Abstract
Background and Objectives
Brain organoids have the potential to improve our understanding of brain development and neurological disease. Despite the importance of brain organoids, the effect of vascularization on brain organoids is largely unknown. The objective of this study is to develop vascularized organoids by assembling vascular spheroids with cerebral organoids.
Methods and Results
In this study, vascularized spheroids were generated from non-adherent microwell culture system of human umbilical vein endothelial cells, human dermal fibroblasts and human umbilical cord blood derived mesenchymal stem cells. These vascular spheroids were used for fusion with iPSCs induced cerebral organoids. Immunostaining studies of vascularized organoids demonstrated well organized vascular structures and reduced apoptosis. We showed that the vascularization in cerebral organoids up-regulated the Wnt/β-catenin signaling.
Conclusions
We developed vascularized cerebral organoids through assembly of brain organoids with vascular spheroids. This method could not only provide a model to study human cortical development but also represent an opportunity to explore neurological disease.
Keywords: Organoid, Brain, Cerebral organoid, iPSCs, Vascularization
Introduction
Understanding of the development of the human brain requires the ability to construct and deconstruct developing structures, but this has been challenging due to its complexity and poor accessibility during development (1-5). Therefore, there is a need to develop functional and realistic models of the developing human brain to understand its architecture and functions. To solve this problem, several recent advances have provided opportunities to study early human brain development in structures known as brain organoids. Among these advances, previous studies have generated cerebral organoids from human embryonic stem cells (hESCs) or induced pluripotent stem cells (iPSCs) that recapitulate in vivo human cortical development and structure (2, 3, 6, 7). Therefore, brain organoids, compared to traditional 2D culture, have advantages because brain organoids can be cultured in a dish for more than a year and have recapitulated many features in the development of the human brain, providing access to previously inaccessible developmental features, such as functionality and the gene expression, in the human brain (4, 8).
However, there are several limitations that prevent in vivo functionality, such as these organoids not having a vascular system. The lack of functional blood vessels inhibits the supply of oxygen and nutrients to the core of the organoids; as a result, the center of the organoid becomes necrotic, and functional features are observed on the side of the organoid (9-11). Additionally, blood vessels are important components of the neurogenic niche that regulate neurogenesis and differentiation of neural progenitor cells (12, 13). In the neurogenic niche, the formation of blood vessels in the brain is initiated by various pathways, such as Wnt/β-catenin signaling, which is one of the key developmental signaling pathways that plays pivotal roles in neurogenesis and the differentiation of neural stem cells (13-15). To overcome these limitations, a previous study generated vascularized brain organoids through the fusion of human umbilical vein endothelial cells (HUVECs) with brain organoids to generate vascularized brain organoids (11). In addition, another study established a method to generate vascularized human cerebral organoids by ectopically expressing ETV2 in the organoids (10). All of these studies indicated that vascularization of brain organoids is an important feature for establishing a human brain model to investigate human brain development accurately and show that there is a need for further advances to recapitulate the human brain.
The objective of this study was to investigate the vascularization function of brain organoids. In this study, we developed a method for vascularizing of cerebral organoids by coculturing HUVEC spheroids with cerebral organoids in vitro. HUVEC spheroids formed a developed vascular system in the spheroid, and these well-developed vascularized spheroids induced angiogenesis in the cerebral organoid when cocultured together. Furthermore, despite several studies regarding vascularization of brain organoids, no published vascularized brain organoid models have demonstrated consistent expression of the Wnt/β-catenin pathway that increases neuronal proliferation and differentiation. Here, we used a method to assemble brain organoids with vascular spheroids, which led to the expression of the Wnt/β-catenin pathway. We further demonstrated the function of vascularization in brain organoids in regulating apoptosis, neural proliferation and differentiation.
Material and Methods
Maintenance of cells
All cells were cultured and maintained in incubators at 37℃ and 5% CO2. Human iPSCs were cultured on vitronectin (A31804, Thermo Fisher Scientific, USA)-coated dishes and maintained in E8TM-based hPSC medium (A1517001, Thermo Fisher Scientific, USA) supplemented with 1X penicillin/streptomycin. Culture medium was changed every day. Human iPSCs were detached from the dish by treatment with ReLeSRTM passaging reagent (StemcellTM) and were dissociated into smaller pieces by tapping. The iPSCs used throughout the study, CMC-hiPSC-003 and CMC-hiPSC-011, were provided by the National Stem Cell Bank of Korea (Korea National Institute of Health), which were originally provided by Catholic University. Human dermal fibroblasts (HDFs) were maintained in FGM2 (CC-3132, Lonza, Switzerland) medium supplemented with 10% fetal bovine serum (FBS) and 1X penicillin/streptomycin. Culture medium was changed every other day. Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) were obtained and approved for use by the Boramae Hospital Institutional Review Board (IRB) as previously described. hUCB-MSCs were maintained in EGM-2 endothelial growth medium supplemented with 10% FBS and 1X penicillin/streptomycin. HUVECs (ATCC, USA) were cultured in EGM-2 medium (CC-3162, Lonza, Switzerland) supplemented with 10% FBS and 1X penicillin/streptomycin.
Generation of brain organoids
To generate cerebral organoids from hiPSCs, we used methods from a previous study (7). Briefly, hiPSCs were dissociated and singularized using TrypLe (12604013, Thermo Fisher Scientific, USA) and resuspended at 12,000 cells/well in EB medium (E8 media, 4 ng/ml bFGF and 50 μM ROCKi) in ultralow attached plates (CLS7007, Corning, USA). Next, EB medium was replaced with neural induction medium (DMEM/F12, 20% KSR, 1% NEAA, 0.5%, 1% penicillin/streptomycin, 0.1 mM 2-mercaptoethanol, 5 μM dorsomorphin and 10 μM SB431542) for 5 days. On day 6, the media replaced with neural differentiation media (neurobasal A medium, 2% B27 without vitamin A, 1% GlutaMAX, 20 ng/ml EGF and FGF2). After neural induction, organoids were transferred to 96-well ultralow attachment plates and assembled with vascular spheroids in neural differentiation media. On day 25, the medium was replaced with generation medium (neurobasal A medium, 2% B27 without vitamin A, 1% GlutaMAX, 20 ng/ml BDNF and NT3). On day 44, the medium was changed, and cells were maintained in maturation medium (neurobasal A medium, 2% B27 without vitamin A, 1% GlutaMAX).
Formation of vascular spheroids
To induce the formation of vascular spheroids, each cell combination (HUVEC/HDF/hUCB-MSCs) and ratio (1:4:5) were used as described in a previous study (16). To form the aggregate, premixed solutions of each cell type were suspended into 96-well low attachment dishes in EGM-2 medium supplemented with 10% FBS and 1X penicillin/streptomycin. On day 10, vascular spheroids were used for fusion with brain organoids.
Histology and immunofluorescence
Assembloids or organoids were fixed in 4% paraformaldehyde overnight at 4℃, dehydrated in 30% sucrose solution and embedded in OCT compound (Tissue Tek, Germany). Cryosections were cut into 10 μm thick sections, washed with phosphate-buffered saline (PBS), deparaffinized, rehydrated and stained with hematoxylin/eosin (HE) and picrosirius red.
For immunohistochemistry, cryosections were cut into 20 μm thick sections and mounted onto silane-coated slides (Muto, Japan). Samples were washed 3 times with PBS, blocked and permeabilized for 1 h at room temperature (RT) in 5% normal goat serum and 0.5% Triton X-100 diluted in PBS. Primary antibodies were incubated overnight at 4℃ followed by incubation with appropriate secondary antibodies and 4’,6-diamidino-2-phenylindole (DAPI) for nuclear staining. Samples were mounted using mounting media and then dried at RT overnight. All samples were imaged using an Eclipse TE200 confocal microscope (Nikon, Japan).
Analysis of cell death
For quantification of cell death in organoids, cryosectioned samples were immunostained for cleaved caspase-3 (#9664, Cell Signaling, MA) and subjected to a TUNEL assay (S7165, Merck, USA). For quantification of the percentage of apoptotic cells, randomly selected images of a 400×400 μm areas containing both the progenitor and neuronal layers were analyzed. The number of cells positive for cleaved caspase-3 was counted and divided by the total number of DAPI-positive cells.
Immunoblotting
Organoid proteins were extracted using PRO-PREPTM (iNtRON, Korea). Western blotting was performed on 12∼15% acrylamide gels, and proteins were transferred onto nitrocellulose membranes. Then, the membranes were incubated with 5% bovine serum albumin (GenDEPOT, USA) and probed with antibodies against γH2AX, cleaved caspase-3, GAPDH, β-catenin, Wnt7a, GSK3β, AXIN2, and GFAP. Membranes were then washed with TRIS-buffered saline with 0.1% Tween (TBS-T) and subsequently probed with the appropriate HRP-conjugated secondary antibodies (Invitrogen, CA, USA). The blots were exposed to ECL reagent, and immunoreactive bands were analyzed using ChemiDOC (FluorChem HD2, Proteinsimple, USA). Data were quantified using ImageJ 1.38 software (NIH, Bethesda, MD).
RT-PCR analysis
Total RNA was isolated from the spinal cord using TRIzol (INvitron, Waltham, USA) according to the manufacturer’s protocol. The extracted RNA was quantified by a spectrophotometer (ASP-2680, ACTgene, USA). To generate cDNA, reverse transcription was performed using SuperScript III First-Strand (Invitrogen, CA, USA) according to the manufacturer’s protocol. The resulting cDNA was used for real-time PCR using SYBR green PCR master mix (Thermo Fisher Scientific, MA, USA). Expression data of the duplicated results were used for 2-ΔΔCt statistical analysis, and GAPDH expression was used for normalization.
Quantification and statistical analysis
All statistical analyses were performed using GraphPad Prisms software (version 5). One-way ANOVA followed by Newman-Keuls post hoc test and t-test were used to investigate the significance of each data. All data are presented as the mean±standard error of the mean (SEM). Significance values are indicated as *p<0.05, **p<0.01, and ns (no significance).
Results
Phenotypical characterization of HUVEC spheroids
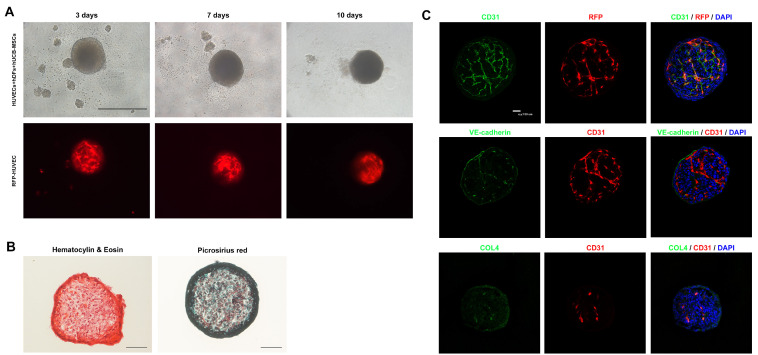
A previous study investigated whether the 1:9 ratio of the cell combination resulted in stable spheroids formation during the entire culture period of 10 days (16). For the formation of HUVEC spheroids, a cell combination of HUVECs, hUCB-MSCs, and HdF and a cell ratio of 1/4:5/4:5 were used. To assess the aggregation of spheroids, premixed cells were seeded onto the microwells, and cells began to aggregate after one day in culture. Cellular debris was observed after one day in culture. However, these combinations exhibited stable spheroids throughout the entire culture period of 10 days. The spheroids were round, and less cellular debris was observed 10 days after coculture of the cells. The organization of HUVECs in the spheroids was assessed using RFP-expressing HUVECs, and we observed that RFP expression was well organized and maintained in the spheroids (Fig. 1A). To assess ECM production, picrosirius red staining was performed and showed a large positive area of collagen fibers in the spheroids, suggesting increased ECM production. Hematoxylin eosin staining revealed circular organized nuclei in the center (Fig. 1B). We next examined whether cocultured HUVECs formed vascularization in the spheroids. We observed that RFP-positive HUVECs were colocalized with CD31-positive cells and VE-cadherin. These vessel structures were enveloped by a basement membrane, which was identified using immunostaining for collagen type IV (Fig. 1C). These results indicate that HUVECs can stably form vessel-like structures in coculture spheroids.
Fig. 1.
Phenotypical characterization of vascular spheroids. (A) Representative images of vascular spheroids. HUVECs, hDFs and hUCB-MSCs were cultured directly for 10 days. Angiogenesis is shown using RFP fluorescence in the vascular spheroid (Scale bar: 500 μm). (B) Representative H&E and picrosirius staining images of vascular spheroids. Collagen deposition is shown in the vascular spheroid (Scale bar: 100 μm). (C) Representative images from day 10 spheroids immunostained with CD31, VE-cadherin, vWF and collagen IV. Nuclei are stained with DAPI (Scale bar: 100 μm).
Characterization of vascular structure in assembloids of HUVEC spheroids and cortical organoids
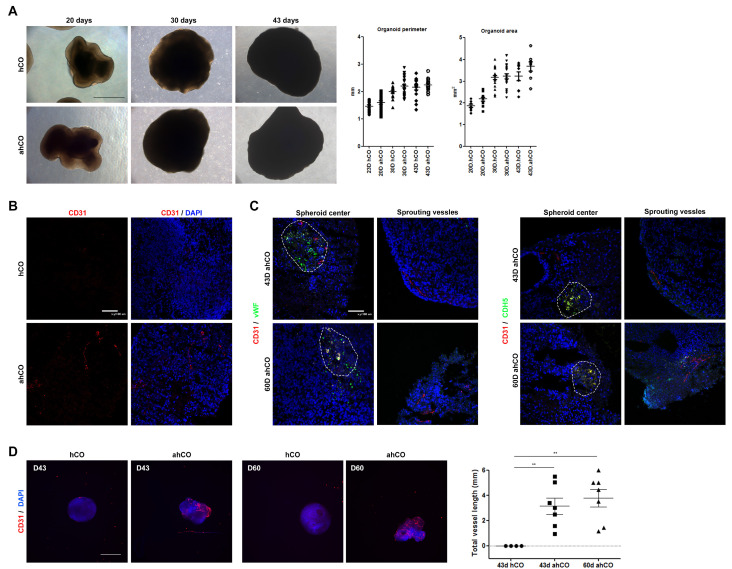
We hypothesized that the assembly of HUVEC spheroids with cerebral organoids, called assembloid human cerebral organoids (ahCO), induces the formation of vessel-like structures in cerebral organoids. Thus, we assembled HUVEC spheroids with cerebral organoids (hCO) on day 13, which is the end point of neural induction, to avoid interrupting neural induction with the assembly of HUVEC spheroids. To investigate the effect of HUVEC spheroids during the generation of organoids, cerebral organoids assembled with or without HUVEC spheroids were observed for 43 days. The growth rate of each organoid was measured during this period. We found that HUVEC spheroids effectively assembled into hCO and that both groups of organoids, hCO and ahCO, were similar in parameter and area until day 43 (Fig. 2A). To investigate vascularization in ahCO, the organization of vessel-like structures was observed by staining for several endothelial markers. On day 43, the CD31 endothelial marker was expressed in ahCO but not hCO. Furthermore, Von willebrand factor (vWF) and Cadherin 5 (CDH5) were effectively expressed in the assembled spheroids through day 60, and sprouting vessels were marked by CD31 expression, whereas hCO lacked these endothelial markers. These results demonstrate that the assembled endothelial structures were stably formed in cerebral organoids until day 60 (Fig. 2B and C). Quantitative PCR results also supported the immunostaining results, exhibiting elevated expression of calponin1, CDH5, vWF and α-SMA in vascular organoids compared to control organoids on day 43 (Supplementary Fig. S1). Furthermore, whole-mount immunostaining demonstrated that CD31-positive vessel-like structures were formed and maintained until day 60. Quantification of total vessel length demonstrated that ahCO displayed significantly increased vessel length than control organoids (Fig. 2D and Supplementary Fig. S2).
Fig. 2.
Characterization of vascular structure in the assembloid of vascular spheroids and cortical organoids. (A) Morphology and size of organoids on days 20, 30 and 43. Quantification of diameter and area from each group at different stages (Scale bar: 500 μm). (B) Representative CD31 immunostaining images of organoids on day 43 (Scale bar: 100 μm). (C) Immunostaining for CD31 and endothelial markers vWF and CDH5 in sectioned organoids at different time points (days 43 and 60). Expression of CD31 is shown in the vascular spheroid and cerebral organoid. The endothelial markers CDH5 and vWF are expressed in the vascular spheroid. White dash line indicates the location of the vascular spheroid (Scale bar: 100 μm). One-way ANOVA analysis. (D) Immunostaining images of whole-mount organoids at different time points (days 43 and 60) showing vascular-like structures in the organoids. Total vessel length was quantified by angiogenesis analysis (Scale bar: 1 mm). **p<0.01.
Neuronal characterization of vascular organoids
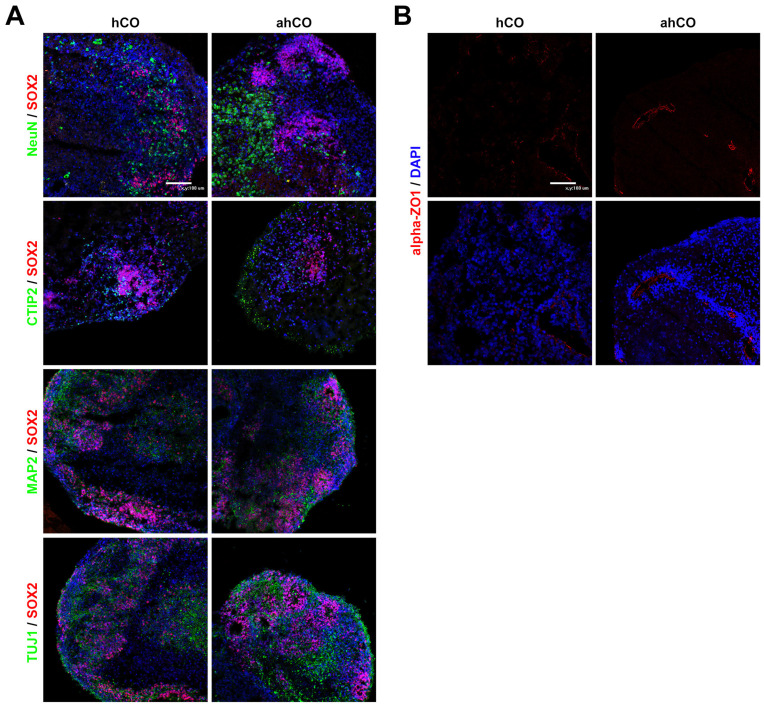
Both control and vascular organoids exhibited well-organized SOX2-positive ventricular zones. NeuN and MAP2, which mark mature neurons, were detected outside of the SOX2 expression domain. Also, the expression of neural marker (TUJ1) and cortical layer marker (CTIP2) in both organoids were identified (Fig. 3A). Consistently, high population of positive cells with TUJ1, CTIP2 and MAP2 were expressed in both organoids at day 43. Moreover, the mature neuronal marker, NeuN, was further identified in vascularized organoids relative to the control organoids (Supplementary Fig. S3). In addition to the expression of neuronal markers, we identified the tight junction marker α-ZO1 in the lumens. On day 60, we identified that the lumens of the ventricular zone (VZ) in ahCO were stained with α-ZO1 compared to those of hCO,which were only weakly stained for α-ZO1 in the lumens (Fig. 3B).
Fig. 3.
Neuronal characterization of cerebral organoids. (A) Representative images of each organoid on day 43 stained with NeuN, CTIP2, TUJ1 and MAP2. Nuclei are stained with DAPI (Scale bar: 100 μm). (B) Immunostaining images of α-ZO1 in each group on day 60 (Scale bar: 100 μm). Student’s t-test, n=3, independent organoids from 2 different batches. *p<0.05; n.s. not significant.
Vascularization of cortical organoids reduces apoptosis
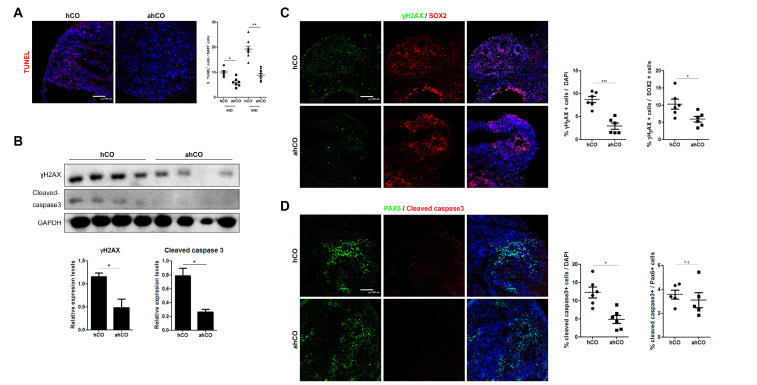
To determine the effects of vascularization on DNA integrity and cell survival in organoids, we performed western blots for rH2AX and cleaved caspase-3, markers of DNA double strand breaks and apoptosis, respectively, and performed TUNEL assays. We observed significantly decreased apoptotic cell death (TUNEL positive) in vascular organoids compared to control organoids (Fig. 4A). We also observed an increase in the protein levels of rH2AX and cleaved caspase-3 in cerebral organoids on day 60, and the protein levels of both were decreased in vascularized organoids (Fig. 4B). Furthermore, immunostaining of rH2AX and cleaved caspase-3 revealed significantly fewer positive cells in vascularized organoids than in control organoids. To determine whether DNA damage and apoptosis occurred significantly in differentiating or precursor positions, we quantified the population of rH2AX and cleaved caspase-3 cells in the ventricular zone and outer layers of both organoids after 60 days. We identified a significant increase in the population of rH2AX- and cleaved caspase-3-positive cells in control organoids compared to vascularized organoids, similar to the western blot data. Since a majority of SOX2+ cells resided in the ventricular zone, we further quantified the number of SOX2+ cells with rH2AX and cleaved caspase3 in the VZ, which were significantly decreased in vascular organoids compared to control organoids (Fig. 4C and D).
Fig. 4.
Vascularization of hCOs with vascular spheroids reduces apoptosis. (A) Representative images of the TUNEL assay for detecting apoptotic cells. Quantification showing the percentage of TUNEL+ cells that were positive for DAPI in each group (Scale bar: 100 μm). (B) Protein levels of rH2AX and cleaved caspase-3. (C) Immunostaining images and quantification of rH2AX and SOX2 in each group on day 60 (Scale bar: 100 μm). (D) Immunostaining images and quantification of cleaved caspase-3 and PAX6 in each group on day 60 (Scale bar: 100 μm). Student’s t-test, n=4, independent organoids from 2 different batches. *p<0.05, ***p<0.001; n.s. not significant.
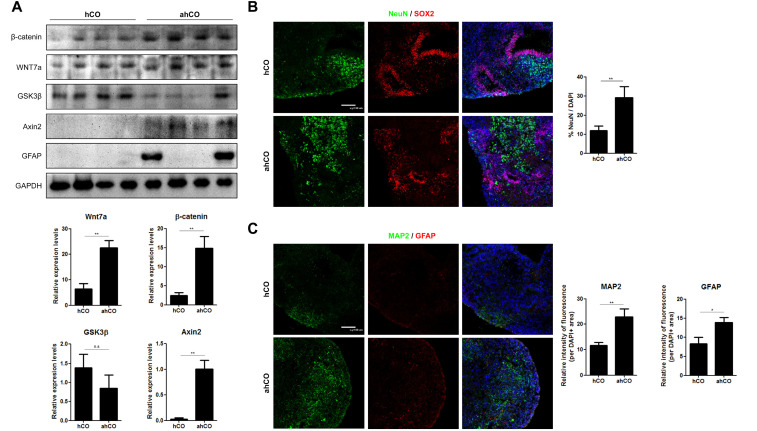
Wnt/β-catenin signaling is unregulated in ahCO
Wnt/β-catenin signaling is important for the formation of blood vessels, especially CNS-specific angiogenesis. Wnts may specifically regulate CNS vessel formation and function. Specifically, Wnt7a is expressed in the forebrain and ventral neural tube (15, 17). Effective activation of Wnt/β-catenin signaling activity was confirmed by western blots. We found that Wnt7a and β-catenin protein levels were elevated in vascular organoids compared to control organoids. Additionally, binding of Wnt ligands to Frizzled/LRP receptor complexes causes stabilization of β-catenin. Therefore, we assessed GSK3β and axin2 protein levels and identified activation axin2 along with activation of Wnt7a and β-catenin. Although no significant difference was confirmed in the case of GSK3β, we identified up-regulation of Axin2 (Fig. 5A). Endothelial cells signal to neural stem cells, regulating their self-renewal and differentiation into neurons. In particular, the Wnt/β-catenin pathway also regulates the proliferation of neural precursor cells during CNS development. The proliferation marker Ki67 was increased in the vascular organoids compared to the control organoids (Supplementary Fig. S4). Furthermore, Wnt7a plays critical roles in neurogenesis by activating β-catenin signaling and the specific transcription factors correlated with neural differenti-ation. To determine the difference between vascular and control organoids, we immunostained each organoid with mature neuronal markers and astrocytes. In vascular organoids, NeuN- and MAP2-positive cells, which are mature neuronal markers, were identified, and GFAP-positive cells, which are astrocytic markers, were detected more often in the vascular organoids than in the control organoids (Fig. 5B and C).
Fig. 5.
Wnt/β-catenin signaling are upregulated in ahCOs. (A) Western blot of Wnt7a, β-catenin, GSK3β, AXIN2 and GFAP in each group of organoids. Western blot analysis revealed that decrease tendency of GSK3β and up-regulation of axin2 along with Wnt7a activation and that β-catenin was increased in the ahCO group compared to the hCO group. (B) Immunostaining images and quantification of SOX2 and NeuN in each group on day 60 (Scale bar: 100 μm). (C) Immunostaining images and quantification of MAP2 and GFAP in each group on day 60 (Scale bar: 100 μm). Student’s t-test, n=4, independent organoids from 2 different batches. **p<0.01; n.s. not significant.
Discussion
Proper development of the brain relies on mutual crosstalk between neural and vascular cells, which is called the neurovascular unit (NVU). The NVU is an organized multicellular system comprised of both neural and vascular cells that is necessary for the delivery of nutrients and oxygen from the circulatory system to the brain (18, 19). Therefore, modeling the neurovasculature in brain organoids is critical for inducing proper development, as well as characterizing the molecular mechanisms of brain development (9, 20).
Although many studies have advanced brain organoids for studying the development and function of the human brain, many challenges remain in current culture methods, such as the occurrence or cellular necrosis in the center of organoids, because nutrients and oxygen cannot be supplied due to the absence of the circulatory system (11, 21, 22). To solve these problems, many studies have reported methods for generating human brain organoids with functional vascular systems. For example, Cakir and colleagues described a method that engineered human brain organoids with a vascular system by ectopically expressing ETV2 in hESCs (10), and Shi and colleagues (11) reported a reproducible system for generating vascularized cerebral organoids by coculturing hiPSCs with HUVECs.
Here, we established a reproducible system for the generation of vascularized cerebral organoids by coculturing vascular spheroids and cerebral organoids. Vascular spheroids are vascularized microtissues containing a vascular network. These spheroids have potential applications as an in vitro model of angiogenesis or as a platform for drug screening research (23, 24). In spheroids, HUVECs, which are derived from the veins of the umbilical cord, have been used to characterize angiogenesis. Because HUVECs alone are unable to build spheroids, the combination of other cell types, such as fibroblasts, pericyte-like cells or smooth muscle cells, is necessary for the establishment of round spheroids (16). In this study, we used fibroblasts and hUCB-MSCs as stromal cell types. In particular, hUCB-MSCs can enhance angiogenesis, producing proangiogenic cytokines and growth factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factors (bFGF), which promote EC proliferation and vascularization (25-27). Additionally, HUVECs, which we used, are distinct from human brain microvascular endothelial cells (HBMECs), which are endothelial cells in the human brain. However, Shi and colleagues determined that coculture of HUVECs with neural cells can induce HUVECs toward brain-like ECs (11). Although we could not identify whether the integrity of HUVECs was transferred to HBMECs in this study, we found that vascular spheroids containing HUVECs could induce angiogenesis in cerebral organoids.
A previous study demonstrated that the transplantation of iPSC-derived brain organoids into immunodeficient mice induced angiogenesis and that there were significantly reduced TUNEL-positive cells in the grafted organoid (28). We also observed reduced DNA damage and fewer cleaved caspase-3-positive cells in vascular organoids than in control organoids. These results indicate that the vascular system generated by vascular spheroids may supply even greater levels of oxygen and nutrients for neural progenitors and neurons in the vascular organoids. The vascular system generated herein may also promote cell proliferation and neurogenesis, which could account for the enhanced expression of Ki67- and NeuN-positive cells observed in the vascular organoids. Given the limitations of traditional organoid culture, which lacks a vascular system, our coculture of cerebral organoids with vascular spheroids may solve the problem of necrosis in the center of organoids to some extent.
Furthermore, the interaction between cerebral organoids and vascular spheroids induced angiogenic signals through physical association in what is called the neurovascular niche (29). Endothelial cells signal to neural stem cells, regulating their self-renewal and differentiation into neurons, whereas neural stem cells have been implicated in regulating the resistance of endothelial tight junctions through various pathways (30). Among these various pathways, a previous study identified that inhibition of Wnt/β-catenin signaling in vivo induced severe CNS-specific angiogenesis defects, and these signals regulated the expression of GLUT-1, which is a BBB-specific transporter (31, 32). Another study showed that Wnt signaling is active in neural progenitors during neurogenesis and that constitutive activation of Wnt signaling induces the expansion of neural precursor cells, while inhibition of Wnt signaling leads to depletion of neural precursor cells (33, 34). Our findings also implicate that Wnt/β-catenin signaling in vascular organoids is correlated with the interaction between neural and vascular cells, as we found that the expression of angiogenic signaling in the developing CNS, represented by Wnt/β-catenin, was increased in vascular organoids compared to control organoids. In this study, the increase in proliferation and neurogenesis observed in vascular organoids is likely explained not only by the endothelial signals required to support neural stem cells but also by the activation of Wnt/β-catenin signaling.
Here, we described a novel method to vascularize cerebral organoids by assembling organoids with vascularized spheroids. In the vascularized organoids, angiogenesis was detected that sprouted from the vascular spheroids. Furthermore, we observed inhibition of necrosis in the vascular organoids and an increase in Wnt/β-catenin signaling in the vascular organoids, which in turn resulted in the promotion of proliferation and neurogenesis of neural progenitor cells. Therefore, our results illustrate that the vascular system in our platform activates signaling pathways to promote the development of cerebral organoids. Vascularization is a potential vital factor for the survival of organoids, as it not only promotes cell growth but also plays a pivotal role in the development of cerebral organoids. Taken together, our study demonstrates the potential of assembling spheroids to vascularize cerebral organoids, and we speculate that our model may be widely applicable for future 3D organoid culture with respect to improving survival rates and achieving functional reconstruction.
Supplementary Materials
Supplementary data including four figures can be found with this article online at https://doi.org/10.15283/ijsc21157.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A4A4078907). The funders had no role in the study design, analysis, interpretation and submission of data.
Footnotes
Potential Conflict of Interest
The authors have no conflicting financial interest.
References
- 1.Marshall JJ, Mason JO. Mouse vs man: organoid models of brain development & disease. Brain Res. 2019;1724:146427. doi: 10.1016/j.brainres.2019.146427. [DOI] [PubMed] [Google Scholar]
- 2.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 2014;9:2329–2340. doi: 10.1038/nprot.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian X, Song H, Ming GL. Brain organoids: advances, applications and challenges. Development. 2019;146:dev166074. doi: 10.1242/dev.166074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 6.Luo C, Lancaster MA, Castanon R, Nery JR, Knoblich JA, Ecker JR. Cerebral organoids recapitulate epigenomic signatures of the human fetal brain. Cell Rep. 2016;17:3369–3384. doi: 10.1016/j.celrep.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sloan SA, Andersen J, Pașca AM, Birey F, Pașca SP. Generation and assembly of human brain region-specific three-dimensional cultures. Nat Protoc. 2018;13:2062–2085. doi: 10.1038/s41596-018-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koo B, Choi B, Park H, Yoon KJ. Past, present, and future of brain organoid technology. Mol Cells. 2019;42:617–627. doi: 10.14348/molcells.2019.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsui TK, Tsuru Y, Hasegawa K, Kuwako KI. Vasculari-zation of human brain organoids. Stem Cells. 2021;39:1017–1024. doi: 10.1002/stem.3368. [DOI] [PubMed] [Google Scholar]
- 10.Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, Raredon MSB, Dengelegi J, Kim KY, Sun P, Zhong M, Lee S, Patra P, Hyder F, Niklason LE, Lee SH, Yoon YS, Park IH. Engineering of human brain organoids with a functional vascular-like system. Nat Methods. 2019;16:1169–1175. doi: 10.1038/s41592-019-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Sun L, Wang M, Liu J, Zhong S, Li R, Li P, Guo L, Fang A, Chen R, Ge WP, Wu Q, Wang X. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 2020;18:e3000705. doi: 10.1371/journal.pbio.3000705.66f6d290c2f84ede886185d58cd7368f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Licht T, Keshet E. The vascular niche in adult neurogenesis. Mech Dev. 2015;138 Pt 1:56–62. doi: 10.1016/j.mod.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Karakatsani A, Shah B, Ruiz de Almodovar C. Blood vessels as regulators of neural stem cell properties. Front Mol Neurosci. 2019;12:85. doi: 10.3389/fnmol.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chenn A. Wnt/beta-catenin signaling in cerebral cortical development. Organogenesis. 2008;4:76–80. doi: 10.4161/org.4.2.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Moor L, Merovci I, Baetens S, Verstraeten J, Kowalska P, Krysko DV, De Vos WH, Declercq H. High-throughput fabrication of vascularized spheroids for bioprinting. Biofabrication. 2018;10:035009. doi: 10.1088/1758-5090/aac7e6. [DOI] [PubMed] [Google Scholar]
- 17.Mulligan KA, Cheyette BN. Wnt signaling in vertebrate neural development and function. J Neuroimmune Phar-macol. 2012;7:774–787. doi: 10.1007/s11481-012-9404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muoio V, Persson PB, Sendeski MM. The neurovascular unit - concept review. Acta Physiol (Oxf) 2014;210:790–798. doi: 10.1111/apha.12250. [DOI] [PubMed] [Google Scholar]
- 19.Bell AH, Miller SL, Castillo-Melendez M, Malhotra A. The neurovascular unit: effects of brain insults during the perinatal period. Front Neurosci. 2020;13:1452. doi: 10.3389/fnins.2019.01452.469ce704fd7343508e166500e1437879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, Nolta JA, Waldau B. Generation of human vascularized brain organoids. Neuroreport. 2018;29:588–593. doi: 10.1097/WNR.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X, Xu Z, Xiao L, Shi T, Xiao H, Wang Y, Li Y, Xue F, Zeng W. Review on the vascularization of organoids and organoids-on-a-chip. Front Bioeng Biotechnol. 2021;9:637048. doi: 10.3389/fbioe.2021.637048.d9603ef2f6584acfa5016be2d649b8c3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rademakers T, Horvath JM, van Blitterswijk CA, LaPointe VLS. Oxygen and nutrient delivery in tissue engineering: approaches to graft vascularization. J Tissue Eng Regen Med. 2019;13:1815–1829. doi: 10.1002/term.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heiss M, Hellström M, Kalén M, May T, Weber H, Hecker M, Augustin HG, Korff T. Endothelial cell spheroids as a versatile tool to study angiogenesis in vitro. FASEB J. 2015;29:3076–3084. doi: 10.1096/fj.14-267633. [DOI] [PubMed] [Google Scholar]
- 24.Pfisterer L, Korff T. Spheroid-based in vitro angiogenesis model. Methods Mol Biol. 2016;1430:167–177. doi: 10.1007/978-1-4939-3628-1_11. [DOI] [PubMed] [Google Scholar]
- 25.Serra J, Alves CPA, Brito L, Monteiro GA, Cabral JMS, Prazeres DMF, da Silva CL. Engineering of human mesenchymal stem/stromal cells with vascular endothelial growth factor-encoding minicircles for angiogenic ex vivo gene therapy. Hum Gene Ther. 2019;30:316–329. doi: 10.1089/hum.2018.154. [DOI] [PubMed] [Google Scholar]
- 26.Arutyunyan I, Fatkhudinov T, Kananykhina E, Usman N, Elchaninov A, Makarov A, Bolshakova G, Goldshtein D, Sukhikh G. Role of VEGF-A in angiogenesis promoted by umbilical cord-derived mesenchymal stromal/stem cells: in vitro study. Stem Cell Res Ther. 2016;7:46. doi: 10.1186/s13287-016-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maacha S, Sidahmed H, Jacob S, Gentilcore G, Calzone R, Grivel JC, Cugno C. Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int. 2020;2020:4356359. doi: 10.1155/2020/4356359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018;36:432–441. doi: 10.1038/nbt.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau M, Li J, Cline HT. In vivo analysis of the neurovascular niche in the developing Xenopus brain. eNeuro. 2017;4:ENEURO.0030-17.2017. doi: 10.1523/ENEURO.0030-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang M, Gao G, Rueda CB, Yu H, Thibodeaux DN, Awano T, Engelstad KM, Sanchez-Quintero MJ, Yang H, Li F, Li H, Su Q, Shetler KE, Jones L, Seo R, McConathy J, Hillman EM, Noebels JL, De Vivo DC, Monani UR. Brain microvasculature defects and Glut1 deficiency syndrome averted by early repletion of the glucose transporter-1 protein. Nat Commun. 2017;8:14152. doi: 10.1038/ncomms14152.cfe083483d664856ac5c4ced1381ccce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang M, Park SH, Petri S, Yu H, Rueda CB, Abel ED, Kim CY, Hillman EM, Li F, Lee Y, Ding L, Jagadish S, Frankel WN, De Vivo DC, Monani UR. An early endothelial cell-specific requirement for Glut1 is revealed in Glut1 deficiency syndrome model mice. JCI Insight. 2021;6:e145789. doi: 10.1172/jci.insight.145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wexler EM, Paucer A, Kornblum HI, Palmer TD, Geschwind DH. Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells. 2009;27:1130–1141. doi: 10.1002/stem.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kriska J, Janeckova L, Kirdajova D, Honsa P, Knotek T, Dzamba D, Kolenicova D, Butenko O, Vojtechova M, Capek M, Kozmik Z, Taketo MM, Korinek V, Anderova M. Wnt/β-catenin signaling promotes differentiation of ischemia-activated adult neural stem/progenitor cells to neuronal precursors. Front Neurosci. 2021;15:628983. doi: 10.3389/fnins.2021.628983.abcd8bb230064c7b80ad86ff6baa7608 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.