Abstract
Background:
This study aimed to evaluate the sensitivity and specificity of the leukocyte esterase (LE) band in two groups of patients receiving and not receiving antibiotics and compare the results.
Methods:
This prospective cross-sectional study was conducted on 105 joints with clinical suspicion of infectious arthritis (based on Kocher criteria) admitted in Shohada Hospital, Tabriz, Iran, within 2017-2018. Patients were divided into two groups, including receiving antibiotics (n=29; group 1) and not receiving antibiotics (n=76; group 2). Articular fluid aspiration was performed under sterile conditions with an 18-gauge angiocath with at least 1 ml volume of the hip, knee, ankle, elbow, and shoulder joints. Polymorphonuclear cell percentage count, cell count, Gram staining (GS), culture, and leukocyte esterase test were performed immediately after the aspiration of the specimens.
Results:
Levels of synovial fluid white blood cell count, serum white blood cell count, PMN, serum glucose, erythrocyte sedimentation rate, C-reactive protein, and time of aspiration (TOA) were significantly higher in the group receiving antibiotics (P<0.05). Synovial glucose levels were significantly lower in the group receiving antibiotics. Furthermore, the positive frequency of glucose esterase, blood culture, GS, serum culture, and ultimate diagnosis of septic arthritis tests were significantly lower in the antibiotic receiving group (P<0.05). The sensitivity, and positive predictive value of the leukocyte esterase test were obtained at 100%, and 96.55% in the antibiotic receiving group, respectively. Moreover, in the group not receiving antibiotics, the sensitivity, specificity, positive predictive value, and negative predictive value of the leukocyte esterase test were estimated at 72.22%, 92.50%, 89.66%, 78.72%, respectively.
Conclusion:
Antibiotic use and the prolongation of TOA lead to increased inflammatory products, which is interfering with lab variables. As a result, they increase the sensitivity of the test. The sensitivity and specificity of the leukocyte esterase test in patients who did not receive antibiotics showed that this was a suitable and reliable laboratory method for the rapid detection of infectious arthritis that required an emergency rescue procedure.
Key Words: Leukocyte esterase test, Infectious arthritis, Septic arthritis, Antibiotic
Introduction
Septic arthritis is a common clinical problem associated with disability and mortality, the most common cause of which is bacteria and is more common in people younger than 20 years (1, 2). Despite classic clinical symptoms, such as joint pain and swelling (3), the diagnosis of septic arthritis is challenging due to the lack of standardized criteria (4, 5). Diagnosis is often based on determining the causative organism by means of articular fluid culture, Gram staining, purulence appearance of articular fluid, response to treatment, increased erythrocyte sedimentation rate (ESR), and increased C-reactive protein (CRP). An increase in leukocytes count and the ratio of neutrophils in the synovial fluid are also associated with infection risk (1, 2, 6). Kocher criteria are based on a retrospective analysis of patients and provide an estimate of the probability of infectious arthritis; however, they do not provide a definitive diagnosis (1). A definitive diagnosis is obtained with a polymorph count of > 50,000 cells/mm3, a Gram staining or positive articular fluid culture, and a positive culture is a gold standard for detection (1, 4). Given the disabilities and destruction of articular cartilage by inflammatory factors, as well as the effect of tamponade and ischemia caused by increased joint volume as a result of infusion (1), rapid diagnosis and rejection of septic arthritis or appropriate surgical treatment are crucial, which can prevent future disabilities and complications and mortality. In recent studies, the use of leukocyte esterase (LE) with a sensitivity of 100%, the positive predictive value of 50%, and negative predictive value of 100% have been suggested as the appropriate test for the diagnosis of septic arthritis (3, 5, 7, 8). The alpha-defensin immunoassay method also has a positive predictive value of about 100%; nevertheless, it requires an advanced laboratory and is expensive. On the other hand, urine dipstick is a low-cost method with a response time of about 120 sec. Although the specificity of urine dipstick is lower than that of alpha-defensin immunoassay, both have a sensitivity of 100% (1, 3, 8). Given that patients with infectious arthritis are usually delayed and sometimes have already received antibiotic therapy that makes the clinical manifestations and even the appearance of articular fluid out of the typical situation, waiting until the preparation of culture response gives the bacteria the opportunity to deliver articular cartilage to the point of no return. To the best of our knowledge, no studies have been dedicated to investigating the effect of antibiotic intake on leukocyte esterase test results. Therefore, this study aimed to evaluate the sensitivity and specificity of the leukocyte esterase test in two groups of patients, namely, those with and without septic arthritis receiving antibiotics, and compare the results to identify a method that could quickly and reliably diagnose septic arthritis and ultimately prevent time-wasting in initiating treatment as much as possible.
Materials and Methods
This prospective cross-sectional study, after being approved by the Ethics Committee of Tabriz University of Medical Sciences, Tabriz, Iran (IR.TBZMED.R.EC.1396.325), was performed on 105 patients admitted to Shohada Hospital in Tabriz with a diagnosis of septic arthritis based on clinical symptoms and ESR and CRP values (based on Kocher criteria). The patient's history of recent use or non-use of antibiotics was questioned and the patients were divided into two groups of receiving antibiotics (n=29, group A) and not receiving antibiotics (n=76, group B). Articular fluid aspiration was performed in the emergency department through the skin under standard procedures and sterile conditions with an 18-gauge angiocath. Samples were aspirated with at least 1 ml of the hip, knee, ankle, elbow, and shoulder joints. Samples less than 1 ml (n=13) were excluded from the study, and articular fluid was re-sampled through the skin or with arthrotomy to proceed treatment procedure. Patients with infectious arthritis were also divided into two groups based on positive (n=5) or negative (n=100) diabetes mellitus, and serum glucose (SE-G) and synovial fluid glucose levels were compared.
Immediately after aspiration, samples were sent to the laboratory for polymorphonuclear (PMN) count, cell count, Gram staining, culture, and leukocyte esterase test. To prevent abnormalities in blood tests, 1 ml of articular fluid was centrifuged at a speed of up to 6,600 rpm for 120-180 sec. The sample was removed after centrifugation by a sampler, and to reduce the error in the interpretation of the test, each sample was drip simultaneously with two similar LE strips and compared with standard color after 120 sec.
The results of the experiments were interpreted as follows:
Negative leukocyte esterase: leukocyte esterase indicator on the urine dipstick is very pale purple
Leukocyte esterase 1+: leukocyte esterase indicator on the urine dipstick is pale purple
Leukocyte esterase 2+: leukocyte esterase indicator on the urine dipstick is dark purple
In the present study, leukocyte esterase 2+ was considered a positive response and the other results were negative for the probability of infection.
The final patient outcome (ultimate diagnosis of septic arthritis [UDSA]) was based on the purified appearance of the aspirated fluid, laboratory findings, and Gram staining. The inclusion criteria were having at least two points of Kocher criteria and discomfort in the affected joint. The criteria can be used on multiple joints with the hip being the most tested due to its diagnosis frequency and importance to the patient's mobility. The knee and the ankle can also experience these symptoms and the criteria can be applied to similar symptomatic joints (13). On the other hand, patients with a history of intra-articular injection for a maximum of 1 month, an external device (e.g., prosthesis inside the articular), and cellular immunodeficiency were excluded from the study. Moreover, cases in which the aspirated specimen was completely bloody and less than 1 ml volume after articular fluid centrifugation was not included in the research.
Statistical analysis
Data were analyzed using descriptive statistics (frequency-percentage), Chi-square test, or Fisher's exact test. Sensitivity, specificity, negative and positive predictive values, and likelihood ratio (LR) were calculated to evaluate the diagnostic value. All analyses were performed in SPSS 25 software and the significance level was considered at P < 0.05.
The analysis was performed in two parts. In the first part, the mean and relative frequency indices were used to express the demographic characteristics of the subjects. Mann-Whitney U and Chi-square tests were used for quantitative variables analysis. In the second part, sensitivity, specificity, positive predictive value, negative predictive value, positive exponential ratio, and negative exponential ratio were employed to evaluate diagnostic tests for infectious arthritis if the test response was classified. In case that the test response was continuous, the receiver operating characteristic (ROC) and area under the curve were calculated, and according to this curve, the best cutoff point was determined. Afterward sensitivity, specificity, positive and negative predictive values, and positive and negative exponential ratios were estimated. It should be noted that all descriptive results with a 95% confidence interval and all analytical results were expressed at a significant level of 0.05.
Results
A total of 105 joints were examined, including knees (n=65), pelvis (n=28), ankles (n=8), shoulders (n=2), and elbows (n=2). No significant difference was observed between the two groups in terms of mean age and gender distribution (P>0.05). It was also revealed that synovial fluid white blood cell count (SyWBC), PMN, ESR, CRP, and time of aspiration (TOA) levels were significantly higher in the group receiving antibiotics (P<0.05). Synovial glucose level was significantly lower in the antibiotic receiving group. Furthermore, the positive frequency of glucose esterase, blood culture (BC), serum culture (SC), and UDSA tests were significantly lower in the group receiving antibiotics (P<0.05). There was no significant difference between mean serum white blood cell count (SWBC), SE-G, and positive frequency of GS test in the two groups (P>0.05) (Table 1).
Table 1.
Demographic information of the patients
| P-value | Group B (n=76) | Group A (n=29) | Variables | |
|---|---|---|---|---|
| 0.072 | 49.24±19.30 | 41.72±21.83 | Age | |
| 0.429 | 38 (50) | 12 (41.4) | Men | Gender |
| 38 (50) | 17 (58.6) | Women | ||
| <0.001* | 37.41±20.97 | 338.11±1479.99 | SyWBC (cells/mm 3 ) | |
| 0.188 | 12.45±3.28 | 14.04±4.51 | SWBC (cells/mm 3 ) | |
| <0.001* | 55.51±23.74 | 80.51±6.71 | PMN (%) | |
| <0.001* | 49.96±18.71 | 33.31±10.84 | Sy-G (mmol/L) | |
| 0.37 | 89.37±19.91 | 93.83±22.59 | SE-G (mmol/L) | |
| <0.001* | 32.67±24.43 | 89.89±24.62 | ESR (mm/hour) | |
| <0.001* | 36.31±26.39 | 68.72±31.68 | CRP (mg/L) | |
| <0.001* | 74.40±62.84 | 172.58±56.68 | TOA | |
| <0.001* | 43 (56.58) | - | Negative | LET |
| 4 (5.26) | - | 1+ | ||
| 29 (38.2) | 29 (100) | 2+ | ||
| <0.001* | 35 (46.1) | 26 (89.7) | Negative | GT |
| 40 (25.6) | - | 1+ | ||
| 1 (1.3) | 3 (10.3) | 2+ | ||
| 0.013* | 14 (18.42) | - | Positive | BC |
| 62 (81.58) | 29 (100) | Negative | ||
| 0.314 | 26 (33.2) | 13 (44.8) | Positive | GS |
| 50 (65.8) | 16 (55.2) | Negative | ||
| 0.040* | 10 (13.16) | - | Positive | SC |
| 66 (86.84) | 29 (100) | Negative | ||
| <0.001* | 36 (47.37) | 28 (96.55) | Positive | UDSA |
| 40 (52.63) | 1 (3.45) | Negative | ||
Data are expressed as mean ± SD or numbers (%).
The t-test was used for continuous data and the Chi-square test for qualitative data.
*P<0.05 is considered as a significant level.
SyWBC: Synovial fluid white blood cell count×1000/l; PMN: Polymorphonuclear cell percentage; Sy-sE G: Synovial fluid-concomitant serum glucose mg/dl; SWBC: Serum white blood cell count; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; PAb: Previous antibiotic use; TOA: Time of aspiration after beginning of symptoms; LET: Leukocyte esterase test; GT: Glucose esterase test; DM: Diabetes mellitus; BC: Blood culture; GS: Gram stain; SC: Serum culture; UDSA: Ultimate diagnosis of septic arthritis
The comparison of the final results of patient diagnosis and leukocyte esterase test results led to the identification of sensitivity of 84.38%, specificity of 90.24%, positive predictive value of 93.10%, and negative predictive value of 78.72%. Additionally, comparing the results of the final diagnosis of patients and the positive or negative leukocyte esterase test response in group 1, the sensitivity of 100%, specificity of 0%, and positive predictive value of 96.55% were obtained, and in group 2, the sensitivity of 72.22%, specificity of 92.50%, positive predictive value of 89.66%, and negative predictive value of 78.72% were determined. The sensitivity and specificity are presented in Table 2 based on the final diagnostic results of patients and the results obtained from other blood factors.
Table 2.
Diagnostic power of the Leukocyte esterase test and other blood factors
| Groups | Sensitivity | Specificity | Positive LR | Negative LR | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| LET | Value | 84.38% | 90.24 % | 8.65 | 0.17 | 93.10% | 78.72% |
| 95% CI | 73.14-92.24% | 76.87-97.28% | 3.39-22.07 | 0.10-0.31 | 84.10-97.18% | 67.48-86.84% | |
| SC | Value | 15.62% | 100% | - | 0.84 | 100% | 43.16% |
| 95% CI | 7.76-26.86% | 91.40-100% | - | 0.76-0.94 | - | 40.59-45.76% | |
| GS | Value | 59.38% | 97.56% | 24.34 | 0.42 | 97.44% | 60.61% |
| 95% CI | 46.37-71.49% | 87.14-99.94% | 3.48-170.51 | 0.31-0.56 | 84.44-99.63% | 53.26-67.50% | |
| BC | Value | 21.88% | 100% | - | 0.78 | 100% | 45.05% |
| 95% CI | 12.51-33.97% | 91.40-100% | - | 0.69-0.89 | - | 41.87-48.28% | |
| SyWBC | Value | 76.69% | 95.12% | 16.34 | 0.21 | 96.23% | 75.00% |
| 95% CI | 67.77-88.72% | 83.47-99.40% | 4.20-63.48 | 0.13-0.35 | 86.78-99.00% | 64.76-83.04% | |
| SWBC | Value | 57.81% | 58.54% | 1.39 | 0.72 | 68.52% | 47.06% |
| 95% CI | 44.82-70.06% | 42.11-73.68% | 0.92-2.12 | 0.49-1.06 | 58.86-76.80% | 37.68-56.65% | |
| ESR | Value | 59.38% | 78.05% | 2.70 | 0.52 | 80.85% | 55.17% |
| 95% CI | 46.37-71.49% | 62.39-89.44% | 1.47-4.99 | 0.37-0.73 | 69.61-88.62% | 46.75-63.31% | |
| CRP | Value | 76.56% | 63.41% | 2.09 | 0.37 | 76.56% | 63.41% |
| 95% CI | 64.31-86.25% | 46.94-77.88% | 1.37-3.20 | 0.22-0.61 | 68.10-83.33% | 51.25-74.08% | |
LR: Likelihood ratio; PPV: Positive predictive value; NPV: Negative predictive value
SyWBC: Synovial fluid white blood cell count×1000/l; Sy-sE G: Synovial fluid-concomitant serum glucose mg/dl; SWBC: Serum white blood cell count; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; PAb: Previous antibiotic use; TOA: Time of aspiration after beginning of symptoms; LET: Leukocyte esterase test; GT: Glucose esterase test; DM: Diabetes mellitus; BC: Blood culture; GS: Gram stain; SC: Serum culture; UDSA: Ultimate diagnosis of septic arthritis
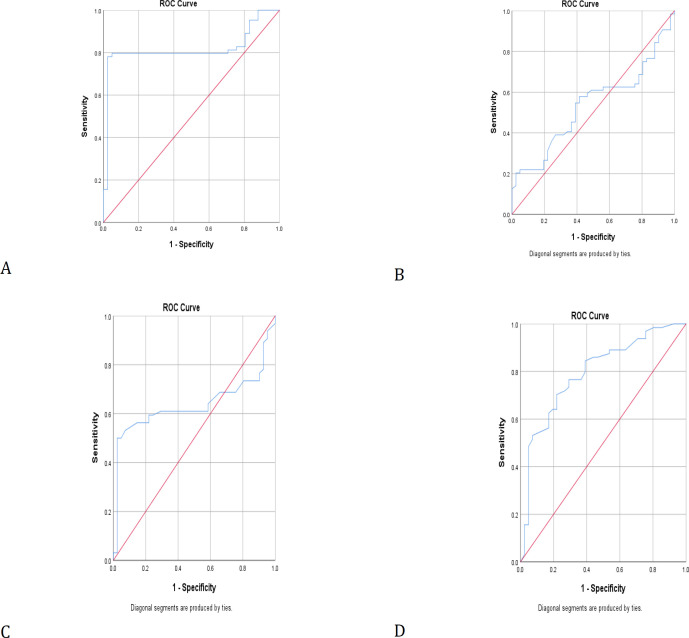
The cut-off points of the ROC curve for SyWBC, SWBC, ESR, and CRP tests were 45.74, 12.33, 43.50, and 27.5, respectively (Figure 1). In groups A and B, 3 and 2 patients had diabetes, respectively. Serum glucose and synovial fluid glucose were compared in patients having infectious arthritis with and without diabetes, and both factors were significantly higher in those with positive diabetes (P<0.05) (Table 3).
Figure 1.
Receiver operating characteristic curve for (A) SyWBC, (B) SWBC, (C) ESR, and (D) CRP tests
Table 3.
Comparison of serum glucose and synovial fluid glucose in patients with infectious arthritis with and without diabetes
| P-value | Negative DM (n=100) | Positive DM (n=5) | Variables | |
| 0.099 | 26 (89.66) | 3 (10.34) | Group A | DM |
| 74 (97.37) | 2 (2.63) | Group B | ||
| 0.015* | 3.39±5.92 | 55.40±13.77 | Sy-G (mmol/L) | |
| <0.0001* | 85.34±14.67 | 152±28.14 | SE-G (mmol/L) | |
Data are expressed as mean ± SD or numbers (%).
The t-test test was used for continuous data and Chi-Square test for qualitative data.
*P<0.05 is considered as significant level.
DM: Diabetes mellitus; Sy-G: Synovial glucose; Se-G: serum glucose
Discussion
According to the 2018 International Consensus Meeting (ICM) on Musculoskeletal Infection with Delphi methodology is used a general agreement on a clinical problem that could be the basis for future studies focused on surgical site infection and periprosthetic joint infection (PJI). Topics discussed in ICM 2018 included antibiotics, research caveats, host immunity, acute versus chronic infection, diagnosis, and modifiable factors. Moreover, it is specified in ICM 2018 that such factors as autoimmune disease, infection in a distal limb after surgery, and kidney disease can alter the systemic markers used to diagnose infection (false positive) and factors that may cause false negative of systemic markers, such as chemotherapy, human immunodeficiency virus, neutropenia, and infection, with low- virulence organism (e.g., Cutibacterium acnes and coagulase negative staphylococci) expressed. Importantly serologic tests are currently recommended as the first-line screening for PJI in ICM 2013 and the American Academy of Orthopedic Surgeons. Two positive cultures from the joint identifying the same organism by tissue or fluid have remained as one of the major criteria for the diagnosis of PJI; however, in 7-35% of patients, no organism can be isolated. Consequently, the UK standard for microbiology investigation has recommended the exploration of culture plates with a plate microscope to detect small colonies (14, 15).
In a study conducted by the Management of Resistant, Atypical, and Culture-negative Periprosthetic Joint Infections after Hip and Knee Arthroplasty, the combination of the two tests of CRP and α-defensin demonstrated a sensitivity of 97% and specificity of 100% for PJI. It is noteworthy that the results were not influenced by the concurrent antibiotic. Perhaps the most important cause of negative culture is the administration of antibiotics before receiving aspiration fluids or tissue samples from the joint. Berberi et al. found that 53% of patients that had negative cultures had received antibiotics before culture or tissue culture. Culture yields can be improved if antimicrobial therapy is been used at least 2 weeks before surgical intervention or aspiration. In addition, prophylactic antibiotics therapy before the definitive diagnosis alters the synovial fluid leukocyte count and differential, which will lead to further complications and potentially delay in the administration of the appropriate treatment. Bacteriostatic agents, such as local anesthetics and saline, should not be employed before obtaining fluid or tissue samples. The use of aerobic and anaerobic blood culture flasks can improve the culture specificity (16).
Multiple pathologies, especially crystal-induced diseases and rheumatic diseases, may have similar manifestations to SA. In a patient suspected of SA, it is difficult and important to decide whether he/she should receive antibiotic treatment. To the best of our knowledge, no studies have been dedicated to investigate the specificity of the relevant clinical symptoms, and data on laboratory results are highly limited (3, 9, 10). In addition to a thorough physical examination and blood test, arthrocentesis and then synovial fluid analysis are central to the diagnosis of septic arthritis (4, 6, 10). Normal synovial fluid has a glucose concentration approximately equal to plasma. A sharp decrease in glucose concentration indicates an infectious process in which microbial glucose metabolism occurs (8, 11); nevertheless, glucose is less frequently used as a unique marker of infection than other parameters (10). Microbiological examination alone is not sufficient to diagnose or rule out this disease. The sensitivities of the Gram staining and culture methods were reported to be 50% and 67%, respectively (1). High levels of synovial fluid leukocytes with a high percentage of PMN are more likely to suggest septic arthritis; however, the results of other researchers have reported that these values are not able to differentiate septic arthritis and other types of inflammatory arthritis (1). Since leukocyte levels are reduced within a few hours, it is important to have a fast and accurate procedure in this area. Among these, the leukocyte esterase test can be used to diagnose urinary tract infections, peritonitis, meningitis, and joint infections (7, 8). Testing for leukocyte esterase may distinguish inflammatory and non-inflammatory arthritis; nonetheless, it may not be effective in differentiating septic arthritis from other inflammatory processes (e.g., the metabolic form of arthritis) that lead to increased neutrophil accumulation (2, 4, 10, 12). This study aimed to evaluate the sensitivity and specificity of the leukocyte esterase strip in two groups of patients receiving and not receiving antibiotics and compare the results. The results of this study showed that the measurement of leukocyte esterase in the synovial fluid has certain advantages for the diagnosis of septic arthritis. Leukocyte esterase concentration showed a significant difference between the two groups in terms of SyWBC, SWBC, PMN%, SE-G, synovial glucose, ESR, CRP, and TOA (P<0.05). The positive frequency of GT, BC, GS, SC, and UDSA was also significantly different between the two groups (P<0.05). Serum glucose and synovial fluid glucose were compared in patients having infectious arthritis with and without diabetes, and both factors were significantly higher in those with positive diabetes (P<0.05). Since this study was the first to investigate the role of antibiotic use in the final response of the leukocyte esterase test, there were no similar studies to compare their results with those of the present research; nevertheless, the results from the second group (not receiving antibiotics) were in line with those of the previous studies.
Omar et al. conducted a similar study on patients in the septic (n=19) and aseptic (n=127) groups. Sensitivity, specificity, positive predictive value, and negative predictive value of leukocyte esterase were obtained at 89.5%, 99.2%, 94.4%, and 98.4%, respectively (3). In the present study, the sensitivity and specificity of the leukocyte esterase test were 84.38% and 90.24%, respectively, which were similar to those of the study carried out by Omar et al. The larger sample size and differences in inclusion and exclusion criteria in the study conducted by Omar et al. may have resulted in higher sensitivity and specificity and lower positive and negative predictive values. In another study, Colvin et al. examined a leukocyte esterase test in 5 patients. The results of this test for sensitivity, specificity, positive, and negative predictive values were 100%, 50%, 33.33%, and 100%, respectively, which due to the small sample size in that study, their results were not compared with those of the present study (8). Tischler studied leukocyte esterase among patients with musculoskeletal infections. The results of their study reported the sensitivity, specificity, positive predictive value, and negative predictive value of the test as 79.2%, 65.9%, 80.8%, and 61.8%, respectively. They also concluded that although the type of disease affected the diagnostic value of the leukocyte esterase test, this test showed acceptable results in the field of musculoskeletal infections (10).
In the present study, the sensitivity of the leukocyte esterase test in the group with a history of antibiotic use was 100%, which was higher than that in the previous studies, and the specificity was 0%, which was much lower than that in similar studies. These two differences caused by the leukocyte esterase test can be attributed to different reasons. The significant difference in TOA in the two groups and the prolongation of time between symptom onset and diagnosis due to antibiotic treatment in group A increased inflammatory products, SWbc, and PMN%, which in turn boosted leukocyte esterase concentration. It was articulated that it might have increased susceptibility in group A. On the other hand, one of the major reasons for the differences in the results obtained for specificity in group 1 with those in previous studies may be due to the small sample size in group A. Patients with aseptic arthritis receiving antibiotic treatment also referred to the emergency room for symptoms less mild than septic arthritis; therefore, the true negative cases, and consequently specificity, were less in this group than in group B.
Based on the results of the leukocyte esterase test and other tests in this study, it can be concluded that blind antibiotic use, together with increased TOA, increased inflammatory products and interference with laboratory variables. As a result, it increased the sensitivity and impaired the specificity of the test. The sensitivity and specificity of the leukocyte esterase test in patients that did not receive antibiotics were similar to those in the previous studies, suggesting that this is an appropriate and reliable laboratory method for the rapid detection of septic arthritis that requires an emergency rescue procedure. The level of articular fluid glucose is higher in diabetic patients than in normal individuals and is not a suitable criterion for diagnosing infectious arthritis in such people. Since the present study was the first to evaluate the ability of this test in patients with antibiotic use, to confirm the results, it is suggested to perform studies with larger sample sizes, more confounding factors, and long-term follow-up.
Acknowledgment
The present study was derived from an orthopedic assistant thesis. The authors would also thank the staff for their assistance and cooperation in this research project.
References
- 1.Horowitz DL, Katzap E, Horowitz S, Barilla-LaBarca M-L. Approach to septic arthritis. American family physician. 2011;84(6):653–60. [PubMed] [Google Scholar]
- 2.Kelly E, Cashman J. Leucocyte esterase in the rapid diagnosis of paediatric septic arthritis. Medical Hypotheses. 2013;80(2):191–3. doi: 10.1016/j.mehy.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Baldassare AR, Chang F, Zuckner J. Markedly raised synovial fluid leucocyte counts not associated with infectious arthritis in children. Annals of the Rheumatic Diseases. 1978;37(5):404–9. doi: 10.1136/ard.37.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margaretten ME, Kohlwes J, Moore D, Bent S. Does this adult patient have septic arthritis? Jama. 2007;297(13):1478–88. doi: 10.1001/jama.297.13.1478. [DOI] [PubMed] [Google Scholar]
- 5.Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for musculoskeletal infection society criteria. JBJS. 2014;96(22):1917–20. doi: 10.2106/JBJS.M.01591. [DOI] [PubMed] [Google Scholar]
- 6.Omar M, Ettinger M, Reichling M, Petri M, Lichtinghagen R, Guenther D, et al. Preliminary results of a new test for rapid diagnosis of septic arthritis with use of leukocyte esterase and glucose reagent strips. JBJS. 2014;96(24):2032–7. doi: 10.2106/JBJS.N.00173. [DOI] [PubMed] [Google Scholar]
- 7.Colvin OC, Kransdorf MJ, Roberts CC, Chivers FS, Lorans R, Beauchamp CP, et al. Leukocyte esterase analysis in the diagnosis of joint infection: can we make a diagnosis using a simple urine dipstick? Skeletal radiology. 2015;44(5):673–7. doi: 10.1007/s00256-015-2097-5. [DOI] [PubMed] [Google Scholar]
- 8.McGillicuddy DC, Shah KH, Friedberg RP, Nathanson LA, Edlow JA. How sensitive is the synovial fluid white blood cell count in diagnosing septic arthritis? The American journal of emergency medicine. 2007;25(7):749–52. doi: 10.1016/j.ajem.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Li SF, Henderson J, Dickman E, Darzynkiewicz R. Laboratory tests in adults with monoarticular arthritis: can they rule out a septic joint? Academic emergency medicine. 2004;11(3):276–80. doi: 10.1111/j.1553-2712.2004.tb02209.x. [DOI] [PubMed] [Google Scholar]
- 10.Torun S, Dolar E, Yilmaz Y, Keskin M, Kiyici M, Sinirtas M, et al. Evaluation of leukocyte esterase and nitrite strip tests to detect spontaneous bacterial peritonitis in cirrhotic patients. World Journal of Gastroenterology: WJG. 2007;13(45):6027. doi: 10.3748/wjg.v13.45.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Booth RE, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clinical Orthopaedics and Related Research®. 2015;473(1):198–203. doi: 10.1007/s11999-014-3722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foocharoen C, Sarntipipattana C, Foocharoen T, Mahakkanukrauh A, Paupairoj A, Teerajetgul Y, et al. Synovial fluid adenosine deaminase activity to diagnose tuberculous septic arthritis. Southeast Asian Journal of Tropical Medicineand Public Health. 2011;42(2) [PubMed] [Google Scholar]
- 13.R Singhal D C, Perry C E. The Diagnostic Utility of Kocher's Criteria in the Diagnosis of Septic Arthritis in Children: An External Validation Study. J Bone Joint Surg. 2012;94:XXXV –6. [Google Scholar]
- 14.Saeed K, McLaren AC, Schwarz EM, et al. The 2018 international consensus meeting on musculoskeletal infection: summary from the Biofilm Workgroup and consensus on biofilm related musculoskeletal infections. J Orthop Res. 2019 doi: 10.1002/jor.24229. [DOI] [PubMed] [Google Scholar]
- 15.Edward M, Javad Parvizi. 2018 International Consensus Meeting on Musculoskeletal Infection: Research Priorities from the General Assembly Questions. 2019;37:997–1006. doi: 10.1002/jor.24293. [DOI] [PubMed] [Google Scholar]
- 16.Alexander S, McLawhorn H, Amar S. Hospital for Special Surgery. The Open Orthopaedics Journal. 2016;10:615–632. doi: 10.2174/1874325001610010615. [DOI] [PMC free article] [PubMed] [Google Scholar]