Abstract
Background:
Cemented Total Knee Arthroplasty (TKA) provides excellent long-term survival rates and functional results, however, radiolucent lines (RLLs) often appear during early post-operative follow-up and their incidence and clinical significance are unknown. The primary aim was to establish the incidence, location, frequency, and time taken for RLLs to appear within the first year after a primary cemented TKA with an anatomic tibial baseplate (Smith and Nephew, LEGION Total Knee System).
Methods:
This was a retrospective analysis of 135 primary cemented TKA in 131 patients over three years. We compared demographics, serial radiographs, and early clinical and functional outcomes.
Results:
There were 65 TKAs (48%) in 62 patients who had RLLs within the first year post-operatively. Most were females (58.8%). Mean age was 68.3 ± 7.9 years. There were 88 RLLs, with the most and second commonest location at the medial tibial baseplate (38%) and anterior femoral flange (23%). 89% were in the bone-cement interface. The largest average length of RLLs were at the anterior flange of the femoral component (1.98 ± 1.33 mm). The average time to development was 6.5 ± 4.1 months. None of these patients had infections nor required revision. Patients with RLLs did not do worse in functional and clinical scoring at 1-year.
Conclusion:
There was a 48% incidence of physiological RLLs after cemented TKA, with the highest occurrence at the medial tibial baseplate at 38%. These radiolucent lines did not affect early post-operative clinical and functional outcomes of patients.
Key Words: Radiolucency, Total Knee Arthroplasty, Total Knee Replacement, Cemented
Introduction
Total Knee Arthroplasty (TKA) is a popular treatment option for osteoarthritis of the knees, especially in end-stage arthritis, achieving excellent long-term survival rates(1–5). Despite its longevity, revisions of TKA are increasing in prevalence due to the increase in primary TKAs performed(6). The most common cause of revision, especially in late revision, is attributed to aseptic loosening. Aseptic loosening is associated with the presence of radiolucent lines (RLL)(2,7,8). Additionally, there are several studies demonstrating the early development of RLLs, appearing within the first 2 years of primary implantation(9–11). These early RLLs are postulated to be physiological(12–15).
Previous reports on the characterization of physiological RLLs are based on symmetrical tibial baseplates(2,9–11). With a shift towards using asymmetric or anatomic tibial baseplates in modern knee designs, including in our centre, we observed a larger proportion of medial tibial baseplate RLLs in our patients who received anatomic tibial baseplates, as compared to patients who received symmetric tibial baseplates.
Thus far, there have been no reports on the incidence of RLLs in anatomic tibial baseplates. Hence, the primary aim of this study was to establish the incidence, location, frequency, and time taken for RLLs to appear within the first 12 months after a primary cemented TKA with an anatomic tibial baseplate (Smith and Nephew, LEGION Total Knee System). The secondary aim was to determine if there was correlation with the development of RLLs and early functional outcomes.
Materials and Methods
Study design and patient selection
This was a single centre, retrospective analysis on a prospective database on all patients who had undergone total knee replacement at our institution. From our database, we identified all patients who had undergone cemented primary TKA between 1 January 2017 and 31 May 2019. All surgeries were performed by six different fellowship-trained arthroplasty surgeons.
The inclusion criteria were patients who had primary knee osteoarthritis, at least 1-year of follow-up, and TKAs performed using the Smith and Nephew LEGION Total Knee System. Implants were either of cruciate-retaining or posterior stabilized designs (Smith and Nephew, Memphis, TN, USA). Patients who had TKAs other than the study implant were excluded. Patients who also did not have serial radiographs during the follow-up period were excluded. There were 135 primary cemented TKAs (131 patients). Demographic data such as age, gender, body mass index (BMI), pre-surgery and follow-up data including clinical data, knee X-rays, and knee function scores were retrieved from our institutional knee registry. The review of medical records was approved by the local ethical committee, DSRB 2019/01037.
Surgical procedure
All 6 surgeons at our institution perform TKA in a similar fashion. Surgery is performed either under spinal or general anaesthesia, with a midline skin incision and a medial parapatellar approach. A thigh tourniquet is used in all cases. All surgeries were performed with navigation using the KneeAlign 2 System (OrthAlign Inc, Aliso Viejo, CA, USA). After performing all bone cuts, the bone surface is irrigated with 0.9% saline with a high-pressure pulsatile lavage. The bone surface is then carefully dried. The operating room temperature is kept between 20 and 21 degrees Celsius. A single packet of high viscosity bone cement (PALACOS® R+G or SMARTSET™) is prepared using MIXIGUN® from Zimmer Biomet. After the cement is vacuum mixed, pressurization of cement is performed during application to the tibia (including the keel). The tibial component is then inserted and impacted. The femoral component is likewise implanted with the same mix of cement. Tranexamic acid is given intravenously or through intra-articular injection during the surgery.
Post-operatively, patients are placed on continuous passive motion from post-operative day zero, and allowed to full weight bear from post-operative day zero. All patients were discharged between three to ten days post-operatively and referred to an outpatient rehabilitation program.
Radiologic assessment
Post-operative radiographs are performed on post-operative day zero, 3, 12 and 24 months after surgery. In addition, full length lower limb radiographs (hip-knee-ankle radiographs) are taken at 3 and 12 months follow-up to assess the post-operative leg alignment. All radiographs were taken according to a standardized protocol, consisting of a) anteroposterior (AP) weight-bearing view, b) lateral view at 30-degrees of knee flexion, and c) full length lower limb standing radiographs. To ensure correct positioning of all anatomic landmarks the patient was informed to keep the knee in full extension with his/her feet in slight internal rotation.
For this study, serial radiographs taken during the patients’ follow-up period were retrospectively analysed for the presence of RLLs. A radiolucent zone of any size between cement and bone, and the implant and cement, were considered to be RLLs(10,11).
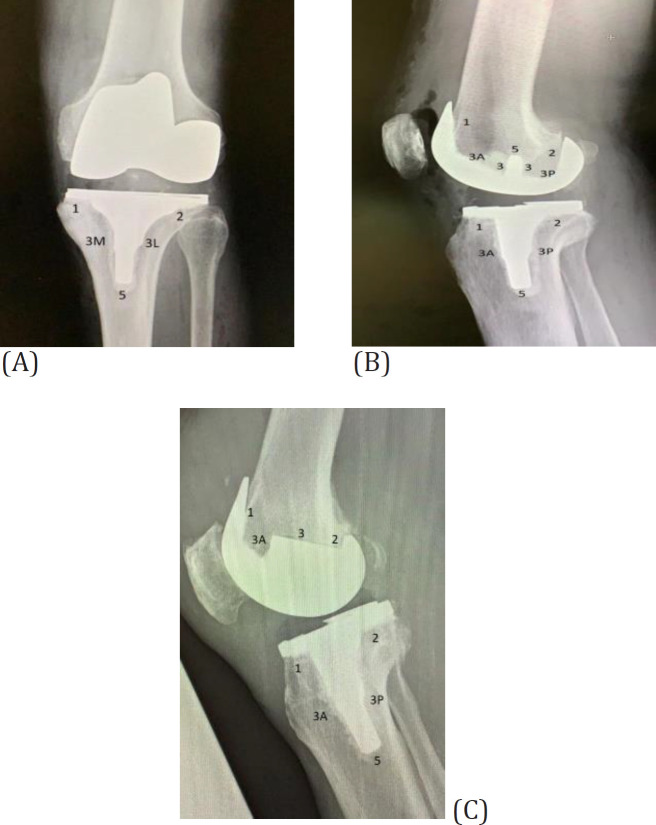
The radiographic assessment was conducted independently by three authors, blinded to the medical history of the patients. The average of the three measurements by the three authors was used as the final RLL size. The assessments were performed using Modern Knee Society Radiographic Evaluation System to document the location of the RLLs(16). Using this evaluation system, the components of the implants were divided into different zones to document the location of RLLs [Figure 1].
Figure1.
(A) AP views of the tibial component; (B and C) lateral views of the tibial and femoral component showing zone classification
Outcomes
Functional outcome measures were the Oxford Knee Score (OKS) and the 1989 Knee Society Clinical Rating System Score (KSS) (clinical and functional scores), collected pre-operatively, and at 3-months and 1-year post-operatively(17–19). Clinical outcome measures were complications in terms of the rate of revisions, re-operations, peri-prosthetic fractures or any infections.
Statistical Analysis
Descriptive variables were reported as mean ± standard deviation (SD) or n (%) prevalence. Continuous variables were compared with independent sample t-tests, whilst categorical variables were compared with chi-square test or Fisher’s exact test when appropriate. Statistical significance was taken where p-value < 0.05. All analyses were performed using SPSS version 23.0 (IBM, Chicago, IL, USA).
Results
Demographic data
A total of 131 patients underwent 135 cemented primary TKAs for osteoarthritis of the knee and were included in this study. The patient demographics in patients who had RLL and without RLL were similar, including age, BMI, gender, laterality, and follow-up period [Table 1]. None of the patients had re-operations or underwent revision surgery during the follow-up period. None had superficial infections, nor prosthetic joint infections. No patient complained of knee pain during follow-up in both groups. There were no peri-prosthetic fractures.
Table 1.
Demographic data
| Total | With RLL | Without RLL | p -value | |
|---|---|---|---|---|
| No. of TKAs | 135 | 65 | 70 | NA |
| No. of patients | 131 | 62 | 69 | NA |
| Mean age (years) | 68.2 ± 7.5 | 68.3 ± 7.9 | 68.2 ± 7.2 | 0.92 |
| BMI (kg/m2) | 28.5 ± 6.7 | 27.5 ± 4.2 | 27.7 ± 4.3 | 0.78 |
| Males (%) | 43 (32.6) | 26 (41.2) | 18 (26.1) | 0.06 |
| Laterality (left : right) | 61 : 74 | 29: 36 | 32: 38 | 0.90 |
| Follow-up period (months) | 15.9 ± 5.3 | 16.4 ± 4.9 | 16.2 ± 5.1 | 0.82 |
| Pre-operative anatomical knee alignment () [varus (-), valgus (+)] | -3.7 ± 9.5 | -5.1 ± 6.4 | -2.4 ± 11.5 | 0.15 |
| 3-month anatomical knee alignment () [varus (-), valgus (+)] | 5.6 ± 2.7 | 5.9 ± 2.6 | 5.4 ± 2.8 | 0.31 |
| 1-year anatomical knee alignment () [varus (-), valgus (+)] |
5.1 ± 3.5 | 5.2 ± 3.2 | 5.1 ± 3.7 | 0.89 |
| 1-year Hip-Knee-Ankle angle () [varus (-), valgus (+)] | 0.02 ± 3.8 | 0.18 ± 3.1 (range -13.6 – 11.9) | -0.12 ± 4.3 (range -7.2 – 7.6) | 0.68 |
| 1-year tibial keel alignment () [varus (-), valgus (+)] | 0.72 ± 2.1 | 0.93 ± 2.3 (range -6 – 8.3) | 0.53 ± 1.8 (range -3 – 5.8) | 0.31 |
Legend: TKA (Total Knee Arthroplasty); RLL (Radiolucent Line); BMI (Body Mass Index)
Radiolucent lines
We found 65 TKAs (48%) in 62 patients (47%) had RLLs post-operatively, including 4 patients who had RLLs bilaterally. There was a total of 88 RLLs. The most frequent location for RLLs was found to be at the medial tibial baseplate (n = 33, 38%), while the second most common location was at the anterior flange of the femoral component (n = 20, 23%). The frequency and location of RLLs are presented in [Table 2]. In terms of the interface of development of RLLs, 78 (89%) were found between the bone and cement interface, and 10 (11%) were found between the cement and implant (IC) interface.
Table 2.
Frequency, location and associated length of RLL
| AP Tibial | Lateral Tibial | Lateral Femur | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | 1 | 2 | 3M | 3L | 5 | 1 | 2 | 3A | 3P | 5 | 1 | 2 | 3 | 3A | 3P | 5 |
| Frequency | 33 | 4 | 0 | 0 | 0 | 11 | 4 | 0 | 0 | 0 | 20 | 14 | 2 | 0 | 0 | 0 |
| Average Length/mm | 1.28 | 1.38 | - | - | - | 1.75 | 1.1 | - | - | - | 1.98 | 1.35 | 1.05 | - | - | - |
| SD/mm | 0.59 | 0.71 | - | - | - | 0.62 | 0.71 | - | - | - | 1.33 | 0.66 | 0.65 | - | - | - |
Legend: RLL (Radiolucent Line); AP (Antero-posterior); SD (Standard Deviation)
RLLs were found to appear at an average of 6.5 ± 4.1 months post-operatively, with an average length of 1.5 ± 0.9 mm on first presentation. Within the first 3 months post-operatively, 24 TKAs developed RLLs, while at 4-6 months post-operatively, 12 further TKAs developed RLLs. The remaining RLLs developed within 6-12 months post-operatively.
A total of 6 RLLs in 5 patients progressed in length. The average increase was 0.41 mm. In 4 patients, the progressive RLL was located at the medial tibial baseplate (1). In 1 patient, there was progression of 2 RLLs, 1 located at the anterior tibial baseplate (1), and 1 at the anterior flange [1] of the femur component.
Outcomes
There was no significant difference of KSS and OKS scores between the two groups at all time points [Table 3].
Table 3.
Outcome scores of patients
| Outcome scores (% available at follow-up) | TKAs with RLLs (n=65) | TKAs without RLLs (n=70) | p -value |
|---|---|---|---|
| Pre-operative KSS | 46.0 ± 15.9 (80.0 %) |
42.8 ± 20.8 (80.0 %) |
0.38 |
| 3-month post-operative KSS | 86.5 ± 8.5 (78.5 %) |
82.8 ± 13.8 (72.9 %) |
0.11 |
| 1-year post-operative KSS | 75.6 ± 22.9 (70.8 %) |
83.4 ± 13.8 (72.9 %) |
0.06 |
| Pre-operative KSS Functional Score | 47.1 ± 20.6 (76.9 %) |
48.6 ± 18.9 (82.9 %) |
0.69 |
| 3-month post-operative KSS Functional Score | 68.1 ± 19.9 (76.9 %) |
66.3 ± 18.8 (74.3 %) |
0.66 |
| 1-year post-operative KSS Functional Score | 82.1 ± 14.3 (50.8 %) |
73.0 ± 15.0 (70.0 %) |
0.007 |
| Pre-operative OKS | 7.4 ± 10.4 (87.7 %) |
5.7 ± 8.7 (91.4 %) |
0.31 |
| 3-month post-operative OKS | 39.6 ± 5.0 (89.2 %) |
38.4 ± 5.4 (84.3 %) |
0.12 |
| 1-year post-operative OKS | 42.8 ± 5.7 (49.2 %) |
43.2 ± 4.5 (51.4 %) |
0.36 |
Legend: TKA (Total Knee Arthroplasty); RLL (Radiolucent Line); KSS (Knee Society Clinical Rating System Score); OKS (Oxford Knee Score)
There was no significant difference of pre-operative and 3-month post-operative KSS functional scores between the 2 groups. However, TKAs with RLLs had higher 1-year post-operative KSS functional score (82.1 ± 14.3), as compared to TKAs without RLLs (73.0 ± 15.0), which was significant (p = 0.007).
Discussion
The most important finding of our study was that a high incidence of radiolucent lines were present at one year post-operatively, with the reported incidence ranging from 6.7% to 35.1%(2,9,20–26). While there was progression of 6 RLLs in 5 patients, this may be due to intra or inter-observer variability, or rotation of the radiographs, rather than actual progression of the RLLs. Despite this high incidence of RLLs, our patients were clinically asymptomatic and no revisions were required during the follow-up period.
Of concern, there was a high incidence of RLLs on the medial tibial baseplate, at 38%. While early radiolucency development under the lateral tibial baseplate can be explained by inadequate compression during cementing which can occur in a knee with severe pre-operative varus, adequate compression in the medial compartment after implantation is to be expected. Other studies have similarly found that RLLs commonly develop at the tibial component during early follow-up post-operatively, with the medial baseplate to be where RLLs develop most frequently(2,8,9).
There have been several theories, but no definite consensus, to account for the development of RLLs, which can be due to surgical technique or prosthesis related issues. It is possible that a high incidence of RLLs on the tibial aspect was from an irregular surface of the tibia due to inaccurate tibia cut. Theoretically, these irregularities should have been compensated for by the cement. Thermal necrosis to the bone due to the heat generated by cement polymerization may have resultant bony resorption and cement loosening due to micromotion, although lavage may reduce this incidence(9,13,27). Alternatively, the tibial keel preparation with additional preparation for the cement mantle and movement during the inter-locking phase may increase the force on the tibial baseplate, causing prosthesis loosening(2).
We also found that the highest incidence of RLLs was at the bone-cement interface. This may be related to the cementing technique, where the ideal cementing technique is still unclear(27–30). Bone-cement radiolucencies have also been hypothesized to be due to inadequate bony preparation such as the lack of use of a high-volume, high-pressure lavage or with finger packing of the cement; bony hemostasis where active bleeding reduces shear strength of bone-cement interface by 50%; and poor cement pressurization(31,32).
In our institution, meticulous care is taken in the preparation of cementing to reduce early post-operative RLLs on radiographs. All TKAs are performed with a thigh tourniquet, with a high-pressure lavage used prior to cementation. Suction drying is carried out, and cement is pressurized. We postulate that the higher incidence of early RLL formation, especially at the medial tibial baseplate, may be due to the design of the tibial baseplate.
The LEGION tibial baseplate has an asymmetric design, with a smaller lateral component to avoid tibial overhang. The stem and keel are medialized to align with the centre of the tibial canal. Anatomic designs have been shown to demonstrate meaningful increases in tibia coverage with accurate rotational alignment(33–35). However, asymmetrical tibial baseplate designs may cause greater stress shielding, leading to greater asymptomatic tibial bone loss(36, 37).
From a technical perspective, the design of the keel is long and narrow, with short, low profile fins on each side. Comparatively, in older designs, the keel is large in diameter with broader fins. In sclerotic medial tibial bone, broaching and impaction during preparation of the fin slots can be inadequate, leading to uneven impaction of the tibial baseplate during implantation, a problem not encountered in older designs. This would result in immediate RLL on the post-operative radiograph. We suggest that this can be mitigated by performing additional preparation using a reciprocating saw to widen the medial fin slot.
Interestingly, the largest average RLL interval in this study was reported at the femoral component anterior flange [1], with an average length of 1.9 ± 1.3 mm. This was also the second most common location for RLL development (23%). We propose that this finding is likely related to implant design. The LEGION femoral component has a built in 3 degree divergence at the anterior flange to minimize notching. If the femur is implanted in flexion, a gap, or appearance of “RLL” at the anterior flange of the femur may occur. The anterior flange by design is also longer and broader than other knee implants e.g. Zimmer NexGen or the Stryker Triathlon systems. Achieving a fully cemented contact point under the anterior flange given the curvature of the anterior distal femur can be technically challenging. Similarly, Kraay et al found that RLLs were most frequently seen at the most proximal area of the anterior flange, speculating that this was due to the nonconformity of the Miller-Galante femoral component to the anterior bone cut of the femur(38). As such, we postulate that this finding is likely insignificant to the longevity and performance of the implant. However, we propose that care should be taken by ensuring a sufficient amount of cement on the anterior flange.
Implant design may be associated with developing physiological RLLs, but further work exploring other factors such as positioning factors (implant position, tibial slope, and rotation), the balancing of the knee, and even osteoporosis, is needed to draw definite conclusions.
Despite the high incidence of RLLs in our study, we found that there was no significant association between RLL development to hip-knee-ankle angle, nor to tibial keel alignment. While other authors have found an association between malalignment and aseptic loosening, none of our patients had early complications or had revision surgery(39–41). It is also reassuring that the development of RLLs did not affect our patients functional outcomes. These findings are consistent with other studies that found that non-progressive RLLs were not associated with poorer clinical outcomes(10,42).
Limitations
All demographic and functional outcome data were collected in a prospective manner into our knee registry. However, this study and the analysis of the presence of RLLs was done retrospectively, which has the inherent limitations of a retrospective study design, and may introduce selection bias. Furthermore we examined the incidence of RLLs in only one type of implant, namely the Smith and Nephew LEGION Total Knee System. A comparison study against other implants with or without an anatomic tibial baseplate could better illustrate the differences in outcomes.
In conclusion, there was a 48% incidence of physiological RLLs after cemented TKA with Smith and Nephew LEGION implants, with the highest occurrence at the medial tibial baseplate at 38%. While are technical reasons that can explain their early development, more work is needed to determine if implant factors or implant positioning make a significant difference. Reassuringly, these RLLs do not affect early post-operative clinical and functional outcomes of patients.
Further long term follow-up is needed to determine if these RLLs progress and affect clinical and functional outcomes.
Declarations
Competing interests: All authors declare that they have no competing interests
Funding:
No funding was required for this study, nor was any received for this study
References
- 1.Egol KA, Koval KJ, Zuckerman JD. Handbook of fractures. Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 2.Papagelopoulos PJ, Partsinevelos AA, Themistocleous GS, Mavrogenis AF, Korres DS, Soucacos PN. Complications after tibia plateau fracture surgery. Injury. 2006;37(6):475–84. doi: 10.1016/j.injury.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Young MJ, Barrack R. Complications of internal fixation of tibial plateau fractures. Orthopaedic review. 1994;23(2):149–54. [PubMed] [Google Scholar]
- 4.Tscherne H, Lobenhoffer P. Tibial plateau fractures. Management and expected results. Clinical orthopaedics and related research. 1993;(292):87–100. [PubMed] [Google Scholar]
- 5.Lansinger O, Bergman B, Körner L, Andersson G. Tibial condylar fractures A twenty-year follow-up. The Journal of bone and joint surgery American volume. 1986;68(1):13–9. [PubMed] [Google Scholar]
- 6.Schatzker J, Mcbroom R, Bruce D. The tibial plateau fracture: the Toronto experience 1968–1975. Clinical Orthopaedics and Related Research®. 1979(138):94–104. [PubMed] [Google Scholar]
- 7.Ali AM, El-Shafie M, Willett KM. Failure of fixation of tibial plateau fractures. J Orthop Trauma. 2002;16(5):323–9. doi: 10.1097/00005131-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Stevens DG, Beharry R, McKee MD, Waddell JP, Schemitsch EH. The long-term functional outcome of operatively treated tibial plateau fractures. J Orthop Trauma. 2001;15(5):312–20. doi: 10.1097/00005131-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Biyani A, Reddy NS, Chaudhury J, Simison AJ, Klenerman L. The results of surgical management of displaced tibial plateau fractures in the elderly. Injury. 1995;26(5):291–7. doi: 10.1016/0020-1383(95)00027-7. [DOI] [PubMed] [Google Scholar]
- 10.Elsoe R, Johansen MB, Larsen P. Tibial plateau fractures are associated with a long-lasting increased risk of total knee arthroplasty a matched cohort study of 7,950 tibial plateau fractures. Osteoarthritis and Cartilage. 2019;27(5):805–9. doi: 10.1016/j.joca.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 11.Oladeji LO, Dreger TK, Pratte EL, Baumann CA, Stannard JP, Volgas DA, et al. Total knee arthroplasty versus osteochondral allograft: prevalence and risk factors following tibial plateau fractures. The journal of knee surgery. 2019;32(04):380–6. doi: 10.1055/s-0038-1641593. [DOI] [PubMed] [Google Scholar]
- 12.Pinter Z, Jha AJ, McGee A, Paul K, Lee S, Dombrowsky A, et al. Outcomes of knee replacement in patients with posttraumatic arthritis due to previous tibial plateau fracture. European journal of orthopaedic surgery & traumatology : orthopedie traumatologie. 2020;30(2):323–8. doi: 10.1007/s00590-019-02575-4. [DOI] [PubMed] [Google Scholar]
- 13.Roerdink WH, Oskam J, Vierhout PA. Arthroscopically assisted osteosynthesis of tibial plateau fractures in patients older than 55 years. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2001;17(8):826–31. doi: 10.1016/s0749-8063(01)90005-2. [DOI] [PubMed] [Google Scholar]
- 14.Su EP, Westrich GH, Rana AJ, Kapoor K, Helfet DL. Operative treatment of tibial plateau fractures in patients older than 55 years. Clinical Orthopaedics and Related Research®. 2004;421:240–8. doi: 10.1097/01.blo.0000119247.60317.bc. [DOI] [PubMed] [Google Scholar]
- 15.Timmers TK, van der Ven DJ, de Vries LS, van Olden GD. Functional outcome after tibial plateau fracture osteosynthesis: a mean follow-up of 6 years. The Knee. 2014;21(6):1210–5. doi: 10.1016/j.knee.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Stevenson I, McMillan TE, Baliga S, Schemitsch EH. Primary and Secondary Total Knee Arthroplasty for Tibial Plateau Fractures. The Journal of the American Academy of Orthopaedic Surgeons. 2018;26(11):386–95. doi: 10.5435/JAAOS-D-16-00565. [DOI] [PubMed] [Google Scholar]
- 17.Boureau F, Benad K, Putman S, Dereudre G, Kern G, Chantelot C. Does primary total knee arthroplasty for acute knee joint fracture maintain autonomy in the elderly? A retrospective study of 21 cases. Orthopaedics & Traumatology: Surgery & Research. 2015;101(8):947–51. doi: 10.1016/j.otsr.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Ries MD. Primary arthroplasty for management of osteoporotic fractures about the knee. Current osteoporosis reports. 2012;10(4):322–7. doi: 10.1007/s11914-012-0122-3. [DOI] [PubMed] [Google Scholar]
- 19.Bohm E, Tufescu T, Marsh J. The operative management of osteoporotic fractures of the knee: to fix or replace? The Journal of Bone and Joint Surgery British volume. 2012;94(9):1160–9. doi: 10.1302/0301-620X.94B9.28130. [DOI] [PubMed] [Google Scholar]
- 20.Frattini M, Vaienti E, Soncini G, Pogliacomi F. Tibial plateau fractures in elderly patients. La Chirurgia degli organi di movimento. 2009;93(3):109–14. doi: 10.1007/s12306-009-0038-y. [DOI] [PubMed] [Google Scholar]
- 21.Levy O, Salai M, Ganel A, Mazor J, Oran A, Horoszowski H. The operative results of tibial plateau fractures in older patients: a long-term follow-up and review. Bulletin (Hospital for Joint Diseases (New York, NY)) 1993;53(1):15–6. [PubMed] [Google Scholar]
- 22.Hsu CJ, Chang WN, Wong CY. Surgical treatment of tibial plateau fracture in elderly patients. Archives of orthopaedic and trauma surgery. 2001;121(1-2):67–70. doi: 10.1007/s004020000145. [DOI] [PubMed] [Google Scholar]
- 23.Ebrahimzadeh MH, Makhmalbaf H, Birjandinejad A, Soltani-Moghaddas SH. Cross-cultural adaptation and validation of the persian version of the oxford knee score in patients with knee osteoarthritis. Iranian journal of medical sciences. 2014;39(6):529. [PMC free article] [PubMed] [Google Scholar]
- 24.Montazeri A, Goshtasebi A, Vahdaninia M, Gandek B. The Short Form Health Survey (SF-36): translation and validation study of the Iranian version. Quality of life research. 2005;14(3):875–82. doi: 10.1007/s11136-004-1014-5. [DOI] [PubMed] [Google Scholar]
- 25.Doosti-Irani A, Nedjat S, Nedjat S, Cheraghi P, Cheraghi Z. Quality of life in Iranian elderly population using the SF-36 questionnaire: systematic review and meta-analysis. Eastern Mediterranean Health Journal. 2018;24:11. doi: 10.26719/2018.24.11.1088. [DOI] [PubMed] [Google Scholar]
- 26.Ali AM, Saleh M, Eastell R, Wigderowitz CA, Rigby AS, Yang L. Influence of bone quality on the strength of internal and external fixation of tibial plateau fractures. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2006;24(11):2080–6. doi: 10.1002/jor.20270. [DOI] [PubMed] [Google Scholar]
- 27.Cheung WH, Miclau T, Chow SK, Yang FF, Alt V. Fracture healing in osteoporotic bone. Injury. 2016:47 Suppl 2:S21–6. doi: 10.1016/S0020-1383(16)47004-X. [DOI] [PubMed] [Google Scholar]
- 28.Karpouzos A, Diamantis E, Farmaki P, Savvanis S, Troupis T. Nutritional aspects of bone health and fracture healing. Journal of osteoporosis. 2017:2017. doi: 10.1155/2017/4218472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holstein JH, Becker SC, Fiedler M, Scheuer C, Garcia P, Histing T, et al. Exercise enhances angiogenesis during bone defect healing in mice. Journal of Orthopaedic Research. 2011;29(7):1086–92. doi: 10.1002/jor.21352. [DOI] [PubMed] [Google Scholar]
- 30.Marques EA, Mota J, Carvalho J. Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. AGE. 2012;34(6):1493–515. doi: 10.1007/s11357-011-9311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shahrezaee M, Oryan A, Bastami F, Hosseinpour S, Shahrezaee MH, Kamali A. Comparative impact of systemic delivery of atorvastatin, simvastatin, and lovastatin on bone mineral density of the ovariectomized rats. Endocrine. 2018;60(1):138–50. doi: 10.1007/s12020-018-1531-6. [DOI] [PubMed] [Google Scholar]
- 32.Oldroyd A, Dubey S. The association between bone mineral density and higher body mass index in men. International journal of clinical practice. 2015;69(1):145–7. doi: 10.1111/ijcp.12523. [DOI] [PubMed] [Google Scholar]
- 33.Hannan MT, Felson DT, Dawson‐Hughes B, Tucker KL, Cupples LA, Wilson PW, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. Journal of Bone and Mineral Research. 2000;15(4):710–20. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- 34.Greenstein AS, Gorczyca JT. Orthopedic Surgery and the Geriatric Patient. Clinics in geriatric medicine. 2019;35(1):65–92. doi: 10.1016/j.cger.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9(4):273–7. doi: 10.1097/00005131-199509040-00001. [DOI] [PubMed] [Google Scholar]
- 36.Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee Long-term results. J Bone Joint Surg Br. 1990;72(4):634–8. doi: 10.1302/0301-620X.72B4.2380219. [DOI] [PubMed] [Google Scholar]
- 37.Wasserstein D, Henry P, Paterson JM, Kreder HJ, Jenkinson R. Risk of total knee arthroplasty after operatively treated tibial plateau fracture: a matched-population-based cohort study. J Bone Joint Surg Am. 2014;96(2):144–50. doi: 10.2106/JBJS.L.01691. [DOI] [PubMed] [Google Scholar]
- 38.Sarzaeem MM, Omidian MM, Kazemian G, Manafi A. Acute Primary Total Knee Arthroplasty for Proximal Tibial Fractures in Elderly. Arch Bone Jt Surg. 2017;5(5):302–7. [PMC free article] [PubMed] [Google Scholar]
- 39.Sivasubramanian H, Kini SG, Ang KY, Sathappan S. Use of tantalum cones in primary arthroplasty of acute proximal tibial fractures. Acta Orthop Belg. 2016;82(3):593–8. [PubMed] [Google Scholar]
- 40.Huang J-F, Shen J-J, Chen J-J, Tong P-J. Primary total knee arthroplasty for elderly complex tibial plateau fractures. Acta orthopaedica et traumatologica turcica. 2016;50(6):702–5. doi: 10.1016/j.aott.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong MT, Bourget-Murray J, Johnston K, Desy NM. Understanding the role of total knee arthroplasty for primary treatment of tibial plateau fracture: a systematic review of the literature. Journal of Orthopaedics and Traumatology. 2020;21:1–7. doi: 10.1186/s10195-020-00546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rademakers M, Kerkhoffs G, Sierevelt I, Raaymakers E, Marti R. Operative treatment of 109 tibial plateau fractures: five-to 27-year follow-up results. Journal of orthopaedic trauma. 2007;21(1):5–10. doi: 10.1097/BOT.0b013e31802c5b51. [DOI] [PubMed] [Google Scholar]
- 43.Gerich T, Blauth M, Witte F, Krettek C. [Osteosynthesis of fractures of the head of the tibia in advanced age A matched-pair analysis] Der Unfallchirurg. 2001;104(1):50–6. doi: 10.1007/s001130050687. [DOI] [PubMed] [Google Scholar]
- 44.van Dreumel RL, van Wunnik BP, Janssen L, Simons PC, Janzing HM. Mid- to long-term functional outcome after open reduction and internal fixation of tibial plateau fractures. Injury. 2015;46(8):1608–12. doi: 10.1016/j.injury.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 45.Lachiewicz PF, Funcik T. Factors Influencing the Results of Open Reduction and Internal Fixation of Tibial Plateau Fractures. Clinical Orthopaedics and Related Research®. 1990:259. [PubMed] [Google Scholar]
- 46.Rommens PM. Paradigm shift in geriatric fracture treatment. European journal of trauma and emergency surgery : official publication of the European Trauma Society. 2019;45(2):181–9. doi: 10.1007/s00068-019-01080-x. [DOI] [PubMed] [Google Scholar]