Abstract
Oxidative stress is the downstream of various adverse stresses which impairs meat quality of broiler chickens. Yet, the specific molecular mechanisms of oxidative stress in meat quality of broiler thigh muscle remains unclear. This study investigated the effects and mechanisms of H2O2-induced oxidative stress on meat quality of broiler thigh muscle, with particular emphasis on apoptosis and autophagy and the ROS/NF-κB signaling pathway. The results showed that 10%H2O2-treated broilers exhibited significantly higher drip loss and shear force and lower pH24h and muscle weight. Moreover, the ROS formation, the contents of oxidation products, the expressions of caspases (3, 6, 8, 9), Beclin1, and LC3-II/LC3-I were significantly increased, whereas the levels of antioxidation products and the expression of phosphorylation of NF-κBp65 were significantly decreased. These findings from the present study indicating that H2O2-induced oxidative stress significantly impaired the meat quality by inducing apoptosis and abnormal autophagy via ROS/NF-κB signaling pathway in the broiler thigh muscle.
Key words: oxidative stress, cell death, ROS, NF-κB, thigh muscle
INTRODUCTION
Poultry production in commercial settings is associated with various stressors (e.g., environmental, nutritional and biological factors) which causes adverse impact such as decreased productive performance and meat quality (Liu et al., 2019 Surai et al., 2019a; Guo et al., 2020;).Oxidative stress, defined as an imbalance between the generation of reactive oxygen species (ROS) and endogenous antioxidant defense systems, are the primary causes of the negative consequences of stress in commercial broiler production (Estévez, 2015 Liu et al., 2020;). Recent researches and our previous study have demonstrated that oxidative damage decreased breast meat quality and growth performance of broilers, as indicated by the increased pH, meat lightness and drip loss, and by the decreased BW gain and muscle weight (Zhang et al., 2011 Chen et al., 2017; Chen et al., 2018;). Yet, the role and molecular mechanism of oxidative damage in the broiler thigh muscle remains unclear.
Generally, the oxidation is typically initiated by ROS, which are produced by living systems as byproducts of cellular metabolism (Nathan and Cunningham-Bussel, 2013). It has previously been demonstrated that hydrogen peroxide (H2O2) caused the generation of ROS, and excessive ROS molecules would trigger oxidative reaction in a feedback mechanism involving a series of complex cellular processes, such as apoptosis and autophagy (Kaminskyy and Zhivotovsky, 2014 Weidinger and Kozlov, 2015; Sies et al., 2017; Guo et al., 2021;). Cell apoptosis, which can be induced by oxidative stress, is a process of regulated cell death mechanism. Moreover, researches show that excessive apoptosis disrupt the tissue growth dynamics, thereby impairing meat quality in the liver and breast of broilers exposed to H2O2 (Chen, et al., 2017, 2018). On the other hand, autophagy is a physiologically process for the degradation and recycling of macromolecules and organelles damaged by oxidative stress. It has been reported that abnormal autophagy triggered by accumulated ROS can lead to a quick loss in the skeletal muscle of chickens, eventually causing myopathy. However, whether H2O2-induced oxidative stress could activate apoptosis and autophagy and then function in meat quality through ROS/NF-κB signaling in broiler thigh muscle has not been researched. To address this question, we will investigate the roles of oxidative damage, with a focus on its molecular effects on the meat quality of broiler thigh muscles, the results would provide experimental evidence for possible molecular mechanisms of oxidative damage in the thigh muscles of broilers.
MATERIALS AND METHODS
The current study was approved by the Animal Ethics Committee of Nanjing Agricultural University (approve no. SYXK2017-0007), Nanjing, China.
Animals and Treatment
One-day-old male Arbor Acres broiler chickens (n = 320; 50.12 ± 0.05g) were obtained from a commercial hatchery (Hewei Agricultural Development Co. Ltd, Xuancheng, China). The chicks were randomly distributed to five groups (64 birds per group, n = 8): control group, no injection; saline treatment, intraperitoneal saline injection (0.75%, 1.0 mL/kg in body weight); H2O2 treatments, intraperitoneal injection of 2.5%, 5.0%, and 10.0%H2O2, with a dosage of 1.0 mL/kg BW, respectively. Birds in saline and H2O2 treatment groups received injection at day 16 and 37 during the experimental period. The time and dose of H2O2 injections were according to the reports by Kaiser et al. and Yin et al. (Kaiser et al., 2012 Jie et al., 2015;).The basal diet was formulated according to the NRC- recommended nutrient requirements for Arbor Acres broilers at the age of 1 to 21 days and 22 to 42 days, and the diet formula is presented in Supplemental Table S1.
Sample Collection and Storage
At the end of the experimental period, one bird from each replicate with a BW similar to the mean BW was selected to be euthanized by cervical dislocation and immediately necropsied for thigh muscle collection. The samples were divided into 2 portions, one was divided for ROS detection and meat quality measurement, the other slice was immediately frozen in liquid nitrogen and stored at −80°C for biochemical, mRNA, and protein assays.
Meat Quality Measurement
Muscle pH at 24 h (pH24h) postmortem was measured using a pH meter (HI9125 portable waterproof pH/ORP meter, HANNA Instrument, Cluj-Napoca, Romania) according to the procedure described by Bendall (1979). Color measurements for thigh muscles (L* = lightness, a* = redness, and b* = yellowness) was conducted using a colorimeter (Minolta CR200portable color difference meter, Minolta Co. Ltd., Osaka, Japan) at 24 h postmortem. To assess the drip loss of the muscle, an approximately 5 g muscle rectangle was initially weighed and suspended in a vacuum bag at 4°C for 24 h. Then, thigh muscle was weighed again, and the difference between the final and initial weights was calculated. After drip loss determination, cooking loss (CL) was measured, the thigh sample was weighed (initial weight) and packed in polyethylene bags to be cooked by immersion in a water bath (TW20, JULABO Labortechnik GmbH, Seelbach, Germany) at 70°C for 15 min. Then, the cooked sample was followed by cooling and reweighing (final weight). The difference between final and initial weights was calculated. For shear force measurement, the cooked sample was cut into three strips parallel to the muscle fiber. Then each strip was measured using a Digital Meat Tenderness Meter (Model C1LM3, Northeast Agricultural University, Harbin, China).
Oxidative Parameters Determination
The levels of total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) were measured in the homogenate supernatant of thigh muscle by corresponding assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's protocol. The protein carbonyl content was assayed basing on spectrophotometric detection of the reaction between the protein carbonyl with 2, 4-dinitrophenylhydrazine (2, 4-DNPH) to form protein hydrazone with a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The 8-hydroxy-2′-deoxyguanosine (8-OHdG) concentration and the advanced oxidation protein products (AOPPs) were measured using commercial enzyme-linked immunosorbent assay kits (Nanjing Angle Gene Biotechnology Institute, Nanjing, China). Furthermore, the total protein content of thigh muscle was determined by the method of coomassie brilliant blue using the commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the manufacturer's instructions.
Detection of Reactive Oxygen Species
The concentration of intracellular ROS was detected using an ROS measurement kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer's instructions and visualized under a fluorescence microscope (Olympus, Tokyo, Japan). The fluorescence was monitored using a FACS Calibur flow cytometer (FC500 MCL/MPL, Beckman Coulter Inc., Brea, CA) with excitation at 488 nm and emission at 525 nm. ROS generation was quantified by the mean fluorescence intensity over that of the control.
Real-Time Quantitative PCR Analysis
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA), the purity and quantity of RNA were measured by a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA). cDNA was synthesized using a Prime Script TM RT Master Mix (TaKaRa, Japan) according to the manufacturer's protocols. Real-time quantitative PCR was performed using standard protocols on an Applied Biosystem's 7500 H T Sequence detection system by SYBR Premix Ex Taq (TaKaRa, Japan). The PCR program used was as follows: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 30 s, then 95°C for 15 s, 60°C for 1 min, 95°C for 15 s. Gene expression data were normalized to the glyceraldehyde -3-phosphate dehydrogenase (GAPDH) by employing an optimized comparative Ct (2−ΔΔCt) value method. The primer sense and antisense sequences were as follows: caspase-3: forward 5′- ACAGCAAGCGAAGCAGTTTT-3′, reverse 5′- TCACCTCTGAAAAGGCTGGT -3′; caspase-6: forward 5′- CAGCAAACCTACACCAACCA -3′, reverse 5′- CGCAACTCCTCTTCTCTGATG -3′; caspase-8: forward 5′- TCTCCTACAGAAGCCCAAGC -3′, reverse 5′- TCTTGCTGCTCACCTCTTGA -3′; caspase-9: forward 5′- ATTCCTTTCCAGGCTCCATC -3′, reverse 5′- CACTCACCTTGTCCCTCCAG -3′; Beclin 1: forward 5′- CTTGGACTGTGTGCAGCAAT -3′, reverse 5′- ACCACCACTGCCACCTGTAT -3′; LC3-I: forward 5′- GCTGGACAAGACCAAGTTCC -3′, reverse 5′- TTCACCAGCAGGAAGAAAGC -3′; LC3-II: forward 5′- ATCCGAGATCAGCATCCAAC -3′, reverse 5′- GTGATCTGGCACCAGGAACT -3′; GAPDH: forward 5′-GAGGGTAGTGAAGGCTGCTG-3′, reverse 5′- CATCAAAGGTGGAGGAATGG -3′.
Western Blot Analysis
To determine the protein expression level, frozen samples of thigh muscle were crushed into powder in liquid nitrogen with a mortar and pestle, and the homogenates were centrifuged to collect the supernatants. Protein concentration was quantified using a BCA protein assay kit (Beyotime, China). Proteins were separated by SDSPAGE and transferred to the PVDF membrane. After being blocked, the membranes were incubated overnight with primary antibodies against caspase-3 (1:1000, Cell Signaling Technology Inc., Beverly, MA), LC3-II (1:1000, Cell Signaling Technology Inc.) and β-actin (1:2000, Cell Signaling Technology Inc.). The blots were incubated HRP-conjugated secondary antibodies and signals detected by enhanced chemiluminescence (ECL) Western blot detection reagents (Pierce, Rockford, IL). Immunoblots were scanned and densitometry was performed by using ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical Analysis
The data on all indexes in the present study were analyzed using the individual broiler as the experimental unit (n = 8). Differences between the mean values obtained in each treatment were evaluated by the analyses of variance (ANOVA; SAS 9.2) to test the homogeneity of variances via Levene's test, followed by Tukey's multiple comparison test. P < 0.05 was considered a significant difference.
RESULTS
Relative Weight of Organs in Broilers
Broilers were treated with H2O2 injection and sampled on 21 and 42 days post-hatch. Data on relative organ weights were presented in Table 1, which showed that the organ index of liver was significantly decreased in 5% and 10.0% H2O2 treatment group than the control and saline groups on 21 days post-hatch (P < 0.01), whereas the pancreas index was significantly higher in 10.0%H2O2 treatment group when compared with the control and saline groups on 42 days post-hatch (P < 0.05).
Table 1.
Effects of H2O2-induced oxidative stress on relative organ weight of broilers (%).
| Items | Treatments1 |
SEM2 | P value | ||||
|---|---|---|---|---|---|---|---|
| Control | Saline | 2.5%H2O2 | 5%H2O2 | 10%H2O2 | |||
| 21 d | |||||||
| Heart | 0.68 | 0.68 | 0.68 | 0.66 | 0.66 | 0.01 | 0.50 |
| Liver | 2.96a | 2.93a | 2.89a | 2.63b | 2.52b | 0.04 | <0.01 |
| Proventriculus | 0.72 | 0.69 | 0.68 | 0.68 | 0.67 | 0.01 | 0.13 |
| Gizzard | 3.14 | 3.00 | 2.99 | 2.91 | 3.03 | 0.06 | 0.10 |
| Pancreas | 0.41 | 0.40 | 0.39 | 0.39 | 0.41 | 0.01 | 0.45 |
| 42 d | |||||||
| Heart | 0.46 | 0.45 | 0.44 | 0.45 | 0.44 | 0.02 | 1.00 |
| Liver | 2.28 | 2.28 | 2.27 | 2.26 | 2.24 | 0.08 | 1.00 |
| Proventriculus | 0.32 | 0.32 | 0.32 | 0.33 | 0.33 | 0.01 | 0.81 |
| Gizzard | 1.65 | 1.64 | 1.65 | 1.68 | 1.67 | 0.03 | 0.87 |
| Pancreas | 0.23bc | 0.22c | 0.24bc | 0.26ab | 0.28a | 0.01 | <0.01 |
Control is the noninjected treatment. Saline is 1 mL/kg BW physiological saline (0.75%) injected treatment. 2.5%H2O2, 5%H2O2, and 10%H2O2 are H2O2 treatments injected with 0.74, 1.48, and 2.96 mmol/kg BW H2O2 solution per bird.
SEM: pooled standard error of means.
Means within a row with no common superscripts differ significantly (P < 0.05), n = 8.
Thigh Muscle Weight and Meat Quality Measurement
The effects of H2O2-induced oxidative stress on relative weight and meat quality in thigh muscle of broilers on 42 days post-hatch is shown in Table 2. Results showed that the thigh meat of broilers in 5.0% and 10.0%H2O2 injection groups had a marked lower pH24h value than those in the control and saline groups (P < 0.01). Whereas, compared to the control and saline groups, significant increase in drip loss of the thigh meat were observed in 10.0%H2O2 group (P < 0.01), and the value of shear force in 5.0%H2O2 and 10.0%H2O2 injection groups were also significantly increased (P < 0.01). Besides, a remarkable decrease in the thigh muscle weight were observed after 10.0%H2O2 injection (P < 0.05).
Table 2.
Effects of H2O2-induced oxidative stress on relative weight and meat quality in thigh muscle of broilers on 42 days post-hatch.
| Items | Treatments |
SEM1 | P value | ||||
|---|---|---|---|---|---|---|---|
| Control | Saline | 2.5%H2O2 | 5%H2O2 | 10%H2O2 | |||
| pH24 h | 6.07a | 6.06a | 6.06a | 5.85b | 5.82b | 0.02 | <0.01 |
| a* | 6.57 | 6.58 | 6.52 | 6.24 | 5.83 | 0.22 | 0.29 |
| b* | 17.69 | 17.75 | 17.81 | 18.29 | 19.05 | 0.40 | 0.31 |
| L* | 47.47 | 47.45 | 47.48 | 48.09 | 48.32 | 0.46 | 0.74 |
| drip loss, % | 1.25b | 1.26b | 1.28b | 1.35b | 1.62a | 0.02 | <0.01 |
| Cooking loss, % | 13.52 | 13.68 | 13.65 | 13.91 | 14.10 | 0.11 | 0.06 |
| Shear force value, N | 15.36b | 15.59b | 15.56b | 19.33a | 20.09a | 0.44 | <0.01 |
| Thigh muscle weight, % | 13.76a | 13.78a | 13.64a | 13.63a | 12.85b | 0.19 | <0.01 |
Control is the noninjected treatment. Saline is 1 mL/kg BW physiological saline (0.75%) injected treatment. 2.5%H2O2, 5%H2O2, and 10%H2O2 are H2O2 treatments injected with 0.74, 1.48, and 2.96 mmol/kg BW H2O2 solution per bird.
1SEM: pooled standard error of means.
Means within a row with no common superscripts differ significantly (P < 0.05), n = 8.
Redox Status and Antioxidants in the Thigh Muscle
The redox status and antioxidants in the thigh muscle of broilers on 21 and 42 days post-hatch is shown in Table 3. Compared with the control and saline groups, there were significant decreases in the levels of T-AOC, T-SOD, GSH-Px, and Total sulfhydryl (P < 0.05), but remarkable increases in the contents of Carbonyl, 8-OHdG, AOPPs and MDA were observed after 10.0%H2O2 injection on both days 21 and 42 post-hatch (P < 0.05). Furthermore, the activity of T-SOD, GSH-Px and Total sulfhydryl was significantly decreased (P < 0.05), and the content of MDA was markedly increased (P < 0.05) in thigh muscle of broilers exposed to 5%H2O2 treatment on days 42 post-hatch.
Table 3.
Effects of H2O2-induced oxidative stress on antioxidant indices in thigh muscle of broilers on 21 and 42 days post-hatch.
| Items | Treatments |
SEM1 | P value | ||||
|---|---|---|---|---|---|---|---|
| Control | Saline | 2.5%H2O2 | 5%H2O2 | 10%H2O2 | |||
| 21 d | |||||||
| T-AOC, U/mg of protein | 0.11a | 0.11a | 0.11a | 0.06b | 0.06b | 0.01 | <0.01 |
| T-SOD, U/mg of protein | 91.76a | 91.19a | 89.71ab | 82.59ab | 78.66b | 5.20 | 0.01 |
| GSH-Px, U/mg of protein | 28.38a | 28.16a | 26.03a | 21.73b | 14.15c | 0.65 | <0.01 |
| Protein canbonyl, nmol/mg of protein | 1.68b | 1.74b | 1.81b | 1.95b | 2.35a | 0.07 | <0.01 |
| Total sulfhydryl, μmol/g of protein | 74.30a | 72.21ab | 70.97ab | 64.98ab | 61.60b | 5.45 | 0.03 |
| 8-OHdG, pg/g of protein | 27.43b | 27.52b | 27.77b | 29.01ab | 35.49a | 0.67 | <0.01 |
| AOPPs, nmol/g of protein | 85.33b | 85.56b | 85.88b | 88.53ab | 94.91a | 1.85 | <0.01 |
| MDA, nmol/mg of protein | 0.44b | 0.46b | 0.49b | 0.55ab | 0.63a | 0.05 | <0.01 |
| 42 d | |||||||
| T-AOC, U/mg of protein | 0.31a | 0.30a | 0.31a | 0.27a | 0.20b | 0.01 | <0.01 |
| T-SOD, U/mg of protein | 88.95a | 90.95a | 89.94a | 77.13b | 69.34c | 1.40 | <0.01 |
| GSH-Px, U/mg of protein | 29.42a | 28.24a | 27.14a | 20.45b | 17.71b | 1.24 | <0.01 |
| Protein canbonyl, nmol/mg of protein | 3.14b | 3.16b | 3.27b | 3.50b | 4.20a | 0.14 | <0.01 |
| Total sulfhydryl, μmol/g of protein | 88.64a | 88.59a | 86.54a | 76.59b | 72.84b | 1.53 | <0.01 |
| 8-OHdG, pg/g of protein | 30.98b | 30.90b | 31.07b | 32.58ab | 36.03a | 1.06 | <0.01 |
| AOPPs, nmol/g of protein | 80.18b | 80.31b | 80.43b | 83.04ab | 89.67a | 3.57 | <0.01 |
| MDA, nmol/mg of protein | 0.54c | 0.55c | 0.56c | 0.65b | 0.74a | 0.02 | <0.01 |
Control is the noninjected treatment. Saline is 1 mL/kg BW physiological saline (0.75%) injected treatment. 2.5%H2O2, 5%H2O2, and 10%H2O2 are H2O2 treatments injected with 0.74, 1.48, and 2.96 mmol/kg BW H2O2 solution per bird.
1SEM: pooled standard error of means.
Means within a row with no common superscripts differ significantly (P < 0.05), n = 8.
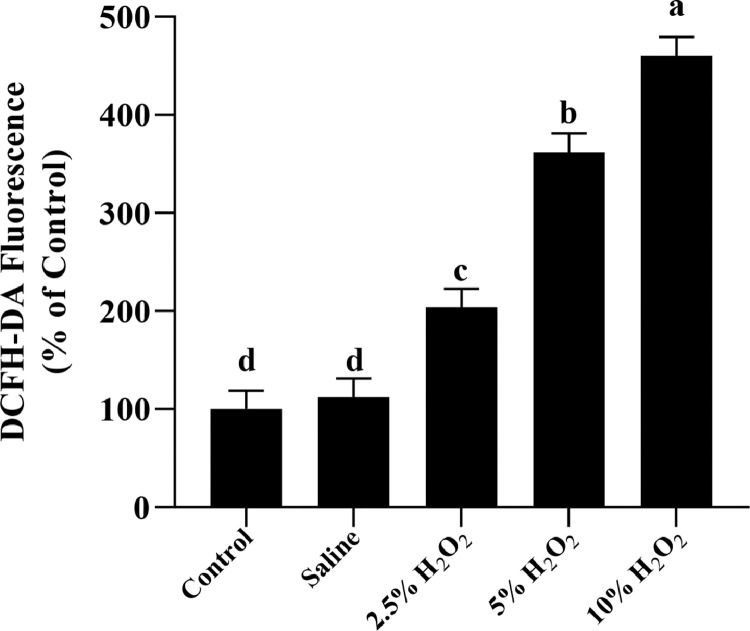
Besides, we also detected ROS production in thigh muscle of broilers on 42 days post-hatch using DCFH-DA probe after exposure to H2O2 (Figure 1). Results showed that an increase (P < 0.05) of ROS formation was detected after treatment with H2O2 in comparison with the control and saline groups.
Figure 1.
Effects of H2O2-induced oxidative stress on the level of ROS in thigh muscle of broilers on 42 days post-hatch. Control is the noninjected treatment. Saline is 1 mL/kg BW physiological saline (0.75%) injected treatment. 2.5%H2O2, 5%H2O2 and 10%H2O2 are H2O2 treatments injected with 0.74, 1.48, and 2.96 mmol/kg BW H2O2 solution per bird; all data were represented as the mean value ± SE of eight sample birds per treatment; a,b,c,d means within a row with no common superscripts differ significantly (P < 0.05).
Effects of H2O2-Induced Oxidative Stress on cell Apoptosis in the Thigh Muscle of Broilers
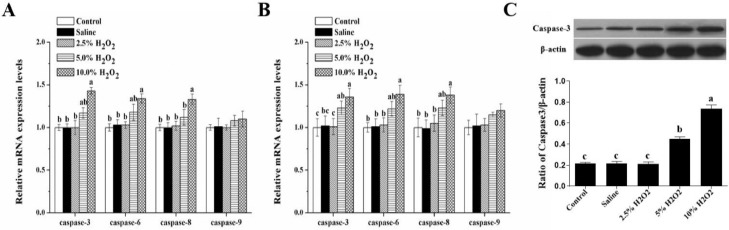
Results showed that broilers exposed to 10.0%H2O2 had higher mRNA expressions of caspase-3, 6, 8, and 9 in comparison with the control and saline groups (P < 0.05, Figure 2A, B). And the protein expression of caspase 3 were significantly increased in groups treated with 5% and 10%H2O2 (Figure 2C). These findings show that cell apoptosis is activated by H2O2 in thigh muscle of broilers.
Figure 2.
Effects of H2O2-induced oxidative stress on the mRNA and protein expression of the caspases in the thigh muscle of broilers. (A) Thigh muscle of broilers on 21 days post-hatch; (B, C) thigh muscle of broilers on 42 days post-hatch. Control is the noninjected treatment. Saline is 1 mL/kg BW physiological saline (0.75%) injected treatment. 2.5%H2O2, 5%H2O2, and 10%H2O2 are H2O2 treatments injected with 0.74, 1.48, and 2.96 mmol/kg BW H2O2 solution per bird. All data were represented as the mean value ± SE of eight sample birds per treatment; a,b,c means within a row with no common superscripts differ significantly (P <0.05).
Effects of H2O2-Induced Oxidative Stress on Cell Autophagy in the Thigh Muscle of Broilers
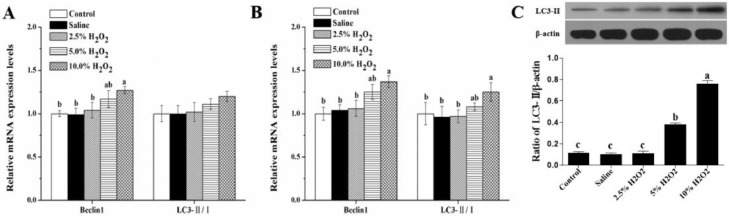
It turns out that the administration of 10.0%H2O2 significantly up-regulated the mRNA expressions of beclin1 and LC3-Ⅱ/LC3-Ⅰ (Figure 3A, B), and increased the protein expression of total LC3-Ⅱ in comparison with the control and saline groups (Figure 3C). These results suggest that H2O2-induced oxidative stress can lead to accumulation of abnormal autophagy in thigh muscle of broilers.
Figure 3.
Effects of H2O2-induced oxidative stress on the mRNA and protein levels of autophagy response in the thigh muscle of broilers. (A) Twenty one days post-hatch; (B, C) 42 days post-hatch. Control is the non-injected treatment. Saline is 1 mL/kg BW physiological saline (0.75%) injected treatment. 2.5%H2O2, 5%H2O2 and 10%H2O2 are H2O2 treatments injected with 0.74, 1.48, and 2.96 mmol/kg BW H2O2 solution per bird. All data were represented as the mean value ± SE of eight sample birds per treatment; a,b,c means within a row with no common superscripts differ significantly (P < 0.05).
Effects of H2O2-Induced Oxidative Stress on NF-κB Pathway in the Thigh Muscle of Broilers
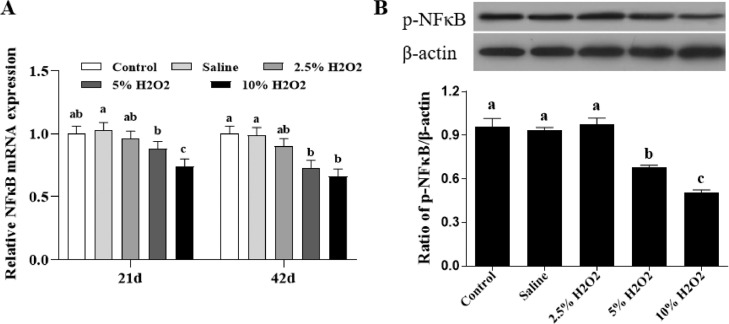
Results showed that broilers exposed to 10.0%H2O2 had much lower (P < 0.05) mRNA expressions of NF-κB than the control and saline groups (Figure 4A). Moreover, compared with the control and saline groups, the protein expression of phospho-NF-κB P65 were significantly decreased in 5% and 10%H2O2 treated groups (Figure 4B). These findings indicate that the activation of NF-κB pathway is inhibited by H2O2-treatment in thigh muscle of broilers.
Figure 4.
Effects of H2O2-induced oxidative stress on the mRNA (A) and protein (B) levels of the NF-κB pathway in the thigh muscle of broilers. (A) 21 and 42 days post-hatch; (B) 42 days post-hatch. Control is the noninjected treatment. Saline is 1 mL/kg BW physiological saline (0.75%) injected treatment. 2.5%H2O2, 5%H2O2, and 10%H2O2 are H2O2 treatments injected with 0.74, 1.48, and 2.96 mmol/kg BW H2O2 solution per bird. All data were represented as the mean value ± SE of eight sample birds per treatment; a,b,c means within a row with no common superscripts differ significantly (P < 0.05).
DISCUSSION
In the current study, we focused on the negative impacts of H2O2-induced oxidative stress on meat quality and cell death signaling activation in the thigh muscle of broilers. The experimental results here demonstrated that intraperitoneal injection of 10%H2O2 significantly impaired the meat quality of broiler thigh muscle by inducing cell apoptosis and abnormal autophagy via ROS/NF-κB pathway, which is consistent with our previous study on broiler breast muscle (Chen et al., 2017). Furthermore, these findings provide additional explanation for how oxidative stress plays an important role in broiler production.
Nowadays, poultry production on a large scale has already been accomplished, the emphasis is now being laid on improving meat quality by modifying varieties of characteristics of broiler meat. Oxidative stress is the downstream of various adverse stresses which contributes remarkably to the impairment of poultry meat quality (Surai et al., 2019b). In the present study, meat quality of the broiler thigh muscle was indicated by muscle weight, pH24h, meat color, drip loss, cook loss and shear force. pH has a direct relevance to meat quality, poultry meat with low pH can lead to increased cook-loss, drip loss and shear force (Cai et al., 2018). Herein, we found that injection of 10%H2O2 strikingly decreased thigh muscle weight and pH24h value and increased drip loss and shear force of thigh meat (Table 2), the results are partly consistent with the previous research by Hu et al. (Huang et al., 2015 Hu et al., 2020;), which showed that high-temperature induced oxidative damage significantly impaired meat quality of the thigh muscle. Our results revealed that oxidative stress induced by H2O2 had a negative impact on meat quality of broilers thigh muscle
It is now widely accepted that administration of H2O2 is able to directly cause oxidative stress, and broilers can be significantly impacted by oxidative stress. ROS are continuously generated during the physiological oxygen metabolism, and excessive production of ROS leads to oxidative stress, which is distinguished by remarkable changes in ROS-mediated damage and redox balance (Rehman et al., 2018 Mishra and Jha, 2019;). To handle the oxidative stress, birds have a multilayered antioxidant system, including SOD, T-AOC, and GSH-Px. Thus, we investigated the antioxidant capacity and the accumulation of ROS levels in the thigh muscle of broilers. Our results showed that H2O2 treatment increased the level of ROS production and MDA content, whereas decreased the levels of T-AOC, T-SOD, GSH-Px, and Total sulfhydryl in the thigh muscle of broilers (Table 3, Figure 1). These results are consistent with the research results obtained previously (Chen et al., 2017 Chen et al., 2021;). Collectively, our findings combined with recent data suggest that H2O2-induced oxidative stress lead to the excessive production of ROS, destroyed antioxidant system and altered macromolecules including proteins and lipids in the thigh muscle of broilers.
Oxidative stress that results from an imbalance between the production of free radical and endogenous antioxidant defense leads to lipid peroxidation, protein nitration, DNA damage and cell death (Ryter et al., 2007). Furthermore, the oxidative damage to lipids, proteins and nucleic acids is associated with the stimulation of programmed cell death, including cell apoptosis and autophagy (Allen and Mieyal, 2012). It has been validated that oxidative stress could induce apoptosis characterized by activated caspase-cascaded signaling and disrupted mitochondrial membrane potential and mitochondrial function proteins like B-cell lymphoma-2 (Bcl-2) family antiapoptotic proteins (Chen et al., 2017 Wang et al., 2018; Xing et al., 2021;). Consistent with previous studies, our data showed that the expression of caspase-3, 6, 8, 9 was upregulated after H2O2 treatment in broiler thigh muscle (Figure 3), suggesting that apoptosis was triggered in the thigh muscle of broilers exposed to H2O2, and the initiation of apoptosis is probably attributed to elevated ROS level. What's more, the occurrence of autophagy induced by oxidative stress was also largely attributed to ROS accumulation (Higgins et al., 2012 Dando et al., 2013;). Autophagy is a catabolic process for degrading and recycling cytoplasmic substrates to protect cells against diverse conditions of stress, for instance, in response to oxidative stress, a certain degree of autophagy can decrease ROS concentration and alleviate the oxidative damage to biomolecules and organelles, whereas dysregulated and excessive autophagy may facilitate cell death and increased tissue damage (Song et al., 2017). In this study, we found the expression of LC3-II, an essential protein and monitor in the process of autophagy, was markedly increased in H2O2-treated broiler thigh muscle, which was consistent with the studies obtained previously (Yin et al., 2015). These data indicate that abnormal autophagy was induced by H2O2 administration in the broiler thigh muscle. Taken together, our results revealed that H2O2-induced oxidative stress may function as an activator of apoptosis and abnormal autophagy via ROS accumulation, damaging the thigh muscle in broilers.
NF-κB proteins are a family of transcription factors and are of main importance in regulating cell death through transcriptional induction of genes encoding apoptotic and autophagic cell death proteins (Baldwin, 2012). Several recent studies have shown the role of NF-κB in regulating cell death, the work of Li et al. was reported that H2O2 triggered apoptosis through ROS-mediated oxidative stress via NF-κB signaling pathway, and besides, the initiation of autophagy was mediated by NF-κB signal pathway in the intestine of piglets exposed to H2O2 (Jie, et al., 2015 Li et al., 2018; Liu et al., 2021a;,b). Moreover, crosstalk between NF-κB signaling and ROS has been widely studied. On the one hand, the transcription of NF-κB dependent genes affects the production of ROS, on the other hand, the activity of NF-κB activity is also mediated by ROS production (Morgan and Liu, 2011). In accordance with the previous study, we found that the expressions of NF-κB was significantly decreased in H2O2-treated broiler thigh muscle (Figure 4), indicating that oxidative stress induced by H2O2 treatment triggers apoptosis and autophagy by suppressing the activation of NF-κB via ROS production in the thigh muscle of broilers.
CONCLUSION
Collectively, our results provide that H2O2-induced oxidative stress have a negative impact on meat quality of broilers thigh muscle, which could be caused by the apoptosis and autophagy processes initiated by excessive ROS accumulation that inhibit the NF-κB signaling pathway. These findings from the present study indicate an essential role and possible molecular mechanism of oxidative damage in the broiler thigh muscle.
DISCLOSURES
The authors declared that they have no conflicts of interest to this work.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.101759.
Appendix. Supplementary materials
References
- Allen E.M., Mieyal J.J. Protein-thiol oxidation and cell death: regulatory role of glutaredoxins. Antioxid. Redox Signal. 2012;17:1748–1763. doi: 10.1089/ars.2012.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A.S. Regulation of cell death and autophagy by IKK and NF-κB: critical mechanisms in immune function and cancer. Immunol. Rev. 2012;246:327–345. doi: 10.1111/j.1600-065X.2012.01095.x. [DOI] [PubMed] [Google Scholar]
- Bendall J.R. Relations between muscle pH and important biochemical parameters during the postmortem changes in mammalian muscles. Meat Sci. 1979;3:143–157. doi: 10.1016/0309-1740(79)90016-0. [DOI] [PubMed] [Google Scholar]
- Cai K., Shao W., Chen X., Campbell Y.L., Nair M.N., Suman S.P., Beach C.M., Guyton M.C., Schilling M.W. Meat quality traits and proteome profile of woody broiler breast (pectoralis major) meat. Poult. Sci. 2018;97:337–346. doi: 10.3382/ps/pex284. [DOI] [PubMed] [Google Scholar]
- Chen X., Gu R., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Induction of nuclear factor-κB signal-mediated apoptosis and autophagy by reactive oxygen species is associated with hydrogen peroxide-impaired growth performance of broilers. Animal. 2018;12:2561–2570. doi: 10.1017/S1751731118000903. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang L., Li J., Gao F., Zhou G. Hydrogen peroxide-induced change in meat quality of the breast muscle of broilers is mediated by ROS generation, apoptosis, and autophagy in the NF-κB signal pathway. J Agric. Food Chem. 2017;65:3986–3994. doi: 10.1021/acs.jafc.7b01267. [DOI] [PubMed] [Google Scholar]
- Chen Z., Xing T., Li J., Zhang L., Jiang Y., Gao F. Hydrogen peroxide-induced oxidative stress impairs redox status and damages aerobic metabolism of breast muscle in broilers. Poult. Sci. 2021;100:918–925. doi: 10.1016/j.psj.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando I., Fiorini C., Pozza E.D., Padroni C., Costanzo C., Palmieri M., Donadelli M. UCP2 inhibition triggers ROS-dependent nuclear translocation of GAPDH and autophagic cell death in pancreatic adenocarcinoma cells. Biochim. Biophys. Acta. 2013;1833:672–679. doi: 10.1016/j.bbamcr.2012.10.028. [DOI] [PubMed] [Google Scholar]
- Estévez M. Oxidative damage to poultry: from farm to fork. Poult. Sci. 2015;94:1368–1378. doi: 10.3382/ps/pev094. [DOI] [PubMed] [Google Scholar]
- Guo Y., Balasubramanian B., Zhao Z.H., Liu W.C. Marine algal polysaccharides alleviate aflatoxin B1-induced bursa of Fabricius injury by regulating redox and apoptotic signaling pathway in broilers. Poult. Sci. 2021;100:844–857. doi: 10.1016/j.psj.2020.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Zhao Z.H., Pan Z.Y., An L.L., Balasubramanian B., Liu W.C. New insights into the role of dietary marine-derived polysaccharides on productive performance, egg quality, antioxidant capacity, and jejunal morphology in late-phase laying hens. Poult. Sci. 2020;99:2100–2107. doi: 10.1016/j.psj.2019.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins G.C., Devenish R.J., Beart P.M., Nagley P. Transitory phases of autophagic death and programmed necrosis during superoxide-induced neuronal cell death. Free Radic. Biol. Med. 2012;53:1960–1967. doi: 10.1016/j.freeradbiomed.2012.08.586. [DOI] [PubMed] [Google Scholar]
- Hu H., Dai S., Li J., Wen A., Bai X. Glutamine improves heat stress-induced oxidative damage in the broiler thigh muscle by activating the nuclear factor erythroid 2-related 2/Kelch-like ECH-associated protein 1 signaling pathway. Poult. Sci. 2020;99:1454–1461. doi: 10.1016/j.psj.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Jiao H., Song Z., Zhao J., Wang X., Lin H. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 2015;93:2144–2153. doi: 10.2527/jas.2014-8739. [DOI] [PubMed] [Google Scholar]
- Jie Y., Dua J., Cui Z., Ren W., Yin Y. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Adv. 2015;5:15479–15486. [Google Scholar]
- Kaiser M.G., Block S.S., Ciraci C., Fang W., Sifri M., Lamont S.J. Effects of dietary vitamin E type and level on lipopolysaccharide-induced cytokine mRNA expression in broiler chicks. Poult. Sci. 2012;91:1893–1898. doi: 10.3382/ps.2011-02116. [DOI] [PubMed] [Google Scholar]
- Kaminskyy V.O., Zhivotovsky B. Free radicals in cross talk between autophagy and apoptosis. Antioxid. Redox Signal. 2014;21:86–102. doi: 10.1089/ars.2013.5746. [DOI] [PubMed] [Google Scholar]
- Li Y., Xia J., Jiang N., Xian Y., Ju H., Wei Y., Zhang X. Corin protects H(2)O(2)-induced apoptosis through PI3K/AKT and NF-κB pathway in cardiomyocytes. Biomed. Pharmacother. 2018;97:594–599. doi: 10.1016/j.biopha.2017.10.090. [DOI] [PubMed] [Google Scholar]
- Liu W., Yuan Y., Sun C., Balasubramanian B., Zhao Z., An L. Effects of dietary betaine on growth performance, digestive function, carcass traits, and meat quality in indigenous yellow-feathered broilers under long-term heat stress. Animals (Basel) 2019;9:506. doi: 10.3390/ani9080506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Guo Y., Zhao Z.H., Jha R., Balasubramanian B. Algae-derived polysaccharides promote growth performance by improving antioxidant capacity and intestinal barrier function in broiler chickens. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.601336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Ou B.H., Liang Z.L., Zhang R., Zhao Z.H. Algae-derived polysaccharides supplementation ameliorates heat stress-induced impairment of bursa of Fabricius via modulating NF-κB signaling pathway in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Zhu Y.R., Zhao Z.H., Jiang P., Yin F.Q. Effects of dietary supplementation of algae-derived polysaccharides on morphology, tight junctions, antioxidant capacity and immune response of duodenum in broilers under heat stress. Animals (Basel) 2021;11:2279. doi: 10.3390/ani11082279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B., Jha R. Oxidative stress in the poultry gut: potential challenges and interventions. Front. Vet. Sci. 2019;6:60. doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M.J., Liu Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat. Rev. Immunol. 2013;13:349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman Z.U., Meng C., Sun Y., Safdar A., Pasha R.H., Munir M., Ding C. Oxidative stress in poultry: lessons from the viral infections. Oxid. Med. Cell Longev. 2018 doi: 10.1155/2018/5123147. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter S.W., Kim H.P., Hoetzel A., Park J.W., Nakahira K., Wang X., Choi A.M. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- Sies H., Berndt C., Jones D.P. Oxidative Stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- Song S., Tan J., Miao Y., Li M., Zhang Q. Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J. Cell Physiol. 2017;232:2977–2984. doi: 10.1002/jcp.25785. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Kochish I.I., Fisinin V.I., Kidd M.T. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants (Basel) 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P.F., Kochish I.I., Romanov M.N., Griffin D.K. Nutritional modulation of the antioxidant capacities in poultry: the case of vitamin E. Poult. Sci. 2019;98:4030–4041. doi: 10.3382/ps/pez072. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao H., Shao Y., Liu J., Li J., Luo L., Xing M. Copper (II) and/or arsenite-induced oxidative stress cascades apoptosis and autophagy in the skeletal muscles of chicken. Chemosphere. 2018;206:597–605. doi: 10.1016/j.chemosphere.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Weidinger A., Kozlov A.V. Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules. 2015;5:472–484. doi: 10.3390/biom5020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T., Chen X., Li J., Zhang L., Gao F. Dietary taurine attenuates hydrogen peroxide-impaired growth performance and meat quality of broilers via modulating redox status and cell death signaling. J. Anim. Sci. 2021;99:1–9. doi: 10.1093/jas/skab089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Duan J., Cui Z., Ren W., Li T., Yin Y. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Adv. 2015;5:15479–15486. [Google Scholar]
- Zhang W., Xiao S., Lee E.J., Ahn D.U. Consumption of oxidized oil increases oxidative stress in broilers and affects the quality of breast meat. J. Agric. Food Chem. 2011;59:969–974. doi: 10.1021/jf102918z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.