Abstract
Splenic infarction in COVID-19 patients is a rare entity with few documented cases. We report a case of symptomatic complete splenic infarction and discuss COVID-19 related thrombosis, splenic infarction, diagnostic imaging for splenic infarction, and the management. Thrombotic events related to COVID-19 have been reported in unusual locations, and our case highlights an example of one such location, the splenic artery. Contrast enhanced Computed Tomography (CT) is the standard diagnostic modality and will typically reveal foci of hypo-enhancement, peripheral and wedge-shaped. CT angiography can be performed to evaluate the arteries and diagnose thrombosis. The primary treatment is aimed at addressing the underlying cause and includes supportive care. It is important that physicians consider splenic infarction as an explanation for abdominal pain in COVID-19 patients.
Keywords: Splenic infarction, COVID-19, Artery, Thrombosis, CT
Introduction
Severe acute respiratory syndrome from corona virus 2 (Sars-CoV-2) has greatly impacted the world. Infection with this virus results in Coronavirus disease 2019 (COVID-19). This presents primarily as a respiratory illness with a spectrum of multisystem involvement [1]. Data continues to delineate an association between the disease and hypercoagulable states [2]. Micro- and macro-vascular angiopathy has been observed in severe disease with multiple proposed mechanisms [3]. These proposed mechanisms include cytokine storm with endothelial involvement, hypoxia, massive immune response, antiphospholipid antibodies, and others [4], [5], [6], [7]. This relationship is significant because hypercoagulability in COVID-19 is associated with increased mortality [8]. Cases of both venous and arterial thromboses have been reported, with arterial thrombosis often occurs in unusual locations [1]. We present a case of splenic artery thrombosis in a 44-year-old male leading to infarction of the entire spleen. Our case brings to light this observed phenomenon of splenic infarction in COVID-19, for which there are few documented cases in the literature.
Case report
A 44-year-old African American male presented to the emergency department for shortness of breath, cough, and body aches which gradually worsened over several days. He reported exposure to COVID-19 patient and had not been vaccinated against the disease. His medical history includes hypertension, prior gastrointestinal hemorrhage, and obesity. Physical exam revealed diminished breathing sounds bilaterally. A chest X-ray was ordered and showed interstitial and alveolar opacities in both lungs consistent with COVID-19 pneumonia. A rapid COVID-19 test was positive, and the patient was treated supportively and discharged home later the same day after improvement of symptoms. Since the patient became symptomatic at the time of the peak of Delta variant activity in the United States, it was assumed to be the cause of his infection. Vaccines were available for about 6 months before the presentation of this unvaccinated patient.
Four days later, the patient returned to the emergency department with severe abdominal pain. Prior to coming to the emergency department, he had a syncopal episode at home while trying to have a bowel movement. After failing to improve with supportive measures, patient was admitted to the hospital for further evaluation of his intractable generalized abdominal pain.
The patient's respiratory status deteriorated to acute hypoxic respiratory failure. The patient was started on Remdesivir and eventually also received steroids. Workup for abdominal pain included EKG, troponin I, lipase, CBC, and urinalysis which were all unremarkable. A CT abdomen and pelvis with contrast was ordered and showed hypo-enhancement of the entire spleen, mild hepatomegaly, and a focus of atherosclerotic narrowing at the origin of the splenic artery (Figure 1). A subsequent CT angiogram showed a large filling defect within the celiac trunk that had progressed into the splenic artery and resulted in splenic artery occlusion and infarction (Figure 2). Patient was treated supportively and discharged in good condition, after a total of 2 days in the hospital. He was referred to vascular surgery for follow up.
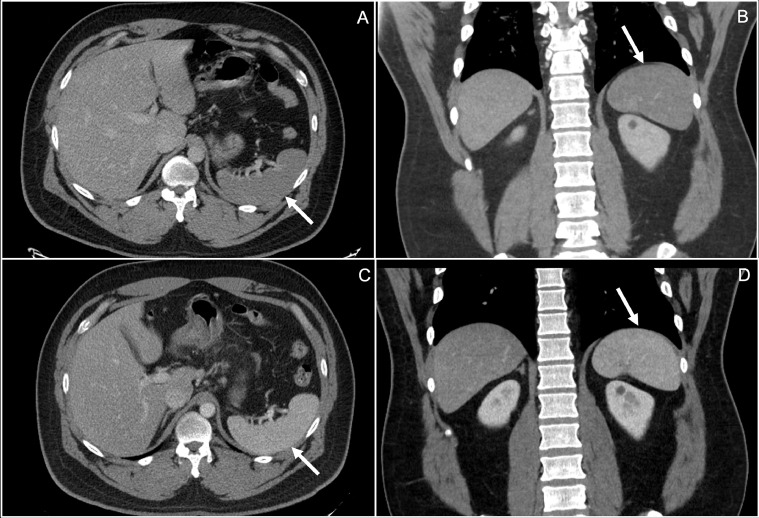
Fig. 1.
Axial and coronal CT images (A and B) showing hypo-enhancement of the entire spleen in the portal venous phase, a subtle finding when compared to the prior study, 2 mo before presentation, which shows normal enhancement of the spleen in the corresponding axial and coronal imaging (C and D).
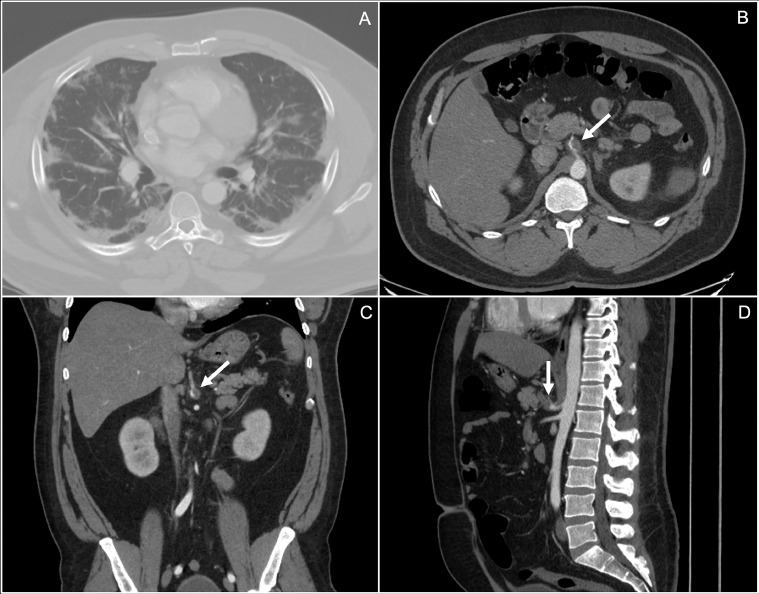
Fig. 2.
Axial image (A), in the lung window, showing characteristic peripheral ground glass, multifocal lung opacities bilaterally, consistent with COVID-19 pneumonia. Axial, coronal, and sagittal images (B, C, and D respectively) from the CT angiogram showing an occluding thrombus at the origin of the splenic vein.
Discussion
On March 11, 2020, the World Health Organization declared COVID-19 a global pandemic [9]. The disease has led to significant morbidity, death, and detrimental consequences around the world [3]. The primary target of the illness tends to be the lungs. In addition to acute lung injury, respiratory failure and multiorgan dysfunction may occur [1,2].
COVID-19 is well associated with a hypercoagulable state, especially in more severe disease [3]. A few detailed and interrelated mechanisms have been proposed which include the fundamentals of Virchow's triad of endothelial injury as well as abnormal coagulation parameters, low Factor XII levels, positive lupus anticoagulant, and others [1]. Further characterization of the exact processes that lead to hypercoagulability in COVID-19 continue to be presented in the literature. COVID-19 associated coagulopathy appears to be related to intrinsic proinflammatory immune response rather than a specific component of the virus [10]. It initially presents with elevation of D-dimer and fibrin/fibrinogen degradation products. In contrast, platelet count, prothrombin time, and partial thromboplastin time tend to exhibit minimal changes. Elevated D-dimer at the time of admission, sepsis, and microvascular thrombotic events are associated with increased mortality [10].
Pulmonary embolism is the most common thrombotic complication in COVID-19 [11]. Venous thromboembolism has been well described in the setting of COVID-19. The reported incidence of venous thromboembolism ranges from 20% to 27% [1]. In ICU patients, this is estimated to be as high as 69%. A study including 184 ICU patients from the Netherlands reported an overall 31% rate. Of this, venous thromboembolism comprised 27% of thromboses while arterial thromboses comprised 3.7% [12].Arterial thrombosis in the context of COVID-19 has also been reported less than venous thrombosis. Both thrombotic and embolic etiologies are possible as causes of arterial thrombosis [13].
Abdominal vessel thrombosis in the setting of COVID-19 is even less studied currently. It is vital to consider the possibility of visceral vessel involvement when investigating abdominal pain in COVID-19 patients. There are a handful of reported cases of splenic infarct, splanchnic vein thrombosis, and large vessel mesenteric arterial thrombosis [1,11,14,15].
Splenic infarction has been studied in association with many medical conditions. It is most associated with hematological disorders such as sickle cell anemia, lymphoma, leukemia, or with thromboembolic disorders such as atrial fibrillation or endocarditis [16]. It may arise from various etiologies such as hypercoagulability, structural heart disease, inflammation, infection, and trauma [11]. Additional causes include vascular injury following transcatheter embolization of splenic hemorrhage, collagen vascular disorders, malignancy, oral contraceptive use, antiphospholipid syndrome, aneurysm, torsion, wandering spleen, pancreatic disease, portal hypertension, and others [11,17]. Most cases are thought to resolve without sequelae such as rupture or abscess [16]. Usually, only 1 segment of the spleen is affected but global infarction of the organ is possible [11]. The classic clinical presentation of splenic infarction is acute abdominal pain and tenderness in the left upper quadrant or flank [18]. Nausea/vomiting may also be seen [11]. In up to 37.5% of cases, a left pleural effusion and/or flat atelectasis can be seen on chest X-ray at the time of admission [19].
It is difficult to determine the exact number of documented cases of splenic infarction in COVID-19. More cases are being documented, however, some of these cases are silent infarctions and many are multisystemic infarctions [20], [21], [22]. Our case is a symptomatic patient with an isolated complete splenic infarction secondary to arterial thrombosis. In a recent study from the American Journal of the Medical Sciences, Sztajnbok et al. reported 7 cases of splenic infarction in the context of COVID-19 and found that 5 of them had a complaint of abdominal pain [11]. Atrial fibrillation and hypercoagulability are predisposing factors to thrombotic events. These risk factors are often seen in patients with COVID-19 [11]. This offers at least a partial explanation for the increased thrombotic events in patients with COVID-19. Atrial fibrillation has been detected in 19%-21% of COVID-19 patients and up to 36% of cardiac patients [23]. The increased prevalence of atrial fibrillation in COVID-19 patients could increase the chance of thrombotic events such as splenic infarction with COVID-19 [11].
Splenic infarction appears differently on imaging in the acute phase versus the chronic phase [17]. In the acute phase, splenic parenchyma displays edema in areas of infarction due to inflammation and necrosis [17]. This results in poorly circumscribed areas of diminished attenuation/echogenicity [17]. As time passes, the region turns to more sharply demarcated areas of volume loss in the chronic phase with possible fibrosis and calcification [17]. The first line imaging in evaluating splenic parenchyma is ultrasound [11]. However, areas of infarction are frequently isoechoic and difficult to characterize in the acute phase meaning a lower sensitivity in diagnosis. Because of low diagnostic yield of sonography, CT is the preferred method of diagnosis [24]. Ultrasound is more useful in following up with patients to detect complications of splenic infarction such as abscess, peritoneal or subcapsular hemorrhage, or pseudocyst formation [24]. Contrast may be included with ultrasound which allows for better visualization of the infarct. This may be very beneficial in ICU patients who cannot be transported as ultrasound is portable and fast to a skilled sonographer [11]. Overall, however, the benefits of CT outweigh those of ultrasound in a patient who has no contraindications to CT. Contrast-enhanced CT allows for diagnosis and subsequent CT angiography can confirm [16].
CT performed during the portal venous phase is the ideal method of diagnosing splenic infarction during the acute phase [11]. The venous phase is recommended to avoid confusion with the characteristic enhancement of the splenic parenchyma in the arterial phase [13]. The benefits of contrast-enhanced CT are numerous. It can show infarction in the spleen, and other target organs, along with the extent of thrombosis [24]. The underlying cause of infarction may be identified on CT as well. Classically, a peripheral, wedge-shaped, hypo-enhancing lesion with the apex pointing toward the hilum and base parallel with the convex capsule is seen on imaging [11]. Often, imaging will instead reveal peripheral round or linear-shaped areas of hypodensity in the spleen [24]. With multiple infarcts, hypodense non-enhancing lesions with interposed normally enhancing splenic tissue may be observed [11].
Management of splenic infarction is primarily conservative and based on addressing the underlying cause. Data for non-COVID-19 patients reveals that majority of infarcts seen on initial CT will resolve without complications [16]. With cases of COVID-19 associated splenic infarction, close follow-up should be pursued. Analgesics, antiemetics, hydration, and other means of supportive treatment are appropriate [11]. In some instances, anticoagulation may be considered [11,24]. Rarely, surgical intervention or splenectomy may be required for complications or persistent symptoms [25]. In our case, the patient was treated supportively and discharged after 2 days with a referral to see vascular surgery.
Conclusion
As the COVID-19 pandemic progresses, more information regarding the virus and its effects is becoming available. Outside of lung disease, thrombosis is a primary area of concern. Thrombosis at unusual cites continues to be seen in hospitals and reported in the literature. Physicians should carefully consider the possibility of infarction of abdominal viscera, especially in cases with abdominal pain. Splenic infarction should be explored and considered on the differential for all COVID-19 patients presenting with abdominal pain [17]. CT is the best modality of diagnosis. Treatment should be aimed at addressing the underlying cause. We hope our case aids in outlining 1 of the few documented cases of splenic infarction associated with COVID-19. Treatment strategies should continue to be scrutinized for efficiency through the lens of data and literature.
Authorship
The authors declare that this is their original work and they all approve the content of this manuscript. They confirm that this manuscript has not been published previously, in any language, in whole or in part, and is not currently under consideration elsewhere.
Patient consent
A written informed consent was obtained from the patient for the publication of this case report.
Ethical clearance
This project did not involve any research and no ethical clearance was required.
Footnotes
Acknowledgments: This project did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests: The authors declare that they have no conflicting interests and have not been supported or funded by any drug company or authority.
References
- 1.Ghalib N., Pophali P., Chamorro-Pareja N., Jayarangaiah A., Kumar A. Incidental asymptomatic splenic infarct in a COVID-19 patient. Cureus. 2021;13(2):e13065. doi: 10.7759/cureus.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanff T.C., Mohareb A.M., Giri J., Cohen J.B., Chirinos J.A. Thrombosis in COVID-19. Am J Hematol. 2020;95(12):1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh B., Kaur P., Patel P., Nabati C., Ayad S., Shamoon F., et al. COVID-19 and arterial thrombosis: report of 2 cases. Radiol Case Rep. 2021;16(7):1603–1607. doi: 10.1016/j.radcr.2021.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez Canas E., Gimenez Gaibar A., Rodriguez Lorenzo L., Castro Rios J.G., Martinez Toiran A., Bella Cueto M.R., et al. Acute peripheral arterial thrombosis in COVID-19. role of endothelial inflammation. Br J Surg. 2020;107(10) doi: 10.1002/bjs.11904. e444-e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thachil J. Hypoxia-An overlooked trigger for thrombosis in COVID-19 and other critically ill patients. J Thromb Haemost. 2020;18(11):3109–3110. doi: 10.1111/jth.15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pineton de Chambrun M., Frere C., Miyara M., Amoura Z., Martin-Toutain I., Mathian A., et al. High frequency of antiphospholipid antibodies in critically ill COVID-19 patients: a link with hypercoagulability? J Intern Med. 2021;289(3):422–424. doi: 10.1111/joim.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruip M., Cannegieter S.C., Ten Cate H., van Gorp E.C.M., Juffermans N.P., Klok F.A., et al. Caging the dragon: research approach to COVID-19-related thrombosis. Res Pract Thromb Haemost. 2021;5(2):278–290. doi: 10.1002/rth2.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lango M.N. How did we get here? Short history of COVID-19 and other coronavirus-related epidemics. Head Neck. 2020;42(7):1535–1538. doi: 10.1002/hed.26275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sztajnbok J., Brasil L., Romero L.A., Ribeiro A.F., Vidal J.E., Figueiredo-Mello C., et al. Splenic infarction with aortic thrombosis in COVID-19. Am J Med Sci. 2021;362(4):418–423. doi: 10.1016/j.amjms.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Posada-Arango A.M., Garcia-Madrigal J., Echeverri-Isaza S., Alberto-Castrillon G., Martinez D., Gomez A.C., et al. Thrombosis in abdominal vessels associated with COVID-19 infection: a report of three cases. Radiol Case Rep. 2021;16(10):3044–3050. doi: 10.1016/j.radcr.2021.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Hoyo J., Lopez-Munoz P., Fernandez-de la Varga M., Garrido-Marin A., Valero-Perez E., Prieto M., et al. Hepatobiliary and Pancreatic: A fatal case of extensive splanchnic vein thrombosis in a patient with Covid-19. J Gastroenterol Hepatol. 2020;35(11):1853. doi: 10.1111/jgh.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasseh S., Trabelsi M.M., Oueslati A., Haloui N., Jerraya H., Nouira R. COVID-19 and gastrointestinal symptoms: a case report of a mesenteric large vessel obstruction. Clin Case Rep. 2021;9(6):e04235. doi: 10.1002/ccr3.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller L.A., Mirvis S.E., Shanmuganathan K., Ohson A.S. CT diagnosis of splenic infarction in blunt trauma: imaging features, clinical significance and complications. Clin Radiol. 2004;59(4):342–348. doi: 10.1016/j.crad.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Unal E., Onur M.R., Akpinar E., Ahmadov J., Karcaaltincaba M., Ozmen M.N., et al. Imaging findings of splenic emergencies: a pictorial review. Insights Imaging. 2016;7(2):215–222. doi: 10.1007/s13244-016-0467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brett A.S., Azizzadeh N., Miller E.M., Collins R.J., Seegars M.B., Marcus M.A. Assessment of clinical conditions associated with splenic infarction in adult patients. JAMA Intern Med. 2020;180(8):1125–1128. doi: 10.1001/jamainternmed.2020.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schattner A., Adi M., Kitroser E., Klepfish A. Acute splenic infarction at an academic general hospital over 10 years: presentation, etiology, and outcome. Med (Baltimore) 2015;94(36):e1363. doi: 10.1097/MD.0000000000001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennison J.J., Carlson S., Faehling S., Phelan H., Tariq M., Mubarik A. Splenic infarction and spontaneous rectus sheath hematomas in COVID-19 patient. Radiol Case Rep. 2021;16(5):999–1004. doi: 10.1016/j.radcr.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos Leite Pessoa M., Franco Costa Lima C., Farias Pimentel A.C., Godeiro Costa J.C., Bezerra Holanda J.L. Multisystemic Infarctions in COVID-19: Focus on the Spleen. Eur J Case Rep Intern Med. 2020;7(7) doi: 10.12890/2020_001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Mashdali A.F., Alwarqi A.F., Elawad S.M. Simultaneous renal infarction and splenic infarction as a possible initial manifestation of COVID-19: A case report. Clin Case Rep. 2021;9(11):e04819. doi: 10.1002/ccr3.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gawalko M., Kaplon-Cieslicka A., Hohl M., Dobrev D., Linz D. COVID-19 associated atrial fibrillation: Incidence, putative mechanisms and potential clinical implications. Int J Cardiol Heart Vasc. 2020;30 doi: 10.1016/j.ijcha.2020.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antopolsky M., Hiller N., Salameh S., Goldshtein B., Stalnikowicz R. Splenic infarction: 10 years of experience. Am J Emerg Med. 2009;27(3):262–265. doi: 10.1016/j.ajem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Nores M., Phillips E.H., Morgenstern L., Hiatt J.R. The clinical spectrum of splenic infarction. Am Surg. 1998;64(2):182–188. [PubMed] [Google Scholar]