Abstract
INTRODUCTION
Under the European Union (EU) Tobacco Products Directive (2014/40/EU) (TPD), manufacturers and importers of tobacco products are required to report information to the European Commission (EC) and Member States (MS) on products intended to be placed on the market. We describe the distribution of notifications to the EU Common Entry Gate (EU-CEG) and identify key fields for improvement on reporting cigarettes or roll-your-own (RYO) tobacco.
METHODS
A cross-sectional analysis of secondary data reported in the EU-CEG was conducted for tobacco products notified within EU-CEG between June 2016 and October 2019 for 12 EU MS. Analysis of compliance to specific regulations for priority additives that refer to cigarettes and RYO was conducted for 10 EU countries.
RESULTS
Overall, 39170 tobacco products were notified. This included 16762 (42.8%) notifications of cigars, followed by cigarettes 11242 (28.7 %), waterpipes 3291 (8.4%), cigarillos (n=1783), pipe (n=1715), RYO (n=1635), chewing tobacco (n=1021), novel tobacco products (n=839), herbal products for smoking (n=535), other (n=258), nasal (n=74) and oral tobacco (n=15). In cigarettes and RYO tobacco products, the proportion of ingredients notified in all countries that contained an unknown Chemical Abstract Services (CAS) number was 3.8% and 2.1%, respectively. The proportion of underreporting flagging of priority additives ranged from 15.9% in Malta to 41.3% in Lithuania, the mean proportion of underreporting of the variable ‘priority additive’ for the 10 countries together was 24.7%.
CONCLUSIONS
In the EU-CEG data base, for the period of analysis, a significant number of product notifications took place while large variations in the number of types of tobacco products notified across EU countries was noted. The timely monitoring of these data is needed so that products non-compliant within the EU-CEG system are assessed.
Keywords: ingredients, Tobacco Products Directive, priority additives, EU-CEG, CAS number, cigarette characterizing flavor
INTRODUCTION
The Tobacco Products Directive (TPD) (2014/40/ EU) is a directive of the European Union (EU) which places limits on the sale and merchandising of tobacco and tobacco related products in the EU. The TPD aims to improve the functioning of the internal market for tobacco and related products, while ensuring a high level of health protection for European citizens. This Directive entered into force on 19 May 2014 and became applicable in the EU Member States (MS) on 20 May 20161. Under the EU-TPD, manufacturers and importers of tobacco products, electronic cigarettes (e-cigarettes) and refill containers are required to report comprehensive information to the European Commission (EC) and (MS) on products which they intend to place on the market1, through the European Union Common Entry Gate (EU-CEG). The required information includes, but is not limited to, ingredients, emissions, and toxicological data, with the specific parameters defined in the Annexes to Commission Implementing Decision (EU) 2015/2186 for tobacco products and Commission Implementing Decision (EU) 2015/2183 for e-cigarettes and refill containers2. Following the EU-TPD in October 2017, a Joint Action on Tobacco Control (JATC) was launched by the EC. The general objective of the JATC was to provide support for the implementation of the TPD by the use of the EU-CEG.
EU-CEG is the information technology tool developed to provide a common format for manufacturers and importers to report the information required under the TPD3. EU-CEG was designed to facilitate a harmonized reporting system that lessens the administrative burden for submitters, as well as enhances the EC and MS ability to compare data and ultimately regulate tobacco products on the EU market. Since May 2016, manufactures and importers are required to submit information through EU-CEG on any new or modified tobacco product including e-cigarettes and refills, six months prior to being placed on the market. Once data is uploaded and successfully passes a technical validation process, the data is directed to the relevant national data repository that is accessible to the EC and the relevant competent national authority4. EU-CEG data are only available for analysis for those countries having signed a Data Sharing Agreement within the JATC. In our study, only 12 out of 27 EU MS signed this Agreement. EU-CEG adheres to the Regulation 45/2001 on the protection of personal data5.
Our study aims to describe the distribution of notifications of 12 types of tobacco products [chewing, cigar, cigarette, cigarillo, herbal products for smoking, nasal, novel tobacco products (NTP), oral, pipe, roll-your-own (RYO), water-pipe, and other tobacco products] for 12 EU countries. Distribution of notifications refers to the number and percentage of notifications of each tobacco product introduced in EU-CEG by country and type of tobacco product from 22 June 2016 to 21 October 2019.
This study also aims to analyze compliance to specific regulations on priority additives in cigarettes and RYO for 10 EU countries. Specifically, paragraphs 2 (a) and 2 (b) of Article 6 of the TPD1 prescribe that MS shall require manufacturers and importers of cigarettes and RYO containing a priority additive, to carry out comprehensive studies, which shall examine for each additive whether: 1) it contributes to the toxicity or addictiveness of the products concerned, and 2) it results in a characterizing flavor. The Commission Implementing Decision (EU) 2016/787 of 18 May 20166 refers to the following 15 priority additives: carob bean, cocoa, diacetyl, fenugreek, fig-extract, geraniol, glycerol, guaiacol, guar-gum, liquorice, maltol, menthol, propylene glycol, sorbitol, and titanium dioxide.
A third aim of this study is to identify areas of improvement in the process of notification within the EU-CEG to ensure compliance of the TPD by manufacturers. Consequently, the findings of our study should contribute to an increased awareness of MS and EU-CEG administrators in order to improve compliance of the TPD.
METHODS
Study design
Cross-sectional analysis of the data reported in the EU-CEG for 12 tobacco products from 22 June 2016 to 21 October 2019 for 12 EU countries. The analysis of compliance to specific regulations on priority additives in cigarettes and RYO was conducted for 10 EU countries.
Data
Within the JATC project funded by the EC during 2017-2020 (https://jaotc.eu), two Work Packages referred to the use of the EU-CEG7. EU-CEG data were only available for analysis for those countries having signed the Data Sharing Agreement within the JATC. In our study, only 12 out of 27 EU MS signed this Agreement and hence our analysis was performed for 12 EU countries: Belgium (BE), Czechia (CZ), Denmark (DK), Spain (ES), France (FR), Greece (GR), Italy (IT), Malta (MT), Lithuania (LT), Latvia (LV), Slovenia (SI) and The Netherlands (NL). Two countries (Spain and Greece) contributed to the first part of the study along with the other 10 countries, but for the second part of this study, the variables ‘priority additive’ and ‘cigarette characterizing flavor’ could not be retrieved because these two variables were not included in the initial data request, therefore, these two countries were excluded from this analysis.
Data are introduced in EU-CEG by manufacturers and most of the fields have fixed response options ensuring standardization of responses. For example, some fields can be filled with numerical values only. Most of the fields are not compulsory to fill. Finally, open fields for comments are also present in the data set3,4. All EU-CEG variables are described in the Data dictionary for the Proposed Common EU Reporting Format for Tobacco Products8. The data were acquired in xml and/or pdf files, downloaded from the portal. The data used for this descriptive study were downloaded on 20 February 2020.
Definitions
The fields/variables: ‘CAS number’, ‘priority additive’, ‘cigarette characterizing flavor’, ‘toxicological data available’ and ‘existence of studies on carcinogenic, mutagenic and reprotoxic chemicals (tox-CMR)’ of the EU-CEG database were the fields used to assess compliance to the relevant paragraphs of Article 6 of the TPD with regard to priority additives and characterizing flavors in cigarettes and RYO. Within our analyses, the definitions of the TPD data dictionary were used8:
Chemical Abstract Service (CAS) number is the CAS registry number used to identify the ingredient.
Priority additive is the indication if the ingredient is a priority additive (Responses include: yes; no; not published yet). Until the ‘Priority additive list’ of the TPD is provided, all responses shall be noted as ‘not published yet’.
Cigarette characterizing flavor is the classification of the cigarette as having a characterizing flavor as referred to in Article 7(14) of Directive 2014/40/ EU.
Toxicological data available is the existence of toxicological data available, for either as an individual substance or as part of a mixture and in burnt or unburnt form.
Existence of studies on carcinogenic, mutagenic and reprotoxic chemicals (tox-CMR) is existence of any CMR related study, including but not limited to: in vitro toxicological assays to evaluate potential genotoxic and cytotoxic properties. Assays to determine the effect of the ingredient on the reproductive system and its potential to cause birth defects. Assays to determine whether the ingredient affects the tumorigenic properties of the product (the analyses should be based on either inhalation or dermal exposure for the latter).
Statistical analysis
For the first objective, a descriptive analysis of selected variables was conducted for the 12 types of tobacco products that were notified and not flagged as withdrawn within EU-CEG for each of the 12 countries.
For the second and third objectives, we assessed/ verified the completeness of selected key variables within the notifications of ingredients. This means that important variables such as, for example, CAS number, should not have a missing value. We first assessed the completeness of the variable ‘CAS number’ across EU MS and described the proportion of priority additives identified from ‘CAS number’ over all ingredients across 12 EU MS. Moreover, we described the distribution of each priority additive overall and for each of the 12 EU MS. For the 10 countries for which the ‘priority additive’ variable was available, we compared the number of priority additives with the number of priority additives for which the variable ‘CAS number’ was completed. We also assessed whether for those ingredients notified as ‘priority additive’=true, the informed ‘CAS number’ was within the list of the Commission Implementing Decision (EU) 2016/7878. Finally, we assessed in cigarettes and RYO whether the variables ‘toxicological data available’ (true/false) and ‘existence of studies on toxicity or CMR properties of tobacco’ (true/false) were completed and computed all ingredients (priority additives and other) as not having toxicological data available, for which the variable ‘toxicological data available’ was completed.
Data cleaning and graphics were done with Excel 2010 and data were analyzed in Stata 14 (StataCorp, College Station, TX, USA).
RESULTS
From 22 June 2016 to 21 October 2019, a total of 39170 tobacco products were notified in the EU-CEG database for the 12 EU MS that were studied. Most of the notifications belong to cigars 16762 (42.8%), followed by cigarettes 11242 (28.7 %), and waterpipes 3291 (8.4%). The remaining (20%) notifications were distributed as follows: cigarillos (n=1783), pipe (n=1715), RYO (n=1635), chewing (n=1021), NTP (n=839), herbal products for smoking (n=535), other (n=258), nasal (n=74), and oral (n=15).
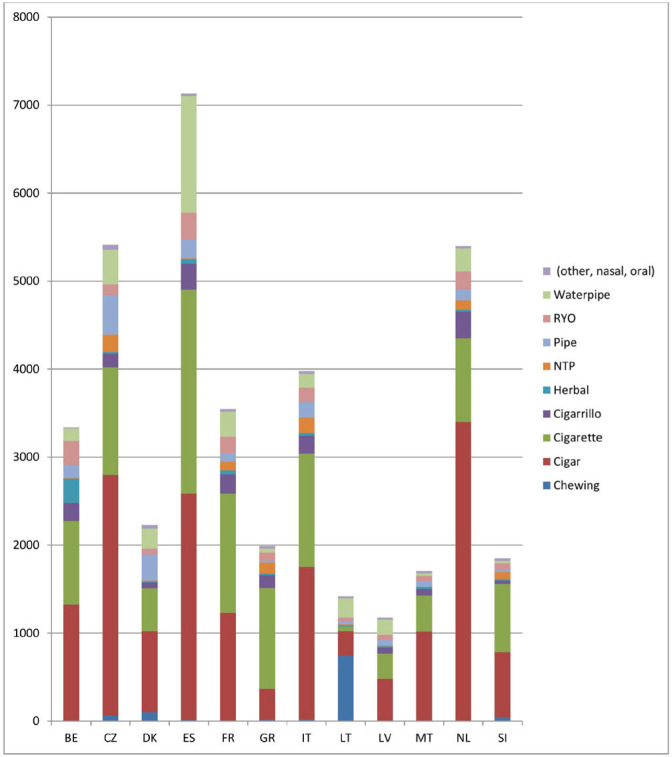
The proportional distribution of notifications of tobacco products was slightly different across EU MS. In the majority of the EU MS, cigars were the most frequently notified category of tobacco product. This was the case in Belgium, Czechia, Denmark, Spain, Italy, Latvia, Malta and the Netherlands. The highest number of notifications in Greece, France and Slovenia was for cigarettes, while in Lithuania the most frequently notified type of tobacco product was chewing tobacco.
Cigarettes were the second product most frequently notified in Belgium, Czechia, Denmark, Spain, Italy, Latvia, Malta and the Netherlands, and cigars were the second product in France, Greece, Lithuania, and Slovenia. Waterpipes were the third tobacco product notified in Spain, France, Lithuania, and Latvia. Pipes were the third tobacco product notified in Czechia and Denmark. Herbal products were the third in Belgium and NTPs in Slovenia. Finally, cigarillos were the third tobacco product notified in Greece, Italy, Malta, and the Netherlands. (Figure 1).
Figure 1.
Number of notifications of 12 types of tobacco products active in EU-CEG from 22 June 2016 to 21 October 2019, by country (N=39170)
Spain, Czechia and the Netherlands were the countries with highest number of notifications (above 5000), substantially higher than Latvia, Lithuania, Slovenia, Malta and Greece with <2000 notifications. In the remaining countries, Denmark, Belgium, France and Italy, the number of notifications ranged from 2229 to 3979 (Figure 1).
Completeness of the variable ‘CAS number’
In cigarettes, the proportion of ingredients notified as unknown or with missing CAS number, over all reported ingredients, ranged from 0.9% in Latvia to 4.9% in Spain, with a mean proportion of 3.8%. For RYO products this proportion ranged from 0.7% in Lithuania and Czechia to 4.8% in Greece with a mean proportion of 2.1% (Table 1).
Table 1.
Ingredients notified with no information on CAS number among notifications of cigarettes and RYO active in EU-CEG from 22 June 2016 to 21 October 2019 by country
| Country | Cigarettes | Roll-your-own | ||
|---|---|---|---|---|
| Reported ingredients in active notifications in EU-CEG n | Ingredients with an unknown or missing CAS number among notifications active in EU-CEG n (%) | Reported ingredients in active notifications in EU-CEG n | Ingredients with an unknown or missing CAS number among notifications active in EU-CEG n (%) | |
| BE | 72595 | 3485 (4.8) | 4116 | 84 (2.0) |
| CZ | 100859 | 3429 (3.4) | 1873 | 14 (0.7) |
| DK | 40571 | 1501 (3.7) | 1283 | 22 (1.7) |
| ES | 175811 | 8677 (4.9) | 4489 | 85 (1.9) |
| FR | 99186 | 3670 (3.7) | 2631 | 57 (2.2) |
| GR | 92335 | 3840 (3.6) | 1375 | 66 (4.8) |
| IT | 105317 | 2844 (2.7) | 2335 | 38 (1.6) |
| LT | 23285 | 373 (1.6) | 964 | 7 (0.7) |
| LV | 23594 | 212 (0.9) | 1532 | 15 (1.0) |
| MT | 30644 | 1440 (4.7) | 1331 | 35 (2.6) |
| NL | 74345 | 2899 (3.9) | 2558 | 79 (3.1) |
| SI | 64861 | 2011 (3.1) | 887 | 31 (3.5) |
| Total | 903403 | 34381 (3.8) | 25374 | 533 (2.1) |
Proportion and distribution of priority additives among ingredients
The proportion of priority additives over all reported ingredients, in notifications of cigarettes, ranged from 2.7% in Greece to 16.2% in Denmark, with a mean proportion of 12.7%. For RYO, this proportion ranged from 5% in Greece to 24.2% in the Netherlands, with a mean proportion of 18.4%. The proportion of priority additives reported within cigarettes was less than those reported for RYO (Table 2).
Table 2.
Priority additivesa in notifications of cigarettes and RYO active in EU-CEG from 22 June 2016 to 21 October 2019 by country
| Country | Cigarettes | Roll-your-own | ||
|---|---|---|---|---|
| Notifications active in EU-CEG for ingredients n | Priority additives from CAS b n (%) | Notifications active in EU-CEG for ingredients n | Priority additives from CAS b n (%) | |
| BE | 72595 | 9432 (13.0) | 4116 | 890 (21.6) |
| CZ | 100859 | 14215 (14.1) | 1873 | 361 (19.3) |
| DK | 40571 | 6559 (16.2) | 1283 | 226 (17.6) |
| ES | 175811 | 21651 (12.3) | 4489 | 809 (18.0) |
| FR | 99186 | 13999 (14.1) | 2631 | 596 (22.7) |
| GR | 92335 | 2457 (2.7) | 1375 | 69 (5.0) |
| IT | 105317 | 15391 (14.6) | 2335 | 422 (18.1) |
| LT | 23285 | 3646 (15.7) | 964 | 103 (10.7) |
| LV | 23594 | 3726 (15.8) | 1532 | 167 (10.9) |
| MT | 30644 | 3973 (13.0) | 1331 | 215 (16.2) |
| NL | 74345 | 10157 (13.7) | 2558 | 618 (24.2) |
| SI | 64861 | 9156 (14.1) | 887 | 189 (21.3) |
| Total | 903403 | 114362 (12.7) | 25374 | 4665 (18.4) |
From summary of listed CAS numbers corresponding to a priority additive.
Priority additives identified from CAS number in notifications active in EU-CEG for ingredients.
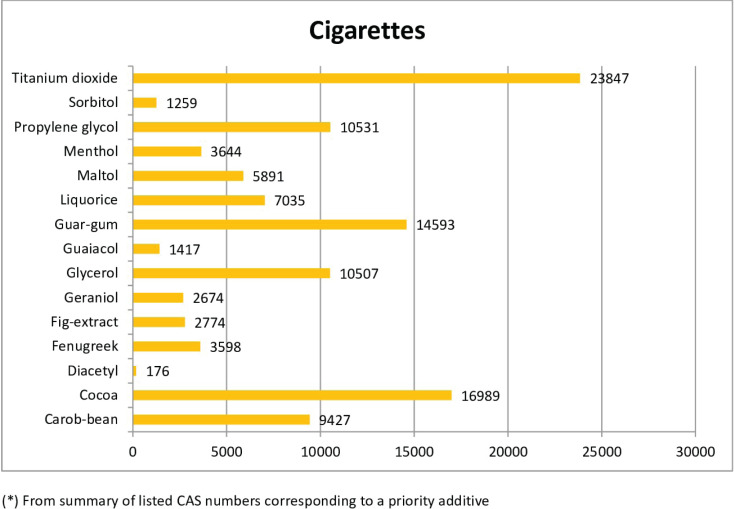
The most frequently notified priority additive among cigarettes was titanium dioxide, followed by cocoa, guar-gum, propylene glycol and glycerol with over 10000 notifications (Figure 2A). By country (Table 3), notifications of titanium dioxide in cigarettes ranges from 0.5% of total ingredients in Greece to 4% in Denmark, with a mean proportion of 2.6% of all reported ingredients for all countries combined. Cocoa, the second most frequently notified priority additive, ranged from 0.7% in Greece to 2.4% in Denmark, with a mean overall proportion of 1.9%. Notifications of guar gum were more evenly distributed across countries, ranging from 1.5% in Lithuania to 1.9% in Denmark, France, Malta and Slovenia, with a mean proportion of 1.6% for all countries.
Figure 2A.
Overall reports of priority additives* in cigarettes in EU-CEG from 22 June 2016 to 21 October 2019, by type of priority additive
Table 3.
Number of notifications of priority additivesa in cigarettes and RYO in EU-CEG from 22 June 2016 to 21 October 2019 by country
| Priority additive | Frequency of notifications (n) for cigarettes per country | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BE | CZ | DK | ES | FR | GR | IT | LT | LV | MT | NL | SI | Total | |
| Carob-bean | 794 | 1073 | 516 | 1469 | 1080 | 773 | 1163 | 328 | 338 | 361 | 819 | 713 | 9427 |
| Cocoa | 1396 | 2097 | 988 | 3062 | 1833 | 618 | 2369 | 499 | 510 | 599 | 1569 | 1449 | 16989 |
| Diacetyl | 13 | 42 | 0 | 27 | 26 | 0 | 28 | 2 | 0 | 0 | 0 | 38 | 176 |
| Fenugreek | 187 | 382 | 246 | 348 | 472 | 334 | 664 | 166 | 201 | 54 | 222 | 322 | 3598 |
| Fig-extract | 274 | 342 | 170 | 522 | 246 | 0 | 398 | 50 | 52 | 121 | 331 | 268 | 2774 |
| Geraniol | 250 | 360 | 188 | 360 | 332 | 129 | 296 | 74 | 79 | 128 | 298 | 180 | 2674 |
| Glycerol | 887 | 1378 | 529 | 2106 | 1321 | 0 | 1510 | 363 | 363 | 378 | 921 | 751 | 10507 |
| Guaiacol | 76 | 186 | 103 | 189 | 130 | 0 | 242 | 82 | 94 | 34 | 110 | 171 | 1417 |
| Guar-gum | 1293 | 1848 | 767 | 3041 | 1863 | 0 | 1929 | 344 | 368 | 581 | 1359 | 1200 | 14593 |
| Liquorice | 593 | 991 | 348 | 1389 | 835 | 0 | 808 | 273 | 254 | 226 | 637 | 681 | 7035 |
| Maltol | 504 | 762 | 281 | 1199 | 716 | 0 | 957 | 141 | 139 | 181 | 508 | 503 | 5891 |
| Menthol | 137 | 469 | 304 | 728 | 569 | 84 | 366 | 189 | 157 | 73 | 340 | 228 | 3644 |
| Propylene glycol | 828 | 1440 | 467 | 2041 | 1380 | 0 | 1524 | 357 | 357 | 327 | 897 | 913 | 10531 |
| Sorbitol | 44 | 206 | 51 | 289 | 149 | 0 | 236 | 68 | 62 | 17 | 75 | 62 | 1259 |
| Titanium dioxide | 2156 | 2639 | 1601 | 4881 | 3047 | 519 | 2901 | 710 | 752 | 893 | 2071 | 1677 | 23847 |
| Total | 9432 | 14215 | 6559 | 21651 | 13999 | 2457 | 15391 | 3646 | 3726 | 3973 | 10157 | 9156 | 114362 |
| Priority additive | Frequency of notifications (n) for RYO per country | ||||||||||||
| BE | CZ | DK | ES | FR | GR | IT | LT | LV | MT | NL | SI | Total | |
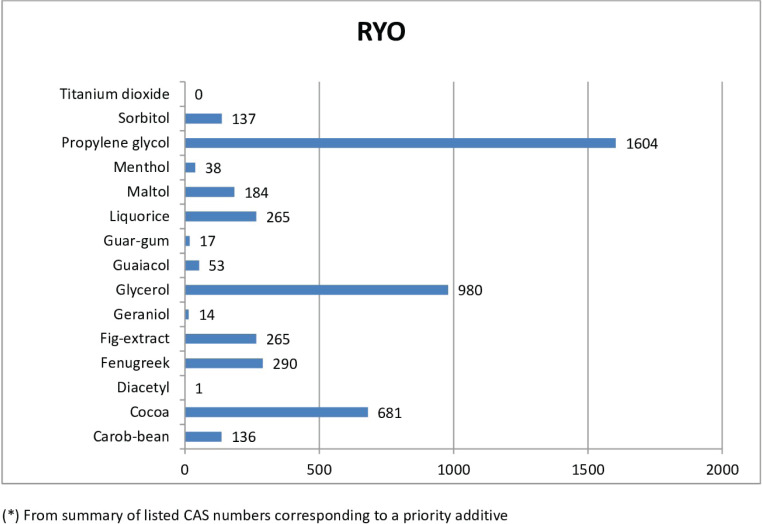
| Carob-bean | 36 | 4 | 11 | 20 | 15 | 5 | 4 | 1 | 3 | 2 | 33 | 2 | 136 |
| Cocoa | 113 | 61 | 34 | 95 | 92 | 39 | 63 | 12 | 25 | 33 | 80 | 34 | 681 |
| Diacetyl | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Fenugreek | 63 | 11 | 13 | 43 | 32 | 24 | 22 | 5 | 11 | 8 | 49 | 9 | 290 |
| Fig-extract | 41 | 11 | 13 | 45 | 44 | 0 | 32 | 4 | 6 | 18 | 39 | 12 | 265 |
| Geraniol | 2 | 1 | 0 | 4 | 0 | 0 | 3 | 1 | 3 | 0 | 0 | 0 | 14 |
| Glycerol | 187 | 87 | 51 | 172 | 132 | 0 | 79 | 22 | 35 | 32 | 144 | 39 | 980 |
| Guaiacol | 7 | 3 | 4 | 6 | 3 | 0 | 7 | 4 | 6 | 6 | 7 | 0 | 53 |
| Guar-gum | 3 | 0 | 0 | 3 | 2 | 0 | 6 | 0 | 0 | 3 | 0 | 0 | 17 |
| Liquorice | 55 | 25 | 17 | 38 | 48 | 0 | 32 | 5 | 6 | 5 | 28 | 6 | 265 |
| Maltol | 36 | 7 | 3 | 50 | 14 | 0 | 20 | 8 | 9 | 19 | 8 | 10 | 184 |
| Menthol | 10 | 4 | 0 | 6 | 2 | 1 | 4 | 1 | 3 | 2 | 5 | 0 | 38 |
| Propylene glycol | 302 | 126 | 76 | 310 | 203 | 0 | 136 | 33 | 54 | 78 | 209 | 77 | 1604 |
| Sorbitol | 35 | 21 | 4 | 17 | 9 | 0 | 13 | 7 | 6 | 9 | 16 | 0 | 137 |
| Titanium dioxide | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 890 | 361 | 226 | 809 | 596 | 69 | 422 | 103 | 167 | 215 | 618 | 189 | 4665 |
From summary of listed CAS numbers corresponding to a priority additive.
For RYO, the most frequently reported priority additives among RYO were propylene glycol, glycerol and cocoa, with over 600 notifications (Figure 2B). Propylene glycol was the most frequently notified priority additive, ranging from 3.4% in Lithuania to 8.2% in the Netherlands, with a mean proportion of 6.3% of all reported ingredients for all countries. Glycerol was the second priority additive most frequently notified in RYO, ranging from 2.3% in Lithuania and Latvia to 5.6% in the Netherlands, with a mean proportion of 3.9% of all notifications on ingredients for all countries.
Figure 2B.
Overall reports of priority additives* in RYO in EU-CEG from 22 June 2016 to 21 October 2019, by type of priority additive
Completeness of the variables: ‘CAS number’ compared to flagged ‘Priority additive’
In order to identify the real number of priority additives notified we used the variable ‘CAS number’ and compared with the variable ‘priority additive’ (true/false). Table 4 shows the differences in the completeness of the variables ‘CAS number’ and flagging as a ‘priority additive’ (true/false). The latter was underreported in most of the notifications of cigarettes and RYO. In summary, the proportion of underreporting flagging of priority additives ranged from 15.9% in Malta to 41.3% in Lithuania, the mean proportion of underreporting of the variable ‘priority additive’ for the 10 countries together was 24.7%.
Table 4.
Comparison of number of priority additives identified from ‘CAS number’ variable versus ‘Priority additive’ variable in notifications active in EU-CEG for ingredients in cigarettes and RYO from 22 June 2016 to 21 October 2019 in 10 countries
| Country | Priority additives from CAS a n | Priority additives from the variable ‘priority additive’ b n | Unknown valuesc % |
|---|---|---|---|
| BE | 10322 | 8436 | 18.3 |
| CZ | 14576 | 11165 | 23.4 |
| DK | 6785 | 5173 | 23.8 |
| FR | 14595 | 10828 | 25.8 |
| IT | 15813 | 10958 | 30.7 |
| LT | 3749 | 2199 | 41.3 |
| LV | 3893 | 2415 | 38.0 |
| MT | 4188 | 3524 | 15.9 |
| NL | 10775 | 8580 | 20.4 |
| SI | 9345 | 7534 | 19.4 |
| Total | 94041 | 70812 | 24.7 |
Number of priority additives identified from CAS number in notifications active in EU-CEG for ingredients in cigarettes and RYO.
Number of priority additives identified from the variable ‘priority additive’ (true/false) in notifications active in EU-CEG for ingredients in cigarettes and RYO.
Proportion of missing values for the variable ‘priority additive’ (true/false) compared to CAS in notifications active in EU-CEG for ingredients in cigarettes and RYO.
Completeness of the variable ‘cigarette characterizing flavor’ in all reported ingredients in notifications of cigarettes
The variable ‘cigarette characterizing flavor’ was almost 100% complete in notifications of ingredients of all countries (with the exception of Italy that had 291 missing values) out of 105317 notifications active in EU-CEG for ingredients in cigarettes.
Completeness of the variables ‘toxicological data available’ and ‘having studies on toxicity and CMR properties’ among products containing priority additives active in EU-CEG in cigarettes and RYO
The completeness of both variables informing about the existence or not of toxicological data or studies on toxicity and CMR properties was 100%. For cigarettes, all 10 countries mentioned to some degree an absence of toxicological data or absence of studies on toxicity and CMR properties, ranging from 16.7% not having information on toxicological data and 16.6% not having studies on toxicity and CMR properties in Malta to 48.8% and 46.9% in Latvia, respectively. For RYO, the proportion of notifications among priority additives as ‘not having toxicological data available’ and ‘not having studies on Tox-CMR’ across countries, ranged from 17.9% not having ‘toxicological data available’ nor ‘having studies on toxicity and CMR properties’ in Slovenia, to 100% without toxicological data nor studies in Lithuania (Table 5).
Table 5.
Number and proportion of notifications on priority additives as ‘not having toxicological data available’ or ‘not having studies on Tox-CMR’ in notifications active in EU-CEG for ingredients in cigarettes and RYO from 22 June 2016 to 21 October 2019 in 10 countries
| Countries | Cigarettes | Roll-your-own | ||||
|---|---|---|---|---|---|---|
| Priority additives identified from the variable ‘priority additive’a n | Not having tox data b n (%) | Not having studies on Tox-CMRc n (%) | Priority additives identified from the variable ‘priority additive’a n | Not having tox data b n (%) | Not having studies on Tox-CMR c n (%) | |
| BE | 7771 | 1434 (18.4) | 1390 (17.9) | 665 | 172 (25.9) | 172 (25.9) |
| CZ | 10931 | 2643 (24.2) | 2567 (23.5) | 234 | 44 (18.8) | 44 (18.8) |
| DK | 4966 | 1389 (28.0) | 1343 (27.0) | 207 | 101 (48.8) | 101 (48.8) |
| FR | 10310 | 2827 (27.4) | 2748 (26.6) | 518 | 108 (20.8) | 108 (20.8) |
| IT | 10654 | 2935 (27.5) | 2782 (26.1) | 304 | 113 (37.2) | 113 (37.2) |
| LT | 2139 | 826 (38.6) | 774 (36.2) | 60 | 60 (100.0) | 60 (100) |
| LV | 2307 | 1125 (48.8) | 1081 (46.9) | 108 | 77 (71.3) | 77 (71.3) |
| MT | 3348 | 558 (16.7) | 555 (16.6) | 176 | 58 (32.9) | 58 (32.9) |
| NL | 8062 | 1594 (19.8) | 1551 (19.2) | 518 | 131 (25.3) | 131 (25.3) |
| SI | 7367 | 2175 (29.5) | 2158 (29.3) | 167 | 30 (17.9) | 30 (17.9) |
| Total | 67855 | 17506 (25.8) | 16949 (25.0) | 2957 | 894 (30.2) | 894 (30.2) |
Number of priority additives identified from the variable ‘priority additive’ (true/false) in notifications active in EU-CEG for ingredients in cigarettes.
Number of priority additives identified from the variable ‘priority additive’ (true/false) for which the variable ‘toxicological data available’ is ‘No toxicological data available’.
Number of priority additives identified from the variable ‘priority additive’ (true/false) for which the variable ‘Ingredient Tox-CMR’ is ‘No existence of any CMR related study’.
DISCUSSION
To the best of our knowledge, this is the first study describing tobacco products notifications in the EU-CEG database among a large number of EU MS. EU-CEG data on tobacco products may have been analyzed at the country level but not many publications are available, except for some analysis of e-cigarette liquids and flavors marketed in the Netherlands and the United Kingdom9-11 and a descriptive article of the products’ notifications in Spain12.
Noticeably, there is much variability of tobacco product types across the European countries. Whilst the tobacco market is led by a few transnational companies with national subsidiaries, our analysis of 12 countries has shown that notifications of products vary at large between countries. This variation could be an indicator of different dynamics in the notification of tobacco products and their introduction to the market, possibly in reaction to different and country-specific policies to place tobacco products in the market, or to production or composition differences.
Cigars were the most frequently notified tobacco product in 8 out of 12 EU MS. This finding is not aligned with the Eurobarometer 2020 special report for 27 countries on attitudes of Europeans towards tobacco and e-cigarettes13 which identified boxed cigarettes as the most popular choice among smokers while only relatively small proportions smoke cigarillos, cigars, or pipes. It is possible that the high frequency of notifications for cigars also reflects production changes within this product category.
Our study identified misreporting in the flagging of priority additives. Overall, the mean proportion of ingredients notified with an unknown or missing CAS number were small, while in the contrary products studied in our assessment indicated that on average 1 in 4 CAS numbers which refer to a priority additive were not flagged as such by the notifying party. Future effort should focus on automatically flagging as priority additives ingredients for which the submissions within EU-CEG indicate a CAS number that corresponds to one of the priority additives under regulatory scrutiny. We also noted a high cross-country variability of the notification of priority additives in cigarettes and RYO. Our analysis indicated that Greece is a clear outlier with very low proportions of priority additives, as identified by their CAS number (2.7% in cigarettes and 5% in RYO). This indicates the need for quality control mechanisms at either the central or EU MS level.
The number of priority additives flagged by the manufacturers or distributors answering ‘true’ to the variable ‘priority additive’ is much lower than the number obtained searching for the 30 CAS numbers9 linked to the 15 priority additives within the EU-CEG dataset. This indicates that the introduction of data within the EU-CEG disregarded the flagging of the variable ‘priority additive (true/false)’. This situation may lead to an increased workload for regulatory authorities responsible for analyzing and monitoring notifications in the EU-CEG database as regular data analyses based on binary variables such as ‘priority additive, (true/false)’ are quicker than having to handle complex files with the selection of all CAS numbers accounting for priority additives. One positive finding was that almost all the CAS numbers identified after selecting from the variable priority ‘additive= true’ were truly priority additives.
It is also required by the EU-TPD to have ‘toxicological data available’ on priority additives in cigarettes and RYO1. Our analyses revealed a high number of absences of toxicological data for these additives. It is not clear whether this absence of toxicological data is due to an actual lack of data available to the manufacturers or due to manufacturers responding negatively to this question, regardless of the availability of data that they may provide in another area of the reporting system by submitting specific files but not in parallel properly flagging the variable ‘toxicological data available’. Since these fields (CAS number, priority additive, cigarette characterizing flavor, toxicological data available, and existence of studies on CMR properties) are mandatory, we recommend that the EU-CEG system should be improved to not allow submissions including empty fields (i.e. missing information) or information not coherent with the TPD (e.g. ‘cigarette characterizing flavor’ should always be responded as false and ‘toxicological data available’ should always be responded as true).
Limitations
Among the limitations of the present study, we have not presented the trends by any defined time period (e.g. by years). Such an analysis for a long period of time of several years could provide useful information to infer future tendencies. Moreover, such an analysis comparing trends between countries could also be of help to advance the introduction or modification of tobacco products if early trends could be identified in some countries. Another important task to verify compliance to the TPD is the qualitative analysis of the files inserted as ‘attachments’ within the EU-CEG system. However, this task was not part of the objectives of the JATC and the current analysis. This analysis might have allowed assessing if reported inexistence of toxicological data or studies on toxicity or CMR properties among priority additives was real or just a reporting error related to the specific variables analyzed. The handling of the large data files beyond the direct outputs that the EU-CEG platform can provide, requires high level programming and computing skills. Moreover, the merging of files from different countries is complex and secondary to complex agreements on confidentiality. These situations have, however, been overcome thanks to the framework established by the JATC that has made a complex analysis of a large set of EU-CEG data possible.
CONCLUSIONS
This descriptive analysis shows that there are differences among EU MS in the number of types of tobacco products notified. The timely monitoring of the EU-CEG data could be used by government officials to assess, at the first instance, product compliance to the TPD. Additional filters and automated checks before submission would further increase the utility of EU-CEG for regulatory purposes.
ACKNOWLEDGEMENTS
We acknowledge the MS and partners of the JATC that agreed to share the national EU-CEG data for joint analyses. The authors thank Jon Aldazábal for helping with data handling and formatting.
CONFLICTS OF INTEREST
The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none was reported.
FUNDING
This study is derived from the Joint Action on Tobacco Control funded by the European Union's Health Program (2014-2020) under the grant agreement - 761297. The Tobacco Control Research Group at ICO-IDIBELL (DCP, OT, EF) is partly supported by the Ministry of Universities and Research, Government of Catalonia (2017SGR319). DCP, OT and EF also thank CERCA Programme Generalitat de Catalunya for the institutional support to IDIBELL. The authors declare that the content of this publication represents the views of the authors only and is their sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the Consumers, Health, Agriculture and Food Executive Agency or any other body of the European Union. The European Commission and the Agency do not accept any responsibility for use that may be made of the information it contains.
ETHICAL APPROVAL AND INFORMED CONSENT
Ethical approval and informed consent were not required for this study, as existing data from the EU-CEG for 12 tobacco products from 22 June 2016 to 21 October 2019 for 12 EU countries were used.
DATA AVAILABILITY
The data supporting this research are available from the authors on reasonable request.
AUTHORS’ CONTRIBUTIONS
DCP, OT and EF conceptualized the manuscript. DCP analyzed the data and discussed the results with OT and EF. DCP wrote the initial draft. All the authors provided comments and suggestions to the versions of the manuscript and approved the final version. EF is the guarantor.
PROVENANCE AND PEER REVIEW
Not commissioned; externally peer reviewed.
REFERENCES
- 1.Directive 2014/40/EU of the European Parliament and the Council of April 2014 on the approximation of the laws, regulations and administrative provisions of the Member States concerning the manufacture, presentation and sale of tobacco and related products and repealing Directive 2001/37/EC. Official Journal of the European Union. 2014;127:1–38. Accessed December 10, 2021. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014L0040&from=EN. [Google Scholar]
- 2.European Commission Providing information on tobacco products, e-cigarettes and refill containers - The EU Common Entry Gate (EU-CEG) 2021. Accessed December 10, 2021. https://ec.europa.eu/health/eu-common-entry-gate-eu-ceg/providing-information-tobacco-products-e-cigarettes-and-refill-containers-eu-common-entry-gate-eu_en.
- 3.Commission Implementing Decision (EU) 2015/2186 of 25 November 2015 establishing a format for the submission and making available of information on tobacco products (notified under document C(2015) 8162) Official Journal of the European Union. 2015;312:5–18. Accessed December 10, 2021. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015D2186&from=EN. [Google Scholar]
- 4.European Commission, Consumers, Health, Agriculture and Food Executive Agency . Study on the assessment of available evidence on toxicity, addictiveness and attractiveness of ingredients contained in tobacco and related products on the basis of information submitted by the industry in the context of reporting obligations introduced by Directive 2001/37/EC. Vardavas C, Behrakis P; 2017. Accessed December 10, 2021. https://data.europa.eu/doi/10.2818/65608. [Google Scholar]
- 5.Regulation 45/2001 of the European Parliament and the Council of December 2000 on the protection of individuals with regard to the processing of personal data by the Community institutions and bodies and on the free movement of such data. Accessed December 10, 2021. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02001R0045-20010201&from=EN.
- 6.Commission Implementing Decision (EU) 2016/787 of 18 May 2016 laying down a priority list of additives contained in cigarettes and roll-your-own tobacco subject to enhanced reporting obligations. Official Journal of the European Union. 2016;131:88–90. Accessed December 10, 2021. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016D0787&from=EN. [Google Scholar]
- 7.European Commission . Joint Action on Tobacco Control. Accessed December 10, 2021. https://jaotc.eu/ [Google Scholar]
- 8.European Commission TPD submission data dictionary tobacco products. Version 1.1.1. 2017. Accessed December 10, 2021. https://circabc.europa.eu/faces/jsp/extension/wai/login.jsp.
- 9.Havermans A, Krüsemann EJZ, Pennings J, de Graaf K, Boesveldt S, Talhout R. Nearly 20 000 e-liquids and 250 unique flavour descriptions: an overview of the Dutch market based on information from manufacturers. Tob Control. 2021;30(1):57–62. doi: 10.1136/tobaccocontrol-2019-055303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krüsemann EJZ, Havermans A, Pennings JLA, et al. Comprehensive overview of common e-liquid ingredients and how they can be used to predict an e-liquid’s flavour category. Tob Control. 2021;30:185–191. doi: 10.1136/tobaccocontrol-2019-055447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyakutsikwa B, Britton J, Bogdanovica I, Boobis A, Langley T. Characterising vaping products in the United Kingdom: an analysis of Tobacco Products Directive notification data. Addiction. 2021;116(9):2521–2528. doi: 10.1111/add.15463. [DOI] [PubMed] [Google Scholar]
- 12.Rebollar A, Perea MD, Doncel JC, Panero J, Gómez-Chacón MC. [Tobacco products and related: EU-CEG European Portal]. Article in Spanish. Rev Esp Salud Publica. 2019;93:e201908077. Accessed December 10, 2021. https://www.sanidad.gob.es/biblioPublic/publicaciones/recursos_propios/resp/revista_cdrom/VOL93/ORIGINALES/RS93C_201908077.pdf. [PubMed] [Google Scholar]
- 13.European Commission, Directorate-General for Communication, Directorate-General for Health and Food Safety Attitudes of Europeans towards tobacco and electronic cigarettes. 2018. Accessed December 10, 2021. https://data.europa.eu/doi/10.2875/804491.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this research are available from the authors on reasonable request.