Abstract
The goal of this study is to use the TLC-densitometric method to determine the concentration of catechin, pyrocatechol, and quercetine in gambir block extracts in a reliable and efficient manner. The best eluent is a mixture of chloroform: ethyl acetate: glacial acetic acid (4:4:2). The concentration of catechin, pyrocatechol and quercetine in gambir block was found to be 25.50 ± 3.13, 0.91 ± 0.60 and 0.83 ± 0.34% (w/w) w/w respectively. The linearity was obtained between 750-2500, 50–350, and 50–350 μg/spot. 12.49–41.63, 0.48–1.60, 3,85–12.83 μg/spots were found to represent the LOD and LOQ, respectively. The proposed approach exhibited great sensitivity, precision, and accuracy, as well as strong linearity.
Keywords: Gambir block, Pesisir Selatan, Simultaneous analysis, TLC densitometry, Uncaria gambir
Gambir block; Pesisir Selatan; Simultaneous analysis; TLC densitometry; Uncaria gambir.
1. Introduction
Recently, natural compounds have gained immense popularity because they can be used to improve health. Various studies have shown that these natural compounds can be used in preventing disease. Scientists have endeavored extensively to identify chemical constituents that have potential medicinal uses [1]. In continuation of our research on Sumatran medicinal plants [2, 3, 4, 5, 6, 7, 8, 9, 10], simultaneous analysis of gambir extract was carried out.
Gambir (Uncaria gambir Roxb) is an economically important natural product of Indonesia. This plant has been planted for years in West Sumatra Province, mainly in Lima Puluh Kota and Pesisir Selatan District. The leaves and young branches of gambir are treated into gambir blocks or powder, which has a special odor and stimulates a fresh bitter flavor on the tongue. About 80% of gambir block transactions on the earth come from West Sumatra Province [11]. It has some chemical compounds, one of them is catechin. Catechin has many biological activities [12, 13], such as antimicrobial, antioxidant, nitric oxide [14], cytotoxic activity [15].
Gambir block was found by evaporating the aqueous extract of young twigs and leaves of U. gambir. It is used in coloring, browning, and astringent in pharmacy. Gambir extract contains several flavonoid components, namely catechin (7–33%), pyrocatechol (20–30%), quercetine (2–4%) [16]. This block is rich in catechin, which showed hepatoprotective and antiulcer activities as well as strengthened connective tissues.
Since gambir block is used for industrial raw materials of catechin and as it must be differentiated from black catechu. It is very important to find a reliable, fast, and inexpensive method to identify the main components of this gambir block (catechin) from other components (pyrocatechol and quercetine).
In this study concentration of catechin, quercetine, and pyrocatechol in gambir block from the production center of gambir in Pesisir Selatan District will be determined by TLC densitometric method.
2. Materials and methods
2.1. Chemicals
All chemicals and reagents were of AR grade. The marker compound of catechin, quercetine, and pyrocatechol were procured from Sigma. Cylindrical gambir blocks used in this study were obtained from Pesisir Selatan regency Blocks were crumpled to get a powdered substance for further use. The gambir block extract was analyzed using TLC pre-coated plates of silica gel 60 F 254 and analytical grade methanol obtained from Merck. Ethyl acetate, glacial acetic acid, chloroform, methanol, and distilled water were all procured from the same source for TLC investigations.
2.2. TLC analysis
2.2.1. Chromatographic conditions for thin layer chromatography
As the stationary phase, precoated silica gel 60 F254 TLC plates (20 cm. 20 cm) with a coating thickness of 0.2 mm (E. Merck, Germany) were employed. Using a CAMAG Nanomat 4 TLC sampler, 1 μl of standard and sample liquids were spotted onto chromatographic plates. The linear ascending development was eluted in a saturated mode of twin through the chamber at room temperature using the eluent system chloroform: ethyl acetate: glacial acetic acid (4:4:2). The plate was then dried at room temperature before being scanned using densitometry. The TLC plate was scanned at 275 nm (catechin and pyrocatechol) and 263 nm wavelengths (quercetine). CAMAG TLC scanner 4 was used to analyze the plate. The CAMAG winCATS program displayed a densitogram. A peak zone with linear regression was used to assess the situation.
2.2.2. Preparation of stock solution of catechin, pyrocatechol and quercetine
The stock solution of catechin, pyrocatechol and quercetine references standard were made by precisely weighing 100 mg of standards quantitatively put into a measuring container and be sufficient to the desired amount with methanol to achieve concentration of 10.000 μg/mL.
2.2.3. Preparation of sample for TLC analysis
The gambir block extract was perfectly balanced and poured into a 10 mL volumetric flask. (final concentration 100.000 μg/mL).
2.2.4. Validation of method
The approach was validated in accordance with the International Conference on Harmonization's guidelines [17]. The technique's linearity, precision, accuracy, specificity, limit of detection (LOD), and limit of quantitation have all been demonstrated (LOQ).
2.2.5. Statistical analysis
Values are stated as a mean ± SD. Tukey's test (P < 0.05) was used to determine statistical significance.
3. Result
3.1. TLC optimization
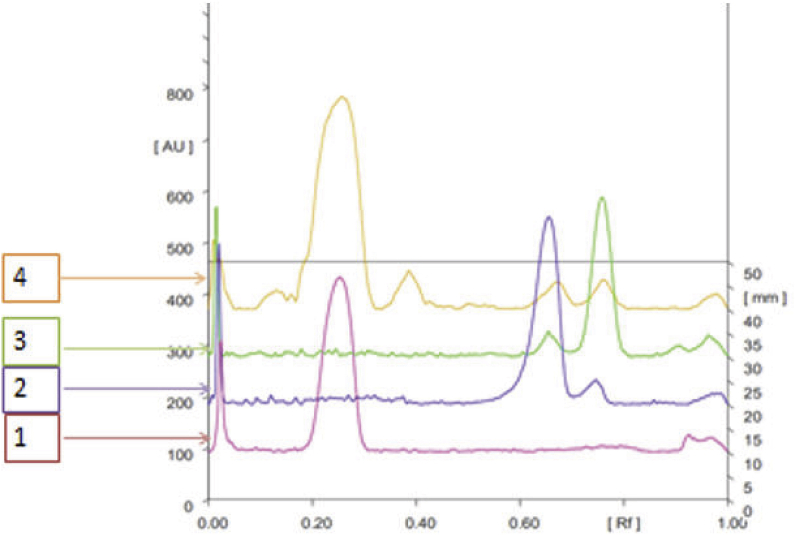
The eluent and wavelength of examination were improved to provide accurate, precise, selective, and repeatable procedures for measuring catechin, pyrocatechol, and quercetine concentrations. Different tests for optimization of the eluent system ensued in the mixture of chloroform: ethyl acetate: glacial acetic acid (4:4:2) as the best for the separation of catechin, pyrocatechol, and quercetine. The best wavelength was 275 nm (catechin and pyrocatechol) and 263 nm (quercetine). The separation of samples on TLC was illustrated in Figure 1. It showed very good separation of catechin, pyrocatechol and quercetine. It was found that the resolution was very good (resolution value >1.5). The Rf values of catechin, pyrocatechol and, quercetine were found to be 0.22 ± 0.05, 0.69 ± 0.01 and 0.92 ± 0.01 respectively. For catechin, pyrocatechol, and quercetine, there is a linear relationship between the area under the curve of each peak and the concentration of the compound and the match. The linear regression of catechin, pyrocatechol and quercetine is shown in (Table 1). The compound structure of catechin, pyrocatechol and, quersetine is shown in Figure 1. It's separation (Figure 2).
Figure 1.
Structure of catechin, pyrocatechol and quercetine
Table 1.
Characteristic parameters for the regression equations of the TLC methods for the determination of catechin, quercetine and pyrocatechol in gambir cube extract.
| No | Parameters | catechin | quercetine | pyrocatechol |
|---|---|---|---|---|
| 1 | Calibration range (μg/spot for TLC) | 750–2500 μg/spot) | 50–350 μg/spot) | 50–350 μg/spot) |
| 2 | Regression equation (Y)∗ | y = 4,3394x + 3119,1 | y = 27,312x -820,3 | y = 13,394x – 226,84 |
| 3 | Slope (b) | 4.339 | 27.312 | 13.394 |
| 4 | Standard deviation of the slope (Sb) | 0.06 | 0.29 | 0.01 |
| 5 | Relative standard deviation of the slope (Sb) | 23.04 | 3.66 | 7.48 |
| 6 | Confidence Limit of the slope∗∗ | 0.07 | 0.33 | 0.06 |
| 7 | Intercept (a) | 3119.1 | 820.3 | 226.84 |
| 8 | Standard deviation of the intercept (Sa) | 126.26 | 88.38 | 53.42 |
| 9 | Confidence Limit of the intercept∗∗ | 142.88 | 100.01 | 60.45 |
| 10 | Correlation coefficient (r) | 0.9995 | 0.9977 | 0.9991 |
| 11 | Limit of Detection (μg/spot for HPTLC) | 12.489 μg/spot | 0.482 μg/spot | 3.849 μg/spot |
| 12 | Limit of Quantitation (μg/spot for HPTLC) | 41.63 μg/spot | 1.60 μg/spot | 12.833 μg/spot |
Figure 2.
Overlay TLC chromatogram of standard catechin (1), quercetine (2), pyrocatechol (3) and gambir block extract from Pesisir Selatan (4).
3.2. Validation of the methods
3.2.1. Linearity
The linearity of the procedure was tested by looking at a series of different quantities of catechin, pyrocatechol, and quercetine in a solution. Using the TLC method, rational linearity was attained for catechin in the range of 750–2500 μg/spot, 50–350 μg/spot, and 50–350 μg/spot. Every level was replicated three times to give data on the difference area under curve among of the same concentration of samples. The linearity of every standard graph was confirmed by coefficient correlation (Table 1).
3.2.2. Precision
Precision expresses the degree of agreement between several tests derived from multiple tests of the same homogenous sample under specific conditions. Repeatability, intermediate precision, and reproducibility are the three steps in which precision was usually achieved. The technique's accuracy was demonstrated by lower percentage relative standard deviation (percent RSD) values for intraday and interday accuracy. For each catechin, pyrocatechol, and quercetine, the precision of the procedure was determined using percent RSD values. This study looked at intraday and interday precision. The intraday precision data came from three replications of the sample over the entire plate, whereas the interday precision data came from three replications of the sample across the entire plate once daily for three days. Table 2 shows the precision research for catechin, pyrocatechol, and quercetine using the indicated TLC procedures.
Table 2.
Precision data of the proposed TLC method.
| Actual concentration | Measured concentration |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra day | Inter day | |||||||||||||
| catechin μg/spot |
quercetine μg/spot |
pyrocatechol μg/spot |
catechin μg/spot |
quercetine μg/spot |
pyrocatechol μg/spot |
%RSD C | %RSD Q | %RSDP | catechin μg/spot | quercetine μg/spot |
pyrocatechol μg/spot |
%RSD C | %RSD Q | %RSD P |
| 750 ± 3.01 | 50 ± 4.40 | 50 ± 1.16 | 706.92 ± 3.43 | 49.01 ± 4.404 | 43.31 ± 1.156 | 0.485 | 8.98 | 2.670 | 707.50 ± 1.35 | 49.010 ± 3.50 | 43.13 ± 0.318 | 0.191 | 7.142 | 0.739 |
| 1500 ± 0.09 | 200 ± 10.24 | 200 ± 0.91 | 1520.02 ± 0.09 | 202.43 ± 10.24 | 207.37 ± 0.91 | 0.005 | 5.06 | 0.439 | 1532.68 ± 10.96 | 200,76 ± 2,75 | 206.935 ± 1.106 | 0.7156 | 1.370 | 0.53 |
| 2500 ± 2.71 | 350 ± 6.15 | 350 ± 1.02 | 2500 ± 0.64 | 275 ± 68.47 | 345.66 ± 1.02 | 0.025 | 0.025 | 0.295 | 2483 ± 11.60 | 349,45 ± 1,22 | 347.08 ± 0.480 | 0.467 | 0.310 | 0.138 |
3.2.3. Range
The calibration series was required to provide reliable, exact, and linear values for catechin, pyrocatechol, and quercetine concentrations detected in the Gambir block extract. The calibration scale of the suggested techniques is shown in Table 3.
Table 3.
Catechin, pyrocatechol and quercetine content in gambir cube extract determined by TLC methods.
| Samples | Content of catechin (%w/w) | Content of pyrocatechol (%w/w) | Content of Quercetine (%w/w) |
|---|---|---|---|
| Gambir cube Pesisir Selatan | 25.50 ± 3.13 | 0.91 ± 0.60 | 0.83 ± 0.34 |
3.2.4. Limit of detection
The limit of detection (LOD) refers to the lowest concentration of analyte that can be detected but not quantitatively determined with sufficient precision and accuracy, whereas the limit of quantification (LOQ) refers to the lowest quantity of analyte in an exceeding sample that can be quantitatively determined with sufficient precision and accuracy [17].
According to the ICH guidelines, the LOD and LOQ were computed using the following equations [17]:
| Equation I: LOD = 3.3. σ/S (Table 1 no 11) |
| Equation II: LOQ = 10. σ/S (Table 1 no 12) |
where:
σ is the SD of the response.
S is the slope of the standardization curve.
3.2.5. Robustness
Robustness method on catechin, pyrocatechol and, quercetine using the same concentration, 0.5 and 1 microliter/spot, and eluent volume 10 and, 20 mL (Table 4).
Table 4.
Robustness measurement results.
| compound | eluent volume (10 mL) |
eluent volume (20 mL) |
||||||
|---|---|---|---|---|---|---|---|---|
| 0,5 μl/spot |
1 μl/spot |
0,5 μl/spot |
1 μl/spot |
|||||
| AUC | %RSD | AUC | %RSD | AUC | %RSD | AUC | %RSD | |
| catechin | 5066 ± 11.85 | 0.23 | 12031 ± 7.46 | 0.06 | 3725.07 ± 26.22 | 0.7 | 6380.23 ± 60.33 | 0.935 |
| pyrocatechol | 188.33 ± 1.53 | 0.81 | 271 ± 1.99 | 0.74 | 3760.53 ± 19.86 | 0.53 | 6262.1 ± 17.52 | 0.28 |
| quercetine | 1588 ± 0.17 | 0.166 | 3015.8 ± 2.18 | 0.07 | 5199.8 ± 35.31 | 0.67 | 13137 ± 46.03 | 0.35 |
4. Discussion
Determination of the levels of catechin, pyrocatechol, and quercetine in the extract of the gambir block was carried out using the described procedure. Bhardward P has reported his research on the separation of catechin compounds in Acacia catechu using ethyl acetate: distilled water: formic acid: glacial acetic acid (100:23:11:11) as mobile phase. The same mobile phase was also tried for the separation of the main polyphenolic components in the gambir block (catechin, pyrocatechol, and quercetine), but this condition could not separate catechin from other polyphenolic compounds properly. will be disinfected simultaneously (catechin, pyrocatechol and, quercetine), then the results of the best separation research is to use a solvent of chloroform: ethyl acetate and, acetic acid (4:4:2).
The content of catechin in the gambir block from Pesisir Selatan is 25.50%. Quite a lot compared to other components in gambir, such as 0.91% pyrocatechol and 0.83% quercetine. From the results obtained, there is a great opportunity for catechin from the gambir block produced to become raw materials for the development of the pharmaceutical collar industry. This opportunity is very good because West Sumatra is famous as a center.
The amount of catechin, pyrocatechol, and quercetine in the extract of the gambir block was determined using standard curves of catechin, pyrocatechol, and quercetine which were chromatographed under the same conditions. Table 5 shows the amounts of catechin, pyrocatechol, and quercetine. There was no significant difference in the mean content of catechin, pyrocatechol, and quercetine, according to Tukey's test (P < 0.05). Therefore, the TLC method can be used to determine the content of catechin, pyrocatechol and quercetine in the gambir block extract.
Table 5.
Other compound peaks identified in TLC spectrum of gambir cube extract.
| Peak | Maximum Rf | Assigned Substance |
|---|---|---|
| 1 | 0,01 | unknown |
| 2 | 0,04 | unknown |
| 3 | 0,11 | unknown |
| 4 | 0,16 | unknown |
| 5 | 0,22 | catechin |
| 6 | 0,37 | unknown |
| 7 | 0,58 | unknown |
| 8 | 0,60 | unknown |
| 19 | 0,69 | Quercetine |
| 10 | 0,82 | Pyrocatechol |
The content of catechin in the gambir block from Pesisir Selatan is 25.50%. This is much larger than the content of catechin in A. catechu. So that gambir block has very good potential to be used as a source of catechin as raw material for the pharmaceutical industry.
5. Conclusion
According to the statistical interpretation of results obtained from validation, the developed TLC techniques are precise, sensitive, accurate, and specific for the determination of catechin, pyrocatechol, and quercetine. Therefore, the proposed method can be used to analyze catechin, pyrocatechol, and quercetine in both qualitative and quantitative ways in extracts which may be useful for standardization purposes.
Declarations
Author contribution statement
Sefrianita Kamal: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Meri Susanti: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Febriyenti, Erizal Zaini: Conceived and designed the experiments.
Dachriyanus Hamidi: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Directorate of Resources, Directorate General of Higher Education, Ministry of Education, Culture (021/E4.1/AK.04.PT/2021) and (T/4/UN.16.17/PT.01.03/PDD-Kesehatan/2021).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Bhardwaj P., Banarjee A., Jindal D., Kaur C. Validation of TLC-densitometry method for estimation of catechin in Acacia catechu heartwood. Pharm. Chem. J. 2020;54(2):184–189. [Google Scholar]
- 2.Susanti M., Lena D.I., Dachriyanus Development and validation of a HPLC method for determination and quantification of rubraxanthone in stem bark extract of mangosteen. Indian J. Pharmacol. 2014;25(4):237–244. [Google Scholar]
- 3.Susanti M., Ibrahim S., Harahap Y., Dachriyanus Comparison between high performance thin layer chromatography and high performance liquid chromatography methods for determination of rubraxanthone in the stem bark extract of Garcinia cowa Roxb. Pharmacog. J. 2018;10(6):s42–s47. doi: 10.4103/pr.pr_144_16. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Susanti M., Harahap Y., Itam A., Dachriyanus Development and validation of UPLC-UV method for the determination of rubraxanthone in human plasma. J. Pharm. Pharmacog. Res. 2019;7(5):381–388. [Google Scholar]
- 5.Wahyuni F.S., Shaari K., Stanslas J., Lajis N.H., Hamidi D. Cytotoxic compounds from the leaves of Garcinia cowa Roxb. J. Appl. Pharmaceut. Sci. 2015;5(2):6–11. [Google Scholar]
- 6.Wahyuni F.S., Shaari K., Stanslas J., Lajis N.H., Hamidi D. Cytotoxic properties and complete nuclear magnetic resonance assignment of isolated xanthones from the root of Garcinia cowa Roxb. Phcog. Mag. 2016;12(45):S52–S56. doi: 10.4103/0973-1296.176115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahyuni F.S., Ali D.A., Lajis N.H., Dachriyanus Anti-inflammatory activity of isolated compounds from the stem bark of Garcinia cowa Roxb. Pharmacog. J. 2017;9(1):55–57. [Google Scholar]
- 8.Wahyuni F.S., Stanslas J., Lajis N.H., Dachriyanus Cytotoxicity studies of tetraprelyltoluquinone, a prenylated hydroquinone from Garcinia cowa Roxb on H-460, MCF-7 and DU-145. Int. J. Pharm. Pharmaceut. Sci. 2015;7(3):60–63. 2015. [Google Scholar]
- 9.Arbain D., Byrne L.T., Dachriyanus Evrayoza N., Sargent M.V. Bracteatine, a quaternary glucoalkaloid from Ophiorrhiza bracteata. Aust. J. Chem. 1997;50:1111–1112. [Google Scholar]
- 10.Andayani R., Wahyuni F.S., Wirasti Y., Dachriyanus Development and validation of RP-HPLC method for quantitative estimation of α-Mangostin in the rind extract and fractions of Garcinia mangostana L. and their cytotoxic activity on T47D breast cancer cell. Int. J. Pharm. Pharmaceut. Sci. 2015;7(2):174–178. [Google Scholar]
- 11.Nandika D., Syamsu K., Arinana A., Kusumawardani D.T., Fitriana Y. Bioactivities of catechin from gambir (Uncaria gambir Roxb.) against wood-decaying fungi. Bioresources. 2019;14(3):5646–5656. [Google Scholar]
- 12.Bae J., Kim N., Shin Y., Kim S., Kim Y. Activity of catechin and their applications. Biomed. Derm. 2020;4(1):1–10. [Google Scholar]
- 13.Isemura M. Catechin in human health and disease. Molecules. 2019;24:528. doi: 10.3390/molecules24030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iglesias D., Bombicino S., Boveris A., Valdez L. (+)-catechin inhibits heart mitochondrial complex and nitric oxide synthase: functional consequences on membrane potential and hydrogen peroxide production. Food Funct. 2019;10:2528–2537. doi: 10.1039/c8fo01843j. [DOI] [PubMed] [Google Scholar]
- 15.Gutiérrez C.L.M., Damián J.H., Chaverri J.P., Legarreta I.G., Téllez D.I., Flores M.E.J. Antioxidant capacity and cytotoxic effects of catechin and resveratrol oligomers produced by enzymatic oxidation against T24 human urinary bladder cancer cells. Antioxidants. 2019;8:214. doi: 10.3390/antiox8070214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marlinda Identification of catechin concentration in gambir. J. Optimal. 2019;4(1):47–53. [Google Scholar]
- 17.International Conference on Harmonization . International Conference on Harmonisation; USA: 2019. Guidance for Industry, Q2B: Validation of Analytical Procedures: Methodology. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.