Abstract
Background:
Non-fatal opioid-related overdoses have increased significantly over the past two decades and there have been increasing reports of brain injuries and/or neurocognitive impairments following overdose events. Limited preclinical research suggests that opioid overdoses may cause brain injury; however, little is known about such injuries in humans. The purpose this systematic review is to summarize existing studies on neurocognitive impairments and/or brain abnormalities associated with an opioid-related overdose in humans.
Methods:
PubMed, Web of Science, Ovid MEDLINE and PsyINFO were searched, without year restrictions, and identified 3099 articles. An additional 24 articles were identified by reviewing references. Articles were included if they were published in English, reported study findings in humans, included individuals 18 years of age or older, and reported an objective measure of neurocognitive impairments and/or brain abnormalities resulting from an opioid-related overdose. Six domains of bias (selection, performance, attrition, detection (two dimensions) and reporting were evaluated and themes were summarized.
Results:
Seventy-nine journal articles, published between 1973–2020, were included in the review. More than half of the articles were case reports (n = 44) and there were 11 cohort studies, 18 case series, and 6 case-control studies. All of the studies were categorized as at-risk of bias, few controlled for confounding factors, and methodological differences made direct comparisons difficult. Less than half of the studies reported toxicology results confirming an opioid-related overdose; 64.6 % reported brain MRI results and 27.8 % reported results of neuropsychological testing. Only two studies had within subject comparative data to document changes in the brain possibly associated with an overdose. Despite these limitations, existing publications suggest that brain injuries and neurocognitive impairments are associated with opioid overdose. Additional research is needed to establish the incidence of overdose-related brain injuries and the potential impact on functioning, as well as engagement in treatment of substance use disorders.
Conclusions:
Respiratory depression is a defining characteristic of opioid overdose and prolonged cerebral hypoxia may cause brain injuries and/or neurocognitive impairments. The onset, characteristics, and duration of such injuries is variable and additional research is needed to understand their clinical implications.
Keywords: Opioid-related overdose, Neurocognitive impairments, Brain abnormalities, Systematic review
1. Introduction
Worldwide, the large majority of deaths associated with illicit drug use has been attributed to opioids (80 %; World Drug Report, 2020). The United States (U.S.) is leading the world in rates of illicit opioid use and its associated morbidity and mortality. These increases in drug overdose deaths in the U.S. have occurred in “waves.” The first wave beginning in the late 1990s was characterized by increased prescribing and subsequent illicit use of pharmaceutically manufactured opioids, the second wave starting in 2010 was related to a shift to heroin use, and the third wave starting in 2013 was due to increased sales and use of illicitly manufactured fentanyl and its chemical analogs (Ciccarone, 2019). Most recently, a fourth wave appears to be emerging with an increase in psychostimulants, such as methamphetamines, involved in overdose deaths (Cano and Huang, 2021). Opioids have been identified in many of these stimulant-related fatalities. In 2020, the provisional number of drug-related overdose deaths in the U.S. is 88,295; the highest number ever recorded (Ahmad et al., 2021; O’Donnell et al., 2020).
Deaths resulting from opioid-related overdose are only the tip of the iceberg however, as there are significantly more non-fatal overdoses. Estimates of non-fatal overdoses are variable and likely underestimate the true prevalence because survivors and bystanders or lay responders often do not seek medical care (Seal et al., 2003). Australian researchers have estimated that 20–30 non-fatal overdoses occur for every overdose death (Darke et al., 2003). More recent U.S.-based studies estimate that 4–18 % of opioid-related overdoses treated in the pre-hospital or hospital setting result in a fatality (Chang et al., 2020; Lasher et al., 2019; Lowder et al., 2020; Dunn et al., 2010). Further, 46–92 % of people who use opioids illicitly either have experienced a non-fatal overdose or have witnessed an overdose during their lifetime (Winstanley et al., 2020; Bennett et al., 2011; Doe-Simkins et al., 2009). The influx of illicitly manufactured fentanyl, with markedly higher potency and low cost, has led to a greater risk of opioid overdose. In addition, early data suggest that opioid overdoses increased during the COVID-19 pandemic (Slavova et al., 2020). Despite the high incidence of non-fatal overdoses, the associated morbidity has not been adequately characterized by empirical research.
An opioid overdose is classically defined as exposure to an opioid compound that results in the clinical signs of depressed mental status and/or unconsciousness, slow and shallow breathing, and constricted pupils. Opioid-induced respiratory depression may cause cerebral hypoxia (Kiyatkin, 2019; White and Irvine, 1999) and if untreated, it could lead to cardiorespiratory arrest and/or death. For the purposes of this review, however, we will define an overdose as the use of an opioid in an amount that results in over-sedation and/or respiratory depression requiring intervention by medical or non-medical persons. The necessary interventions usually include reversal of the opioid agonist effects by naloxone administration, respiratory support, and monitoring of mental status and breathing. Because the effects of naloxone are of short duration, a recurrence of opioid-induced respiratory depression may emerge. In the absence of effective interventions, fatalities may occur over a period of time that varies across cases depending mainly on opioid potency and efficacy and individual tolerance to opioids, with the likely critical time window for interventions to be administered ranging from a few minutes to just over an hour (Boyer, 2012).
Exogenous opioids induce respiratory depression through various sites in the cerebral cortex, subcortical regions and brainstem, targeting both voluntary and involuntary breathing neural circuits. Some of the respiratory pathways are parallel to, and overlap with analgesic pathways, likely increasing the propensity to overdose, as someone seeking pain relief may be inadvertently inhibiting their respiration (Montandm and Slutsky, 2019). Additionally, fentanyl and its analogs appear to have a greater propensity to produce chest wall rigidity that contributes to respiratory depression (Gill et al., 2019). It is likely that the hypoxic period before overdose reversal causes toxic injuries to multiple organs including the central nervous system (CNS), even when a fatal outcome is averted (Feng et al. 2015). In animal models, a large intravenous challenge dose of heroin produces a rapid and pronounced drop in oxygen levels in the brain (Solis et al., 2017). Several case studies in humans have suggested that opioid overdose can cause acute or delayed onset of leukoencephalopathy, as well as damage to brain areas sensitive to hypoxic ischemia including the hippocampus and cerebellum (Milroy and Parai, 2011; Salgado et al., 2010). Reduced oligodendroglia and myelin, and white matter damage and vacuolation have also been described (Milroy and Parai, 2011; Salgado et al., 2010; Barnett et al., 2001; Huisa et al., 2013).
In addition to histopathological changes in the brain, neurocognitive impairments have been reported following opioid overdose. One study showed memory and motor impairments in opioid-naïve rats after acute methadone-induced apnea that persisted for several days (Ahmad-Molaei et al., 2018). Neurocognitive sequelae from opioid overdoses in humans have not been rigorously or systematically studied; however, evidence of potential brain injuries have been documented as early as 1969 (Brust and Richter, 1976). In case studies, overdoses involving methadone or heroin resulted in a constellation of neurocognitive impairments that persisted for over three months or up to more than a year (Salgado et al., 2010; Barnett et al., 2001; Huisa et al., 2013). Investigators have described various findings after overdose reversal that ranged from amnesia, inattention and forgetfulness, to gait impairment and incontinence. The purpose of this systematic review is to summarize existing studies on brain abnormalities and neurocognitive impairments associated with opioid-related overdoses.
2. Methods
2.1. Search strategy
This systematic review followed the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines (Moher et al., 2009) and the review protocol was registered (https://osf.io/v7ujg) on the Open Science Framework (OSF) Registry. PubMed, Web of Science, Ovid MEDLINE and PsyINFO were used to search for research articles indexed by August 2020. The search terms included 1) overdose, poisoning, drug-related death, toxicity or intoxication; 2) cognition, cognitive, hypoxia/hypoxic, anoxic/anoxia, brain injury, brain damage, neurocognition, neurocognitive, neurological, or encephalopathy; and 3) opioid, opiate, heroin, fentanyl, methadone, opium, hydrocodone, oxycodone, tramadol, narcotic or carfentanil (see Supplement, Table 1 for complete reporting of search strategy). Other related articles were identified by reviewing the references of articles identified in the search. Articles were included in the review if 1) the article was published in English, 2) reported data from human research studies or clinical cases, 3) included data on individuals 18 years of age or older, 4) reported data on cognition or brain abnormalities based on neuropsychological testing, neuroimaging or clinical diagnosis, and 5) the cognitive impairments or brain abnormalities were described as occurring subsequent to a suspected or confirmed overdose involving opioids. Adolescents were excluded from this study because more than 95 % of individuals that present to the emergency department for a non-fatal opioid-related overdose or die of an opioid-related overdose are older than 18 years of age (Vivolo-Kanter et al., 2020; Scholl et al., 2019). Suspected intentional and unintentional overdoses were defined as respiratory depression reversed by naloxone administration, self-reported use of opioids prior to the overdose, bystander reports of opioid use or evidence of opioid use observed at the scene of the overdose. Articles were excluded if data were based only on histology and if the article was classified as a non-systematic review, a commentary, or news. Studies that focused only on neurocognitive impairments associated with opioid use, without specification of overdose, were excluded. Similarly, articles that focused on neurocognitive impairments or brain abnormalities resulting from overdoses not involving opioids were excluded.
2.2. Quality assessment
Existing tools to assess bias of studies included in systematic reviews are study design specific; few apply to multiple study designs and none can be used to assess case reports/series, cohort, and case-control studies (Sanderson et al., 2007; Viswanathan et al., 2012). Hence, six recommended domains of bias were assessed including selection (does the design or analysis control for confounding factors), performance (did the researchers rule out concurrent intervention or unintended exposure(s) that could bias results), attrition (differential nonresponse, dropout, or loss to follow-up), detection (two dimensions: overdose detection using toxicology and brain injury detection using imaging or neurocognitive assessments), and reporting (conclusion supported by study design & objective measures) (Viswanathan et al., 2012). The scale used to rank bias risk in the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) was applied to each domain (high risk, low risk, unclear) (Whiting et al., 2011). The QUADAS-2 is not scored, rather if all domains are rated as ‘low risk’ then the study is overall rated as ‘low risk’. If one or more domains are rated as ‘high risk’ or ‘unclear’, then the study overall is rated as ‘at risk of bias’ (Whiting et al., 2011).
2.3. Data synthesis
Search engine results were imported into EndNote, which was used to identify and delete duplicate articles. One author (ELW) initially screened the titles and abstracts to identify those potentially meeting the inclusion criteria. After initial screening, eligible articles were imported into COVIDENCE systematic review software (COVIDENCE Systematic Review Software). At least two authors (ELW, JJM, FC) reviewed every article abstract to determine eligibility and a third author resolved disagreements. Articles requiring full review were uploaded into COVIDENCE and at least two authors (ELW, JJM, FC) independently reviewed the articles and reason for exclusion was coded using pre-specified criteria. If consensus was not achieved, a third or fourth author (SDC) would review and make a final determination.
An Excel case extraction form was used to facilitate synthesis of the study findings and it captured information on the year of publication, country in which the study was conducted, the sample size (overall and cases relevant to this review), study design (based on classifications used elsewhere (Zara et al., 2000)), diagnosis (when relevant), reported behavioral symptoms exhibited among cases, the measurement of cognitive impairments or brain abnormalities (MRI, CT scan, neuropsychological testing or clinical observation), positive drug toxicology reports (drugs and number positive), outcome and time to outcome. Reported symptoms were categorized as cognitive, dyskinesia, dysautonomic, mutism, unresponsiveness or emotional/psychiatric. These characteristics are reported at the study level because diagnosis, symptoms, drug/alcohol use and outcomes were not systematically reported across relevant cases/subjects. Three authors (ELW, JJM, FC) reviewed the case extraction form to ensure agreement on content and coding. The Excel data were imported into Stata/SE Version 15.1 (StataCorp., 2017) in order to generate descriptive statistics. The overall characteristics of the articles are reported and other factors were thematically organized into the following categories: 1) drugs used at time of the overdose, 2) assessment of cognitive impairments and brain abnormalities, 3) diagnosis and symptoms, and 4) outcomes.
3. Results
A total of 3099 articles were identified in the search (see Supplement, Table 1 for details) and 24 articles were identified by reviewing reference lists (see Fig. 1). Duplicate articles and those reporting the same study results, were excluded. There were several case reports of sudden onset of amnesia that were summarized in two articles by Barash et al. (Barash and Kofke, 2018; Barash et al., 2020). Articles summarized in Barash et al. (2018) were excluded (Benoilid et al., 2013; Small et al., 2016; Barash et al., 2017; Haut et al., 2017; Duru et al., 2018). Barash et al. (2020) expands upon their 2018 review to define opioid-associated amnestic syndrome and minimal details were provided on the cases. Hence any articles not included in the Barash et al. 2018 paper that met the inclusion criteria for this review, were included (Taylor et al., 2019); noting that most had already been identified by our search (Butler et al., 2019; Jasne et al., 2019; Ramirez-Zamora et al., 2015). One study reported baseline (Dassanayake et al., 2012) and follow-up results (Oxley et al., 2015) in two articles and both articles were included as the results were non-duplicative. After reviewing 2270 abstracts, 2067 articles were excluded and an additional 124 articles were excluded after reviewing the full text. Two hundred thirty-nine articles were excluded because they did not describe research results or were non-systematic reviews. The vast majority of the articles were excluded because they either were not relevant to overdose (n = 1072), the overdose did not involve opioids or were reported in aggregate as drug overdoses (n = 165), or brain abnormalities or cognitive functioning were not reported (n = 103). Seventy-nine articles were included this review.
Fig. 1.
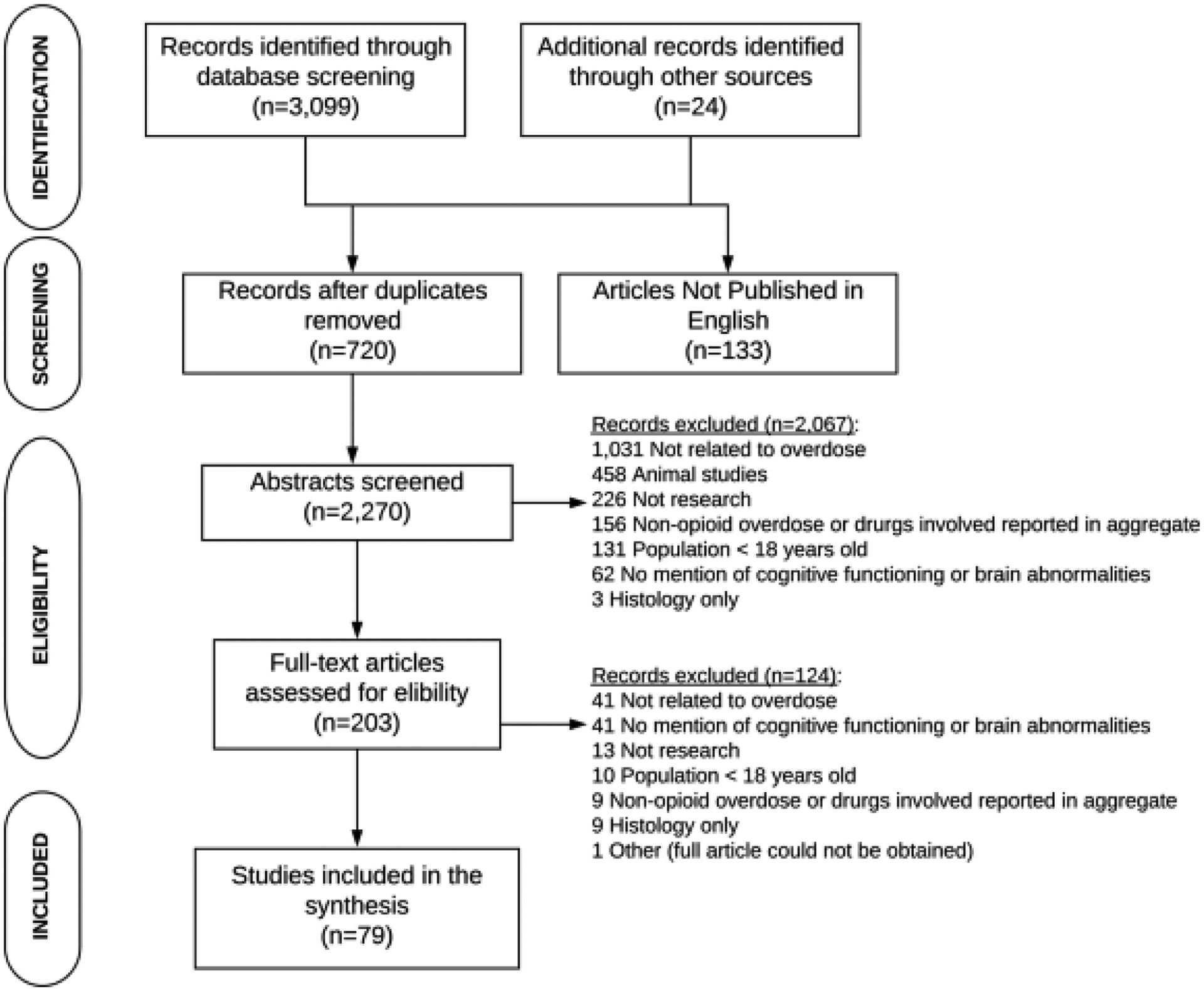
PRISMA Flow Diagram of Studies of Neurocognition and/or Brain Abnormalities Resulting from Opioid-Related Overdoses.
The 79 studies (see Table 1) reported results for 16,330 subjects and, after excluding control cases, 3498 subjects remained. The vast majority of cases (n = 2433) were from a single retrospective cohort study conducted in Canada (Morrow et al., 2019). The earliest publication was in 1973 (Richter et al., 1973) and three years later two other studies were reported (Ginsberg et al., 1976; Pearson et al., 1975); the next study was not published until more than a decade later (Revesz and Geddes, 1988). The majority of studies (60.8 %) were published in 2010 or later. Twenty-one countries were represented in the studies; 41.8 % of the articles reported studies conducted in the U.S. and 11.4 % reported studies conducted in Australia. More than half of the articles were case reports (n = 44) and there were 18 case series, 11 cohort studies, and 6 case-control studies. Six studies reported only post-mortem results of forensic brain autopsies of decedents presumed to die of drug (opioid) or overdose-related causes of death (Alturkustani et al., 2017; Andersen and Skullerud, 1999; Muller et al., 2018; Pearson et al., 1975; Oehmichen et al., 1996; Yarid and Harruff, 2015). Fifty-two studies reported brain MRI results, whereas only 23 studies reported the results of neurocognitive testing.
Table 1.
Study characteristics.
| First author (Pub. year)/ Country | Methods | Overall sample (Sample with opioids & OD) | Diagnosis (Symptoms) | Measure (s) impairment | Drugs confirmed by toxicology (Num. of subjects) | Outcomes (Time frame) | Key findings/Summary |
|---|---|---|---|---|---|---|---|
| Achamallah et al. (2019)/ USA | Case series | 3 (3) | Acute leukoencephalopathy (seizures, tremors, dysautonomia, rigidity, hyperthermia, tachycardia, fever, hypertension) | Brain MRI, CT scan | Opioids (3) Amphetamine (1) Benzodiazepines (1) Cannabis (1) Cocaine (1) |
Persistent impairments (Month 2-Year 5) |
|
| Adrish et al. (2014)/ USA | Case report | 1 (1) | Renal failure, rhabdomyolysis, gluteal compartment syndrome (lower extremity weakness) | CT scan | Opioids (1) | No brain injury |
|
| Alquist et al. (2012)/ USA | Case report | 1 (1) | Leukoencephalopathy (N/A) | Brain autopsy | Oxymorphone (1) Cocaine (1) Benzodiazepines (1) Cannabis (1) |
Death (Day 5) |
|
| Alturkustani et al. (2017)/ England | Retrospective cohort | 5 (5) | Leukoencephalopathy (N/A) | Brain autopsy | Opiates (4) Cocaine (3) Cannabis (1) Benzodiazepines (1) |
N/A |
|
| Andersen and Skullerud (1999)/ Norway | Retrospective cohort | 100 (92) | Brain abnormalities (N/A) | Brain autopsy | Unavailable | N/A |
|
| Arciniegas et al. (2004)/ USA | Case report | 1 (1) | Delayed encephalopathy (memory impairments, speed processing, and executive functioning; incontinence, ideational dyspraxia, agraphesthesia) | Brain MRI, NP | Unavailable | Improved (Month 5) |
|
| Barash and Kofke (2018)/ USA | Case series | 22 (21) | Sudden onset of amnesia (memory impairment, inattention, impaired executive functioning, problems with orientation) |
Brain MRI, NP | Opioids (9) Cocaine (5) Cannabis (2) Cannabinoids (2) Benzodiazepine (3) Amphetamines (3) Buprenorphine (1) Barbiturates (1) Fentanyl (4) Norfentanyl (1) |
Persistent impairments (4) (Week 8-Month 22) |
|
| Barnett et al. (2001)/ Australia | Case report | 1 (1) | Delayed post-anoxic encephalopathy (rhabdomyolysis, incontinence, mutism, immobility) | Brain MRI, CT scan, NP | Unavailable | Partial recovery (Month 9) |
|
| Beeskow et al. (2018)/ German | Case report | 1 (1) | Delayed leukoencephalopathy (odd behavior, agitation, apathy, myoclonus, mutism) | Brain MRI | Unavailable | Partial recovery (Month 9) |
|
| Bileviciute-Ljungar et al. (2014)/ Sweden | Case report | 1 (1) | Delayed leukoencephalopathy (cognitive impairment, gait impairment, spasticity, mania, aggressive, disorientation, incontinence) | Brain MRI, CT scan, NP | Methadone (1) Benzodiazepines (1) |
Partial recovery (Month 7) |
|
| Butler et al. (2019)/ USA | Case report | 1 (1) | Acute-onset anterograde amnesia (tachycardia, disorientation, memory impairment) | Brain MRI, CT scan, NP | None | Persistent impairments (Month 4) |
|
| Carroll et al. (2012)/ Switzerland | Case report | 1 (1) | Delayed leukoencephalopathy (inappropriate behavior, apathy, incontinence, gait impairment, impaired executive functioning) | Brain MRI, CT scan, NP | Unavailable | Recovered (Month 6) |
|
| Cerase et al. (2011)/ Italy | Case report | 1 (1) | Leukoencephalopathy (comatose, severe physical & cognitive impairment) | Brain MRI, CT scan | Methadone (1) | Recovered (Month 3) |
|
| Chang et al. (2009)/ Taiwan | Case report | 1 (1) | Delayed leukoencephalopathy (comatose, delirium, bradykinesia, rigidity, incontinence, mutism, memory impairment) | Brain MRI, CT scan, NP | Unavailable | Partial recovery (Month 5) |
|
| Cheng et al. (2019)/ Taiwan | Case series | 2 (2) | Delayed leukoencephalopathy (mutism, bradykinesia, bradyphrenia, odd behavior, incontinence, confusion) | Brain MRI, CT scan | Heroin (2) | 1 partial recovery (Month 1), 1 partial recovery (Month 2), |
|
| Chute and Smialek (2002)/ USA | Case series Case report |
10 (5) 1 (1) |
Subarachnoid hemorrhage, encephalopathy, cerebral edema, cardiorespiratory arrest (comatose) | CT scan, brain autopsy | Opiates (5), Cocaine (1), Alcohol (1) Unavailable |
Death (Day 1–6) |
|
| Cohen and Hack (2019)/ USA | Brain abnormalities (unresponsive, rigidity) | CT scan & brain MRI | Recovery (Day 5) |
|
|||
| Corliss et al. (2013)/ USA | Case report | 1 (1) | Brain abnormalities, rhabdomyolysis (N/A) | Brain autopsy | Benzodiazepines (1) Cannabinoids (1) Methadone (1) |
Death (24 h) |
|
| Corre et al. (2013)/France | Case report | 1 (1) | Brain abnormalities (comatose) | CT Scan & brain MRI | Methadone (1), Cannabis (1), Alcohol (1), Benzodiazepines (1) | Recovered (Unknown) |
|
| Darke et al. (2000)/ Australia | Case-control | 60 (30) | N/A | NP | Unavailable | Unknown |
|
| Dassanayake et al. (2012)/ Australia | Case-control | 175 (25) | N/A | NP | Unavailable | Unknown |
|
| Fatovich et al. (2008)/ Australia | Prospective cohort | 224 (224) | N/A | Clinical observation | Unavailable | Variable |
|
| Ferrari et al. (2020)/ Italy | Case report Case report |
1 (1) 1 (1) |
Overdose (unresponsive, liver failure, polyneuropathy) | CT scan | Tramadol (1) Methadone (1) |
Unknown |
|
| Gheuens et al. (2010)/ Belgium | Spongiform leukoencephalopathy (hypertonic quadriparesis, catatonia, mutism, unable to eat) | Brain MRI, NP | Persistent impairments (Month 3) |
|
|||
| Ginsberg et al. (1976)/ USA | Case series | 3 (1) | Leukoencephalopathy (N/A) | Brain autopsy | Unavailable | Death (Day 23) |
|
| Gottfried et al. (1997)/ USA | Case report | 1 (1) | Anterograde and retrograde amnesia (odd behavior, confusion, incontinence, myoclonic jerks) | Brain MRI | Benzodiazepines (1) Amphetamines (1) Opiates (1) |
Persistent Impairments (Month 6) |
|
| Grigorakos et al. (2010)/ Greece | Retrospective cohort | 42 (42) | N/A | Clinical observation | Heroin (42) | Variable (ICU discharge) |
|
| Gupta et al. (2009)/ Bahrain | Case report | 1 (1) | Spongiform leukoencephalopathy (unresponsive, tachypnea, altered mental status, bradycardia, facial paresis) | CT scan, Brain MRI | Opioids (1), Benzodiazepines (1) | Partial (Month 6) |
|
| Haghighi-Morad et al. (2020)/ Iran | Case series | 10 (6) | Encephalopathy (intoxication, confusion, neurological deficits) | Brain MRI | Methadone (6), Opiates (2) | Unknown |
|
| Hill et al. (2000)/ Canada | Case report | 1 (1) | Leukoencephalopathy (comatose) | CT Scan, Brain MRI | Opioid (1) | Death (Day 26) |
|
| Holyoak et al. (2014)/ Australia | Case series | 2 (1) | Toxic leukoencephalopathy (hypoxia, hypoglycemia) | CT scan, Brain MRI | Unavailable | Death (Day 60) |
|
| Hsu et al. (2009)/ Taiwan | Case report | 1 (1) | Rhabdomyolysis and stroke (cognitive impairments, aphasia, limb weakness) | Brain MRI | Opioids (1) | Persistent impairments (Week 2) |
|
| Huisa et al. (2013)/ USA | Case series | 2 (2) | Delayed leukoencephalopathy (comatose, confusion, spasticity, myoclonus, insomnia, hallucinations) | Brain MRI, NP | Methadone (1) | 1 Full recovery, 1 Persistent impairments (Year 1) |
|
| Jasne et al. (2019)/ USA | Case series | 6 (4) | Encephalopathy, amnesia, acute cerebellar edema, Obstructive hydrocephalus (unconscious, hypoxic, cyanosis) | Brain MRI, CT scan | Unavailable | 1 Death (Hospital), 1 Partial recovery (Month 1), 1 Persistent impairments (Month 6) |
|
| Jensen et al. (1990)/ Denmark | Case series | 2 (2) | Ischemic stroke (comatose, cyanosis, paralysis, aphasia, hemianopia) | Brain scintigraphy, CT scan | Unavailable | 1 Persistent impairment (Year 1), 1 Persistent impairment (Month 15) |
|
| Khot et al. (2007)/ USA | Case report | 1 (1) | Delayed post-hypoxic demyelination (bradyphrenia, memory deficits, abulia, akinetic, mutism, rigidity) | CT scan, Brain MRI, Brain autopsy |
Morphine (1), Codeine (1) | Death (Day 66) |
|
| King et al. (2015)/ USA | Case report | 1 (1) | Delayed post hypoxic leukoencephalopathy (odd behavior, memory deficits incontinence, parkinsonism, bradyphrenia, apathy, mutism) | Brain MRI, CT scan | Unavailable | Persistent impairments (Week 10) |
|
| Koch et al. (2018)/ German | Case report | 1 (1) | Cerebral edema (Comatose, tachycardia, and hypothermia) | CT scan | U-47,700 (1) Benzodiazepines (1) |
Death (Day 6) |
|
| Koksel et al. (2019)/ USA | Case series | 87 (20) | Leukoencephalopathy | Brain MRI | Unavailable | N/A |
|
| Kuhlman and Gwathmey (2018)/ USA | Case series | 26 (1) | Gluteal compartment syndrome (leg pain, numbness) | Brain MRI | Opiates (1) | No brain injury |
|
| Landais (2014)/ France | Case report | 1 (1) | Memory impairment (Hypoxemia, partial consciousness & memory deficits) | Brain MRI, NP | Unavailable | Partial recovery (Month 10) |
|
| Lefaucheur et al. (2017)/ France | Case report | 1 (1) | Leukoencephalopathy (cognitive impairments, anosognosia, apraxia, memory impairment) | Brain MRI, NP | Unavailable | Recovery (Month 4) |
|
| Loftsgard et al. (2017)/ USA | Case report | 1 (1) | Hypoxic-ischemic brain injury (multiorgan failure, respiratory failure) | CT scan, brain autopsy | Opioids (1) Benzodiazepines (1) |
Death (Day 4) |
|
| Long et al. (2013)/ China | Case report | 1 (1) | Leukoencephalopathy (comatose, hydrocephalus) | Brain MRI, CT scan | Opiates (1) | Recovered (Month 1) |
|
| McDonald et al. (2013)/ Australia | Case-control | 225 (148) | N/A | NP | Unavailable | Unknown |
|
| Meyer (2013)/ USA | Case report | 1 (1) | Delayed leukoencephalopathy (incontinence, lethargic, socially withdrawn) | CT scan, Brain MRI | Unavailable | Recovered (Year 1) |
|
| Molloy et al. (2006)/ England | Case report | 1 (1) | Delayed leukoencephalopathy (confusion, inattention) | Brain MRI, NP | Unavailable | Recovered (Month 9) |
|
| Morales Odia et al. (2010)/ USA | Case report | 1 (1) | Encephalopathy, severe cerebellitis, hydrocephalus (confusion, gait impairment, delirium, agitation, disorientation) | Brain MRI, CT scan | Morphine (1) Benzodiazepines (1) |
Persistent impairments (Month 3) |
|
| Morrow et al. (2019)/ Canada | Retrospective cohort | 14,011 (14,011) | Encephalopathy (N/A) | Clinical observation | Unavailable | Variable (During hospitalization) |
|
| Muller et al. (2018)/ Germany | Case control | 26 (14) | Brain abnormalities (N/A) | Brain autopsy | Unavailable | N/A |
|
| Mumma et al. (2009)/ USA | Case report | 1 (1) | Cardiac arrest (comatose, mild dysarthria, cognitive impairment, rhabdomyolysis, slurred speech) | CT Scan, Brain MRI, NP | Opiates (1), Benzodiazepines (1) | Recovered (Month 8) |
|
| O’Brien et al. (2009)/ Ireland | Retrospective cohort | 43 (21) | N/A | NP | Unavailable | Variable (Months 20–37) |
|
| O’Brien and Todd (2009)/ Australia | Case series | 10 (10) | Brain injury (N/A) | CT scans, Brain MRI, NP | Unavailable | Variable (Year 2–4) |
|
| Oehmichen et al. (1996)/ Germany | Retrospective cohort | 180 (168) | N/A | Brain autopsy | Unavailable | N/A |
|
| Ormseth et al. (2019)/ USA | Retrospective cohort | 300 (15) | N/A | Brain MRI, CT scan | Opioids (15) Polysubstance (10) Cocaine (3) |
Variable (During hospitalization) |
|
| Oxley et al. (2015)/ Australia | Case-control | 36 (21) | N/A | NP | Unavailable | Variable (During hospitalization) |
|
| Pearson et al. (1975)/ USA | Case-control | 11 (11) | N/A | Brain autopsy | Morphine (8) Methadone (3) |
N/A |
|
| Pfister et al. (2016)/ USA | Retrospective cohort | 178 (178) | N/A | Clinical observation | Opioids (178) | Deaths - 18 (During hospitalization) |
|
| Pirompanich and Chankrachang (2015)/ Thailand | Case report | 1 (1) | Spongiform leukoencephalopathy (confusion, mutism, akinetic, spastic, hyperreflexive) | Brain MRI | Heroin (1) Morphine (1) | Persistent impairments (Month 6) |
|
| Quinn and Abbott (2014)/ USA | Case report | 1 (1) | Delayed leukoencephalopathy (incontinence, nausea, confusion, odd behavior) | Brain MRI | Opioids (1) | Persistent impairments (Year 1) |
|
| Ramirez-Zamora et al. (2015)/ USA | Case report | 1 (1) | Brain abnormalities (confusion, apathy, inattention, memory deficits) | Brain MRI | Opioids (1) Cannabinoids (1) |
Persistent impairments (Week 1) |
|
| Revesz and Geddes (1988)/ England | Case report | 1 (1) | Encephalopathy (cardiac arrest, hypothermia, pulmonary edema) | CT scan, brain autopsy | Methadone (1), Amphetamines (1) | Death (Day 6) |
|
| Richter et al. (1973)/ USA | Case series | 42 (Undetermined) | Delayed encephalopathy, dementia, Parkinsonian syndrome (hemiballistic movements) | Unknown | Unavailable | Unknown |
|
| Rizzuto et al. (1997)/ Italy | Case report | 1 (1) | Delayed spongiform encephalopathy (comatose, heart failure, rhabdomyolysis, renal failure) | Brain MRI, brain autopsy | Unavailable | Death (Week 6) |
|
| Salazar and Dubow (2012)/ USA | Case report | 1 (1) | Delayed leukoencephalopathy (confusion, lethargy, akinetic mutism) | CT scan, Brain MRI | Morphine (1) | Partial recovery (Day 40) |
|
| Shprecher et al. (2008)/ USA | Case series | 3 (3) | Delayed leukoencephalopathy, pneumonia (hypotension, memory deficits, disorientation, gait impairment, delusional, catatonia, automatisms, incontinence, mutism, loss of interest, fatigue, inattention, catatonia, imbalance) | Brain MRI, NP | Unavailable | 1 Partial recovery (Year 1), 1 Partial recovery (Week 38), 1 Persistent impairments (Week 8) |
|
| Shu et al. (2016)/ Australia | Case report | 1 (1) | Dystonia-Parkinsonism (involuntary movements, gait impairment, jaw spasms, dystonia, bradykinesia) | Brain MRI | Unavailable | Persistent declines (Year 3) |
|
| Singh and Saini (2015)/ Singapore | Case report | 1 (1) | Leukoencephalopathy (inattention, stiffness, mutism, developed bradykinesia, tremors) | Brain MRI | None | Persistent impairments (Week 6) |
|
| Switzer et al. (2020)/ Canada | Case report | 1 (1) | Delayed leukoencephalopathy, amnesia (rigidity, tremor, gait impairments, inattention, unconscious, memory deficits) | Brain MRI, NP | Fentanyl (1) | Partial recovery (Month 5) |
|
| Taheri et al. (2011)/ Iran | Retrospective cohort | 403 (108) | Brain abnormalities (comatose) | Clinical observation, CT scan | Unavailable | Variable (During hospitalization) |
|
| Taylor et al. (2019)/ Canada | Case report | 1 (1) | Amnesia (unconscious, confusion) | Brain MRI, NP | Opiates (1) | Persistent deficits (Month 4) |
|
| Torralba-Moron et al. (2017)/ Spain | Case series | 3 (2) | Delayed leukoencephalopathy (unresponsive, amnesia, bradypnea, muscle spasm, inattention, fever) | Brain MRI, CT scan | Methadone (2), Alprazolam (1), Alcohol (1) | 1 Persistent deficits (Discharge), 1 Death (Day 40) |
|
| Vendrame and Azizi (2007) /USA | Case report | 1 (1) | Brain abnormalities (comatose, cyanosis, gait impairment) | Brain MRI | Opiates (1) Cocaine (1) Cannabis (1) |
Partial improvements (Month 3) |
|
| Vila and Chamorro (1997)/ Spain | Case series | 2 (2) | Stroke (comatose, ballistic movements) | Brain MRI, CT scan | Unavailable | Recovered (Day 3 & Month 9) |
|
| Villella et al. (2010)/ Italy | Case report | 1 (1) | Spongiform leukoencephalopathy (comatose) | Brain MRI | None | Partial improvements (Year 2) |
|
| Voigt (2013)/ Germany | Case report | 1 (1) | Brain damage (hypoventilation, bradycardia, hypotension, miosis) | CT scan, Brain MRI | None | No improvements (Day 30) |
|
| Wijdicks (2005)/ USA | Case report | 1 (1) | Encephalopathy (comatose, apathy, memory deficits, rigidity, monotonic, gait impairment) | Brain MRI, CT scan | Opioids (1) Benzodiazepines (1) |
No improvements (Month 3) |
|
| Yarid and Harruff (2015)/ USA | Retrospective cohort | 27 (10) | N/A | Brain autopsy | Opiates (8) Benzodiazepines (2) Methadone (2) Cocaine (2) Methamphetamines (1) |
N/A |
|
| Zamora et al. (2015)/ USA | Case series | 5 (4) | Delayed leukoencephalopathy (incontinence, mutism, ataxia, odd behaviors, executive functioning impairment) | Brain MRI | Unavailable | Death -1 Recovery -2, Persistent impairments 1 (Week 2-Week 5) |
|
NOTES: AMA = against medical advice; ATL = acute toxic leukoencephalopathy; CNS-D = Central Nervous System Depressants; CNS-ND = Central Nervous System non-depressant; DPHL = delayed post hypoxic leukoencephalopathy; ECT = electroconvulsive therapy; ED = emergency department; ICU = intensive care unit; MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment; OUD = opioid use disorder; NP = neuropsychological testing; ROSC = return of spontaneous circulation (ROSC); SSRI = selective serotonin reuptake inhibitor; TMT-B = Trail making test, part B; WASI-III = Wechsler Abbreviated Scale of Intelligence 3rd Edition.
3.1. Quality of studies
All of the studies were determined to have at least one source of bias (see Table 2). Ninety-one percent of the articles were categorized as being at high risk of selection bias, which in part reflects the study designs and that randomization was not used in any of the studies. Among the six case-control studies, only three (Darke et al., 2000; McDonald et al., 2013; Muller et al., 2018) used matching to reduce selection bias. Eighty-two percent of the articles were categorized as being at high risk of performance bias because there were inadequate controls for exposures, other than a drug overdose, that could have contributed to the observed cognitive impairments or brain abnormalities. In terms of detection bias, 48.7 % of the studies were rated as being at high risk of bias due to their measurement of an overdose and only 11.4 % were rated as being at high risk of measuring either cognitive functioning or brain abnormalities. Four studies reported brain abnormalities or cognitive impairment based only on clinical observation (Morrow et al., 2019; Fatovich et al., 2008; Grigorakos et al., 2010; Pfister et al., 2016). Only two studies (Yarid and Harruff, 2015; Zamora et al., 2015) fully acknowledged the potential for confounding and/or bias and hence did not make diagnostic conclusions.
Table 2.
Study quality assessment.
| First author (Pub. year) | Study design (Sample size) | Selection biasa | Performance biasb | Attrition biasc | Detection bias (overdose)d | Detection bias (brain abnormalities/CF)d | Reporting biase |
|---|---|---|---|---|---|---|---|
| Achamallah et al. (2019) | Case series (N = 3) | High risk | High risk | High risk | Low risk | Low risk | Unclear |
| Adrish et al. (2014) | Case report (N = 1) | High risk | High risk | High risk | Low risk | Unclear | High risk |
| Alquist et al. (2012) | Case report (N = 1) | High risk | High risk | Low risk | Low risk | Low risk | Unclear |
| Alturkustani et al. (2017) | Retrospective cohort (N = 5) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Andersen and Skullerud (1999) | Retrospective cohort (N = 100) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Arciniegas et al. (2004) | Case report (N = 1) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Barash and Kofke (2018) | Case series (N = 22) | High risk | High risk | High risk | Low risk | Low risk | Unclear |
| Barnett et al. (2001) | Case report (N = 1) | High risk | Low risk | Low risk | High risk | Low risk | High risk |
| Beeskow et al. (2018) | Case report (N = 1) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Bileviciute-Ljungar et al. (2014) | Case report (N = 1) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Butler et al. (2019) | Case report (N = 1) | Low risk | High risk | Low risk | High risk | Low risk | Unclear |
| Carroll et al. (2012) | Case report (N = 1) | High risk | Low risk | Low risk | High risk | Low risk | High risk |
| Cerase et al. (2011) | Case report (N = 1) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Chang et al. (2009) | Case report (N = 1) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Cheng et al. (2019) | Case series (N = 2) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Chute and Smialek (2002) | Case series (N = 5) | High risk | High risk | Low risk | High risk | High risk | High risk |
| Cohen and Hack (2019) | Case report (N = 1) | High risk | High risk | High risk | High risk | Low risk | High risk |
| Corliss et al. (2013) | Case report (N = 1) | High risk | Unclear | Low risk | Low risk | Low risk | High risk |
| Corre et al. (2013) | Case report (N = 1) | High risk | High risk | High risk | Low risk | Low risk | High risk |
| Darke et al. (2000) | Case-control (N = 60) | Low risk | Low risk | Low risk | High risk | Low risk | High risk |
| Dassanayake et al. (2012) | Case-control (N = 175) | Low risk | Low risk | Low risk | Unclear | Low risk | High risk |
| Fatovich et al. (2008) | Prospective observational (N = 224) | Unclear | High risk | Low risk | High risk | High risk | High risk |
| Ferrari et al. (2020) | Case report (N = 1) | High risk | High risk | High risk | Low risk | High risk | High risk |
| Gheuens et al. (2010) | Case report (N = 1) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Ginsberg et al. (1976) | Case series (N = 3) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Gottfried et al. (1997) | Case report (N = 1) | High risk | High risk | Low Risk | Low risk | Low risk | High risk |
| Grigorakos et al. (2010) | Retrospective cohort (N = 42) | High risk | High risk | Low risk | Low risk | High risk | High risk |
| Gupta et al. (2009) | Case report (N = 1) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Haghighi-Morad et al. (2020) | Case series (N = 10) | High risk | High risk | High risk | Low risk | Low risk | High risk |
| Hill et al. (2000) | Case report (N = 1) | High risk | Low risk | Low risk | Low risk | Low risk | High risk |
| Holyoak et al. (2014) | Case series (N = 2) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Hsu et al. (2009) | Case report (N = 1) | High risk | Low risk | High risk | Low risk | Low risk | High risk |
| Huisa et al. (2013) | Case series (N = 2) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Jasne et al. (2019) | Case series (N = 6) | High risk | High risk | High risk | High risk | Low risk | High risk |
| Jensen et al. (1990) | Case series (N = 2) | High risk | High risk | High risk | High risk | High risk | High risk |
| Khot et al. (2007) | Case report (N = 1) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| King et al. (2015) | Case report (N = 1) | High risk | Low risk | Low risk | High risk | Low risk | High risk |
| Koch et al. (2018) | Case report (N = 1) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Koksel et al. (2019) | Case series (N = 87) | High risk | High risk | High risk | High risk | Low risk | High risk |
| Kuhlman and Gwathmey (2018) | Case series (N = 26) | High risk | High risk | High risk | High risk | Low risk | High risk |
| Landais (2014) | Case report (N = 1) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Lefaucheur et al. (2017) | Case report (N = 1) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Loftsgard et al. (2017) | Case report (N = 1) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Long et al. (2013) | Case report (N = 1) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| McDonald et al. (2013) | Case-control (N = 225) | Low risk | Low risk | Low risk | High risk | Low risk | High risk |
| Meyer (2013) | Case report (N = 1) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Molloy et al. (2006) | Case report (N = 1) | High risk | Low risk | Low risk | High risk | Low risk | High risk |
| Morales Odia et al. (2010) | Case report (N = 1) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Morrow et al. (2019) | Retrospective cohort (N = 14,011) | High risk | High risk | Low risk | High risk | High risk | High risk |
| Muller et al. (2018) | Case control (N = 25) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Mumma et al. (2009) | Case Report (N = 1) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| O’Brien et al. (2009) | Retrospective cohort (N = 43) | High risk | High risk | Low risk | Unclear | Low risk | High risk |
| O’Brien and Todd (2009) | Case series (N = 10) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Oehmichen et al. (1996) | Cohort (N = 180) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Ormseth et al. (2019) | Retrospective cohort (N = 300) | High risk | High risk | Low risk | High risk | High risk | High risk |
| Oxley et al. (2015) | Prospective case-control (N = 36) | Low risk | High risk | High risk | Unclear | Low risk | Unclear |
| Pearson et al. (1975) | Case-control (N = 11) | Low risk | High risk | Low risk | High risk | Low risk | Unclear |
| Pfister et al. (2016) | Retrospective cohort (N = 178) | High risk | High risk | Low risk | Low risk | High risk | High risk |
| Pirompanich and Chankrachang (2015) | Case report (N = 1) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Quinn and Abbott (2014) | Case report (N = 1) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Ramirez-Zamora et al. (2015) | Case report (N = 1) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Revesz and Geddes (1988) | Case report (N = 1) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Richter et al. (1973) | Case series (N = 42) | High risk | High risk | High risk | High risk | High risk | High risk |
| Rizzuto et al. (1997) | Case report (N = 1) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Salazar and Dubow (2012) | Case report (N = 1) | High risk | Low risk | Low risk | Low risk | Low risk | High risk |
| Shprecher et al. (2008) | Case series (N = 3) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Shu et al. (2016) | Case report (N = 1) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Singh and Saini (2015) | Case report (N = 1) | High risk | High risk | Low risk | High risk | Low risk | High risk |
| Switzer et al. (2020) | Case report (N = 1) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Taheri et al. (2011) | Cohort (N = 403) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Taylor et al. (2019) | Case report (N = 1) | High risk | Low risk | High risk | Low risk | Low risk | High risk |
| Torralba-Moron et al. (2017) | Case series (N = 3) | High risk | High risk | High risk | High risk | Low risk | High risk |
| Vendrame and Azizi (2007) | Case report (N = 1) | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Vila and Chamorro (1997) | Case series (N = 2) | High risk | High risk | High risk | High risk | Low risk | High risk |
| Villella et al. (2010) | Case report (N = 1) | High risk | Low risk | Low risk | High risk | Low risk | High risk |
| Voigt (2013) | Case report (N = 1) | High risk | High risk | High risk | Low risk | Low risk | High risk |
| Wijdicks (2005) | Case report (N = 1) | High risk | Unclear | Low risk | Low risk | Low risk | High risk |
| Yarid and Harruff (2015) | Retrospective cohort (N = 27) | High risk | High risk | Low risk | Unclear | Low risk | Low risk |
| Zamora et al. (2015) | Retrospective case series (N = 5) | High risk | High risk | High risk | Low risk | Low risk | Low risk |
Selection bias: does the design or analysis control for confounding factors.
Performance bias: did the researchers rule out concurrent intervention or unintended exposure that could bias results.
Attrition bias: was there differential nonresponse, dropout, or loss to follow-up.
Detection bias: was toxicological confirmation of opioid-related overdose reported and was neuroimaging, psychological testing or autopsy used to confirm brain abnormalities/ cognitive impairments.
Reporting bias: conclusion supported by study design & objective measures CF = cognitive functioning.
3.2. Themes
3.2.1. Drugs used at the time of overdose
Forty-one studies reported toxicological drug results at the time of the overdose. Among the 330 subjects with drug toxicology results reported, only 3 were opioid negative. The majority reported the result as positive for opiates/opioids, without specification of opioid compounds. Eighteen subjects had a positive toxicology for methadone, 5 for fentanyl, 1 norfentanyl, and 1 reported a novel opioid synthetic compound (U-4770) (Koch et al., 2018). Non-opioid positive toxicology results that were reported included 17 subjects who tested positive for benzodiazepines, 15 subjects who tested positive for cocaine, 12 who tested positive for cannabis/cannabinoids, and 7 who tested positive for amphetamines/methamphetamines. The majority of articles (59.5 %) did not report on whether the cases had used alcohol, had a history of alcohol use, or had toxicology results positive for alcohol. Only eight articles reported toxicological testing for alcohol (Adrish et al., 2014; Barash and Kofke, 2018; Butler et al., 2019; Cerase et al., 2011; Cheng et al., 2019; Corliss et al., 2013; Corre et al., 2013; Pearson et al., 1975) and of these only two studies reported positive toxicology (Corre et al., 2013; Pearson et al., 1975).
3.2.2. Assessment of neurocognitive impairments or brain abnormalities
Forty-four percent of articles reported results from CT scans, 65.8 % reported brain MRI results and 29.1 % reported results of neuropsychological testing. Fourteen case reports/series used postmortem brain autopsy results to confirm brain abnormalities in decedents. Only two studies reported having pre-overdose brain imaging or neurocognitive testing which could be used to accurately measure change after the overdose (Butler et al., 2019; Molloy et al., 2006). Eighteen studies reported both brain MRI and neuropsychological testing. Of the 22 studies that reported findings from neuropsychological testing, 18 % did not specify the names of the tasks administered (only specifying the domain assessed). For studies that did report the specific tasks, there was a wide range in the extensiveness of the neuropsychological evaluations across the studies: some included only screening measures (e.g. Mini-Mental State Exam, Montreal Cognitive Assessment; 7 studies), some administered select measures (5 studies), and others utilized more comprehensive batteries (6 studies). When multiple neuropsychological measures were administered and specified, task selection also greatly varied as no more than two studies utilized the same neuropsychological measure. Few of the studies reported the specific neuropsychological test performed and none utilized the same test.
3.2.3. Diagnoses and symptoms
Sixty-seven studies reported subjects’ diagnoses which included encephalopathy/leukoencephalopathy (40 studies), amnesia (7 studies), stroke (3 studies), other brain injury/damage/abnormalities (17 studies) and other medical conditions (7 studies). Seven articles reported subjects having amnesia, most of which were summarized by Barash et al. (2019; 2020) and three additional articles were identified in this review (Gottfried et al., 1997; Switzer et al., 2020; Torralba-Moron et al., 2017). Several other studies reported memory deficits that were not specifically diagnosed as amnesia (Arciniegas et al., 2004, Chang et al., 2009; Cheng et al., 2019; Khot et al., 2007; King et al., 2015; Landais, 2014; Lefaucheur et al., 2017; Meyer, 2013; Shprecher et al., 2008; Switzer et al., 2020; Wijdicks, 2005). Across these 67 studies, the most frequently reported symptoms included the following: cognitive symptoms (n = 58 subjects), dyskinesia (n = 40 subjects), dysautonomia (n = 27 subjects), unresponsiveness (n = 23 subjects), emotional/psychiatric symptoms (n = 18 subjects), and mutism (n = 12 subjects). Specific cognitive deficits were related to: confusion/disorientation (n = 15), memory (n = 13), attention (n = 6), executive functions (n = 4), processing speed (n = 4), and speech (n = 3); 4 additional papers described unspecified cognitive deficits. Specific emotional/psychiatric symptoms included “odd” behavior (n = 6), apathy (n = 5), agitation/aggression (n = 3), inappropriate behavior, abulia, social withdrawal, and mania (n = 1 each). Two case reports found that two individuals did not have brain abnormalities following an overdose and no clinical observations suggesting cognitive impairment were reported (Adrish et al., 2014; Kuhlman and Gwathmey, 2018).
3.3. Outcomes and follow-up
The studies investigated clinical recovery after an overdose and the follow-up periods ranged from hospital discharge to five years. Eight studies reported outcomes at a year or greater follow-up (Achamallah et al., 2019; Barash and Kofke, 2018; Huisa et al., 2013; Jensen et al., 1990; Meyer, 2013; O’Brien et al., 2009; O’Brien and Todd, 2009; Quinn and Abbott, 2014; Shu et al., 2016; Villella et al., 2010). Among the forensic autopsy studies, data were not available on the timing of the overdose and the subjects’ death. Thirteen subjects were reported to have fully recovered during the follow-up window, which ranged from three days to one year (Carroll et al., 2012; Cerase et al., 2011; Cohen and Hack, 2019; Corre et al., 2013; Huisa et al., 2013; Koch et al., 2018; Lefaucheur et al., 2017; Long et al., 2013; Meyer, 2013; Molloy et al., 2006; Mumma et al., 2009; Vila and Chamorro, 1997; Zamora et al., 2015). Sixteen had a partial recovery at follow-up, which ranged from three to nine months (Arciniegas et al., 2004; Barnett et al., 2001; Beeskow et al., 2018; Bileviciute-Ljungar et al., 2014; Cheng et al., 2019; Chang et al., 2009; Gupta et al., 2009; Jasne et al., 2019; Landais, 2014; Salazar and Dubow, 2012; Shprecher et al., 2008; Switzer et al., 2020; Vendrame and Azizi, 2007; Villella et al., 2010). The studies reported 36 subject deaths that occurred either prior to hospital discharge or within 6 weeks of the overdose.
4. Discussion
Seventy-nine articles, reflecting studies conducted in 21 countries, documented 3496 subjects that possibly had neurocognitive impairments and/or brain abnormalities associated with an opioid-related overdose. Two case reports found no evidence of post-overdose brain injuries based on neuroimaging (Adrish et al., 2014; Kuhlman and Gwathmey, 2018). As overdose deaths have increased over the past two decades, so have the number of published articles documenting brain injuries. Significant methodological differences across these studies limit direct comparisons and all of the studies were categorized as being at risk of bias. The primary methodological weaknesses of these studies included limited control of confounding factors that could explain the observed neurocognitive impairments and/or brain abnormalities, limited availability of objective measures to confirm that opioid-related overdose occurred, and variable follow-up periods. The vague specification of study inclusion criteria and the limited number of opioid-positive toxicology reports are problematic. It is possible that some of the subjects in the studies were presumed to have experienced an opioid-related overdose because they had a history of opioid use and/or documented opioid use disorder without definitive evidence for an opioid overdose. The majority of individuals demonstrated persistent impairments at follow-up, and the overall number of deaths reported was 36 across all of the studies.
Twenty-nine of the studies reported a diagnosis of leukoencephalopathy, which is a form of encephalopathy that specifically affects white matter (Lyon et al., 2006). Spongiform leukoencephalopathy, a form of toxic leukoencephalopathy, occurs secondary to exposure to a wide variety of agents, including carbon monoxide poisoning and drugs of abuse, which subsequently damage white-matter tracts devoted to higher cerebral function (Filley and Kleinschmidt-DeMasters, 2001). Leukoencephalopathy can have an acute onset or a delayed onset that can occur 2–180 days after a hypoxic-ischemic brain injury (Arciniegas et al., 2004). Acute and delayed leukoencephalopathy, with and without spongiform, has been associated with inhalation of heroin fumes, which is known as ‘chasing the dragon’ (Wolters et al., 1982; Cordova et al., 2014; Kass-Hout et al., 2011; Blasel et al., 2010; Keogh et al., 2003). Most of the studies in this review reported a delayed onset of leukoencephalopathy; however, one recent case series reported sudden onset (Achamallah et al., 2019). Delayed-onset cases were less likely to report toxicological confirmation of opioid-related overdoses and it is largely unknown if an alternative exposure could have caused the observed brain injury. Some of the articles postulated that neurological impairments resulted from a particular method of opioid administration (e.g., inhalation or injection) rather than from an opioid overdose (Wijdicks, 2005; Filley and Kleinschmidt-DeMasters, 2001). Others have speculated that spongiform leukoencephalopathy is caused by an unidentified heroin adulterant or contaminant; however, there is insufficient evidence to support a causal mechanism (Cordova et al., 2014). Leukoencephalopathy associated with opioid use and/or overdose has been reported in multiple case reports dating as early as 1981 (Wolters et al., 1982), across multiple countries, involving different opioid compounds and various routes of administration (Alambyan et al., 2018).
Case reports of an “amnestic syndrome” associated with use of novel opioid synthetics have been reported in Massachusetts (Barash and Kofke, 2018; Small et al., 2016; Barash et al., 2017), California (Butler et al., 2019), West Virginia (Haut et al., 2017; Duru et al., 2018), and in France (Benoilid et al., 2013). Two cases were identified in this review that were not included in the amnesia cluster reported by Barash et al. (Barash and Kofke, 2018; Barash et al., 2020). Gottfried et al. (1997) reported a case of persistent anterograde and retrograde amnesia in a patient that experienced an overdose involving opioids, benzodiazepines, and amphetamines. A recent case report involving self-reported use of fentanyl, that was not confirmed by toxicology, described acute onset of anterograde amnesia (Butler et al., 2019). While Barash et al. make a strong case for a fentanyl-specific cause of the amnesia (Barash and Kofke, 2018), there is limited toxicological testing to confirm fentanyl as the explanatory factor. Several other articles reported that subjects experienced memory deficits, but few clinical details were provided on these deficits. Eight articles reported cases with memory problems lasting more than one month. Standard urine toxicology testing used in clinical practice infrequently differentiates opioid compounds and rarely includes novel opioid synthetics.
The period after a non-fatal overdose is not well studied in humans, yet it is a critical one when interventions to decrease morbidity and mortality can be made. These potential treatment options would likely have to be adequate for patients who may have developed overdose-related cognitive impairments. Questions remain over appropriate post-overdose clinical management and the role of neuropsychological testing when initiating treatment for OUD. The studies in this review provide insufficient evidence to estimate the prevalence of brain injuries among individuals who experience a non-fatal overdose. Given that the majority of the studies were case reports/series, it is likely that bias exists in reporting high acuity injuries and hence even less is known about mild cognitive impairments that may occur. Dassanayake et al.’ case control study (n = 175) found evidence of sufficient neurocognitive impairment among patients hospitalized for CNS-D-related overdose to conclude that they had an increased risk of motor vehicle accidents upon discharge (Dassanayake et al., 2012; Oxley et al., 2015).
Studies in this review rarely controlled for confounding factors that could explain brain injuries or neurocognitive impairments. Individuals who use drugs have a higher risk of traumatic brain injuries (Corrigan and Deutschle, 2008; McHugo et al., 2017), largely due to injuries sustained during periods of intoxication. Drugs may be adulterated or contaminated by substances known to be neurotoxic (Langston et al., 1983) or due to co-use of drugs known to be associated with neurocognitive deficits including alcohol and methamphetamines. Many individuals with OUD experience, on average, 3–6 non-fatal overdoses (Doe-Simkins et al., 2009; Heale et al., 2003; Winstanley et al., 2020; Sherman et al., 2008) and the effects of these overdoses could compound over time. In a rodent model, Zhu et al. (2005) found that the immature brain can tolerate longer periods of oxygen deprivation and hence age may influence the risk of overdose-related brain injuries. While a few case reports ruled out infectious diseases such as HIV or Hepatitis C (Barnett et al., 2001; Carroll et al., 2012; Hill et al., 2000; Oehmichen et al., 1996; Salazar and Dubow, 2012; Torralba-Moron et al., 2017; Villella et al., 2010), three reported cases did test positive for Hepatitis C (Carroll et al., 2012; Hsu et al., 2009; King et al., 2015); however, such testing was infrequently mentioned. Neurocognitive impairments in drug users, including extra-medical use of prescription opioids and heroin, may result from chronic use (Baldacchino et al., 2012; Gruber et al., 2007; Kroll et al., 2018) and it is difficult to disentangle compound-specific deficits in the context of polydrug use. There is limited research on the prevalence of neurocognitive impairments in individuals with OUD; one small cross-sectional study estimated that 39 % of patients seeking buprenorphine treatment had neurocognitive impairments (Arias et al., 2016). Four case reports tested for a “pseudodeficiency” of arylsulfatase A, which is caused by a genetic mutation that may make individuals more susceptible to post-hypoxic demyelination, and two of the cases tested positive (Barnett et al., 2001; Gottfried et al., 1997). There is also speculation that a cytochrome P4502D6 genetic polymorphism may increase the risk of leukoencephalopathy (Bach et al., 2012). However, there is insufficient evidence to understand the role that genes may play in increasing the risk of overdose-related brain injuries. Ideally, confirmation of an opioid-related overdose would be determined by a respiration rate less than 12 breaths per minute (Boyer, 2012) and an opioid-positive toxicology. Articles were included in this review if participants experienced an opioid-related overdose and if drug toxicology data was missing, other objective measures were considered (e.g., reversal of respiratory depression after naloxone administration, secondary reports of opioid use from bystanders). For example, one study reported a case of post-hypoxic leukoencephalopathy following a suicide attempt involving multiple drugs (Loftsgard et al., 2017). This study was excluded because it was unknown whether the drug overdose involved opioids and they reported that the patient was not responsive to naloxone. It would have been overly-restrictive to exclude studies without opioid-positive toxicology and yet relevant studies may have been excluded. Terminology has changed over time and across disciplines. For example, some of the older articles used the term ‘heroin intoxication’ which was described in such a way to be consistent with what we now define as an opioid overdose (Oehmichen et al., 1996). The lack of standardized language on this topic, as demonstrated by the variation in the diagnoses and symptoms described in this review, complicate identification of relevant studies. Importantly, this review was unable to accurately characterize the symptoms of overdose-related brain injuries as symptoms were not systematically reported across the studies.
Despite the limitations of the articles included in this review, we know that opioid overdose can cause cerebral hypoxia and anoxia and that brain injury can occur within 3 – 6 min of oxygen deprivation. There is increasing awareness of the potential for overdose-related brain injuries, as evidenced by a 2019 U.S. Department of Health and Human Services (DHHS) report on health outcomes associated with non-fatal overdose (Zibbel et al., 2019). Variability in the onset and duration of symptoms described in these studies may complicate timely identification of brain injuries in patients that received medical treatment for an overdose. Initial management often occurs in the pre-hospital setting. Time with inadequate respiration likely predicts the extent of brain injuries and it is unknown if that critical information is systematically captured in the patients’ electronic medical records (EMR). Emergency departments do not routinely conduct comprehensive toxicology on patients presenting with overdose and neurocognitive impairments in this population may be attributed to ongoing drug use, rather than associated specifically with an overdose. Because of the potential delayed onset of neurocognitive impairments, more complete documentation of the overdose event (e.g., Glasgow Coma Score; time, dose & route of naloxone administration; length of time unconscious) in the EMR may help determine whether the brain injury is related to cerebral hypoxia or anoxia. Clinicians may want to consider screening for acute brain injuries and/or neurocognitive impairments in individuals known to have experienced a prolonged period of hypoxia or anoxia due to an opioid overdose and consider monitoring for delayed onset for those with the highest risk. In clinical settings, comprehensive drug toxicology following an overdose could help identify specific opioid compounds and non-opioid drugs involved. These steps would be critical to determining, for example, whether fentanyl-related overdoses cause amnesia.
The findings of this review provide a strong rationale and directionality for future research. Rigorously designed prospective case control studies, that control for confounding factors, are needed to empirically measure changes in the brain and cognition that occur following opioid-related overdose. Importantly, standardized measures should be established to quantify overdose-related brain injuries to ensure comparability across studies. The incidence and prevalence of opioid-related brain injuries is needed to inform clinical care and post-overdose management, particularly in terms of whether screening for brain injuries is warranted in this population. The pharmacodynamic effects of opioid compounds are different and it is plausible that this explains differential risk of brain injuries. For example, fentanyl has a rapid onset that may increase the period of inadequate respiration and chest wall rigidity structurally restricts respiration (Suzuki and El-Haddad, 2017). Concomitant use of CNS depressants will impair reflexes and asphyxiation can occur in tandem with respiratory depression. Any fall or head trauma in the peri-overdose period may also affect overdose outcomes. In all, there is a high level of variability seen in overdose events. In order to adequately control for confounding factors, within-subject study designs are needed to compare baseline and post-overdose neurocognitive functioning.
5. Conclusions
This is the first study to systematically summarize existing research on brain injuries and neurocognitive impairment associated with opioid overdoses. Non-fatal opioid-related overdoses are becoming more common in the context of the current opioid epidemic and there is a high probability that these hypoxic events result in neurocognitive impairments that in turn increase the risk of poor treatment outcomes. The fourth wave of the opioid epidemic may increase the incidence of overdose related brain injuries due to the known neurotoxicity of methamphetamine. Yet, to date, there is no systematic empirical evidence on the incidence of overdose-related neurocognitive impairments and no definitive evidence stating whether or not neurocognitive impairments that result in poor treatment outcomes are attributable to overdose. The reviewed studies lack standardized inclusion criteria, failed to adequately control for confounding factors, and do not harmonize outcome measures. The results reported are not yet sufficient to draw conclusive evidence on the incidence, magnitude, and consequences of opioid overdose-related brain injuries. Therefore, further investigation into these types of impairments is warranted.
Supplementary Material
Role of funding source
Nothing declared.
Footnotes
Declaration of Competing Interest
The authors report no declarations of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.drugalcdep.2021.108838.
References
- Achamallah N, Wright RS, Fried J, 2019. Chasing the wrong dragon: a new presentation of heroin-induced toxic leukoencephalopathy mimicking anoxic brain injury. J Intensive Care Soc 20 (1), 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrish M, Duncalf R, Diaz-Fuentes G, Venkatram S, 2014. Opioid overdose with gluteal compartment syndrome and acute peripheral neuropathy. Am. J. Case Rep 15, 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad FB, Rossen LM, Sutton P, 2021. Provisional Drug Overdose Death Counts. National Center for Health Statistics. [Google Scholar]
- Ahmad-Molaei L, Hassanian-Moghaddam H, Farnaghi F, Tomaz C, 2018. Delayed-dependent impairments in memory and motor functions after acute methadone overdose in rats. Front. Pharmacol 9, 1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alambyan V, Pace J, Miller B, Cohen ML, Gokhale S, Singh G, Shun M, Hammond A, Ramos-Estebanez C, 2018. The emerging role of inhaled heroin in the opioid epidemic: a review. JAMA Neurol. 75 (11), 1423–1434. [DOI] [PubMed] [Google Scholar]
- Alquist CR, McGoey R, Bastian F, Newman W 3rd, 2012. Bilateral globus pallidus lesions. J. State Med. Soc 164 (3), 145–146. [PubMed] [Google Scholar]
- Alturkustani M, Ang LC, Ramsay D, 2017. Pathology of toxic leukoencephalopathy in drug abuse supports hypoxic-ischemic pathophysiology/etiology. Neuropathology 37 (4), 321–328. [DOI] [PubMed] [Google Scholar]
- Andersen SN, Skullerud K, 1999. Hypoxic/ischemic brain damage, especially pallidal lesions, in heroin addicts. Forensic Sci. Int 102, 51–59. [DOI] [PubMed] [Google Scholar]
- Arciniegas DB, Frey KL, Anderson CA, Brousseau KM, Harris SN, 2004. Amantadine for neurobehavioral deficits following delayed post-hypoxic encephalopathy. Brain Inj. 18 (12), 1309–1318. [DOI] [PubMed] [Google Scholar]
- Arias F, Arnsten JH, Cunningham CO, Coulehan K, Batchelder A, Brisbane M, Segal K, Rivera-Mindt M, 2016. Neurocognitive, psychiatric, and substance use characteristics in opioid dependent adults. Addict. Behav 60, 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach AG, Jordan B, Wegener NA, Rusner C, Kornhuber M, Abbas J, Surob A, 2012. Heroin spongiform leukoencephalopathy (HSLE). Clin. Neuroradiol 22, 345–349. [DOI] [PubMed] [Google Scholar]
- Baldacchino A, Balfour DJK, Passetti F, Humphris G, Matthews K, 2012. Neuropsychological consequences of chronic opioid use: a quantitative review and meta-analysis. Neurosci. Biobehav. Rev 36, 2056–2068. [DOI] [PubMed] [Google Scholar]
- Barash JA, Kofke WA, 2018. Connecting the dots: an association between opioids and acute hippocampal injury. Neurocase 24 (2), 124–131. [DOI] [PubMed] [Google Scholar]
- Barash JA, Somerville N, DeMaria A, 2017. Cluster of an unusual amnestic syndrome—Massachusetts, 2012–2016. MMWR 66, 76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash JA, Whitledge J, Watson CJ, Boyle K, Lim C, Lev MH, DeMaria A Jr., Ganetsky M, 2020. Opioid-associated amnestic syndrome: description of the syndrome and validation of a proposed definition. J Neurol Sci Oct 15 (417), 117048. [DOI] [PubMed] [Google Scholar]
- Barnett MH, Miller LA, Reddel SW, Davies L, 2001. Reversible delayed leukoencephalopathy following intravenous heroin overdose. J. Clin. Neurosci 8 (2), 165–167. [DOI] [PubMed] [Google Scholar]
- Beeskow AB, Oberstadt M, Saur D, Hoffman K, Lobsien D, 2018. Delayed post-hypoxic leukoencephalopathy (DPHL)—an uncommon variant of hypoxic brain damage in adults. Front. Neurol 9, 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AS, Bell A, Tomedi L, Hulsey EG, Kral AH, 2011. Characteristics of an overdose prevention, response and naloxone distribution program in Pittsburgh and Allegheny county, Pennsylvania. J. Urban Health 88 (6), 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoilid A, Collongues N, de Sez e J., Blanc F, 2013. Heroin inhalation-induced unilateral complete hippocampal stroke. Neurocase 19 (4), 313–315. [DOI] [PubMed] [Google Scholar]
- Bileviciute-Ljungar I, Haglund V, Carlsson J, von Heijne A, 2014. Clinical and radiological findings in methadone-induced delayed leukoencephalopathy. J. Rehabil. Med 46 (8), 828–830. [DOI] [PubMed] [Google Scholar]
- Blasel S, Hattingen E, Adelmann M, Nichtweiss M, Zanella F, Weidauer S, 2010. Toxic leukoencephalopathy after heroin abuse with heroin vapor inhalation: MR imaging and clinical features in three patients. Clin. Neuroradiol 20 (1), 48–53. [DOI] [PubMed] [Google Scholar]
- Boyer EW, 2012. Management of opioid analgesic overdose. N. Engl. J. Med 367 (2), 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust JCM, Richter RW, 1976. Stroke associated with addiction to heroin. J. Neurol. Neurosurg. Psychol 39, 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PM, Barash JA, Casaletto KB, Cotter DL, La Joie R, Geschwind MD, et al. , 2019. An opioid-related amnestic syndrome with persistent effects on hippocampal structure and function. J. Neuropsychiatry Clin. Neurosci 31 (4), 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano M, Huang Y, 2021. Overdose deaths involving psychostimulants with abuse potential, excluding cocaine: state-level differences and the role of opioids. Drug Alcohol Depend. 1 (January (218)), 108384. [DOI] [PubMed] [Google Scholar]
- Carroll I, Heritier Barras AC, Dirren E, Burkhard PR, Horvath J, 2012. Delayed leukoencephalopathy after alprazolam and methadone overdose: a case report and review of the literature. Clin. Neurol. Neurosurg 114 (6), 816–819. [DOI] [PubMed] [Google Scholar]
- Cerase A, Leonini S, Bellini M, Chianese G, Venturi C, 2011. Methadone-induced toxic leukoencephalopathy diagnosis and follow-up by magnetic resonance imaging including diffusion-weighted imaging and apparent diffusion coefficient maps. J. Neuroimaging 21 (3), 283–286. [DOI] [PubMed] [Google Scholar]
- Chang WL, Chang YK, Hsu SY, Lin GJ, Chen SC, 2009. Reversible delayed leukoencephalopathy after heroin intoxication with hypoxia: a case report. Acta Neurol. Taiwan 18 (3), 198–202. [PubMed] [Google Scholar]
- Chang HY, Ferris L, Eisenberg M, Krawczyk N, Schneider KE, Lemke K, et al. , 2020. The impact of various risk assessment time frames on the performance of opioid overdose forecasting models. Med. Care 58 (11), 1013–1021. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Chin SC, Chang YC, Wu T, Lim SN, Hsieh HY, et al. , 2019. Different routes of heroin intake cause various heroin-induced leukoencephalopathies. J. Neurol 266 (2), 316–329. [DOI] [PubMed] [Google Scholar]
- Chute DJ, Smialek JE, 2002. Pseudo-subarachnoid hemorrhage of the head diagnosed by computerized axial tomography: a postmortem study of ten medical examiner cases. J. Forensic Sci 47 (2), 360–365. [PubMed] [Google Scholar]
- Ciccarone D, 2019. The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. Int. J. Drug Policy 71, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Hack JB, 2019. Opioid overdose with parkinsonian features. Clin. Pract. Cases Emerg. Med 3 (4), 440–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova JP, Balan S, Romero J, Korniyenko A, Alviar CL, Paniz-Mondolfi A, Jean R, 2014. Chasing the dragon: new knowledge for an old practice. Am. J. Ther 21, 52–55. [DOI] [PubMed] [Google Scholar]
- Corliss RF, Mandal R, Soriano BJ, 2013. Bilateral acute necrosis of the globi pallidi and rhabdomyolysis due to combined methadone and benzodiazepine toxicity. Am. J. Forensic Med. Pathol 34 (1), 1–4. [DOI] [PubMed] [Google Scholar]
- Corre J, Pillot J, Hilbert G, 2013. Methadone-induced toxic brain damage. Case Rep. Radiol 2013, 602981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan JD, Deutschle JJ, 2008. The presence and impact of traumatic brain injury among clients in treatment for co-occurring mental illness and substance abuse. Brain Inj. 22 (3), 223–231. [DOI] [PubMed] [Google Scholar]
- Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org.
- Darke S, Sims J, McDonald S, Wickes W, 2000. Cognitive impairment among methadone maintenance patients. Addiction 95 (5), 687–695. [DOI] [PubMed] [Google Scholar]
- Darke S, Mattick RP, Degenhardt L, 2003. The ratio of non-fatal to fatal heroin overdose. Addiction 98 (8), 1169–1171. [DOI] [PubMed] [Google Scholar]
- Dassanayake TL, Michie PT, Jones A, Carter G, Mallard T, Whyte I, 2012. Cognitive impairment in patients clinically recovered from central nervous system depressant drug overdose. J. Clin. Psychopharmacol 32 (August (4)), 503–510. [DOI] [PubMed] [Google Scholar]
- Doe-Simkins M, Walley AY, Epstein A, Moyer P, 2009. Saved by the nose: bystander-administered intranasal naloxone hydrochloride for opioid overdose. Am. J. Public Health 99 (5), 788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. , 2010. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann. Intern. Med 152 (2), 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duru UB, Pawar G, Barash JA, Miller LE, Thiruselvam IK, Haut MW, 2018. An unusual amnestic syndrome associated with combined fentanyl and cocaine use. Ann. Intern. Med 168, 747–748. [DOI] [PubMed] [Google Scholar]
- Fatovich DM, Bartu A, Daly FFS, 2008. A prospective study of non-fatal heroin overdose. J. Subst. Use 13 (5), 299–307. [Google Scholar]
- Feng G, Luo Q, Guo E, Yao Y, Yang F, Zhang B, Longxuan L, 2015. Multiple organ dysfunction syndrome, an unusual complication of heroin intoxication: a case report and review of the literature. J. Clin. Exp. Pathol 8 (9), 11826–11830. [PMC free article] [PubMed] [Google Scholar]
- Ferrari F, Carletti A, Peroni N, Mongodi S, Esposito P, Orlando A, Mojoli F, Ronco C, Belliato M, A lotti G, 2020. Brief report: a case of tramadol overdose: extracorporeal life support and hemoperfusion as life-saving treatment. Blood Purif. 49 (4), 509–512. [DOI] [PubMed] [Google Scholar]
- Filley CM, Kleinschmidt-DeMasters BK, 2001. Toxic leukoencephalopathy. N. Engl. J. Med 345 (6), 425–432. [DOI] [PubMed] [Google Scholar]
- Gheuens S, Michotte A, Flamez A, De Keyser J, 2010. Delayed akinetic catatonic mutism following methadone overdose. Neurotoxicology 31 (6), 762–764. [DOI] [PubMed] [Google Scholar]
- Gill H, Kelly E, Henderson G, 2019. How the complex pharmacology of the fentanyls contributes to their lethality. Addiction 114 (9), 1524–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD, Hedley-Whyte ET, Richardson EP Jr., 1976. Hypoxic-ischemic leukoencephalopathy in man. Arch. Neurol 33 (1), 5–14. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Mayer SA, Shungu DC, Chang Y, Duyn JH, 1997. Delayed posthypoxic demyelination Association with arylsulfatase A deficiency and lactic acidosis on proton MR spectroscopy. Neurology 49 (5), 1400–1404. [DOI] [PubMed] [Google Scholar]
- Grigorakos L, Sakagianni K, Tsigou E, Apostolakos G, Nikolopoulos G, Veldekis D, 2010. Outcome of acute heroin overdose requiring intensive care unit admission. J. Opioid Manag 6 (3), 227–231. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Silveri MM, Yurgelun-Todd DA, 2007. Neuropsychological consequences of opiate use. Neuropsychol. Rev 17 (3), 299–315. [DOI] [PubMed] [Google Scholar]
- Gupta PK, Krishnan PR, Sudhakar PJ, 2009. Hippocampal involvement due to heroin inhalation-“chasing the dragon”. Clin. Neurol. Neurosurg 111 (3), 278–281. [DOI] [PubMed] [Google Scholar]
- Haghighi-Morad M, Naseri Z, Jamshidi N, Haghighi-Morad H, Zamani N, Ahmad-Molaei L, 2020. Methadone-induced encephalopathy: a case series and literature review. BMC Med. Imaging 20 (1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut MW, Hogg JP, Marshalek PJ, Suter BC, Miller LE, 2017. Amnesia associated with bilateral hippocampal and bilateral basal ganglia lesions in anoxia with stimulant use. Front. Neurol 8, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heale P, Dietze P, Fry C, 2003. Intentional overdose among heroin overdose survivors. J. Urban Health 80 (2), 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MD, Cooper PW, Perry JR, 2000. Chasing the dragon – neurological toxicity associated with inhalation of heroin vapour: case report. CMAJ 162 (2), 236–238. [PMC free article] [PubMed] [Google Scholar]
- Holyoak AL, Trout MJ, White RP, Prematuranga S, Senthuran S, 2014. Toxic leukoencephalopathy in the intensive care unit. Anaesth. Intensive Care 42 (6), 782–788. [DOI] [PubMed] [Google Scholar]
- Hsu WY, Chiu NY, Liao YC, 2009. Rhabdomyolysis and brain ischemic stroke in a heroin-dependent male under methadone maintenance therapy. Acta Psychiatr. Scand 120 (1), 76–79. [DOI] [PubMed] [Google Scholar]
- Huisa BN, Gasparovic C, Taheri S, Prestopnik JL, Rosenberg GA, 2013. Imaging of subacute blood-brain barrier disruption after methadone overdose. J. Neuroimaging 23, 441–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasne AS, Alsherbini KH, Smith MS, Pandhi A, Vagal A, Kanter D, 2019. Cerebellar hippocampal and basal nuclei transient edema with restricted diffusion (CHANTER) syndrome. Neurocrit. Care 31 (2), 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R, Olsen TS, Winther BB, 1990. Severe non-occlusive ischemic stroke in young heroin addicts. Acta Neurol. Scand 81 (4), 354–357. [DOI] [PubMed] [Google Scholar]
- Kass-Hout T, Kass-Hout OM, Darkhabani MZ, Mokin M, Mehta B, Radovic V, 2011. “Chasing the dragon” – heroin-associated spongiform leukoencephalopathy. J. Med. Toxicol 7, 240–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh CF, Andrews GT, Spacey SD, Forkheim KE, Graeb DA, 2003. Neuroimaging features of heroin inhalation toxicity: “chasing the dragon”. AJR Am. J. Roentgenol 190 (3), 847–850. [DOI] [PubMed] [Google Scholar]
- Khot S, Walker M, Lacy JM, Oakes P, Longstreth WT Jr., 2007. An unsuccessful trial of immunomodulatory therapy in delayed posthypoxic demyelination. Neurocrit. Care 7 (3), 253–256. [DOI] [PubMed] [Google Scholar]
- King F, Morris NA, Schmahmann JD, 2015. Delayed posthypoxic leukoencephalopathy: improvement with antioxidant therapy. Case Rep. Neurol 7 (3), 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, 2019. Respiratory depression and brain hypoxia induced by opioid drugs: morphine, oxycodone, heroin and fentanyl. Neuropharm 151, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K, Auwarter V, Hermanns-Clausen M, Wilde M, Neukamm MA, 2018. Mixed intoxication by the synthetic opioid U-47700 and the benzodiazepine flubromazepam with lethal outcome: pharmacokinetic data. Drug Test. Anal 10 (8), 1336–1341. [DOI] [PubMed] [Google Scholar]
- Koksel Y, Ozutemiz C, Rykken J, Ott F, Cayci Z, Oswood M, McKinney A, 2019. “CHOICES”: an acronym to aid in delineating potential causes of non-metabolic, non-infectious acute toxic leukoencephalopathy. Eur. J. Radiol. Open 6, 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll SL, Nikolic E, Bieri F, Soyka M, Baumgartner MR, Quednow BB, 2018. Cognitive and socio-cognitive functioning of chronic non-medical prescription opioid users. Psychopharmacology (Berl.) 235 (12), 3451–3464. [DOI] [PubMed] [Google Scholar]
- Kuhlman GD, Gwathmey KG, 2018. Gluteal compartment syndrome with neurologic impairment: report of 2 cases and review of the literature. Muscle Nerve 57 (2), 325–330. [DOI] [PubMed] [Google Scholar]
- Landais A, 2014. Severe memory impairment following acute morphine intoxication. J. Neurol. Sci 343 (1–2), 424–444. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I, 1983. Chronic parkinsonism in humans due to product of meperdine-analog synthesis. Science 219 (4587), 979–980. [DOI] [PubMed] [Google Scholar]
- Lasher L, Rhodes Jason, M.P.A., A. C, Viner-Brown S, 2019. Identification and description of non-fatal opioid overdoses using Rhode Island EMS data, 2016–2018. Rhode Island Med. J 102 (2), 41–45. [PubMed] [Google Scholar]
- Lefaucheur R, Lebas A, Gerardin E, Ozkul-Wermester O, Aubier-Girard C, Martinaud O, Maltete D, 2017. Leucoencephalopathy following abuse of sniffed heroin. J. Clin. Neurosci 35, 70–72. [DOI] [PubMed] [Google Scholar]
- Loftsgard TO, Newcome MD, Hanneman MR, Patch RK 3rd, Seelhammer TG, 2017. Management of neurogenic pulmonary edema and differential hypoxemia in an adult supported on venoarterial extracorporeal membrane oxygenation. J. Cardiothorac. Vasc. Anesth 31 (6), 2170–2174. [DOI] [PubMed] [Google Scholar]
- Long H, Zhou J, Zhou X, Xie Y, Xiao B, 2013. Acute hydrocephalus following heroin induced leukoencephalopathy. Neurol. Sci 34 (6), 1031–1032. [DOI] [PubMed] [Google Scholar]
- Lowder EM, Amlung J, Ray BR, 2020. Individual and county-level variation in outcomes following non-fatal opioid-involved overdose. J. Epidemiol. Comm. Health 74 (4), 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon G, Fattal-Valevski A, Kolodny EH, 2006. Leukodystrophies:clinical and genetic aspects. Top. Magn. Reson. Imaging 17 (4), 219–242. [DOI] [PubMed] [Google Scholar]
- McDonald S, Darke S, Kaye S, Torok M, 2013. Deficits in social perception in opioid maintenance patients, abstinent opioid users and non-opioid users. Addiction 108 (3), 566–574. [DOI] [PubMed] [Google Scholar]
- McHugo GJ, Krassenbaum S, Donley S, Corrigan JD, Bogner J, Drake RE, 2017. The prevalence of traumatic brain injury among people with co-occurring mental health and substance use disorders. J. Head Trauma Rehabil 32 (3), E65–E74. [DOI] [PubMed] [Google Scholar]
- Meyer MA, 2013. Delayed post-hypoxic leukoencephalopathy: case report with a review of disease pathophysiology. Neurol. Int 5 (33), e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milroy CM, Parai JL, 2011. The histopathology of drugs of abuse. Histopathology 59, 579–593. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med 151 (4), 264–270. [DOI] [PubMed] [Google Scholar]
- Molloy S, Soh C, Williams TL, 2006. Reversible delayed posthypoxic leukoencephalopathy. Am. J. Neuroradiol 27, 1763–1765. [PMC free article] [PubMed] [Google Scholar]
- Montandm G, Slutsky AS, 2019. Solving the opioid crisis: respiratory depression by opioids as critical end point. Chest 156 (4), 653–658. [DOI] [PubMed] [Google Scholar]
- Morales Odia Y, Jinka M, Ziai WC, 2010. Severe leukoencephalopathy following acute oxycodone intoxication. Neurocrit. Care 13 (1), 93–97. [DOI] [PubMed] [Google Scholar]
- Morrow RL, Bassett K, Maclure M, Dormuth CR, 2019. Outcomes associated with hospital admissions for accidental opioid overdose in British Columbia: a retrospective cohort study. BMJ Open 9 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller UJ, Mawrin C, Frodl T, Dobrowolny H, Busse S, Bernstein H, Bogerts B, Truebner K, Steiner J, 2018. Reduced volumes of the external and internal globus pallidus in male heroin addicts: a postmortem study. Eur. Arch. Psychiatry Clin. Neurosci 269 (3), 317–324. [DOI] [PubMed] [Google Scholar]
- Mumma BE, Shellennarger D, Callaway CW, Katz KD, Guyette FX, Rittenberger JC, 2009. Neurologic recovery following cardiac arrest due to benzodiazepine and opiate toxicity. Resuscitation 80 (12), 1446–1447. [DOI] [PubMed] [Google Scholar]
- O’Brien P, Todd J, 2009. Hypoxic brain injury following heroin overdose. Brain Impair. 10 (2), 169–179. [Google Scholar]
- O’Brien BP, Murphy D, Conrick-Martin I, Marsh B, 2009. The functional outcome and recovery of patients admitted to an intensive care unit following drug overdose: a follow-up study. Anaesth. Inten. Care 37, 802–806. [DOI] [PubMed] [Google Scholar]
- O’Donnell J, Gladden RM, Mattson CL, Hunter CT, Davis NL, 2020. Vital Signs: characteristics of drug overdose deaths involving opioids and stimulants – 24 states and the District of Columbia, January – June 2019. MMWR Morb. Mortal. Wkly. Rep 69, 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehmichen M, Meissner C, Reiter A, Birkholz M, 1996. Neuropathology in non-human immunodeficiency virus-infected drug addicts: hypoxic brain damage after chronic intravenous drug abuse. Acta Neuropathol. 91, 642–646. [DOI] [PubMed] [Google Scholar]
- Ormseth CH, Maciel CB, Zhou SE, Barden MM, Miyares LC, Beekman RB, Gilmore EJ, Greer DM, 2019. Differential outcomes following successful resuscitation in cardiac arrest due to drug overdose. Resuscitation 139, 9–16. [DOI] [PubMed] [Google Scholar]
- Oxley SOC, Dassanayake TL, Carter GL, Whyte I, Jones AL, Cooper G, Michie PT, 2015. Neurocognitive recovery after hospital-treated deliberate self-poisoning with central nervous system depressant drugs a longitudinal cohort study. J. Clin. Psychopharmacol 35 (6), 672–680. [DOI] [PubMed] [Google Scholar]
- Pearson J, Baden MB, Richter RW, 1975. Neuronal depletion in the globus pallidus of heroin addicts. Drug Alcohol Depend. 1, 349–356. [DOI] [PubMed] [Google Scholar]
- Pfister GJ, Burkes RM, Guinn B, Steele J, Kelley RR, Wiemken TL, Saad M, Ramirez J, Cavallazzi R, 2016. Opioid overdose leading to intensive care unit admission: epidemiology and outcomes. J. Crit. Care 35, 29–32. [DOI] [PubMed] [Google Scholar]
- Pirompanich P, Chankrachang S, 2015. Intravenous heroin-associated delayed spongiform leukoencephalopathy: case report and reviews of the literature. J. Med. Assoc. Thai 98 (7), 703–708. [PubMed] [Google Scholar]
- Quinn DK, Abbott CC, 2014. Catatonia after cerebral hypoxia: do the usual treatments apply? Psychosomatics 55 (6), 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Zamora A, Ramani H, Pastena G, 2015. Bilateral pallidal and medical temporal lobe ischemic lesions after opioid overdose. J. Neurol. Neuroscurg. Psychiatry 86, 1383–1384. [DOI] [PubMed] [Google Scholar]
- Revesz T, Geddes JF, 1988. Symmetrical columnar necrosis of the basal ganglia and brain stem in an adult following cardiac arrest. Clin. Neuropathol 7 (6), 194–298. [PubMed] [Google Scholar]
- Richter RW, Pearson J, Bruun B, Challenor YB, Brust JC, Baden MM, 1973. Neurologicla complications of addiction of heroin. Bull. N. Y. Acad. Med 49 (1), 3–21. [PMC free article] [PubMed] [Google Scholar]
- Rizzuto N, Morbin M, Ferrari S, Cavallaro T, Sparaco M, Boso G, Gaetti L, 1997. Delayed spongiform leukoencephalopathy after heroin abuse. Acta Neuropathol. 94 (1), 87–90. [DOI] [PubMed] [Google Scholar]
- Salazar R, Dubow J, 2012. Delayed posthypoxic leukoencephalopathy following a morphine overdose. J. Clin. Neurosci 19 (7), 1060–1062. [DOI] [PubMed] [Google Scholar]
- Salgado RA, Jorens PG, Baar I, Cras P, Hans G, Parizel PM, 2010. Methadone-inducted toxic leukoencephalopathy: MR imaging and MR proton spectroscopy. AJNR Am. J. Neuroradiol 31 (3), 565–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson S, Tatt ID, Higgins JPT, 2007. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int. J. Epidemiol 36 (3), 666–676. [DOI] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G, 2019. Drug and opioid-involved overdose deaths – United States, 2013–2017. MMWR 67, 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Downing M, Kral AH, Singleton-Banks S, Hammond J, Lorvik J, Ciccarone D, Edlin BR, 2003. Attitudes about prescribing take-home naloxone to injection drug users for the management of heroin overdose: a survey of street-recruited injectors in the San Francisco Bay area. J. Urban Health 80 (2), 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SG, Gann DS, Scott G, et al. , 2008. A qualitative study of overdose responses among Chicago IDUs. Harm Reduct. J 5, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprecher DR, Flanigan KM, Smith GA, Smith SM, Schenkenberg T, Steffens J, 2008. Clinical and diagnostic features of delayed hypoxic leukoencephalopathy. J. Neuropsychiatry Clin. Neurosci 20 (4), 473–477. [DOI] [PubMed] [Google Scholar]
- Shu SL, Thompson PD, Kimber TE, 2016. Dystonia-parkinsonism due to pallidal and nigral necrosis following heroin overdose: long-term evaluation. Mov. Discord. Clin. Pract 3 (2), 188–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Saini M, 2015. Toxic leucoencephalopathy after ‘chasing the dragon’. Singapore Med. J 56 (6), e102–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavova S, Rock P, Bush HM, Quesinberry D, Walsh SL, 2020. Signal of increased opioid overdose during COVID-19 from emergency medical services data. Drug Alcohol Depend. 214, 108176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JE, Butler PM, Zabar Y, Barash JA, 2016. Complete, bilateral hippocampal ischemia: a case series. Neurocase 22 (5), 411–415. [DOI] [PubMed] [Google Scholar]
- Solis E, Cameron-Burr KT, Kiyatkin EA, 2017. Heroin contaminated with fentanyl dramatically enhances brain hypoxia and induces brain hypothermia. eNeuro 4 (5). ENEURO.0323–17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp, 2017. Stata Statistical Software: Release 15. StataCorp LLC, College Station, TX. [Google Scholar]
- Suzuki J, El-Haddad S, 2017. A review: fentanyl and non-pharmaceutical fentanyls. Drug Alcohol Depend. 171, 107–116. [DOI] [PubMed] [Google Scholar]
- Switzer AR, Beland B, Sarna JR, Walzak A, Pfeffer G, 2020. Fentanyl overdose causing hippocampal ischemia followed by delayed leukoencephalopathy. Can. J. Neurol. Sci 47 (3), 398–399. [DOI] [PubMed] [Google Scholar]
- Taheri MS, Noori M, Shakiba M, Jalali AH, 2011. Brain CT-scan findings in unconscious patients after poisoning. Int. J. Biomed. Sci 7 (1), 1–5. [PMC free article] [PubMed] [Google Scholar]
- Taylor RG, Budhram A, Lee DH, Mirsattari SM, 2019. Opioid-associated amnestic syndrome observed with fentanyl patch use. CMAJ 191, E337–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralba-Moron A, Ortiz-Imedio J, Morales-Conejo M, Ruiz-Morales J, Geurra-Vales J, 2017. Delayed leukoencephalopathy: three case reports and a literature review. Eur. J. Case Rep. Intern. Med 4 (2), 000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrame M, Azizi SA, 2007. Pyramidal and extrapyramidal dysfunction as a sequela of hypoxic injury: case report. BMS Neurol. 27 (June (7)), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila N, Chamorro A, 1997. Ballistic movements due to ischemic infarcts after intravenous heroin overdose: report of two cases. Clin. Neurol. Neurosurg 99 (4), 259–262. [DOI] [PubMed] [Google Scholar]
- Villella C, Iorio R, Conte G, Batocchi AP, Bria P, 2010. Toxic leukoencephalopathy after intravenous heroin injection: a case with clinical and radiological reversibility. J. Neurol 257, 1924–1926. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters LM, et al. , 2012. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. Agency for Healthcare Research and Quality Methods Guide for Comparative Effectiveness Reviews. March 2012. Available at:. AHRQ Publication No. 12-EHC047-EF www.effectivehealthcare.ahrq.gov/. [PubMed] [Google Scholar]
- Vivolo-Kanter AM, Seth P, Gladden RM, et al. , 2020. Trends in emergency department visits for suspected opioid overdoses – United States, July 2016 – September 2017. MMWR 67, 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt I, 2013. Fatal overdose due to confusion of an transdermal fentanyl delivery system. Case Rep. Crit. Care 2013, 154143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Irvine RJ, 1999. Mechanisms of fatal opioid overdose. Addiction 94 (7), 961–972. [PubMed] [Google Scholar]
- Whiting PF, Rutjes AW, Westwood ME, Mallet S, Reistsma JB, Leeflang MM, et al. , 2011. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med 155 (8), 529–536. [DOI] [PubMed] [Google Scholar]
- Wijdicks EFM, 2005. Drug overdose in a young man: a not-so-happy face. Rev. Neurol. Dis 2 (4), 217–219. [PubMed] [Google Scholar]
- Winstanley EL, Stover AN, Feinberg J, 2020. Concurrent alcohol and opioid use among harm reduction clients. Addict. Behav 100, 106027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters EC, van Wijngaarden GK, Stam FC, Rengelink H, Lousberg RJ, Schipper ME, Verbeeten B, 1982. Leucoencephalopathy after inhaling “heroin” pyrolysate. Lancet 2 (8310), 1233–1237. [DOI] [PubMed] [Google Scholar]
- World Drug Report, 2020. United Nations publication, Sales No. E.20.XI.6.
- Yarid NA, Harruff RC, 2015. Globus pallidus necrosis unrelated to carbon monoxide poisoning: retrospective analysis of 27 cases of basal ganglia necrosis. J. Forensic Sci 60 (6), 1484–1487. [DOI] [PubMed] [Google Scholar]
- Zamora CA, Nauen D, Hynecek R, Ilica AT, Izbudak I, Sair HI, Gujar S, Pillai JJ, 2015. Delayed posthypoxic leukoencephalopathy: a case series and review of the literature. Brain Behav. 5 (8), e00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zara S, Wright-De A, Briss PA, Truman BI, Hopkins DP, Hennessy MH, et al. , 2000. Data collection instrument and procedure for systematic reviews in the guide to community preventive services. Am. J. Prev. Med 18 (1S), 44–74. [DOI] [PubMed] [Google Scholar]
- Zhu C, Wang X, Bahr BA, Shibata M, Uchiyama Y, Hagberg H, Blomgren K, 2005. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 12, 162–176. [DOI] [PubMed] [Google Scholar]
- Zibbel J, Howard J, Clarke SD, Ferrell A, Karon SL, 2019. Non-fatal Opioid Overdose and Associated Health Outcomes: Final Summary Report. Retrieved 5 April 2021 from. https://aspe.hhs.gov/basic-report/non-fatal-opioid-overdose-and-associated-health-outcomes-final-summary-report.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


