Abstract
Akkermansia muciniphila (AM) is a mucin-degrading anaerobe, exerting beneficial effects on gut integrity improvement, inflammatory alleviation, and metabolic regulations in humans. Excess amounts of mucin and mucogenesis in the gut facilitate the development of necrotic enteritis (NE) in chickens. The study aimed to evaluate the effects of oral inoculation of AM on NE prevention and gut modulation in a NE-reproduced model coinfecting with Clostridium perfringens (CP) and Eimeria parasites. A total of 105 commercial 1-day-old broilers were randomly allocated into 5 groups, respectively challenged with Eimeria (Eimeria group), Eimeria and CP (Eimeria+CP group), Eimeria and CP with AM (Eimeria+CP+AM group), Eimeria and AM (Eimeria+AM group), and a placebo (Noninfected group). The treatment of AM exhibited a low degree of amelioration on NE severity. The application neither protected broilers from NE by decreasing NE-positive numbers nor reached a significant reduction in lesion scores in the small intestines. The development of NE reduced species diversity in jejunal microbiota; the pretreatments of AM exacerbated the consequence by losing species richness and promoted the similarity of the jejunal microbial community presented in the Eimeria+CP group. The participation of AM enhanced the increments of genera Clostridium sensu stricto 1 and Escherichia_Shigella and decreased the number of Lactobacillus. The significant variations of genera Clostridium sensu stricto 1 and Lactobacillus in jejunal microbiota were associated with NE development and promotion. In conclusion, oral inoculation of AM promoted the development of NE and modulated the jejunal microbiota favorable for CP overgrowth in broilers. The application of AM as a probiotic in broilers should be cautious on account of the effects to predispose NE.
Key words: necrotic enteritis, Akkemansia muciniphila, mucin, gut microbiota, 16S rRNA metagenomics
INTRODUCTION
Necrotic enteritis (NE), an economically devastating disease caused by Clostridium perfringens (CP), contributes to the annual cost of US $6 billion in broiler production losses (Wade and Keyburn, 2015). The costs mainly resulted from 10 to 40% mortality in broiler flocks, poor growth performance, and low feed efficiency (McDevitt et al., 2006; Paiva and McElroy, 2014). Regularly, NE was prevented through the use of antibiotic growth promoters (AGPs) in feeds. Due to the trend of exclusion of AGPs used in broiler production, the prevalence of this enteric disease was continuously increased (Van Immerseel et al., 2009). Based on the demands of alternative supplements to replace AGPs, various supplements to prevent the development of NE were widely studied. However, NE is a complex disease with involvements of predisposing factors to modulate the intestinal environment desirable for the pathogen replication and toxin production (Van Immerseel et al., 2009; Stanley et al., 2014). Among those factors, high protein diets and Eimeria parasites were critical factors to facilitate NE development (Prescott et al., 2016b; Yang et al., 2019b). In particular, the intestinal damages caused by Eimeria spp. promoted mucogenesis and the leakage of plasma proteins from the host (Collier et al., 2008), providing the eco-environment and nutrients favorable for CP multiplications (Moore, 2016; Prescott et al., 2016b).
Microbial populations in the gastrointestinal tract of chickens play a role in host defense mechanisms and enteric disease development. Some beneficial bacteria were demonstrated to protect the intestinal mucosa against pathogens by regulating host response to prevent or compete against the colonization of pathogens (Rehman et al., 2007). Additionally, gut microbiota was demonstrated to be associated with the maturation of the gut immune system (Crhanova et al., 2011), modulation of intestinal gene expression (Yin et al., 2010), and T cell-mediated immunity (Mwangi et al., 2010). Adverse disturbance of gut microbiota was shown to be correlated with the development of NE (Stanley et al., 2012; Yang et al., 2019a). Based on those pieces of evidence, the dominance of beneficial microorganisms over pathogens in the gut was considered as a practical strategy to secure gut health and prevent dysbiosis predisposing disease development.
Akkemansia muciniphila (AM) is a gram-negative and strictly anaerobic bacterium distributed in the intestines of humans and some animals (Belzer and de Vos, 2012; Lagier et al., 2015). The bacterium mainly resides in the outer mucus layer of intestines, exerting the mucolytic ability to degrade the mucin as the source of nutrients and release beneficial by-products to maintain microbial balance (Derrien et al., 2008, 2017). Besides, it also exhibited competitive inhibition on other mucin-degrading pathogens (Belzer and de Vos, 2012). AM was perceived to normalize the mucus thickness and promote intestinal barrier by enhancing goblet cell numbers, stimulating the turnover of the mucus layer, and up-regulating the expression of tight-junction proteins (Ganesh et al., 2013; Shin et al., 2014; Zhong et al., 2015; Grander et al., 2018). Moreover, AM participated in the regulations of host immune function and metabolism, providing physiological benefits and promoting health and disease prevention (O'Toole et al., 2017; Zhai et al., 2019a). AM is considered as a promising candidate for a next-generation probiotic in humans; however, little is known to use AM as a probiotic to prevent and control enteric diseases in poultry.
Several studies have demonstrated that the manifestation of NE in chickens accompanied significant overgrowth of CP and microbial shifts in the gut (Huang et al., 2019; Yang et al., 2019a). The methodologies to inhibit the multiplication, colonization, and invasion of CP were considered as keys to prevent and control the disease. According to promising results of AM on human health, this symbiont was hypothesized to mitigate the development of NE through degrading mucin to restrict the nutrient source for CP, competing for the intestinal site for colonization, and strengthening gut barrier integrity. Furthermore, the applications of several probiotics were shown to be beneficially ameliorated gut microbial disturbance and NE severity in chickens (Rajput et al., 2020). The present study aimed to evaluate the effects of oral inoculation of AM on NE prevention and control in a NE-reproduced model coinfecting CP with Eimeria. Microbial diversity, abundance, and shifts in gut microbiota between treatment and control groups were compared to elucidate the effects of AM on the development of NE.
MATERIALS AND METHODS
Experimental Design and Treatments
The experimental design and procedures were approved by the Mississippi State University Committee on Ethics in the Care and Use of Laboratory Animals (IACUC 16-439). A total of 105 male and female 1-day-old broiler chicks (Cobb strain) were obtained from a local commercial hatchery. Chicks were physically examined on receiving to ensure the healthy condition and then randomly allocated into 5 groups: 1) Eimeria group, broilers challenged with Eimeria; 2) Eimeria+CP group, broilers challenged with Eimeria and CP; 3) Eimeria+CP+AM group, broilers pretreated with AM and challenged with Eimeria and CP; 4) Eimeria+AM group, broilers treated with AM and Eimeria; 5) Noninfected group, broilers treated with a placebo. Broilers in groups were raised in separate iron tanks with temperature control and fresh litter. The water and feed were provided ad libitum. All broilers were fed with the same formula of diets at each period. From d 1 to 7 and d 8 to 19, broilers received the wheat diets and the wheat diets supplemented with 50% fishmeal (w/w), respectively.
NE was reproduced by coinfecting with netB-positive CP (CP1 strain) and multispecies of Eimeria parasites, following the procedures described in the previous study (Yang et al., 2019a). The NE cases were determined and validated by histological lesions and intestinal gross lesion scores reaching 2 or more. Chickens in AM-treated groups (Eimeria+AM and Eimeria+CP+AM groups) were daily gavaged with 1 mL of AM cultured broth with the concentration of 109 colony-forming units (CFU)/mL from d 8 to 18. For Eimeria-treated groups a 10-fold dose of anticoccidial vaccine as oral inoculum was provided to chickens on d 10. Afterward, 3 mL of CP1 inoculum with the concentration of 2.5 × 108 CFU/mL was orally administered to chickens in coinfected groups (Eimeria+CP and Eimeria+CP+AM groups) with the frequency of 3 times a day from d 15 to 18. All chickens were monitored for clinical signs and health status twice a day and humanely euthanized at d 19 using carbon dioxide.
Inocula Preparation
The ATCC A. muciniphila BAA-835 strain was used and cultured in Brain Heart Infusion (BHI) broth (Sigma-Aldrich, St. Louis, MO) at 37°C for 18 h under an anaerobic environment to reach the concentration of 109 CFU/mL. An anticoccidial live vaccine (Coccivac-B52, Merck Animal Health, Madison, NJ) containing oocysts of E. acervulina, E. maxima, E. maxima MFP, E. mivati, and E. tenella was used for the Eimeria challenge. A 10-fold dose of a commercial coccidial vaccine was prepared from the original bottle containing 10,000 doses of oocysts in an unspecified proportion of Eimeria species. The vaccines used in the study were at least 6 months before the expiration. The inocula of CP were prepared from a clinical NE strain (CP1) carrying netB gene obtained from Dr. John F. Prescott (Ontario Agricultural College, University of Guelph, Canada). The CP1 strain was reactivated on blood agar plates and then was transferred in fluid thioglycollate (FTG) medium (Himedia, Mumbai, Maharashtra, India) to reach approximately 2.5 × 108 CFU/ml for CP challenges. The bacterial concentration (CFU/mL) of the inocula was enumerated using plate counting in BHI agar (Sigma-Aldrich) to ensure the adequate bacteria number used for the challenge.
Lesion Scoring and Sample Collection
Broilers in this study were euthanized on d 19. Histological examination was conducted on small intestines (duodenum, jejunum, and ileum). NE lesion scores in the small intestinal segments were single-blinded evaluated and recorded based on the criteria of Keyburn et al., 2006. The highest score in all segments was recorded as the final lesion score for statistical analysis and the broiler with a lesion score ≥2 was recognized as the NE positive. As for 16 s rRNA metagenomics, 4 jejunal contents (1 content per broiler) were randomly collected from each group and immediately frozen at −80°C for further analysis.
DNA Extraction and 16S rRNA Pyrosequencing
Genomic DNA was extracted from 250 mg of the jejunal content using QIAamp PowerFecal DNA Kit (Qiagen, Germantown, MD) according to the manufacturer's instructions. The DNA was subsequently checked for concentration and quality using ultraviolet spectroscopy (Fisher Scientific, Pittsburgh, Pennsylvania) and 0.8% agarose gel (BD Biosciences, San Jose, CA). Then, the DNAs were used to prepare 16S rRNA libraries. The amplicon targeting the V3-V4 regions of bacterial 16S rRNA was amplified in 25 μL reaction mixtures, containing 12.5 μL Clontech Labs 3P CLONEAMP HIFI PCR PREMIX (Fisher Scientific, Pittsburgh, PA), 2.5 μL of each 10-μm Illumina primer with adapter sequences (Forward, CGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG; Reverse, GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC), 4 μL of DNA template, and 3.5 μL of nuclease-free water by an initial step at 95˚C (3 min), followed by 25 cycles of 95˚C (30 s), 55˚C (30 s), and 72˚C (30 s), and a final step at 72˚C (5 min) on Applied Biosystems GeneAmp PCR System 9700 (Applied Biosystems Inc., Foster City, CA). After the clean-up step by Monarch DNA Gel Extraction Kit (New England Biolabs, Ipswich, MA), an index PCR was conducted using NexterabXT Index Kit (Illumina, San Diego, CA) by adding 12.5 μL KAPA HiFi HotStartbReady Mix (Kapa Biosystems, Wilmington, MA), 2.5μL of each index primer, and 1 μL of 16S rRNA amplicon. The thermal cycling conditions were 95˚C for 3 min, 8 cycles of 95˚C (30 s), 55˚C (30 s), 72˚C for (30 s), and 72˚C for 5 min on Mastercycler pro (Eppendorf AG, Hamburg, Germany). The PCR products were purified by Agencourt AMPure XP beads (Beckman Coulter, Indianapolis, IA). The size and concentration were determined by Bioanalyzer with DNA 1000 chip (Agilent, Santa Clara, CA) and Qubit 2.0 Fluorometer with Qubit dsDNA HS Assay Kit (Fisher Scientific). Finally, libraries were sequenced on the Illumina MiSeq platform and 2 × 300 bp paired-end reads were generated.
Processing of Sequencing Data and Bioinformatics Analyses
Paired-end sequences were merged into tags by fast length adjustment of short reads (FLASH) v1.2.11. and then demultiplexed and filtered by Quantitative Insights into Microbial Ecology (Qiime) software v1.9.1 to have effective tags meeting the quality requirement and a threshold Phred quality score of Q ≥20. Sequences with ≥97% similarity were assigned to the same operational taxonomic unit (OTU) by the UPARSE algorithm in USEARCH. The OTU was selected and annotated for taxonomic information using RDP Classifier v2.11 with a cut-off of 80%. The clustered OTUs and taxa information were used for statistical analyses by Qiime v1.9.1 and R package v.3.3.1. Analyses of diversities were performed based on the output data of OTUs abundance information by indices of Shannon, Simpson, Abundance-based coverage estimator (ACE), and Chao1 for alpha diversity, and unifarc distance matrices by principal coordinate analysis (PCoA) for beta diversity. Differences in taxonomic profiles between groups were compared using Statistical Analysis Metagenomic Profiles (STAMP) software v2.1.3 with Welch's t test. An algorithm of linear discriminant analysis effect size (LEfSe) was used to determine the significant feature taxa between groups with linear discriminant analysis (LDA) scores of 3.5. Differential abundance of OTU among treatments was evaluated by MetagenomeSeq.
Statistical Analyses
Significant differences in NE incidence and severity were determined by the Chi-square test (or Fisher exact test if cell frequency was zero) and Tukey test using SAS software version 9.4 (SAS Institute, Inc., Cary, NC). The differences in taxa abundance and diversities were compared by Welch's t test, Tukey test, and multiple response permutation procedure (MRPP) by SAS v9.4, Qiime v1.9.1, and R package v.3.3.1. Statements of statistical significance were based on the level of P ≤ 0.05.
RESULTS
Effects of AM on the Incidence and Severity of NE
The incidence and lesion scores of NE in different treatments were demonstrated in Table 1. Gross lesions were shown in Figure 1. The coinfection of CP1 and Eimeria (Eimeria+CP group) produced significant NE cases (P ≤ 0.05) and remarkable lesions (P ≤ 0.01) when compared to the Noninfected group, authenticating the successful reproduction of NE in this study. By comparison with the Eimeria+CP group, oral pretreatments of AM from d 8 to 18 did not significantly reduce the number of NE after sequential challenges of Eimeria and CP1 in the Eimeria+CP+AM group. Although some degree of amelioration on NE severity was noted, the difference was not significant. Without CP1 challenge, the treatment of AM with Eimeria or Eimeria alone did not produce any NE cases in the Eimeria+AM or Eimeria groups. Some cases of diarrhea due to coccidiosis were still observed. When the netB-positive CP (CP1) was incorporated into the ecosystem containing AM and Eimeria (Eimeria+CP+AM group), the combination significantly enhanced the incidence and severity of NE when compared to the Noninfected group (P ≤ 0.01) or the Eimeria+AM group (P ≤ 0.01 for the incidence; P ≤ 0.05 for the severity).
Table 1.
Incidence and severity of NE in different treatments.
| Group | N | NE case | Frequency |
Lesion score (Mean ± SD) | Incidence | ||
|---|---|---|---|---|---|---|---|
| Score 2 | Score 3 | Score 4 | |||||
| Noninfected | 21 | 0 | 0 | 0 | 0 | 0.71 ± 0.46b | 0.00%b |
| Eimeria+CP | 19 | 5 | 0 | 4 | 1 | 1.57 ± 1.01a | 26.32%a |
| Eimeria+CP+AM | 20 | 7 | 5 | 2 | 0 | 1.45 ± 0.67a | 35.00%a |
| Eimeria+AM | 21 | 0 | 0 | 0 | 0 | 1 ± 0b | 0.00%b |
| Eimeria | 21 | 0 | - | - | - | - | 0.00%b |
Three chicks were dead and identified as early mortality after necropsy in Eimeria+CP (n = 2) and Eimeria+CP+AM (n = 1) groups.
Lesion score ≥ 2 was NE positive.
Lesion scores: 0 (no gross lesions), 1 (congested intestinal mucosa), 2 (small focal necrosis or ulceration; 1 to 5 foci), 3 (focal necrosis or ulceration; 6 to 15 foci), and 4 (focal necrosis or ulceration; 16 or more foci).
Unitary treatment of Eimeria spp. caused pale mucus membrane in all segments of small intestines. The scoring was not conducted on the Eimeria group to avoid the bias of observations and comparisons.
The lesion score was transformed to ranked data and then analyzed by Tukey's test. The difference in NE frequency was evaluated by Chi-square or Fisher exact tests.
A different superscript (a,b) in the same column means significantly different (P ≤ 0.05).
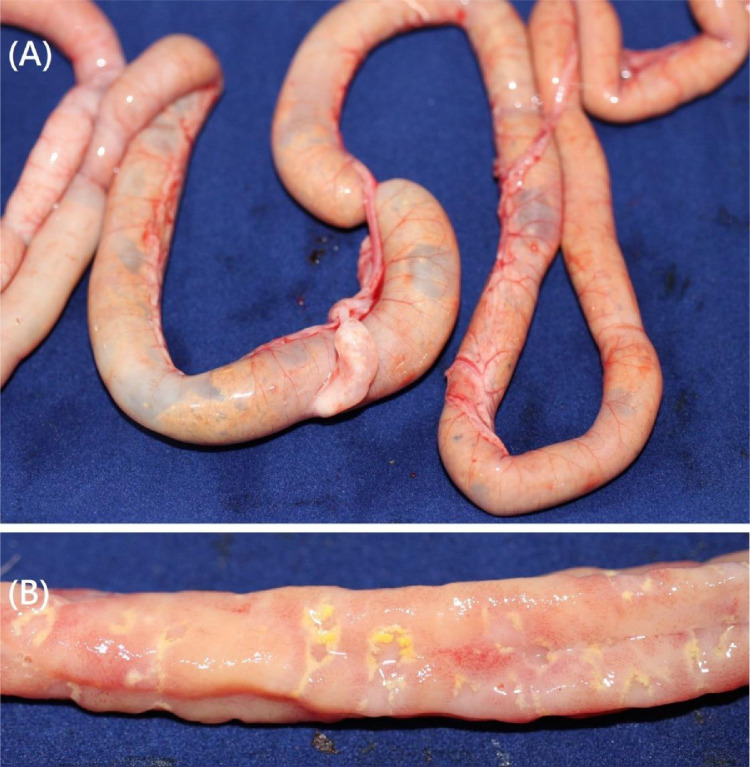
Figure 1.
Gross lesions in broilers classified as NE cases (lesion score ≥ 2). (A) Gas-filled and dilated small intestines. (B) Multifocal necrosis in the duodenum (lesion score 3).
Microbial Diversity in Response to Treatments
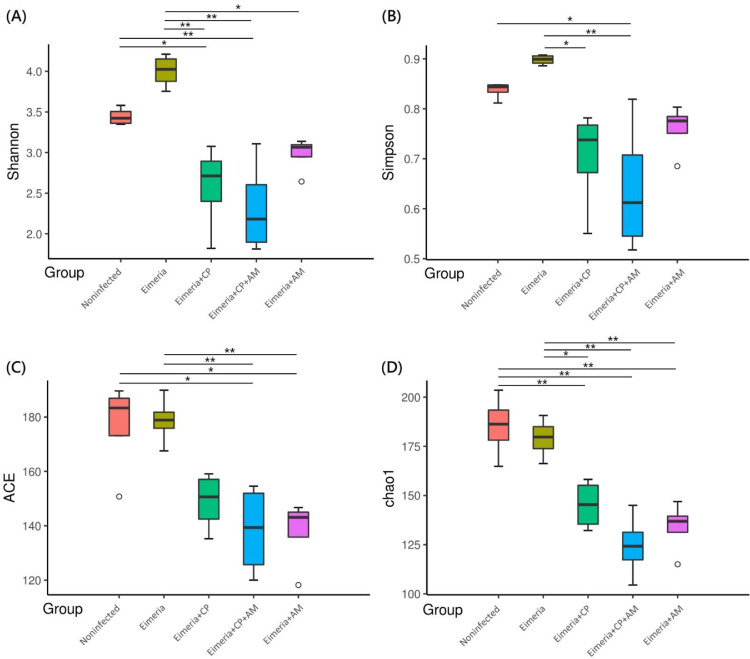
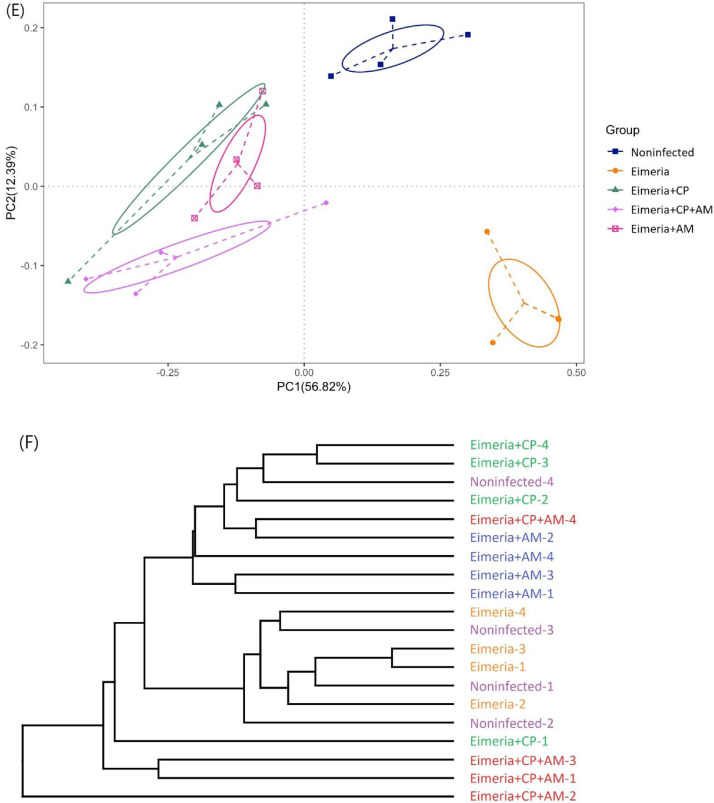
Challenges of Eimeria and CP1 (Eimeria+CP group) significantly reduced species richness and evenness in the jejunal microbiota (Figure 2) by Shannon and Chao 1 indices when compared to the Noninfected group (P ≤ 0.05). The treatment of AM with a coinfection of CP1 and Eimeria (Eimeria+CP+AM group) enhanced the decrement of species diversity observed in the Eimeria+CP group by comparing it to the Noninfected group (P ≤ 0.01). Oral inoculations of AM and Eimeria (Eimeria+AM group) significantly reduced species richness but it did not affect the species evenness in comparison to the Noninfected group. The development of NE reduced species diversity in jejunal microbiota and the pretreatments of AM exacerbated the consequence, particularly species richness. PCoA further demonstrated distinct microbial community profiles for each group, presenting the compositional differences or dissimilarities between groups (Figure 2E). On the other hand, cluster analysis by the unweighted paired-group method using arithmetic means (UPGMA) revealed that samples in the same group have similarities in microbial structures and aggregated at the adjacent hierarchical cluster, showing the consistency within the group (Figure 2F). Most of the pairwise-compared groups were significantly different in the species composition by multiple response permutation procedure (MRPP) (P ≤ 0.05; shown in Table 2), except for comparisons between Eimeria+CP+AM and Eimeria+AM groups or Eimeria+CP+AM and Eimeria+CP groups.
Figure 2.
Microbial diversity and structure in the jejunum evaluated by alpha diversity and beta diversity. (A) Shannon-Wiener diversity index; (B) Simpson index; (C) Abundance-based coverage estimator (ACE) index; (D) Chao1 index. (E) Bray-Curtis principal coordinate analysis (PCoA) for evaluating the compositions and similarities of jejunal microbiota. (F) Cluster analysis by the unweighted paired-group method using arithmetic means (UPGMA). Results are shown as mean ± SEM. Tukey test: * P ≤ 0.05, ** P ≤ 0.01.
Table 2.
Pairwise comparison of the species composition between groups by multiple response permutation procedure (MRPP).
| Group | A | Observed-delta | Expected-delta | P value |
|---|---|---|---|---|
| Noninfected - Eimeria+CP | 0.2597 | 0.244 | 0.3295 | 0.028 |
| Noninfected - Eimeria+AM | 0.3426 | 0.1774 | 0.2698 | 0.033 |
| Noninfected - Eimeria | 0.2923 | 0.2262 | 0.3196 | 0.029 |
| Noninfected - Eimeria+CP+AM | 0.3308 | 0.2364 | 0.3533 | 0.027 |
| Eimeria - Eimeria+AM | 0.5135 | 0.1806 | 0.3712 | 0.026 |
| Eimeria+CP+AM - Eimeria+AM | 0.1616 | 0.1909 | 0.2277 | 0.057 |
| Eimeria - Eimeria+CP+AM | 0.4542 | 0.2397 | 0.4391 | 0.03 |
| Eimeria -Eimeria+CP | 0.4483 | 0.2472 | 0.448 | 0.039 |
| Eimeria+CP - Eimeria+AM | 0.1341 | 0.1984 | 0.2291 | 0.031 |
| Eimeria+CP - Eimeria+CP+AM | 0.01012 | 0.2574 | 0.2601 | 0.306 |
A represents the effect size of within-group homogeneity as compared to the random expectation.
A > 0 indicates the difference between groups is higher than the difference within-groups, and vice versa for A < 0.
Observed-delta and expected-delta represent the level of difference within-groups and between groups, respectively.
Compositions of the Jejunal Microbiota and Their Modulations by AM
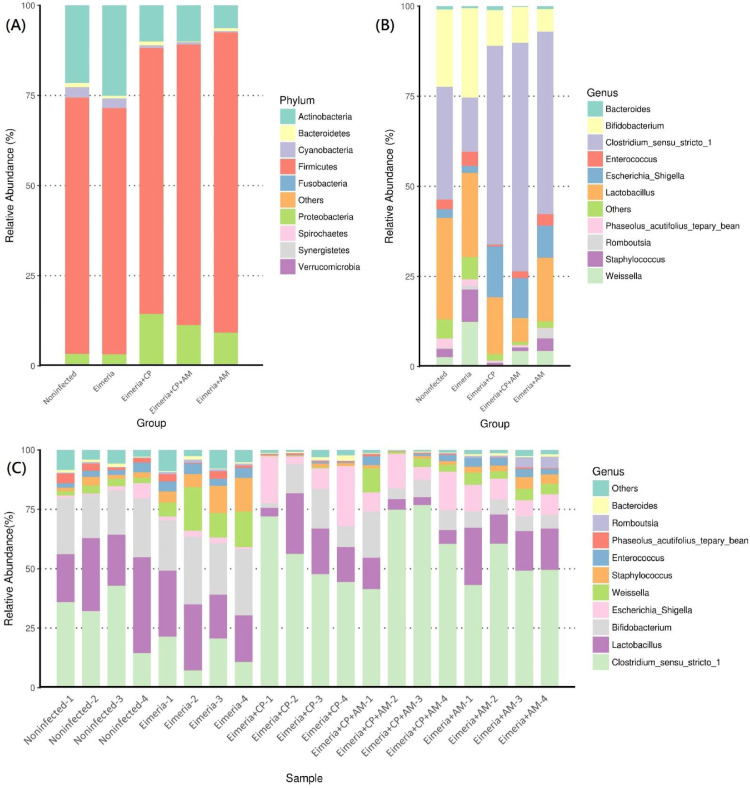
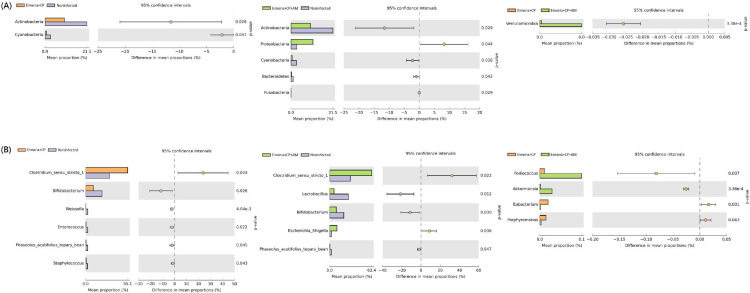
The jejunal microbiota in 19 days old broilers was mainly composed of phyla Firmicutes (71.1%), Actinobacteria (21.5%), Proteobacteria (3.1%), Cyanobacteria (2.7%), and Bacteroidetes (1.2%), shown in Figure 3A. The coinfection of CP1 and Eimeria (Eimeria+CP group) significantly reduced the abundance of Actinobacteria (10.0%) and Cyanobacteria (0.7%) when compared to the Noninfected group (P ≤ 0.05). Pretreatments of AM with a coinfection of CP1 and Eimeria (Eimeria+CP+AM group) further increased the amount of Proteobacteria (11.29%) and decreased the abundance of Bacteroidetes (0.24%) (P ≤ 0.05). A significant difference in phyla abundance of Verrucomicrobia was noted between Eimeria+CP and Eimeria+CP+AM groups (P ≤ 0.05; Figure 4A).
Figure 3.
Microbial composition in the jejunum in response to different treatments. (A) The distribution of the 10 most abundant phyla in the jejunum by groups. (B) Microbial structure at the level of the genus by groups. (C) The distribution of most abundant genera in jejunal samples. Each bar represents the average relative abundance of each taxon within a group or a sample.
Figure 4.
Differentially abundant clades identified by STAMP analysis (Eimeria+CP vs. Noninfected, Eimeria+CP+AM vs. Noninfected, and Eimeria+CP vs Eimeria+CP+AM). (A) The differential abundance of phyla between groups. (B) The differential abundance of genera between groups. Statistical significance (P ≤ 0.05) was detected by Welch's t test.
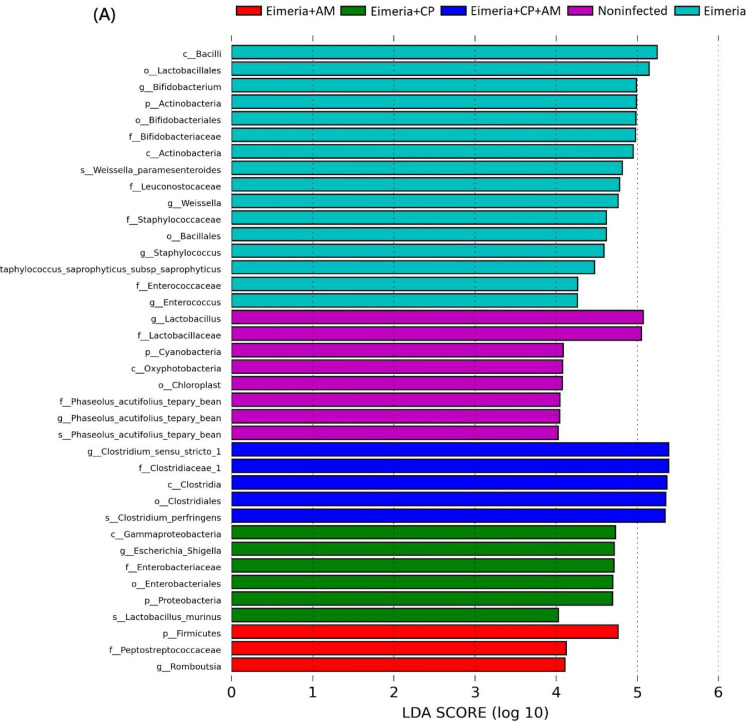
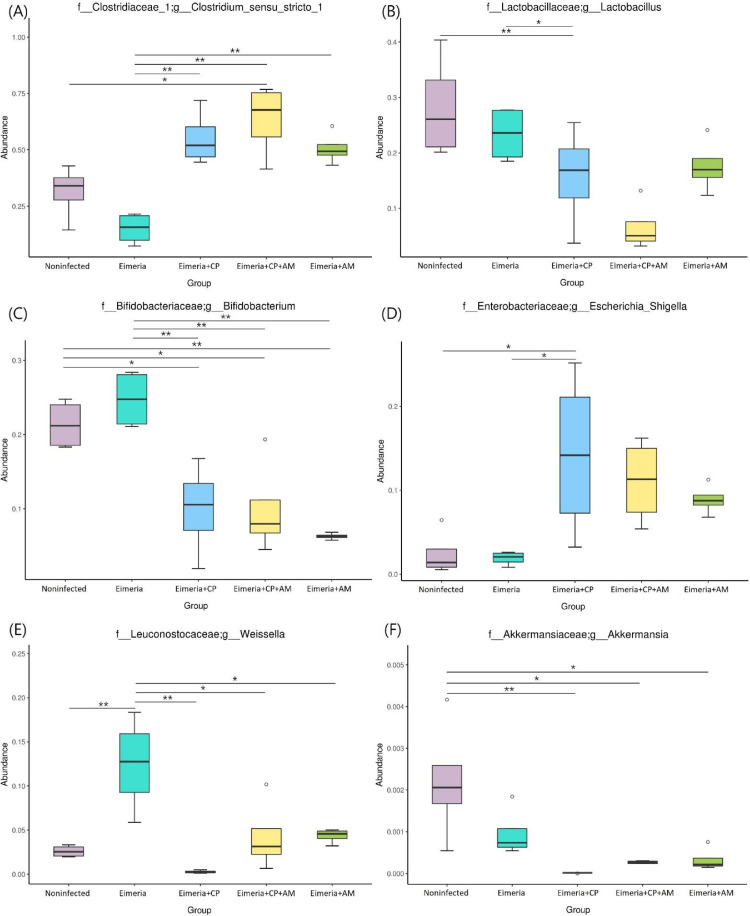
At the genus level, Clostridium sensu stricto 1 was the most abundant genus (31.35%) followed by Lactobacillus (28.16%), Bifidobacterium (21.37%), Enterococcus (2.62%), and Weissella (2.60%) in placebo-treated broilers (Figure 3B). Microbial community structures and the distribution of the most abundant genera in each sample were shown in Figure 3C. After challenges of Eimeria and CP1, the jejunal microbiota exhibited the significant increment of Clostridium sensu stricto 1 (55.12%) but remarkable decrements of Bifidobacterium (9.95%), Weissella (0.27%), Enterococcus (0.43%), Phaseolus_acutifolius_tepary_bean (0.39%), and Staphylococcus (0.76%) in comparison to the microbiota in the Noninfected group (P ≤ 0.05). The decrease of Lactobacillus (from 28.16% to 15.74%) was noted without significance. The treatment of AM with a coinfection of CP1 and Eimeria promoted significant increments of Clostridium sensu stricto 1 (63.41%) and Escherichia_Shigella (11.06%), accompanying significant decrements of Lactobacillus (6.64%), Bifidobacterium (9.96%), and Phaseolus_acutifolius_tepary_bean (0.49%) in comparison to the Noninfected group (P ≤ 0.05). Notably, increments of Clostridium sensu stricto 1 and Escherichia_Shigella and the decrement of Lactobacillus were enhanced by the treatment of AM. When the Eimeria+CP+AM group was compared to the Eimeria+CP group, significant increments of Prediococcus and Akkermansia were noted (Figure 4B).
LEfSe analysis on 5 treatment groups demonstrated that Lactobacillus and Phaseolus_acutifolius_tepary_bean were the most differential genera in the Noninfected group. Bifidobacterium, Weissella, Staphylococcus, and Enterococcus were the most differential genera in the Eimeria group. As for Eimeria+CP+AM, Eimeria+CP, and Eimeria+AM groups, the differential abundance of genera Clostridium sensu stricto 1, Escherichia_Shigella, and Romboutsia were noted, respectively (Figure 5A). The difference in abundance of those differential genera between groups was demonstrated in Figure 5B.
Figure 5.
Differential taxa and their abundant differences at the genus level between groups. (A) The most differentially abundant clades at all taxonomic levels between groups were identified by LEfSe using the LDA score of 4. (B) MetagenomeSeq analyses of Clostridium sensu stricto 1, Lactobacillus, Bifidobacterium, Escherichia_Shigella, Weissella, and Romboutsia between groups. Results are shown as mean ± SEM. Tukey test: * P ≤ 0.05, ** P ≤ 0.01.
DISCUSSION
According to the advantages of improving intestinal health and competitive inhibition of other mucin-utilized pathogens, AM was selected as a probiotic to treat broilers in this study (Belzer and de Vos, 2012; Derrien et al., 2017). As a result, the pretreatments of AM produced a higher number of NE cases under the coinfection of CP1 and Eimeria. The development of NE involves the significant overgrowth and colonization of CP in the epithelial layer (Prescott, et al., 2016a). Since CP and AM both required amino acids, such as threonine, and by-products from mucin degradation for their multiplications (Shimizu et al., 2002; Ottman et al., 2017), efficient enzyme machinery or rate for utilizing the nutrients decided the competitive advantage. In vitro, AM was noted by strict culture condition and loner cultivation time than CP to reach the concentration needed for further augmentation when the same BHI medium was used. The difference in bacterial generation time potentially generated the competitive advantage of CP on nutrients. Subsequent overgrowth led to the predominance of CP in the gut, thereby increasing the possibility to develop NE. Additionally, the function of the gut mucosal layer not only protects epithelial cells from microbial invasions but only provides a source of nutrients for intestinal bacteria. AM was known to be abundant in the mucosal layer and regularly colonized the ileum and cecum in mice (Derrien et al., 2011), with the largest amount in the cecum (Zhang et al., 2019). In contrast to AM, CP was highly abundant in the jejunum of broilers (Yang et al., 2019a) and its colonization mainly targeted the segments of small intestines, causing focal and multifocal necrotic lesions in the submucosa (Prescott et al., 2016a). The differential abundance and different colonized locations in the gut segments may contribute to the nonsignificant effect of AM on the competitive exclusion of CP. However, the information on AM distribution and its status of colonization in chickens was very limited. Further studies are suggested for clarifying the speculation.
In this study, the severity of intestinal lesions was measured based on the number of focal necrosis on the mucosa. By treatments of AM, a low degree of alleviation on NE lesions was noted. The development of enteric diseases was regularly initiated when the intestinal mucosa was damaged, facilitating pathogens to invade the host (Quintana-Hayashi et al., 2018). For CP, the invasion was through the destructions on extracellular matrix and cellular junctions (Timbermont et al., 2011). The increment of AM has been demonstrated to promote the expression of tight junction protein in rats (Wang et al., 2018), playing a role in protecting the tight junction and intestinal barrier. It was speculated that the invasions of CP were resisted to a certain degree on the strength of securing tight junction by AM. In addition, AM was shown to ameliorate gut inflammation in mice by diminishing the lipopolysaccharides-binding protein, reducing the expression of the proinflammatory cytokines, and upregulating anti-inflammatory factors (Zhao et al., 2017; Zhai et al., 2019b). Another study further demonstrated that AM relieved intestinal inflammation and mucosal damage caused by S. Pullorum in chicks (Zhu et al., 2020). Those indicated that AM may lower the NE severity by the means of regulating gut inflammation as well. However, there is controversy about the effects of AM in different diseases. Under serious intestinal damage caused by S. Typhimurium, AM aggravated the intestinal injury in gnotobiotic mice (Ganesh et al., 2013). In that case, the lipopolysaccharides (LPS) on the inner wall of AM were demonstrated to participate in the exacerbation.
AM represented 3% to 5% of the microbial community in healthy humans (Belzer and de Vos, 2012). On the contrary, the genus Akkermansia accounted for simply 0.22% of relative abundance in the jejunal microbiota of broilers in the present study. Under the existence of netB-positive CP (Eimeria+CP and Eimeria+CP+AM groups), the relative abundance of Akkermansia in jejunal microbiota decreased to 0.001 and 0.027%, respectively. The results demonstrated that the role of AM in chickens was not as significant as in humans. Even though similar amounts of AM (109 CFU/day/per chicks) and CP1 (8.4 × 108 CFU/day/per chicks) were simultaneously provided to broilers, the abundance of Akkermansia was still decreased with significance, showing the competitive advantage of CP.
In the present study, the results of reduced microbial diversity and the dominance of CP in broilers affected by NE were in line with previous studies (Fasina et al., 2016; Li et al., 2017). The treatments of AM in the Eimeria+CP (NE) group significantly decreased the total number of species in a community (richness) and enhanced the relative abundance of Clostridium sensu stricto 1 when compared to the Noninfected group. The β-diversity revealed that the jejunal microbial communities of five treatment groups were significantly separated except for comparisons between Eimeria+CP+AM and Eimeria+AM groups or Eimeria+CP+AM and Eimeria+CP groups. Taken together, the application of AM favored the overgrowth of Clostridium sensu stricto 1 but the challenge of CP1 did not alter the main community structure in AM group, contributing to the similarities between Eimeria+CP+AM and Eimeria+CP or Eimeria+CP+AM and Eimeria+AM groups.
As for the compositional analysis of jejunal microbiota between Eimeria+CP+AM and Eimeria+CP groups, the provisions of AM significantly increased the abundance of phylum Verrucomicrobia and genus Akkermansia, representing the accomplishment of AM induction. In broilers, the jejunal microbiota was predominant by genera Clostridium sensu stricto 1, Lactobacillus, and Bifidobacterium. Among genus Clostridium sensu stricto 1, CP was well-known as a commensal species in chickens (Wiegel et al., 2006). The extraneous challenge of CP with Eimeria significantly disturbed the microbial homeostasis by the increment of Clostridium sensu stricto 1 and decrements of Lactobacillus, Bifidobacterium, and other minor bacteria. On the other hand, the treatment of AM with a coinfection of CP and Eimeria promoted taxonomic dysbiosis by enhancing the abundance of Clostridium sensu stricto 1 and declining an additional amount of Lactobacillus, leading to more NE cases. It is noteworthy that there was not a NE case by treatments without the participation of CP. When CP was incorporated in the AM group (i.e., Eimeria+CP+AM group), the amount of Lactobacillus was significantly decreased by comparison to the Noninfected group, showing the synergistic effects with AM. Accordingly, microbial shifts of Clostridium sensu stricto 1 and Lactobacillus were recognized as featured steps in the progress of NE development. Another study further demonstrated the trend that the levels of featured shifts (the increment of Clostridium sensu stricto 1 and the decrement of Lactobacillus) followed the severity of NE (Yang et al., 2019a). Although the treatment of AM and Eimeria induced a similar pattern of featured shifts, the degree of variations on Clostridium sensu stricto 1 and Lactobacillus was not significant by metagenomeSeq analysis. The decrement of Bifidobacterium in the jejunum may involve in the development of NE. However, its abundance did not follow the incidence of NE, indicating that the reduction of this taxon in the jejunum may not be necessary for facilitating NE development.
The genera of Lactobacillus, Bifidobacterium, and Pediococcus are lactic acid bacteria (LAB) widely distributed in the mammalian gut microbiota. They could synthesize bacteriocins to protect the host from invasions of pathogenic bacteria (Porto et al., 2017). The treatment of AM was expected to exert preventive effects on enteric diseases. It was not merely based on the actions of AM itself, but also relied on other short-chain fatty acids (SCFAs)-producing bacteria, affecting gut motility and immunity in the host. The abundance of Pediococcus, conceived as a probiotic in humans, was significantly elevated after AM treatments. Romboutsia, shown as an SCFA-producing bacterium (Li et al., 2019), was noted as the most differentially abundant genus in the AM group by LEfSe analysis. These results were consistent with other studies showing that AM promoted the growth of other SCFA-producing bacteria (Tamanai-Shacoori et al., 2017). However, such promotion by AM was not sufficient to achieve the preventive outcome of NE based on the disease incidence and severity in this study. For the Eimeria+CP group, Escherichia_Shigella was the differential taxon in this group when compared to the other 4 groups. This genus is regularly considered as nonpathogenic bacteria in the host. It may convert to pathogenic under the stimulus of stress (Lutful Kabir, 2010). Nevertheless, a lower abundance of Escherichia_Shigella was with the higher NE numbers in the Eimeria+CP+AM group indicated that this genus may not be relevant to NE development.
To conclude, oral inoculations of AM promoted the development of NE and modulated the jejunal microbiota favorable for CP overgrowth in broilers. Although a low degree of amelioration on NE severity was noted, the application of AM as a probiotic in broilers should be cautious on account of its effect to predispose NE.
ACKNOWLEDGMENTS
The authors thank Dr. John F. Prescott for the provision of netB-positive CP1 strain and Dr. Hsinyi Lu and Dr. Yue-Jia Lee for assistance in the trial work and 16S rRNA pyrosequencing. This work was supported by the College of Veterinary Medicine, Mississippi State University.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Belzer C., de Vos W.M. Microbes inside–from diversity to function: the case of Akkermansia. Isme J. 2012;6:1449–1458. doi: 10.1038/ismej.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier C.T., Hofacre C.L., Payne A.M., Anderson D.B., Kaiser P., Mackie R.I., Gaskins H.R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008;122:104–115. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Crhanova M., Hradecka H., Faldynova M., Matulova M., Havlickova H., Sisak F., Rychlik I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect. Immun. 2011;79:2755–2763. doi: 10.1128/IAI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M., Belzer C., de Vos W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017;106:171–181. doi: 10.1016/j.micpath.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Derrien M., Collado M.C., Ben-Amor K., Salminen S., de Vos W.M. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 2008;74:1646–1648. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M., Van Baarlen P., Hooiveld G., Norin E., Müller M., de Vos W.M. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader Akkermansia muciniphila. Front. Microbiol. 2011;2:166. doi: 10.3389/fmicb.2011.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasina Y.O., Newman M.M., Stough J.M., Liles M.R. Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poult. Sci. 2016;95:247–260. doi: 10.3382/ps/pev329. [DOI] [PubMed] [Google Scholar]
- Ganesh B.P., Klopfleisch R., Loh G., Blaut M. Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice. PLoS One. 2013;8:e74963. doi: 10.1371/journal.pone.0074963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grander C., Adolph T.E., Wieser V., Lowe P., Wrzosek L., Gyongyosi B., Ward D.V., Grabherr F., Gerner R.R., Pfister A., Enrich B., Ciocan D., Macheiner S., Mayr L., Drach M., Moser P., Moschen A.R., Perlemuter G., Szabo G., Cassard A.M., Tilg H. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67:891–901. doi: 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- Huang T., Peng X.Y., Gao B., Wei Q.L., Xiang R., Yuan M.G., Xu Z.H. The effect of Clostridium butyricum on Gut Microbiota, immune response and intestinal barrier function during the development of necrotic enteritis in chickens. Front Microbiol. 2019;10:2309. doi: 10.3389/fmicb.2019.02309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutful Kabir S.M. Avian colibacillosis and salmonellosis: a closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Pub. Health. 2010;7:89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A.L., Sheedy S.A., Ford M.E., Williamson M.M., Awad M.M., Rood J.I., Moore R.J. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 2006;74:6496–6500. doi: 10.1128/IAI.00806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier J.C., Hugon P., Khelaifia S., Fournier P.E., Scola B.L., Raoult D. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin. Microbiol. Rev. 2015;28:237–264. doi: 10.1128/CMR.00014-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.W., Fang B., Pang G.F., Zhang M., Ren F.Z. Age- and diet-specific effects of chronic exposure to chlorpyrifos on hormones, inflammation and gut microbiota in rats. Pestic Biochem. Physiol. 2019;159:68–79. doi: 10.1016/j.pestbp.2019.05.018. [DOI] [PubMed] [Google Scholar]
- Li Z., Wang W., Liu D., Guo Y. Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt R., Brooker J., Acamovic T., Sparks N. Necrotic enteritis; a continuing challenge for the poultry industry. Worlds Poult. Sci. J. 2006;62:221–247. [Google Scholar]
- Moore R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016;45:275–281. doi: 10.1080/03079457.2016.1150587. [DOI] [PubMed] [Google Scholar]
- Mwangi W.N., Beal R.K., Powers C., Wu X., Humphrey T., Watson M., Bailey M., Friedman A., Smith A.L. Regional and global changes in TCRalphabeta T cell repertoires in the gut are dependent upon the complexity of the enteric microflora. Dev. Comp. Immunol. 2010;34:406–417. doi: 10.1016/j.dci.2009.11.009. [DOI] [PubMed] [Google Scholar]
- O'Toole P.W., Marchesi J.R., Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017;2:17057. doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- Ottman N., Geerlings S.Y., Aalvink S., de Vos W.M., Belzer C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract. Res. Clin. Gastroenterol. 2017;31:637–642. doi: 10.1016/j.bpg.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Paiva D., McElroy A. Necrotic enteritis: applications for the poultry industry. J. Appl. Poult. Res. 2014;23:557–566. [Google Scholar]
- Porto M.C., Kuniyoshi T.M., Azevedo P.O., Vitolo M., Oliveira R.P. Pediococcus spp.: an important genus of lactic acid bacteria and pediocin producers. Biotechnol. Adv. 2017;35:361–374. doi: 10.1016/j.biotechadv.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Prescott J.F., Parreira V.R., Mehdizadeh Gohari I., Lepp D., Gong J. The pathogenesis of necrotic enteritis in chickens: what we know and what we need to know: a review. Avian Pathol. 2016;45:288–294. doi: 10.1080/03079457.2016.1139688. [DOI] [PubMed] [Google Scholar]
- Prescott J.F., Smyth J.A., Shojadoost B., Vince A. Experimental reproduction of necrotic enteritis in chickens: a review. Avian Pathol. 2016;45:317–322. doi: 10.1080/03079457.2016.1141345. [DOI] [PubMed] [Google Scholar]
- Quintana-Hayashi M.P., Padra M., Padra J.T., Benktander J., Lindén S.K. Mucus-pathogen interactions in the gastrointestinal tract of farmed animals. Microorganisms. 2018;6 doi: 10.3390/microorganisms6020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput D.S., Zeng D., Khalique A., Rajput S.S., Wang H., Zhao Y., Sun N., Ni X. Pretreatment with probiotics ameliorate gut health and necrotic enteritis in broiler chickens, a substitute to antibiotics. AMB Express. 2020;10:220. doi: 10.1186/s13568-020-01153-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman H.U., Vahjen W., Awad W.A., Zentek J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch. Anim. Nutr. 2007;61:319–335. doi: 10.1080/17450390701556817. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Ohtani K., Hirakawa H., Ohshima K., Yamashita A., Shiba T., Ogasawara N., Hattori M., Kuhara S., Hayashi H. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. U S A. 2002;99:996–1001. doi: 10.1073/pnas.022493799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N.R., Lee J.C., Lee H.Y., Kim M.S., Whon T.W., Lee M.S., Bae J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- Stanley D., Keyburn A.L., Denman S.E., Moore R.J. Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Vet. Microbiol. 2012;159:155–162. doi: 10.1016/j.vetmic.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Stanley D., Wu S.B., Rodgers N., Swick R.A., Moore R.J. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanai-Shacoori Z., Smida I., Bousarghin L., Loreal O., Meuric V., Fong S.B., Bonnaure-Mallet M., Jolivet-Gougeon A. Roseburia spp.: a marker of health? Future Microbiol. 2017;12:157–170. doi: 10.2217/fmb-2016-0130. [DOI] [PubMed] [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., Rood J.I., Moore R.J., Titball R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009;17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Wade B., Keyburn A. The true cost of necrotic enteritis. World Poult. 2015;31:16–17. [Google Scholar]
- Wang K., Yang Q., Ma Q., Wang B., Wan Z., Chen M., Wu L. Protective effects of salvianolic acid against dextran odium sulfate-induced acute colitis in rats. Nutrients. 2018;10 doi: 10.3390/nu10060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegel J., Tanner R., Rainey F.A. Pages 654-678 in The Prokaryotes. Springer; New York, NY: 2006. An introduction to the family Clostridiaceae. [Google Scholar]
- Yang W.Y., Lee Y.J., Lu H.Y., Branton S.L., Chou C.H., Wang C. The netB-positive Clostridium perfringens in the experimental induction of necrotic enteritis with or without predisposing factors. Poult. Sci. 2019;98:5297–5306. doi: 10.3382/ps/pez311. [DOI] [PubMed] [Google Scholar]
- Yang W.Y., Lee Y., Lu H., Chou C.H., Wang C. Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS One. 2019;14 doi: 10.1371/journal.pone.0205784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Lei F., Zhu L., Li S., Wu Z., Zhang R., Gao G.F., Zhu B., Wang X. Exposure of different bacterial inocula to newborn chicken affects gut microbiota development and ileum gene expression. Isme J. 2010;4:367–376. doi: 10.1038/ismej.2009.128. [DOI] [PubMed] [Google Scholar]
- Zhai Q., Feng S., Arjan N., Chen W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019;59:3227–3236. doi: 10.1080/10408398.2018.1517725. [DOI] [PubMed] [Google Scholar]
- Zhai R., Xue X., Zhang L., Yang X., Zhao L., Zhang C. Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in mice. Front Cell Infect. Microbiol. 2019;9:239. doi: 10.3389/fcimb.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Li Q., Cheng L., Buch H., Zhang F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019;12:1109–1125. doi: 10.1111/1751-7915.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Liu W., Wang J., Shi J., Sun Y., Wang W., Ning G., Liu R., Hong J. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J. Mol. Endocrinol. 2017;58:1–14. doi: 10.1530/JME-16-0054. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Teixeira C., Marungruang N., Sae-Lim W., Tareke E., Andersson R., Fåk F., Nyman M. Barley malt increases hindgut and portal butyric acid, modulates gene expression of gut tight junction proteins and Toll-like receptors in rats fed high-fat diets, but high advanced glycation end-products partially attenuate the effects. Food Funct. 2015;6:3165–3176. doi: 10.1039/c5fo00150a. [DOI] [PubMed] [Google Scholar]
- Zhu L., Lu X., Liu L., Voglmeir J., Zhong X., Yu Q. Akkermansia muciniphila protects intestinal mucosa from damage caused by S. pullorum by initiating proliferation of intestinal epithelium. Vet. Res. 2020;51:34. doi: 10.1186/s13567-020-00755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]