Abstract
Objectives
SS with childhood onset is a rare autoimmune disease characterized by heterogeneous presentation. The lack of validated classification criteria makes it challenging to diagnose. Evidence-based guidelines for treatment of juvenile SS are not available due to the rarity of disease and the paucity of research in this patient population. This systematic review aims to summarize and appraise the current literature focused on pharmacological strategies for management of SS with childhood onset.
Methods
PubMed and MEDLINE/Scopus databases up to December 2020 were screened for suitable reports highlighting pharmacological treatment of SS with childhood onset using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 reporting checklist. Animal studies were excluded.
Results
A total of 43 studies (34 case reports, 8 mini case series and 1 pilot study) were eligible for analysis. The studies retrieved included girls in 88% (120/137) of cases and had very low confidence levels. HCQ was prescribed for parotid swelling, as well as in association with MTX and NSAIDs in patients with arthritis and arthralgia. Corticosteroids such as long courses of oral prednisone and i.v. methylprednisolone were commonly prescribed for children with severe disease presentations. Rituximab was mainly indicated for mucosa-associated lymphoid tissue lymphoma and renal and nervous system complications. Other conventional DMARDs were prescribed in selected cases with extraglandular manifestations.
Conclusion
Various therapies are used for the management of juvenile SS and are prescribed based on expert clinician’s opinion. There are currently no good-quality studies that allow clinical recommendations for treatment of SS with childhood onset.
Keywords: juvenile Sjögren’s syndrome, treatment, systematic review
Rheumatology key messages.
Sjögren’s syndrome with childhood onset has heterogeneous clinical manifestations.
There is poor-quality evidence for treatment strategies in children with Sjögren’s syndrome.
Future research is required to guide treatment recommendations in this rare disease.
Introduction
SS is characterized by chronic lymphocytic infiltration of the exocrine glands, resulting in progressive glandular destruction, leading to mucosal dryness [1]. In children, the disease is very rare, with a prevalence that is difficult to estimate, as only a few hundred cases have been reported in the literature overall [2]. In children, SS is less well characterized in terms of clinical presentation and long-term outcomes [3]. When the disease starts before age 18 years it is called SS with childhood onset or juvenile SS. Although some recent progress in defining the disease phenotype in children has been achieved [4], SS with childhood onset remains a poorly defined and likely underrecognized and underdiagnosed condition [5]. There are also recognized overlapping clinical features with IgG4-related disease in children that frequently presents as orbital disease [6] despite evidence of distinct underlying pathogenesis [7].
There is no gold standard diagnostic tool for SS with childhood onset and therefore diagnosis is based on expert clinical opinion, which is dependent on findings from the clinical history and examination, functional exocrine gland tests, as well as serological and histological evidence [8]. Although historically the diagnostic label of ‘primary SS’ has been used for children [9], recent reports have highlighted the large spectrum of clinical symptoms children present with, which are difficult to map against the classification criteria for adults with primary SS [5]. Specialists also argue that it is not suitable to classify SS as ‘primary’ or ‘secondary’ to another autoimmune process, as this does not reflect the disease pathogenesis, and they propose the terminology of ‘Sjögren’s disease’ [10]. In this article, to avoid denomination controversies, we decided to use the term ‘SS with childhood onset’ rather than ‘primary SS in children’. Wherever children had features of other autoimmune diseases, details are provided. Therapeutic strategies are similar in children and adults, in particular because of a lack of good-quality evidence for any treatment effectiveness in children with SS, and they will be discussed below.
The purpose of this systematic literature review is to identify and analyse the main publications investigating pharmacological interventions for SS with childhood onset, with a particular focus on their clinical indication and efficacy. We also highlighted the main therapeutic trends in children and adolescents and explored differences in treatment approaches compared with adults with SS.
Methods
A full literature search was conducted using the PubMed and MEDLINE/Scopus databases to identify scientific reports that mention or discuss in detail the treatment of juvenile SS. The following search terms were used: ‘juvenile Sjögren’s syndrome’ OR ‘childhood onset Sjogren’s syndrome’ OR ‘Sjogren’s syndrome with childhood onset’ OR ‘Sjögren’s syndrome in children’ OR ‘paediatric Sjögren’s syndrome’ OR ‘recurrent parotitis’ OR ‘sicca in children’ (PROSPERO registration ref. CRD42021251990).
Inclusion and exclusion criteria
We included original full-text articles describing randomized controlled trials (RCTs), cross-sectional studies, case series and case reports of patients with SS with childhood onset, defined as disease onset before age 18 years. We included articles published through December 2020 and excluded animal studies and abstracts.
Data extraction
Two reviewers (N.M.F. and G.D.) independently screened articles for inclusion in this systematic review. The main reasons for exclusion are recorded in Fig. 1. We grouped the information retrieved into three tables. Table 1 includes all eligible papers (n = 43). Tables 2 and 3 provide information on the clinical use and efficacy of all therapies used in SS with childhood onset.
Fig. 1.
Flow chart of study selection
Table 1.
Clinical manifestations and characteristics of patients with SS with childhood onset included in literature reports describing the use of various treatments
| Author, year [reference] | Level of evidence (Oxford criteria) | N (F:M) | Patient classification criteria used | Age at symptoms onset (years)/age at diagnosis (years) [mean (range) for studies where N > 2] | Associated conditions/ comorbidities, n (%) | Cumulative signs and symptoms, n (%, where applicable) |
|---|---|---|---|---|---|---|
| Singer et al., 2008 [11] | 4 | 7 (7:0) | Not specified | NA/14.2 [10–17] | JIA, 1/7 (14) |
|
| Cimaz et al., 2003 [12] | 4 | 40 (35:5) | Variable classification criteria. Not specified | 10.7/12.4 | NA |
|
| Schuetz et al., 2010 [13] | 4 | 8 (7:1) | Not specified (diagnosis based on histological evidence of salivary gland involvement with or without positive autoantibodies) | 6.5 [0.5–12]/10.6 [6–15] |
|
|
| Kobayashi et al., 1996 [14] | 4 | 4 (4:0) | Not specified | 8.75 [7–10]/10.75 [10–12] |
|
|
| Tomiita et al., 2010 [15] | 3B | 5 (5:0) | Japanese SS diagnostic criteria (1999) | NA/13.6 [9–16] |
|
|
| Franklin et al., 1986 [16] | 4 | 5 (4:1) | At least two of the following three criteria: keratoconjunctivitis sicca, histological evidence of salivary gland involvement with SS and association with well-defined connective tissue disorder | NA/12.6 [5–17] |
|
|
| Saad-Magalhães et al., 2011 [17] | 4 | 8 (6:2) | AECG-2002 (only 3/8 patients fulfilled the criteria) | 5–13 years/NA | NA |
|
| Hamzaoui et al., 2010 [18] | 4 | 3 (3:0) | AECG 2002 | 15.66 [15–16]/15.66 [15–16] | NA |
|
| Yang et al., 2009 [19] | 4 | 4 (4:0) | Revised International Classification for SS (2002) | NA/9–17 years | NA |
|
| Hammett et al., 2020 [20] | 4 | 4 (4:0) | A combination of 2017 ACR/EULAR and expert opinion | 16/16 | N/A | Case 1: abnormal behaviour, tremors, insomnia, polyphagia, polyuria, and suicidal ideation |
| 16/12 | Case 2: 4 year history of severe anxiety, OCD, and tic disorder presented with an abrupt and severe worsening of anxiety, OCD and new auditory hallucinations | |||||
| 19/19 (adult- onset) | Case 3: progressively altered behaviour, incoherent speech, insomnia, headache, and tangential thoughts | |||||
| 17/17 | Case 4: new-onset suicidal ideation, paranoia, confusion and emotional lability | |||||
| Pessler et al., 2006 [21] | 5 | 2 (2:0) | Expert opinion | F, 0.7/10 | NA | Case 1: purpura, polyarthritis, uveitis, RTA, sialadenitis |
| F, 6/10 | NA | Case 2: sialadenitis, RTA/GN | ||||
| Tesher et al., 2019 [22] | 5 | 2 (1:1) | Revised International Classification for SS (2002) | F, 15/15 | NA | Case 1: MALT parotid gland |
| M, 15/15 | Case 2: MALT parotid gland, arthritis | |||||
| De Souza et al., 2012 [23] | 5 | 1 (1:0) | Revised International Classification for SS (2002) | F, 8/16 | NA | Dry eyes, dry mouth |
| Houghton et al., 2005 [24] | 5 | 2 (2:0) | Expert opinion | F, 14 | NA | Case 1: parotid swelling, dental caries, keratitis, xerostomia, LIP |
| F, 14 | Case 2: parotid swelling | |||||
| Berman et al., 1990 [25] | 5 | 1 (1:0) | Expert opinion with histological evidence (parotid biopsy) | F, 10 | NMOSD, hypothyroidism | NMOSD (presented with weakness, decreased sensation in right arm, headache, dizziness, vomiting and low-grade fever) |
| Kornitzer et al., 2016 [26] | 5 | 1 (1:0) | Expert opinion with histological evidence (salivary gland biopsy) | F, 6/9 | NMOSD | NMOSD (presented with fever, headache, progressive right-side weakness and altered mental status) |
| Ostuni et al., 1996 [27]; specific details only given for 2 of 10 patients | 4 | 10 (8:2) | Copenhagen criteria | 11(4–14)/14.6 (11–17) |
|
|
| Baszis et al., 2011 [28] | 4 | 4 (3:1) | Not specified | NA/12 [9–17] | NA |
|
| Gmuca et al., 2017 [29] | 5 | 2 (2:0) | Expert opinion with histological evidence (lip biopsy) | F, 11 | NMOSD | NMOSD (presented with optic neuritis), sicca symptoms |
| F, 13 | NMOSD (presented with optic neuritis) | |||||
| Flaitz et al., 2001 [30] | 5 | 1 (1:0) | Expert opinion with histological evidence (labial lip biopsy) | F, 11/14 | NA | Bilateral parotid swelling, dental problems |
| Nathavitharana et al., 1995 [31] | 5 | 1 (0:1) | Expert opinion with histological evidence (salivary gland biopsy) | M, 5 | NA | Tooth decay, fever, weight loss, bilateral parotid enlargement |
| Siamopoulou-Mavridou et al., 1989 [32] | 5 | 2 (1:1) | Expert opinion with histological evidence (labial salivary gland and lip biopsy, respectively) | M, 8/12 | NA | Recurrent parotid swelling enlargement, keratoconjunctivitis sicca |
| F, 3 | JRA | Arthritis, parotic gland enlargement, dry eyes and dry mouth | ||||
| Civilibal et al., 2007 [33] | 5 | 1 (1:0) | Expert opinion with histological evidence (salivary gland biopsy) | F, 9/13 | N/A | Recurrent bilateral swelling, arthralgia |
| Pessler et al., 2006 [34] | 5 | 1 (1:0) | Expert opinion with histological evidence (salivary gland biopsy) | F, 1/11 | RTA | Purpura, polyarthritis, uveitis, severe dental caries |
| De Oliveira et al., 2011 [35] | 5 | 1 (1:0) | American-European Consensus Group classification criteria for SS | F, 2.6 | NA | Xerostomia, xeropthalmia, bilateral parotic gland enlargement |
| Ohlsson et al., 2006 [36] | 5 | 1 (1:0) | Expert opinion | F, 8 | dRTA | Arthritis |
| Nikitakis et al., 2003 [37] | 5 | 1 (1:0) | Expert opinion with histological evidence (parotid biopsy and labial minor salivary glands biopsy) | F, 4 | NA | Bilateral parotid gland enlargement |
| Ohtsuka et al., 1995 [38] | 5 | 1 (1:0) | Japanese criteria (1980–85) | F, 9 | CNS manifestations | SS with CNS involvement (other symptoms included fever, nausea, xerostomia, parotid gland enlargement) |
| Zhang et al., 2007 [39] | 5 | 1 (1:0) | Expert opinion with histological evidence (minor salivary gland biopsy) | F, 6/9 | PHTN | Recurrent parotid enlargement, xerostomia, purpura, exertional dyspnoea |
| Skalova et al., 2008 [40] | 5 | 1 (1:0) | Expert opinion | F, 16 | dRTA | Rapid-onset muscle weakness, dysphagia, dysphonia, significant wasting |
| Moy et al., 2014 [41] | 5 | 1 (1:0) | Expert opinion with histological evidence (labial gland biopsy) | F, 9 | NA | Recurrent parotid swelling |
| Ladino et al. 2015 [42] | 5 | 1 (0:1) | Expert opinion with histological evidence (salivary gland biopsy) | M, 9/12 | NA | Arthralgia |
| Thouret et al. 2002 [43] | 5 | 1 (1:0) | Expert opinion with histological evidence (labial gland biopsy) | F, 9/13 | NA | Bilateral parotid swelling |
| Shahi et al., 2011 [44] | 5 | 1 (1:0) | Expert opinion with histological evidence (salivary minor gland biopsy) | F, 10 | NA | Recurrent arthralgia, foot swelling |
| Sardenberg et al., 2010 [45] | 5 | 1 (0:1) | Expert opinion | M, 10 | NA | Recurrent parotitis, xerostomia, dental caries |
| Bogdanovic et al., 2013 [46] | 5 | 1 (1:0) | Expert opinion with histological evidence (kidney biopsy) | F, 13 | TIN (manifested as dRTA) | Nephrocalcinosis (incidental finding), parotid gland swelling |
| Zhao et al., 2020 [47] | 5 | 1 (1:0) | 2012 ACR criteria | F, 12 | TIN | Arthritis, glucosuria |
| Aburiziza et al., 2020 [48] | 5 | 1 (1:0) | Expert opinion | F, 3/5 | NA | Bilateral parotid gland enlargement, severe teeth decay, painful micturition |
| Gottfried et al., 2011 [49] | 5 | 1 (1:0) | European Classification Criteria (1996) | F, 4/9 | CNS involvement | Bilateral conjunctival injection and ptosis, lip and cheek swelling, parotid gland enlargement, dry eyes and mouth |
| Fidalgo et al., 2016 [50] | 5 | 1 (1:0) | Expert opinion | F, 12 | JRA | Dry mouth, tooth sensitivity, dental pain, recurrent parotic gland enlargement |
| Majdoub et al., 2017 [51] | 5 | 1 (1:0) | Expert opinion with histological evidence (labial gland biopsy) | F, 4/7 | NA | Recurrent parotid gland swelling |
| Vermylen et al., 1985 [52] | 5 | 1 (1:0) | Expert opinion with histological evidence (parotid gland biopsy) | F, 2/2 | NA | Combination of parotid gland enlargement, hyperglobulinaemia and interstitial infiltrations of chest radiography suggestive of SS |
| Marino et al., 2017 [53] | 5 | 1 (0:1) | Expert opinion and evidence of cystic changes on parotid gland MRI | M, 2/1 | NMOSD | Left vision loss, right hemiparesis and lethargy associated with dry mouth |
AECG: American European Consensus Criteria; AIH: autoimmune hepatitis; JRA: juvenile RA; NA: not available.
Table 2.
Evidence of efficacy for the use of NSAIDs, corticosteroids, HCQ and topical treatments in SS with childhood onset
| Treatment | Reference | Acute symptoms/signs associated with SS targeted by treatment | Response | Background medications | Symptoms/signs targeted by background medications | Response |
|---|---|---|---|---|---|---|
| NSAIDs | Kobayashi et al., 1996 [14] | Secondary SS to mixed connective tissue disease, no details of symptoms targeted by treatment | No clear benefit or further details. Liver functions remained abnormal | NA | NA | NA |
| De Souza et al., 2012 [23] | Arthritis, parotitis | Controlled arthritis with no systemic evolution of SS after a 12 month follow-up. No further parotitis episodes | NA | NA | NA | |
| Oral corticosteroids | Schuetz et al., 2010 [13] | Sicca syndrome, fever, abdominal pain, parotid swelling | Good response | NA | NA | NA |
| Houghton et al., 2005 [24] | LIP | Clinical and radiographic improvement | HCQ | Not specified | Not specified | |
| Flaitz et al., 2001 [30] | Parotitis and fever | Cessation of pyrexia, decrease in size of parotid gland swelling and improved appetite. Two months after treatment, there was evidence of improvement in non-specific markers of inflammation but hypergammaglobulinemia persisted | NA | NA | NA | |
| Nathavitharana et al., 1995 [31] | Parotitis | Evidence of clinical improvement after 2 months of treatment | Not specified | Rampant caries | Not specified | |
| Saad-Magalhães et al., 2011 [17] | Recurrent orbital swelling | Prompt response | NA | NA | NA | |
| Yang et al., 2009 [19] | Kidney involvement | Relieved symptoms (non-specific) | NA | NA | NA | |
| Siamopoulou-Mavridou et al., 1989 [32] | Juvenile RA and SS | Evidence of clinical improvement after 2 months | Aspirin 90 mg/kg/day | Not specified | Not specified | |
| Civilibal et al., 2007 [33] | Parotid swelling, arthralgias, local oedema and purpura | Follow-up at 6 months: patient reported only one parotid swelling attack; arthralgias, local oedema and purpura disappeared completely | MTX 10 mg/m2/week | Same symptoms | Improvement, as mentioned | |
| Zhao et al., 2020 [47] | Tubular interstitial damage | Treatment with prednisone (5–10 mg/day) for half a year (for persistent renal glycosuria). At 1.5 years follow-up there was stable renal function | Celebrex (200 mg/day) and HCQ (100 mg/day) for the first week. HCQ (200 mg/day) and SSZ enteric-coated tablets (400 mg/day) for the next 6 months | Joint pain, increased ESR | Complete remission of joint pain, normal complete blood counts and ESR at 2 months follow-up | |
| Kobayashi et al., 1996 [14] | Primary SS. Presented with aseptic meningoencephalitis | Symptoms resolved and condition has been stable on low-dose prednisolone (5 mg/day) | Acetylsalicylic acid, diclofenac | High-grade fever, headache, nausea and skin rash | Symptoms resolved | |
| Methylprednisolone i.v. | Kobayashi et al., 1996 [14] | Primary SS complicated with overt dRTA | Good response to treatment. Patient’s condition and renal function have remained stable during 5 years of follow-up | CYC, sodium citrate | Same symptoms | Good overall response |
| Houghton et al., 2005 [24] | LIP | Clinical and radiographic improvement | 3 daily pulses of i.v. methylprednisolone (1 g/day) followed by prednisone (1 mg/kg/day), additional HCQ | Same symptoms | Clinical and radiographic improvement | |
| Ohtsuka et al., 1995 [38] | CNS involvement: hemiparesis, diffuse swelling of the cervical cord and increased signal intensity on MRI | Resolution of symptoms occurred progressively after i.v. methylprednisolone | Corticosteroids for 28 days; prednisolone (2 mg/kg/day then tapered to 0.2 mg/kg/day), followed by i.v. methylprednisolone 30 mg/kg/day for 3 days | Same symptoms | Four months after being discharged from hospital, patient developed nausea, headache and new-onset left hemiparesis despite being on prednisolone (0.2 mg/kg/day), requiring i.v. methylprednisolone | |
| Gottfried et al., 2011 [49] | Orofacial swelling, facial nerve palsy or stroke-like symptoms | Rapid improvement of diplopia, disequilibrium and ataxia, less prominent ptosis while facial diplegia remain unchanged after i.v. methylprednisolone therapy | Oral prednisolone (2 mg/kg/day) then slowly tapered over the next 3 months following i.v. methylprednisolone for 5 days | Same symptoms | MRI showed full resolution of midbrain lesion at a the 6 month follow-up. Patient continued to improve with full conjugate extraocular movements, minimal ptosis and stable facial diplegia | |
| HCQ | Schuetz et al., 2010 [13] | Not specified | 2/3 (66.6%) clinically stable, 1/3 (33.3%) not specified (patient later diagnosed with SS with overlapping SLE and started on AZA) |
1/3 steroids 1/3 NSAIDs |
Arthritis and “skin eruption,” asthenia, fever, arthritis of toes and forefeet | Good response. Controlled symptoms for 1 year until development of asthenia and jaundice—diagnosed with AIH with underlying diagnosis of SS with overlapping SLE. Responded partially to NSAIDs |
| Moy et al. 2014 [41] | Parotitis | Patient still had recurrent bilateral/unilateral parotid swelling in the subsequent 3 years despite HCQ therapy | Antibiotics | Episodes of parotitis lasting 1 week were treated with antibiotics | Still recurrent symptoms | |
| Hamzaoui et al., 2010 [18] | Inflammatory arthralgia | Good | NA | NA | NA | |
| Ladino et al., 2015 [42] | Joint pain and fatigue | Prednisone and HCQ associated with good response in terms of joint pain and fatigue | Prednisolone (7.5 mg/day), artificial tears, oral mucolytic | Eye dryness, xerostomia | Artificial tears associated with benefit for eye dryness, oral mucolytic treatment beneficial for xerostomia | |
| Thouret et al., 2002 [43] | Parotid swelling | Clinical improvement of bilateral parotid swelling, although no impact on serological markers | NA | NA | NA | |
| Shahi et al., 2011 [44] | Recurrent arthralgia | Stable clinical features and laboratory values at 6 months follow-up. No mention of response to HCQ therapy | A | N/A | NA | |
| Majdoub et al., 2017 [51] | Parotid swelling | HCQ was effective in preventing parotid swelling (at 2 year follow-up, no flares were reported since starting HCQ) | Artificial tears | Dry eyes | Effective | |
| Treatments for dryness-related symptoms | ||||||
| Pilocarpine | Tomiita et al., 2010 [15] | Xerostomia | Improved in 5/5 (100%) patients. Specified as ‘improved’ in 1/5 (20%), ‘slightly improved’ in 4/5 (80%) | NA | NA | NA |
| De Souza et al., 2012 [23] | Dryness | Adequate control of SS symptoms | NA | NA | NA | |
| Bromhexine | Hamzaoui et al., 2010 [18] | Dryness | Not specified | NA | NA | NA |
| Artificial tears | Hamzaoui et al., 2010 [18] | Eye dryness | Not specified | NA | NA | NA |
| Oral balance gel | Nikitakis et al., 2003 [37] | Xerostomia | No new cavities at 10 months follow-up | NA | No systemic symptoms | Stable clinical features and laboratory values with no evidence of connective tissue disease |
| Plaque control, diet modification, regular fluoride application, restorative treatment | Sardenberg et al., 2010 [45] | Xerostomia and dental problems | No complications or new carious lesions at 2 year follow-up | NA | NA | NA |
| Oral hygiene instructions, vulvar moisturizer, 1% hydrocortisone cream for intermittent use | Aburiziza et al. 2020 [48] | Dental problems, vulvar dryness | Patient continued to have new dental caries. Vulvar itchiness and irritation became a prominent clinical problem 2 years after presentation | Short course of oral prednisolone given once with antibiotics | Parotitis | Resolved |
| Artificial saliva, dental treatment | Fidalgo et al., 2016 [50] | Dry mouth, tooth sensibility and dental pain | Artificial saliva: improved hydration of the tissues of the oral cavity, in particular the oral mucosa. Successful endodontic treatment and dental restorations | Corticoid therapy | Additional diagnosis of RA, parotitis | No details |
AIH: autoimmune hepatitis; NA: not available.
Table 3.
Evidence of efficacy for the use of conventional and biologic DMARDs in SS with childhood onset
| Treatment | Reference | Acute symptoms/signs associated with SS targeted by treatment | Response | Background medications | Symptoms/signs targeted by background medications | Response |
|---|---|---|---|---|---|---|
| MTX | Hamzaoui et al., 2010 [18] | Arthritis | Excellent response. Stopped following diagnosis of SS and maintained on low-dose corticosteroids | Low-dose corticosteroids | Uveitis, maintenance (following MTX) | Good control. Clinically stable |
| De Oliveira et al. 2011 [35] | Myalgia and arthralgia (very low dose 2.5 mg weekly associated with oral methylprednisolone) | No mention of treatment response for methylprednisolone and MTX | Oral methylprednisolone 9 mg every 48 h. 1% neutral sodium fluoride every 3 months since diagnosis | Myalgia and arthralgia, oral dryness | At 6 year follow-up, patient has well-controlled oral health | |
| Ohlsson et al., 2006 [36] | Arthritis, dRTA | No details provided | HCQ. No further information on treatments | NA | NA | |
| Civilibal et al., 2007 [33] | Severe arthralgia | Symptoms resolved at 6 month follow-up | Methylprednisolone (1 mg/kg/day) | Local oedema and purpura, bilateral parotid swelling | 6 months after discharge the patient had only one episode of parotid swelling; local oedema and purpura disappeared completely | |
| MMF | Pessler et al., 2006 [21] | Primary SS complicated with overt dRTA | No mention of treatment response | Electrolyte supplementation | Same manifestations | Not available |
| CYC | Berman et al., 1990 [25] | Optic neuropathy and CNS involvement associated with primary SS | Visual acuity improved. Patient stable with no new cerebral infarcts | Oral corticosteroids followed by i.v. steroids in combination with i.v. CYC therapy | Same manifestations | As mentioned |
| Gmuca et al., 2017 [29] | NMOSD | No mention of treatment response in case 1. Visual symptoms worsened in case 2 following treatment and thus the patient received apheresis and RTX | Case 1: i.v. methylprednisolone, RTX in association with CYC, apheresis | Same manifestations | As mentioned | |
| Case 2: HCQ, i.v. methylprednisolone, CYC, switched to mycophenolate as maintenance | ||||||
| Zhang et al., 2007 [39] | SS associated with pulmonary hypertension | At 1 month follow-up, exertional dyspnoea improved dramatically (assessed by walk test). Patient remained stable after prednisolone was tapered and diltiazem was stopped | Prednisolone (0.5 mg/kg/day then gradually tapered), diltiazem, anticoagulant therapy | Same manifestations | As mentioned | |
| Kobayashi et al., 1996 [14] | SS secondary to SLE, membranous and mesangial glomerulonephritis (lupus nephritis class 2, 3) and interstitial nephritis | Good response to treatment; 24 h urinary excretion of protein decreased. Patient’s condition and renal function remained stable during 7 years of follow-up | Methylprednisolone orally followed by prednisolone orally | Initially presented with arthralgia, RP, sicca symptoms, photophobia, facial rash and recurrent parotitis | Good response. Sicca symptoms resolved without using artificial tear or saliva | |
| Ciclosporin | Skalova et al., 2008 [40] | SS associated with hypokalaemia paralysis | Good response | Methylprednisolone (4 mg every other day), potassium chloride (2.5 g/day), Shohl’s solution (9 ml twice daily) | Same manifestations | As mentioned |
| AZA | Bogdanovic et al., 2013 [46] | dRTA/TIN | Significant improvement at 6 months follow-up | Potassium citrate (for dRTA), prednisone (1 mg/kg/day) for 6 months then tapered to 0.5–0.25 mg/kg/day (for TIN). After 3.5 years, MMF replaced AZA for several months | Same manifestations | At 6 years follow-up there was no evidence of xerostomia, xerophthalmia or any other SS-related symptoms |
| Singer et al., 2008 [11] | SS overlapping with SLE with autoimmune hepatitis | Improvement | HCQ | Not specified | Not specified | |
| Biologic treatments | ||||||
| IVIG | Hamzaoui et al., 2010 [18] | Hepatitis, myositis, pericarditis, oral dryness | Clinically stable | Corticosteroids (short course) | Not specified | Not specified |
| Etanercept | Pessler et al., 2006 [21] | Arthritis | At the 4 year follow-up, arthritis responded well to etanercept (disappearance of tender and swollen joints) | HCQ (200 mg daily), MTX (25 mg s.c. weekly) | Renal tubular dysfunction | Normal urinalyses and serum creatinine levels but unchanged renal tubular dysfunction (evidenced by stable requirements for oral sodium citrate (3 mEq/kg/24 h), potassium (3 mEq/kg/24 h) and phosphate supplementation) |
| Infliximab switched to etanercept because of loss of response | Pessler et al., 2006 [34] (likely the same case as reported in the paper above) | Chronic polyarthritis | Initial good response to infliximab, loss of response after 7 months despite dose increase and 3 weeks of infliximab administration. Good response to etanercept after 18 months | NSAIDs, corticosteroids, MTX (0.5 mg/kg once weekly s.c.) and topical steroid eye drops for presumed JRA with uveitis | Xerostomia, uveitis, optic neuritis, RTA | Systemic symptoms developed during treatment with infliximab and not influenced by subsequent treatment with etanercept |
| Rituximab | Tesher et al., 2019 [22] | MALT lymphoma | Both patients achieved remission of MALT lymphoma, with one case having no recurrence of symptoms associated with SS at the 2 year follow-up | Case 1: additional pulsed 1 g i.v. methylprednisolone, HCQ daily | Medication mainly targeted at MALT | As mentioned |
| Case 2: parotidectomy; bendamustine after a course of RTX (due to anaphylaxis to RTX) followed by HCQ monotherapy | ||||||
| Kornitzer et al., 2016 [26] | NMOSD | Clinically improved but not clear if this was related to RTX treatment. Only residual subtle right-sided weakness and mild abducens and facial nerve weakness on examination 3 years after presentation | NA | NA | NA | |
| Hammett et al., 2020 [20] | Psychosis | Psychiatric symptoms improved with RTX infusions in all four patients (at 4–6 month intervals). One patient allergic to RTX was switched to obinutuzumab with maintained benefit | Case 1: pulse methylprednisolone 1000 mg daily for 3 days followed by a prednisone taper over 24 weeks, olanzapine | Various psychiatric manifestations including: insomnia; increase in hallucinations, tics and anxiety after starting an oral contraceptive; catatonia; suicidal ideation; fluctuating coherence; delusions; slow psychomotor responses and echolalia, echopraxia and posturing | All patients improved and were able to go back to a normal life | |
| Case 2: aripiprazole and obinutuzumab, as the patient developed an allergic reaction to RTX | ||||||
| Case 3: MMF 1500 mg twice a day, oral prednisone 2.5 mg/day, risperidone along with benztropine and clonazepam | ||||||
| Case 4: pulse methylprednisolone for 3 days followed by oral prednisone taper, HCQ 200 mg daily | ||||||
| Tocilizumab | Marino et al., 2017 [53] | NMOSD | Neurological manifestations: left vision loss, right hemiparesis and lethargy not well controlled by RTX and i.v. methylprednisolone | No concomitant medication. Previous treatment with CYC and RTX followed by MMF. Despite complete depletion of CD19+ B lymphocytes, the patient continued flaring | Same manifestations | Clinical remission |
AIH: autoimmune hepatitis; NA: not available.
Quality assessment
To assess the quality of studies, we used the Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence (cebm.net) (Table 1). The assessment bias was evaluated as high, as most of the studies identified were small case series and case reports, with only a very few cohort studies [11, 12].
Results
Study selection
An algorithm detailing the number of studies included and excluded, with reasons for exclusions, is included in Fig. 1. In total, 43 studies were identified as eligible. Cohen’s κ coefficient for interrater agreement was 0.87 (95% agreement). Our research did not identify any interventional studies.
The papers retrieved included between 1 and 40 patients and 88% (120/127) of cases reported were girls. The patient age distribution across all eligible papers was 2.6–17 years; however, the youngest patient’s age at the onset of SS symptoms was 5 months (Table 1).
Below, we present the main findings related to various therapeutic strategies used in the management of SS with childhood onset.
Evidence for use of NSAIDs
A small proportion of children with SS were prescribed NSAIDs [10% (12/118)]. From the data available for five patients, we learned that the mean age at SS diagnosis was 11 years (range 6–17) and 80% (4/5) were females. Of the 12 cases, 2 children had an overlapping diagnosis of juvenile RA (JRA) [16, 23] and another child had a diagnosis of aseptic meningoencephalitis with an old intracranial haemorrhage [14]. The main clinical indications were arthritis, asthenia and fever [13, 23, 32], and there was evidence of clinical benefit.
Evidence for use of corticosteroids in various preparations
Corticosteroids, irrespective of the dose and route of administration, were prescribed to 52% (72/137) of children with SS. From the available data, children treated with steroids had a mean age of 8.5 years at diagnosis (with an age range between 2 and 19 years) and 88% were females (38/43).
Only a small proportion of children [15% (11/72)] received i.v. methylprednisolone in combination with oral prednisone/prednisolone for various clinical indications, such as lymphocytic interstitial pneumonia (LIP) [24], central nervous system (CNS) involvement associated or not with ocular neurological manifestations [25, 29, 38, 49], SS presenting with psychiatric symptoms [20] and mucosa-associated lymphoid tissue (MALT) lymphoma [22], with overall benefit (Table 2). One patient with SS with distal renal tubular acidosis (dRTA) was treated with i.v. methylprednisolone 100 mg/day for 3 days, followed by maintenance therapy of oral methylprednisolone 4 mg every other day in combination with ciclosporin A (Table 2) [40]. Other clinical indications for oral methylprednisolone use were recurrent parotitis [52] and parotid swelling and arthralgia in combination with MTX [33] (Tables 1 and 2).
A total of 27 children (37.5%) were prescribed oral steroid treatment for JRA [16, 30, 32], tubulointerstitial nephritis (TIN) [14, 21, 46, 47], SLE [16], aseptic meningoencephalitis [14], severe isolated pulmonary hypertension (PH) [39], dRTA [46], mesangial glomerulonephritis [27], parotitis [11, 28, 30, 31, 48] or orbital swelling [17] (Tables 2 and 3).
The response to oral steroid treatment was only described in 14/27 of these patients and all reported clinical improvement, which is difficult to attribute to steroids alone, as some patients were treated with additional DMARDs (Tables 2 and 3).
Evidence for use of conventional DMARDS
Based on the available data, 85/137 (62%) children with SS were treated with DMARDs (conventional and biologic).
HCQ
HCQ treatment was prescribed in 34% of children with SS (46/137) (Table 2); 68% were girls and had a mean age at diagnosis of 13 years (range 7–17). Some children had overlapping phenotypes, including SLE and JIA (Table 2).
The most frequent clinical indications for HCQ were unilateral or bilateral parotid swelling in 15% (7/46) of children [28, 41, 43, 51], arthralgia [4.3% (2/46)] [18, 44], renal involvement [4.3% (2/46)] [27] and a combination of arthralgia and fatigue [2% (1/46)] [42] (Table 2). HCQ was also prescribed as maintenance therapy for SS presenting with psychiatric symptoms [2% (1/46)] (Table 3). In a large proportion of reports [72% of patients (33/46)] the symptoms/signs targeted by HCQ treatment were not mentioned. The doses prescribed varied from 200 to 400 mg/day.
The response to HCQ treatment was favourable in 39% (18/46) of children (Table 2). Because HCQ was used in combination therapy, either with Celebrex, SSZ, prednisone, naproxen, AZA, rituximab (RTX) or bendamustine, it is difficult to attribute the clinical improvement to treatment with HCQ alone. The reports also identified a lack of improvement or side effects from HCQ in 15% (7/46) of children with SS (e.g. worsened visual symptoms [29], hypersensitivity [11], recurrence of parotitis [28] or a lack of clinical benefit [11, 41]). However, data on HCQ efficacy were lacking in 32% (15/46) of children.
MTX
MTX was prescribed in 5.8% (8/137) of children with SS. The mean age at SS diagnosis was 10 years (range 2–17) with an equal distribution of sexes (4 females, 4 males).
Among this small number of children treated with MTX, 25% (2/8) had an overlapping diagnosis of JIA [11, 13] and 25% (2/8) presented with RTA at disease onset [21, 36]. The main clinical indications were arthralgia and purpura [33, 35], polyarthritis [21] and JIA [11]. The weekly MTX dose varied from 2.5 mg (age 2 years 7 months) [35] to 10 mg/m2 (age 13 years) [33] to 25 mg once a week (age 10 years) [21]. Where reported (4/8 patients), MTX was associated with clinical benefit.
AZA
Only a very small proportion of children with SS [2% (3/137)] were prescribed AZA. All three patients were female with an average age at SS diagnosis of 15 years [13, 14, 16, 23, 32]. The clinical indications were overlapping JIA and SLE with autoimmune hepatitis phenotypes [11, 13] as well as TIN, in which case AZA was used as the initial therapy, followed by MMF after a loss of efficacy [46].
CYC
Nine girls with a mean age at SS diagnosis of 11.5 years [9–14, 16, 23, 32] were treated with CYC for a diagnosis of TIN [14, 21], neuromyelitis optica spectrum disorder (NMOSD) [29], isolated PH [39], SS presenting with psychiatric symptoms [20] or CNS involvement associated with thyroiditis [25]. The most detailed case was that of a 9-year-old girl with symptoms onset at age 6 years, who experienced recurrent parotid enlargement, xerostomia, purpura and exertional dyspnoea and was eventually diagnosed as severe isolated PH associated with SS based on clinical manifestations, hyperglobulinemia, positive ANA, SSA and SSB and an abnormal Schirmer test (Table 3). The patient received CYC at a dose of 100 mg every other day alongside prednisolone, diltiazem and anticoagulant therapy with significant improvement [39].
MMF
MMF was used to treat four patients with SS and an additional diagnosis of NMOSD [29] or TIN [46] or as maintenance therapy for severe renal involvement evident on biopsy [21] (Table 3). MMF was also given as maintenance therapy in combination with RTX for one patient with SS manifesting with psychiatric symptoms, with good response [20].
Ciclosporin A
Ciclosporin A was given to three patients but a favourable response to treatment was only documented in one case report of a 16-year-old female with a diagnosis of SS with dRTA [40].
SSZ
One female patient, age 12 years, was prescribed SSZ 400 mg/day in combination with HCQ for arthritis associated with SS and achieved remission of joint pain after 2 months of treatment [47].
Evidence for use of biologic DMARDs
Biologic treatments such as RTX and etanercept or infliximab were prescribed in a minority of patients. Of the nine patients that were prescribed RTX, two patients had a diagnosis of MALT lymphoma [22], three patients had NMOSD [26, 29] and four adolescent patients had SS presenting with severe psychiatric symptoms [20].
One patient (female, age 15 years) who was diagnosed with MALT lymphoma achieved remission of >3 years following four 375 mg/m2 once-weekly doses of RTX, alongside pulsed i.v. methylprednisolone and regular HCQ at a dose of 200 mg/day [22], while another patient reported an anaphylactic reaction with the second RTX infusion [22] (Table 3). Both patients (a boy and a girl) had parotid gland involvement and were diagnosed at age 15 years. The boy had additional features of arthritis and was treated with parotidectomy and bendamustine after a course with RTX associated with anaphylaxis; he was discharged on HCQ monotherapy. The girl received additional treatment with i.v. methylprednisolone and HCQ daily. Both patients achieved remission of MALT lymphoma (Table 3).
There is no mention of response to RTX in two cases of NMOSD [29], while good clinical outcome was reported in the third case [26] (Table 3). Of the four patients who were given RTX for SS with psychiatric involvement, three patients experienced significant improvement in symptoms and were able to be weaned off antipsychotics [20]. RTX was discontinued in the remaining patient due to the development of RTX-induced serum sickness and obinutuzumab was commenced as an alternative, with good response [20].
Treatment with etanercept was initiated in one child with SS and juvenile arthritis was reported extensively in the literature [11, 21, 34] and was associated with clinical benefit (Table 3).
Other immunomodulatory therapies
Intravenous immunoglobulin (IVIG) was successfully prescribed for indications such as hepatitis, myositis, pericarditis and oral dryness in one patient [13] and was also given in combination with steroids and MTX to treat CNS manifestations in another patient with good clinical outcomes [11].
There was also evidence of therapeutic success with tocilizumab in a boy with neurological manifestations compatible with NMOSD (left vision loss, right hemiparesis and lethargy) who was subsequently diagnosed with SS at age 14 years based on a combination of dry mouth, positive serology and the presence of parotid cysts on MRI [53]. Treatment with tocilizumab was initiated after four courses of RTX and i.v. methylprednisolone over 2 years that were not successful in controlling neurological relapses. Treatment with tocilizumab was associated with no further relapses after 3 years.
Evidence for use of topical treatments and other therapies for oral dryness
A total of 17 of 137 children (12.4%) were prescribed treatments for their oral sicca symptoms. Their mean age at SS diagnosis was 11.5 years [2–14, 16, 23] and 76% (13/17) were females.
A significant proportion of children [35% (6/17)] were prescribed oral pilocarpine as treatment for their oral sicca symptoms [15, 23]. There was evidence of clinical benefit in all patients.
Other treatments were also prescribed for oral symptoms or as prophylactic treatment against dental caries, ranging from bromhexine [18], sodium fluoride topical treatment for enamel protection [35, 45], fluoride varnish for dental caries [31], artificial saliva [37, 50] and oral mucolytic treatment [42], with evidence of overall benefit, with the exception of oral bromhexine, for which the outcome of treatment was not specified [18].
Evidence for use of topical treatments and other therapies for ocular symptoms
A total of 19% (26/137) of children were prescribed treatments for their ocular sicca symptoms. Data about the patients’ ages were retrievable in 81% (21/26) of children: the mean age at SS diagnosis was 13 years (range 7–17) and 77% (20/26) were females. The most used therapeutic interventions for eye dryness were humidifier eye drops [17], artificial tears [18, 22, 27, 28, 32, 42, 51] and topical lubricants [25, 28], however, the response to treatment was not specified in the majority of cases.
Conclusion
This methodologically robust systematic review of the literature demonstrates that there is currently no standardized treatment regime for childhood-onset SS and that the therapeutic decisions are based on clinicians’ expertise and preference and very likely derived from adult SS studies. In addition, there are no validated disease activity outcome measures for use in children with SS, thus the response to treatment was assessed based entirely on clinician opinion.
The British Society for Rheumatology (BSR) guidelines for the management of adults with SS [54] recommend a few general strategies for treatment, including conservation of oral and ocular secretions and replacement of tears and saliva, therapies for stimulating oral and ocular secretions (including pilocarpine, cevimeline and nizatidine) and treatment of severe ocular complications (topical ciclosporin) and glandular swelling (short courses of oral steroids or i.m. depomedrone). Corticosteroids, including pulse therapy, are recommended for lung, haematological, renal and neurological manifestations in combination with other DMARDs, while low-dose oral prednisolone (5–7.5 mg/day) could provide a modest benefit for sicca symptoms.
HCQ is recommended as the first-line treatment in adults with systemic disease manifestations, specifically skin disease, joint disease or fatigue [54]; MTX for inflammatory arthritis; AZA for lung disease, myelopathy and cytopaenia; and MMF for lung disease and cytopaenia, while CYC may be considered in patients with organ-threatening systemic complications such as CNS, renal or lung disease. The BSR guidelines recommend the use of RTX in patients with systemic disease manifestations refractory to other immunosuppressant agents and in those with particular manifestations such as lymphoma, immune thrombocytopenia, vasculitic neuropathy or cryoglobulinaemia [54]. There is no current evidence to suggest RTX efficacy in the treatment of sicca symptoms and non-specific symptoms including fatigue [55].
The most frequently used treatments in children with SS were corticosteroids, HCQ and NSAIDs, with conventional and biologic DMARDs being reserved for selected cases. From the 43 studies that were included in this systematic literature review, a few general therapeutic trends emerged.
HCQ was predominantly used to treat parotid swelling and as a background treatment for other manifestations such as fatigue, LIP and MALT lymphoma, while inflammatory arthritis and arthralgia were frequently treated with NSAIDs, MTX or anti-TNF blockade, especially in the case of patients with overlapping phenotypes with juvenile arthritis.
Corticosteroids were frequently given in children with SS, particularly in those with systemic flares and severe disease presentations. Methylprednisolone i.v. was often prescribed as pulse therapy alongside other immunosuppressive agents to treat children with acute clinical deterioration and severe disease phenotypes, which included CNS involvement, MALT lymphoma and severe renal disease. Oral prednisolone was also used in the treatment of parotitis with good clinical response. Biologic treatments such as RTX were only reserved for children with MALT lymphoma and NMOSD, while etanercept was only given to children with various types of juvenile arthritis.
Treatment with RTX and CYC was reserved for severe cases of SS with childhood onset, with CNS involvement, MALT lymphoma, severe renal disease and PH. The clinical observations derived from this systematic review conclude that the paediatric practice aligns with the current BSR guidance for treatment of adults with SS, where RTX and CYC are reserved for patients with organ-threatening systemic complications [54].
Regarding the efficacy of various treatments used in SS with childhood onset, most reports described good or stable outcomes following various expert clinician treatment choices, except for a handful of cases that described no clinical improvement or adverse effects to treatment. This may be explained by the well-recognized effect of publication bias, as clinicians reporting therapeutic success are more likely to publish their observations. Treatment with MTX, AZA, ciclosporin A and NSAIDs predominantly reported stable outcomes and improvement of symptoms overall.
It is important to note that a large proportion of treatments were given as combination therapy and therefore it is difficult to attribute the efficacy of a certain treatment Some children also had overlapping clinical phenotypes that could have driven the treatment decisions.
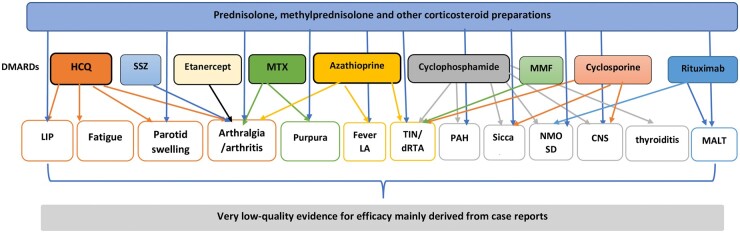
We summarize in Fig. 2 the main treatment choices and clinical indications for which low-quality evidence for efficacy was available. In addition, there was also an abundance of missing information from some of the case reports, including treatment dose, clinical indication for starting treatment, symptoms targeted by treatment and clinical benefit. Another limitation of case reports and case series is the lack of representability, precluding any assumption of therapeutic efficacy based on individual cases. Due to the rarity and heterogeneity in presentation of SS in children, there are currently no RCTs reported in the literature to assess the evidence for efficacy of any of the treatments available.
Fig. 2.
Treatments associated with low evidence of efficacy in SS with childhood onset and their clinical indications
LAD: lymphadenopathy.
The poor quality of the literature data extracted by this systematic review is one of the major limitations of this report, as no reliable conclusion regarding the efficacy of available therapies for SS with childhood onset can be drawn.
In conclusion, this systematic literature review demonstrates the high heterogeneity in presentation of SS in children and adolescents as well as in the use of various therapies and combinations of treatments to address their clinical manifestations.
Based on available evidence and evidence from adult disease, we can recommend the use or oral NSAIDs and corticosteroids for less severe manifestations, such as rashes, arthralgia, as first-line therapy for arthritis and for recurrent parotitis, as there is evidence of some benefit. In addition, steroid treatment was effective in treating renal disease [19, 47] or neurological manifestations [14, 38, 49] without additional DMARD therapy in some selected cases. Treatment with HCQ is recommended in patients with persistent arthralgia, myalgia and recurrent parotitis, while the use of MTX and anti-TNF blockade could be beneficial in children with persistent inflammatory arthritis. Some of the severe disease manifestations affecting kidneys, lungs and CNS are likely to benefit from pulse corticosteroid followed by gradually tapered oral therapy, in addition to stronger immunosuppressive treatments such as CYC, MMF, AZA and RTX. RTX seems to be effective in controlling psychiatric symptoms associated with SS in children and adolescents. The use of topical treatments for dryness is also recommended for symptomatic relief.
At present, there are no good-quality studies in SS with childhood onset to enable any clinical recommendations for strict selection of therapies. As the disease is rare in children compared with adults, further research into establishing validated classification criteria and disease outcome measures tailored for young people with SS is required.
In conclusion, we recommend clinicians have a higher level of suspicion for a potential diagnosis of SS in children and adolescents presenting with recurrent parotid swelling and heterogeneous clinical manifestations not explained by an alternative diagnosis at any age. Pursuing a tissue biopsy to guide the diagnosis or performing extensive SS-specific investigations is required in many cases because of the lack of validated diagnostic or classification criteria in children. Clinicians have a reasonably large therapeutic armamentarium to choose from based on patients’ clinical presentation and evolution. The severe organ involvement associated with SS is likely to respond to a combination of strong immunosuppression and high steroid doses. Future research is required to establish the long-term outcomes of children with SS into adulthood. Children should also be included in adult RCTs to investigate new therapeutic strategies before good evidence-based treatment recommendations for this patient population can be made.
Funding: This research was funded by the National Institute of Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre (BRC525/III/CC/191350, BRC 773III/CC/101350) and Lupus UK. This work was performed within the Centre for Adolescent Rheumatology Versus Arthritis at UCLH and Great Ormond Street Hospital supported by grants from Versus Arthritis (21593 and 20164). The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
Data extraction files are available upon request.
References
- 1. Bowman SJ. Primary Sjögren’s syndrome. Lupus 2018;27:32–5. [DOI] [PubMed] [Google Scholar]
- 2. Virdee S, Greenan-Barrett J, Ciurtin C.. A systematic review of primary Sjögren’s syndrome in male and paediatric populations. Clin Rheumatol 2017;36:2225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ciurtin C, Cho Y, Al-Obaidi M, EC J, Price EJ.. Barriers to translational research in Sjögren’s syndrome with childhood onset: challenges of recognising and diagnosing an orphan rheumatic disease. Lancet Rheumatol 2021;3:e138–48. [DOI] [PubMed] [Google Scholar]
- 4. Ramos-Casals M, Acar-Denizli N, Vissink A. et al. Childhood-onset of primary Sjogren’s syndrome: phenotypic characterization at diagnosis of 158 children. Rheumatology (Oxford) 2021;60:4558--67. [DOI] [PubMed] [Google Scholar]
- 5. Hammenfors DS, Valim V, Bica Berg Pasoto SG. et al. Juvenile Sjögren’s syndrome: clinical characteristics with focus on salivary gland ultrasonography. Arthritis Care Res 2020;72:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tucker L, Ciurtin C.. Sjögren’s syndrome and immunoglobulin-G4 disease. In: Petty RE, Laxer RA, Lindsley CB. et al. (eds.). Textbook of pediatric rheumatology. 8th ed.Amsterdam: Elsevier, 2020:417–28. [Google Scholar]
- 7. Tsuboi H, Honda F, Takahashi H. et al. Pathogenesis of IgG4-related disease. Comparison with Sjögren’s syndrome. Mod Rheumatol 2020;30:7–16. [DOI] [PubMed] [Google Scholar]
- 8. Basiaga ML, Stern SM, Mehta JJ. et al. Childhood Sjögren syndrome: features of an international cohort and application of the 2016 ACR/EULAR classification criteria. Rheumatology 2021;60:3144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bartůňková J, Šedivá A, Vencovský J, Tesař V.. Primary Sjogren’s syndrome in children and adolescents: proposal for diagnostic criteria. Clin Exp Rheumatol 1999;17:381–6. [PubMed] [Google Scholar]
- 10. Weisman MH. Sjogren’s disease. Rheum Dis Clin N Am 2016;42:xi–xii. [DOI] [PubMed] [Google Scholar]
- 11. Singer NG, Tomanova-Soltys I, Lowe R.. Sjögren’s syndrome in childhood. Curr Rheumatol Rep 2008;10:147–55. [DOI] [PubMed] [Google Scholar]
- 12. Cimaz R, Casadei A, Rose C. et al. Primary Sjogren syndrome in the paediatric age: a multicentre survey. Eur J Pediatr 2003;162:661–5. [DOI] [PubMed] [Google Scholar]
- 13. Schuetz C, Prieur AM, Quartier P.. Sicca syndrome and salivary gland infiltration in children with autoimmune disorders: when can we diagnose Sjögren’s syndrome? Clin Exp Rheumatol 2010;28:434–9. [PubMed] [Google Scholar]
- 14. Kobayashi I, Furuta H, Tame A. et al. Complications of childhood Sjogren syndrome. Eur J Pediatr 1996;155:890–4. [DOI] [PubMed] [Google Scholar]
- 15. Tomiita M, Takei S, Kuwada N. et al. Efficacy and safety of orally administered pilocarpine hydrochloride for patients with juvenile-onset Sjögren’s syndrome. Mod Rheumatol 2010;20:486–90. [DOI] [PubMed] [Google Scholar]
- 16. Franklin DJ, Smith RJ, Person DA, Pflugfelder SC.. Sjögren’s syndrome in children. Otolaryngol Head Neck Surg 1986;94:230–5. [DOI] [PubMed] [Google Scholar]
- 17. Saad-Magalhães C, De Souza Medeiros PB, de Oliveira Sato J, Custódio-Domingues MA.. Clinical presentation and salivary gland histopathology of paediatric primary Sjögren’s syndrome. Clin Exp Rheumatol 2011;29:589–93. [PubMed] [Google Scholar]
- 18. Hamzaoui A, Harzallah O, Attia S. et al. Le syndrome de Goujerot-Sjogren juvénile: à propos de 3 cas. Archives de Pediatrie 2010;17:1531–34. [DOI] [PubMed] [Google Scholar]
- 19. Yang L, Wang L, Peng S. [Primary Sjogren’s syndrome in children: a case report of 4 case]. Zhongguo Dang Dai Er Ke Za Zhi 2009;11:233–4. [PubMed] [Google Scholar]
- 20. Hammett EK, Fernandez-Carbonell C, Crayne C. et al. Adolescent Sjogren’s syndrome presenting as psychosis: a case series. Pediatr Rheumatol 2020;18:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pessler F, Emery H, Dai L. et al. The spectrum of renal tubular acidosis in paediatric Sjögren syndrome. Rheumatology 2006;45:85–91. [DOI] [PubMed] [Google Scholar]
- 22. Tesher MS, Esteban Y, Henderson TO, Villanueva G, Onel KB.. Mucosal-associated lymphoid tissue (MALT) lymphoma in association with pediatric primary Sjogren syndrome: 2 cases and review. J Pediatr Hematol Oncol 2019;41:413–6. [DOI] [PubMed] [Google Scholar]
- 23. De Souza TR, Silva IHM, Carvalho AT. et al. Juvenile Sjögren syndrome: distinctive age, unique findings. Pediatr Dent 2012;34:427–30. [PubMed] [Google Scholar]
- 24. Houghton KM, Cabral DA, Petty RE, Tucker LB.. Primary Sjögren’s syndrome in dizygotic adolescent twins: one case with lymphocytic interstitial pneumonia. J Rheumatol 2005;32:1603–6. [PubMed] [Google Scholar]
- 25. Berman JL, Kashii S, Trachtman MS, Burde RM.. Optic neuropathy and central nervous system disease secondary to Sjögren’s syndrome in a child. Ophthalmology 1990;97:1606–9. [DOI] [PubMed] [Google Scholar]
- 26. Kornitzer V, Kimura Y, Ginger J.. Primary Sjögren syndrome in a child with a neuromyelitis optica spectrum disorder. J Rheumatol 2016;43:1260–1. [DOI] [PubMed] [Google Scholar]
- 27. Ostuni PA, Ianniello A, Sfriso P. et al. Juvenile onset of primary Sjögren’s syndrome: report of 10 cases. Clin Exp Rheumatol 1996;14:689–93. [PubMed] [Google Scholar]
- 28. Baszis K, Toib D, Cooper M, French A, White A.. Recurrent parotitis as a presentation of primary pediatric Sjögren syndrome. Pediatrics 2012;129:e179–82. [DOI] [PubMed] [Google Scholar]
- 29. Gmuca S, Lieberman SM, Mehta J.. Pediatric neuromyelitis optica spectrum disorder and Sjögren syndrome: more common than previously thought? J Rheumatol 2017;44:959–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flaitz CM. Parotitis as the initial sign of juvenile Sjögren’s syndrome. Pediatr Dent 2001;23:140–42. [PubMed] [Google Scholar]
- 31. Nathavitharana KA, Tarlow MJ, Bedi R, Southwood TR.. Primary Sjogren’s syndrome and rampant dental caries in a 5‐year‐old child. Int J Paediatr Dent 1995;5:173–6. [DOI] [PubMed] [Google Scholar]
- 32. Siamopoulou-Mavridou A, Drosos AA, Andonopoulos AP.. Sjogren syndrome in childhood: report of two cases. Eur J Pediatr 1989;148:523–4. [DOI] [PubMed] [Google Scholar]
- 33. Civilibal M, Canpolat N, Yurt A. et al. A child with primary Sjögren syndrome and a review of the literature. Clin Pediatr 2007;46:738–42. [DOI] [PubMed] [Google Scholar]
- 34. Pessler F, Monash B, Rettig P. et al. Sjögren syndrome in a child: favorable response of the arthritis to TNFα blockade. Clin Rheumatol 2006;25:746–8. [DOI] [PubMed] [Google Scholar]
- 35. de Oliveira MA, de Rezende NPM, Maia CMF, Gallottini M.. Primary Sjögren syndrome in a 2-year-old patient: role of the dentist in diagnosis and dental management with a 6-year follow-up. Int J Paediatr Dent 2011;21:471–5. [DOI] [PubMed] [Google Scholar]
- 36. Ohlsson V, Strike H, James-Ellison M, Tizard EJ, Ramanan AV.. Renal tubular acidosis, arthritis and autoantibodies: primary Sjögren’s syndrome in childhood. Rheumatology 2006;45:238–40. [DOI] [PubMed] [Google Scholar]
- 37. Nikitakis NG, Rivera H, Lariccia C, Papadimitriou JC, Sauk JJ.. Primary Sjögren syndrome in childhood: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;96:42–7. [DOI] [PubMed] [Google Scholar]
- 38. Ohtsuka T, Saito Y, Hasegawa M. et al. Central nervous system disease in a child with primary Sjögren syndrome. J Pediatr 1995;127:961–3. [DOI] [PubMed] [Google Scholar]
- 39. Zhang X, Zeng X.. Severe pulmonary hypertension in pediatric primary Sjögren syndrome: a case report. J Clin Rheumatol 2007;13:276–7. [DOI] [PubMed] [Google Scholar]
- 40. Skalova S, Minxova L, Slezak R.. Hypokalaemic paralysis revealing Sjogren’s syndrome in a 16-year old girl. Ghana Med J 2008;42:124–8. [PMC free article] [PubMed] [Google Scholar]
- 41. Moy MM, Mandel L.. Identifying primary Sjögren syndrome in children: case report. J Oral Maxillofac Surg 2014;72:2485–90. [DOI] [PubMed] [Google Scholar]
- 42. Ladino RM, Gasitulli OA, Campos MX.. Síndrome de Sjögren: caso clínico. Rev Chil Pediatr 2015;86:47–51. [DOI] [PubMed] [Google Scholar]
- 43. Thouret MC, Sirvent N, Triolo V. et al. Syndrome de Gougerot-Sjögren primitif chez une fille de 13 ans. Arch Pediatr 2002;9:142–6. [DOI] [PubMed] [Google Scholar]
- 44. Shahi E, Donati C, Gattinara M, Pontikaki I, Gerloni V.. Primary Sjögren syndrome: report of a 10 years old girl with local edema and positivity of anti SS-A and anti SS-B autoantibodies. Reumatismo 2011;63:97–100. [DOI] [PubMed] [Google Scholar]
- 45. Sardenberg F, Goursand D, Polletto LT. et al. Oral manifestations and treatment of a child with Sjögren’s syndrome. J Dent Child 2010;77:102–5. [PubMed] [Google Scholar]
- 46. Bogdanović R, Basta-Jovanović G, Putnik J, Stajić N, Paripović A.. Renal involvement in primary Sjogren syndrome of childhood: case report and literature review. Mod Rheumatol 2013;23:182–9. [DOI] [PubMed] [Google Scholar]
- 47. Zhao J, Chen Q, Zhu Y. et al. Nephrological disorders and neurological involvement in pediatric primary Sjogren syndrome: a case report and review of literature. Pediatr Rheumatol Online J 2020;24:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aburiziza AJ. Primary juvenile Sjögren’s syndrome in a 3-year-old pediatric female patient: diagnostic role of salivary gland ultrasonography: case report. Open Access Rheumatol 2020;12:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gottfried JA, Finkel TH, Hunter JV, Carpentieri DF, Finkel RS.. Central nervous system Sjögren’s syndrome in a child: case report and review of the literature. J Child Neurol 2001;16:683–5. [DOI] [PubMed] [Google Scholar]
- 50. Fidalgo TKDS, Nogueira C, Andrade MRTC, Valente AGLR, Tannure PN.. Oral rehabilitation and management for secondary Sjögren’s syndrome in a child. Case Rep Dent 2016;2016:3438051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Majdoub I, Kallel S, Hsairi M. et al. Syndrome de Goujerot-Sjögren primitif de l’enfant: à propos d’un cas. Arch Pediatr 2017;24:49–52. [DOI] [PubMed] [Google Scholar]
- 52. Vermylen C, Meurant A, Noël H, Claus D, Cornu G.. Sjögren’s syndrome in a child. Eur J Pediatr 1985;144:266–9. [DOI] [PubMed] [Google Scholar]
- 53. Marino A, Narula S, Lerman MA.. First pediatric patient with neuromyelitis optica and Sjogren syndrome successfully treated with tocilizumab. Pediatr Neurol 2017;73:e5–6. [DOI] [PubMed] [Google Scholar]
- 54. Price EJ, Rauz S, Tappuni AR. et al. The British Society for Rheumatology guideline for the management of adults with primary Sjögren’s syndrome. Rheumatology 2017;56:1828. [DOI] [PubMed] [Google Scholar]
- 55. Brown S, Navarro Coy N, Pitzalis C. et al. The TRACTISS protocol: a randomised double blind placebo controlled clinical TRial of Anti-B-Cell therapy in patients with primary Sjögren’s syndrome. BMC Musculoskelet Disord 2014;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data extraction files are available upon request.