Abstract
The ability to selectively introduce diverse functionality onto hydrocarbons is of substantial value in the synthesis of both small molecules and polymers. Herein, we report an approach to aliphatic C–H diversification via radical chain transfer featuring an easily prepared O-alkenylhydroxamate reagent, which upon mild heating facilitates a range of challenging or previously undeveloped aliphatic C–H functionalizations of small molecules and polyolefins. This broad reaction platform enabled the functionalization of post-consumer polyolefins in infrastructure used to process plastic waste. Furthermore, the chemoselective placement of ionic functionality onto a branched polyolefin via C–H functionalization upcycled the material from a thermoplastic into a tough elastomer with the tensile properties of high-value polyolefin ionomers.
One Sentence Summary:
A simple reagent enables the broad diversification of unactivated, aliphatic C–H bonds in small molecules and post-consumer plastic waste.
The direct transformation of unreactive aliphatic C–H bonds to useful functionality is a streamlined and sustainable approach to accessing complex molecules and materials with enhanced properties from readily available compounds (1–4). Late-stage diversification of drug-like molecules, wherein complex substrates are modified selectively to alter their function, has emerged as a powerful strategy to access new lead compounds for medicinal chemistry and structure-activity relationship (SAR) studies without resorting to de novo synthesis (5). Despite significant progress, there remains a pressing need for new aliphatic C–H diversification platforms that facilitate the site-selective introduction of a range of desirable functionality to small molecule substrates with substrate as the limiting reagent.
An estimated 95% of the economic value of plastics is lost after a single use (6). Specifically, branched polyolefins represent >35% of polymers produced worldwide, but undergo deleterious chain scission events during mechanical reprocessing or polymer functionalization, which degrades their thermomechanical properties and contributes to their poor recycling rate (<5% in the US) (7, 8). Developing synthetic methods to place desired functionality on post-consumer branched polyolefins would lead to performance-advantaged thermoplastics derived from single-stream or mixed plastic waste (9, 10). The new materials realized from such platform methods could serve as sustainable substitutes to current high-value materials that are derived from petrochemical resources, thus representing an example of polymer upcycling (11).
Currently, a number of transformations of aliphatic C–H bonds exist and are used for the late-stage diversification of drug-like molecules and commodity polymers, but the vast majority of these use either nearby directing groups to control reaction site selectivity or involve promiscuous reactive intermediates which limit the scope of these approaches (12, 13). A notable exception that employs substrate as the limiting reagent is the use of high-valent transition metal-oxo complexes in aliphatic C–H functionalization, but this approach is limited by the scope of accessible transformations owing to the use of highly oxidizing intermediates (14, 15). Intermolecular alkylation or borylation of C–H bonds using rhodium catalysis is also well-developed, but the requirement for donor–acceptor diazo reagents for alkylation limits overall scope, and the use of a precious metal limits high-volume applications in polymer science (16, 17). Singlet carbenes generated from the photo- or thermal-decomposition of diazirines represent an efficient C–H functionalization strategy for polymer crosslinking and biopolymer photoaffinity labeling, but the required substitution pattern of the diazirine and limited functional group tolerance hinders broad applicability (18, 19). Furthermore, several valuable C–H transformations, such as aliphatic C–H iodination and C–H methylation, remain limited regardless of approach. A universal strategy for aliphatic C–H functionalization, wherein a wide array of functionality can be placed site-selectively in an intermolecular transformation on both complex organic substrates and commodity polymers, remains a grand challenge (Fig. 1A) (20).
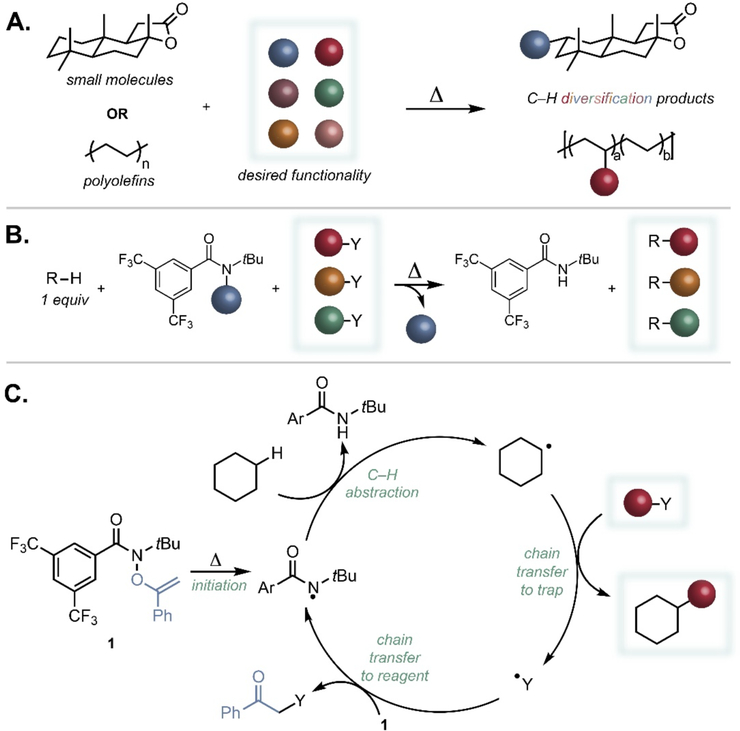
Fig. 1. Aliphatic C–H diversification using N-functionalized amides.
(A) A universal approach to C–H diversification would enable the introduction of a range of desired functionality onto small molecules and commodity polyolefins. (B) An N-functionalized amide and a diverse set of chain transfer agents constitute a general platform for C–H diversification. (C) The mechanistic hypothesis for C–H diversification using O-alkenylhydroxamates separates the HAT reagent from the chain transfer agent. Ar = 3,5-bis(trifluoromethyl)phenyl.
Recent studies have demonstrated the utility of heteroatom-centered radicals to facilitate site-selective, intermolecular functionalizations of unactivated aliphatic C–H bonds on a variety of small molecules and materials, constituting a complementary strategy to metal-catalyzed methods (21–26). These reactions principally harness the capacity of a tuned nitrogen-centered radical to achieve facile hydrogen atom transfer (HAT) from strong, unactivated aliphatic C–H sites. A critical drawback to these previous studies is the requirement for direct group transfer of the functionality appended to nitrogen, which greatly restricts the diversity of products accessible via the HAT platform. With this in mind, we hypothesized that decoupling the formation of the nitrogen-centered radical responsible for HAT from the chain transfer step would unlock a universal C–H diversification manifold applicable to a vast range of transformations (Fig. 1B). We identified an O-alkenylhydroxamate (1) as an ideal reagent capable of forming reactive nitrogen-centered radicals, while also manifesting slow enough chain transfer kinetics for external radical traps to outcompete it in substrate functionalization (Fig. 1C). We hypothesized that such a versatile C–H diversification strategy would encompass many important transformations, including ones inaccessible with current synthetic technology, and extend to areas ranging from the late-stage C–H diversification of complex molecules to applications in the transformation of post-consumer plastic waste to functional polyolefins.
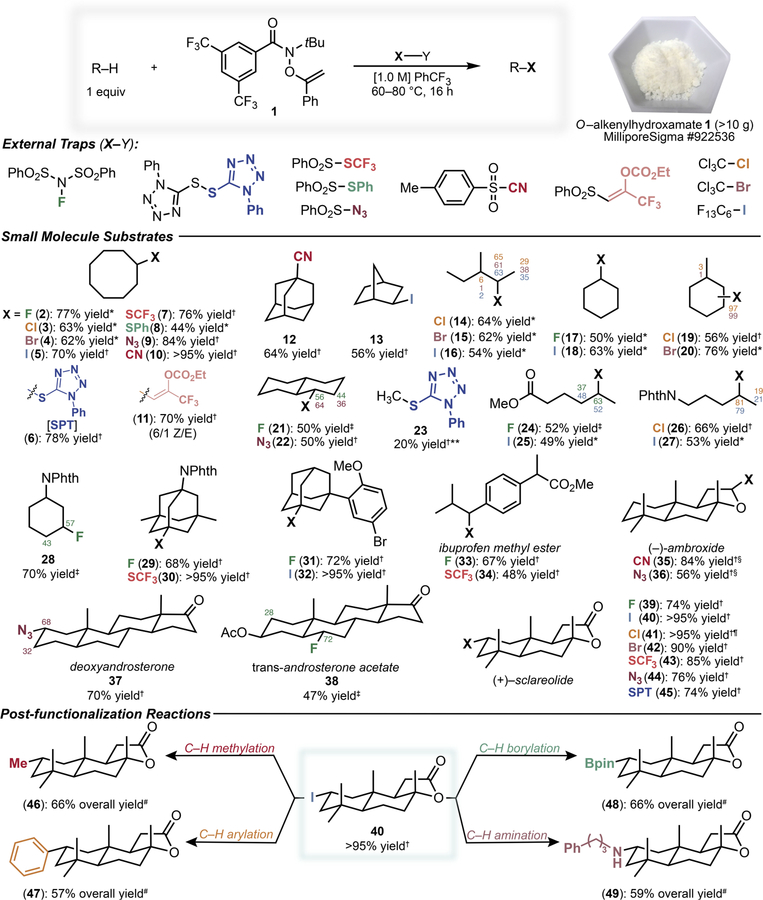
Our initial studies demonstrated the versatility of easily accessed, shelf-stable O-alkenylhydroxamate 1 for the intermolecular, aliphatic C–H diversification of a range of small molecules (Fig. 2). The C–H functionalizations promoted by reagent 1 proceeded simply upon mild heating (70° C) or visible light irradiation without the need for an exogenous initiator, which is an enabling aspect of the approach. The C–H diversification of cyclooctane with substrate as limiting reagent was successful using 10 diverse trapping agents in good to excellent yield, establishing the broad scope of the platform (2–11). Practical intermolecular, aliphatic C–H iodination sets the stage for a range of challenging C–H transformations (vide infra) (27). While there are extant methods available for a subset of these reactions, examples using substrate as limiting reagent remain quite rare; commonly, the alkane is used in large excess (>5 equiv) and often times as reaction solvent. Furthermore, there are no platforms for aliphatic C–H functionalization that rival the synthetic scope demonstrated herein with respect to both the diversity of accessible transformations and the viable substrates ranging from small molecules to post-consumer waste. While we targeted many synthetically valuable C–H transformations, additional processes are easily envisioned upon the use of alternative radical traps.
Fig. 2. C–H diversification of small molecules using reagent 1.
Yields refer to combined isolated products, or were determined by either GC, 1H NMR or 19F NMR with the addition of internal standard. Percent functionalizations are provided in examples involving minor regioisomers; see the Supplementary Materials for reaction details. *GC yield. †1H NMR yield using internal standard. ‡19F NMR yield using internal standard. §3:1 diastereoselectivity. ¶Irradiated with blue light. #Isolated yield. **Reaction performed under 50 atm methane.
We next applied the C–H diversification to several representative small molecule substrates. Diverse cyclic and linear hydrocarbons react efficiently using substrate as limiting reagent (12–22). The sterically dictated site selectivities controlled by the bulky N-tBu amidyl radical favor accessible secondary C–H sites over weaker, tertiary C–H bonds which are commonly the most reactive in C–H functionalizations (14–16, 19–22). For comparison, prior efforts towards C–H diversification via HAT using photoredox catalysis strongly favored tertiary functionalization; such tertiary-selective functionalization is also characteristic of reactions involving HAT with sulfate radicals (28, 29). The transformation of the unreactive C–H bond of gaseous methane remains a considerable challenge for any C–H functionalization. The strong N–H bond (110.7 kcal/mol) of the parent amide of 1 suggested that methane HAT (C–H bond ~105 kcal/mol) could be viable (30). As a demonstration of the notable reactivity of the amidyl radical in HAT, we successfully performed the (phenyltetrazole)thiolation of methane under our standard conditions to deliver 23 in 20% yield with respect to 1. Functionalized substrates containing electronwithdrawing groups (24–28) exhibit strong polar effects in discriminating between methylene sites, with sites distal to the electron-withdrawing group preferred (31). With respect to the mechanism of reactions involving 1, a C–H iodination competition experiment between cyclohexane and d12-cyclohexane proceeded with a kH/kD of 6.4, consistent with an irreversible aliphatic C–H HAT. Additionally, the C–H (phenyltetrazole)thiolation reaction produces the α-SPT acetophenone byproduct, consistent with the chain-transfer mechanism outlined in Fig. 1C. The notable sterically and electronically dictated site selectivities characteristic of this platform, when combined with the breadth of accessible C–H transformations, enable a wealth of valuable late-stage diversifications of complex molecules as described below.
We next examined the C–H functionalization of several representative natural products and drug derivatives to highlight the scope of our approach. The reactions of adamantyl substrates were highly efficient (29–32). The benzylic functionalization of ibuprofen methyl ester provided fluorination and trifluoromethylthiolation products 33 and 34, respectively, as single regioisomers in contrast to previous C–H functionalizations of this substrate (28). Functionalization of terpenoid and steroid natural products, complex molecules with a multitude of aliphatic C–H sites, favors the activated C–H site a to the ether oxygen atom of (–)-ambroxide (35–36) while reaction of deoxyandrosterone favored functionalization of the C2 position of the A-ring (37) and reaction of trans-androsterone acetate afforded a single diastereomer of a B-ring fluoride (38). For comparison, a previous C–H fluorination of this substrate with Selectfluor yielded greater than seven alkyl fluorides, with none formed in greater than 6% yield (32). Lastly, we performed several C–H functionalizations of the terpenoid natural product (+)–sclareolide, favoring the most reactive A-ring methylene site (39–45, for reaction optimization studies see Table S1). In each case, a single regioisomer was obtained in good to excellent yield with high (>10:1) diastereoselectivity, including the C–H iodination which delivers iodide 40 in virtually quantitative yield. The present platform thus offers a powerful tool for the late-stage introduction of fluorinated groups at unactivated aliphatic sites in complex molecules for modulating the absorption, distribution, metabolism, and excretion (ADME) properties of drug-like compounds.
With respect to late-stage diversification, the versatility of our approach enables more valuable, yet rare, C–H transformations from now easily accessible, functionalized compounds. As a second step following the highly efficient iodination of sclareolide (>95% yield), reaction with Me2CuLi delivers the formal C–H methylation product 46 in good yield as a single diastereomer, furnishing a two-step protocol to investigate “magic methyl” effects via late-stage, intermolecular methylation of unactivated aliphatic C–H bonds (33, 34). Alternatively, iron-catalyzed cross-coupling of 40 with PhMgBr leads to the C–H arylation product 47; previous C–H arylation of this substrate required the use of superstoichiometric amounts of (+)–sclareolide (35). Facile borylation of 40 using B2cat2 followed by transesterification yielded 48 as a single product, another transformation with very limited precedent using substrate as limiting reagent (36–38). Finally, the copper-catalyzed cross-coupling of 40 with a primary alkyl amine delivered C–H amination product 49, constituting a formal dehydrogenative alkane-amine coupling (39). Other attractive C–H transformations are also easily envisioned capitalizing on the versatility of the phenyltetrazole sulfone group which can be easily accessed from product 45 (40).
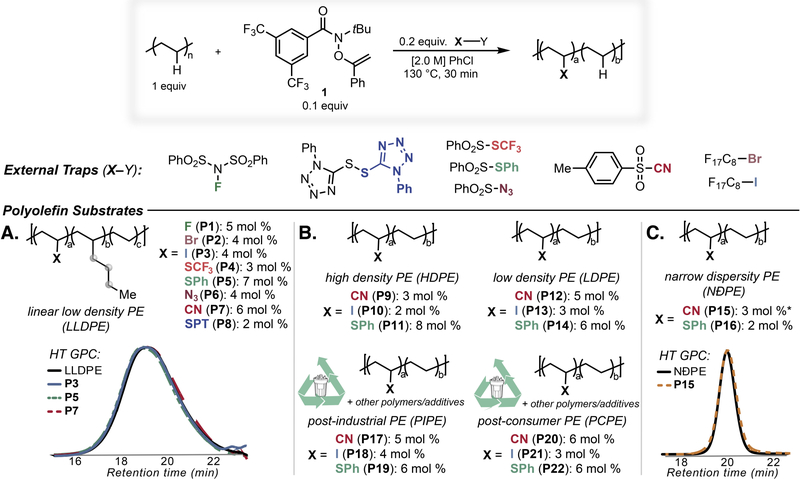
We envisioned that this versatile C–H diversification strategy could unlock numerous transformations on branched polyolefins. Commercial approaches to polyolefin functionalization proceed through high-energy radical processes that selectively abstract tertiary C–H bonds in branched polymers, resulting in β-scission processes that deteriorate thermomechanical properties. We hypothesized that the high regioselectivity of HAT involving reagent 1 favoring methylene sites would prevent polymer chain-scission by eliminating the formation of tertiary radicals during reactive processing, and the generality of this method would enable access to a range of branched polyolefins with polar functionality. Such polar polyolefins, which are inaccessible using traditional Ziegler-Natta or metallocene catalysis, enhance interfacial adhesion and provide sites for controlled polymer deconstruction (41). LLDPE (Dow DNDA-1081) was chosen as a model branched polyolefin to exemplify this method (melting temperature of 122 °C; 19 branches per 100 carbons). As a representative transformation to introduce polar functionality incompatible with early transition metal catalysts, cyanation of LLDPE with 1 under homogeneous conditions (130 °C in chlorobenzene) proceeded efficiently with selectivity for methylene sites and involved no discernable chain-scission as confirmed by size exclusion chromatography (SEC) as well as a variety of 1D and 2D NMR techniques (Fig. 3A; Fig. S1, S12–S15). More precise analysis of selectivity was obtained through the use of a narrow dispersity PE (NÐPE), made through the reduction of polybutadiene. The SEC chromatogram was virtually identical before and after functionalization, demonstrating the lack of chain scission or long-chain branching accompanying polymer functionalization (Fig. 3C). In contrast, an analogous cyanation using dicumyl peroxide as a radical initiator in place of 1 yielded no functionalization and a decrease in polymer molecular weight. All polymer functionalizations target a maximum of 10 mol % repeat-unit modification in order to add functionality while maintaining the beneficial semicrystalline nature of the material.
Fig. 3. C–H diversification of polyolefins using reagent 1.
Polymer functionalization is indicated as mol % compared to repeat unit and were determined by 1H NMR on the isolated product. Grey spheres indicate minor regioisomers, see the Supplementary Materials for reaction details. High temperature GPC (HT GPC) was conducted at 140 °C in trichlorobenzene. *Reaction time was 10 min.
In addition to polyolefin cyanation, the installations of fluoride, bromide, iodide, trifluoromethylthiol, thiophenyl, azido, and (phenyltetrazole)thiol groups onto LLDPE exemplified the versatility of this approach. Several of these polyolefin C–H transformations deliver products inaccessible by other means (24, 25, 42, 43). To further extend the scope, C–H cyanation, thiophenylation, and iodination were successful on complementary substrates, including highly crystalline high-density PE (HDPE), highly branched LDPE (49 branches per 100 carbons), post-industrial waste PE (PIPE) remnants from packaging forms, and post-consumer waste PE (PCPE) obtained from PE foam packaging (Fig. 3B). Furthermore, thiophenylation of isotactic polypropylene (50 branches per 100 carbons) proceeded successfully without discernable chain scission (Figure S9), demonstrating the value of this method for these tough and highly branched thermoplastics. It is notable that functionalization proceeded efficiently even with an undefined mix of oxidation byproducts and/or additives in PCPE evident by IR and 1H NMR spectroscopy, indicating the tolerance of this method to common impurities in plastic waste.
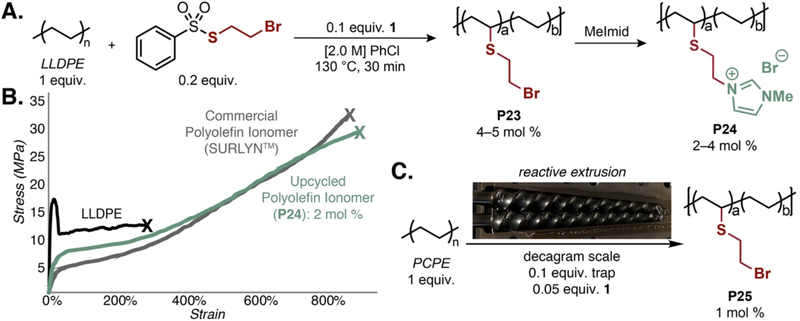
The ability to place diverse functionality onto polyolefins through this universal approach provides an opportunity to substitute current high-value plastics, and create new ones, using post-consumer waste as a starting material. Polyolefin ionomers such as SURLYN™ are a high-value class of thermoplastics toughened by ionic crosslinks, with applications ranging from structural adhesives to ion-conducting membranes (44). However, SURLYN™ is synthesized through radical copolymerization of acrylic acid and ethylene, which limits polymer architecture to a highly branched microstructure, precludes use of α-olefins as comonomers, and limits functional group identity to a carboxylate. These limitations compromise the potential strength, toughness, and transport properties of the materials. There are currently limited strategies to prepare polyolefin ionomers on materials made through Ziegler–Natta or related catalytic approaches (i.e., LLDPE or HDPE). Given the structural fidelity and lack of long-chain branching of our polyolefin functionalization approach, we envisioned creating ionomers from polyolefins through late-stage functionalization. The generality of the C–H functionalization mediated by 1 enabled the development of a 2-bromoethyl thiosulfonate radical trapping reagent that installed a primary bromide onto the polyolefin (P23, Fig 4A). Displacement of the bromide by methyl imidazole yielded imidazolium-functionalized LLDPE (P24), which represents a formal copolymerization of α-olefins with an ion-containing vinyl monomer. The ionomers had distinct properties from the parent LLDPE, including solubility in polar aprotic solvents, a decreased melting temperature, and enhanced clarity (Fig. S25). Introduction of the imidazolium to only 2 mol % of the repeat units dramatically changed the material from a thermoplastic to a tough elastomer (Fig. 4B). While yield stress and Young’s modulus (E) of P24 decreased compared to the parent LLDPE, the strain at break (εB) quadrupled and the stress at break (σB) more than doubled, leading to an increase in the tensile toughness (UT) of >550%. These tensile properties compare favorably to a commercial sample of Dow SURLYN™, demonstrating the dramatic effect that a small amount of targeted functionalization can have on material properties.
Fig. 4. Access to polyolefin ionomers through C–H functionalization.
(A) Polyolefin C–H functionalization enabled the production of ionomers from commercial plastics in a two-step approach. (B) Tensile tests demonstrate the change in polymer properties upon functionalization and how they compare to a commercial sample of Dow SURLYN™. Strain rate = 1.0 mm/s. (C) Reactive extrusion was performed on PCPE at a decagram scale.
Collectively, the ability to produce an ionomer from a post-consumer waste stream with functional equivalence to the thermomechanical properties of a high-value commercial material make this upcycled material a potentially environmentally sustainable substitute for polyolefin ionomers (45). The translational potential of this method was further demonstrated through C–H functionalization of PCPE in a twin-screw extruder, which is the infrastructure used for processing plastic waste. Reacting reagent 1 with 5 mol % 2-bromoethyl thiosulfonate radical trapping reagent, we procured seven grams of 1 mol % bromoethylthiolated PCPE (P25, Fig 4C). Reaction of the extruded material with methyl imidazole afforded a large-scale synthesis of the polyolefin ionomer. While further reagent development is required to make this material an economically sustainable substitute, this C–H functionalization platform enables access to a library of polyolefin ionomers–among other materials–from plastic waste. These ionomers can be systematically studied to assess the impact of ion identity, ion content, and polymer branching on polyolefin properties and circularity, and could ultimately contribute to a more sustainable plastics economy.
Supplementary Material
Funding:
This work was supported by Award No. R35 GM131708 from the National Institute of General Medical Sciences (E.J.A.) and the Air Force Office of Scientific Research award number 17RT0487 under the Young Investigator Program (F.A.L). J.W.A. thanks the Henry G. Luce Foundation and UNC for a Clare Boothe Luce Graduate Student Fellowship and the UNC Chemistry Department for the Venable Award. The UNC Department of Chemistry’s Mass Spectrometry Core Laboratory provided expertise and instrumentation that enabled this study with support from the National Science Foundation (CHE-1726291) and the National Institute of General Medical Sciences of the National Institutes of Health (R35GM118055). The UNC Department of Chemistry’s NMR Core Laboratory provided expertise and instrumentation that enabled this study with support from National Science Foundation (CHE-1828183 and CHE-0922858). We thank the Dow Chemical Company for supplying samples of SURLYN™ ionomers and HighCube LLC for providing samples of post-industrial and post-consumer plastic waste.
Footnotes
Competing interests: E.J.A. and F.A.L. are inventors on US provisional patent application 63/188,215 covering the diversification of C–H bonds in small molecules and polyolefins, including the upcycling of polymers, filed by the University of North Carolina at Chapel Hill.
Data and materials availability:
Experimental and characterization data are available in the supplementary information.
References and Notes:
- 1.Hartwig JF, Evolution of C–H Bond Functionalization from Methane to Methodology. J. Am. Chem. Soc. 138, 2–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies HML, Finding Opportunities from Surprises and Failures. Development of Rhodium-Stabilized Donor/Acceptor Carbenes and Their Application to Catalyst-Controlled C–H Functionalization. J. Org. Chem. 84, 12722–12745 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White MC, Adding Aliphatic C-H Bond Oxidations to Synthesis. Science. 335, 807–809 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Hong B, Luo T, Lei X, Late-Stage Diversification of Natural Products. ACS Cent. Sci. 6, 622–635 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cernak T, Dykstra KD, Tyagarajan S, Vachal P, Krska SW, The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016). [DOI] [PubMed] [Google Scholar]
- 6.MacArthur Dame Ellen; Dominic Waughray; Stuchtey Martin R., The New Plastics Economy Rethinking the Future of Plastics. World Economic Forum (2016), (available at http://www3.weforum.org/docs/WEF_The_New_Plastics_Economy.pdf).
- 7.Rahimi AliReza; García Jeanette M., Chemical recycling of waste plastics for new materials production. Nature Reviews Chemistry. 1, 1–11 (2017). [Google Scholar]
- 8.Hamielec AE, Gloor PE, Zhu S, Kinetics of, free radical modification of polyolefins in extruders – chain scission, crosslinking and grafting. The Canadian Journal of Chemical Engineering. 69, 611–618 (1991). [Google Scholar]
- 9.Williamson JB, Lewis SE, Johnson RR, Manning IM, Leibfarth FA, C−H Functionalization of Commodity Polymers. Angewandte Chemie International Edition. 58, 8654–8668 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Britt Phillip F.; Coates Geoffrey W.; Winey Karen I., Basic Energy Sciences Roundtable: Chemical Upcycling of Polymers. Office of Science and Technical Information (2019), (available at https://www.osti.gov/servlets/purl/1616517).
- 11.Korley LTJ, Epps TH, Helms BA, Ryan AJ, Toward polymer upcycling—adding value and tackling circularity. Science. 373, 66–69 (2021). [DOI] [PubMed] [Google Scholar]
- 12.An Q, Wang Z, Chen Y, Wang X, Zhang K, Pan H, Liu W, Zuo Z, Cerium-Catalyzed C–H Functionalizations of Alkanes Utilizing Alcohols as Hydrogen Atom Transfer Agents. J. Am. Chem. Soc. 142, 6216–6226 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Boaen NK, Hillmyer MA, Post-polymerization functionalization of polyolefins. Chem. Soc. Rev. 34, 267–275 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Ravelli D, Fagnoni M, Fukuyama T, Nishikawa T, Ryu I, Site-Selective C–H Functionalization by Decatungstate Anion Photocatalysis: Synergistic Control by Polar and Steric Effects Expands the Reaction Scope. ACS Catal. 8, 701–713 (2018). [Google Scholar]
- 15.White MC, Zhao J, Aliphatic C–H Oxidations for Late-Stage Functionalization. J. Am. Chem. Soc. 140, 13988–14009 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao K, Yang Y-F, Li Y, Sanders JN, Houk KN, Musaev DG, Davies HML, Design of catalysts for site-selective and enantioselective functionalization of non-activated primary C–H bonds. Nature Chem. 10, 1048–1055 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo Y, García-Cuadrado D, Hartwig JF, Boaen NK, Wagner NL, Hillmyer MA, Rhodium-Catalyzed, Regiospecific Functionalization of Polyolefins in the Melt. J. Am. Chem. Soc. 124, 1164–1165 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Lepage ML, Simhadri C, Liu C, Takaffoli M, Bi L, Crawford B, Milani AS, Wulff JE, A broadly applicable cross-linker for aliphatic polymers containing C–H bonds. Science. 366, 875–878 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Ge S-S, Chen B, Wu Y-Y, Long Q-S, Zhao Y-L, Wang P-Y, Yang S, Current advances of carbene-mediated photoaffinity labeling in medicinal chemistry. RSC Adv. 8, 29428–29454 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blakemore DC, Castro L, Churcher I, Rees DC, Thomas AW, Wilson DM, Wood A, Organic synthesis provides opportunities to transform drug discovery. Nature Chemistry. 10, 383–394 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Schmidt VA, Quinn RK, Brusoe AT, Alexanian EJ, Site-Selective Aliphatic C–H Bromination Using N -Bromoamides and Visible Light. Journal of the American Chemical Society. 136, 14389–14392 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Quinn RK, Könst ZA, Michalak SE, Schmidt Y, Szklarski AR, Flores AR, Nam S, Horne DA, Vanderwal CD, Alexanian EJ, Site-Selective Aliphatic C–H Chlorination Using N -Chloroamides Enables a Synthesis of Chlorolissoclimide. Journal of the American Chemical Society. 138, 696–702 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czaplyski WL, Na CG, Alexanian EJ, C–H Xanthylation: A Synthetic Platform for Alkane Functionalization. Journal of the American Chemical Society. 138, 13854–13857 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plummer CM, Zhou H, Zhu W, Huang H, Liu L, Chen Y, Mild halogenation of polyolefins using an N -haloamide reagent. Polym. Chem. 9, 1309–1317 (2018). [Google Scholar]
- 25.Williamson JB, Czaplyski WL, Alexanian EJ, Leibfarth FA, Regioselective C−H Xanthylation as a Platform for Polyolefin Functionalization. Angewandte Chemie International Edition. 57, 6261–6265 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Williamson JB, Na CG, Johnson RR, Daniel WFM, Alexanian EJ, Leibfarth FA, Chemo- and Regioselective Functionalization of Isotactic Polypropylene: A Mechanistic and Structure–Property Study. J. Am. Chem. Soc. 141, 12815–12823 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Artaryan A, Mardyukov A, Kulbitski K, Avigdori I, Nisnevich GA, Schreiner PR, Gandelman M, Aliphatic C–H Bond Iodination by a N -Iodoamide and Isolation of an Elusive N -Amidyl Radical. J. Org. Chem. 82, 7093–7100 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Margrey KA, Czaplyski WL, Nicewicz DA, Alexanian EJ, A General Strategy for Aliphatic C–H Functionalization Enabled by Organic Photoredox Catalysis. J. Am. Chem. Soc. 140, 4213–4217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H, Xiao Z, Wu J, Guo Y, Xiao J-C, Liu C, Chen Q-Y, Direct Trifluoromethylthiolation of Unactivated C(sp3)-H Using Silver(I) Trifluoromethanethiolate and Potassium Persulfate. Angewandte Chemie International Edition. 54, 4070–4074 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Tierney MM, Crespi S, Ravelli D, Alexanian EJ, Identifying Amidyl Radicals for Intermolecular C–H Functionalizations. J. Org. Chem. 84, 12983–12991 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitts CR, Bloom S, Woltornist R, Auvenshine DJ, Ryzhkov LR, Siegler MA, Lectka T, Direct, Catalytic Monofluorination of sp 3 C–H Bonds: A Radical-Based Mechanism with Ionic Selectivity. J. Am. Chem. Soc. 136, 9780–9791 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Chambers RD, Nakano T, Parsons M, Sandford G, Batsanov AS, Howard JAK, Elemental fluorine Part 22. Fluorination of 3b-acetoxy-5a-androstan-17-one using fluorine and Selectfluor. Journal of Fluorine Chemistry, 811–816 (2008). [Google Scholar]
- 33.Schönherr H, Cernak T, Profound Methyl Effects in Drug Discovery and a Call for New C-H Methylation Reactions. Angewandte Chemie International Edition. 52, 12256–12267 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Feng K, Quevedo RE, Kohrt JT, Oderinde MS, Reilly U, White MC, Late-stage oxidative C(sp3)–H methylation. Nature. 580, 621–627 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perry IB, Brewer TF, Sarver PJ, Schultz DM, DiRocco DA, MacMillan DWC, Direct arylation of strong aliphatic C–H bonds. Nature. 560, 70–75 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oeschger R, Su B, Yu I, Ehinger C, Romero E, He S, Hartwig J, Diverse functionalization of strong alkyl C–H bonds by undirected borylation. Science. 368, 736–741 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shu C, Noble A, Aggarwal VK, Metal-free photoinduced C(sp3)–H borylation of alkanes. Nature. 586, 714–719 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Cheng Y, Mück-Lichtenfeld C, Studer A, Metal-Free Radical Borylation of Alkyl and Aryl Iodides. Angewandte Chemie International Edition. 57, 16832–16836 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matier CD, Schwaben J, Peters JC, Fu GC, Copper-Catalyzed Alkylation of Aliphatic Amines Induced by Visible Light. J. Am. Chem. Soc. 139, 17707–17710 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merchant RR, Edwards JT, Qin T, Kruszyk MM, Bi C, Che G, Bao D-H, Qiao W, Sun L, Collins MR, Fadeyi OO, Gallego GM, Mousseau JJ, Nuhant P, Baran PS, Modular radical cross-coupling with sulfones enables access to sp 3 -rich (fluoro)alkylated scaffolds. Science. 360, 75–80 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Häußler M, Eck Marcel, Rothauer D, Mecking S, Closed-loop recycling of polyethylene-like materials. Nature. 590, 423–427 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Liu D, Bielawski CW, Direct azidation of isotactic polypropylene and synthesis of ‘grafted to’ derivatives thereof using azide–alkyne cycloaddition chemistry. Polymer International. 66, 70–76 (2017). [Google Scholar]
- 43.Plummer CM, Li L, Chen Y, The post-modification of polyolefins with emerging synthetic methods. Polym. Chem. 11, 6862–6872 (2020). [Google Scholar]
- 44.Grady BP, Review and critical analysis of the morphology of random ionomers across many length scales. Polymer Engineering & Science. 48, 1029–1051 (2008). [Google Scholar]
- 45.Vadenbo C, Hellweg S, Astrup TF, Let’s Be Clear(er) about Substitution: A Reporting Framework to Account for Product Displacement in Life Cycle Assessment. Journal of Industrial Ecology. 21, 1078–1089 (2017). [Google Scholar]
- 46.Alewood PF, Calder IC, Richardson RL, An Improved Preparation of N-(t-Butyl)-N-(3,5-dinitrobenzoyl)-nitroxyl. Synthesis. 2, 121–122 (1981). [Google Scholar]
- 47.Piou T, Rovis T, Rh(III)-Catalyzed Cyclopropanation Initiated by C–H Activation: Ligand Development Enables a Diastereoselective [2 + 1] Annulation of N-Enoxyphthalimides and Alkenes. J. Am. Chem. Soc. 136, 11292–11295 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer D, Jangra H, Walther F, Zipse H, Renaud P, A third generation of radical fluorinating agents based on N-fluoro-N-arylsulfonamides. Nature Communications. 9, 1–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chavez-Flores D, Salvador JM, Facile conversion of racemic ibuprofen to (S)-ibuprofen. Tetrahedron: Asymmetry. 23, 237–239 (2012). [Google Scholar]
- 50.Koch V, Bräse S, Pd-mediated cross-coupling of C-17 lithiated androst-16-en-3-ol – access to functionalized arylated steroid derivatives. Org. Biomol. Chem. 15, 92–95 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Fredo Naciuk F., do Nascimento Faria J, Gonçalves Eufrásio A, Torres Cordeiro A, Bruder M, Development of Selective Steroid Inhibitors for the Glucose-6-phosphate Dehydrogenase from Trypanosoma cruzi. ACS Med. Chem. Lett. 11, 1250–1256 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H, Shan C, Tung C-H, Xu Z, Dual gold and photoredox catalysis: visible light-mediated intermolecular atom transfer thiosulfonylation of alkenes. Chem. Sci. 8, 2610–2615 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyer D, Renaud P, Enantioselective Hydroazidation of Trisubstituted Non-Activated Alkenes. Angewandte Chemie International Edition. 56, 10858–10861 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Anthore L, Zard SZ, A Convergent Radical Based Route to Trifluoromethyl Ketones and to α,β-Unsaturated Trifluoromethyl Ketones. Org. Lett. 17, 3058–3061 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Li X, Liu L, Liu G, Rong Y, Yang Y, Wang H, Ku Z, Xu M, Zhong C, Han H, Efficient Dye-Sensitized Solar Cells with Potential-Tunable Organic Sulfide Mediators and Graphene-Modified Carbon Counter Electrodes. Advanced Functional Materials. 23, 3344–3352 (2013). [Google Scholar]
- 56.Yadav AK, Srivastava VP, Yadav LDS, An easy access to fluoroalkanes by deoxygenative hydrofluorination of carbonyl compounds via their tosylhydrazones. Chemical Communications. 49, 2154–2156 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Kennedy N, Liu P, Cohen T, Fundamental Difference in Reductive Lithiations with Preformed Radical Anions versus Catalytic Aromatic Electron-Transfer Agents: N,N-Dimethylaniline as an Advantageous Catalyst. Angewandte Chemie International Edition. 55, 383–386 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Van Oystaeyen A, Oliveira RC, Holman L, van Zweden JS, Romero C, Oi CA, d’Ettorre P, Khalesi M, Billen J, Wackers F, Millar JG, Wenseleers T, Conserved Class of Queen Pheromones Stops Social Insect Workers from Reproducing. Science. 343, 287–290 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Bérubé Sm., Kamal F, Roy J, Poirier D, A Dehydrohalogenation Methodology for Synthesizing Terminal Olefins under Mild Conditions. Synthesis, 3085–3091 (2006). [Google Scholar]
- 60.Levy LM, de Gonzalo G, Gotor V, Resolution of N-protected cis- and trans-3-aminocyclohexanols via lipase-catalyzed enantioselective acylation in organic media. Tetrahedron: Asymmetry, 2051–2056 (2004). [Google Scholar]
- 61.Zeng C, Shen G, Yang F, Chen J, Zhang X, Gu C, Zhou Y, Fan B, Rhodium-Catalyzed Generation of Anhydrous Hydrogen Iodide: An Effective Method for the Preparation of Iodoalkanes. Org. Lett. 20, 6859–6862 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Kamijo S, Watanabe M, Kamijo K, Tao K, Murafuji T, Synthesis of Aliphatic Azides by Photoinduced C(sp3)–H Azidation. Synthesis, 115–121 (2016). [Google Scholar]
- 63.Kamijo S, Hoshikawa T, Inoue M, Photochemically Induced Radical Transformation of C(sp 3 )–H Bonds to C(sp 3 )–CN Bonds. Organic Letters. 13, 5928–5931 (2011). [DOI] [PubMed] [Google Scholar]
- 64.Kropp PJ, Adkins RL, Photochemistry of alkyl halides. 12. Bromides vs. iodides. J. Am. Chem. Soc. 113, 2709–2717 (1991). [Google Scholar]
- 65.Chambers RD, Kenwright AM, Parsons M, Sandford G, Moilliet JS, Elemental fluorine. Part 14.1 Electrophilic fluorination and nitrogen functionalisation of hydrocarbons. J. Chem. Soc., Perkin Trans. 1, 2190–2197 (2002). [Google Scholar]
- 66.Huang X, Bergsten TM, Groves JT, Manganese-Catalyzed Late-Stage Aliphatic C–H Azidation. Journal of the American Chemical Society. 137, 5300–5303 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Toda N, Asano S, Barbas CF, Rapid, Stable, Chemoselective Labeling of Thiols with Julia–Kocieński-like Reagents: A Serum-Stable Alternative to Maleimide-Based Protein Conjugation. Angewandte Chemie International Edition. 52, 12592–12596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halperin SD, Fan H, Chang S, Martin RE, Britton R, A Convenient Photocatalytic Fluorination of Unactivated C-H Bonds. Angewandte Chemie International Edition. 53, 4690–4693 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Larkina MS, Ozerskaya AV, Podrezova EV, Belousov MV, Tolmachev V, Zhdankin VV, Yusubov MS, Efficient Synthesis of ω-[18F]Fluoroaliphatic Carboxylic Esters and Acids for Positron Emission Tomography. European Journal of Organic Chemistry. 2020, 6375–6381 (2020). [Google Scholar]
- 70.Nielsen MK, Ahneman DT, Riera O, Doyle AG, Deoxyfluorination with Sulfonyl Fluorides: Navigating Reaction Space with Machine Learning. J. Am. Chem. Soc. 140, 5004–5008 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Hua AM, Mai DN, Martinez R, Baxter RD, Radical C–H Fluorination Using Unprotected Amino Acids as Radical Precursors. Org. Lett. 19, 2949–2952 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Xu W, Wang W, Liu T, Xie J, Zhu C, Late-stage trifluoromethylthiolation of benzylic C-H bonds. Nat Commun. 10, 1–8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, Ji S, Wei K, Lin J, Epiandrosterone-derived prolinamide as an efficient asymmetric catalyst for Michael addition reactions of aldehydes to nitroalkenes. RSC Adv. 4, 30850–30856 (2014). [Google Scholar]
- 74.Liu W, Huang X, Cheng M-J, Nielsen RJ, Iii WAG, Groves JT, Oxidative Aliphatic C-H Fluorination with Fluoride Ion Catalyzed by a Manganese Porphyrin. Science. 337, 1322–1325 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Köhler F, Gais H-J, Raabe G, Asymmetric Synthesis of Highly Substituted γ-Amino Acids from Allyltitanium Sulfoximines. Org. Lett. 9, 1231–1234 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Nakamura M, Matsuo K, Ito S, Nakamura E, Iron-Catalyzed Cross-Coupling of Primary and Secondary Alkyl Halides with Aryl Grignard Reagents. J. Am. Chem. Soc. 126, 3686–3687 (2004). [DOI] [PubMed] [Google Scholar]
- 77.Reiss T, Breit B, Total Synthesis of (+)-Bourgeanic Acid Utilizing o-DPPB-Directed Allylic Substitution. Org. Lett. 11, 3286–3289 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Experimental and characterization data are available in the supplementary information.