Abstract
Objectives
RA is a chronic autoimmune disease characterized by joint inflammation and tissue destruction. Immune responses mediated by T cells and autoantibodies are known to play critical roles in RA. Collagen type II (CII)-induced arthritis (CIA) is a commonly used animal model of human RA. We have previously reported the identification of a new T cell inhibitory molecule CD300c. Here we investigate the ability of recombinant CD300c-IgG2a Fc (CD300c-Ig) fusion protein to prevent and treat CIA.
Methods
Mice were induced to develop CIA by CII and injected with CD300c-Ig or control Ig protein before or after CIA symptoms occur. The mice were examined for CIA clinical and pathological scores, and analysed for the expression of proinflammatory cytokines, the percentage and activation of CD4 T cells and regulatory T cells, CII-specific T cell proliferation and cytokine production, and CII-specific autoantibody production.
Results
In a prevention model, CD300c-Ig significantly decreases CIA incidence, and reduces clinical and pathological arthritis scores. In the treatment model, CD300c-Ig ameliorates established CIA. The beneficial effects of CD300c-Ig are related to decreased expansion and activation of T cells in the spleen and reduced expression of proinflammatory cytokines in the joints. CD300c-Ig also inhibits CII-specific T cell proliferation and Th1 and Th17 cytokine production. In addition, CD300c-Ig treatment reduced the production of CII autoantibodies in the serum. Furthermore, CD300c-Ig inhibits the proliferation and activation of T cells from RA patients in vitro.
Conclusion
CD300c-Ig protein has the potential to be used in the treatment of patients with RA.
Keywords: rheumatoid arthritis, collagen-induced arthritis, T cell inhibitory molecule, CD300c, T cells, regulatory T cells, autoantibody, cytokine
Rheumatology key messages.
CD300c protein inhibits T cell response and autoantibody production and ameliorates CIA in mice.
CD300c inhibits the proliferation and activation of T cells from RA patients in vitro.
CD300c protein has the potential to be used in the treatment of patients with RA.
Introduction
RA is one of the most common human systemic autoimmune diseases, affecting 0.5%–1% of the population worldwide [1]. RA is characterized by inflammation of the synovial tissue and formation of rheumatoid pannus, which erodes adjacent cartilage and bone, causing joint destruction [2]. Type II collagen (CII) is a major protein in cartilage, the target tissue of RA [3]. CIA, induced by CII, is a commonly used mouse model for human RA [3]. CIA shares several pathological features with RA, including synovial hyperplasia, mononuclear cell infiltration and cartilage degradation [3]. The CIA model has been used to identify potential pathogenic mechanisms of autoimmune disease, and to test and develop new biologically based therapeutics for human RA [3, 4].
T cells are known to play a pivotal role in the pathogenesis of RA [5–8]. T cells are regulated by costimulatory and coinhibitory molecules. T cell coinhibitory molecules are critical for maintaining peripheral tolerance to avoid autoimmune disease. Among the T cell regulators, the B7 family members are of central importance [9–11].
We have previously reported the identification of a novel T cell inhibitory molecule CD300c that shares significant sequence and structural similarities with existing B7 family members [9]. CD300c protein is expressed on professional antigen-presenting cells (APCs), including B cells, monocytes, macrophages and dendritic cells [9]. The CD300c receptor is expressed on CD4 and CD8 T cells, and the expression levels are upregulated upon activation although the identity of the CD300c receptor is not yet known [9]. Both recombinant murine and human CD300c-Ig proteins significantly inhibit the proliferation, activation and cytokine production of CD4 and CD8 T cells in vitro [9]. Administration of CD300c-Ig attenuates acute graft-vs-host disease in mice, which is associated with the inhibition of T cell proliferation, activation and cytokine production [9]. In this study, we determined the ability of CD300c-Ig protein to prevent and treat CIA.
Methods
Mice
DBA/1j mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Age-matched mice, 7–10 weeks old, were used for all experiments. The mice were used in accordance with a protocol approved by the Institutional Animal Care and Use Committee of the University of Connecticut.
Production of recombinant human CD300c-Ig fusion protein
The extracellular domains of human CD300c were cloned into a pCMV6-AC-FC-S expression vector containing the constant region of mouse IgG2a (ORIGENE, Rockville, MD, USA). The vector was transfected into HEK293F cells to produce recombinant CD300c-IgG2a (CD300c-Ig) fusion protein that was then purified from supernatant of cultured cells using Protein G Sepharose 4 Fast Flow. Purified protein was verified by SDS–PAGE, Coomassie Staining and western blot as described [9]. The endotoxin level was <0.01 EU/ml of 1 µg of purified CD300c-Ig as determined by a Limulus Amebocyte Lysate assay [9].
Induction of CIA
Male DBA/1 mice were injected intradermally at the base of the tail with 200 µg of bovine CII (Chondrex Inc., Woodinville, WA, USA) emulsified in complete Freund adjuvant (CFA) (Sigma-Aldrich, St. Louis, MO, USA). Twenty-one days after the primary immunization, the mice were boosted with the same concentration of CII emulsified in incomplete Freund adjuvant (IFA). Mice were examined for the onset of CIA and clinical scores. The swelling of four paws was graded from 0 to 4 as follows: grade 0, no evidence of erythema and swelling; grade 1, erythema and mild swelling confined to the tarsals or ankle joint; grade 2, erythema and mild swelling extending from the ankle to the tarsals; grade 3, erythema and moderate swelling extending from the ankle to metatarsal joints; and grade 4, erythema and severe swelling encompass the ankle, foot and digits, or ankylosis of the limb [3]. Each paw was graded, and the four scores were totalled so that the maximal score per mouse was 16.
Histological assessment of arthritis
The hind paws were fixed in formalin, decalcified in 10% EDTA, embedded in paraffin, sectioned, and stained with haematoxylin and eosin. All slides were evaluated by blinded investigators regarding the experimental conditions. The extent of synovitis, pannus formation and bone and cartilage destruction was determined using a graded scale, as follows [12]: grade 0, no signs of inflammation; 1, mild inflammation with hyperplasia of the synovial lining without cartilage destruction; 2–4, increasing degrees of inflammatory cell infiltration and cartilage/bone destruction. The tissue sections were also stained with Safranin-O; the cartilage destruction was measured with a 0–4 score system as described [13]: 0 = no destruction; 1 = minimal erosion; 2 = slight to moderate erosion in a limited area; 3 = more extensive erosion; 4 = general destruction.
CII-specific T cell proliferation and cytokine production
Erythrocyte-depleted splenocytes or purified splenic CD4+ T cells were seeded in 96-well plates and cultured in the presence or absence of denatured (60°C, 30 min) bovine CII for 72 h. In the culture with purified CD4+ T cells, splenocytes from the CIA mice treated with mitomycin C (50 µg/ml, 37°C, 30 min; Sigma-Aldrich) were added into the cultures and used as APCs. Proliferative response was assessed by pulsing the culture with [3H]thymidine (1 µCi/well) (PerkinElmer, Inc., Downers Grove, IL, USA) 12 h before harvest. Incorporation of [3H]thymidine was measured by liquid scintillation spectroscopy (PerkinElmer, Inc.). The supernatants from similar cultures were collected after 72 h and assessed for the contents of cytokines by ELISA (BioLegend, San Diego, CA, USA).
Flow cytometry analysis
The single cell suspension of organs was stained with the fluorochrome-conjugated antibodies protein as described [14–17]. For intracellular staining, the cells were first permeabilized with a BD Cytofix/Cytoperm solution for 20 min at 4°C. Direct or indirect staining of fluorochrome-conjugated antibodies included: CD4, CD25, CD19, Foxp3, CD44, CD62L and CD69 (BioLegend, , San Jose, CA, USA or BD Biosciences, San Diego, CA, USA). CD300c-Ig were biotinylated with sulfo-NHS-LC-Biotin (Pierce, Waltham, MA, USA) as described [9]. The samples were analysed on a LSRFortessa X-20 Cell Analyzer (BD Biosciences). Data analysis was done using FlowJo software (Ashland, OR, USA).
Real-time qualitative RT-PCR
Total RNA from cells was isolated from tissues or cells, and cDNA was synthesized as described [18]. Equal amounts of cDNAs were used for RT-PCR. Real-time qualitative RT-PCRs were performed with the Power SYBR green mastermix (Applied Biosystems, UK) using the 7500 real-time PCR system (Applied Biosystems, UK) [10].
Measurement of serum anti-CII antibody and cytokine levels
Bovine CII (1 µg/ml) was coated onto microtiter plates overnight at 4°C. After blocking with 1% BSA in PBS, serum samples were added and incubated for 1 h at room temperature. After washing, biotin-conjugated anti-mouse IgG1, IgG2a or IgG2b antibody (Ab) (BioLegend) was added, followed by HRP-labelled avidin (BioLegend). The Ab binding was detected using the TMB Substrate Set according to the manufacturer’s instructions (BioLegend). The concentration of TNFα, IL-1β and IL-6 proteins was determined by ELISA Kit (BioLegend) according to the manufacturer’s instructions.
In vitro human T cell proliferation assay
Peripheral blood mononuclear cells (PBMCs) were purchased from Sanguine Biosciences (Valencia, CA, USA). The PBMCs were labelled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (ThermoFisher Scientific) and then stimulated with anti-CD3 antibody (BioLegend) in the presence of CD300c-Ig or control Ig for 3 days. CFSE dilution by CD4 or CD8 T cells was analysed by flow cytometry.
Statistical analysis
P-values were based on the two-sided Student’s t test. For comparing means of multiple groups, significance was determined using one-way ANOVA with Dunnett test. A confidence level above 95% (P < 0.05) was determined to be significant.
Results
In vivo administration of CD300c-Ig protein reduces CIA development
To examine the effect of CD300c on the development of experimental arthritis, DBA/1 mice were immunized with CII emulated in CFA on day 0 and received a booster injection of CII in IFA on day 21. The immunized mice were randomly divided and injected intraperitoneally with the either control Ig or CD300c-Ig protein (12.5, 25 or 50 µg) every 3 days for 10 times beginning from the same day as the second immunization (day 21). In our preliminary experiment, we observed no obvious differences among the mice treated with the three doses of control Ig protein. We therefore selected the dose 50 µg for a control group.
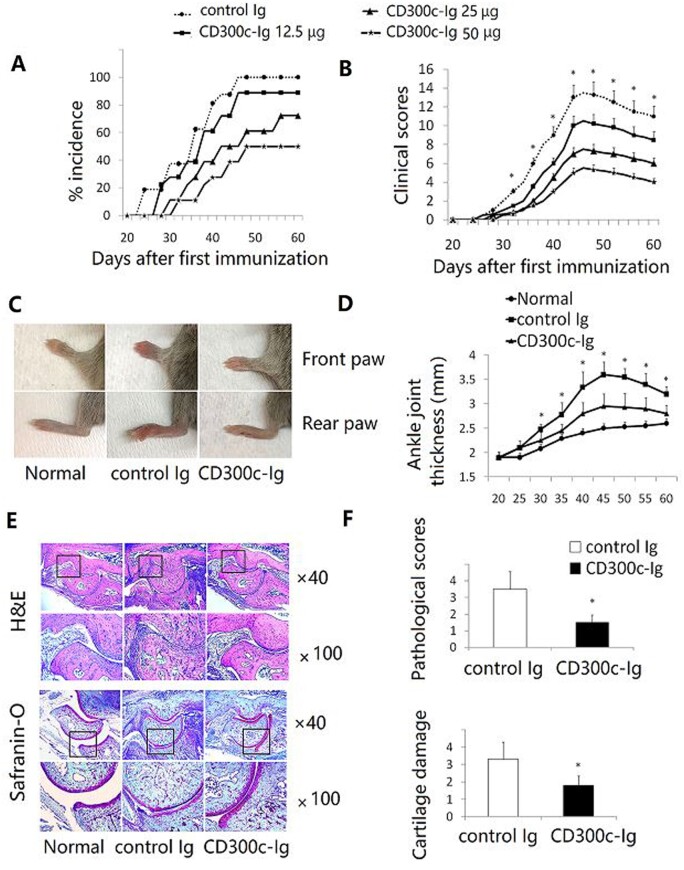
In comparison with control Ig, CD300c-Ig significantly decreased the incidence of CIA (Fig. 1A), delayed the onset and reduced the clinical score of arthritis in a dose-dependent manner (Fig. 1B). Fig. 1C and D show that CD300c-Ig decreased the severity of joint swelling, as compared with control Ig treatment. Untreated normal mice were also used as a control.
Fig. 1.
CD300c-Ig protein reduces CIA development
CIA was induced in DBA/1 mice by immunization with CII/CFA on day 0 and boosted with CII/IFA on day 21. The mice were injected intraperitoneally with control Ig (50 µg) or CD300c-Ig protein (12.5, 25 or 50 µg) every 3 days for 10 times beginning from day 21. (A) CIA incidence and (B) clinical score are shown. The data are pooled from three independent experiments (n = 15–18). (C) Representative images of front and rear paws from untreated normal mice, control Ig- or CD300c-Ig protein-treated CIA mice. (D) Statistical analysis of the thickness of angle joint in the rear paws. (E) The rear paws were harvested from the mice on day 60. H&E (upper panel) and Safranin-O (lower panel) staining of representative hind paws from untreated normal mice, and control Ig- or CD300c-Ig protein-treated CIA mice (50 µg groups). (F) Pathological scores that were evaluated in a blinded fashion are shown. (D, F) The data are representative of three independent experiments with similar results (n = 5–6 for each group). CFA: complete Freund adjuvant; H&E: haematoxylin and eosin; IFA: incomplete Freund adjuvant.
We then performed histological examination. Haematoxylin and eosin staining of arthritic paw joints from control Ig-treated CIA mice revealed severe infiltration of mononuclear cells, synovial hyperplasia, pannus formation, cartilage destruction and bone erosion that are characteristic features of arthritis (Fig. 1E and data not shown). In contrast, the levels of inflammation, infiltration and cartilage erosion were markedly alleviated in CD300c-Ig-treated mice (Fig. 1E). Safranin-O staining also showed that CD300c-Ig-treated CIA mice had reduced cartilage destruction (Fig. 1E). Consequently, the pathological scores in CD300c-Ig-treated mice were significantly decreased, as compared with those in control Ig-treated mice (Fig. 1F). Taken together, our results suggest that in vivo administration of CD300c-Ig protein prevents the development of CIA.
Cd300c-Ig protein reduces the expression of proinflammatory cytokines in the joints
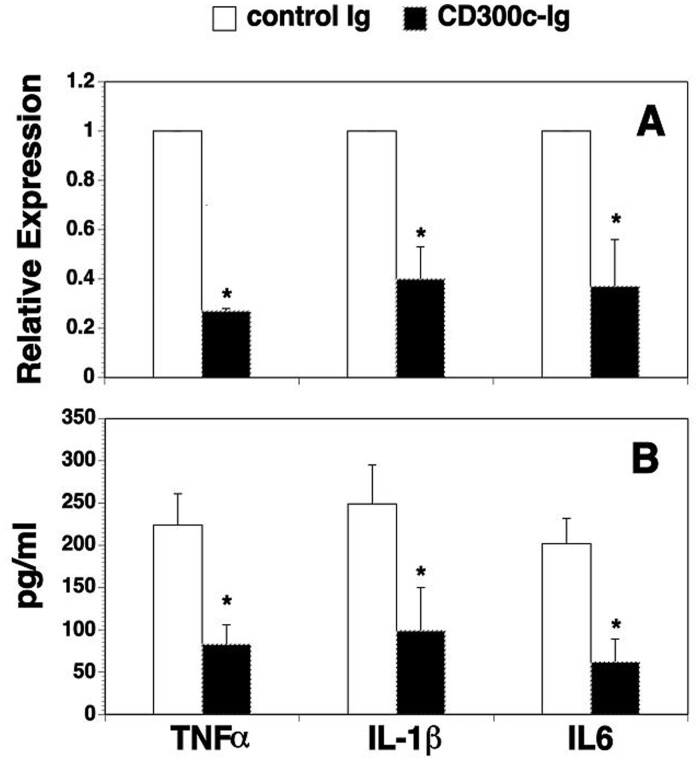
It has been reported that high levels of TNFα, IL-1β and IL-6 are detected in the synovial tissues of RA patients, and these inflammatory cytokines play a critical role in the development of arthritis [19–21]. We determined whether CD300c-Ig affected the expression of the proinflammatory cytokines in the synovium and the serum. As shown in Fig. 2, CD300c-Ig significantly reduced the expression of these proinflammatory cytokine mRNAs in the synovium and the proteins in the serum, as compared with control Ig-treated mice.
Fig. 2.
CD300c-Ig protein reduces the expression of proinflammatory cytokines in the joints and the serum
CIA was induced in DBA/1 mice and injected with control Ig or CD300c-Ig protein (50 µg) as in Fig. 1. The joints of hind paws and serum were collected on day 60. The expression levels of TNFα, IL-1β, and IL-6 mRNA in the synovium and the proteins in the serum were examined by (A) qRT-PCR and (B) ELISA. (A) The expression levels of the genes in control Ig-treated mice are defined as 1. The data are expressed as mean (+ s.d.) from one of three independent experiments with similar results (5–6 mice per group in each experiment). *P < 0.05 versus control Ig group. qRT-PCR: real-time qualitative RT-PCR.
Synovial fibroblasts are regarded to be a major source of IL-6 [22]. Therefore, we determined whether CD300c directly affect synovial fibroblasts. Studies have shown that human SW982 synovial cells share similar physiological and immunological properties with primary synovial fibroblasts and that IL-1β stimulation of SW982 cells can mimic the inflammatory status of synovial cells typically seen in RA patients [23]. We found that CD300c-Ig did not inhibit IL-1β-induced IL-6 secretion by SW982 cells, as compared with control Ig, suggesting that CD300c does not directly affect synovial fibroblasts (supplementary Fig. S1, available at Rheumatology online).
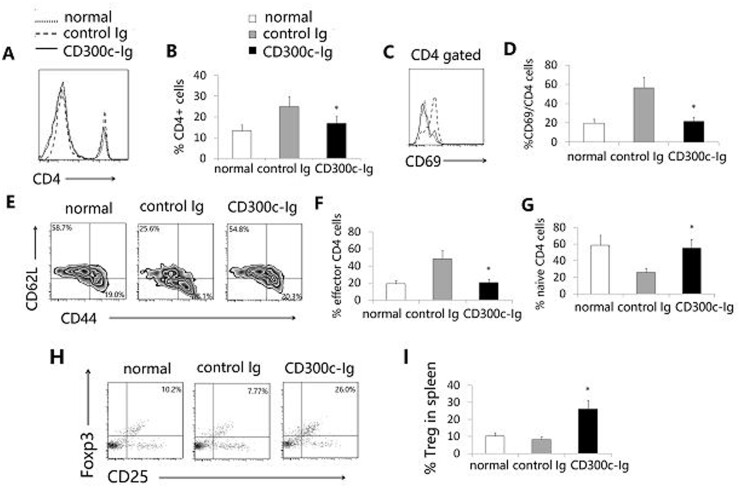
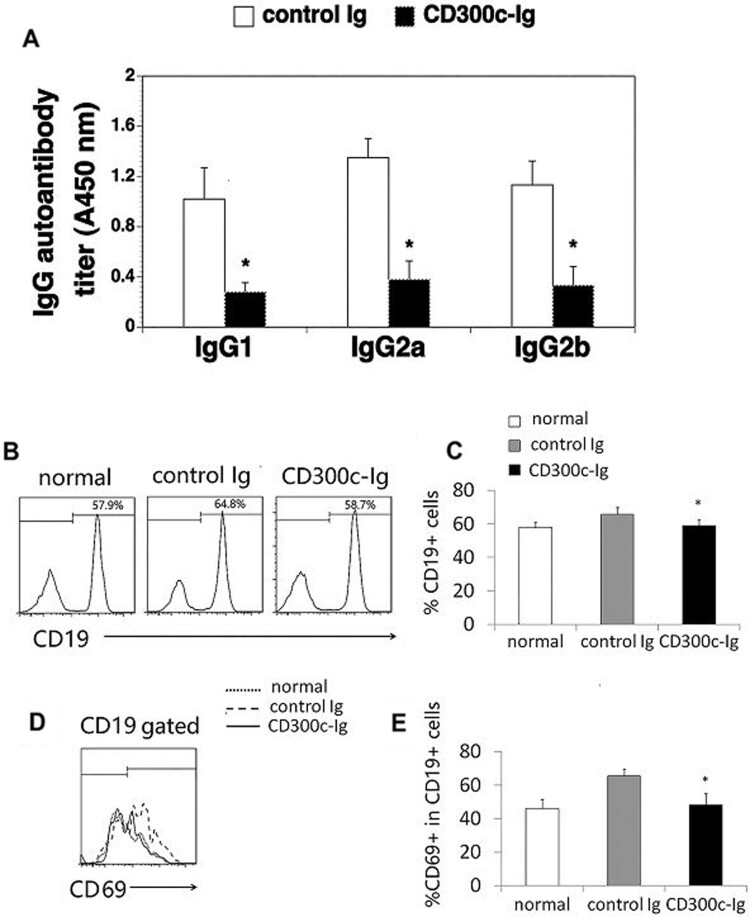
Cd300c-Ig protein decreases the percentage and activation of T cells, but increases the percentage of Tregs, in the spleen
Because CIA is primarily mediated by CD4 T cells, we analysed CD4+ T cells in the spleen of the mice. CD300c-Ig significantly decreased the percentage of CD4+ T cells (Fig. 3A and B), and reduced the expression of CD69, an activation maker, by CD4+ T cells (Fig. 3C and D). T cells can be divided into naïve and effector memory T cells based on the expression levels of CD44 and CD62L. We next analysed these T cell subsets. CD300c-Ig increased the percentage of CD44loCD62Lhi naïve CD4 T cells but decreased the percentage of CD44hiCD62Llo effector memory CD4 T cells (Fig. 3E–G). Consistent with our previous report [9], our results suggest that CD300c-Ig inhibits the expansion and activation of T cells in CIA mice.
Fig. 3.
CD300c-Ig protein decreases the percentage and activation of CD4 T cells but increases the percentage of Tregs in the spleen
DBA/1 mice were induced to develop CIA and injected with control Ig or CD300c-Ig protein as in Fig. 2. Untreated normal (no CIA) mice were also used as a control. The splenocytes were analysed for (A, B) the percentage of CD4+ T cells, (C, D) the expression of CD69 by CD4+ T cells, and (E–G) the percentages of CD44loCD62Lhi naive and CD44hiCD62Llo effector memory CD4 T cells, as well as (H, I) the percentage of CD4+CD25+Foxp3+ Tregs. (A, C, E, H) Representative flow cytometric profiles, and (B, D, F, G, I) data from statistical analyses are shown. The data are expressed as mean (+ s.d.) from one of three independent experiments with similar results (5–6 mice per group in each experiment). *P < 0.05 versus control Ig group.
It is well known that Tregs are involved in immune tolerance induction, and that CD4+CD25+FoxP3+ cells are the most thoroughly characterized Tregs [24]. We evaluated CD4+CD25+FoxP3+ Tregs in the spleen and found that CD300c-Ig significantly increased the percentage of Tregs (Fig. 3H and I). To determine whether Tregs play a role in the amelioration of CIA by CD300c-Ig, we deleted Tregs by an anti-CD25 antibody. We and others have shown that anti-CD25 antibody efficiently deletes Tregs [25, 26]. As shown in supplementary Fig. S2, available at Rheumatology online, deletion of Tregs partly abolished the effect of CD300c-Ig on CIA.
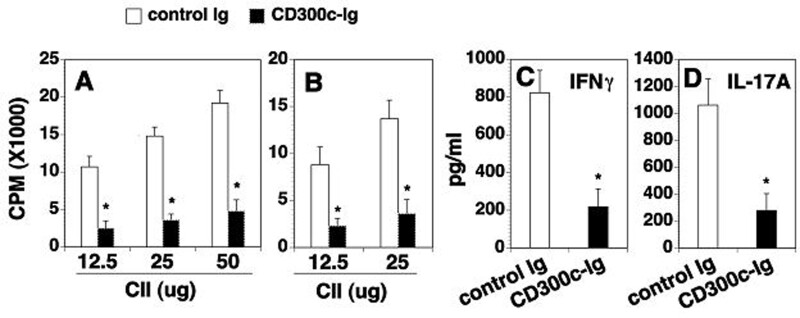
Cd300c-Ig protein inhibits CII-specific T cell proliferation and cytokine production
We also determined whether CD300c modulates antigen-specific T cell responses. Splenocytes and splenic CD4 T cells were isolated from the CIA mice. The proliferative responses and cytokine production against CII were then examined. Splenocytes from the control Ig-treated mice proliferated well in response to CII in vitro stimulation (Fig. 4A). In contrast, splenocytes from CD300c-Ig-treated mice showed significantly reduced proliferative responses (Fig. 4A). A similar inhibitory effect of CD300c-Ig was observed when splenic CD4+ T cells were used as responder cells (Fig. 4B). The results suggest that CD300c inhibits the expansion of CII-specific T cells.
Fig. 4.
CD300c-Ig reduces CII-specific T cell proliferation and cytokine production
DBA/1 mice were induced to develop CIA and injected with control Ig or CD300c-Ig protein as in Fig. 2. (A) The splenocytes (normalized to 2 × 105 T cells/well) and (B) purified CD4+ T cells (1 × 105 cells/well) were stimulated with indicated dose of CII for 3 days in vitro. Mitomycin C-treated immunized splenocytes (1 × 105 cells/well) were also added as APCs in the purified CD4+ T cell cultures. [3H]thymidine (1 µCi/well) was added to the cultures 12 h before harvest. T cell proliferation was measured by [3H]thymidine incorporation. Results are expressed as counts per minute (CPM). (C, D) The supernatants of the cultured splenocytes stimulated with 25 µg/ml CII were analysed for the levels of IFNγ and IL-17A by ELISA. The data are expressed as mean (+ s.d.) from one of three independent experiments with similar results (5–6 mice per group in each experiment). *P < 0.05 versus control Ig group. APCs: antigen-presenting cells.
We next examined cytokine production. Splenocytes from control Ig- or CD300c-Ig-treated mice were stimulated with CII for 72 h, and the supernatants were analysed for cytokine content. Splenic cells from the control Ig-treated mice produced high levels of IFNγ and IL-17A in response to CII, whereas the production of IFNγ and IL-17A was greatly reduced from the cells of CD300c-Ig-treated mice (Fig. 4C and D). The results suggest that CD300c-Ig reduces CII-specific T cell proliferation and Th1 and Th17 cytokine production.
Cd300c-Ig protein reduces CII-specific autoantibody production
Since CII autoantibodies also play an important role in the pathogenesis of CIA [21, 27], we analysed the levels of anti-CII antibodies in the serum. The levels of anti-CII IgG1, IgG2a and IgG2b in the serum of CD300c-Ig-treated mice were dramatically decreased, as compared with those in control Ig-treated mice (Fig. 5A). The results suggest that the reduced production of anti-CII Abs might also be responsible for the ameliorating effect of CD300c-Ig.
Fig. 5.
CD300c-Ig reduces CII-specific IgG1, IgG2a and IgG2b production and decreases the percentage and activation of B cells in CIA mice
DBA/1 mice were induced to develop CIA and injected with control Ig or CD300c-Ig protein as in Fig. 2. (A) The serum was harvested and analysed for anti-CII IgG1, IgG2a and IgG2b levels by ELISA. (B, C) The splenocytes were analysed for the percentage of CD19+ B cells. (D, E) The splenocytes were stimulated with anti-IgM (10 μg/ml) for 24 h. The expression of CD69 by CD19+ B cells were analysed by flow cytometry. (B–E) Untreated normal mice were also used as control. The data are expressed as mean (+ s.d.) from one of three independent experiments with similar results (5–6 mice per group in each experiment). *P < 0.05 versus control Ig group.
We then determined whether CD300c-Ig affected B cells in the CIA mice. The percentage of CD19+ B cells in the spleen of CD300c-Ig-treated CIA mice was decreased as compared with that in control Ig-treated CIA mice (Fig. 5B and C). Furthermore, CD300c-Ig inhibited the expression of CD69 by B cells in the CIA mice (Fig. 5D and E). The results suggest that CD300c also inhibits B cell proliferation and activation in CIA mice.
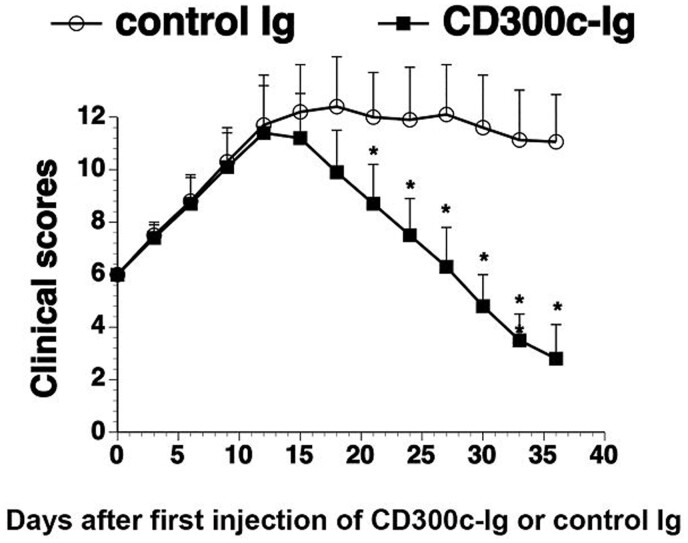
In vivo administration of CD300c-Ig protein ameliorates established CIA
We next determined whether CD300c-Ig protein could be used to treat established CIA. Mice were immunized with CII on day 0 and day 21, as shown in Fig. 1. When the mice had a clinical score of 6, they were injected with 50 µg control Ig or CD300c-Ig once every 3 days for 11 times. As shown in Fig. 6, CD300c-Ig also significantly decreased the clinical score of arthritis, as compared with control Ig treatment. The results suggest that CD300c-Ig can also attenuate established CIA.
Fig. 6.
CD300c-Ig protein attenuates established CIA
CIA was induced in DBA/1 mice by immunization with CII/CFA on day 0 and boosted with CII/IFA on day 21. When the CIA clinical score was 6, the mice were injected intraperitoneally with control Ig (50 µg) or CD300c-Ig protein (50 µg) every 3 days for 11 times. The CIA clinical scores for days after infection of CD300c-Ig or control Ig were recorded. The data were pooled from of two independent experiments (n = 15 for each group). CFA: complete Freund adjuvant; IFA: incomplete Freund adjuvant.
Cd300c-Ig inhibits the proliferation and activation of T cells from RA patients
Since our data suggest that CD300c has the potential to be used in the treatment of RA patients, we determined the ability of CD300c-Ig to affect the functions of T cells from RA patients in vitro. PBMCs from RA patients were labelled with CFSE and then stimulated with anti-CD3 antibody in the presence of CD300c-Ig or control Ig for 3 days. T cell proliferation was measured by CFSE fluorescent dilution in CD4 and CD8 T cells. As shown in supplementary Fig. S3A and B, CD300c-Ig inhibited the proliferation of both CD4 and CD8 T cells. Furthermore, CD300c-Ig inhibited the activation of the T cells as indicated by reduced expression of CD69 on T cells (supplementary Fig. S3C and D).
To determine whether the CD300c receptor expression on T cells in RA patients is altered, we compared the expression levels between RA patients and healthy donors by analysing the binding of biotinylated CD300c-Ig to T cells as described [9]. Although the expression levels of the CD300c receptor on CD4 and CD8 T cells between RA patients were slightly increased as compared with those from healthy donors, the differences did not reach statistical significance (supplementary Fig. S3E–H).
Discussion
We show here that in vivo administration of CD300c-Ig not only prevents CIA development, but also attenuates established CIA. Studies have shown that autoreactive T cells, especially CD4 T cells, play a critical role in CIA pathogenesis. We have previously shown that the CD300c putative receptor is expressed on CD4 T cells, and the expression level is upregulated upon activation. CD300c-Ig protein significantly inhibits the proliferation, activation and cytokine production of CD4 T cells in vitro. In this study, we show that CD300c-Ig treatment decreases the percentage of CD4 T cells in the spleen, inhibits T cell activation as indicated by reduced expression of CD69, decreases the percentage of effector T cells, but increases the percentage of native T cells in CIA mice. Importantly, CD300c-Ig inhibits CII-specific T cell proliferation and Th1/Th17 cytokine production. Therefore, CD300c-mediated inhibition of the proliferation, activation and cytokine production of effector T cells, especially autoreactive T cells, likely plays a key role in the attenuation of CIA.
In addition to the CII-specific immune response, locally produced proinflammatory cytokines also play an important role in the development of arthritis. Among such cytokines, TNFα, IL-1β and IL-6 are crucial; blockage of these cytokines has beneficial effects on CIA [19, 21, 28–30]. We have shown that CD300c-Ig treatment reduces the expression of proinflammatory cytokines including TNFα, IL-1β and IL-6 in the joints and the serum. We have also shown that CD300c-Ig does not directly affect synovial fibroblasts to produce IL-6. It has been reported that interactions of T cells or B cells with synovial fibroblasts play an important role in RA pathogenesis [31, 32]. It is possible that CD300c indirectly affect synovial fibroblasts by inhibiting the activation and proliferation of T and B cells.
In contrast to inhibiting effector T cell proliferation and activation, CD300c-Ig treatment increased the percentage of Tregs. Deletion of Tregs partly abolished the effect of CD300c-Ig on CIA. These data suggest that the increased Tregs also contribute to the beneficial effects of CD300c in CIA. It is well known that natural Tregs develop in the thymus, whereas induced Tregs develop in the periphery. It remains to be determined whether CD300c increases the production of natural Tregs and/or induced Tregs.
In addition to T cells, B-cell-mediated autoimmune responses also play an important role in CIA pathogenesis [27]. Our data show that the serum levels of anti-CII IgG1, IgG2a and IgG2b antibodies are dramatically decreased in CD300c-Ig-treated CIA mice as compared with control Ig-treated mice, suggesting that CD300c also reduces the production of CII-specific autoantibodies from B cells. Since the CD300c receptor is expressed on B cells [9], it is possible that CD300c directly inhibits B cells to produce autoantibodies. Indeed, we have shown that CD300c-Ig significantly reduces B cell proportion and activation in CIA mice. It has been reported that CD300c deficient B cells had an enhanced proliferation in response to B cell receptor and CpG stimulation. In contrast, restoration of the CD300c expression in CD300c deficient B cells suppressed B cell receptor- and CpG-mediated proliferation [33, 34]. Our results are consistent with these reports. Since CD4 T cells are essential in the activation of B cells to produce antibodies [35, 36], it is also possible that the reduced production of CII-specific autoantibodies in CD300c-Ig-treated CIA mice is partly due to the effect of CD300 on T cells.
In summary, we have demonstrated that CD300c-Ig treatment significantly ameliorates CIA. This is associated with decreased expansion of effector CD4 T cells, but increased production of Tregs. CD300c also inhibits CII-specific T cell proliferation and Th1/Th17 cytokine production. In addition, CD300c reduces the expression of proinflammatory cytokines including TNFα, IL-1β and IL-6 in the joints, and reduces the production of CII-specific autoreactive antibodies in the serum. Furthermore, CD300c significantly inhibits the proliferation and activation of T cells from RA patients. Therefore, CD300c protein has the potential to be used in the treatment of patients with RA.
Supplementary Material
Acknowledgements
H.L., J.Z and M.S performed major experiments and analysed the experimental data. X.T. analysed the experimental data. L.L. designed the study, performed experiments and drafted the manuscript. All authors read the manuscript critically and approved the final version.
Funding: This work was supported by grants from the National Institutes of Health (1R01AI123131 and 1R21AI156779) and Connecticut Regenerative Medicine Research Fund (16-RMB-UCONN-02).
Disclosure statement: There is a patent application pending related to this work.
Data availability statement
Data are available upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature 2003;423:356–61. [DOI] [PubMed] [Google Scholar]
- 2. Feldmann M, Brennan FM, Maini RN.. Rheumatoid arthritis. Cell 1996;85:307–10. [DOI] [PubMed] [Google Scholar]
- 3. Brand DD, Latham KA, Rosloniec EF.. Collagen-induced arthritis. Nat Protoc 2007;2:1269–75. [DOI] [PubMed] [Google Scholar]
- 4. Luo L, Zhu G, Xu H. et al. B7-H3 promotes pathogenesis of autoimmune disease and inflammation by regulating the activity of different T cell subsets. PLoS One 2015;10:e0130126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brand DD, Kang AH, Rosloniec EF.. Immunopathogenesis of collagen arthritis. Springer Semin Immunopathol 2003;25:3–18. [DOI] [PubMed] [Google Scholar]
- 6. VanderBorght A, Geusens P, Raus J, Stinissen P.. The autoimmune pathogenesis of rheumatoid arthritis: role of autoreactive T cells and new immunotherapies. Semin Arthritis Rheum 2001;31:160–75. [DOI] [PubMed] [Google Scholar]
- 7. Cope AP, Schulze-Koops H, Aringer M.. The central role of T cells in rheumatoid arthritis. Clin Exp Rheumatol 2007;25(Suppl 46):S4–11. [PubMed] [Google Scholar]
- 8. Weyand CM, Bryl E, Goronzy JJ.. The role of T cells in rheumatoid arthritis. Arch Immunol Ther Exp 2000;48:429–35. [PubMed] [Google Scholar]
- 9. Cui C, Su M, Lin Y, Lai L.. A CD300c Fc fusion protein inhibits T cell immunity. Front Immunol 2018;9:2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin Y, Cui C, Su M. et al. Skint8, a Novel B7 family-related molecule, negatively regulates T cell responses. J Immunol 2019;203:400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Su M, Lin Y, Cui C, Tian X, Lai L.. ERMAP is a B7 family-related molecule that negatively regulates T cell and macrophage responses. Cell Mol Immunol 2020; doi: 10.1038/s41423-020-0494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen W, Wang J, Xu Z. et al. Apremilast ameliorates experimental arthritis via suppression of Th1 and Th17 cells and enhancement of CD4(+)Foxp3(+) regulatory T cells differentiation. Front Immunol 2018;9:1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han Z, Chang L, Yamanishi Y, Karin M, Firestein GS.. Joint damage and inflammation in c-Jun N-terminal kinase 2 knockout mice with passive murine collagen-induced arthritis. Arthritis Rheum 2002;46:818–23. [DOI] [PubMed] [Google Scholar]
- 14. Jin J, Goldschneider I, Lai L.. In vivo administration of the recombinant IL-7/hepatocyte growth factor beta hybrid cytokine efficiently restores thymopoiesis and naive T cell generation in lethally irradiated mice after syngeneic bone marrow transplantation. J Immunol 2011;186:1915–22. [DOI] [PubMed] [Google Scholar]
- 15. Lai L, Zhang M, Goldschneider I.. Recombinant IL-7/HGFβ efficiently induces transplantable murine hematopoietic stem cells. J Clin Invest 2012;122:3552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lai L, Zhang M, Song Y, Rood D.. Recombinant IL-7/HGFβ hybrid cytokine enhances T cell recovery in mice following allogeneic bone marrow transplantation. PloS One 2013;8:e82998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song Y, Su M, Panchatsharam P, Rood D, Lai L.. c-Met signalling is required for efficient postnatal thymic regeneration and repair. Immunology 2015;144: 245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yan Y, Su M, Song Y. et al. Tbx1 modulates endodermal and mesodermal differentiation from mouse induced pluripotent stem cells. Stem Cells Dev 2014;23:1491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feldmann M, Brennan FM, Maini RN.. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 1996;14:397–440. [DOI] [PubMed] [Google Scholar]
- 20. Müssener Å, Litton MJ, Lindroos E, Klareskog L.. Cytokine production in synovial tissue of mice with collagen-induced arthritis (CIA). Clin Exp Immunol 1997;107:485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iwai H, Kozono Y, Hirose S. et al. Amelioration of collagen-induced arthritis by blockade of inducible costimulator-B7 homologous protein costimulation. J Immunol 2002;169:4332–9. [DOI] [PubMed] [Google Scholar]
- 22. Zhang F, Wei K, Slowikowski K. et al. ; Accelerating Medicines Partnership Rheumatoid Arthritis and Systemic Lupus Erythematosus (AMP RA/SLE) Consortium. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol 2019;20:928–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirpotina LN, Schepetkin IA, Hammaker D. et al. Therapeutic effects of tryptanthrin and tryptanthrin-6-oxime in models of rheumatoid arthritis. Front Pharmacol 2020;11:1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakaguchi S, Yamaguchi T, Nomura T, Ono M.. Regulatory T cells and immune tolerance. Cell 2008;133:775–87. [DOI] [PubMed] [Google Scholar]
- 25. Chen M, Su W, Lin X. et al. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum 2013;65:1181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Su M, Lin Y, Cui C. et al. ESC-derived thymic epithelial cells expressing MOG prevents EAE by central and peripheral tolerance mechanisms. Cell Immunol 2017;322:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Myers LK, Rosloniec EF, Cremer MA, Kang AH.. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci 1997;61:1861–78. [DOI] [PubMed] [Google Scholar]
- 28. Williams RO, Feldmann M, Maini RN.. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci USA 1992;89:9784–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berg WB, Joosten LAB, Helsen M, Loo FAJ.. Amelioration of established murine collagen-induced arthritis with anti-IL-1 treatment. Clin Exp Immunol 2008;95:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takagi N, Mihara M, Moriya Y. et al. Blockage of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum 1998;41:2117–21. [DOI] [PubMed] [Google Scholar]
- 31. Bishop GA, Hostager BS.. B lymphocyte activation by contact-mediated interactions with T lymphocytes. Curr Opin Immunol 2001;13:278–85. [DOI] [PubMed] [Google Scholar]
- 32. Wang Q, Ma Y, Liu D, Zhang L, Wei W.. The roles of B cells and their interactions with fibroblast-like synoviocytes in the pathogenesis of rheumatoid arthritis. Int Arch Allergy Immunol 2011;155:205–11. [DOI] [PubMed] [Google Scholar]
- 33. Borrego F. The CD300 molecules: an emerging family of regulators of the immune system. Blood 2013;121:1951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakano-Yokomizo T, Tahara-Hanaoka S, Nakahashi-Oda C. et al. The immunoreceptor adapter protein DAP12 suppresses B lymphocyte-driven adaptive immune responses. J Exp Med 2011;208:1661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parker DC. The functions of antigen recognition in T cell-dependent B cell activation. Semin Immunol 1993;5:413–20. [DOI] [PubMed] [Google Scholar]
- 36. Parker DC. T cell-dependent B cell activation. Annu Rev Immunol 1993;11:331–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.