whether the risk of disease among the elderly relates to waning population immunity to this bacterium or to age-related increases in other predisposing factors is not known, but one study identified increasing age as being associated with risk even after adjusting for specific chronic illnesses which are more frequent among older persons (87). Deficiency in anticapsular antibody may be common among healthy adults (76, 77) as well as newborns and the elderly, but the potential for anticapsular antibody to protect against disease in the elderly has not yet been explored. Demonstration of the burden of disease among adults with chronic illness suggests the need to articulate the immunology of GBS infections in this population.
FIG. 5.
Age-specific incidence of invasive GBS disease. Data are based on active surveillance in four geographic areas of the United States in 1993 and were obtained from the CDC.
The Very Young
Most infants with invasive GBS disease are ill within hours following birth, consistent with the particular role played by exposures unique to labor and delivery in pathogenesis of the infection. Aspiration of amniotic fluid laden with bacteria probably provides a larger inoculum than could be achieved through other routes. Passage through the birth canal, where GBS colonization is prevalent, can also lead to neonatal infections. The prevalence of GBS infections in infants during the first 4 to 6 weeks of life, while placentally derived antibodies are still available, without a subsequent increase in disease rates among older infants suggests that immunologic protection against GBS infection differs from that related to other encapsulated bacteria including Haemophilus influenzae serotype b, S. pneumoniae, and Neisseria meningitidis. Maturation of the immune system during the first months of life may make only the youngest infants susceptible to invasive GBS infection (48, 162).
Recent reports of very late infections in infants and young children suggest other immune system defects may be important. Although reports of GBS infection in HIV-infected children have appeared (52), these are of nominal concern compared with that posed by agents such as pneumococcus or salmonella among children with HIV infection.
The Very Old
Because even healthy adults are unlikely to have substantial levels of antibody to capsular polysaccharide from most GBS types (77), it is not clear whether waning antibody levels play a role in the increased risk of invasive GBS disease among the elderly. In newborns, lower levels of maternally acquired anticapsular antibody characterized infants with early-onset disease compared with asymptomatic infants born to colonized mothers. At present, little is known about the immunology of GBS disease among the elderly, such as whether elderly persons produce antibody following natural infection with GBS or whether cell-mediated immunity or local factors may be relevant for protecting the elderly from invasive illness. Regardless of the mechanism of increased risk, it is still possible that enhancement of humoral antibody levels, such as by immunization, will protect elderly adults against invasive disease due to GBS.
Patients with Immunocompromising Conditions
Studies of risk factors for invasive GBS disease among nonpregnant adults suggest a role for several conditions in the pathogenesis of disease in this population (87). The risk of GBS disease among survivors of neurologic disease suggests that aspiration may be the route of infection in some persons lacking a gag reflex and that urinary catheterization required by a neurogenic bladder may lead to invasive disease via urosepsis in others. The association between decubitus ulcers and invasive GBS suggests that endogenous skin colonization, aided by integument breakdown, is the source of infection. Destruction of lymphatic drainage in women who have had mastectomies for breast cancer may lead to local infections, such as cellulitis, which apparently lead to bacteremic illness in certain cases. Although adults with invasive GBS disease have a range of chronic medical conditions, there is no clear evidence for a major role for deficient humoral or cell-mediated immunity in risk of this disease. GBS disease occurs more frequently in HIV-infected individuals than would be expected (59), but persons on corticosteroid therapy, transplant recipients, and those with hematologic cancers do not appear to be at greatly increased risk (87). The sample size in previous studies was insufficient to exclude increased risk due to splenectomy (87).
THE AGENT
Serotype Distribution
At present, the following serotypes of GBS are recognized: Ia, Ib, Ia/c, II, III, IV, V, and VI. Studies in the 1970s suggested that type III isolates had unique features, in that they caused the majority of meningitis cases and late-onset infections despite accounting for a smaller percentage of colonizing isolates (20). Multistate surveillance in the 1980s suggested that type III isolates accounted for 36% of early-onset and 71% of late-onset GBS infections (180), compared with 20% of infections in adults. During the 1990s, several reports of disease due to serotype V suggest that this recently described serotype accounts for a substantial proportion of adult disease (33, 81, 141). In Atlanta, type V isolates caused 21% of all cases, including 14% of early-onset infections and 31% of infections in nonpregnant adults (33). Although not yet recognized within the United States, serotypes VI and VIII have been identified frequently from pregnant women in Japan (97). Tracking the serotypes associated with GBS cases is important, since multivalent vaccines are being developed against the serotypes known to cause most invasive cases.
Molecular Epidemiology of Pathogenic Strains
Several subtyping methods have been applied to GBS strains, and they suggest that relatively little heterogeneity exists among strains within a serotype (32, 73, 82, 135). Since most adult infections are due to the endogenous flora and since most infant disease is acquired through maternal exposure, molecular epidemiology has been less critical for GBS studies than for other pathogens for which it is necessary to distinguish common-source exposures through subtyping. A few nosocomial GBS clusters have been reported when subtyping was performed.
Antimicrobial Susceptibility
GBS isolates remain susceptible to penicillin, and this is the agent of choice for GBS prophylaxis and therapy. Reports of penicillin-tolerant isolates have not been accompanied by evidence that this in vitro observation has clinical relevance (31, 46, 156).
A greater concern about antimicrobial resistance associated with GBS is whether increased use of antimicrobial prophylaxis in obstetric care for GBS prevention will lead to the emergence of antimicrobial resistance among other perinatal pathogens. Although GBS isolates thus far seem resilient to antibiotic pressure from penicillin or ampicillin, perhaps other enteric organisms such as Escherichia coli will become increasingly resistant as the obstetric use of antibiotics increases. Meyn and Hillier recently reported no change in the susceptibility to ampicillin among vaginal isolates of E. coli collected after an increase in the intrapartum use of ampicillin prophylaxis at one hospital (113). Additional efforts to monitor trends in neonatal sepsis and resistance patterns among perinatal pathogens will be important as antimicrobial prophylaxis becomes more widely used.
Organism Virulence
The capsular polysaccharide antigens of GBS are recognized as key virulence factors; type-specific anticapsular antibody opsonizes homologous GBS strains (56a). This observation provided the basis for development of vaccines consisting of capsular polysaccharide antigen and, later, polysaccharide-protein conjugate vaccines. More recent investigations focusing on surface proteins (e.g., protein C and rib protein) suggest that these are common to multiple capsular types and that they also play a role in virulence (90, 104, 163).
ROLE OF CLINICAL PRACTICES IN DISEASE
Obstetric Practices
Obstetric practices have changed substantially in the United States during this century, as relationships between women and the health care system have evolved and as new technologies arrived touting potential advances in pain control, risk reduction, or information management (3, 130). Relevant to GBS disease, obstetric practices can be divided into those which may increase and those which may reduce the risk of disease.
For most of this century, deliveries had shifted from the home to the hospital (51). Along with the medicalization of childbirth, numerous manipulations during labor and delivery entered routine practice. Since GBS is a common colonizer of the genital and rectal area, any manipulation which promotes the ascent of GBS from the vagina to the amniotic fluid would be likely to increase the risk of disease. In concert with this hypothesis, Yancey et al. have described the risk of maternal amnionitis or neonatal GBS disease associated with increased vaginal examinations and longer durations of monitoring by intrauterine pressure catheters (187). Reports of GBS abscesses following the use of fetal monitors (49, 50) sounded an early caution about these devices. Although rare, GBS infection following chorionic villus sampling has been reported, and this has led practitioners in some areas to routinely culture for GBS before this procedure. The use of procedures such as bladder catherization and enemas during the intrapartum period theoretically increase the risk of GBS, but these procedures have not been linked to disease as yet. Although rates of early-onset GBS disease were more common among women undergoing cesarean section (151), indications for cesarean delivery include factors associated with GBS disease risk; therefore, the link is unlikely to be causal. Since longer intervals between membrane rupture and delivery are associated with increased risk of GBS, practices which reduce the intrapartum period could potentially lead to a reduced risk. However, decisions to use labor induction, augmentation, and cesarean section should be based on more common risks and benefits rather than consideration of GBS infection. Health care providers and prenatal patients need to know that women colonized with GBS do not require or necessarily benefit from cesarean delivery. GBS can cross intact membranes, and interventions less invasive than surgery (i.e., intrapartum antibiotic prophylaxis) are more effective means of blocking vertical transmission of GBS.
Obstetric attitudes have been an important factor in whether GBS prevention is practiced. Although clinical trial data in the mid-1980s (36, 106, 120, 122, 170) suggested that GBS disease among newborns could be prevented through maternal antibiotic prophylaxis, several provider surveys in the early 1990s suggested that clinicians were not implementing strategies as proposed, were concerned about the cost-effectiveness of prevention efforts, and were often motivated to adopt prevention protocols only after they had had direct experience with delivering a baby who had complications from GBS infection (71, 88, 110, 141). Conflicting statements on prevention issued in 1992 were a barrier to implementation by many obstetricians. By 1994, a multistate survey revealed that only 38% of hospital obstetric programs had GBS prevention policies and only 12% had committed their policies to writing (141). Controversy about prevention strategies raged (171).
Obstetric attitudes regarding prenatal cultures were initially an obstacle to their use. The timing of screening studied in the main clinical trial (36), 26 to 28 weeks gestation, proved to be a cumbersome schedule for many obstetricians. Concerns that this visit was not usually associated with a pelvic examination were voiced. However, optimal detection of GBS colonization requires culture of the lower third of the vagina and the rectum (i.e., through the anal sphincter) (17, 129, 131); therefore, a pelvic examination and use of speculum are not needed for specimen collection. In fact, Mercer et al. demonstrated that women could collect their own vaginal and rectal specimens, since culture yields were slightly higher from self-collected specimens than from specimens collected by nurses (112).
The extent to which obstetric care is separated from pediatric care, due to either practitioner specialty or practice setting, may also affect the success of GBS prevention efforts. Although pediatric training and research have highlighted GBS disease since the 1970s, only relatively recently have obstetricians focused on the disease and its prevention. Increasing attention to preventive care in obstetrics, as well as liability concerns (134), may have enhanced awareness of GBS among obstetric care providers.
Pediatric Practices
Pediatric practices also have influenced the epidemiology of neonatal GBS disease. The dramatic decrease in deaths among infants with GBS disease observed during the 1980s is attributed to improved recognition and prompt therapy of newborns suspected to have sepsis (99, 133).
Pediatric practices unrelated to GBS disease actually provided insight into GBS pathogenesis and prevention opportunities. Clinicians at Mt. Sinai hospital noted that their GBS rate was substantially lower than at comparable facilities, and they credited this to their practice of providing penicillin prophylaxis to newborns to prevent ophthalmitis neonatorum, while other centers used topical medications (164). This observation was investigated formally in clinical trials conducted in Dallas (159, 160) and Chicago (132). Siegel et al. found a significant decline in early-onset GBS disease among infants treated with intramuscular penicillin, although rates of total neonatal sepsis and death were not reduced in the penicillin group (159, 160). In contrast, Pyati et al. (132) found no reduction in GBS disease when intramuscular penicillin was given in a randomized trial to low-birth-weight infants. Subsequent observational data from Dallas suggest that lower rates of GBS disease among term infants occurred when postnatal penicillin was routinely used (158). Since most GBS infections occur within the first hours of birth, the applicability of the Dallas experience to other centers is difficult to assess. Some authorities have suggested the use of postnatal intramuscular penicillin among term infants born to mothers who are asymptomatic GBS carriers, saving intrapartum (maternal) antimicrobial prophylaxis for women with labor complications (75). However, obstetricians have been reluctant to implement selective intrapartum prophylaxis (71, 88, 110, 171), and the impact on antibiotic efficacy of delaying antibiotic prophylaxis until after labor complications occur has not yet been assessed. Furthermore, dividing responsibilities for prophylaxis between obstetrics and pediatrics might result in reduced compliance and increased confusion (79).
Much information about the epidemiology of GBS disease is dependent on isolation of the bacteria from sterile sites, so diagnostic practices among pediatricians can affect the detection of invasive cases. Whether pediatricians routinely collect blood cultures from high-risk infants (e.g., preterm infants and infants born to women with amnionitis) can have a substantial effect on disease estimates. Recent study of 245 early-onset cases in four geographic areas suggested that 21% (144) of infants had asymptomatic bacteremia; cultures were collected to follow up maternal complications. Although these infants had good outcomes, prompt diagnosis and treatment probably were responsible for the mild clinical course.
One difficulty in interpreting data from clinical trials which include the use of intrapartum and postnatal antimicrobial prophylaxis is the possibility of culture-negative clinical sepsis; that is, illness is not prevented but the ability to diagnose its etiology is reduced. The proportion of infants with clinical sepsis and negative culture results whose disease should be attributed to GBS is not known. While for many years pediatricians may have urged obstetricians to avoid administering antibiotics to women in labor unless necessary for therapy of maternal infection, so as to avoid complicating the diagnosis of infection in the newborn, recent consensus guidelines suggest that obstetric prophylaxis of high-risk women is preferable to delaying maternal antibiotics (e.g., until after the cord is clamped) until newborns can be evaluated. Still, surveys of pediatric providers conducted before the consensus guidelines were issued suggested that GBS colonization of the mother may be associated with increased diagnostic testing, length of stay, and therapy of the newborn even in the absence of clinical symptoms (111, 182). To minimize excessive work-up and therapy of infants whose mothers received prophylactic antibiotics, the AAP developed a sample clinical algorithm which could be followed for infants born to mothers who received antimicrobial prophylaxis for GBS (8). Of great concern to parents, providers, and administrators is the optimal in-hospital observation period required for asymptomatic infants whose mothers received prophylactic antibiotics. Although this issue has not been evaluated in randomized trials, the AAP algorithm suggested a 48-h observation period. Since nearly 80% of early-onset GBS infections are evident during the first day of life, early discharge might be relatively safe. However, the possibility exists that maternal antimicrobial prophylaxis will delay but not prevent the onset of clinical illness in some infants. Until more data are available to refute this concern, observation periods longer than 24 h appear prudent.
Clinical Microbiology Laboratory Practices
The cornerstone of many GBS prevention efforts is identifying the bacteria from pregnant women. Numerous investigators have compared methods to isolate the bacteria from the genital tract (17, 23, 62, 74, 129, 131, 147). In addition to site of sampling (combined swab of both vagina and rectum being preferable to other sites), the use of selective broth media improves recovery by 50% (147). Although clinicians often send genital tract specimens to laboratories for culture without specifying the purpose, GBS is unlikely to be detected unless selective methods are used. Consensus prevention guidelines from the CDC, ACOG, and AAP recommended that swabs be collected from both vagina and rectum and labelled “for GBS culture” to alert laboratory personnel of the need for special methods. Information on laboratory results must be communicated to prenatal caregivers, who can inform patients, and to the site of labor and delivery, to ensure access to culture results among personnel who will administer prophylaxis if needed. Improvements in laboratory information systems may be needed in some areas to ensure prenatal laboratory results are available at the labor and delivery site.
The procedure for culture of GBS from prenatal specimens with selective broth media is as follows. (i) One or two swabs of the vaginal introitus and anorectum are obtained. Cervical cultures are not acceptable; a speculum should not be used for culture collection. (ii) The swabs are placed in a container of transport medium. In transport medium, the swabs will maintain GBS viability for up to 4 days at room temperature or under refrigeration. Appropriate nonnutritive moist swab transport systems (e.g., Amies’ system) are commercially available. (iii) The swabs are removed from the transport medium, and both swabs are inoculated together into selective broth medium. Todd-Hewitt broth supplemented either with colistin (10 μg/ml) and nalidixic acid (15 μg/ml) or with gentamicin (8 μg/ml) and nalidixic acid (15 μg/ml) may be used; appropriate commercially available options include Lim or SBM broth. (iv) The selective broth is incubated for 18 to 24 h. It is then subcultured to a sheep blood agar plate. (v) Organisms suggestive of GBS (beta-hemolytic or nonhemolytic, gram-positive, and catalase-negative organisms) are inspected and identified. If GBS is not identified after incubation for 18 to 24 hours on a sheep blood agar plate, the plate is reincubated and inspected at 48 h to identify suspected organisms. (vi) Various slide agglutination tests or other tests for GBS antigen detection (e.g., genetic probe or fluorescent antibody) are used for specific identification; the CAMP test may be used for presumptive identification.
GBS bacteriuria is a recognized risk factor for GBS disease, although the portion of cases attributable to this factor is probably small. Clinical laboratories frequently reserve identification of bacteria from urine cultures to specimens in which high colony counts (e.g., >105 organisms) are observed and rarely have information pertaining to the pregnancy status of the patient. Thus, laboratories are likely to detect and report GBS bacteriuria due to high levels but less likely to routinely recognize GBS when it is present in the urine at lower concentrations. The magnitude and cost of changes which would be necessary to permit clinical laboratories to routinely detect low levels of GBS bacteriuria are unlikely to exceed their value in preventing GBS disease. Patients with lower concentration of GBS in urine are likely to be detected subsequently on 35- to 37-week vaginorectal cultures and should then be identified as potentially benefiting from intrapartum prophylaxis. Although lower levels of GBS bacteriuria may turn out to be a risk factor for preterm low-birth-weight delivery, it is unclear whether such outcomes can be prevented with antenatal antibiotics (94). Rapid tests may eventually find a niche in the detection of heavily colonized women early in gestation or as an aid to the evaluation of urine specimens in certain circumstances.
Culture of GBS with selective broth media is the current standard for identification of GBS from prenatal patients (12, 153). Several methods for the rapid detection of GBS were evaluated during the past decade, with the goal of permitting prompt recognition of GBS carriers at the time of labor or membrane rupture, when intervention could be initiated. Unfortunately, methods available to date are insensitive compared with the use of selective broth media, and their timeliness is insufficient to justify their use instead of broth culture on prenatal specimens. Clinicians should not decide against intrapartum prophylaxis based on a negative rapid-detection test performed during labor. Rapid-detection methods have been evaluated in comparative studies (18, 177, 185), and the Food and Drug Administration recently considered the issue formally (12). The consensus at present is that detection of GBS isolated from pregnant women should be performed via culture with selective broth media (12, 153).
Although this is the new consensus, a survey of more than 200 clinical microbiology laboratories during 1994 suggested that few were using selective broth media at that point (181). Following increased attention to this issue (12, 83, 153), practices may be improving. Follow-up assessment of laboratory practices is under way.
Institutional Factors
Most babies in the United States are delivered in hospitals, and the institution remains an important variable in the GBS equation. Individual women have different risks of delivering an infant with GBS disease, depending on colonization, demographic and obstetric factors. Individual obstetric caregivers differ in routine prenatal and intrapartum practices. Hospitals could theoretically provide a convenient unit for monitoring GBS disease, incorporating information on both patient population profiles and obstetric practice routines. Hospitals are also critical sites for the implementation of preventive strategies.
Since GBS neonatal disease rates are generally <3 per 1,000 births, most hospitals will have too few deliveries to rely exclusively on disease outcome in tracking GBS prevention at the hospital level. Furthermore, important shifts in patient populations at single hospitals are occurring, since changes in Medicaid reimbursement have led to increased competition among private institutions for patients who previously used public facilities for obstetric care. Of tremendous concern for effective perinatal management is the practice of low-income patients seeking prenatal care at public clinics but delivering at unrelated private institutions, without adequate linkages being established to ensure that prenatal care information is available to labor and delivery personnel.
Evaluating GBS disease at multiple hospitals, such as those affiliated with a managed-care organization or unaffiliated hospitals within defined geographic areas (181), can provide valuable information on trends in perinatal GBS disease and can identify hospital factors predictive of disease rates. Comparing the rate of GBS infection among deliveries in two different hospitals may not be valid if the patient populations differ substantially or if, for example, one hospital serves as a referral center for high-risk pregnancies.
Whitney et al. identified hospital characteristics associated with GBS disease in more than 118 hospitals, with 129,000 total deliveries, in a multistate area where GBS surveillance was under way (181). Several factors were associated with GBS cases in univariate analysis, including the number of deliveries occurring among women who had received no prenatal care, were on medical assistance, or had low-birth-weight babies, as well as the number of deliveries in African American patients; whether the hospital had a neonatal intensive care unit; and whether the hospital was affiliated with a medical school. By multivariate analysis, which controlled for the number of births and the other factors, hospitals with more African American obstetric patients and more obstetric patients lacking prenatal care had more GBS disease, while hospitals with GBS screening policies had significantly less disease.
Although initial GBS prevention education was directed at individual caregivers (7, 9), the role of institutions—hospitals, managed-care organizations, university systems, and insurance plans—in implementing prevention strategies is increasingly evident. System-wide changes are more effective mechanisms to alter clinical practice than are efforts directed at individual clinicians. Clearly, an obstetrician or nurse midwife who collects appropriate prenatal specimens will be ineffective at preventing GBS disease unless labor and delivery staff are aware of the prevention protocol and satellite laboratories provide efficient reporting of results to the labor site. Furthermore, labor and delivery staff may be confused about implementation when individual clinicians have discordant strategies. For this reason, ACOG, AAP, and CDC recommended that institutions adopt prevention strategies and facilitate communication among relevant personnel. Managed-care organizations are uniquely poised to implement prevention strategies and monitor their performance (149).
While experience with each of the recommended prevention strategies is still relatively limited, a few reports provide encouraging results. The University of Miami Medical Center at Jackson Memorial implemented the risk-based strategy in 1992 and found that early-onset GBS disease decreased from 1.8 per 1,000 births in 1992 to 0.2 per 1,000 births in 1995 (58). This decline was evident even after adjusting for changes in the risk profile of deliveries (i.e., fewer deliveries to African Americans and more deliveries to Hispanic women). The decline in cases occurred while there was a documented increase in intrapartum antibiotic use among women with preterm delivery and among women with membrane rupture of ≥18 h. Katz et al. have reported successful implementation of prenatal screening and treatment of women who are colonized or deliver preterm (93).
Managed-Care Organizations
The shift in health care toward increasing use of managed care has important implications for GBS disease. Many managed-care organizations consider the economic implications of diagnostic, therapeutic, and preventive measures and restrict provider access to procedures or interventions which are costly without adequate clinical justification. Both the risk-based and prenatal screening-based strategies were identified as cost-saving compared with treating GBS cases (116, 145, 186). However, pediatric management of infants born to mothers who received intrapartum prophylaxis has not been included in previous cost analyses. An algorithm for newborns of mothers receiving intrapartum antimicrobial prophylaxis was included in CDC and AAP recommendations as a sample for clinicians to follow, although both organizations suggested that other strategies developed by local institutions may also be appropriate (8, 153). In the CDC and AAP algorithms, infants without symptoms, born after 35 weeks gestation, whose mothers received two or more doses (8) or more than 4 h (153) of antibiotic prophylaxis before delivery needed no extra treatment or diagnostic testing, but a 48-h observation period was suggested. Since some managed-care organizations recommend earlier discharge policies, this issue has potentially important cost implications. Although the effect of different discharge schedules on GBS disease has not been studied, the recognition that 20% of early-onset cases present on day 2 of life or later and concern that intrapartum antimicrobial prophylaxis could potentially delay onset rather than prevent some infections, led to this conservative recommendation for a 48-h observation period. The passage of state and, subsequently, federal legislation prohibiting insurance companies from mandating “drive-through deliveries” in recent years leaves the discharge timing up to clinicians and patients.
Managed-care organizations track key health delivery indicators such as through Health Plan Employer Data and Information Set (86), which become available to purchasers of health care as an objective means of comparing plans. Providing feedback to clinicians about their performance is a well-proven means of motivating improved compliance. Managed-care organizations with strong performance monitoring and quality assurance systems may have an advantage in implementing and tracking compliance with GBS prevention. CDC is currently supporting research with managed-care organizations to investigate whether suitable simple indicators of overall compliance with GBS prevention protocols can be identified for use in other facilities.
SHIFTING PARADIGMS
Disease Control: from Treatment to Prevention
During the decade following recognition of the new role of GBS disease in newborns, tremendous advances in treating infections and improving survival were achieved (99, 133). During the second decade, the 1980s, several clinical trials evaluated approaches to prevention (36, 55, 67, 105, 119, 159, 160, 174) but practices did not change. During the third decade since the emergence of GBS, prevention is being taken seriously. The 1990s have seen debate and decisions regarding GBS prevention brought to courtrooms, state legislatures, and executive boards of major organizations representing health professionals. Parents and their advocacy groups, including the Group B Strep Association (Chapel Hill, N.C.), ensured that consumer concerns were addressed. CDC, ACOG, AAP, and others reached consensus that prevention made sense, was achievable by economic and safe methods, and should be adopted by all institutions serving obstetric patients in the United States (8, 10, 153). Consideration regarding this emerging infection transcended initial questions about why it had arrived to address how it could be controlled; prevention protocols for perinatal disease became the standards of care systemwide. Shifts from treatment to prevention models have been widespread in clinical medicine, from cardiac disease to cancer. Although GBS disease has traditionally been outside the public health realm, prevention guidelines should now place this infection squarely among traditional maternal-child health program concerns.
Intrapartum Antimicrobial Prophylaxis: from “Whether” to “How”
As noted above, for many years after demonstration of the efficacy of intrapartum antimicrobial prophylaxis through clinical trials, health care providers and researchers debated whether intrapartum antimicrobial prophylaxis should be performed. Critical issues were whether the practice was more costly than could be justified (116, 145, 186) and whether problems with implementation were worth the trouble (16, 70, 95, 100, 127, 171). The consensus guidelines now require obstetric providers to replace the question of whether they should use intrapartum antimicrobial prophylaxis with how they will administer this. The CDC, ACOG, and AAP recommended the use of either a screening-based approach or a risk-based approach (Fig. 3 and 4). The screening-based approach provides intrapartum antibiotics to women with GBS colonization identified by late-gestation cultures and to those without culture results who are delivering at <37 weeks gestation. The risk-based approach provides intrapartum antibiotics to women delivering either at <37 weeks gestation, after an interval of 18 h or more of ruptured membranes or with an intrapartum temperature of ≥38°C (100.4°F). Both strategies provide intrapartum antibiotics to women with GBS bacteriuria identified during the pregnancy, as well as to women who previously delivered an infant with GBS disease.
Institutional committees and health care providers may choose between these strategies based on a variety of considerations. The screening-based strategy addresses prevention of early-onset disease among asymptomatic carriers or women with GBS colonization who do not have fever, preterm delivery, or prolonged rupture of the membranes. A recent review of maternal and infant records from early-onset GBS cases identified through multistate active surveillance suggested that nearly half of the early-onset cases in 1995 occurred in infants born to women without one of these risk factors (144). The strategy also provides antibiotics relatively early in labor, since women identified as carriers can be offered prophylaxis on admission, without waiting until 18 h has elapsed after membrane rupture or until a fever or amnionitis is evident. Although there is little direct data to assess whether longer intervals of antibiotic treatment before delivery will reduce the chances that infants will develop early-onset disease, many mothers of the infants in whom cases that occurred in 1995 despite maternal antibiotic prophylaxis had received antibiotics for <4 h or only after fever developed (144). One report of GBS colonization among newborns whose mothers were colonized suggested that only 1% of infants were colonized when their carrier mothers received ampicillin 4 h or more before delivery, whereas more than 40% were still colonized when antibiotics were administered within 1 h of delivery (45). The main disadvantage of the screening-based strategy is its complexity, since it depends on collection of prenatal cultures and on ensuring that results are available and acted upon at the labor and delivery setting.
The risk-based strategy is potentially easier for providers to implement, since its only prenatal component is educating patients about the strategy (a component of both approaches). Recognition of risk factors should be relatively straightforward, although this strategy requires education of providers, including nursing staff, to ensure compliance. This strategy may be more appropriate for hospitals where a high proportion of patients have no prenatal care. The main disadvantage of the risk-based strategy is that even if perfectly applied, it will prevent only early-onset cases which occur in women with risk factors. Based on the 1995 case review described earlier (144), it was estimated that up to 79% of early-onset disease could be prevented by the screening-based strategy compared with less than 40% of early-onset disease by the risk-based strategy. These figures are theoretical estimates, though, and the effectiveness of both strategies in clinical practice might be considerably lower. Nevertheless, encouraging reports from individual hospitals (58, 93) and larger populations (42) suggest that substantial declines in early-onset GBS disease can be achieved.
Prenatal Screening: Who, Where, When, How
Prenatal screening for GBS has been under consideration for 15 years, but perspectives on this practice have changed.
Who.
A large-scale study of GBS colonization (140) showed that selective approaches to screening prenatal patients were not justified. Since no combination of clinical and demographic information could identify a subset of women whose screening would identify the majority of carriers, these data suggested that in practices and institutions where screening programs are used, the test should be applied universally (i.e., to all prenatal patients).
Where.
Investigators also compared collection of specimens from different sites; cervical cultures are poor predictors of GBS colonization, and collection of samples from both the vagina and rectum provides a significantly higher yield than does collection of vaginal samples alone (83). Therefore, unlike many genital pathogens for which speculum examination and cervical specimens are recommended, the consensus recommendations for GBS screening are that swabs from the vagina and rectum should be collected. These may be collected by use of a single swab (swabbing the vagina first) or with two separate swabs. If two swabs are used, both should be inoculated into a single broth for culture, since determining the site of colonization is not necessary and this will reduce the cost to the laboratory.
When.
Based on an initial documentation of the importance of timing on the accuracy of antenatal cultures (35), Boyer and Gotoff conducted a randomized clinical trial based on the collection of screening cultures at 26 to 28 weeks gestation (36), whereas Garland and Fliegner selected 32 weeks gestation for a large observational study in Australia (67). Both studies showed effectiveness of intrapartum prophylaxis, but subsequent criticism focused on the sensitivity and predictive value of a 26- to 28-week culture and the problem with preterm deliveries occurring before the 32 week screening could be performed. The consensus process led to the decision to separate the concern about preterm deliveries from selection of the optimal timing of antenatal cultures. Since the most accurate cultures are those collected within 5 weeks of delivery (189) and since preterm deliveries can be addressed empirically if negative culture results are not yet available, collection of prenatal cultures at 35 to 37 weeks gestation is now recommended (8, 10, 153). One problem associated with earlier cultures was the pressure on clinicians to provide antenatal antibiotics to women identified as carriers at 26 to 28 weeks. Despite knowledge that this practice is not effective in preventing GBS transmission, many providers reported perceiving the need to offer women some response to the GBS test result obtained several weeks before delivery (88). Since the 35- to 37-week culture result should be available relatively close to the patient’s due date, describing a plan for intrapartum antibiotics at this point may be adequate to relieve patient concerns without providers feeling obligated to recommend antenatal antibiotics.
How.
Since appropriate samples for GBS culture are obtained from the rectum and vagina, many other bacteria will be present, and selective media are needed to ensure recovery of GBS. Comparative studies have assessed the optimal methods to recover GBS from these specimens. Exclusive reliance on recovery from blood agar plates will miss up to 50% of carriers. Although this practice was frequent (181), it is no longer acceptable (12) for laboratories to process prenatal screening cultures for GBS without using appropriate media. Since few clinicians are aware of the culture methods used by the laboratory and since laboratories were rarely aware that swabs from the genital tract or rectum were being sent for identification of GBS and not all bacteria, the use of nonselective methods seems to have continued for many years. Clinicians cannot interpret negative results from a GBS culture which did not use selective broth media. Laboratory personnel will not know how to process genital specimens from prenatal patients without appropriate labelling that the culture is for GBS isolation. Improved communication among these personnel is needed for prevention programs to work.
Vaccines: Type and Target
Substantial reduction in early-onset GBS disease is likely to occur through improved use of intrapartum antimicrobial prophylaxis. However, the consensus prophylaxis strategies are associated with frequent antibiotic use, which might lead to the emergence of resistant infections by GBS or other agents during the perinatal period. Furthermore, antimicrobial prophylaxis is unlikely to prevent most late-onset infections, GBS-related stillbirths, or prematurity and does not address GBS disease in nonpregnant adults.
GBS vaccine development began almost as soon as the emergence of disease due to this pathogen was recognized, as Baker and colleagues explored the immunologic basis for protection against GBS disease (24). Type-specific antibody to capsular polysaccharide is protective, yet such antibodies are rare. Initial reports of GBS disease suggested that type III isolates were causing the majority of meningitis and a substantial proportion of all disease, and so vaccine efforts initially concentrated on this antigen. Additional studies have suggested that multiple serotypes are important causes of perinatal and adult disease (33, 81, 141, 180), and multivalent GBS vaccines became the objective. Through a series of studies by Kasper, Baker, and others, GBS capsular polysaccharides were purified and the immune response to a type III polysaccharide vaccine was studied in women (25, 26). After fewer than 60% of women immunized during the third trimester produced antibody to type III, investigators suspected that the response could be enhanced by conjugation of capsular polysaccharide to a protein carrier, following the successful method used to improve the immune response to H. influenzae b vaccines. Excellent response to a GBS type III polysaccharide conjugated to tetanus toxoid was reported recently (91). Clinical trials are currently evaluating polysaccharide-protein conjugate vaccines for the other serotypes.
The initial target of GBS vaccine development was the use of vaccines in pregnant women, during the second trimester, after organogenesis was complete but early enough to permit antibody production before most preterm deliveries occur. With the exception of tetanus toxoid vaccine, which is administered to millions of women around the world annually, the use of vaccines during pregnancy has been controversial. GBS vaccines could potentially be given to women of reproductive age before pregnancy occurs, since conjugate vaccines are likely to lead to long-term protection in adults. The recent introduction of a routine adolescent immunization visit (41) might offer the ideal opportunity to administer GBS vaccines prior to pregnancy. Thus, the target population for GBS vaccines to prevent perinatal GBS disease may have shifted from pregnant women to women of reproductive age, perhaps adolescents. Some investigators have even suggested that GBS vaccines be administered with childhood immunizations, since vaccine delivery systems in the United States are most efficient in reaching this age group (142). Since hepatitis B vaccine has now been integrated into the childhood immunization schedule, the concept of vaccinating infants to protect adults is no longer radical.
The definition of substantial disease burden among nonpregnant adults (59, 87, 123, 154) suggests that others may also benefit from GBS vaccines. Until more information on the immunology of GBS disease in adults is available, consideration of GBS vaccines for prevention of nonperinatal disease remains speculative. Nevertheless, investigators have defined risk groups (87) and disease burden (59), and cost-effectiveness analyses based on these data are in progress. If enhancing antibodies to GBS capsular polysaccharides can protect adults from invasive GBS disease, the growing population of elderly persons and others with chronic illnesses at increased risk of GBS disease, as well as the substantial costs associated with treating patients for this condition, suggests that the market for GBS vaccines could eventually be much broader than women contemplating pregnancy.
Further studies are needed to assess whether mucosal immunity is achieved through GBS polysaccharide-protein conjugates. If products under study had an important effect on genital colonization with GBS, similar to the effect on nasopharyngeal colonization afforded by H. influenzae b vaccines (117, 169), GBS vaccines might be more effective than would be predicted from antibody levels in serum. Furthermore, field trials of GBS vaccines could potentially use colonization as a key outcome measure, which would permit studies to be completed with fewer subjects.
CONCLUSIONS
The epidemiology of GBS disease in the United States has undergone radical changes since this pathogen emerged in hospital nurseries 25 years ago. While other perinatal infectious agents continued to emerge, GBS remained the leading cause of sepsis and meningitis in newborns. GBS infections now are less often fatal, but may require treatment with expensive new technologies like extracorporeal membrane oxygenation. Children are more likely to survive GBS infections, but whether survivors are less likely to be disabled is not yet clear. Initially a concern of pediatricians alone, GBS disease now has a presence in the fields of obstetrics, geriatrics, microbiology, public health, and vaccinology. Tackling emerging infections during the phase of their recognition can be frightening and is sometimes adventuresome. Reflection on the decades of cumulative illness, disability, and death attributable to GBS since its emergence can be daunting. While many pathogens like GBS undergo the transformation from exotic emerging infection to endemic disease burden, a long-term commitment to the prevention of infectious diseases is needed for real solutions to be identified.
A decade elapsed between dramatic results of clinical trials testing intrapartum antibiotic prophylaxis (36) and the adoption of consensus prevention protocols by the relevant professional and public health communities (8, 10, 153). This can be contrasted with immediate translation of clinical trial data on antiretroviral agents in pregnancy to their routine use in the United States to interrupt perinatal HIV transmission. Now that important declines have been reported in both perinatal GBS disease and perinatally acquired HIV infection (40, 42), perhaps the lessons of emerging infections like GBS disease are finally being learned. Permitting other preventive strategies to suffer such delays between their identification and implementation would be repeating tragedy.
“Those who cannot remember the past are condemned to repeat it.” —George Santayana (The Life of Reason [1905], vol. 1).
REFERENCES
- 1.Adams W G, Kinney J S, Schuchat A, Collier C L, Papasian C J, Kilbride H W, Riedo F X, Broome C V. Outbreak of early-onset group B streptococcal sepsis. Pediatr Infect Dis J. 1993;12:565–570. doi: 10.1097/00006454-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Aharoni A, Potasman I, Levitan Z, Golan D, Sharf M. Postpartum maternal group B streptococcal meningitis. Rev Infect Dis. 1990;12:273–276. doi: 10.1093/clinids/12.2.273. [DOI] [PubMed] [Google Scholar]
- 3.Albers L L, Krulewitch C J. Electronic fetal monitoring in the United States in the 1980s. Obstet Gynecol. 1993;82:8–10. [PubMed] [Google Scholar]
- 4.Alger L S, Lovchik J C, Hebel J R, Blackmon L R, Crenshaw M C. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynecol. 1988;159:397–404. doi: 10.1016/s0002-9378(88)80093-0. [DOI] [PubMed] [Google Scholar]
- 5.Allardice J G, Baskett T F, Seshia M M K, Bowman N, Malazdrewicz R. Perinatal group B streptococcal colonization and infection. Am J Obstet Gynecol. 1982;142:617–620. doi: 10.1016/s0002-9378(16)32429-2. [DOI] [PubMed] [Google Scholar]
- 6.Alsoub H, Najma F, Robida A. Group B streptococcal endocarditis in children beyond the neonatal period. Pediatr Infect Dis J. 1997;16:418–420. doi: 10.1097/00006454-199704000-00020. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics. Guidelines for prevention of group B streptococcal infection by chemoprophylaxis. Pediatrics. 1992;90:775–778. [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics and COID/COFN. Revised guidelines for prevention of early-onset group B streptococcal (GBS) infection. Pediatrics. 1997;99:489–496. doi: 10.1542/peds.99.3.489. [DOI] [PubMed] [Google Scholar]
- 9.American College of Obstetricians and Gynecologists. Group B streptococcal infections in pregnancy. ACOG technical bulletin 170, July 1992. Washington, D.C: American College of Obstetricians and Gynecologists; 1992. [Google Scholar]
- 10.American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Prevention of early-onset group B streptococcal disease in newborns. ACOG Committee opinion (June, no. 173). Washington, D.C: American College of Obstetricians and Gynecologists; 1996. [PubMed] [Google Scholar]
- 11.Ancona R J, Ferrieri P, Williams P P. Maternal factors that enhance the acquisition of group B streptococci by newborn infants. J Med Microbiol. 1980;3:273–280. doi: 10.1099/00222615-13-2-273. [DOI] [PubMed] [Google Scholar]
- 12.Anonymous. Safety alert re risk of misdiagnosis of group B streptococcal infection. JAMA. 1997;277:1343. [Google Scholar]
- 13.Anthony B F, Okada D M. The emergence of group B streptococci in infections of the newborn infant. Annu Rev Med. 1977;28:355–369. doi: 10.1146/annurev.me.28.020177.002035. [DOI] [PubMed] [Google Scholar]
- 14.Anthony B F, Okada D M, Hobel C J. Epidemiology of group B Streptococcus: longitudinal observations during pregnancy. J Infect Dis. 1978;137:524–530. doi: 10.1093/infdis/137.5.524. [DOI] [PubMed] [Google Scholar]
- 15.Anthony B F, Okada D M, Hobel C J. Epidemiology of the group B streptococcus: maternal and nosocomial sources for infant acquisitions. J Pediatr. 1979;95:431–436. doi: 10.1016/s0022-3476(79)80530-2. [DOI] [PubMed] [Google Scholar]
- 16.Apgar B S, Green L A. Preventing group B streptococcal sepsis in the newborn. Am Fam Physician. 1994;49:315–316. , 318. [PubMed] [Google Scholar]
- 17.Badri M S, Zawaneh S, Cruz A C, Mantilla G, Baer H, Spellacy W N, Ayoub E M. Rectal colonization with group B streptococcus: relation to vaginal colonization of pregnant women. J Infect Dis. 1977;135:308–312. doi: 10.1093/infdis/135.2.308. [DOI] [PubMed] [Google Scholar]
- 18.Baker C J. Inadequacy of rapid immunoassays for intrapartum detection of group B streptococcal carriers. Obstet Gynecol. 1996;88:51–55. doi: 10.1016/0029-7844(96)00111-1. [DOI] [PubMed] [Google Scholar]
- 19.Baker C J, Barrett F F. Transmission of group B streptococci among parturient women and their neonates. J Pediatr. 1973;83:919–925. doi: 10.1016/s0022-3476(73)80524-4. [DOI] [PubMed] [Google Scholar]
- 20.Baker C J, Barrett F F. Group B streptococcal infections in infants: the importance of various serotypes. JAMA. 1974;230:1158–1160. [PubMed] [Google Scholar]
- 21.Baker C J, Barrett F F, Gordon R C, Yow M D. Suppurative meningitis due to streptococci of Lancefield group B: a study of 33 infants. J Pediatr. 1973;82:724–729. doi: 10.1016/s0022-3476(73)80606-7. [DOI] [PubMed] [Google Scholar]
- 22.Baker C J, Edwards M S, Kasper D L. Role of antibody to native type III polysaccharide of group B streptococcus in infant infection. Pediatrics. 1981;68:544–549. [PubMed] [Google Scholar]
- 23.Baker C J, Goroff D K, Alpert S, Crockett V A, Zinner S H, Evrard J R, Rosner B, McCormack W M. Vaginal colonization with group B streptococcus: a study of college women. J Infect Dis. 1977;135:392–397. doi: 10.1093/infdis/135.3.392. [DOI] [PubMed] [Google Scholar]
- 24.Baker C J, Kasper D L. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976;294:753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- 25.Baker C J, Rench M A, Edwards M S, Carpenter R J, Hays B M, Kasper D L. Immunization of pregnant women with a polysaccharide vaccine of group B streptococcus. N Engl J Med. 1988;319:1180–1185. doi: 10.1056/NEJM198811033191802. [DOI] [PubMed] [Google Scholar]
- 26.Baker C J, Rench M A, Kasper D L. Response to type III polysaccharide in women whose infants have had invasive group B streptococcal infection. N Engl J Med. 1990;322:1857–1860. doi: 10.1056/NEJM199006283222606. [DOI] [PubMed] [Google Scholar]
- 27.Baker C J, Webb B J, Kasper D L, Yow M D, Beachler C W. The natural history of group B streptococcal colonization in the pregnant women and her offspring. II. Determination of serum antibody to capsular polysaccharide from type III group B Streptococcus. Am J Obstet Gynecol. 1980;137:39–42. doi: 10.1016/0002-9378(80)90383-x. [DOI] [PubMed] [Google Scholar]
- 28.Barton L L, Feigin R D, Lins R. Group B beta hemolytic streptococcal meningitis in infants. J Pediatr. 1973;82:719–723. doi: 10.1016/s0022-3476(73)80605-5. [DOI] [PubMed] [Google Scholar]
- 29.Bayer A S, Chow A W, Anthony B F, Guze L B. Serious infections in adults due to group B streptococci: clinical and serotypic characterization. Am J Med. 1976;61:498–503. doi: 10.1016/0002-9343(76)90329-6. [DOI] [PubMed] [Google Scholar]
- 30.Berkowitz G S, Papiernik E. Epidemiology of preterm birth. Epidemiol Rev. 1993;15:414–443. doi: 10.1093/oxfordjournals.epirev.a036128. [DOI] [PubMed] [Google Scholar]
- 31.Betriu C, Gomez M, Sanchez A, Cruceyra A, Romero J, Picazo J J. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother. 1994;38:2183–2186. doi: 10.1128/aac.38.9.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blumberg H M, Stephens D S, Licitra C, Pigott N, Facklam R, Swaminathan B, Wachsmuth I K. Molecular epidemiology of group B streptococcal infections: use of restriction endonuclease analysis of chromosomal DNA and DNA restriction fragment polymorphisms of ribosomal RNA genes. J Infect Dis. 1992;166:574–579. doi: 10.1093/infdis/166.3.574. [DOI] [PubMed] [Google Scholar]
- 33.Blumberg H M, Stephens D S, Modansky M, Erwin M, Elliott J, Facklam R, Schuchat A, Baughman W, Farley M M. Invasive group B streptococcal disease: the emergence of serotype V. J Infect Dis. 1996;173:365–373. doi: 10.1093/infdis/173.2.365. [DOI] [PubMed] [Google Scholar]
- 34.Boyer K M, Gadzala C A, Burd L I, Fisher D E, Paton J B, Gotoff S P. Selective intrapartum chemoprophylaxis of neonatal group B streptococcal early-onset disease. I. Epidemiologic rationale. J Infect Dis. 1983;148:795–801. doi: 10.1093/infdis/148.5.795. [DOI] [PubMed] [Google Scholar]
- 35.Boyer K M, Gadzala C A, Kelly P D, Burd L I, Gotoff S P. Selective intrapartum chemoprophylaxis of neonatal group B streptococcal early-onset disease. II. Predictive value of prenatal cultures. J Infect Dis. 1983;148:802–809. doi: 10.1093/infdis/148.5.802. [DOI] [PubMed] [Google Scholar]
- 36.Boyer K M, Gotoff S P. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med. 1986;314:1665–1669. doi: 10.1056/NEJM198606263142603. [DOI] [PubMed] [Google Scholar]
- 37.Braun T I, Pinover W, Sih P. Group B streptococcal meningitis in a pregnant woman before the onset of labor. Clin Infect Dis. 1995;21:1042–1043. doi: 10.1093/clinids/21.4.1042. [DOI] [PubMed] [Google Scholar]
- 38.Broughton D D, Mitchell W G, Grossman M, Hadley W K, Cohen M S. Recurrence of group B streptococcal infection. J Pediatr. 1976;89:183–185. doi: 10.1016/s0022-3476(76)80441-6. [DOI] [PubMed] [Google Scholar]
- 39.Carstensen H, Christensen K K, Grennert L, Persson K, Polberger S. Early-onset neonatal group B streptococcal septicaemia in siblings. J Infect. 1988;17:201–204. doi: 10.1016/s0163-4453(88)96426-2. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. AIDS among children—United States, 1996. Morbid Mortal Weekly Rep. 1996;45:1005–1010. [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Immunization of adolescents: recommendations of the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics, the American Academy of Family Physicians, and the American Medical Association. Morbid Mortal Weekly Rep. 1996;45(RR-13):1–13. [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention. Decreasing incidence of perinatal group B streptococcal disease—United States, 1993–1995. Morbid Mortal Weekly Rep. 1997;46:473–477. [PubMed] [Google Scholar]
- 42a.Centers for Disease Control and Prevention. Unpublished data.
- 43.Chin K C, Fitzhardinge P M. Sequelae of early-onset group B streptococcal meningitis in infants. J Pediatr. 1985;106:819–822. doi: 10.1016/s0022-3476(85)80365-6. [DOI] [PubMed] [Google Scholar]
- 44.Cochi S L, Feldman R A. Estimating national incidence of group B streptococcal disease: the effect of adjusting for birth weight. Pediatr Infect Dis J. 1983;2:414–415. [PubMed] [Google Scholar]
- 45.Cueto M, Sanchez M J, Sampedro A, Miranda J A, Herruzo A, Alvarez M, Rosa-Fraile M. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Relationship between timing of intrapartum ampicillin administration and its effectiveness in preventing vertical transmission of group B streptococci, abstr. K-193. [Google Scholar]
- 46.Cunningham R, Walker C, Ridgway E. Prosthetic hip-joint infection associated with a penicillin-tolerant group B streptococcus. J Infect. 1992;25:77–81. doi: 10.1016/0163-4453(92)93609-t. [DOI] [PubMed] [Google Scholar]
- 47.Daugaard H O, Thomsen A C, Henriques U, Ostergaard A. Group B streptococci in the lower urogenital tract and late abortions. Am J Obstet Gynecol. 1988;158:28–31. doi: 10.1016/0002-9378(88)90769-7. [DOI] [PubMed] [Google Scholar]
- 48.Davis C A, Vallota E H, Forristal J. Serum complement levels in infancy: age-related changes. Pediatr Res. 1979;13:1043–1046. doi: 10.1203/00006450-197909000-00019. [DOI] [PubMed] [Google Scholar]
- 49.Davis J P, Gutman L T, Higgins M V, et al. Infant nasal colonization with group B streptococcus associated with intrauterine pressure transducers. J Infect Dis. 1978;138:804–810. doi: 10.1093/infdis/138.6.804. [DOI] [PubMed] [Google Scholar]
- 50.Davis J P, Moggio M V, Klein D, Tiosejo L L, Welt S I, Wilfert C M. Vertical transmission of group B streptococcus: relation to intrauterine fetal monitoring. JAMA. 1979;242:42–44. [PubMed] [Google Scholar]
- 51.Devitt N. The transition from home to hospital birth in the United States, 1930–1960. Birth Fam J. 1977;4:47. [Google Scholar]
- 52.Di John D, Krasinski K, Lawrence R, Borkowsky W, Johnson J P, Schieken L S, Rennels M B. Very late onset of group B streptococcal disease in infants infected with the human immunodeficiency virus. Pediatr Infect Dis J. 1990;9:925–928. [PubMed] [Google Scholar]
- 53.Dillon H C, Khare S, Gray B M. Group B streptococcal carriage and disease: a 6-year prospective study. J Pediatr. 1987;110:31–36. doi: 10.1016/s0022-3476(87)80283-4. [DOI] [PubMed] [Google Scholar]
- 54.Duma R J, Weinberg A N, Medrek T F, Kunz L J. Streptococcal infections: a bacteriologic and clinical study of streptococcal bacteremia. Medicine. 1969;48:87–127. [Google Scholar]
- 55.Easmon C S F, Hastings M J G, Deeley J, Bloxham B, Rivers R P A, Marwood R. The effect of intrapartum chemoprophylaxis on the vertical transmission of group B streptococci. Br J Obstet Gynaecol. 1983;90:633–635. doi: 10.1111/j.1471-0528.1983.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 56.Edwards M S, Jackson C V, Baker C J. Increased risk of group B streptococcal disease in twins. JAMA. 1981;245:2044–2046. [PubMed] [Google Scholar]
- 56a.Edwards M S, Baker C J, Kasper D L. Opsonic specificity of human antibody to the type III polysaccharide of group B streptococcus. J Infect Dis. 1979;140:1004–1008. doi: 10.1093/infdis/140.6.1004. [DOI] [PubMed] [Google Scholar]
- 57.Edwards M S, Rench M A, Haffar A A M, et al. Long-term sequelae of group B streptococcal meningitis in infants. J Pediatr. 1985;106:717–722. doi: 10.1016/s0022-3476(85)80342-5. [DOI] [PubMed] [Google Scholar]
- 58.Factor, S. H., O. Levine, A. Fajardo, A. Nassar, J. Potter, M. O’Sullivan, and A. Schuchat. Impact of a risk-based prevention policy on neonatal group B streptococcal disease. Am. J. Obstet. Gynecol., in press. [DOI] [PubMed]
- 59.Farley M M, Harvey R C, Stull T, Smith J D, Schuchat A, Wenger J D, Stephens D S. A population-based assessment of invasive disease due to group B streptococcus in nonpregnant adults. N Engl J Med. 1993;328:1807–1811. doi: 10.1056/NEJM199306243282503. [DOI] [PubMed] [Google Scholar]
- 60.Faxelius G, Bremme K, Christensen K K, Christensen P, Ringertz S. Neonatal septicemia due to group B streptococci—perinatal risk factors and outcome of subsequent pregnancies. J Perinat Med. 1988;16:423–430. doi: 10.1515/jpme.1988.16.5-6.423. [DOI] [PubMed] [Google Scholar]
- 61.Fejgin M, Amiel A, Kaneti H, Ben-Nun I, Beyth Y. Fulminant sepsis due to group B beta-hemolytic streptococci following transcervical chorionic villi sampling. Clin Infect Dis. 1993;17:142–143. doi: 10.1093/clinids/17.1.142-a. [DOI] [PubMed] [Google Scholar]
- 62.Ferrieri P, Blair L L. Pharyngeal carriage of group B streptococci: detection by three methods. J Clin Microbiol. 1977;6:136–139. doi: 10.1128/jcm.6.2.136-139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fox B C. Delayed-onset postpartum meningitis due to group B streptococcus. Clin Infect Dis. 1994;19:350. doi: 10.1093/clinids/19.2.350. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 64.Franciosi R A, Knostman J D, Zimmerman R A. Group B streptococcal neonatal and infant infections. J Pediatr. 1973;82:707–718. doi: 10.1016/s0022-3476(73)80604-3. [DOI] [PubMed] [Google Scholar]
- 65.Gallagher P G, Watanakunakorn C. Group B streptococcal bacteremia in a community teaching hospital. Am J Med. 1985;78:795–800. doi: 10.1016/0002-9343(85)90285-2. [DOI] [PubMed] [Google Scholar]
- 66.Gallagher P G, Watanakunakorn C. Group B streptococcal endocarditis: report of seven cases and review of the literature, 1962–1985. Rev Infect Dis. 1986;8:175–188. doi: 10.1093/clinids/8.2.175. [DOI] [PubMed] [Google Scholar]
- 67.Garland S M, Fliegner J R. Group B streptococcus and neonatal infections: the case for intrapartum chemoprophylaxis. Aust N Z J Obstet Gynaecol. 1991;31:119–122. doi: 10.1111/j.1479-828x.1991.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 68.Gerards L J, Cats B P, Hoogkamp-Korstanje J A A. Early neonatal group B streptococcal disease: degree of colonisation as an important determinant. J Infect. 1985;2:119–124. doi: 10.1016/s0163-4453(85)91955-3. [DOI] [PubMed] [Google Scholar]
- 69.Gibbs R S, Blanco J D, St. Clair P J, Castaneda Y S. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145:1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 70.Gibbs R S, McDuffie R S, McNabb F, Fryer G E, Miyoshi T, Merenstein G. Neonatal group B streptococcal sepsis during 2 years of a universal screening program. Obstet Gynecol. 1994;84:496–500. [PubMed] [Google Scholar]
- 71.Gigante J, Hickson G B, Entman S S, Oquist N L. Universal screening for group B streptococcus: recommendations and obstetricians’ practice decisions. Obstet Gynecol. 1995;85:440–443. doi: 10.1016/0029-7844(94)00422-A. [DOI] [PubMed] [Google Scholar]
- 72.Glass P, Wagner A E, Papero P H, Rajasingham S R, Civitello L A, Kjaer M S, Coffman C E, Getson P R, Short B L. Neurodevelopmental status at age five years of neonates treated with extracorporeal membrane oxygenation. J Pediatr. 1995;127:447–457. doi: 10.1016/s0022-3476(95)70082-x. [DOI] [PubMed] [Google Scholar]
- 73.Gordillo M E, Singh K V, Baker C J, Murray B E. Typing of group B streptococci: comparison of pulsed-field gel electrophoresis and conventional electrophoresis. J Clin Microbiol. 1993;31:1430–1434. doi: 10.1128/jcm.31.6.1430-1434.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gordon J S, Sbarra A J. Incidence, technique of isolation, and treatment of group B streptococci in obstetric patients. Am J Obstet Gynecol. 1976;126:1023–1026. doi: 10.1016/0002-9378(76)90695-5. [DOI] [PubMed] [Google Scholar]
- 75.Gotoff S P, Boyer K M. Prevention of early-onset neonatal group B streptococcal disease. Pediatrics. 1997;99:866–869. doi: 10.1542/peds.99.6.866. [DOI] [PubMed] [Google Scholar]
- 76.Gray B M, Egan M L, Pritchard D G. The group B streptococci: from natural history to the specificity of antibodies. Semin Perinatol. 1990;14(4 Suppl. 1):10–21. [PubMed] [Google Scholar]
- 77.Gray B M, Pritchard D G, Dillon H C. Seroepidemiology of group B streptococcus type III colonization at delivery. J Infect Dis. 1989;159:1139–1142. doi: 10.1093/infdis/159.6.1139. [DOI] [PubMed] [Google Scholar]
- 78.Hall E L, Dwyer D, Billmann L, Schuchat A, Harrison L H. Abstracts of the American Public Health Association 124th Annual Meeting. Washington, D.C: American Public Health Association; 1996. Invasive group B streptococcal disease in Maryland nursing home residents; p. 327. [Google Scholar]
- 79.Halsey N A, Schuchat A, Oh W, Baker C J. The 1997 AAP guidelines for prevention of early-onset group B streptococcal disease. American Academy of Pediatrics. Pediatrics. 1997;100:383–384. doi: 10.1542/peds.100.3.383. [DOI] [PubMed] [Google Scholar]
- 80.Harrison L H, Ali A, Dwyer D M, Libonati J P, Reeves M W, Elliott J A, Billmann L, Lashkerwala T, Johnson J A. Relapsing invasive group B streptococcal infection in adults. Ann Intern Med. 1995;123:421–427. doi: 10.7326/0003-4819-123-6-199509150-00004. [DOI] [PubMed] [Google Scholar]
- 81.Harrison L H, Dwyer D M, Johnson J A. Emergence of serotype V group B streptococcal infection among infants and adults. J Infect Dis. 1995;171:513. doi: 10.1093/infdis/171.2.513. [DOI] [PubMed] [Google Scholar]
- 82.Helmig R, Uldbjerg N, Boris J, Kilian M. Clonal analysis of Streptococcus agalactiae isolated from infants with neonatal sepsis or meningitis and their mothers and from healthy pregnant women. J Infect Dis. 1993;168:904–909. doi: 10.1093/infdis/168.4.904. [DOI] [PubMed] [Google Scholar]
- 83.Hillier S, Schuchat A. Preventing neonatal group B streptococcal disease: the role of the clinical microbiology laboratory. Clin Microbiol Newsl. 1997;19:113–116. [Google Scholar]
- 84.Hillier S L, Martius J, Krohn M, Kiviat N, Holmes K K, Eschenbach D A. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 85.Hillier S L, Nugent R P, Eschenbach D A, Krohn M A, Gibbs R S, Martin D H, Cotch M F, Edelman R, Pastorek J G, Vijaya Rao A, McNellis D, Regan J A, Carey J C, Klebanoff M A. Association between bacterial vaginosis and preterm delivery of a low birth weight infant. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 86.Holman P B, Harris J R, Jones W K. Managed care—issues and opportunities: a view from the CDC. J Am Med Women’s Assoc. 1997;52:85–88. [PubMed] [Google Scholar]
- 87.Jackson L A, Hilsdon R, Farley M, Harrison L, Reingold A, Wenger J D, Schuchat A. Risk factors for group B streptococcal disease in adults. Ann Intern Med. 1995;123:415–420. doi: 10.7326/0003-4819-123-6-199509150-00003. [DOI] [PubMed] [Google Scholar]
- 88.Jafari H S, Schuchat A, Hilsdon R, Whitney C, Toomey K, Wenger J. Barriers to prevention of perinatal group B streptococcal disease. Pediatr Infect Dis J. 1995;14:662–667. doi: 10.1097/00006454-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Kasper D L. Designer vaccines to prevent infections due to group B streptococcus. Proc Assoc Am Physicians. 1995;107:369–373. [PubMed] [Google Scholar]
- 90.Kasper D L, Ausubel F M, Michel J L, Madoff L C, Kling D E. Cloned alpha and beta C protein antigens of group B streptococci elicit protective immunity. Infect Immun. 1991;59:2023–2038. doi: 10.1128/iai.59.6.2023-2028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kasper D L, Paoletti L C, Wessels M R, Guttormsen H K, Carey V J, Jennings H J, Baker C J. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Clin Invest. 1996;98:2308–2314. doi: 10.1172/JCI119042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Katz V, Bowes W A. Perinatal group B streptococcal infections across intact amniotic membranes. J Reprod Med. 1988;33:445–449. [PubMed] [Google Scholar]
- 93.Katz V L, Moos M K, Cefalo R C, Thorp J M, Bowes W A, Wells S D. Group B streptococci: results of a protocol of antepartum screening and intrapartum treatment. Am J Obstet Gynecol. 1994;170:521–526. doi: 10.1016/s0002-9378(94)70221-7. [DOI] [PubMed] [Google Scholar]
- 94.Klebanoff M A, Regan J A, Vijaya Rao A, Nugent R P, Blackwelder W C, Eschenbach D A, Pastorek J G, Williams S, Gibbs R S, Carey J C. Outcome of the Vaginal Infections and Prematurity Study: results of a clinical trial of erythromycin among pregnant women colonized with group B streptococci. Am J Obstet Gynecol. 1995;172:1540–1545. doi: 10.1016/0002-9378(95)90493-x. [DOI] [PubMed] [Google Scholar]
- 95.Kleey M J. Group B streptococcal infection: to culture or not to culture? Am Fam Physician. 1994;50:304. , 306. [PubMed] [Google Scholar]
- 96.Krohn M A, Hillier S L, Nugent R P, Cotch M F, Carey C, Gibbs R S, Eschenbach D A, Study Group VIP. The genital flora of women with intraamniotic infection. J Infect Dis. 1995;171:1475–1480. doi: 10.1093/infdis/171.6.1475. [DOI] [PubMed] [Google Scholar]
- 97.Lachenauer C, Kasper D L, Shimada J, Ichiman Y, Ohtsuka H, Kaku M, Paoletti L C, Madoff L C. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Serotypes VI and VIII predominate among group B streptococci isolated from pregnant Japanese women, abstr. K-80. [Google Scholar]
- 98.Lancefield R C, Hare R. The serological differentiation of pathogenic and non-pathogenic strains of hemolytic streptococci from parturient women. J Exp Med. 1935;61:335–349. doi: 10.1084/jem.61.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lannering B, Larsson L E, Rojas J, Stahlman M T. Early onset group B streptococcal disease. Seven year experience and clinical scoring system. Acta Paediatr Scand. 1983;72:597–602. doi: 10.1111/j.1651-2227.1983.tb09777.x. [DOI] [PubMed] [Google Scholar]
- 100.Larsen J W, Dooley S L. Group B streptococcal infections: an obstetrical viewpoint. Pediatrics. 1993;91:148–149. [PubMed] [Google Scholar]
- 101.Lederberg J, Shope R E, Oaks S C J, editors. Emerging infections: microbial threats to health in the United States. Washington, D.C: National Academy Press; 1992. [PubMed] [Google Scholar]
- 102.Lerner P I, Gopalakrishna K V, Wolinsky E, McHenry M C, Tan J S, Rosenthal M. Group B streptococcus (S. agalactiae) bacteremia in adults: analysis of 32 cases and review of the literature. Medicine. 1977;56:457–473. doi: 10.1097/00005792-197711000-00001. [DOI] [PubMed] [Google Scholar]
- 103.Lischke J H, McCreight P H B. Maternal group B streptococcal vertebral osteomyelitis: an unusual complication of vaginal delivery. Obstet Gynecol. 1990;76:489–491. [PubMed] [Google Scholar]
- 104.Madoff L C, Michel J L, Gong E W, Rodewald A K, Kasper D L. Protection of neonatal mice from group B streptococcal infection by maternal immunization with beta C protein. Infect Immun. 1992;60:4989–4994. doi: 10.1128/iai.60.12.4989-4994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matorras R, Garcia-Perea A, Madero R, Usandizaga J A. Maternal colonization by group B streptococci and puerperal infection: analysis of intrapartum chemoprophylaxis. Eur J Obstet Gynecol Reprod Biol. 1990;38:203–207. doi: 10.1016/0028-2243(91)90292-s. [DOI] [PubMed] [Google Scholar]
- 106.Matorras R, Garcia-Perea A, Omenaca F, Diez-Enciso M, Madero R, Usandizaga J A. Intrapartum chemoprophylaxis of early-onset group B streptococcal disease. Eur J Obstet Gynecol Reprod Biol. 1991;40:57–62. doi: 10.1016/0028-2243(91)90045-m. [DOI] [PubMed] [Google Scholar]
- 107.Matorras R, Garcia-Perea A, Omenaca F, Usandizaga J A, Nieto A, Herruzo R. Group B streptococcus and premature rupture of membranes and preterm delivery. Gynecol Obstet Invest. 1989;27:14–18. doi: 10.1159/000293607. [DOI] [PubMed] [Google Scholar]
- 108.McCracken G H. Group B streptococci: the new challenge in neonatal infections. J Pediatr. 1973;82:703–706. doi: 10.1016/s0022-3476(73)80603-1. [DOI] [PubMed] [Google Scholar]
- 109.McDonald H, Vigneswaran R, O’Loughlin J A. Group B streptococcal colonization and preterm labor. Aust N Z J Obstet Gynaecol. 1989;29:291–293. doi: 10.1111/j.1479-828x.1989.tb01745.x. [DOI] [PubMed] [Google Scholar]
- 110.Mercer B M, Ramsey R D, Sibai B M. Prenatal screening for group B streptococcus. I. Impact of antepartum screening on antenatal prophylaxis and intrapartum care. Am J Obstet Gynecol. 1995;173:837–841. doi: 10.1016/0002-9378(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 111.Mercer B M, Ramsey R D, Sibai B M. Prenatal screening for group B streptococcus. II. Impact of antepartum screening and prophylaxis on neonatal care. Am J Obstet Gynecol. 1995;173:842–846. doi: 10.1016/0002-9378(95)90352-6. [DOI] [PubMed] [Google Scholar]
- 112.Mercer B M, Taylor M C, Fricke J L, Baselski V S, Sibai B M. The accuracy and patient preference for self-collected group B streptococcus cultures. Am J Obstet Gynecol. 1995;173:1325–1328. doi: 10.1016/0002-9378(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 113.Meyn L A, Hillier S L. Ampicillin susceptibilities of vaginal and placental isolates of group B streptococcus and Escherichia coli obtained between 1992 and 1994. Antimicrob Agents Chemother. 1997;41:1173–1174. doi: 10.1128/aac.41.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Minkoff H. Prematurity: infection as an etiologic factor. Obstet Gynecol. 1983;62:137–144. [PubMed] [Google Scholar]
- 115.Minkoff H L, Sierra M F, Pringle G F, Schwarz R H. Vaginal colonization with group B beta-hemolytic streptococcus as a risk factor for post-cesarean section febrile morbidity. Am J Obstet Gynecol. 1982;142:992–995. doi: 10.1016/0002-9378(82)90781-5. [DOI] [PubMed] [Google Scholar]
- 116.Mohle-Boetani J, Schuchat A, Plikaytis B D, Smith D, Broome C V. Comparison of prevention strategies for neonatal group B streptococcal infection: a population-based economic analysis. JAMA. 1993;270:1442–1448. [PubMed] [Google Scholar]
- 117.Mohle-Boetani J C, Ajello G, Breneman E, Deaver K A, Harvey C, Plikaytis B D, Farley M M, Stephens D S, Wenger J D. Carriage of Haemophilus influenzae type b in children after widespread vaccination with conjugate Haemophilus influenzae type b vaccines. Pediatr Infect Dis J. 1993;12:589–593. doi: 10.1097/00006454-199307000-00009. [DOI] [PubMed] [Google Scholar]
- 118.Moller M, Thomsen A C, Borch K, Dinesen K, Zdravkovic M. Rupture of fetal membranes and premature delivery associated with group B streptococci in urine of pregnant women. Lancet. 1984;ii:69–70. doi: 10.1016/s0140-6736(84)90242-3. [DOI] [PubMed] [Google Scholar]
- 119.Morales W J, Lim D. Reduction of group B streptococcal maternal and neonatal infections in preterm pregnancies with premature rupture of membranes through a rapid identification test. Am J Obstet Gynecol. 1987;157:13–16. doi: 10.1016/s0002-9378(87)80336-8. [DOI] [PubMed] [Google Scholar]
- 120.Morales W J, Lim D V, Walsh A F. Prevention of neonatal group B streptococcal sepsis by the use of a rapid screening test and selective intrapartum chemoprophylaxis. Am J Obstet Gynecol. 1986;155:979–983. doi: 10.1016/0002-9378(86)90329-7. [DOI] [PubMed] [Google Scholar]
- 121.Newton E R, Butler M C, Shain R N. Sexual behavior and vaginal colonization b group B streptococcus among minority women. Obstet Gynecol. 1996;88:577–582. doi: 10.1016/0029-7844(96)00264-5. [DOI] [PubMed] [Google Scholar]
- 122.Omenaca Teres F, Matorras R, Garcia Perea A, Elorza M D. Prevention of neonatal group B streptococcal sepsis. Pediatr Infect Dis J. 1987;6:874. doi: 10.1097/00006454-198709000-00026. [DOI] [PubMed] [Google Scholar]
- 123.Opal S M, Cross A, Palmer M, Almazan R. Group B streptococcal sepsis in adults and infants. Contrasts and comparisons. Arch Intern Med. 1988;148:641–645. [PubMed] [Google Scholar]
- 124.Pass M A, Gray B M, Khare S, Dillon H C. Prospective studies of group B streptococcal infections in infants. J Pediatr. 1979;95:437–443. doi: 10.1016/s0022-3476(79)80531-4. [DOI] [PubMed] [Google Scholar]
- 125.Pass M A, Khare S, Dillon H C. Twin pregnancies: incidence of group B streptococcal colonization and disease. J Pediatr. 1980;97:635–637. doi: 10.1016/s0022-3476(80)80028-x. [DOI] [PubMed] [Google Scholar]
- 126.Pearlman M, Faix R, Johnson T R B. Early onset neonatal group B strep (EOGBS) infections: do risk factors affect long term outcome? Am J Obstet Gynecol. 1996;174:367. [Google Scholar]
- 127.Peralta-Carcelen M, Fargasan C A J, Cliver S P, Cutter G R, Gigante J, Goldenberg R L. Impact of maternal group B streptococcal screening on pediatric management in full-term newborns. Arch Pediatr Adol Med. 1996;150:802–808. doi: 10.1001/archpedi.1996.02170330028005. [DOI] [PubMed] [Google Scholar]
- 128.Persson K, Christensen K K, Christensen P, Forsgren A, Jorgensen C, Persson P. Asymptomatic bacteriuria during pregnancy with special reference to group B streptococci. Scand J Infect Dis. 1985;17:195–199. doi: 10.3109/inf.1985.17.issue-2.11. [DOI] [PubMed] [Google Scholar]
- 129.Philipson E H, Palermino D A, Robinson A. Enhanced antenatal detection of group B streptococcus colonization. Obstet Gynecol. 1995;85:437–439. doi: 10.1016/0029-7844(94)00412-7. [DOI] [PubMed] [Google Scholar]
- 130.Pitcock C D H, Clark R B. From Fanny to Fernand: the development of consumerism in pain control during the birth process. Am J Obstet Gynecol. 1992;167:581–587. doi: 10.1016/s0002-9378(11)91553-1. [DOI] [PubMed] [Google Scholar]
- 131.Platt M W, McLaughlin J C, Gilson G J, Wellhoner M F, Nims L J. Increased recovery of group B streptococcus by the inclusion of rectal culturing and enrichment. Diagn Microbiol Infect Dis. 1995;21:65–68. doi: 10.1016/0732-8893(95)00022-3. [DOI] [PubMed] [Google Scholar]
- 132.Pyati S P, Pildes R S, Jacobs N M, Ramamurthy R S, Yeh T F, Raval D S, Lilien L D, Amma P, Metzger W I. Penicillin in infants weighing two kilograms or less with early-onset group B streptococcal disease. N Engl J Med. 1983;308:1383–1389. doi: 10.1056/NEJM198306093082303. [DOI] [PubMed] [Google Scholar]
- 133.Pyati S P, Pildes R S, Ramamurthy R S, Jacobs N. Decreasing mortality in neonates with early-onset group B streptococcal infection: reality or artifact. J Pediatr. 1981;98:625–627. doi: 10.1016/s0022-3476(81)80779-2. [DOI] [PubMed] [Google Scholar]
- 134.Queenan J T. GBS prevention: national guidelines at last. Contemp Obstet Gynecol. 1996;41:8–11. [Google Scholar]
- 135.Quentin R, Huet H, Wang F, Geslin P, Goudeau A, Selander R K. Characterization of Streptococcus agalactiae strains by multilocus enzyme genotype and serotype: identification of multiple virulent clone families that cause invasive neonatal disease. J Clin Microbiol. 1995;33:2576–2581. doi: 10.1128/jcm.33.10.2576-2581.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rantz L A, Keefer C S. Distribution of hemolytic streptococci groups A, B, and C in human infections. J Infect Dis. 1941;68:128–132. [Google Scholar]
- 137.Rantz L A, Kirby W M M. Hemolytic streptococcus bacteremia: report of thirteen cases with special reference to serologic groups of etiologic organisms. N Engl J Med. 1942;227:730–733. [Google Scholar]
- 138.Regan J A, Chao S, James L S. Premature rupture of membranes, preterm delivery, and group B streptococcal colonization of mothers. Am J Obstet Gynecol. 1981;141:184–186. doi: 10.1016/s0002-9378(16)32589-3. [DOI] [PubMed] [Google Scholar]
- 139.Regan J A, Klebanoff M A, Nugent R P, Eschenbach D A, Blackwelder W C, Lou Y, Gibbs R S, Rettig P J, Martin D H, Edelman R. Colonization with group B streptococci in pregnancy and adverse outcome. Am J Obstet Gynecol. 1996;174:1354–1360. doi: 10.1016/s0002-9378(96)70684-1. [DOI] [PubMed] [Google Scholar]
- 140.Regan J A, Klebanoff M A, Nugent R P Vaginal Infections and Prematurity Study Group. The epidemiology of group B streptococcal colonization in pregnancy. Obstet Gynecol. 1991;77:604–610. [PubMed] [Google Scholar]
- 141.Rench M A, Baker C J. Neonatal sepsis caused by a new group B streptococcal serotype. J Pediatr. 1993;122:638–640. doi: 10.1016/s0022-3476(05)83554-1. [DOI] [PubMed] [Google Scholar]
- 142.Robbins J B, Schneerson R, Vann W F, Bryla D A, Fattom A. Prevention of systemic infections caused by group B streptococcus and Staphylococcus aureus by multivalent polysaccharide-protein conjugate vaccines. Ann N Y Acad Sci. 1995;754:68–82. doi: 10.1111/j.1749-6632.1995.tb44439.x. [DOI] [PubMed] [Google Scholar]
- 143.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis A P, Hobbins J C. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 144.Rosenstein N, Schuchat A Neonatal GBS Disease Study Group. Opportunities for prevention of perinatal group B streptococcal disease: a multistate surveillance analysis. Obstet Gynecol. 1997;90:901–906. doi: 10.1016/s0029-7844(97)00486-9. [DOI] [PubMed] [Google Scholar]
- 145.Rouse D J, Goldenberg R L, Cliver S P, Cutter G R, Mennemeyer S T, Fargason C A. Strategies for the prevention of early-onset neonatal group B streptococcal sepsis: a decision analysis. Obstet Gynecol. 1994;83:483–494. doi: 10.1097/00006250-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 146.Ruiz-Gomez D, Tarpay M M, Riley H D. Recurrent group B streptococcal infections: report of three cases. Scand J Infect Dis. 1979;11:35–38. doi: 10.3109/inf.1979.11.issue-1.05. [DOI] [PubMed] [Google Scholar]
- 147.Sayahtaheri Altaie S, Dryja D. Detection of group B Streptococcus: comparison of solid and liquid culture media with and without selective antibiotics. Diagn Microbiol Infect Dis. 1994;18:141–144. doi: 10.1016/0732-8893(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 148.Schuchat A. Group B streptococcal disease in newborns: a global perspective on prevention. Biomed Pharmacother. 1995;49:19–25. doi: 10.1016/0753-3322(96)82573-X. [DOI] [PubMed] [Google Scholar]
- 149.Schuchat A. Guidelines for prevention of perinatal group B streptococcal disease. HMO Pract. 1996;10:48–49. [PubMed] [Google Scholar]
- 150.Schuchat A, Deaver-Robinson K, Plikaytis B D, Zangwill K, Mohle-Boetani J, Wenger J D. Multistate case-control study of maternal risk factors for neonatal group B streptococcal disease. Pediatr Infect Dis J. 1994;13:623–629. doi: 10.1097/00006454-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 151.Schuchat A, Oxtoby M, Cochi S, Sikes R K, Hightower A W, Plikaytis B, Broome C V. Population-based risk factors for neonatal group B streptococcal disease: results of a cohort study in metropolitan Atlanta. J Infect Dis. 1990;162:672–677. doi: 10.1093/infdis/162.3.672. [DOI] [PubMed] [Google Scholar]
- 152.Schuchat A, Wenger J D. Epidemiology of group B streptococcal disease: risk factors, prevention strategies and vaccine development. Epidemiol Rev. 1994;16:374–402. doi: 10.1093/oxfordjournals.epirev.a036159. [DOI] [PubMed] [Google Scholar]
- 153.Schuchat A, Whitney C, Zangwill K. Prevention of perinatal group B streptococcal disease: a public health perspective. Morbid Mortal Weekly Rep. 1996;45(RR-7):1–24. [PubMed] [Google Scholar]
- 154.Schwartz B, Jackson L. Invasive group B streptococcal disease in adults. JAMA. 1991;266:3284. [PubMed] [Google Scholar]
- 155.Schwartz B, Schuchat A, Oxtoby M J, Cochi S L, Hightower A, Broome C V. Invasive group B streptococcal disease in adults: a population-based study in metropolitan Atlanta. JAMA. 1991;266:1112–1114. [PubMed] [Google Scholar]
- 156.Severin M J, Wiley J L. Change in susceptibility of group B streptococci to penicillin G from 1968 through 1975. Antimicrob Agents Chemother. 1976;10:380–381. doi: 10.1128/aac.10.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sexton D J, Rockson S G, Hempling R E, Cathey C W. Pregnancy-associated group B streptococcal endocarditis: a report of two fatal cases. Obstet Gynecol. 1985;66:445–465. [PubMed] [Google Scholar]
- 158.Siegel J D, Cushion N B. Prevention of early-onset group B streptococcal disease: another look at single-dose penicillin at birth. Obstet Gynecol. 1996;87:692–698. doi: 10.1016/0029-7844(96)00004-x. [DOI] [PubMed] [Google Scholar]
- 159.Siegel J D, McCracken G H J, Threlkeld N, DePasse B M, Rosenfeld C R. Single-dose penicillin prophylaxis of neonatal group B streptococcal disease. Lancet. 1982;i:1426–1430. doi: 10.1016/s0140-6736(82)92449-7. [DOI] [PubMed] [Google Scholar]
- 160.Siegel J D, McCracken G H J, Threlkeld N, Milvenan B, Rosenfeld C R. Single dose penicillin prophylaxis against neonatal group B streptococcal infections: a controlled trial in 18,738 newborn infants. N Engl J Med. 1980;303:769–775. doi: 10.1056/NEJM198010023031401. [DOI] [PubMed] [Google Scholar]
- 161.Sledge D, Austin E, Walter S, Gerald R. Group B streptococcal endocarditis involving the tricuspid valve in a 7 month old infant. Clin Infect Dis. 1994;19:166–168. doi: 10.1093/clinids/19.1.166. [DOI] [PubMed] [Google Scholar]
- 162.Smith C L, Baker C J, Anderson D C, et al. Role of complement receptors in opsonophagocytosis of group B streptococci by adult and neonatal neutrophils. J Infect Dis. 1990;162:489–495. doi: 10.1093/infdis/162.2.489. [DOI] [PubMed] [Google Scholar]
- 163.Stalhammar-Carlemalm M, Stenberg L, Lindahl G. Protein Rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J Exp Med. 1993;177:1593–1603. doi: 10.1084/jem.177.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Steigman A J, Bottone E J, Hanna B A. Intramuscular penicillin administration at birth: prevention of early-onset group B streptococcal disease. Pediatrics. 1978;62:842–844. [PubMed] [Google Scholar]
- 165.Stewardson-Krieger P B, Gotoff S P. Risk factors in early-onset neonatal group B streptococcal infections. Infection. 1978;6:50–53. doi: 10.1007/BF01642157. [DOI] [PubMed] [Google Scholar]
- 166.Stoll B J, Gordon T, Korones S B, Shankaran S, Tyson J E, Bauer C R, et al. Late-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1996;129:63–71. doi: 10.1016/s0022-3476(96)70191-9. [DOI] [PubMed] [Google Scholar]
- 167.Stoll B J, Gordon T, Korones S B, Shankaran S, Tyson J E, Bauer C R, Fanaroff A A, Lemons J A, Donovan E F, Oh W, Stevenson D K, Ehrenkranz R A, Papile L, Verter J, Wright L L. Early-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1996;129:72–80. doi: 10.1016/s0022-3476(96)70192-0. [DOI] [PubMed] [Google Scholar]
- 168.Sutton G P, Smirz L R, Clark D H, Bennett J E. Group B streptococcal necrotizing fasciitis arising from an episiotomy. Obstet Gynecol. 1985;66:733–736. [PubMed] [Google Scholar]
- 169.Takala A K, Eskola J, Leinonen M, et al. Reduction of oropharyngeal carriage of Haemophilus influenzae type b (Hib) in children immunized with an Hib conjugate vaccine. J Infect Dis. 1991;164:982–986. doi: 10.1093/infdis/164.5.982. [DOI] [PubMed] [Google Scholar]
- 170.Thomsen A C, Morup L, Brogaard Hansen K. Antibiotic elimination of group B streptococci in urine in prevention of preterm labour. Lancet. 1987;i:591–593. doi: 10.1016/s0140-6736(87)90234-0. [DOI] [PubMed] [Google Scholar]
- 171.Towers C V. Group B streptococcus: the U.S. controversy. Lancet. 1995;346:197–199. doi: 10.1016/s0140-6736(95)91262-2. [DOI] [PubMed] [Google Scholar]
- 172.Towers C V, Lewis D F, Asrat T, Gardner K, Perlow J H. The effect of colonization with group B streptococci on the latency phase of patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:1139–1143. doi: 10.1016/0002-9378(93)90270-s. [DOI] [PubMed] [Google Scholar]
- 173.Trager J D, Martin J M, Barbadora K, Green M, Wald E R. Probable community acquisition of group B streptococcus in an infant with late-onset disease: demonstration using field inversion gel electrophoresis. Arch Pediatr Adol Med. 1996;150:766–768. doi: 10.1001/archpedi.1996.02170320112022. [DOI] [PubMed] [Google Scholar]
- 174.Tuppurainen N, Hallman M. Prevention of neonatal group B streptococcal disease: intrapartum detection and chemoprophylaxis of heavily colonized parturients. Obstet Gynecol. 1989;73:583–587. [PubMed] [Google Scholar]
- 175.Verghese A, Mireault K, Arbeit R D. Group B streptococcal bacteremia in men. Rev Infect Dis. 1986;8:912–917. doi: 10.1093/clinids/8.6.912. [DOI] [PubMed] [Google Scholar]
- 176.Wald E R, Bergman I, Taylor H G, et al. Long-term outcome of group B streptococcal meningitis. Pediatrics. 1986;77:217–221. [PubMed] [Google Scholar]
- 177.Walker C K, Crombleholme W R, Ohm-Smith M J, Sweet R L. Comparison of rapid tests for detection of group B streptococcal colonization. Am J Perinatol. 1992;9:304–308. doi: 10.1055/s-2007-999247. [DOI] [PubMed] [Google Scholar]
- 178.Walsh J A, Hutchins S. Group B streptococcal disease: its importance in the developing world and prospect for prevention with vaccines. Pediatr Infect Dis J. 1989;8:271–276. [PubMed] [Google Scholar]
- 179.Weisman L E, Stoll B J, Cruess D F, Hall R T, Merenstein G B, Hemming V G, Fischer G W. Early-onset group B streptococcal sepsis: a current assessment. J Pediatr. 1992;121:428–433. doi: 10.1016/s0022-3476(05)81801-3. [DOI] [PubMed] [Google Scholar]
- 180.Wenger J D, Hightower A W, Facklam R R, Gaventa S, Broome C V. Bacterial meningitis in the United States, 1986: report of a multistate surveillance study. J Infect Dis. 1990;162:1316–1323. doi: 10.1093/infdis/162.6.1316. [DOI] [PubMed] [Google Scholar]
- 181.Whitney C G, Plikaytis B D, Gozansky W S, Wenger J D, Schuchat A. Prevention practices for perinatal group B streptococcal disease: a multi-state surveillance analysis. Obstet Gynecol. 1997;89:28–32. doi: 10.1016/s0029-7844(96)00372-9. [DOI] [PubMed] [Google Scholar]
- 182.Wiswell T E, Stoll B J, Tuggle J M. Management of asymptomatic, term gestation neonates born to mothers treated with intrapartum antibiotics. Pediatr Infect Dis J. 1990;9:826–831. [PubMed] [Google Scholar]
- 183.Wood E G, Dillon H C. A prospective study of group B streptococcal bacteriuria in pregnancy. Am J Obstet Gynecol. 1981;140:515–520. doi: 10.1016/0002-9378(81)90226-x. [DOI] [PubMed] [Google Scholar]
- 184.Yagupsky P, Menegus M S, Powell K R. The changing spectrum of group B streptococcal disease in infants: an eleven-year experience in a tertiary care hospital. Pediatr Infect Dis J. 1991;10:801–808. doi: 10.1097/00006454-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 185.Yancey M K, Armer T, Clark P, Duff P. Assessment of rapid identification tests for genital carriage of group B streptococci. Obstet Gynecol. 1992;80:1038–1047. [PubMed] [Google Scholar]
- 186.Yancey M K, Duff P. An analysis of the cost-effectiveness of selected protocols for the prevention of neonatal group B streptococcal infection. Obstet Gynecol. 1994;83:367–371. [PubMed] [Google Scholar]
- 187.Yancey M K, Duff P, Clark P, Kurtzer T, Frentzen B H, Kubilis P. Peripartum infection associated with vaginal group B streptococcal colonization. Obstet Gynecol. 1994;84:816–819. [PubMed] [Google Scholar]
- 188.Yancey M K, Duff P, Kubilis P, Clark P, Frentzen B H. Risk factors for neonatal sepsis. Obstet Gynecol. 1996;87:188–194. doi: 10.1016/0029-7844(95)00402-5. [DOI] [PubMed] [Google Scholar]
- 189.Yancey M K, Schuchat A, Brown L K, Ventura V L, Markenson G R. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996;88:811–815. doi: 10.1016/0029-7844(96)00320-1. [DOI] [PubMed] [Google Scholar]
- 190.Young N, Finn A, Powell C. Group B streptococcal epiglottitis. Pediatr Infect Dis J. 1996;15:95–96. doi: 10.1097/00006454-199601000-00024. [DOI] [PubMed] [Google Scholar]
- 191.Zangwill K M, Schuchat A, Wenger J D. Group B streptococcal disease in the United States, 1990: report from a multistate active surveillance system. CDC Surveillance Summaries. Morbid Mortal Weekly Rep. 1992;41(SS-6):25–32. [PubMed] [Google Scholar]