Abstract
Organoids show great potential in clinical translational research owing to their intriguing properties to represent a near physiological model for native tissues. However, the dependency of organoid generation on the use of poorly defined matrices has hampered their clinical application. Current organoid culture systems mostly reply on biochemical signals provided by medium compositions and cell-cell interactions to control growth. Recent studies have highlighted the importance of the extracellular matrix (ECM) composition, cell-ECM interactions, and mechanical signals for organoid expansion and differentiation. Thus, several hydrogel systems prepared using natural or synthetic-based materials have been designed to recreate the stem cell niche in vitro, providing biochemical, biophysical, and mechanical signals. In this review, we discuss how recapitulating multiple aspects of the tissue-specific environment through designing and applying matrices could contribute to accelerating the translation of organoid technology from the laboratory to therapeutic and pharmaceutical applications.
Keywords: Stem Cell Niche, Organoid Engineering, Extracellular Matrix, Hydrogel
Introduction
Recent studies of stem cell behavior and stem cell niche have led to the development of complex three-dimensional (3D) organoid culture systems (1, 2). In the presence of specific biochemical and biophysical cues that mimic the in vivo microenvironment, stem cells constantly proliferate; some populations differentiate into multiple cell types, as are observed in organs. These cells then self-assemble into complex structures based on the same intrinsic organization principles of their tissue of origin. Compared with conventional two-dimensional (2D) cultures, 3D organoids better mimic the complex aspects of the target organ in terms of histology, metabolism, and functionality (3). Organoids can be initiated either from tissue-resident adult stem cells (ASCs) or from pluripotent stem cells (PSCs), including embryonic stem cells and induced PSCs (iPSCs).
Stimulation of the stem cell niche established by the extracellular matrix (ECM) and conditioned medium is essential for promoting the self-organization and differentiation of stem cells in culture. Organoids are commonly cultured in a 3D hydrogel system as scaffolds. Hydrogels can have various levels of modulus, topography, permeability, and biodegradability, all of which have profound effects on stem cell fate (4). ECM protein-based hydrogels are often used for organoid expansion, among which Matrigel is recognized as the “golden standard” material. Matrigel is a basement membrane extract, purified from Engelbreth-Holm-Swarm mouse sarcoma, which is mostly composed of laminin and collagen IV (5, 6). The applications of Matrigel over the recent years have greatly exceeded other biomaterials, since long to support organoid growth. However, Matrigel has limited use in translational therapies, chiefly because Matrigel is derived from mouse sarcoma and therefore contains tumor-derived growth factors and enzymes (5, 7, 8). Moreover, Matrigel cannot be easily manipulated to generate specific organoid niches for targeting organs, thereby suffering from limited maturation cell-responsiveness. Additionally, lot-to-lot variability owing to inherent compositional variation also limits the clinical application of this biomaterial. Accordingly, the development of alternative hydrogel systems that are amendable to the clinical setting and closely mimic the in vivo stem cell niche may increase the applications and versatility of organoid technology. Recent studies have evaluated various natural and synthetic hydrogels as alternatives to Matrigel. In this review, we aim at providing an overview on the use of ECM-based hydrogel systems as alternatives of Matrigel for organoid cultures. A variety of scaffolds have been explored for organoid cultures, including decellularized tissue-derived scaffolds, natural polymer- and synthetic polymer-based scaffolds (Table 1). We first briefly summarize key roles of ECM as stem cell niche.
Table 1.
Various types of biomaterials as alternatives of Matrigel for organoid culture
| Materials | Material type | Cell type | Reference |
|---|---|---|---|
| Natural | Collagen | ASC-derived intestinal organoid | (12, 13) |
| ASC-derived stomach organoid | (14) | ||
| ASC-derived colon organoid | (14) | ||
| ESC-derived kidney organoid | (15) | ||
| Primary mammary epithelial cell-derived mammary organoid | (16) | ||
| Alginate | PSC-derived intestinal organoid | (17, 18) | |
| Collagen–laminin–fibronectin–hyaluronan | Primary mammary epithelial cell-derived mammary organoid | (20) | |
| Fibrin–laminin | ASC-derived small intestinal, pancreatic and liver organoids | (22) | |
| Decellularized tissue | Brain | PSC-derived brain organoid | (28, 29) |
| Islet | ASC-derived islet organoid | (32, 33) | |
| Endometrium | ASC-derived endometrium organoid | (36) | |
| Testicle | ASC-derived testicular organoid | (35) | |
| Retina | ASC-derived retinal organoid | (34) | |
| ASC-derived intestinal organoid | (30, 31) | ||
| Intestine | ASC-derived hepatocyte organoid | (31) | |
| ASC-derived pancreatic organoid | (31) | ||
| Synthetic | PEG | PSC-derived cardiac organoid | (38) |
| ASC-derived intestinal enteroid and endometrial organoid | (37, 40) | ||
| PSC-derived intestinal organoid | (37, 39) | ||
| PSC-derived lung organoid | (37, 39) | ||
| PLGA | PSC-derived intestinal organoid | (45, 46) | |
| PSC-derived lung organoid | (45) | ||
| PCL | PSC-derived lung organoid | (45, 47) | |
| pNIPAM | ASC-derived intestinal organoid | (47) | |
| PVA | PSC-derived kidney organoid | (48) | |
| Hybrid | PEG-fibrin | PSC-derived liver organoid | (44) |
| PEG-gelatin | ASC-derived liver organoid | (43) |
Roles of the ECM as a Stem Cell niche
The ECM is a fibrous network of macromolecules that surrounds cells. Although the composition and organization of the ECM vary among organs, the major components include fibrous proteins, glycoproteins, proteoglycans, and glycosaminoglycans. In organs, the ECM has tissue-specific architecture with topographical cues and elastic properties that transmit biochemical and biomechanical signals. These signals regulate cell adhesion, migration, proliferation, and differentiation, thereby guiding cell fate and developmental processes (9). Thus, the specific dynamics of the ECM are almost inseparable from the identity of the cell within the organ. Stem cells can be directly affected by the mechanical properties of the ECM (10) via sensing of such external forces and can exert intrinsic forces accordingly. Interactions between the ECM and cells, mediated by integrins and other receptors, play key roles in transmitting signals to cells, leading to the activation of various signaling cascades (11). The ECM can also store and release growth factors and other signaling molecules, and ECM components can be released through ECM cleavage by proteases that regulate ECM architecture and influence cell behavior. Therefore, when recreating the stem cell niche in vitro by introducing matrices, the design should consider biochemical and mechanical factors. Here, we will introduce a range of biomaterials that have been shown to have applications as matrices for organoids, and we will discuss how these materials contribute to the ECM-like environment required for organoid growth.
Natural polymer-based scaffolds
Natural polymer-based hydrogels can be derived from proteins, such as collagen and fibrin, as well as polysaccharides, such as hyaluronic acid, alginate, chitosan, and cellulose. Hydrogels composed of individual ECM polymers and combinations thereof have been applied to create simple culture systems.
Type I collagen, as the most abundant protein present in mammals, has been extensively investigated, is readily available, and has been approved by the United States Food and Drug Administration (FDA) for several applications. Collagen hydrogels have been applied in ASC-derived small intestinal, stomach, and colonic organoids (12-15) and in human PSC-derived polycystic kidney disease organoids (15). Small intestinal organoids grow as budding cysts in Matrigel droplets, but display a smooth appearance without buds and often form a monolayer with an enteroid pattern when grown in collagen (12, 14). Moreover, when cultured in a floating collagen ring, organoids fuse and form a continuous macroscopic structure. Recently, floating collagen hydrogel culture of single primary human mammary epithelial cells was shown to induce the formation of branched multicellular organoids (16). During branch elongation, cells invade the collagen matrix by branch-internal collective cell migration until branch outgrowth stops, forming alveoli-like end buds. Movement of cells within the branches causes tension that induces the reorganization of the surrounding collagen meshwork and leads to the formation of a mechanically stable cage. This collagen casing was shown to induce more tension and promote branch elongation and collagen deformation, thereby creating spatial confi-nement. In another study by Sachs et al. (13), collagen was used to create macroscopic structures out of organoids by culturing intestinal organoids in a floating collagen ring. They found that the organoids fused and formed a continuous large-scale construct.
Alginate, a seaweed-derived polysaccharide, has also been used as a hydrogel to support intestinal (17) and islet organoid culture (18). Alginate precursor solution was gelled by the ionotropic method (i.e. exposed to CaCl2 (17) or BaCl2 (18) solution). Organoids cultured in alginate hydrogel promoted viability, crypt structure, and protein expression comparable to Matrigel. However, organoid yields were lower for cells cultured with alginate when maintained for a longer term. Because of its inert nature, low toxicity, ease of gelation, and tunable properties, alginate has been approved for use by the FDA and has been widely used for cell encapsulation. However, alginate alone lacks cell recognition sites and signaling proteins provided by the ECM.
Multiple isolated ECM proteins and carbohydrate components can also be used to prepare 3D scaffolds. For example, Curvello et al. (19) designed an ECM multicom-posite hydrogel by mixing collagen with RGD (Arg-Gly-Asp) peptide-functionalized cellulose nanofibers to promote the formation and growth of intestinal orga-noids. RGD peptide was first grafted to cellulose fibers by EDC/NHS coupling, and the resulting RGD-nanocellulose pre-gel solution was then physically mixed with pH-adjusted collagen pre-gel solution. Gelation was then induced via physiological temperature that promoted collagen fibril assembly driven by an increase in entropy (19). Crypts embedded in collagen-RGD-nanocellulose hydrogel were found to form organoids with clear epithelial budding, and the resulting cell viability and metabolic activity of the organoids were comparable to those of organoids cultured in Matrigel. Additionally, Sokol et al. (20) generated ECM hydrogels composed of type I collagen, hyaluronan, laminin, and fibronectin to provide adhesion sites and support breast organoid formation (20). This hydrogel closely mimicked the in vivo environment in a simplistic and defined way. In terms of structural integrity, the proteins of the composite, including collagen, laminin, and fibronectin, provide tensile strength, whereas the carbohydrate hyaluronan resists compressive forces. Overall, this multicomponent ECM hydrogel was found to have a higher swelling ratio and elastic modulus compared with collagen hydrogel alone, likely owing to the inclusion of hyaluronans. When seeded in the ECM hydrogel, mammary epithelial cells were found to self-organize, expand, and form complex ductal and lobular morphologies resembling epithelial structures of human tissues. By contrast, ductal growth was limited when seeded in collagen or Matrigel. More recently, Homan et al. (21) developed human iPSC-derived vascularized kidney organoids by optimizing ECM, medium composition, fluidic shear stress, and coculture with endothelial cells in a millifluidic culture system. Partial embedding of developing kidney organoids in a thick layer of gelatin and fibrin matrices was shown to enhance the peripheral expression of vascular markers compared with a layer composed of fibrin alone or with type I collagen. Broguiere et al. (22) investigated whether well-defined matrices could support various epithelial organoids comparable to Matrigel. The authors modified polyethylene glycol (PEG), hyaluronan, alginate, and fibrin hydrogels to become degradable or deformable, reasoning that the organoids would require physical space as they increased in size during culture (22). PEG and hyaluronan gels contained a matrix metalloproteinase (MMP)-sensitive peptide sequence, whereas alginate was weakly crosslinked, and fibrin was inherently degradable. Among the four scaffolds supplemented with 10% (v/v) Matrigel, fibrin showed small intestinal organoid formation efficiency similar to that of pure Matrigel. In order to completely eliminate the use of Matrigel in the hydrogel system, fibrin was supplemented with the main ECM components of Matrigel: collagen IV, heparin, and laminin-111/entactin complex (22). Fibrinogen and low content of nanofibrillar cellulose was supplemented with various ECM proteins and polymerization was induced by adding thrombin. The nanocellulose was added to increase the viscosity during gelation to prevent the sedimentation of organoids. Out of ECM components, laminin was found to be the critical signaling molecule required for fibrin-laminin hydrogel-supported long-term expansion of small intestinal, pancreatic, and liver organoids comparable to that in pure Matrigel (22).
Decellularized tissue-derived scaffolds
Naturally-derived materials from decellularized tissues have several advantages for hydrogel formation. Dec-ellularized tissue ECM (dECM) preserves important biochemical signals, such as growth factors, glycans, bioactive cryptic peptides, and natural proteins that are fundamental for cell growth, differentiation, and function (23, 24). Decellularized tissues are approved by the FDA and have been already used clinically for tissue regeneration (25). Hydrogels derived from decellularized tissue also have the advantage of being good manufacturing practice-compliant and FDA-approved (26). Decellularization is commonly accomplished by chemical, enzymatic, and/or physical methods (27). After the decellularization step, decellularized tissues are dehydrated, and the resulting powder is then solubilized into protein monomeric components (26). The most prevalent method used to solubilize the powder form of dECM is to use pepsin in an acidic condition. The solubilized dECM is then neutralized to physiologic pH, salt concentration and temperature dominated by collagen assembly (26). dECM-based hydrogels have been used for the culture of brain (28, 29), endodermal (30, 31), islet (32, 33), retinal (34), testicular (35), and endometrial (36) organoids. Additionally, Giobbe et al. (31) demonstrated that endoderm-derived organoids, including small intestinal, gastric, hepatic, and pancreatic organoids, could be cultured in the decellularized porcine small intestine mucosa/submucosa. Compared with endodermal organoids cultured in Matrigel, no significant differences were observed in terms of viability and morphology during the first 3∼4 passages. Transcriptomic analysis of human pediatric small intestinal organoids revealed that the expression of crypt stem cell markers was upregulated in the dECM hydrogel, whereas some of the differentiated intestinal cells were slightly upregulated in Matrigel. The dECM has also been used for brain organoid culture (28, 29). Decellularized human brain ECM (BEM) was shown to provide important biochemical and biophysical cues necessary to guide brain organogenesis at an early phase (29). The authors observed that brain organoids encapsulated in BEM exhibited enhanced proliferation of neural progenitors and increased neuronal differentiation. These organoids displayed well-organized structures with enhanced radial polarity.
The downstream translational application of ECM-derived hydrogels is related to the high variability of laboratory-derived products. Cho et al. (29) investigated the variability of human BEM by examining batch variation (different tissue batches derived from the same donor), sample variation (tissues derived from different donors), and regional variation (tissues derived from different brain regions) by proteomic analysis. The authors found substantial differences in ECM composition between different brain regions, whereas batch-to-batch variation arising from the decellularization process and different donor samples was relatively low. The variability of dECM-derived hydrogels may arise after lyophilization, including solubilization of the dECM powder and gelation of the dECM solution. A study by Dorgau et al. (34) used decellularized neural retina and retinal pigment epithelium ECM as culture medium supplements to guide retinal organogenesis. Supplementation with dECM was shown to enhance the functionality of retinal organoids, including increased ribbon synapse marker expression and light responsiveness. Bi et al. (32) investigated the key ECM components in dECM hydrogels to develop chemically defined materials for islet organoid development and showed that type V collagen was abundantly present in the decellularized pancreatic ECM hydrogel. Type V collagen shows tissue-specific distribution and is highly conserved in the pancreas across species. In the presence of type V collagen in 2D substrates, human iPSCs self-assembled to form islet organoids with enhanced islet-related gene expression and secretion of insulin and glucagon in response to glucose.
Synthetic polymer-based scaffolds
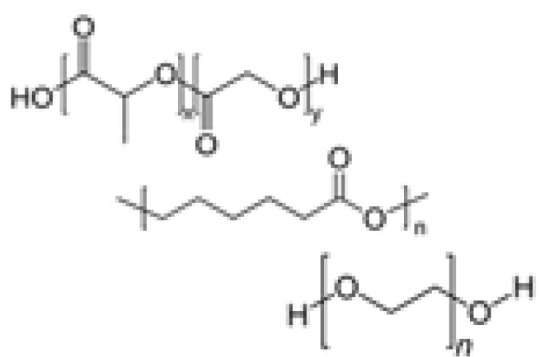
Given limitations in processability and the complexity of natural matrices, chemically defined synthetic polymer hydrogels have emerged. Although synthetic-based materials cannot yet recapitulate the complexity of the ECM in vivo, precise control and consistent fabrication processes confer organoid cultures with high reproducibility. Recently, several hydrogel systems have been developed with tunable properties to mimic mechanical, rheological, and chemical factors. The common synthetic polymers that have been reported include PEG (37-44), poly (lactic-co-glycolic acid) (PLGA) (45, 46), polycaprolactone (PCL) (45, 47), poly (N-isopropylacrylamide) (pNIPAM) (47), and poly (vinyl alcohol) (PVA) (48). Synthetic polymers are often functionalized with cell-adhesive peptides and sequences that can be cleaved by MMP via chemical/enzymatic crosslinking to mimic specific cell-cell interactions relevant to morphogenetic processes.
Gjorevski et al. (41, 42) introduced a PEG-based hydrogel system that can support intestinal stem cell expansion and differentiation into organoids. In this system, RGD and laminin-111 were conjugated to PEG as minimal adhesive ligands. The authors also designed a mechanically dynamic system by blending mechanically static PEG-vinylsulfone and hydrolytically degradable PEG-acrylate. This hybrid hydrogel was found to afford initial high stiffness (≈ 1.3 kPa) to promote the cell expansion and subsequently soften (≈ 200 Pa) to alleviate the accumulation of compressive forces and promoted organoid formation with differentiated phenotype. A subsequent study reported a completely synthetic hydrogel system based on four-arm PEG with maleimide groups at each terminus, and functionalized with bioactive ligands (e.g. RGD adhesive peptide) and crosslinked with the protease-degradable peptide GPQ-W (37, 39). This PEG-based hydrogel supported viability and expansion of intestinal organoids. Delivery of human PSC-derived intestinal organoids to intestinal wounds using a murine colonoscope resulted in successful organoid survival, engraftment, and wound repair (39). This same PEG hydrogel system could also be applied to culture lung organoids. In another study, mouse and human intestinal crypts embedded in functionalized PEG hydrogels have demonstrated to support organoid growth (40). Eight-arm PEG was modified with integrin α2β1 ligand GFOGERRGD and crosslinked with MMP-sensitive peptide. The resulting PEG-based hydrogel supported long term culture of the organoids, but the proliferation rate was relatively lower compared to that in Matrigel (40). A more recent study demonstrated that PEG-based hydrogel could support differentiation of human liver organoids (43). In this study, gelatin and PEG were covalently crosslinked enzymatically via coagulation factor XIIIa (FXIIIa). FXIII-specific amino acid sequence was conjugated to 8 arm PEG which was crosslinked with the native lysine residues on gelatin. Coupling of laminin 111 via naturally occurring lysine residues on the protein into the PEG-based hydrogel improved liver organoid differentiation and function. These studies have demonstrated that both mechanical and biochemical properties were important for the organoid formation and expansion (39, 40, 43). The presence of adhesive sites and degradable sites were the fundamental in these matrices. Other fully synthetic polymers have been applied for intestinal organoid culture. NIPAM-Laponite clay nanoparticles were shown to result in the formation of spherical shapes with no central lumen (47). This hydrogel system seemed to support intestinal organoid growth with buds only after initial culture in Matrigel.
Synthetic polymers can also be used as a nonhydrogel system. For example, a 2D PEG-based micropatterned substrate was used to induce geometric confinement during cardiac differentiation of human iPSCs (38). With the optimal geometric confinement, spatially organized cardiac organoids were generated with cardiomyocytes present in the center, surrounded by stromal cells distributed along the pattern perimeter. Geometric confinement could be provided by a PEG-based micropatterned substrate. PEG can also be used as a 2D substrate coating material to promote attachment of intestinal organoid epithelium on a hydrophobic polydimethylsiloxane-based microdevice (49). In another case, PLGA microfilaments were used as a floating scaffold to generate elongated embryoid bodies, resulting in improved neuroectoderm formation and cortical development in human iPSC-derived cerebral organoids (50).
Conclusions
The development of organoids has been a major breakthrough in simulating physiological organ architecture in vitro. Organoid technology can be used in various applications, including drug development, toxicity screening, disease modeling, and cell therapy. Moreover, the use of organoids can address fundamental biological questions. Matrigel is commonly used as a universal matrix for the generation of almost all types of organoids without considering the effects of tuning tissue-specific factors. Various alternative scaffold systems have recently been designed to meet the needs of organoid culture its use in clinical applications.
Each system has advantages and drawbacks for organoid cultures (Table 2). Natural polymers often provide high biocompatibility, biodegradability, low toxicity, and specific signaling cues. In order to reduce complexity and increase reproducibility, natural single polymers have been employed for organoid culture. These components are derived from the ECM, and some are bioactive; however, further in-depth studies are required to determine whether these systems support long-term organoid growth and differentiation similar to Matrigel. Moreover, these polymers often show limited processability and exhibit relatively poor rheological properties. Naturally-derived dECM hydrogels retain important biochemical signals of the tissue of origin that can support organoid growth and differentiation. Similar to Matrigel, the control of batch-to-batch variation and tunability still need to be addressed. Recently, Cho et al. (29) suggested that the variability of dECM composition may arise from the isolated region of the tissue. Hence, reproducibility may be improved by standardizing the tissue isolation process. Synthetic polymers have several advantages that these systems are xeno-free, chemically defined and reproducible, thus guarantee higher levels of experimental accuracy. In order to increase bioactivity, synthetic matrices can be functionalized to promote cell adhesion and cell-driven matrix remodeling. In contrast to natural polymers, the mechanical properties and chemical composition of synthetic polymers can be precisely modulated and almost independently of each other. These properties offer a powerful platform to deconstruct the complexity of native microenvironments and define their key instructive signals. The effects of individual or combinations of various cues can be probed systematically to study particular biological processes during organogenesis. However, synthetic matrices can only partially reproduce some native ECM features with low number of biologically active sites. Additionally, synthetic systems cannot easily achieve multifactorial parameters for directing cell behaviors in organoid cultures.
Table 2.
Advantages and disadvantages of biomaterials for organoid culture.
|
|
|
|
|
|---|---|---|---|---|
| Matrigel | Natural polymers | dECM | Synthetic polymers | |
| Advantages |
|
|
|
|
| Disadvantages |
|
|
|
|
We have reviewed the use of several hydrogel systems for organoid culture (Table 1). Despite of the rapid progress, the development of the organoid culture systems is still in the infant stage. The ECM is chemically and physically dynamic via numerous binding sites, and mimicry of this complexity for organoid culture remains a challenge. There are several potential strategies to overcome the fundamental limitations of each type of hydrogel systems, but yet have not been validated for organoid culture. For example, the dECM can also be modified with methacrylate (51) and catechol moieties (52) to enhance mechanical and adhesive characteristics. Natural polymers could be combined with synthetic polymers, creating hybrid polymers to provide various physicochemical and biological characteristics, which may compensate the synergic effect between synthetic and natural polymeric constituents. A dual-stage polymerization system, composed of thiolated dECM and PEG-acrylate, having large number of biologically active sties and dynamic mechanical properties (53), could be employed to mimic mechanical changes during tissue development or disease progression. We envisage that ongoing expansive development of advanced hydrogel systems that leads to the generation of multifactorial signals that encode the constraints of dynamic tissue-specific cues, and in depth validation of the reliability of specific composite hydrogels for organoid cultures will be crucial to make practical differences across numerous downstream applications.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea (NRF; grant no. 2021R1 C1C2009131) funded by the Ministry of Science and ICT (MSIT), Republic of Korea. This study was also supported by a new faculty research seed money grant (6-2021-0243) of Yonsei University College of Medicine for 2021. Illustrations in Table 1 were created with BioRender.com.
Footnotes
Potential Conflict of Interest
The authors have no conflicting financial interests.
References
- 1.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 2.Simian M, Bissell MJ. Organoids: a historical perspective of thinking in three dimensions. J Cell Biol. 2017;216:31–40. doi: 10.1083/jcb.201610056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571–584. doi: 10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofer M, Lutolf MP. Engineering organoids. Nat Rev Mater. 2021;6:402–420. doi: 10.1038/s41578-021-00279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Corning Incorporated, author. CorningⓇ MatrigelⓇ Matrix [Internet] Corning; Corning (NY): 2019. [cited 2021 May 7]. Available from: https://www.corning.com/catalog/cls/documents/faqs/CLS-DL-CC-026.pdf . [Google Scholar]
- 7.Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-Q. [DOI] [PubMed] [Google Scholar]
- 8.Gillette KM, Forbes K, Sehgal I. Detection of matrix metalloproteinases (MMP), tissue inhibitor of metalloproteinase-2, urokinase and plasminogen activator inhibitor-1 within matrigel and growth factor-reduced matrigel basement mem-brane. Tumori. 2003;89:421–425. doi: 10.1177/030089160308900415. [DOI] [PubMed] [Google Scholar]
- 9.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840:2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017;18:728–742. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jabaji Z, Sears CM, Brinkley GJ, Lei NY, Joshi VS, Wang J, Lewis M, Stelzner M, Martín MG, Dunn JC. Use of collagen gel as an alternative extracellular matrix for the in vitro and in vivo growth of murine small intestinal epithelium. Tissue Eng Part C Methods. 2013;19:961–969. doi: 10.1089/ten.tec.2012.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachs N, Tsukamoto Y, Kujala P, Peters PJ, Clevers H. Intestinal epithelial organoids fuse to form self-organizing tubes in floating collagen gels. Development. 2017;144:1107–1112. doi: 10.1242/dev.143933. [DOI] [PubMed] [Google Scholar]
- 14.Jee JH, Lee DH, Ko J, Hahn S, Jeong SY, Kim HK, Park E, Choi SY, Jeong S, Lee JW, Cho HJ, Kwon MS, Yoo J. Development of collagen-based 3D matrix for gastrointestinal tract-derived organoid culture. Stem Cells Int. 2019;2019:8472712. doi: 10.1155/2019/8472712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruz NM, Song X, Czerniecki SM, Gulieva RE, Churchill AJ, Kim YK, Winston K, Tran LM, Diaz MA, Fu H, Finn LS, Pei Y, Himmelfarb J, Freedman BS. Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat Mater. 2017;16:1112–1119. doi: 10.1038/nmat4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchmann B, Engelbrecht LK, Fernandez P, Hutterer FP, Raich MK, Scheel CH, Bausch AR. Mechanical plasticity of collagen directs branch elongation in human mammary gland organoids. Nat Commun. 2021;12:2759. doi: 10.1038/s41467-021-22988-2.ca6fd98434244dba96daeb649f0b2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capeling MM, Czerwinski M, Huang S, Tsai YH, Wu A, Nagy MS, Juliar B, Sundaram N, Song Y, Han WM, Takayama S, Alsberg E, Garcia AJ, Helmrath M, Putnam AJ, Spence JR. Nonadhesive alginate hydrogels support growth of pluripotent stem cell-derived intestinal orga-noids. Stem Cell Reports. 2019;12:381–394. doi: 10.1016/j.stemcr.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel SN, Ishahak M, Chaimov D, Velraj A, LaShoto D, Hagan DW, Buchwald P, Phelps EA, Agarwal A, Stabler CL. Organoid microphysiological system preserves pancreatic islet function within 3D matrix. Sci Adv. 2021;7:ea–ba5515. doi: 10.1126/sciadv.aba5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curvello R, Alves D, Abud HE, Garnier G. A thermo-responsive collagen-nanocellulose hydrogel for the growth of intestinal organoids. Mater Sci Eng C Mater Biol Appl. 2021;124:112051. doi: 10.1016/j.msec.2021.112051. [DOI] [PubMed] [Google Scholar]
- 20.Sokol ES, Miller DH, Breggia A, Spencer KC, Arendt LM, Gupta PB. Growth of human breast tissues from patient cells in 3D hydrogel scaffolds. Breast Cancer Res. 2016;18:19. doi: 10.1186/s13058-016-0677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods. 2019;16:255–262. doi: 10.1038/s41592-019-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broguiere N, Isenmann L, Hirt C, Ringel T, Placzek S, Cavalli E, Ringnalda F, Villiger L, Züllig R, Lehmann R, Rogler G, Heim MH, Schüler J, Zenobi-Wong M, Schwank G. Growth of epithelial organoids in a defined hydrogel. Adv Mater. 2018;30:e1801621. doi: 10.1002/adma.201801621. [DOI] [PubMed] [Google Scholar]
- 23.Jin Y, Kim J, Lee JS, Min S, Kim S, Ahn DH, Kim YG, Cho SW. Vascularized liver organoids generated using induced hepatic tissue and dynamic liver-specific microenvironment as a drug testing platform. Adv Funct Mater. 2018;28:1801954. doi: 10.1002/adfm.201801954. [DOI] [Google Scholar]
- 24.Jin Y, Shahriari D, Jeon EJ, Park S, Choi YS, Back J, Lee H, Anikeeva P, Cho SW. Functional skeletal muscle regeneration with thermally drawn porous fibers and reprogrammed muscle progenitors for volumetric muscle injury. Adv Mater. 2021;33:e2007946. doi: 10.1002/adma.202007946. [DOI] [PubMed] [Google Scholar]
- 25.Liao J, Xu B, Zhang R, Fan Y, Xie H, Li X. Applications of decellularized materials in tissue engineering: advantages, drawbacks and current improvements, and future perspectives. J Mater Chem B. 2020;8:10023–10049. doi: 10.1039/D0TB01534B. [DOI] [PubMed] [Google Scholar]
- 26.Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater. 2017;49:1–15. doi: 10.1016/j.actbio.2016.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilpin A, Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to appli-cations. Biomed Res Int. 2017;2017:9831534. doi: 10.1155/2017/9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simsa R, Rothenbücher T, Gürbüz H, Ghosheh N, Emneus J, Jenndahl L, Kaplan DL, Bergh N, Serrano AM, Fogelstrand P. Brain organoid formation on decellularized porcine brain ECM hydrogels. PLoS One. 2021;16:e0245685. doi: 10.1371/journal.pone.0245685.27ddc35147c349cd802f99a4f0c700ec [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho AN, Jin Y, An Y, Kim J, Choi YS, Lee JS, Kim J, Choi WY, Koo DJ, Yu W, Chang GE, Kim DY, Jo SH, Kim J, Kim SY, Kim YG, Kim JY, Choi N, Cheong E, Kim YJ, Je HS, Kang HC, Cho SW. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat Commun. 2021;12:4730. doi: 10.1038/s41467-021-24775-5.4baaa6ea520348a5bdc13ee2f2a1412c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz DM, Pehlivaner Kara MO, Goldstein AM, Ott HC, Ekenseair AK. Spray delivery of intestinal organoids to reconstitute epithelium on decellularized native extracellular matrix. Tissue Eng Part C Methods. 2017;23:565–573. doi: 10.1089/ten.tec.2017.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giobbe GG, Crowley C, Luni C, Campinoti S, Khedr M, Kretzschmar K, De Santis MM, Zambaiti E, Michielin F, Meran L, Hu Q, van Son G, Urbani L, Manfredi A, Giomo M, Eaton S, Cacchiarelli D, Li VSW, Clevers H, Bonfanti P, Elvassore N, De Coppi P. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat Commun. 2019;10:5658. doi: 10.1038/s41467-019-13605-4.a28b06f5539f456b97162079de3a5a5a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bi H, Ye K, Jin S. Proteomic analysis of decellularized pancreatic matrix identifies collagen V as a critical regulator for islet organogenesis from human pluripotent stem cells. Biomaterials. 2020;233:119673. doi: 10.1016/j.biomaterials.2019.119673. [DOI] [PubMed] [Google Scholar]
- 33.Bi H, Karanth SS, Ye K, Stein R, Jin S. Decellularized tissue matrix enhances self-assembly of islet organoids from pluripotent stem cell differentiation. ACS Biomater Sci Eng. 2020;6:4155–4165. doi: 10.1021/acsbiomaterials.0c00088. [DOI] [PubMed] [Google Scholar]
- 34.Dorgau B, Felemban M, Hilgen G, Kiening M, Zerti D, Hunt NC, Doherty M, Whitfield P, Hallam D, White K, Ding Y, Krasnogor N, Al-Aama J, Asfour HZ, Sernagor E, Lako M. Decellularised extracellular matrix-derived peptides from neural retina and retinal pigment epithelium enhance the expression of synaptic markers and light responsiveness of human pluripotent stem cell derived retinal organoids. Biomaterials. 2019;199:63–75. doi: 10.1016/j.biomaterials.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 35.Vermeulen M, Del Vento F, Kanbar M, Pyr Dit Ruys S, Vertommen D, Poels J, Wyns C. Generation of organized porcine testicular organoids in solubilized hydrogels from decellularized extracellular matrix. Int J Mol Sci. 2019;20:5476. doi: 10.3390/ijms20215476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francés-Herrero E, Juárez-Barber E, Campo H, López-Martínez S, de Miguel-Gómez L, Faus A, Pellicer A, Ferrero H, Cervelló I. Improved models of human endometrial organoids based on hydrogels from decellularized endometrium. J Pers Med. 2021;11:504. doi: 10.3390/jpm11060504.e3738e6c805b4812807a4e93f8a15c60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cruz-Acuña R, Quirós M, Huang S, Siuda D, Spence JR, Nusrat A, García AJ. PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nat Protoc. 2018;13:2102–2119. doi: 10.1038/s41596-018-0036-3. Erratum in: Nat Protoc 2019;14:2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoang P, Kowalczewski A, Sun S, Winston TS, Archilla AM, Lemus SM, Ercan-Sencicek AG, Gupta AR, Liu W, Kontaridis MI, Amack JD, Ma Z. Engineering spatial-organized cardiac organoids for developmental toxicity testing. Stem Cell Reports. 2021;16:1228–1244. doi: 10.1016/j.stemcr.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cruz-Acuña R, Quirós M, Farkas AE, Dedhia PH, Huang S, Siuda D, García-Hernández V, Miller AJ, Spence JR, Nusrat A, García AJ. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat Cell Biol. 2017;19:1326–1335. doi: 10.1038/ncb3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez-Gordillo V, Kassis T, Lampejo A, Choi G, Gamboa ME, Gnecco JS, Brown A, Breault DT, Carrier R, Griffith LG. Fully synthetic matrices for in vitro culture of primary human intestinal enteroids and endometrial organoids. Biomaterials. 2020;254:120125. doi: 10.1016/j.biomaterials.2020.120125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, Clevers H, Lutolf MP. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 42.Gjorevski N, Lutolf MP. Synthesis and characterization of well-defined hydrogel matrices and their application to intestinal stem cell and organoid culture. Nat Protoc. 2017;12:2263–2274. doi: 10.1038/nprot.2017.095. [DOI] [PubMed] [Google Scholar]
- 43.Klotz BJ, Oosterhoff LA, Utomo L, Lim KS, Vallmajo-Martin Q, Clevers H, Woodfield TBF, Rosenberg AJWP, Malda J, Ehrbar M, Spee B, Gawlitta D. A versatile biosynthetic hydrogel platform for engineering of tissue analogues. Adv Healthc Mater. 2019;8:e1900979. doi: 10.1002/adhm.201900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Liu H, Zhang M, Wang H, Chen W, Qin J. One-step synthesis of composite hydrogel capsules to support liver organoid generation from hiPSCs. Biomater Sci. 2020;8:5476–5488. doi: 10.1039/D0BM01085E. [DOI] [PubMed] [Google Scholar]
- 45.Dye BR, Youngblood RL, Oakes RS, Kasputis T, Clough DW, Spence JR, Shea LD. Human lung organoids develop into adult airway-like structures directed by physico-chemical biomaterial properties. Biomaterials. 2020;234:119757. doi: 10.1016/j.biomaterials.2020.119757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davoudi Z, Peroutka-Bigus N, Bellaire B, Wannemuehler M, Barrett TA, Narasimhan B, Wang Q. Intestinal organoids containing poly(lactic-co-glycolic acid) nanoparticles for the treatment of inflammatory bowel diseases. J Biomed Mater Res A. 2018;106:876–886. doi: 10.1002/jbm.a.36305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dosh RH, Jordan-Mahy N, Sammon C, Le Maitre CL. Use of l-pNIPAM hydrogel as a 3D-scaffold for intestinal crypts and stem cell tissue engineering. Biomater Sci. 2019;7:4310–4324. doi: 10.1039/C9BM00541B. [DOI] [PubMed] [Google Scholar]
- 48.Kumar SV, Er PX, Lawlor KT, Motazedian A, Scurr M, Ghobrial I, Combes AN, Zappia L, Oshlack A, Stanley EG, Little MH. Kidney micro-organoids in suspension culture as a scalable source of human pluripotent stem cell-derived kidney cells. Development. 2019;146:dev172361. doi: 10.1242/dev.172361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin W, Ambrosini YM, Shin YC, Wu A, Min S, Koh D, Park S, Kim S, Koh H, Kim HJ. Robust formation of an epithelial layer of human intestinal organoids in a polydimethylsiloxane-based gut-on-a-chip microdevice. Front Med Technol. 2020;2:2. doi: 10.3389/fmedt.2020.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, Otani T, Livesey FJ, Knoblich JA. Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol. 2017;35:659–666. doi: 10.1038/nbt.3906. Erratum in: Nat Biotechnol 2018;36:1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravichandran A, Murekatete B, Moedder D, Meinert C, Bray LJ. Photocrosslinkable liver extracellular matrix hydrogels for the generation of 3D liver microenvironment models. Sci Rep. 2021;11:15566. doi: 10.1038/s41598-021-94990-z.491fa025fe4b4c02beea3ed0e7195ee5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JS, Choi YS, Lee JS, Jeon EJ, An S, Lee MS, Yang HS, Cho SW. Mechanically-reinforced and highly adhesive decellularized tissue-derived hydrogel for efficient tissue repair. Chem Eng J. 2022;427:130926. doi: 10.1016/j.cej.2021.130926. [DOI] [Google Scholar]
- 53.Petrou CL, D'Ovidio TJ, Bölükbas DA, Tas S, Brown RD, Allawzi A, Lindstedt S, Nozik-Grayck E, Stenmark KR, Wagner DE, Magin CM. Clickable decellularized extracellular matrix as a new tool for building hybrid-hydrogels to model chronic fibrotic diseases in vitro. J Mater Chem B. 2020;8:6814–6826. doi: 10.1039/D0TB00613K. [DOI] [PMC free article] [PubMed] [Google Scholar]


