Abstract
Background:
Recent studies have shown that human bone marrow-derived mesenchymal stem cells (hBM-MSCs) have several drawbacks in treating critical-sized bone defect (CSD). Secretome may offer considerable advantages over living cells in terms of potency, manufacturing and storing easiness, and potential as a ready-to-go osteoinductive agent. However, thus far, there are no studies regarding the efficacy of secretome in bone healing. The objective of this study is to investigate the effect of the secretome in rat models with CSD.
Methods:
This was an experimental study with post-test only control group design using 60 skeletally mature Sprague Dawley rat which was divided evenly into 5 treatment groups (MSC only, Secretome only, MSC + Secretome, MSC + Secretome + BMP-2, Control group using Normal Saline). We used Bone Marrow derived MSC in this research. The critical-sized bone defect was created by performing osteotomy and defect was treated according to the groups. Rats were sacrificed on 2nd and 4th week and we measured the radiological outcome using Radiographic Union Score for Tibia (RUST) and histomorphometric (callus, osseous, cartilage, fibrous, and void area) evaluation using Image J.
Results:
There was no difference in the weight of rats between groups before and after the intervention. RUST score in all intervention group is significantly higher than the control group, however, the MSC-only group was not statistically significant higher than the control group. There is no statistically significant difference in RUST Score between intervention groups.
Histomorphometric evaluation showed that total callus formation is the widest in the MSC+Secretome+BMP-2 combination group while the osseous area is found highest on the secretome-only group.
Conclusion:
Secretome, whether used solely or combined with BM-MSC and BMP-2, is a novel, potent bone-healing agent for CSD in rat models.
Key Words: Secretome, Mesenchymal Stem Cell, Radiographic, Histomorphometric, Bone healing, Sprague Dawley Rat
Introduction
Critical sized bone defect (CSD) can be caused by a variety of causes such as high-energy trauma with soft tissue and periosteal stripping, an infection that requires massive debridement, and tumour resection. There is no consensus on the definition of a critical-sized bone defect. In the majority of the available literature, CSD is generally defined as a bone defect with a length of 1-2 cm and a loss of circumference of bone > 50%. The long-term functional outcome of CSD is in most cases unsatisfactory because of the high complication rate and the need for reoperation. CSD is undoubtedly a major challenge in orthopaedic surgery due to its clinical impact and economic burden to patients, families, and even the country (1). Despite many recent advances in surgical techniques, implants and biologic agents, there has been no consensus regarding CSD treatment (2).
The diamond concept of fracture healing by Giannoudis(3) in 2007 demonstrated a well-orchestrated mechanism of bone healing, which comprised of four pillars, i.e. osteogenic cells, osteoconductive scaffolds, growth factors and mechanical environment. In contrast to non-union cases, in the case of CSD, the biological cell regeneration capacity and bone stability were adequate, but there was an inability to repair the defect due to complications by soft tissue and patient characteristics.
Human bone marrow-mesenchymal stem cell (hBM-MSC) implantation as a biologic agent have been emerging and initially considered to be a source of osteogenic cells. However, recent literature has elucidated that only small amount of MSCs will differentiate into osteoblast and the osteogenic potential is largely due to the paracrine mechanism (4), in which MSCs secrete various growth factors and cytokines that are called secretome that triggers endogenous MSC recruitment and proliferation (5).
Secretome that is a bioactive factor produced by MSC during the culture process that byproduct has many advantages over the MSCs. Due to its non-cellular characteristics, secretome has several safety advantages including better immune compatibility, less risk for tumorigenicity, emboli formation, and transmission of infection. Besides, secretome is also easier and cheaper in term of production, storage and application. Possibility for mass-production with tailor-made characteristics and availability for acute conditions treatment is one of the main advantages of secretome over MSC (6). However, currently, there has been limited literature that evaluated the secretome effect in comparison with MSC alone or combination with secretome. This study aimed to elaborate the effect of secretome in treating critical-sized defect femur of Sprague Dawley (SD) Rats compared to MSCs and its combination with MSCs and BMP-2.
Materials and Methods
This was an experimental study with post-test only control group design that was performed at our institute. This study was approved by the ethical committee of our institute (No. 784/UN2.F1/ETIK/2017).
Sixty skeletally mature male Sprague Dawley rat weighted 250-350 gram were divided into 5 treatment groups, i.e., Control group (normal saline), MSC only group, secretome only group, MSC+secretome group, and MSC+ secretome +BMP-2 group.
BM-MSC samples preparation
BM-MSC samples were derived from 3 male patients with diverse (<20, 20-55, >55 years old) age group underwent different pelvic or hip orthopaedic procedure. As much as 30 ml of BM obtained using a vertebroplasty VP needle in the iliac crest were prepared for MSC culture in the Stem Cell Integrated Service Unit, Cipto Mangunkusumo Hospital, Jakarta, Indonesia. Combined with the complete medium (alpha MEM containing 10% thrombocyte lysate (Gibco, US)) with a comparison of 1:1, solution then were centrifuged in 2500 RPM speed for 10 minutes. Supernatant of the solution was distributed to T75 cell culture flasks and added 10 ml complete medium, while the pellet was separated to a different T75 cell culture flasks with 10 ml complete medium added (P0). Samples were incubated in a 370 C, 5% CO2 for 7 days, then washed with PBS every 2-3 days while observed under inverted microscope until it reached 80% of confluence. MSCs were harvested after and then replanted until 6th passage (P6) while we used the MSC for transplantation at the 3rd passage (P3).
Secretome collection
Secretome is the collection of conditioned medium used to washed the MSC when it reached confluence during the MSC culture process. BM-MSC that was cultured in penicillin/streptomycin (final concentration of 100 U/mL), amphotericin B (final concentration 2500 ng/mL), 1% L-Glutamine (Lonza 17-605C) containing alpha minimal essential medium (α-MEM) were supplemented with serum (10% thrombocyte concentrate lysate (DMEM (Gibco, US)). For secretome collection, the culture medium was replaced in two different time points. The first was replaced with serum containing culture medium when the cells reached 50% confluence, while the other was replaced with culture medium without serum when the cells reached 70% confluence. The secretome before this medium replacement was collected. After the culture medium was replaced, the cells were incubated for 48 hours in an incubator at 37°C, with 5% CO2. The MSCs were then harvested. The collected secretome samples were filtered and placed in a 15 mL tube in -20oC once before being transferred to 1.5 ml and 5 ml tubes and stored in -20oC.
Surgical Procedure
Prior to intervention, each rat was anesthetized intraperitoneally using a mixture of kethamine and xylazine with a ratio of (60-80mg/kg + 5-10 mg/kg). Afterwards, the rat was positioned in the right lateral decubitus and septic antiseptic procedure was performed on the right thigh using 10% povidone iodine and followed by shaving. Skin incision was performed longitudinally at the lateral side of rat’s femoral diaphysis. Vastus lateralis muscle was elevated cranially, while biceps femoris was retracted caudally.
Critical-sized bone defect model was created by performing osteotomy on the left femur of each rat and using a sharp plier, 5 mm length of bone was eliminated confirmed by a caliper[Figure 1]. Subsequently, 1.2-1.4 mm of threaded k-wire was inserted in a retrograde fashion to fixate the femur. Afterwards, the defect was filled with 0.02 cc hydroxyapatite (HA) from Bongros, Daewong® who has pore size of 300 um, 70-80% porosity and 100% immune to inflammation and cross reaction.
Figure 1.
The intervention procedure. (a, b) Secretome storage. (c) Rat anaesthesia using ketamine. (d) Preparation of left femur (shaving). (e) Draping and positioning. (f) Septic and aseptic procedure. (g) Osteotomy and 5 mm defect creation. (h) Fixation using threaded k-wire. (i) Wound closure
The HA was previously embedded into five different solution for one minute depending on the group. Group 1 was with normal saline, group 2 with MSC, group 3 with
secretome, group 4 with secretome + MSC, group 5 with secrectom + MSC + BMP-2. The 1x105 cells/kg BW dosage of BM-MSC application was selected according to previous research by Decambron et al, which also defined the 11 ng/mm3 BMP-2 dosage used (8). The MSCs in this research were bone marrow derived MSCs (BM-MSC) in the third passage (P3), and we used 1 ml of the secretome that was harvested from the same MSC culture to aid the reproducibility of this technique.
Muscle and skin were then stitched layer by layer using absorbable suture, and the rats were given topical gentamycin and oral amoxicillin 100 mg/kg body weight (BW) and paracetamol 100 mg/kg BW for seven days.
Outcome Measurement
The weights were measured and analyzed before intervention and sacrifice. After two and four weeks, every 30 rats were sacrificed (6 rats in each group), and hip disarticulation was performed to obtain the femur. Conventional radiograph using X-ray was performed to obtain femur anteroposterior and lateral view for further measurement using RUST (Radiographic Union Score for Tibia) score, which evaluated four cortices (anterior, medial, lateral, and posterior) for each formation of bridging callus and visibility of fracture line. The maximum and minimum score was 12 and 4, respectively.
Histological evaluation of bone healing was evaluated by obtaining two axial cuts of 3 m thickness from the widest callus area with a distance between 500 m and 1000 m, which were stained using hematoxylin-eosin (HE) for histomorphometry. Total callus area (TCA), Osseous Area (ArOs), Cartilage Area (ArCr), Fibrous Area (ArF), and Void Area (ArV) were evaluated using Image J.
Statistical Analysis
Data evaluations from five sample groups were processed were processed using Statistical Product and Service Solustion (SPSS) version 20. Shapiro wilk test was used to analyze normality of each group. Further analysis using one-way ANOVA for normal data distribution and Kruskal Wallis for abnormal data distribution was used to compare results between groups. P value < 0.05 was considered statistically significant for this study.
Results
Study Characteristics
A total of 60 rats were examined in this study and divided into 5 groups, 12 rats each. At 2nd and 4th week, 6 rats from each group were sacrificed and analyzed. Sprague Dawley rat femur diameter ranges between 3 and 8 mm, hence the range of CSD, in this case, would be between 4.5 to 6 mm. We performed osteotomy for 5 mm bone defect. The total volume defect was 5x2x2 mm (20 mm3) that necessitated the administration of 0.02 cc HA granules to fill the gap, There was no difference in rats weight between groups before and after intervention both at 2-week and 4-week groups 334.50±12.88 gram (p= 0.113) and 298.33±15.15 (p= 0.500), which indicated that weight did not affect the bone healing process (9).
Radiological Evaluation of treatment Effect in CSD Rats
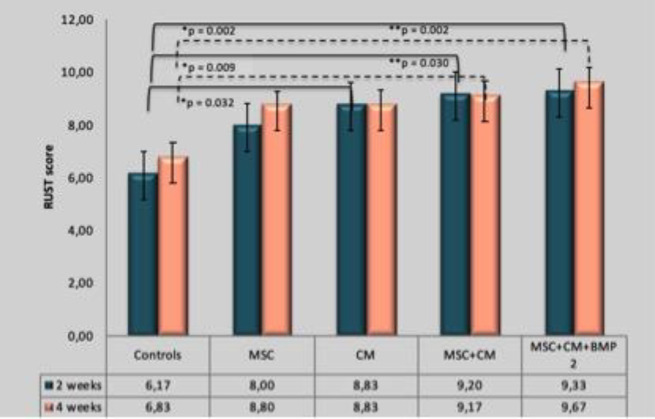
Radiographic Union Score for Tibia (RUST) score evaluation in MSC only, secretome only, MSC+ secretome, MSC+ secretome +BMP-2 groups showed a significantly higher value compared to the control group both in 2nd and 4thweek [Figure 2]. The RUST Score of MSC-only group was not significantly higher than the control group.
Figure 2.
Comparison of RUST score between groups. Group MSC+CM+BMP-2 showed the highest RUST score of all groups. Group CM, MSC+CM and MSC+CM+BMP-2 showed significantly higher RUST score compared to the control group in week 2. Group MSC+CM and MSC+CM+BMP-2 showed significantly higher RUST score compared to the control group in week 4
MSC: Mesenchymal Stem Cell; CM: Conditioned Medium; BMP-2: Bone Morphogenetic Protein-2; RUST: Radiographic Union Scale in Tibial Fracture; *p: showed significant p-value relationship in week 2; **p: showed significant p-value relationship p value in week 4
There is no statistically significant difference in RUST Score between intervention groups that showed that administration of secretome alone is sufficient in enhancing bone healing and showed no significant difference to combination with MSC and BMP. Thus, administration of secretome alone was adequate.
Histomorphometric Evaluation of Treatment Effect in CSD Rats
Radiologic evaluation is an efficient, simple and less-expensive method to evaluate bone healing, but it could not comprehend its process and mechanism, unlike histologic evaluation. Histomorphometric evaluation using Image J software is a relatively simple method that can be performed without modern and expensive types of equipment (10).
Total Callus Formation
Evaluation of total callus area showed a significantly wider area in the MSC+Secretome+BMP-2 group followed with MSC+ secretome, and secretome-only group respectively compared to the MSC and control group both in the 2nd and 4th week [Figure 3].
Figure 3.
Callus area comparison between groups. MSC+CM+BMP-2 group had the highest callus area. A connecting straight line showed a statistically significant correlation in group 2 week. The dotted line showed a statistically significant correlation in group 4 week
MSC: Mesenchymal Stem Cell; CM: Conditioned Medium; BMP-2: Bone Morphogenetic Protein-2
Osseus Area (ArOs)
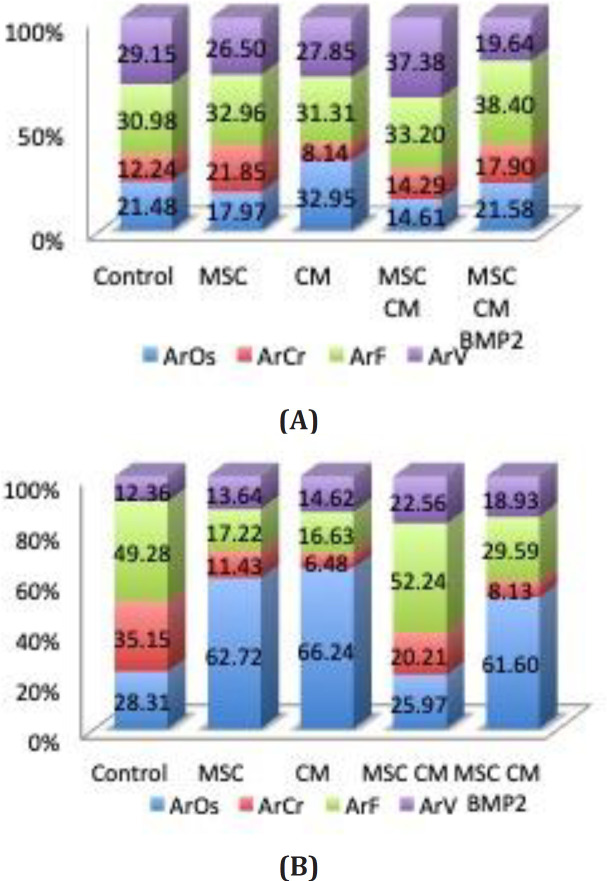
Upon evaluating at the osseous area (ArOs) at 2nd week and 4th week, Secretome-only group showed the highest osseous area formation significantly compared to other
groups (Figure 4). Meanwhile, MSC and MSC+ secretome group showed significantly the lowest osseous area (ArOs) compared to other groups at week 2 and MSC+ secretome group showed significantly the lowest ArOs at week 4 [Figure 4].
Figure 4.
Histomorphometric area comparison between (a) 2nd and (b) 4th week. CM group had the highest osseous area both in week-2 and week-4 compared to other groups
ArOs, osseous area; ArCr, cartilage area; ArF, fibrous area; ArV, void area
Cartilaginous Area (ArCr)
At the second week, cartilaginous area (ArCr) showed the highest value in the MSC+secretome+BMP-2 group significantly compared to secretome-only, MSC-only and MSC+secretome group. In the fourth week, the control group had the highest cartilaginous area compared to other groups. These results showed that normal non-interfered CSD rat healing process was still at the cartilage proliferation phase.
Void Area (ArV) and Fibrosis Area(ArF)
Highest void area (ArV) was showed in the MSC+secretome group and the fibrosis area in the second week was relatively the same. These results showed that at the second week, ossification happens at a significantly faster rate in the secretome-only and MSC+secretome+BMP-2 group; meanwhile, the MSC group still undergo cartilage proliferation.
Discussion
The most interesting result of this study is that we found administration of secretome could accelerate and improve bone healing even in CSD case in rat. Bone defect has long been a problem for orthopaedic surgeon without satisfying solution. Previous alternative such as bone transport procedure was very uncomfortable for patients without satisfying outcome. A newer biologic therapy
such as MSC transplantation was proven effective however carry an ethical controversion. Secretome is the derivate of MSC conditioned medium which is cell-free, able to be stored hence can be produced commercially.
We opted Sprague Dawley (SD) rat as a model due to its standardized bone turnover rat. According to literature, SD rat’s bone healing occurs within 4 to 6 weeks on average, hence it might economically represent results within short period (11). It is hypothesised that bone growth difference is influenced by weight gain. To avoid bias in assessing the bone fracture healing, we measure the rat’s weight differences between groups and there was no weight difference between groups before and after intervention both at 2-week and 4-week groups (p= 0.113 and p= 0.500). (11).
We chose to use Radiological Union Score of Tibia as the outcome measurement evaluation method in this study because of its couple advantages. These advantages are its simplicity and it resembles the continuous process of the fracture healing. RUST score is also more valid and reliable than conventional assessments with its comprehensive measure of cortical bridging and the frequently employed criteria of fracture line visibility. By examining the fracture healing in a complete approach (4 different cortex separately : anterior, posterior, medial, lateral), RUST score measures each unique individualized healing pattern. Also, RUST score examines cortical signs of fracture healing which is why this is very suitable to our study because we use a retrograde K-Wire to fixate the rat
femur which limits us for medullary bone healing assessment. (13).
In an animal study and human application, MSC implantation was proven to enhance fracture healing by recruiting endogenous MSCs to the fracture site due to paracrine effect via secretome, which contains various growth factors, pro-inflammatory cytokines, and anti-inflammatory agents such as TGF-, IGF-1, VEGF and HGF (5,11,12). In the other hand, our study showed that MSC-only group showed no significant difference in RUST Score with the control group and worse histomorphometric evaluation in comparison with another intervention group. Our findings are in accordance with the results of Niemeyer et al study(14), which showed that xenogenic MSC implantation caused worse radiographic, histologic and biomechanic results compared to autologous MSCs or scaffold alone on CSD in rats. This result can be explained by which application of xenogenic MSC in these groups seemed to inhibit or delay bone healing process possibly by host versus graft mechanism, which triggered an inflammatory reaction, prolonged hematoma presence and slowing the callus formation.
Our study showed that the combination of MSC + secretome +BMP-2 groups showed the highest total callus area compared to other groups respectively, which is also consistent with a study performed by Niemeyer et al (14). The prolonged inflammation phase by xenogenic MSCs might probably be reduced by the application of BMP-2 as an osteoinductive factor, and cessation of host versus graft mechanism such as seen in the results of week 4 when xenogenic MSCs were eliminated.
In the meantime, secretome as a non-cellular agent can be used as an alternative of MSCs to support bone healing. Our study showed that the secretome group was significantly superior compared to other groups in all parameters at all time. Application of secretome was proven superior in this research compared to the combined application of secretome, MSC, and BMP-2. Moreover, secretome can prospect as an adjunct in replacement of BMP-2, which require super physiological dose and has a severe inflammatory side effect (15,16).
Limitation of study.
The first limitation of this study was the used of different samples from week 2 and week 4 because the rats had to be sacrificed. Another limitation is the absence of biomechanical study to compare the strength of the callus. Further studies should be performed to evaluate secretome osteogenic potency biomechanically and find the optimum dosage of secretome.
Transplantation of secretome had a positive effect on bone healing, but these incomplete results are only for this present study. Further studies are needed to evaluate the biomechanical strength and find the optimum dosage of secretome.
CONFLICT OF INTERESTS:
None declared
References
- 1.Nauth A, Schemitsch E, Norris B, Nollin Z, Watson JT. Critical-Size Bone Defects: Is there a consensus for diagnosis and treatment? J Orthop Trauma. 2018;32(3):S7–11. doi: 10.1097/BOT.0000000000001115. [DOI] [PubMed] [Google Scholar]
- 2.Roddy E, DeBaun MR, Daoud-Gray A, Yang YP, Gardner MJ. Treatment of critical-sized bone defects: clinical and tissue engineering perspectives. Eur J Orthop Surg Traumatol. 2018;28(3):351–62. doi: 10.1007/s00590-017-2063-0. [DOI] [PubMed] [Google Scholar]
- 3.Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: The diamond concept. Injury. 2007;38(4):3–6. doi: 10.1016/s0020-1383(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 4.Linero I, Chaparro O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. Plos One. 2014;10(3):e0119262. doi: 10.1371/journal.pone.0107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pawitan JA. Prospect of Adipose Tissue Derived Mesenchymal Stem Cells in Regenerative Medicine. Cell & Tissue Transplantation & Therapy. 2009:7–9. [Google Scholar]
- 6.Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell free therapeutic strategies in regenerative medicine. Int J Mol. Sci. 2017;18(9):1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manassero M, Decambron A, Thong BTH, Viateau V, Bensidhoum M, Petite H. Establishment of a segmental femoral critical-size defect model in mice stabilized by plate osteosynthesis. J Vis Exp. 2016;10(116):1–11. doi: 10.3791/52940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decambron A, Fournet A, Bensidhoum M, Manassero M, Sailhan F, Petite H, et al. Low-dose BMP-2 and SPM dual delivery onto coral scaffold for critical-size bone defect regeneration in sheep. J Orthop Res. 2017;35(12):2637–45. doi: 10.1002/jor.23577. [DOI] [PubMed] [Google Scholar]
- 9.Kasman D, Kurniawan A. Histomorphometric analysis of fracture healing using ImageJ software in Sprague-Dawley rat models of fractures with mechanical force to the bone only and to the bone and periosteum. Journal of Physics: Conf. Series. 2018:4:042036. [Google Scholar]
- 10.Holstein JH, Garcia P, Histing T, et al. Advances in the establishment of defined mouse models for the study of fracture healing and bone regeneration. J Orthop Trauma. 2009;23(5):31–38. doi: 10.1097/BOT.0b013e31819f27e5. [DOI] [PubMed] [Google Scholar]
- 11.Kilborn SH, Trudel G, Uhthoff H. Review of growth plate closure compared with age at sexual maturity and lifespan in laboratory animals. Contemp Top Lab Anim Sci. 2002;41(5):21–26. [PubMed] [Google Scholar]
- 12.Marcucio RS, Nauth A, Giannoudis PV, et al. Stem cell therapies in orthopaedic trauma. Journal of Orthopaedic Trauma. 2015;29(12):S24–S27. doi: 10.1097/BOT.0000000000000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kooistra B W. The radiographic union scale in tibial fractures: reliability and validity. J Orthop Trauma. . 2010 ;24(3):81–6. doi: 10.1097/BOT.0b013e3181ca3fd1. [DOI] [PubMed] [Google Scholar]
- 14.Niemeyer P, Szalay K, Luginbuhl R, Sudkamp NP, Kasten P. Transplantation of human mesenchymal stem cells in a non-autogeneous setting for bone regeneration in a rabbit critical-size defect model. Acta Biomater. 2010;6(3):900–8. doi: 10.1016/j.actbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Perri B, Cooper M, Lauryssen C, Anand N. Adverse swelling associated withuse of rh-BMP-2 in anterior cervical discectomy and fusion: a case study. Spine J. 2007;7(2):235. doi: 10.1016/j.spinee.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki K, Aihara M, Honmo J, Sakurai S, Fujimaki Y, Sakamoto K, et al. Effects of recombinant human bone morphogenetic protein-2 on differentiation of cells isolated from human bone, muscle, and skin. Bone. 1998;23(3):223–31. doi: 10.1016/s8756-3282(98)00105-7. [DOI] [PubMed] [Google Scholar]