Abstract
Hydrodynamic cavitation is a new technology used for the treatment of wastewater. Landfill leachates contain a large variety of organic pollutants and inorganic matter, with recalcitrant and bio-refractory compounds. The present study was designed to evaluate the effect of hydrodynamic cavitation on landfill leachate quality indices. Three experimental designs were proposed. First, the influence of collection climate on leachate quality characteristics was analyzed. Second, the best cavitation time was chosen, which promoted the greatest reduction in the effluent pollutant load. Finally, the hydrogen peroxide (H2O2) concentration was evaluated as an adjuvant in the cavitation process. A model TEKMASH TEK-1SL equipment was used. This cavitation unit operated with a flow rate of 30 m3 h−1, a temperature of 75 °C, and an inlet pressure of 3 bar. The cavitation chamber was of the annular flow type. The statistical analyses were run through ANOVA and Tukey's test, with significance α = 0.05. The response variables for the factors were biochemical oxygen demand (BOD5), chemical oxygen demand (COD), total organic carbon (TOC) and total suspended solids (TSS). An influence of the climatic condition on the leachate quality parameters was found, and the difference was marked in COD. In all cases, both for the cavitation process and for the cavitation-oxidant scheme, there was a reduction of 23%–51% BOD5, 30%–53% COD, 12%–21% TOC and 100% removal in TSS. In a 30-minute treatment, the highest COD removal percentage was reached, corresponding to 53.20%. Furthermore, a 200 ppm concentration of hydrogen peroxide enhanced the reduction of BOD5 and COD with proportions of 51.55% and 38.21%, respectively. Hydrodynamic cavitation offers advantages in the treatment of wastewater and can be used as an independent technique or as a hybrid method.
Keywords: Bio-refractory compound, Hydrodynamic cavitation, Landfill, Leachate, Organic matter, Water quality
Graphical abstract
Highlights
-
•
Hydrodynamic cavitation is a useful and efficient technology for treating leachate.
-
•
The annular flow in the reactor (geometry) shows good cavitation performance.
-
•
Hybrid treatment with cavitation and H2O2, improves the reduction of BOD5 and COD.
Bio-refractory compound, Hydrodynamic cavitation, Landfill, Leachate, Organic matter, Water quality.
1. Introduction
In Colombia, wastewater production is close to six million m3/day, of which 10% is discharged into water bodies without any treatment. The most commonly used processes for wastewater treatment are waste stabilization ponds (42%), conventional anaerobic systems (30%), aerobic systems (16%) and other treatment technologies (12%) [1]. With these water treatments, on average, a reduction of 32% in biochemical oxygen demand (BOD5), 31% in chemical oxygen demand (COD) and 26% in total suspended solids (TSS) is achieved [2].
Landfills are designed facilities for the disposal and degradation of urban solid wastes. The decomposition of these wastes through physical, biological and chemical processes generates a liquid byproduct called leachate [3], in which there are four contaminating groups: dissolved organic matter (volatile fatty acids, proteins, carbohydrates), macroinorganic compounds (Ca2+, Mg2+, Na+, K+, NH4+, Fe2+, Mn2+, HCO3-), heavy metals (Cd2+, Cr3+, Cu2+, Pb2+, Ni2+), and xenobiotic compounds at low concentrations (aromatic hydrocarbons, phenols and pesticides) [4, 5].
According to landfill age, there are three kinds of leachate: young landfill leachate (<5 years), intermediate landfill leachate (5–10 years), and mature landfill leachate (>10 years) [6, 7]. The young leachate has a high BOD (4000–13000 mg L−1) and COD (30000–60000 mg L−1) concentration, in addition to a pH value lower than 6.5, with a high biodegradability potential that leads to a 0.2–0.5 ratio of TOC (total organic carbon)/COD and BOD5/COD between 0.5-1.0 [8]. Mature landfill leachate is characterized by a large proportion of high molecular-weight organics, low concentrations of biodegradable substances (COD < 3000 mg L−1), BOD5/COD < 0.1 and high concentrations of ammonia (>1000 mg L−1). In contrast, middle-age landfill leachate shows a 3.0–6.0 COD/TN (total nitrogen) ratio and moderate biodegradability [9].
Landfill effluents can be treated in situ to achieve COD and BOD5 values within the established standard for discharge. Conventional technologies have the disadvantage that they form sludges and/or secondary residues. In recent decades, hydrocavitation has been presented as an efficient alternative process for the oxidation and degradation of organic material content in wastewater. It specifically oxidizes recalcitrant and bio-refractory compounds [7, 10, 11].
Cavitation is a hydrodynamic phenomenon that occurs when the pressure inside the fluid is equal to the vapor pressure, such as pure liquid. The pressure drops necessary to reach the vapor pressure can be obtained through constriction in the flow or under external forces. Cavitation initiates with bubble formation in the low-pressure zone, afterward, the cavities reach their maximum size under isothermal conditions [12]. Finally, the pressure in the system is recovered, and bubbles collapse under adiabatic conditions, reaching a local supercritical state of high temperature and pressure [11]. During this period, called hot spots, highly reactive free radicals and high-speed jets inside the fluid are generated, increasing turbulence within the fluid [13].
Many hydrocavitation applications have been studied in different chemical, physical and biological processes, such as oxidative reaction improvement, biodiesel production, wastewater treatment, active compound extraction, microbial destruction and food processing [14]. This technique reduces the total cost of production, maintaining a high energy efficiency compared to other conventional technologies [6, 12, 13].
Patil et al. [15] evaluated the removal of octanol, dimethyl formamide and cyclohexanol by hydrodynamic cavitation, with a pressure drop between 0.5-5 bar. The vortex-based cavitation device was compared to the linear flow device, intensifying the process with the addition of hydrogen peroxide (H2O2). A reduction of TOC was achieved by 74%. Bis et al. [6] used three configurations of cavitation devices to analyze the effect of constriction on cavitation results. According to their report, by operating with an inlet pressure of 0.7 MPa, a plate with a conical orifice was more conducive to increasing the biodegradability index of these effluents.
Montalvo Andia et al. [16] optimized the application of hydrodynamic cavitation combined with hydrogen peroxide as a promising process for the effective degradation of cyanide in aqueous effluents. The experimental work was conducted using equipment with a venturi device operating at an inlet pressure of 4 bar. The results showed that the degradation of cyanide reached 70% with cavitation alone, and using only H2O2 as an oxidizing agent, a reduction level of 63% was obtained. The efficiency of the combined process was 99% in the removal of cyanide in less than 120 min.
Chakinala et al. [17] applied hydrodynamic cavitation for wastewater treatment, noting a 60%–80% removal of TOC. However, they concluded that the application of cavitation in the treatment of leachates was not very promising with the reduction of COD (less than 10%). According to Gautam et al. [18], the process can be made more effective by changing the experimental configurations with higher pressure, new geometric arrangements or combinations with other advanced oxidation processes (synergistic effect).
Some studies have been conducted to establish the effect of cavitation treatment in reducing COD, BOD and increasing biodegradability in leachate from landfills [19, 20]. However, topics related to the use of annular cavitation reactors, and their combination with strong oxidants has not been reported. A research gap that this study aims to bridge is to explore the possibilities that this type of reactor has in the treatment of wastewater from landfill.
The objective of this study was to assess the hydrocavitation effect on landfill leachate physicochemical characteristics, considering hydrodynamic cavitation and a process assisted with hydrogen peroxide. In addition, a novel annular flow cavitation chamber with three throats was tested. BOD5, COD, TSS and TOC were analyzed to determine the best conditions for the process.
2. Materials and methods
2.1. Leachate samples
The samples were collected from the landfill of a municipality of the Caldas Department, Colombia, following the protocols established by IDEAM [21]. 200 L of leachate were stored in a plastic container that was refrigerated at 4 °C for a maximum of two days. The experimental runs were developed in the Bioprocesos Lab of Universidad de Caldas. Before the experimental runs, the sample was homogenized. The samples were collected during two periods: February (dry season) and April (wet season).
2.2. Equipment used for hydrocavitation process
The experiments were conducted in a TEK-1SL cavitation equipment model from the TEKMASH® Institute. Figure 1 shows its configuration and main components.
Figure 1.
Hydrocavitation equipment description. 1. Main pump, 2. Main tank, 3. Disinfection line, 4. Support, 5. Level adjustment, 6. Cavitation reactor (annular flow cavitation reactor), 7. Feed pump, 8. Control cabinet, 9. Cool water inlet, 10. Hot water outlet, 11. Product inlet, 12. Product outlet, V01–V1–V2–V3–V4–V6 Valves control, M-P1-P2 manometers, D1-D2 Temperatures sensor, SV security valve, SMV Sampling valve, F1–F2 Filters [22].
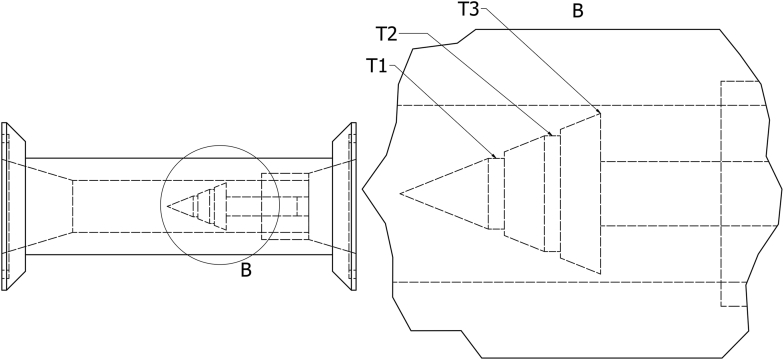
A feed pump was used to charge the equipment. When the main tank (2) was full, the main pump started, and valves 6, 3, 4, 5 and 1 were closed. The fluid was recirculated through the cavitation reactor (6) until the set time was reached. The cavitation chamber of reactor (6) corresponded to an annular flow configuration with three throats in the annulus. The described cavitation chamber novel configuration was tested in this study (see Figure 2). The cavitation reactor can operate with a flow rate between 1.4 and 30 m3 h−1 and a temperature of 40–105 °C. The pressure in the feed of reactor (6) was 3 bar, and the flow rate was 30 m3 h−1. For these operation conditions, the cavitation number in each throat was fixed at 0.07, 0.02 and -0.69.
Figure 2.
Description of the annular flow cavitation chamber (cavitation reactor). T1. Throat 1, T2. Throat 2, T3. Throat 3. B. Plan cut. Adapted from Gutiérrez-Mosquera and Bravo-Hernández [23].
2.3. Experimental runs
The samples were homogenized and placed in the feed tank. Each experimental run was conducted in batches using 60 L of leachate. Different operation times were stablished. The temperature and pressure were set at 75 °C and 3 bar, respectively [16]. After reaching each operation time, the treated leachate was analyzed.
2.4. Quality indices
The selected quality indices to assess the hydrocavitation process were biochemical oxygen demand (BOD5), chemical oxygen demand (COD), total suspended solids (TSS), pH, and total organic carbon (TOC). The BOD5, COD, TSS and pH were measured according to methodology reported by APHA et al. [24]. The TOC concentration was measured using a Parker-Blaston TOC-625 analyzer. This technique was based on acidification, combustion and infrared detection of samples and previous total inorganic carbon removal in the gaseous state [10].
2.5. Experimental description
Three objectives were established for this study: assess the weathering effect on the leachate quality, evaluate the cavitation time effect, and evaluate the hydrogen peroxide concentration effect on the removal of organic material from the leachate (see Table 1). The following response variables were selected: biochemical oxygen demand (BOD5), chemical oxygen demand (COD), total suspended solids (TSS), pH, and total organic carbon (TOC). The experimental runs were made in triplicate.
Table 1.
Experimental description.
| Item | Objective 1 | Objective 2 | Objective 3∗ |
|---|---|---|---|
| Description | Assess the weather effect on the leachate quality. | Evaluate the cavitation time effect on the organic material removal from the leachate. | Hydrogen peroxide (H2O2) concentration effect on the organic material removal from the leachate. |
| Factor | Weather season | Cavitation time | Hydrogen peroxide concentration |
| Factor level | Two levels: Dry Wet |
Three levels: 30 min 60 min 90 min |
Three levels: 50 ppm 125 ppm 200 ppm |
| Response variables | Absolute value of: BOD5, COD, TSS and TOC | Removal percentage of BOD5, COD, TSS and TOC respect to untreated leachate | Removal percentage of BOD5, COD, TSS and TOC respect to untreated leachate |
ppm: parts per million.
To determine the best cavitation time, leachate samples collected in February (dry season) were used. To determine the best hydrogen peroxide concentration, leachate samples collected in April were utilized (wet season).
In each test for determining the best hydrogen peroxide percentage, the optimal cavitation time was used. The H2O2 concentration levels used for the evaluation of the combined method followed the suggestions of Gogate and Bhosale [25], Chen et al. [26], Montalvo Andia et al. [16] and Patil et al. [15].
ANOVA and Tukey tests were applied to the outcomes with a significance of α = 0.05. The homoscedasticity and normality guests were previously validated. In the cases in which the validation was not positive, Kruskal–Wallis and Duncan tests were applied. All statistical analyses were performed using STATGRAPHICS XVI.
3. Results and discussion
3.1. Raw leachates quality indices
Table 2 shows the findings for raw leachates collected in the dry and wet seasons. Based on the outcomes obtained, the leachate can be classified as mature leachate, with pH > 7.5 and a low biodegradability ratio (BOD5/COD <0.1). Likewise, a TOC/COD <0.3 was found. As the landfill age was not known, it was concluded that the evaluated fluid corresponds to leachate over 10 years of age [5, 7].
Table 2.
Physicochemical results of raw leachate.
| Index | Dry season | Wet season |
|---|---|---|
| BOD5 (mg L−1) | 3684.43 ± 214.53 b | 2724.85 ± 12.59 a |
| COD (mg L−1) | 23688.00 ± 432.09 a | 34405.00 ± 332.34 b |
| TOC (mg L−1) | 620.41 ± 22.40 a | 609.77 ± 6.68 a |
| TSS (mg L−1) | 10.00 ± 0.00 a | 10.00 ± 0.00 a |
| pH | 8.92 ± 0.34 b | 7.94 ± 0.00 a |
BOD5: Biochemical oxygen demand, COD: Chemical oxygen demand, TOC: Total organic carbon, TSS Total suspended solids. Average values ±standard deviation, replications per treatment n = 3, significance α = 0.05. Average with the same letter does not present statistically significant difference according to the Tukey Test.
The results were similar to those reported by Xu et al. [27] and Rocha Lebron et al. [28], with the exception of the COD index, which presented characteristics of a young leachate. According to most of the found parameters, the leachate to intervene in this research has a low biodegradability, which is why the use of more powerful and specific unconventional alternatives was prevalent for its treatment [29].
According to IDEAM [30], the local precipitation for February month was 36 mm, whereas for April month, it was 226 mm. This weather behavior created a large difference between the seasons in which the leachates were collected. Therefore, in the wet season, more water percolated into the landfill, favoring the leaching of soluble organic and inorganic materials such as protein and lipidic macromolecules, some recalcitrant compounds (bio-refractory), fulvic and humic acids, tannins, detergents, pesticides, aromatic hydrocarbons, ammonia, chlorides, carbonates, and sulfates [3, 31]. This caused the COD to increase while the TOC, which was a more selective test, decreased.
The TOC method allowed for the estimation of the exact quantity of organic matter in the leachate, whereas the COD test was an indirect technique with some interferences that can react with inorganically reduced species such as ferrous ions, sulfur and manganese, causing errors in the method [32]. This explains the high value for COD (34405.00 mg L−1) and low values for TOC (609.77 mg L−1).
In the wet season, the biodegradable organic matter was diluted, reducing the BOD5 to 2724.85 mg L−1. However, the TOC remained almost constant compared to that in the dry season [33]. This behavior indicates that the biodegradable organic matter remains unaffected.
The increase in COD in the wet season was explained by leachates percolating inorganic compounds such as copper, mercury, nickel, plumb, phosphorus, iron, cyanides, chlorides, and fluorides [34].
3.2. Effect of cavitation time
To find the best operation time during the cavitation process, leachates from landfills collected in February (dry season) were used. Table 3 shows the results obtained at different operation times.
Table 3.
Percent reduction in leachates quality indices treated with hydrocavitation.
| Index reduction | Hydrodynamic cavitation time |
||
|---|---|---|---|
| 30 min | 60 min | 90 min | |
| BOD5 %∗ | 23.75 ± 5.73 a | 24.64 ± 4.41 a | 27.35 ± 8.96 a |
| COD %∗ | 53.20 ± 0.54 b | 44.83 ± 2.91 a | 38.56 ± 4.24 a |
| TOC %∗ | 21.84 ± 6.35 a | 16.75 ± 5.13 a | 20.87 ± 3.50 a |
| TSS %∗∗ | 98.67 ± 0.58 a | 98.67 ± 0.58 a | 99.00 ± 0.00 a |
Average values ±standard deviation, replications per treatment n = 3, significance α = 0.05. Average with the same letter does not present statistically significant difference according to the Tukey Test.
Average values ±standard deviation, replications per treatment n = 3, significance α = 0.05. Average with the same letter does not present statistically significant difference according to the Duncan Test.
All cavitation treatments enhanced the characteristics of the leachates. The chemical and physical effects of cavitation phenomena, such as shock waves, large turbulence, high-pressure jets and the formation of OH− free radicals, favored organic material and suspended particle degradation [14]. Additionally, the basic pH (see Table 2) considerably increased the formation of OH− free radicals, improving the organic matter degradation effect [13].
From a statistical point of view, only the COD parameter was influenced by the cavitation time. According to Patil et al. [15], degradation of pollutants in hydrodynamic cavitation is a function of the constriction device geometry, the pressure drops, the fluid flow rate and the process time. Thirty minutes was the operating time in which a greater decrease in inorganic matter was achieved (COD percentage reduction: 53.20). These results were similar to those reported by Capocelli et al. [35], Gogate and Patil [36] and Badve et al. [37], where 30 min was enough to reach reaction equilibrium and stop the production of free radicals under pressure conditions provoked during the cavitation phenomenon.
According to Wang et al. [29], long process times can lead to decreased fluid flow, causing the entire cavitation device to fill with water, leading to higher static pressure and early collapse of cavities. For this reason, a time of 30 min was sufficient to achieve the maximum degradation of COD under the given conditions [37].
Huo et al. [38] mentioned that a longer operation time drives aromatic polycondensation and increases dissolved organic matter humidification, which caused an increase in the COD and TOC values. This behavior was found for this study, finding a lower removal of COD at longer times.
Wang et al. [29] stated that venturi-type devices, similar to the one used in this research, ensure a higher throat velocity for a given pressure drop, generating a lower cavitation number for the same operating time. The smooth diverging sections also provide sufficient time for the cavities to remain in the low-pressure region until reaching their maximum size before collapse. For this reason and the implementation of a new geometric configuration of the cavitation chamber, the results obtained in this study showed that a shorter process time was required compared to other investigations that explored other geometries (such as orifice plates, rotary drums or cone plates) [39, 40].
TSS removal occurs due to the physical effects of hydrodynamic cavitation, where shock waves of up to 1 GPa pressure and high-speed micro jets of up to 1000 m s−1 strongly hit the suspended particles, causing the surface to break [41]. According to Carpenter et al. [13], asymmetric bubble collapse during the cavitation phenomenon produced a shear force that allows the solid particles within the fluid to breakdown until reaching a particle size of 100 nm or less. Therefore, the technique (vacuum filtration) employed in this study to quantify the suspended solids did not detect the total suspended solid in the leachate, showing a total removal of this suspended contaminant.
The BOD5, COD and TOC concentration reduction was mainly due to the breakdown of some volatile organic macromolecules and the action of free radicals formed during the cavitation phenomenon. Hydroxyl radicals are highly reactive and have great potential for oxidating organic matter. Hydroxyl radicals can also combine to form hydrogen peroxide (H2O2), which favors the organic and inorganic matter degradation present in the leachate [11, 42].
The biodegradability index (BI = BOD5/COD) in the leachate treated with cavitation increased from 0.16 to 0.25. Similar outcomes were obtained by Padoley et al. [43] and Bis et al. [6], who found a BI close to 0.3. It is important to highlight that a high biodegradability index allows that leachate to be treated biotechnologically. According to Perea [10] and Gogate and Kabadi [14], the hydrodynamic cavitation phenomenon was very effective as a pretreatment for wastewater treatment since it improves the subsequent steps of the water treatment process. These results indicate that hydrocavitation can reorganize the molecular structure of organic material and convert organic recalcitrant compounds into substances more biodegradables, improving leachate treatment possibilities [4, 6].
3.3. Effect of hydrogen peroxide concentration on the cavitation process
Table 4 contains the results obtained from cavitating a raw leachate for 30 min. For this study phase, the leachates collated in the wet season. As mentioned above, the increase in COD in the wet season is due to rain water percolating inorganic compounds [34], while according to TOC, the organic matter remains constant.
Table 4.
Percent reduction in leachates quality indices treated with hydrocavitation and hydrogen peroxide.
| Index | Hydrogen peroxide concentration (H2O2) |
||
|---|---|---|---|
| 50 ppm | 125 ppm | 200 ppm | |
| BOD5 %∗ | 35.86 ± 0.35 a | 42.16 ± 0.22 b | 51.55 ± 0.12 c |
| COD %∗ | 30.08 ± 0.18 a | 34.68 ± 0.84 b | 38.21 ± 0.21 c |
| TOC %∗ | 12.95 ± 5.43 a | 15.73 ± 4.56 a | 14.97 ± 5.14 a |
| TSS %∗∗ | 99.50 ± 0.71 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
Average values ±standard deviation, replications per treatment n = 3, significance α = 0.05. Average with the same letter does not present statistically significant difference according to the Tukey Test.
Average values ±standard deviation, replications per treatment n = 3, significance α = 0.05. Average with the same letter does not present statistically significant difference according to the Duncan Test.
The COD reduction with the addition of oxidant (H2O2) was 38.21%, a value that compared the percentage of removal with cavitation without H2O2 (53.2%) was much lower. This result can be explained because hydrogen peroxide was more selective in reducing organic material, diminishing the BOD5 concentration, and affecting inorganic material oxidation [18]. In any case, the results of this study was similar to those obtained by Montalvo Andia et al. [16], who reported a reduction in pollutants between 30-50% for an operating time of 30 min.
The hydrogen peroxide concentration that showed the best result was 200 ppm, Patil and Gogate [44] and Gogate and Bhosale [25] obtained comparable results. Hydrogen peroxide under the cavitation effect dissociates to OH, increasing its formation rate. It has been suggested that taking advantage of the energy from the collapse of the cavitation, effect will make the dissociation of the O–O bond from the H2O2 faster than the O–H bond in the pyrolysis of H2O, thus increasing the generation of OH [16]. Therefore, at a higher oxidant concentration (H2O2), more OH was available inside of the leachate for the oxidation process. However, Carpenter et al. [13] and Gogate and Bhosale [25] state that an H2O2 concentration higher than 224 ppm produces negative effects on organic material oxidation. OH does not interact with organic matter to react with excess H2O2, forming HO2 radicals and water.
At higher H2O2 concentration, the continuous attack of reactive OH radical provokes the opening of some aromatic rings of the target compounds, through cleavage of the C–C bond and several oxidation reactions. Therefore, major addition of hydrogen peroxide is useful in treating mature leachates [45].
The BOD5 percentage reduction was the index that presented the best performance to the treatment combined with hydrocavitation and hydrogen peroxide. This means that peptides, proteins, fatty acids, sugars, and polysaccharides are degraded, and even microbial disinfection is achieved. However, the COD and TOC percentage reduction is due to volatile compounds, organic solvents, phenolic compounds and organochloride substances present in the leachate from landfills being oxidized [33, 46]. Gogate and Patil [36] achieved a degradation of xenobiotic contaminants greater than 80%, combining hydrocavitation with ozone and the Fenton method. However, the present study used H2O2 instead of other oxidizing agents due to its easy handling, low economic cost, potential usability at basic pH and low reaction time [18]. Furthermore, the use of a gaseous oxidant such as ozone significantly reduced the cavitation effect on the fluid, reducing the process efficiency and increasing the cavitation time necessary to achieve a reduction in the COD and TOC in the treated leachate by this technique [47].
Hydrodynamic cavitation technique, initially, generates hydroxyl radicals and hydrogen peroxide, followed by the oxidation of organic contaminants present in the effluent; therefore, any addition of H2O2 as an adjuvant generates a synergistic effect with the conventional process. Previous studies have reported the increase in the concentration of oxidizing species, contributing to the efficient degradation of pollutants [15].
Pressure drops and subsequent implosion of microbubbles, in the cavitation process, contribute to the added H2O2 dissociating and increasing the concentration of OH radicals. The effect on each target species will depend on the reaction rate between the radical and the organic pollutant: if reactivity is high, addition of hydrogen peroxide is expected to show an improvement; otherwise, there may not be an increase in the operation efficiency [15].
4. Conclusions
Hydrodynamic cavitation is presented as a viable alternative for wastewater treatment, including leachates from landfills. In all cases, both for the cavitation process and for the cavitation plus oxidizing agent, a reduction of 23%–51% in BOD5, 30%–53% in COD, 12%–21% in TOC was found and 99% removal in total suspended solids (TSS). In addition, there was an increase in the biodegradability index of the wastewater, from 0.16 to 0.25. The results showed the usefulness and application opportunity of this new technology in landfill leachate treatment.
The leachate physicochemical characteristics show great fluctuations. This type of effluent exhibits, by nature, great variability in its composition and properties. It was possible to verify the influence of the climate precipitation level on the COD index, where the water in excess promotes the dragging of organic and inorganic material to the leachate, making it difficult to degrade.
The best cavitation was 30 min, a sufficient period to reach chemical equilibrium to form free radicals, originating from the fractionation of the water molecule under the cavitation pressure pulse. From this point on, no more hydroxyl radicals were created. At this point, it was necessary to stop the cavitation operation since aromatic condensation and humification reactions can start the synthesis of new substances that were difficult to degrade.
The oxidizing agent concentration of 200 ppm gave the best results in reducing the evaluated quality indices. The dissociation of hydrogen peroxide favors hydroxyl radical formation, which leads to increased inorganic and organic substance degradation.
According to different authors, it is recommended to analyze in future studies the influence of other variables of importance, such as the geometry of the cavitation device, the speed and flow of the fluid, the operating temperature, the number of steps or cycles in the reactor, and the exploration of other oxidizing agents.
Declarations
Author contribution statement
Luis Fernando Gutiérrez-Mosquera, Sebastián Arias-Giraldo & Alejandro Zuluaga-Meza: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported with resources from Universidad de Caldas and Universidad Católica Luis Amigó.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
To Bioprocess and Agro-industry Plant and Research Vice-rectorships of Universidad de Caldas and Universidad Católica Luis Amigó.
References
- 1.Vásquez G.C.E. Universidad Nacional de Colombia; 2013. Panorama del tratamiento de aguas residuales con tecnología anaerobia en la Costa Atlántica Colombiana. [Google Scholar]
- 2.IDEAM . Instituto de Hidrología, Meteorología y Estudios Ambientales de Colombia – IDEAM –; Bogotá, Colombia: 2015. Estudio Nacional de Agua – ENA – 2014; p. 496. [Google Scholar]
- 3.Ocampo M.F., Londoño A., Giraldo G.I., Sanabria N.R. Coeficientes de partición de mercurio en lixiviados del relleno sanitario La Esmeralda. Rev. Fac. Ciencias Básic. 2016;12:56–65. [Google Scholar]
- 4.Morais J., Peralta P. Use of advanced oxidation processes to improve the biodegradability of mature landfill leachates. J. Hazard Mater. 2005;123:181–186. doi: 10.1016/j.jhazmat.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 5.Yao P. Perspectives on technology for landfill leachate treatment. Arab. J. Chem. 2017;10:2567–2574. [Google Scholar]
- 6.Bis M., Montusiewicz A., Ozonek J., Pasieczna-Patkowska S. Application of hydrodynamic cavitation to improve the biodegradability of mature landfill leachate. Ultrason. Sonochem. 2015;26:378–387. doi: 10.1016/j.ultsonch.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Abbas A.A., Jingsong G., Ping L.Z., Ya P.Y., Al-Rekabi W.S. Review on landfill leachate treatments. Am. J. Appl. Sci. 2009;6:672–684. [Google Scholar]
- 8.Alvarez H., Jefferson B., Judd S.J. Membrane bioreactors vs. conventional biological treatment of landfill leachate: a brief review. J. Chem. Technol. Biotechnol. 2005;79:1043–1049. [Google Scholar]
- 9.Jagaba A.H., et al. Sequencing batch reactor technology for landfill leachate treatment: a state-of-the-art review. J. Environ. Manag. 2021;282:1–36. doi: 10.1016/j.jenvman.2021.111946. [DOI] [PubMed] [Google Scholar]
- 10.Perea L.V. Universidad Libre de Colombia; 2016. Evaluación de un reactor de cavitación hidrodinámica a escala de laboratorio para la remoción de Carbono Orgánico Total presente en los lixiviados generados en el relleno sanitario Doña Juana. [Google Scholar]
- 11.Gutiérrez-Mosquera L.F., Arias-Giraldo S., Cardona-Naranjo D.F. Hydrodynamic cavitation: engineering and agribusiness approach. Sci. Tech. 2019;24(2):283–304. [Google Scholar]
- 12.Dindar E. An overview of the application of hydrodinamic cavitation for the intensification of wastewater treatment applications: a review. Innov. Energy Res. 2016;5:137–143. [Google Scholar]
- 13.Carpenter J., Badve M., Rajoriya S., George S., Saharan V.K., Pandit A.B. Hydrodynamic cavitation: an emerging technology for the intensification of various chemical and physical processes in a chemical process industry. Rev. Chem. Eng. 2016;32:433–470. [Google Scholar]
- 14.Gogate P.R., Kabadi A.M. A review of applications of cavitation in biochemical engineering/biotechnology. Biochem. Eng. J. 2009;44:60–72. [Google Scholar]
- 15.Patil P.B., Bhandari V.N., Ranade V.V. Wastewater treatment and process intensification for degradation of solvents using hydrodynamic cavitation. Chem. Eng. Process. - Process Intensif. 2021;166:1–10. [Google Scholar]
- 16.Montalvo Andia J.P., Ticona Cayte A.E., Illachura Rodriguez J.M., Lopez Belon L., Cardenas Malaga M.A., Cesar Teixeira L.A. Combined treatment based on synergism between hydrodynamic cavitation and H2O2 for degradation of cyanide in effluents. Miner. Eng. 2021;171:1–10. [Google Scholar]
- 17.Chakinala A.G., Bremner D.H., Gogate P.R., Namkung K.C., Burgess A.E. Multivariate analysis of phenol mineralisation by combined hydrodynamic cavitation and heterogeneous advanced Fenton processing. Appl. Catal. B Environ. 2008;78:11–18. [Google Scholar]
- 18.Gautam P., Kumar S., Lokhandwala S. Advanced oxidation processes for treatment of leachate from hazardous waste landfill: a critical review. J. Clean. Prod. 2019;237:1–14. [Google Scholar]
- 19.Bis M. Eurasia Symposium Proceedings. 2011. Landfill leachate treatment by hydrodynamic cavitation and ozone; pp. 873–878. [Google Scholar]
- 20.Korniluk M., Ozonek J. Environmental Engineering: The 8th International Conference. 2011. Application of hydrodynamic cavitation for leachate of municipal landfill site; pp. 19–20. [Google Scholar]
- 21.IDEAM . Instituto de Hidrología, Meteorología y Estudios Ambientales de Colombia – IDEAM –; Bogotá, Colombia: 2007. Instructivo para la toma de muestras de aguas residuales; p. 17. [Google Scholar]
- 22.Tekmash . Kiev; Ukraine: 2013. TEK-1SL Hydrodynamic Unit: Technical Description and Operation Unit. [Google Scholar]
- 23.Gutiérrez-Mosquera L.F., Bravo-Hernández M. 2019. Aparato para el rompimiento, alisamiento, pasteurización y homogeneización de la proteína de leche que incluye una combinación de reactores de cavitación en un circuito de flujo. [Google Scholar]
- 24.APHA, AWWA, and WEF . American Public Health Association – APHA –, American Water Works Association – AWWA –, Water Environment Federation – WEF; New York, USA: 2005. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- 25.Gogate P.R., Bhosale G.S. Comparison of effectiveness of acoustic and hydrodynamic cavitation in combined treatment schemes for degradation of dye wastewaters. Chem. Eng. Process. 2013;71:59–69. [Google Scholar]
- 26.Chen W., Wang F., He C., LI Q. Molecular-level comparison study on microwave irradiation-activated persulfate and hydrogen peroxide processes for the treatment of refractory organics in mature landfill leachate. J. Hazard Mater. 2020;397:1–9. doi: 10.1016/j.jhazmat.2020.122785. [DOI] [PubMed] [Google Scholar]
- 27.Xu Q., Siracusa G., Di Gregorio S., Yuan Q. COD removal from biologically stabilized landfill leachate usingAdvanced Oxidation Processes (AOPs) Process Saf. Environ. Protect. 2018;120:278–285. [Google Scholar]
- 28.Rocha Lebron Y.A., et al. A survey on experiences in leachate treatment: common practices, differences worldwide and future perspectives. J. Environ. Manag. 2021;288:1–20. doi: 10.1016/j.jenvman.2021.112475. [DOI] [PubMed] [Google Scholar]
- 29.Wang B., Su H., Zhang B. Hydrodynamic cavitation as a promising route for wastewater treatment – a review. Chem. Eng. J. 2021;412:1–29. [Google Scholar]
- 30.IDEAM . Instituto de Hidrología, Meteorología y Estudios Ambientales de Colombia – IDEAM; Bogotá, Colombia: 2018. Tiempo Y Clima: Boletín Climatológico Mensual Año 2018.http://www.ideam.gov.co/web/tiempo-y-clima/climatologico-mensual/-/document_library_display/xYvlPc4uxk1Y/view/71473013 [Online]. Available: [Google Scholar]
- 31.Montusiewicz A., Bis M., Pasieczna-Patkowska S., Majerek D. Mature landfill leachate utilization using a cost-effective hybrid method. Waste Manag. 2018;76:652–662. doi: 10.1016/j.wasman.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 32.IDEAM . Instituto de Hidrología, Meteorología y Estudios Ambientales de Colombia – IDEAM; Bogotá, Colombia: 2007. Demanda química de oxígeno por reflujo cerrado y volumetría; p. 11. [Google Scholar]
- 33.Rodríguez F.J., Pérez A., Orozco C., González M.N., Ibeas M.V. Biodegradabilidad de la materia orgánica natural del agua y efecto del ozono. Ing. del Agua. 2000;7:271–278. [Google Scholar]
- 34.Julián S. Escuela Universitaria de Ingeniería Técnica Industrial de Zaragoza; 2011. Tratamiento de aguas residuales industriales con materia orgánica no biodegradable. [Google Scholar]
- 35.Capocelli M., Prisciandaro M., Lancia A., Musmarra D. Hydrodynamic cavitation of p-nitrophenol: a theoretical and experimental insight. Chem. Eng. J. 2014;254:1–8. [Google Scholar]
- 36.Gogate P.R., Patil P.N. Combined treatment technology based on synergism between hydrodynamic cavitation and advanced oxidation processes. Ultrason. Sonochem. 2015;25:60–69. doi: 10.1016/j.ultsonch.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Badve M., Gogate P., Pandit A., Csoka L. Hydrodynamic cavitation as a novel approach for wastewater treatment in wood finishing industry. Separ. Purif. Technol. 2013;106:15–21. [Google Scholar]
- 38.Huo S., Xi B., Yu H., He L., Fan S., Liu H. Characteristics of dissolved organic matter (DOM) in leachate with different landfill ages. J. Environ. Sci. 2008;20:492–498. doi: 10.1016/s1001-0742(08)62085-9. [DOI] [PubMed] [Google Scholar]
- 39.Choi J., Cui M., Lee Y., Kim J., Son Y., Khim J. Hydrodynamic cavitation and activated persulfate oxidation for degradation of bisphenol A: kinetics and mechanism. Chem. Eng. J. 2018;338:323–332. [Google Scholar]
- 40.Joshi S.M., Gogate P.R. Intensification of industrial wastewater treatment using hydrodynamic cavitation combined with advanced oxidation at operating capacity of 70L. Ultrason. Sonochem. 2019;52:375–381. doi: 10.1016/j.ultsonch.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Terán R., et al. Hydrodynamic cavitation as an efficient pretreatment method for lignocellulosic biomass: a parametric study. Bioresour. Technol. 2017;235:301–308. doi: 10.1016/j.biortech.2017.03.125. [DOI] [PubMed] [Google Scholar]
- 42.Arrojo S., Benito Y. A theoretical study of hydrodynamic cavitation. Ultrason. Sonochem. 2008;15:203–211. doi: 10.1016/j.ultsonch.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Padoley K.V., Saharan V.K., Mudliar S.N., Pandey S.N., Pandit A.B. Cavitationally induced biodegradability enhancement of a distillery wastewater. J. Hazard Mater. 2012;219–220:69–74. doi: 10.1016/j.jhazmat.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 44.Patil P.N., Gogate P.R. Degradation of methyl parathion using hydrodynamic cavitation: effect of operating parameters and intensification using additives. Separ. Purif. Technol. 2012;95:172–179. [Google Scholar]
- 45.Poblete R., Oller I., Maldonado M.I., Cortés E. Improved landfill leachate quality using ozone, UV solar radiation, hydrogen peroxide, persulfate and adsorption processes. J. Environ. Manag. 2019;232:45–51. doi: 10.1016/j.jenvman.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 46.Sanz J., Lombraña J.I., De Luis A. Estado del arte en la oxidación avanzada a efluentes industriales: nuevos desarrollos y futuras tendencias. Afinidad LXX. 2012;561:25–33. [Google Scholar]
- 47.Wu J., Su K., Wang Y., Gou W.J. Effect of air bubble size on cavitation erosion reduction. Sci. China Technol. Sci. 2017;60:523–528. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.