Abstract
Cardiovascular disease remains the leading cause of death in women. Given accumulating evidence on sex- and gender-based differences in cardiovascular disease development and outcomes, the need for more effective approaches to screening for risk factors and phenotypes in women is ever urgent. Public health surveillance and healthcare delivery systems now continuously generate massive amounts of data that could be leveraged to enable both screening of cardiovascular risk and implementation of tailored preventive interventions across a women’s lifespan. However, healthcare providers, clinical guidelines committees, and health policy experts are not yet sufficiently equipped to optimize the collection of data on women, use or interpret these data, or develop approaches to targeting interventions. Therefore, we provide a broad overview of the key opportunities for cardiovascular screening in women while highlighting the potential applications of artificial intelligence along with digital technologies and tools.
Subject Terms: Cardiovascular Disease, Information Technology, Machine Learning and Artificial Intelligence, Women, Sex, Gender
Central Illustration.
Cardiovascular Disease Screening in Women: Leveraging Artificial Intelligence and Digital Tools
Introduction
Cardiovascular disease (CVD) is the leading cause of death in women accounting for 28% of deaths in the US in 20181 and up to 35% of deaths worldwide in 2019.2 Furthermore, CVD is also the leading cause (approximately 34%) of all pregnancy-related deaths.3, 4 Although up to 90% of women in the United States have at least one cardiovascular risk factor,5 awareness, recognition, and appropriate management of CVD risk in women remains a major opportunity gap. Given the advent of high-throughput data collection and advanced analytical methods for capturing, distilling, and interpreting health information from across multiple sources, there now exist a plethora of opportunities to improve identification of and interventions for CVD risks in women. As such, this review highlights the potential applications of artificial intelligence and digital tools for cardiovascular risk screening in women.
Definitions of Sex and Gender in Relation to Cardiovascular Risk Screening
Whereas sex is a biological construct referring to anatomic and physiologic features categorized as male or female, gender is a multidimensional construct that comprises psychological, social, and cultural identities and roles.6, 7 Although sex and gender are often incorrectly used interchangeably,8 they are two distinct entities that influence cardiovascular disease in women (Figure 1). For instance, there are clear sex-specific effects on cardiovascular risk, such as lower levels of estrogen being associated with increased risk in younger females9 and adverse pregnancy outcomes being associated with higher risk and poor outcomes.10 Gender-specific effects include the association between marital stress and cardiac events among women with ischemic heart disease and a lower socioeconomic status in women being independently associated with CVD and myocardial infarction.7 Recognizing this difference is essential in not just understanding CVD in women, but also in planning targeted interventions such as screening to improve outcomes. Herein, unless otherwise stated, we use the term women in reference to the female sex.
Figure 1.
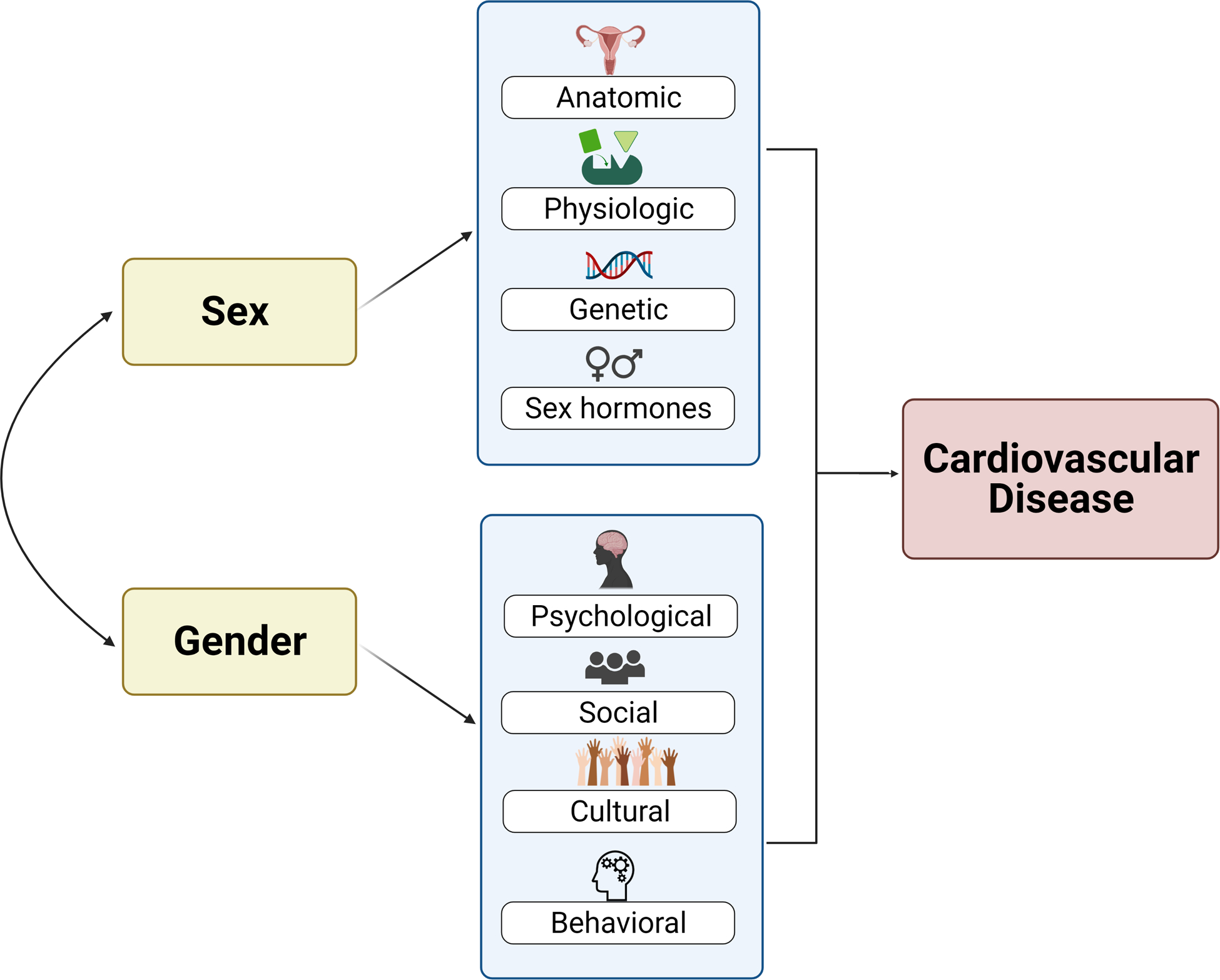
Relationship between sex, gender, and cardiovascular disease.
Comprehensive Screening to Achieve Effective Cardiovascular Disease Prevention
Although CVD is the leading cause of death among women, results from a 2019 survey revealed only 44% of women are aware of this, which is a decline from 65% in 2009.1 Despite recognition that atherosclerotic CVD in women increases not only with traditional factors but also risk-enhancing factors (see Box 1.0),11, 12 the additional risk-enhancing or female-specific factors have unfortunately not been incorporated into any cardiovascular risk assessment tool.13 In addition to these gaps that directly affect women are persistent health disparities – related to race, ethnicity, and social determinants of health – all of which also impact the treatment and outcomes of women. As such, a comprehensive approach is essential for evaluating cardiovascular risk in women. While appropriately targeting all individuals at risk, screening and evaluation tools need to also account for the sex- and gender-specific manifestations of CVD that translated into variations in outcomes. For instance, as discussed in detail within the disease focused sections of this Compendium, women are more likely than men to develop ischemic heart disease that presents as ischemia with nonobstructive coronary arteries13 and atypical symptoms14, 15 that are easily missed or diagnosed late in the disease course. Similarly, women are more likely than men to develop heart failure with preserved rather than reduced ejection fraction and may respond differently to certain treatments.16 Women are also more likely than men to suffer adverse outcomes following stroke.17 Therefore, health institutions should ideally offer individualized, women-centered cardiovascular risk factor assessments, appropriate screening where indicated and effective intervention strategies when needed (Figure 2).18, 19
Box 1.0. Risk Factors for Atherosclerotic Disease in Women.
| Age |
| Menopause |
| Traditional cardiovascular risk factors: hyperlipidemia, diabetes, tobacco use, hypertension, and obesity |
| Risk-enhancing factors: chronic inflammatory conditions, premature menopause, adverse pregnancy outcomes, family history of premature ASCVD (men <55yo and women <65yo), ethnicity (eg, south Asian ancestry), LDL 160–189 mg/dL, chronic kidney disease, metabolic syndrome, elevated high-sensitivity CRP, lipoprotein (a) >50 mg/dL, elevated apolipoprotein B ≥130 mg/dL, and lower extremity peripheral artery disease defined as an ankle-brachial index <0.90. |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CRP, C-reactive protein; LDL, low-density lipoprotein; yo, years old.
Figure 2.
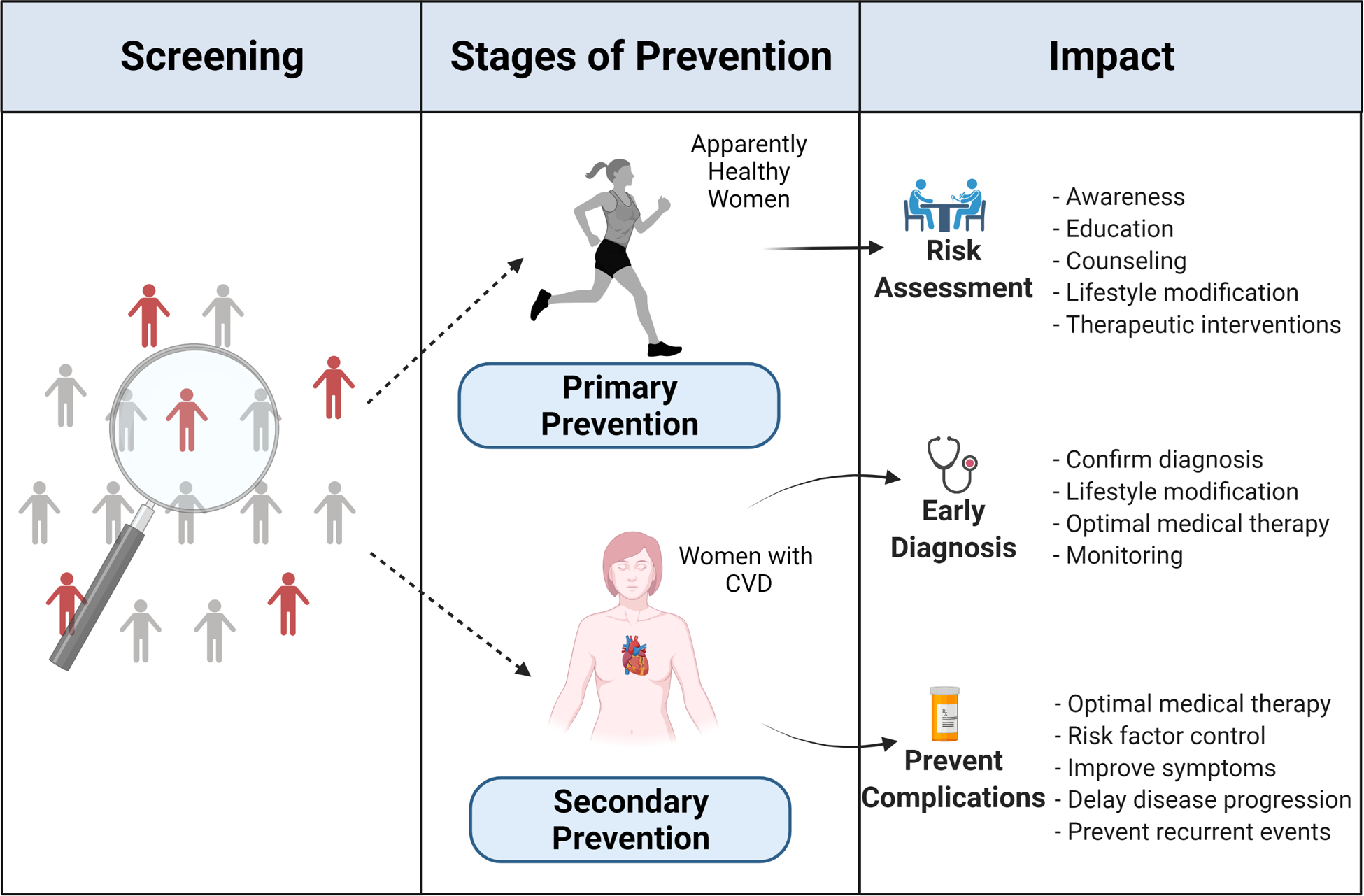
Screening and stages of cardiovascular disease prevention in women.
Tractable approaches to facilitating more comprehensive CVD risk assessment in women are now offered by the ongoing accumulation of readily available health data combined with the rapid development of machine learning (ML) and specifically artificial intelligence (AI) tools for analyzing these data. In fact, the breadth and depth of data and analytical tools can facilitate approaches for primordial and primary as well as secondary prevention of CVD in women. When integrated with evolving personal health data capture systems, in addition to established electronic health records (EHR) systems, AI can be well-positioned to synthesize and analyze the intrinsically complex and rapidly expanding quantities of inter-related data. For primordial prevention, AI could expand the use of currently available personal health and web-based applications by incorporating data from multiple sensors (e.g., activity trackers, digital scales, electronic health records, and fitness apps) to predict women who are at risk for developing CVD risk factors such as hypertension, obesity, and hypercholesterolemia. For primary prevention, digital tools can be used to screen women for known but undiagnosed CVD risk factors such as hypertension, as well as evaluate blood pressure control following intervention. In addition, AI based algorithms can integrate existing clinical or imaging20 data for more accurate risk prediction and aid clinical decision support tools for appropriate early interventions. For secondary prevention, AI algorithms can similarly identify women who already carry a diagnosis of overt CVD but may benefit from more intensive guideline-directed medical therapy or facilitate remote cardiac rehabilitation21 using digital technology.
Leveraging Artificial Intelligence for Cardiovascular Disease Screening in Women
Machine Learning Potential and Purpose
Effectively leveraging artificial intelligence to improve health outcomes, for women as well as for men, requires an understanding of the potential and purpose of machine learning algorithms. The quote, “All models are wrong, but some are useful,” by George Box, is often used to introduce the concept of statistical modeling to learners.22 What has been known for over a century is that statistical models provide a nonunique solution of relating a set of variables to an outcome of interest. The choice of variables, the units of the variables, and the functional form can all influence the model’s fit to the data. Historically, statistical models were written by the analyst guided on knowledge of the clinical domain and statistics. Machine learning (ML) does not remove these elements from the modeling, but rather creates a larger search space for an optimal model by leveraging robust computing capabilities. In a way, ML is allowing for systematic examination of complex relationships using the speed of computers. Deep learning extends many common regression techniques by building a network of complex data extraction and data summarization mathematical operations to provide mechanisms to identify subtle patterns in the data. One particular type of ML, combines these techniques in a way to provide statistical tools that allow for computational tasks that previously required human perception to address. These tasks, sometimes described as human easy-computer hard, are as common as humans using facial and voice recognition to uniquely identify a person. Programming those tasks to be robust into a computer, however, is a challenge.
The use of ML in cardiovascular medicine is increasing, in part due to the expansion of the use of raw data signals, such as electrocardiogram (ECG) or image processing needs, for models based on echocardiogram images.23 Some authors have suggested it is time to move cardiology beyond standard regression approaches,24–26 while others have found little added value in more computationally driven techniques.27, 28 This leaves a few unanswered questions for whether modern ML approaches are necessary, and which, if any, advantages do they provide. To address this, consider the ubiquitous ECG. On a standard clinical system, it is not uncommon to have 10 seconds recorded at 500 Hz over 8 independent leads (with data in a standard 12-lead format derived from those 8 leads).29 These data will produce over 40,000 unique data points for each patient (10 seconds × 500 samplings/second × 8 independent leads) that have both temporal and spatial correlation patterns. Standard regression techniques do not excel with such a data configuration, and thus, alternative methods are needed.
A convolutional neural network is a current ML tool that has proven effective at modeling raw ECG signatures. These include the detection of left ventricular dysfunction,30 hypertrophic cardiomyopathy,31 cardiac amyloidosis,32 aortic stenosis,33 and interestingly, detection of silent atrial fibrillation from an ECG showing normal sinus rhythm.34 The left ventricular dysfunction algorithm has been reevaluated and validated in clinical35 and nonclinical36 settings and in racial and ethnic subgroups37 and has been found to be associated with improved detection of left ventricular dysfunction in a pragmatic clinical trial.38 The basic premise of this model, which is a special type of deep learning model, is that a network architecture is specified to extract repeating patterns from the ECGs through convolutional kernels, and then combine these pattern recognition signals into final classifications using fully connected (dense) neural networks. A convolutional kernel can be conceptualized as a unique digital magnifying glass that slides over the ECG waveform looking for a match. If the input (ie, a small slice of the ECG) matches the convolutional kernel, a large numeric impulse will be generated. The algorithms strive to learn multiple kernels automatically through the training process. The resulting impulses, effectively a pattern recognition process, enables the classification of ECGs into discrete categories, such as left ventricular dysfunction. The advantage of these neural networks is the analyst only needs to provide the basic framework for the network architecture. The algorithm learns the kernels with little human specification. This is not to say the process is automatic as the judicious specification of the network architecture is required to have any training success. Frequently, vast amounts of computing over many competing model architectures are needed to obtain a promising model. A justified concern of such practices is that the final selected model may not be generalizable and may likely be overtrained.
An overtrained model is one that has poor bias-variance trade off. Briefly, bias in this case measures how well the model predictions align with the truth. Ideally, you want the model to predict the truth with little error. Variance measures how robust the model is to small differences in the data. Think of this as how much model performance changes upon an independent validation. A safe model for predicting a person’s risk of left ventricular dysfunction would be to simply use the disease prevalence (eg, 6%). This model would show low variance sample to sample (ie, it is always estimating 6%) but likely high bias as it would not be well calibrated to any of the patient factors that would be expected to influence a person’s risk for left ventricular dysfunction. On the contrary, a model that predicts left ventricular dysfunction with 100% accuracy in a sample is likely over fit to the data and performance in a new sample would be expected to be reduced considerably (high variance). One of the challenges in developing even common statistical models is adjusting for what is regarded as model optimism.39–41 All statistical models fit to data are optimized for the data used for the model development. Should the data change, model performance is expected to change. Validation studies are an essential part of the model development process.
ML models that strive to learn all the unique features in a dataset are at greater risk of bias in the predictions. A more complex model framework combined with the exhaustive techniques used to search for optimal solutions allows the models to develop a highly tailored fit to the data. The risk is that features that are learned do not generalize to additional datasets. This is understood from the more common statistical framework of the bias-variance tradeoff described above. However, additional sources of bias are possible. As with standard epidemiological studies, known and measured confounders can be used to control for confounded results. If these variables are not routinely included in the ML approaches, the algorithms will learn the confounded associations and perpetuate the bias in the sample with future predictions. For example, imagine a scenario where ECGs from healthy controls are retrieved using a standard commercial smart watch and those of affected patients are retrieved from hospital records using standard 12-lead ECGs. The process of selecting only lead II to compare with the consumer watch data introduces the potential for bias in the algorithms due to changes in low-pass filtering, sampling frequency, and likely the positioning of controls (ie, less likely to be supine or resting). An AI algorithm can be expected to easily differentiate the hospital patients from the healthy ambulatory controls. What the algorithm has not learned, however, is differentiating disease from control. Thus, the algorithm is fundamentally flawed. Similarly, imagine a scenario where more complete ECGs are derived solely from male healthy controls, where the purpose of the model is to detect left ventricular hypertrophy from the aVL lead. If the model development in this scenario is assumed to be conducted in a representative population, the resulting algorithm would be insensitive to detecting left ventricular hypertrophy in females. Again, the algorithm would be fundamentally flawed.
In addition to ensuring balanced representation across training and test populations, a key approach to increasing the robustness of a model’s performance is to consider and mirror how clinical decisions are frequently made: decisions are based on a constellation of evidence and judgment. In statistical terms, such a constellation is frequently referred to as an ensemble model. Ensembles represent a key deviation from the selection of the best model. Ensembles recognize that there will be many different models that fit the data well. If you err on the side of simple models (mid to high bias, low variance), you can combine them into a common prediction that will generally have among the lowest bias and variance of any model. Some ML algorithms, such as random forests, are ensembles in of themselves, but in most cases, ensembles are created by summarizing the performance of several competing models into a single prediction. The process of training and validating the summary prediction is the same as any of the ML algorithms.
Contextual Bias in AI Systems
Although AI has the potential to transform cardiovascular medicine by leveraging large amounts of data from clinical trial data, medical records, or data generated from sensors such as ECG rhythm monitors, there are huge concerns related to the likelihood for biased predictions among women and underserved minorities. These groups are underrepresented in cardiology, and the vast majority of clinical evidence and guideline recommendations in the field are largely based on clinical trials that frequently exclude these patient populations. As such, the evidence may not automatically translate or apply to these marginalized populations42.
Recently, some studies have highlighted the potential role of AI to propagate racial and gender bias, reinforce inequalities, and potentially magnify these patterns exponentially,43 termed algorithmic bias,44 which has led to the creation of a field of research called algorithmic fairness.45 AI models are trained to identify patterns in a dataset and are thus fated to reproduce any unfair or discriminating patterns within the datasets they are trained on.45 A compelling example of bias in AI was the Gender Shades study led by Joy Buolamwini, a Black woman computer scientist and researcher at the Massachusetts Institute of Technology. She evaluated commercially available facial recognition algorithms (Microsoft, Face++, and IBM) and demonstrated that these systems were biased with error rates as high as 35% in darker skinned women.46, 47 Following the publication of these findings, the evaluated companies revised their algorithms, and subsequent analysis showed a reduction in error rates; however, a review of other companies not initially evaluated (Amazon and Kairos) showed continued bias with high error rates (31% and 23%, respectively) in darker skinned women.48 The subsequent study emphasizes a key point, eliminating bias and ensuring inclusion is an intentional process that needs to be prioritized.
In the health care space, a commercially available algorithm used by accountable care organizations was found to be biased, resulting in Black patients being less likely to be enrolled in care management programs or to benefit from its resources.49 This algorithm was trained to predict healthcare costs and not actual illness. As such, given Black patients are less likely to utilize or have access to health care services, at a specific threshold risk score predicted by the algorithm, Black patients were sicker than White patients and would have benefited from earlier referral/enrollment in the care management program. Another study demonstrated that an AI algorithm employing medical imaging training datasets that included a lower proportion of female patients performed poorly when used for computer-aided diagnosis in women.50 Another deep learning model developed by DeepMind (a Google company) for prediction of patient deterioration 48 hours in advance, utilized training data from the Veterans Affairs hospital where only 6% of the dataset were women.51 This algorithm has also been criticized for its poor performance in female patient populations.52
Technology companies that dominate the information technology industry in the US (Apple, Amazon, Facebook, Google, and Microsoft)53 and educational and health care institutions that develop AI algorithms and deploy them need to be intentional in addressing bias and make every effort to ensure inclusivity. The AI industry, currently limited to a few technology companies and top tier university laboratories, unfortunately suffers from lack of diversity. Only 15% and 10% of research staff at Facebook and Google are women, and the proportion of Black employees at Facebook and Microsoft is estimated at 4% while Google is even lower at 2.5%.54 Stakeholders (health system administrators, health care organizations, policy makers, and the government) and end-users (health care providers, patients, and insurance companies) of AI algorithms need to be made aware of the potential for bias in these models and the need to focus on awareness and inclusion as key priorities (Figure 3). Recommendations for addressing bias in AI algorithms include ensuring women and minority populations are well represented in datasets,43, 44 appropriate selection of clear and specific training targets for algorithms being developed,44 transparency, rigorous testing, validation, and thorough risk assessments prior to development (Figure 3).54 These efforts can be summarized as responsible AI. In this vein, the world health organization in conjunction with the international telecommunications union have established a team to develop standard evaluation parameters for health related AI models55.
Figure 3.
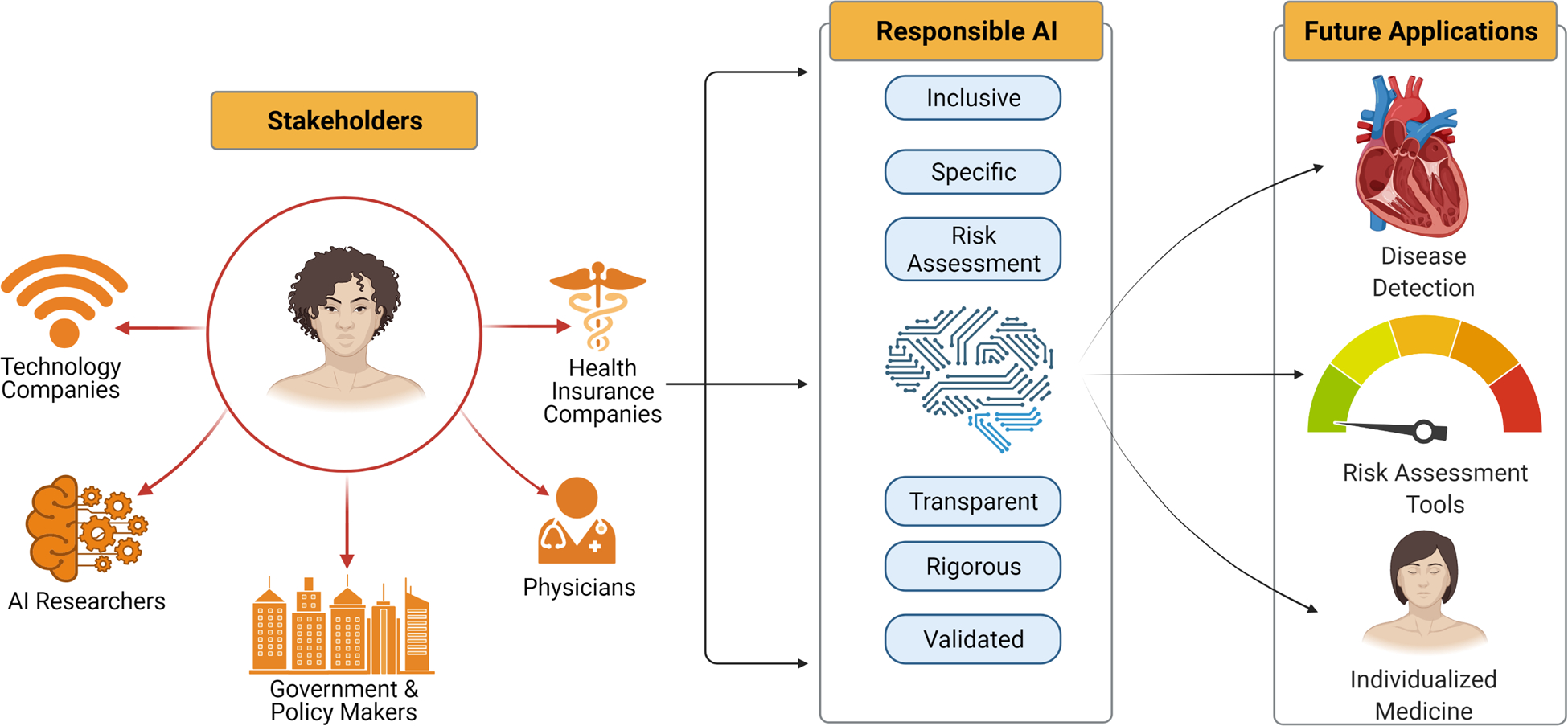
Key stakeholders, responsible AI, and future directions for AI in improving women’s cardiovascular health. AI - artificial intelligence.
A recent article highlighted access to digital tools and digital literacy as social determinants of health,56 and this is likely to become more apparent as we continue to incorporate technology and digital tools in health care delivery. This includes the rapid transition toward telehealth during the start of the pandemic, use of remote patient monitoring technologies, and delivery of other health care interventions using digital technology. Communities without access to digital technology and services are likely to be disadvantaged by this, thus widening the digital divide. To address this, a targeted assessment of digital literacy and tailored patient education when accessing health care should be an important factor in transforming health care delivery for women.
Leveraging Opportunities for Cardiovascular Screening Over the Woman’s Life Course
This section outlines persistent gaps in CVD screening for women, and the ways by which a life course approach can facilitate identifying and addressing these gaps – with many opportunities for improvement that can be potentially aided by AI.
Youth and Preconception
Congenital and familial heart disease recognized early can positively impact clinical outcomes.57, 58 The diagnosis of channelopathies and familial cardiomyopathy is aided by accurate family history, physical examination, and cardiovascular testing. Well child visits and sports pre-participation physicals are important opportunities for capturing these diagnoses – and where clinical screening workflows are imperfect, they may be aided by AI tools. For instance, an ECG-based deep learning algorithm has been demonstrated to be effective in identifying hypertrophic cardiomyopathy in children 18 years of age or younger, with an area under the curve of 0.98.59 AI-aided interpretation of abnormal ECG findings, such as prolonged QT, using data from standard 12-lead ECGs and mobile ECG devices60, 61 can also be of benefit in this patient population. These algorithms could be useful for screening particularly in males as well as females under age 35, in whom sudden death among athletes is a special concern62. In addition, digital health passports that allow patients to store their diagnosis and health management data, including last ECG, chest radiography, genetic test results, and medications, could improve cardiovascular care and the evaluation of family members by providing a more accurate health and family history.63
Unfortunately, many young patients have limited contact with medical providers and may lack knowledge regarding their risk of current or future CVD. In addition, limited time at clinic visits can impact the ability of health care providers to obtain a thorough family history, provide patient education, and recognize abnormalities on testing that may require follow-up. Many electronic health record systems currently send preclinic assessment questionnaires to patients to be completed prior to their health care encounter, and while these can be leveraged to obtain in-depth family history and cardiovascular risk assessments, they also need to be summarized and intelligently incorporated into the health care encounter without increasing physician electronic health record review burden. AI models can address this issue with cognitive computing, such as pattern recognition and natural language processing,64 to facilitate individualized patient care. AI-based analysis of phonocardiograms (recording of heart sounds on auscultation) has also shown promise in the detection of congenital heart disease and valvular heart disease65, 66 and can be deployed in primary care clinics and remote locations (Figure 4). In complex congenital heart disease with single ventricle physiology, digital tools for tracking weights and oxygen saturation have also been successfully used by parents to help manage these complex patients.67, 68
Figure 4.
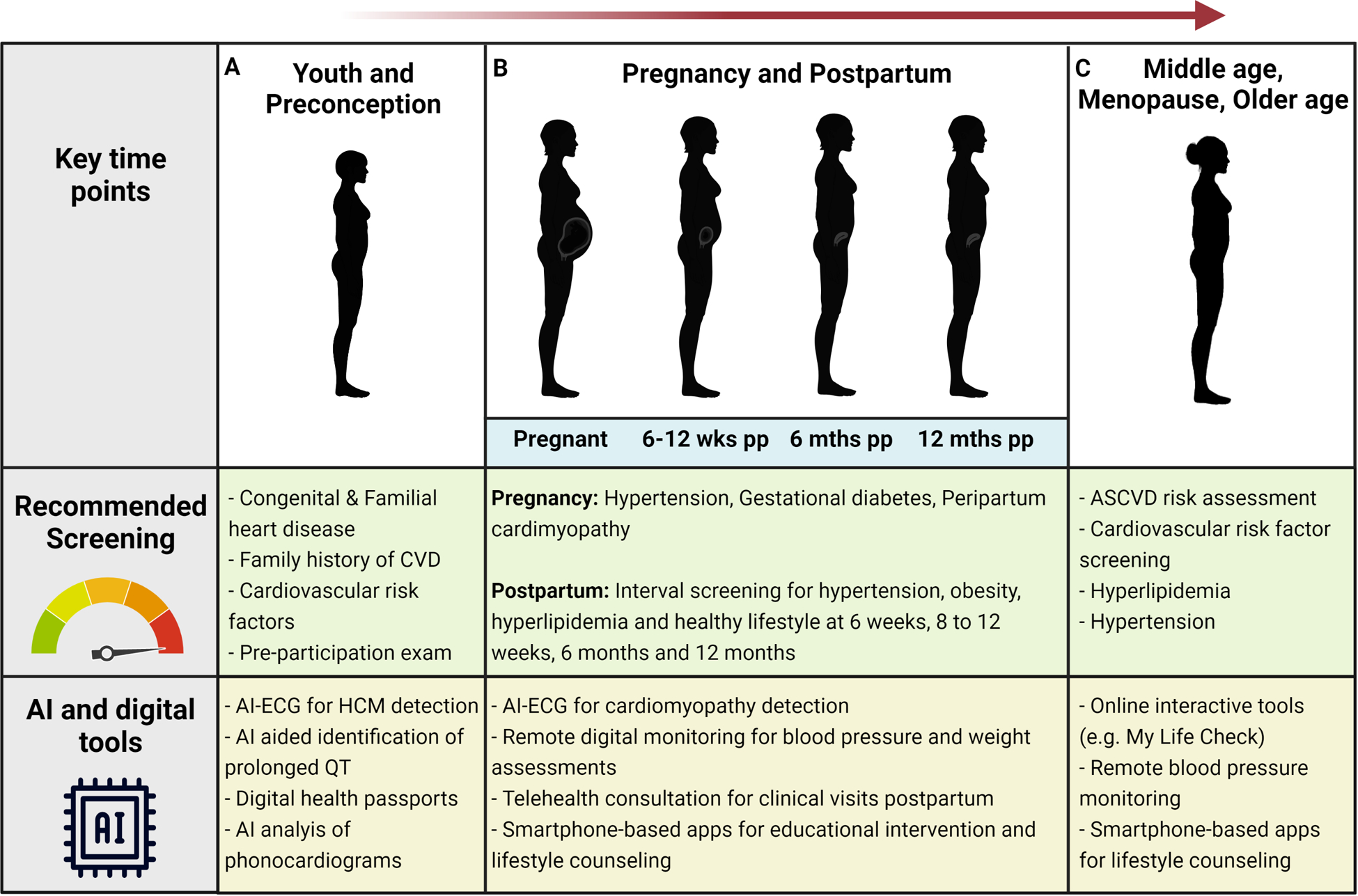
Stages of life and key time points for cardiovascular disease screening: potential opportunities to leverage AI and digital tools. pp - Postpartum; CVD, Cardiovascular disease; HCM, Hypertrophic cardiomyopathy
Children and young adults benefit from screening not only for congenital and familial heart disease, but also to identify risk factors for acquired cardiovascular disease, which creates an opportunity to reinforce healthy habits in young women.69 When common CVD risk factors – such as hypertension, hyperlipidemia, obesity, and impaired fasting glucose – manifest in childhood and young adulthood, the impact on cardiovascular health tends to be much more profound over the lifetime when compared to the later onset of the same risk factors.70–73 Therefore, early recognition, monitoring, and treatment of these risk factors can improve long-term cardiovascular heath, and particularly for women.74 While all women of childbearing age benefit from screening and interventions to promote cardiovascular health, the preconception period when identified is an opportune window for digitally facilitated education and interventions around smoking cessation and cardiometabolic health especially for those with preexisting or a predisposition for hypertension or diabetes. Smoking cessation interventions are important for women of all ages, given evidence of greater associated cardiovascular outcomes in women compared to men; however, children and young adults are especially vulnerable due to their susceptibility to using electronic cigarettes that are known to cause harm especially during pregnancy and in the setting of pre-existing reactive airway disease75.
Although cardiovascular health metrics are important for informing cardiovascular disease prevention in the early years of life, such metrics are not sufficiently captured by current surveillance systems.76 Young women are an ideal target demographic for cardiovascular surveillance and public health education campaigns through the use of digital tools given their avid uptake of novel technologies, and by virtue of being born in the information age, they are at ease with navigating technology-based applications, such as social media, which generate vast amounts of digital data. Large amounts of data obtained from social medial platforms are already being used during public health emergencies for improved surveillance and identification of disease clusters.77 However, it is important to acknowledge the real concerns related to data privacy78, 79 and potential for misuse as previously described with facial recognition systems, or ethical concerns related to consent and minimizing harm.80 Appropriate standards, safeguards, and laws regarding digital surveillance and algorithmic decision making based on these data have yet to be established – under a responsibility that is likely to be shared between government and industry organizations.
Pregnancy and Peripartum
Pregnancy and the peripartum period have been identified as ideal time points to evaluate risk factors and behaviors in women to reduce future cardiovascular risk.81, 82 In fact, young women of childbearing age may already have one or more traditional cardiovascular risk factors, including hypertension, increased body mass index, abnormal lipid profile, and abnormal glucose metabolism.82 If undetected and untreated, these risk factors result in increased risk of adverse events during pregnancy. Even when these risk factors are not manifest prior to pregnancy, the physiologic changes of pregnancy, including insulin resistance, upregulation of inflammatory pathways, and endothelial-dependent alteration in vascular function, can accelerate or unmask cardiovascular risk factors.82–84
Some of the physiological changes in pregnancy can result in physical examination findings that mimic cardiac disease, such as lower extremity edema, dyspnea, and heart murmurs, and distinguishing normal pregnancy-associated changes from cardiac disease is often challenging. Development of left ventricular systolic dysfunction (peripartum cardiomyopathy) or decompensation of previously reduced left ventricular systolic dysfunction is a cause of significant concern in pregnancy and the postpartum period. Compared to similar high-income countries, the US has the highest rate of maternal mortality,85 and cardiomyopathy is the leading cause of cardiovascular-related maternal mortality. Cardiomyopathy is notably difficult to diagnose in pregnancy due to an overlap in the symptoms of normal pregnancy and heart failure due to cardiomyopathy86 and there remains an unmet need for cardiovascular screening in pregnant and postpartum women. We recently evaluated the ability of an AI-enhanced ECG to detect cardiomyopathies during pregnancy and the postpartum period and found it to be effective (Figure 5A).87 Other ML models specifically for use in detecting or predicting the risk for pregnancy-related disorders, such as gestational diabetes and preeclampsia, have also shown promise, albeit with limited external validation.88 Potential AI/digital tools that can be leveraged for screening during the peri-partum period include not only the incorporation of ECG-based AI tools for cardiomyopathy detection87 but also remote monitoring technologies for blood pressure assessments89 and digital scales for weight assessments with automated data capture and transfer such that measurement reporting is less reliant on the patient who may also be navigating the challenges of being a new mother. Data captured from these monitoring tools can be incorporated into a machine learning algorithm to identify uncontrolled hypertension and elevated BMI and incorporated with smartphone-based apps to provide automated educational interventions on lifestyle modification or prompts to ensure medication compliance.
Figure 5.
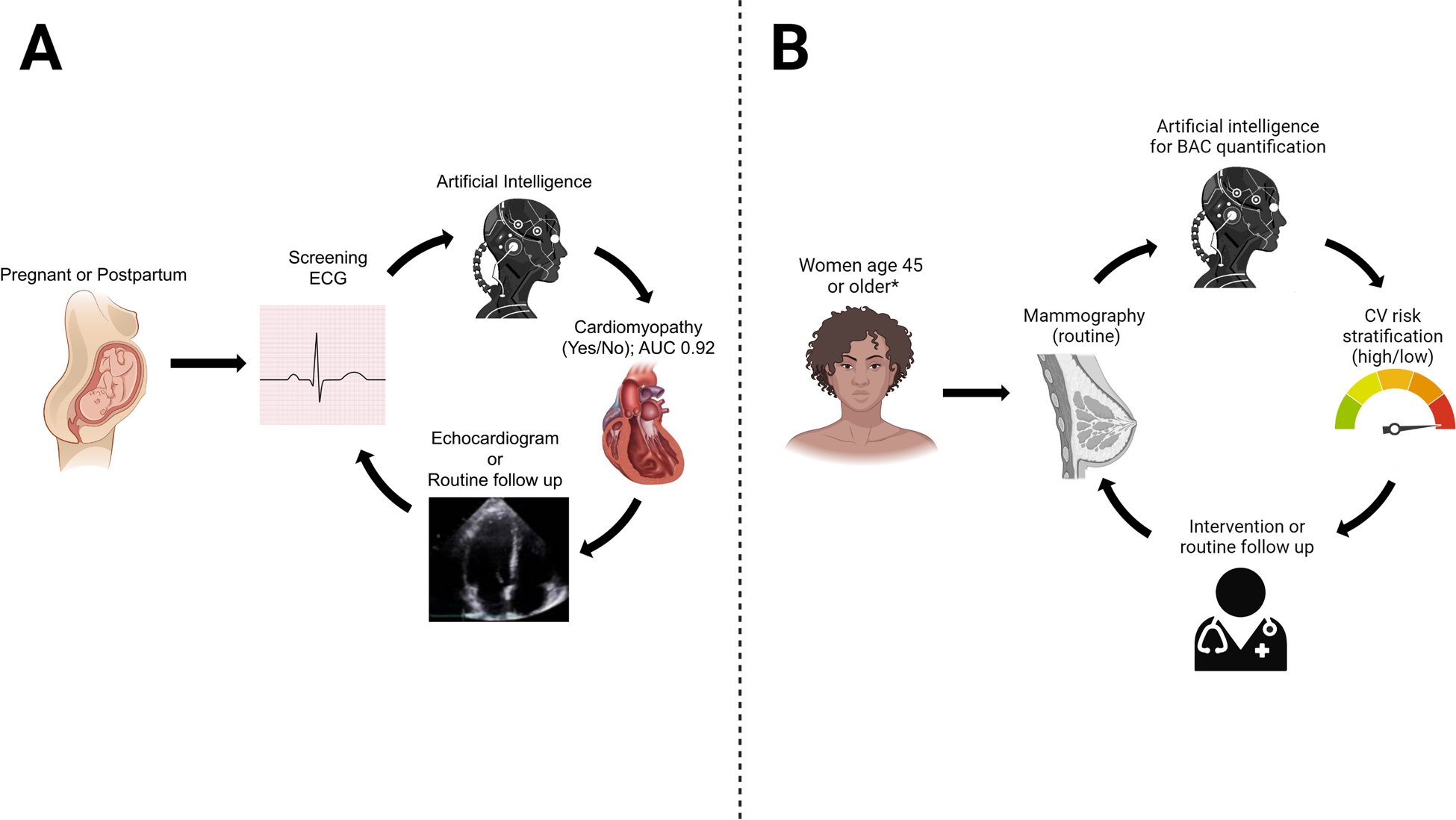
Examples of AI applications to identify cardiovascular disease in women using (a)87 ECG and (b) mammography. BAC - Breast arterial calcification. ECG - Electrocardiogram
We now know that women who experience adverse pregnancy outcomes have an increased risk of future cardiovascular events.10 These obstetrical adverse events are essentially manifestations of abnormal physiologic adaptations to the metabolic and vascular changes required for a successful pregnancy and mirror the abnormal physiology associated with traditional cardiovascular risk factors.82–84 Women with gestational diabetes should have postpartum follow-up screening for abnormal glucose tolerance and receive education regarding lifestyle modifications to reduce the risk of type 2 diabetes and related adverse effects. Preterm delivery is often mediated by inflammatory changes in the cervix and uterus. The association between preterm delivery and cardiovascular disease may be related to a chronic inflammatory state and it is now recognized that cardiovascular disease is mediated in part by inflammation. Recognition of women more prone to increased inflammatory pathways will allow for early intervention strategies to promote improved cardiovascular health.84 Hypertensive disorders of pregnancy are also in part related to abnormal endothelial function, and pre-eclampsia is secondary to abnormal vascular physiology in the placenta, with associated abnormal metabolic adaptation to pregnancy.90, 91 Women with hypertensive disorders of pregnancy should have postpartum screening for hypertension and aggressive cardiovascular risk factor modification.
With cardiovascular disease being the leading cause of death in pregnancy and the postpartum period, targeted cardiovascular screening in the obstetric population is imperative. Specifically, the following timepoints and intervals have been identified as ideal for cardiovascular disease screening in the postpartum period: 6 weeks, 8–12 weeks, 6 months, and 12 months (Figure 4). All these timepoints should include assessments for blood pressure, body mass index, lifestyle counseling, and lipids (except at 6 weeks)92, 93. Following delivery, more than half of women do not receive any routine follow up care with Black women being less likely than White women to receive postpartum care.94, 95 In fact, up to 80% of women who quit tobacco, alcohol and substance use resume postpartum.96
The use of AI algorithms for analysis of repeated blood pressure and weight measurements will limit the additional burden on physicians and other health care providers to review, interpret and provide education/counseling to patients and maximize value-based care. In situations where therapeutic interventions may be indicated, the use of telehealth consultation services can have a huge impact on new mothers who already find it difficult to attend traditional clinic visits.
Middle Age and Menopause
Cardiovascular risk screening should be performed at all routine visits for women ages 40 to 75 years.97 The atherosclerotic cardiovascular disease risk evaluation strategy outlined in the 2019 primary prevention guidelines recognizes how CVD risk factors increase with age, in both women and men, and so provides a comprehensive patient-centered approach to determining which women would benefit from CVD risk reduction strategies such as lipid-lowering therapies.98 Nonetheless, prevention strategies such as exercise programs and lipid-lowering therapies remain under-prescribed in women when compared to men with similar degrees of atherosclerotic risk.99 Given the more extensive complexities associated with risk assessment in women, and the lack of a single tool that incorporates risk factors unique to women, there is an urgent need to develop novel strategies to address the growing gap in physician preparedness to assess cardiovascular risk in women.100
Postmenopausal women represent an especially important demographic for targeted CVD prevention given their increased risk.81 The North American Menopause Society has incorporated the American College of Cardiology/AHA atherosclerotic cardiovascular disease risk score into an app-based clinical decision support tool to assist clinicians in evaluating cardiovascular risk when considering postmenopausal hormone therapy.101 The age at menopause onset and cardiometabolic changes associated with aging have been shown to influence cardiovascular outcomes in women. These data emphasize the need for early intervention and aggressive prevention of cardiometabolic risks during midlife and specifically in the perimenopausal period. For post-menopausal women, comprehensive screening for cardiovascular risk factors and education on lifestyle modification based on the AHA’s Life’s Simple 7 metrics should be encouraged and therapeutic interventions initiated when appropriate. Lipid levels have also been demonstrated to increase in the perimenopausal period,102 highlighting an opportunity for targeted screening and primary prevention strategies to reduce cardiovascular risk in during this critical time point in a woman’s life.
From the patient perspective, there are a variety of ways in which AI algorithms could facilitate addressing these gaps. One way is to improve the provision of appropriate medical education based on atherosclerotic risk following a clinical interaction, and this can be done through an online patient portal or smartphone application linked to the electronic medical records. The online interactive tool, My Life Check,103 is one such tool based on the American Heart Association (AHA)’s Life’s Simple 7 metrics, enables patients to take charge of their cardiovascular health. This readily available tool calculates a heart health score and provides actionable items and recommendations to reduce risk cardiovascular right at the fingertips figuratively and literally. If further extended, digital technologies could help improve women’s access to preventive health services and better integrate health care monitoring into daily work and domestic responsibilities (Figure 4). For example, instead of going into a physician’s office for screening, women might interact with the health system using AI-powered chatbots, electronic patient portals, virtual care, and monitoring devices at home, which provides more flexibility and reduces time pressure.104
On the provider side, health care systems can also leverage AI to personalize the need for and frequency of screening (instead of traditional blanket recommendations) and send notifications and reminders via mobile technologies. This could be particularly beneficial for women as guideline recommendations are often based on randomized controlled trials, though women have been underrepresented in cardiovascular trials.105 An individualized screening approach, powered by big data, could provide more relevant evidence to guide screening in women (Figure 2 & 4). Within a health system, AI algorithms can leverage electronic health records to refine inclusion and exclusion criteria for targeted screening, such that reminders are only sent to patients who would benefit from screening (eg, patients for whom a new diagnosis would change management strategies). Risk enhancing factors can also be integrated into an AI-based clinical decision support tool to assist providers with recommending and prescribing statins for primary prevention. Automated information extraction from patient electronic medical records using natural language processing tools can be used to provide objective additional data for the patient provider discussion when making statin treatment decisions and provide actionable recommendations directly to patients.
Beginning in middle age, both women and men increase the frequency and types of interactions with a health system – contributing enlarging amounts and variety of person-level data that can be harnessed by AI to facilitate individualized CVD risk screening and interventions. Many of these opportunities arise from imaging and biomarker diagnostics, ordered for non-cardiovascular as well as cardiovascular indications. For instance, breast arterial calcifications (BAC) seen on routine mammography has been associated with risk of death and cardiovascular outcomes,20 and neural networks allow for automated detection and quantification of BAC.106 Given the high proportion of aging women who obtain routine mammograms (e.g. 70% of women in Europe), the potential impact of extending CVD risk screening through this modality is huge (Figure 5B). With respect to cardiovascular focused imaging modalities, AI can also augment the quality and quantity of information that can be obtained to refine efficacy and accuracy of CVD screening efforts. For example, AI analysis of computed tomography imaging of type and location of calcified plaques, added to clinical variables, was a better predictor of mortality compared to existing clinical metrics.107, 108 A large and growing body evidence supports the use of AI across all cardiac imaging modalities (including computed tomography, echocardiography, magnetic resonance imaging, nuclear cardiology) for the acquisition and interpretation of imaging data as well as risk prediction algorithms.109, 110 Given that women are more likely to have non-traditional cardiovascular risk factors, as well as unique sex-specific features on cardiovascular imaging, ensemble models (integrating image and clinical data) are likely to improve assessment of cardiovascular risk in women. Comprehensive models, in general, are likely to be especially effective for women. As an example, an ML model based on multiple clinical and demographic variables included in the UK Biobank was a better predictor of cardiovascular outcomes when compared to the Framingham score and also identified unique variables (such as ankle spacing width, self-reported health rating and long-standing illness) as contributing more towards CVD prediction in women compared to men.111
Older Age
With advancing age, the prevalence of subclinical as well as clinical CVD increases substantially in both women and men. As such, the relative benefit of all the individualized screening approaches described above, especially when augmented by AI, can also increase multi-fold so long as the algorithms are trained as well as tested on representative patient populations that include older women with a wider range of potentially influential factors including multisystem co-morbidities and polypharmacy. Advances in digital tools for data capture and in AI algorithms for analytics can be used to stratify risk in secondary prevention and potentially match individual risk profiles with appropriate interventions. These interventions can include prompts to repeat blood pressure checks or lipid profile testing or to initiate or intensify select medication therapies.
In addition to the screening approaches mentioned above, most of which are relevant to multiple age groups, there are digital tools and technologies that are accessible to all and yet potentially of highest yield in older populations including older women given proportionately higher prevalence of under-recognized CVD risk. Notwithstanding initial concerns around technology access barriers for older adults, care delivery transitions during the pandemic have shown that telehealth methods can effectively reach patients of all demographic groups. In fact, telehealth strategies may even augment access to provider care for both women and men from traditionally underserved racial and ethnic groups, albeit with some associated risk for reduced access to follow-up in-person diagnostics112.
Biometric monitoring technologies (BioMETs)113, which are often wearable devices, are another example of technologies that, if accessed appropriately, could offer even greater yield in older than younger populations particularly in areas of secondary prevention114. Due to ease of use, these technologies can improve cardiovascular risk assessment as previously described in the context of hypertension diagnosis and monitoring.115 BioMETs for blood pressure assessment that incorporate a conventional upper arm cuff are preferred as others that use wrist or finger cuffs lack sufficient validation studies and may not provider accurate measurements.116 While screening for hypertension is a classic example, especially given that the age-adjusted prevalence of hypertension is higher in women compared to men,117 additional cardiovascular conditions can be screened for with the use of BioMETs including silent atrial fibrillation118 and asymptomatic or undiagnosed left ventricular dysfunction.119 As AI technology continues to develop in cardiovascular medicine, BioMETs with ECG capabilities soon may be able to provide diagnostic predictions for a myriad of cardiovascular pathologies given the remarkable performance of ECG-based AI models described above.
Women of advancing age are also most likely to benefit from improvements in screening for secondary prevention opportunities. Following the diagnosis of a cardiovascular event, differences in cardiovascular outcomes between women and men are in part related to disparities in the use of guideline-directed medical therapy, such as statins following an acute coronary syndrome.120 Leveraging the strength of AI interfacing with electronic medical record systems using intelligent algorithms and natural language processing tools can provide an avenue for health care institutions to rapidly evaluate the frequency of appropriate prescriptions for guideline-directed optimal medical therapy among women as well as improve medication adherence and compliance by interacting with patients through AI powered chatbots. This in turn makes available important information that can be used to implement provider- or system-based interventions to improve cardiovascular disease management in women.
Another potential intervention strategy is cardiac rehabilitation. Despite a clear mortality benefit for individuals undergoing cardiac rehabilitation following acute coronary syndromes, women are less likely to be referred for,121 participate in,122 or complete123 cardiac rehabilitation compared to men. The delivery of home-based cardiac rehabilitation is not new21 and has been shown to improve participation rates among women and increase physical activity levels.124 Beyond simply tracking physical activity, digital technologies can also be extended to directly engage and facilitate rehabilitation activities in the home. These interventions can involve gamification applications to achieve exercise goals125, 126, food trackers and tools to attain nutritional balance and more accurately summarize dietary consumption compared to diet screener tools127, and algorithms to tailor medication or other therapy adjustments based on heart rate and blood pressure inputs from BioMETs that may be integrated with data from the electronic health record via wired or wireless linkages. In fact, numerous BioMETs and smartphone applications are now commercially available for tracking physical activity. These tools may be especially appealing to women, who are known to be more likely to engage in their medical care, and so can be leveraged to reach women who have qualifying indications for cardiac rehabilitation, although many of these devices still need to be validated for use in cardiac patients.21 Additionally, these tools can play an important role in the longitudinal care of women with cardiovascular disease to provide patient-centered care while accounting for social and gendered structural determinants of health. Women’s heart health clinics with specialty training that integrate the added advantages of digital technologies can serve this growing need.128
Clinical Guideline Implications
The application of AI and digital tools has huge potential to accelerate the collection of sex-specific cardiovascular disease evidence and catalyze the translation of these data into sex-specific cardiovascular clinical practice guidelines. However, at the current time, there are no reports using this methodology, and the challenges with implementation to ensure that representative, comprehensive, and relevant biologic and sociocultural factors are adequately captured are profound. Analysis of big data is a complex process, dependent upon the granularity of the information contained within the specific data banks analyzed, whether they be international, national, regional, public or private, digital or analogue, archived or real-time. For example, sex-specific risk factors for cardiovascular diseases, such as the cardio-obstetric history inclusive of menopause (premature or early), preterm delivery, or gestational hypertensive or diabetic disorders (all of which can increase cardiovascular disease risk later in a woman’s life), are not consistently captured by the Global Burden of Disease Collaborative Network Study, which was used to generate the Lancet Commission on Women and Cardiovascular Disease report.2 Similarly, digital tools and devices can be used individually, or collectively in research trials, to track and report cardiovascular physiologic parameters to generate an evidence base for clinical recommendations. However, it is imperative that such studies have, at a minimum, sex-disaggregated data analysis, and optimally, also include sociocultural, gender, geographic, and generational data, to provide practically applicable information. If such data can be appropriately collected and analyzed, it would likely be of great value in the development of sex-specific guideline-directed management algorithms and contribute to the global improvement of cardiovascular prevention and disease outcomes for women.
Public Health Implications
The growing complex big data environment increasingly exceeds the human cognitive interpretation capability. AI and ML are mathematical techniques used to handle higher dimensional datasets and build algorithms to establish connections between seemingly disparate data elements. In health care, the dizzying expansion of the applications of AI/ML raises both unbridled enthusiasm and concerns about scientific rigor, equity, privacy, and appropriate use of data. For AI/ML, as for all analytical methods, the challenge is to build a robust evidentiary foundation so that results can be used broadly and with confidence. Applied to public health, AI/ML technologies are directly relevant to the emerging agenda of precision population health. This term has been generated by analogy to precision medicine to refer to the delivery of the right interventions to the right populations at the right time.
This, in turn, requires optimizing our understanding of disease patterns by integrating data ranging from biomarkers to environmental factors to improve population health.77 Because precision public health is inherently grounded in a big data ecosystem, the power of AI in public health and in the delivery of health to specific populations requires broad access to large scale datasets and sources of reliable and relevant information. In precision public health, as in all other health domains, an explicit commitment to robust validation and awareness and control of biases in AI-developed algorithms is essential to ensure equitable applications.
As the field is evolving so rapidly, it is not possible to summarize a state-of-the-art static view of the public health applications of AI/ML. Selected examples of potential applications of big data analytics to public health include the identification of individuals with familial hypercholesterolemia129 and the integration of neighborhood characteristics to the analysis of disease patterns.130 The application of AI/ML to population disease surveillance deserves further emphasis. Indeed, precision population surveillance is a prerequisite to effective interventions, and the use and integration of new digital data sources for cardiovascular disease surveillance will surely expand for prevention and management. Specifically, achievement of the AHA’s 2030 Impact Goal131 requires reliance on an effective and nimble population surveillance system that can monitor the burden of disease and the effectiveness of interventions by providing precise assessment in local communities. A recent Policy Statement from the AHA proposed a framework and outlined the powerful model of a digitally based public health surveillance system while also underscoring notable challenges.76
Pragmatic approaches to addressing the challenges of AI applications in health care have been highlighted in a JAMA Viewpoint132 that synthesized the 2019 report of the National Academy of Medicine. The 7 key recommendations are noted in Box 1.1.
Box 1.1. 7 Key Recommendations – Data from Matheny et al. Artificial intelligence in health care: a report from the National Academy of Medicine. JAMA. 2020; 323:509–510.
| • Promoting population-representative data with accessibility, standardization, and quality |
| • Contextualizing the dialogue of transparency and trust requires accepting differential needs |
| • Prioritize ethical, equitable, and inclusive health care AI while addressing explicit and implicit bias |
| • Near-term focus is needed on augmented intelligence vs AI autonomous agents |
| • Develop and deploy appropriate training and educational programs to support health care AI |
| • Leverage frameworks and best practices for learning health care systems, human factors, and implementation science to address the challenges in operationalizing health care AI |
| • Balance innovation with safety via regulation and legislation to promote trust |
Operationalizing the National Academy of Medicine recommendations will undoubtedly be challenging, leading some to predict that “adopting AI in health care will be slow and difficult.”133 These reservations notwithstanding, successful applications of AI/ML offer the promise of a far-reaching impact on public health and clinical medicine, ranging from prevention and prediction to disease management strategies and community surveillance.134
Given the growing applications of AI/ML to clinical decision making, its regulation is of paramount importance. In September 2020, Nature Publishing Group Digital Medicine reported on the status of FDA-approved medical devices and algorithms135 and launched an open access database.136 The authors inventoried 64 devices and algorithms, most developed in the field of radiology, followed by cardiology. In January 2021, the Lancet reporting on the same topic compared regulations in the US and Europe.137 The authors identified 222 devices approved in the US and 240 approved in Europe with a similar preponderance of radiology and cardiology applications. Both reports underscored the need for improved taxonomies, greater transparency, and more explicit regulations of the approval of AI/ML devices and algorithms.
Conclusion
The advances in computer technologies and the exponential growth in the amount of digital data has been mostly responsible for the success seen in the field of AI/ML. In the last few years, there has been a huge jump in the number of scientific articles demonstrating the effectiveness of AI tools or methods in clinical medicine. The field of cardiology has particularly seen tremendous growth in this space likely due to the fact that cardiovascular treatment decisions are based on multiple digitized patient data and diagnostic tests138. We know now that AI algorithms can rapidly synthesize and interpret large amounts of clinical data at levels that vastly exceed human capacity. In addition, the creation and widespread adoption of various digital tools presents potential opportunities to expand our approach to cardiovascular disease screening and prevention. Such tools include patient communication portals (either facilitating clinical care or providing automated support/reminders through text-messaging systems), educational wellness smartphone applications, and a variety of wearable devices that track physical activity, behavior, and biometrics139. The breadth of these technologies can be daunting, but international societies have begun to create online resources that will help clinicians navigate the increasingly rich ecosystem of digital technologies. One such example is the CVD Prevention Toolbox created and maintained by the European Society of Cardiology.140 As the landscape of digital resources becomes more crowded, formal appraisal by trusted third parties, such as cardiovascular professional societies, who may become essential arbitrators of quality, will be increasingly essential.
Given women bear a high burden of cardiovascular disease and are less likely to be diagnosed or treated2, we have a unique opportunity to leverage available AI and digital tools to alter the current landscape of cardiovascular care in women, beginning with screening. These technologies could potentially narrow the disparity gap, as AI algorithms for cardiovascular prevention can be programmed to specifically incorporate sex- and gender-specific factors141.
Despite the potential opportunities for AI in cardiovascular disease screening, future research is needed to examine whether digital technologies fulfill their promise to improve patient outcomes and reduce disparities. Furthermore, the development and implementation of AI-powered decision support and communication tools embedded into existing electronic health record systems come with a cost, and the cost-effectiveness of such strategies needs to be evaluated before broad adoption.142
Acknowledgments
We would like to thank Andrea (Carolina) Morales Lara for her assistance in creating the graphical illustrations used in this manuscript. All illustrations created with BioRender.com
Funding:
Dr. Adedinsewo receives research support from the Mayo Clinic Women’s Health Research Center and the Mayo Clinic Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Program funded by the National Institutes of Health, grant number K12 HD065987. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures. None
References
- 1.Cushman M, Shay CM, Howard VJ, Jiménez MC, Lewey J, McSweeney JC, Newby LK, Poudel R, Reynolds HR and Rexrode KM. Ten-year differences in women’s awareness related to coronary heart disease: results of the 2019 American Heart Association National Survey: a special report from the American Heart Association. Circulation. 2021;143:e239–e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA, Guerrero M, Kunadian V, Lam CSP, Maas A, Mihailidou AS, Olszanecka A, Poole JE, Saldarriaga C, Saw J, Zuhlke L and Mehran R. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet. 2021;397:2385–2438. [DOI] [PubMed] [Google Scholar]
- 3.Petersen EE, Davis NL, Goodman D, Cox S, Syverson C, Seed K, Shapiro-Mendoza C, Callaghan WM and Barfield W. Racial/ethnic disparities in pregnancy-related deaths—United States, 2007–2016. Morbidity and Mortality Weekly Report. 2019;68:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta LS, Warnes CA, Bradley E, Burton T, Economy K, Mehran R, Safdar B, Sharma G, Wood M, Valente AM, Volgman AS, American Heart Association Council on Clinical C, Council on Arteriosclerosis T, Vascular B, Council on C, Stroke N and Stroke C. Cardiovascular Considerations in Caring for Pregnant Patients: A Scientific Statement From the American Heart Association. Circulation. 2020;141:e884–e903. [DOI] [PubMed] [Google Scholar]
- 5.Brown HL, Warner JJ, Gianos E, Gulati M, Hill AJ, Hollier LM, Rosen SE, Rosser ML and Wenger NK. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation. 2018;137:e843–e852. [DOI] [PubMed] [Google Scholar]
- 6.Norris CM, Yip CY, Nerenberg KA, Clavel MA, Pacheco C, Foulds HJ, Hardy M, Gonsalves CA, Jaffer S and Parry M. State of the science in women’s cardiovascular disease: a Canadian perspective on the influence of sex and gender. Journal of the American Heart Association. 2020;9:e015634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connelly PJ, Azizi Z, Alipour P, Delles C, Pilote L and Raparelli V. The importance of Gender to Understand Sex Differences in Cardiovascular Disease. Canadian Journal of Cardiology. 2021. [DOI] [PubMed] [Google Scholar]
- 8.Miller VM. Why are sex and gender important to basic physiology and translational and individualized medicine? American journal of physiology Heart and circulatory physiology. 2014;306:H781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morselli E, Santos RS, Criollo A, Nelson MD, Palmer BF and Clegg DJ. The effects of oestrogens and their receptors on cardiometabolic health. Nature Reviews Endocrinology. 2017;13:352–364. [DOI] [PubMed] [Google Scholar]
- 10.Parikh NI, Gonzalez JM, Anderson CAM, Judd SE, Rexrode KM, Hlatky MA, Gunderson EP, Stuart JJ, Vaidya D, American Heart Association Council on E, Prevention, Council on Arteriosclerosis T, Vascular B, Council on C, Stroke N and the Stroke C. Adverse Pregnancy Outcomes and Cardiovascular Disease Risk: Unique Opportunities for Cardiovascular Disease Prevention in Women: A Scientific Statement From the American Heart Association. Circulation. 2021;143:e902–e916. [DOI] [PubMed] [Google Scholar]
- 11.Arnett DK, Khera A and Blumenthal RS. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Part 1, Lifestyle and Behavioral Factors. JAMA Cardiol. 2019;4:1043–1044. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, De Ferranti S, Faiella-Tommasino J and Forman DE. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2019;73:3168–3209. [DOI] [PubMed] [Google Scholar]
- 13.Garcia M, Mulvagh SL, Merz CNB, Buring JE and Manson JE. Cardiovascular Disease in Women: Clinical Perspectives. Circulation research. 2016;118:1273–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan NA, Daskalopoulou SS, Karp I, Eisenberg MJ, Pelletier R, Tsadok MA, Dasgupta K, Norris CM, Pilote L and Team GP. Sex differences in acute coronary syndrome symptom presentation in young patients. JAMA internal medicine. 2013;173:1863–1871. [DOI] [PubMed] [Google Scholar]
- 15.van Oosterhout RE, de Boer AR, Maas AH, Rutten FH, Bots ML and Peters SA. Sex Differences in Symptom Presentation in Acute Coronary Syndromes: A Systematic Review and Meta‐analysis. Journal of the American Heart Association. 2020;9:e014733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Dungen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP, Investigators P-H and Committees. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 17.Carcel C, Wang X, Sandset EC, Delcourt C, Arima H, Lindley R, Hackett ML, Lavados P, Robinson TG, Muñoz Venturelli P, Olavarría VV, Brunser A, Berge E, Chalmers J, Woodward M and Anderson CS. Sex differences in treatment and outcome after stroke: Pooled analysis including 19,000 participants. Neurology. 2019;93:e2170–e2180. [DOI] [PubMed] [Google Scholar]
- 18.Mensah GA, Dietz WH, Harris VB, Henson R, Labarthe DR, Vinicor F and Wechsler H. Prevention and Control of Coronary Heart Disease and Stroke—Nomenclature for Prevention Approaches in Public Health: A Statement for Public Health Practice from the Centers for Disease Control and Prevention. American Journal of Preventive Medicine. 2005;29:152–157. [DOI] [PubMed] [Google Scholar]
- 19.Murray DM, Cross WP, Simons-Morton D, Engel J, Portnoy B, Wu J, Watson PA and Olkkola S. Enhancing the quality of prevention research supported by the National Institutes of Health. American journal of public health. 2015;105:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trimboli RM, Codari M, Guazzi M and Sardanelli F. Screening mammography beyond breast cancer: breast arterial calcifications as a sex-specific biomarker of cardiovascular risk. European Journal of Radiology. 2019;119:108636. [DOI] [PubMed] [Google Scholar]
- 21.Falter M, Scherrenberg M and Dendale P. Digital Health in Cardiac Rehabilitation and Secondary Prevention: A Search for the Ideal Tool. Sensors (Basel). 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Box GE and Draper NR. Empirical model-building and response surfaces: John Wiley & Sons; 1987. [Google Scholar]
- 23.Lopez-Jimenez F, Attia Z, Arruda-Olson AM, Carter R, Chareonthaitawee P, Jouni H, Kapa S, Lerman A, Luong C, Medina-Inojosa JR, Noseworthy PA, Pellikka PA, Redfield MM, Roger VL, Sandhu GS, Senecal C and Friedman PA. Artificial Intelligence in Cardiology: Present and Future. Mayo Clin Proc. 2020;95:1015–1039. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein BA, Navar AM and Carter RE. Moving beyond regression techniques in cardiovascular risk prediction: applying machine learning to address analytic challenges. European Heart Journal. 2016;38:1805–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamthikar A, Gupta D, Saba L, Khanna NN, Araki T, Viskovic K, Mavrogeni S, Laird JR, Pareek G, Miner M, Sfikakis PP, Protogerou A, Viswanathan V, Sharma A, Nicolaides A, Kitas GD and Suri JS. Cardiovascular/stroke risk predictive calculators: a comparison between statistical and machine learning models. Cardiovasc Diagn Ther. 2020;10:919–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quesada JA, Lopez‐Pineda A, Gil‐Guillén VF, Durazo‐Arvizu R, Orozco‐Beltrán D, López-Domenech A and Carratalá‐Munuera C. Machine learning to predict cardiovascular risk. International journal of clinical practice. 2019;73:e13389. [DOI] [PubMed] [Google Scholar]
- 27.Nusinovici S, Tham YC, Yan MYC, Ting DSW, Li J, Sabanayagam C, Wong TY and Cheng C-Y. Logistic regression was as good as machine learning for predicting major chronic diseases. Journal of clinical epidemiology. 2020;122:56–69. [DOI] [PubMed] [Google Scholar]
- 28.Christodoulou E, Ma J, Collins GS, Steyerberg EW, Verbakel JY and Van Calster B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. Journal of clinical epidemiology. 2019;110:12–22. [DOI] [PubMed] [Google Scholar]
- 29.Popa TR and Mocanu AC. Medical Data Storage, Visualization and Interpretation: A Case Study Using a Proprietary ECG XML Format. Annals of the University of Craiova Series: Automation, Computers, Electronics and Mechatronics. 2011;8:44–49. [Google Scholar]
- 30.Attia ZI, Kapa S, Lopez-Jimenez F, McKie PM, Ladewig DJ, Satam G, Pellikka PA, Enriquez-Sarano M, Noseworthy PA, Munger TM, Asirvatham SJ, Scott CG, Carter RE and Friedman PA. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med. 2019;25:70–74. [DOI] [PubMed] [Google Scholar]
- 31.Ko WY, Siontis KC, Attia ZI, Carter RE, Kapa S, Ommen SR, Demuth SJ, Ackerman MJ, Gersh BJ, Arruda-Olson AM, Geske JB, Asirvatham SJ, Lopez-Jimenez F, Nishimura RA, Friedman PA and Noseworthy PA. Detection of Hypertrophic Cardiomyopathy Using a Convolutional Neural Network-Enabled Electrocardiogram. J Am Coll Cardiol. 2020;75:722–733. [DOI] [PubMed] [Google Scholar]
- 32.Grogan M, Lopez-Jimenez F, Cohen-Shelly M, Dispenzieri A, Attia ZI, Abou Ezzedine OF, Lin G, Kapa S, Borgeson DD, Friedman PA and Murphree DH, Jr. Artificial Intelligence-Enhanced Electrocardiogram for the Early Detection of Cardiac Amyloidosis. Mayo Clin Proc. 2021. [DOI] [PubMed] [Google Scholar]
- 33.Cohen-Shelly M, Attia ZI, Friedman PA, Ito S, Essayagh BA, Ko WY, Murphree DH, Michelena HI, Enriquez-Sarano M, Carter RE, Johnson PW, Noseworthy PA, Lopez-Jimenez F and Oh JK. Electrocardiogram screening for aortic valve stenosis using artificial intelligence. Eur Heart J. 2021;42:2885–2896. [DOI] [PubMed] [Google Scholar]
- 34.Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, Carter RE, Yao X, Rabinstein AA, Erickson BJ, Kapa S and Friedman PA. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394:861–867. [DOI] [PubMed] [Google Scholar]
- 35.Adedinsewo D, Carter RE, Attia Z, Johnson P, Kashou AH, Dugan JL, Albus M, Sheele JM, Bellolio F, Friedman PA, Lopez-Jimenez F and Noseworthy PA. Artificial Intelligence-Enabled ECG Algorithm to Identify Patients With Left Ventricular Systolic Dysfunction Presenting to the Emergency Department With Dyspnea. Circ Arrhythm Electrophysiol. 2020;13:e008437. [DOI] [PubMed] [Google Scholar]
- 36.Attia IZ, Tseng AS, Benavente ED, Medina-Inojosa JR, Clark TG, Malyutina S, Kapa S, Schirmer H, Kudryavtsev AV and Noseworthy PA. External validation of a deep learning electrocardiogram algorithm to detect ventricular dysfunction. International journal of cardiology. 2021;329:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noseworthy PA, Attia ZI, Brewer LC, Hayes SN, Yao X, Kapa S, Friedman PA and Lopez-Jimenez F. Assessing and Mitigating Bias in Medical Artificial Intelligence: The Effects of Race and Ethnicity on a Deep Learning Model for ECG Analysis. Circ Arrhythm Electrophysiol. 2020;13:e007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao X, Rushlow DR, Inselman JW, McCoy RG, Thacher TD, Behnken EM, Bernard ME, Rosas SL, Akfaly A, Misra A, Molling PE, Krien JS, Foss RM, Barry BA, Siontis KC, Kapa S, Pellikka PA, Lopez-Jimenez F, Attia ZI, Shah ND, Friedman PA and Noseworthy PA. Artificial intelligence-enabled electrocardiograms for identification of patients with low ejection fraction: a pragmatic, randomized clinical trial. Nat Med. 2021;27:815–819. [DOI] [PubMed] [Google Scholar]
- 39.Iba K, Shinozaki T, Maruo K and Noma H. Re-evaluation of the comparative effectiveness of bootstrap-based optimism correction methods in the development of multivariable clinical prediction models. BMC Medical Research Methodology. 2021;21:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith GC, Seaman SR, Wood AM, Royston P and White IR. Correcting for optimistic prediction in small data sets. Am J Epidemiol. 2014;180:318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrell FE Jr., Lee KL and Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 42.Tat E, Bhatt DL and Rabbat MG. Addressing bias: artificial intelligence in cardiovascular medicine. The Lancet Digital Health. 2020;2:e635–e636. [DOI] [PubMed] [Google Scholar]
- 43.Paulus JK and Kent DM. Predictably unequal: understanding and addressing concerns that algorithmic clinical prediction may increase health disparities. npj Digital Medicine. 2020;3:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obermeyer Z, Nissan R, Stern M, Eaneff S, Bembeneck EJ, Mullainathan S. Algorithmic Bias Playbook. Center for Applied AI at Chicago Booth. 2021. Jun. URL:https://www.chicagobooth.edu/-/media/project/chicago-booth/centers/caai/docs/algorithmic-bias-playbook-june-2021.pdf [Google Scholar]
- 45.Mitchell S, Potash E, Barocas S, D’Amour A and Lum K. Algorithmic Fairness: Choices, Assumptions, and Definitions. Annual Review of Statistics and Its Application. 2021;8:141–163. [Google Scholar]
- 46.Buolamwini J and Gebru T. Gender shades: Intersectional accuracy disparities in commercial gender classification. Conference on fairness, accountability and transparency. 2018:77–91. [Google Scholar]
- 47.Buolamwini J, Gebru T, Raynham H, Raji D, Zuckerman E. The Gender Shades Project. URL: http://gendershades.org/overview.html. Access Date: September 8, 2021
- 48.Raji ID and Buolamwini J. Actionable auditing: Investigating the impact of publicly naming biased performance results of commercial ai products. Proceedings of the 2019 AAAI/ACM Conference on AI, Ethics, and Society. 2019:429–435. [Google Scholar]
- 49.Obermeyer Z, Powers B, Vogeli C and Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366:447–453. [DOI] [PubMed] [Google Scholar]
- 50.Larrazabal AJ, Nieto N, Peterson V, Milone DH and Ferrante E. Gender imbalance in medical imaging datasets produces biased classifiers for computer-aided diagnosis. Proceedings of the National Academy of Sciences. 2020;117:12592–12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomašev N, Glorot X, Rae JW, Zielinski M, Askham H, Saraiva A, Mottram A, Meyer C, Ravuri S and Protsyuk I. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature. 2019;572:116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lomas N. DeepMind touts predictive healthcare AI ‘breakthrough’ trained on heavily skewed data. 2019;2021. [Google Scholar]
- 53.Herrman J. We’re Stuck with the Tech Giants. But They’re Stuck with Each Other. New York Times Magazine: New York, NY, USA. 2019. [Google Scholar]
- 54.West SM, Whittaker M and Crawford K. Discriminating systems. AI Now. 2019. [Google Scholar]
- 55.Wiegand T, Krishnamurthy R, Kuglitsch M, Lee N, Pujari S, Salathé M, Wenzel M and Xu S. WHO and ITU establish benchmarking process for artificial intelligence in health. The Lancet. 2019;394:9–11. [DOI] [PubMed] [Google Scholar]
- 56.Sieck CJ, Sheon A, Ancker JS, Castek J, Callahan B and Siefer A. Digital inclusion as a social determinant of health. NPJ Digital Medicine. 2021;4:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown KL, Ridout DA, Hoskote A, Verhulst L, Ricci M and Bull C. Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart. 2006;92:1298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters S, Johnson R, Birch S, Zentner D, Hershberger RE and Fatkin D. Familial Dilated Cardiomyopathy. Heart Lung Circ. 2020;29:566–574. [DOI] [PubMed] [Google Scholar]
- 59.Siontis KC, Liu K, Bos JM, Attia ZI, Cohen-Shelly M, Arruda-Olson AM, Zanjirani Farahani N, Friedman PA, Noseworthy PA and Ackerman MJ. Detection of hypertrophic cardiomyopathy by an artificial intelligence electrocardiogram in children and adolescents. Int J Cardiol. 2021. [DOI] [PubMed] [Google Scholar]
- 60.Bos JM, Attia ZI, Albert DE, Noseworthy PA, Friedman PA and Ackerman MJ. Use of Artificial Intelligence and Deep Neural Networks in Evaluation of Patients With Electrocardiographically Concealed Long QT Syndrome From the Surface 12-Lead Electrocardiogram. JAMA Cardiol. 2021;6:532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giudicessi JR, Schram M, Bos JM, Galloway CD, Shreibati JB, Johnson PW, Carter RE, Disrud LW, Kleiman R, Attia ZI, Noseworthy PA, Friedman PA, Albert DE and Ackerman MJ. Artificial Intelligence-Enabled Assessment of the Heart Rate Corrected QT Interval Using a Mobile Electrocardiogram Device. Circulation. 2021;143:1274–1286. [DOI] [PubMed] [Google Scholar]
- 62.Harmon KG, Asif IM, Maleszewski JJ, Owens DS, Prutkin JM, Salerno JC, Zigman ML, Ellenbogen R, Rao AL and Ackerman MJ. Incidence, cause, and comparative frequency of sudden cardiac death in national collegiate athletic association athletes: a decade in review. Circulation. 2015;132:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valente AM, Landzberg MJ, Gianola A, Harmon AJ, Cook S, Ting JG, Stout K, Kuehl K, Khairy P, Kay JD, Earing M, Houser L, Broberg C, Milliren C, Opotowsky AR, Webb G, Verstappen A, Gurvitz M, Alliance for Adult Research in Congenital Cardiology I and Adult Congenital Heart A. Improving heart disease knowledge and research participation in adults with congenital heart disease (the Health, Education and Access Research Trial: HEART-ACHD). Int J Cardiol. 2013;168:3236–40. [DOI] [PubMed] [Google Scholar]
- 64.Krittanawong C, Zhang H, Wang Z, Aydar M and Kitai T. Artificial Intelligence in Precision Cardiovascular Medicine. J Am Coll Cardiol. 2017;69:2657–2664. [DOI] [PubMed] [Google Scholar]
- 65.Ghassemian H and RASOULI KA. EARLY DETECTION OF PEDIATRIC HEART DISEASE BY AUTOMATED SPECTRAL ANALYSIS OF PHONOCARDIOGRAM. 2015.
- 66.Alkhodari M and Fraiwan L. Convolutional and recurrent neural networks for the detection of valvular heart diseases in phonocardiogram recordings. Computer Methods and Programs in Biomedicine. 2021;200:105940. [DOI] [PubMed] [Google Scholar]
- 67.Vergales J, Peregoy L, Zalewski J and Plummer ST. Use of a Digital Monitoring Platform to Improve Outcomes in Infants With a Single Ventricle. World Journal for Pediatric and Congenital Heart Surgery. 2020;11:753–759. [DOI] [PubMed] [Google Scholar]
- 68.Rudd NA, Ghanayem NS, Hill GD, Lambert LM, Mussatto KA, Nieves JA, Robinson S, Shirali G, Steltzer MM and Uzark K. Interstage Home Monitoring for Infants With Single Ventricle Heart Disease: Education and Management: A Scientific Statement From the American Heart Association. Journal of the American Heart Association. 2020;9:e014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC and Rimm EB. Healthy Lifestyle in the Primordial Prevention of Cardiovascular Disease Among Young Women. Journal of the American College of Cardiology. 2015;65:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koskinen J, Juonala M, Dwyer T, Venn A, Thomson R, Bazzano L, Berenson GS, Sabin MA, Burns TL, Viikari JSA, Woo JG, Urbina EM, Prineas R, Hutri-Kahonen N, Sinaiko A, Jacobs D, Steinberger J, Daniels S, Raitakari OT and Magnussen CG. Impact of Lipid Measurements in Youth in Addition to Conventional Clinic-Based Risk Factors on Predicting Preclinical Atherosclerosis in Adulthood: International Childhood Cardiovascular Cohort Consortium. Circulation. 2018;137:1246–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bucholz EM, Gooding HC and de Ferranti SD. Awareness of Cardiovascular Risk Factors in U.S. Young Adults Aged 18–39 Years. Am J Prev Med. 2018;54:e67–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Ferranti SD, Steinberger J, Ameduri R, Baker A, Gooding H, Kelly AS, Mietus-Snyder M, Mitsnefes MM, Peterson AL, St-Pierre J, Urbina EM, Zachariah JP and Zaidi AN. Cardiovascular Risk Reduction in High-Risk Pediatric Patients: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e603–e634. [DOI] [PubMed] [Google Scholar]
- 73.Umer A, Kelley GA, Cottrell LE, Giacobbi P Jr., Innes KE and Lilly CL. Childhood obesity and adult cardiovascular disease risk factors: a systematic review with meta-analysis. BMC Public Health. 2017;17:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McSweeney JC, Rosenfeld AG, Abel WM, Braun LT, Burke LE, Daugherty SL, Fletcher GF, Gulati M, Mehta LS, Pettey C, Reckelhoff JF, American Heart Association Council on C, Stroke Nursing CoCCCoE, Prevention CoHCoL, Cardiometabolic H, Council on Quality of C and Outcomes R. Preventing and Experiencing Ischemic Heart Disease as a Woman: State of the Science: A Scientific Statement From the American Heart Association. Circulation. 2016;133:1302–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kavousi M, Pisinger C, Barthelemy J-C, De Smedt D, Koskinas K, Marques-Vidal P, Panagiotakos D, Prescott EB, Tiberi M, Vassiliou VS and Løchen M-L. Electronic cigarettes and health with special focus on cardiovascular effects: position paper of the European Association of Preventive Cardiology (EAPC). European Journal of Preventive Cardiology. 2020;28:1552–1566. [DOI] [PubMed] [Google Scholar]
- 76.Roger VL, Sidney S, Fairchild AL, Howard VJ, Labarthe DR, Shay CM, Tiner AC, Whitsel LP and Rosamond WD. Recommendations for Cardiovascular Health and Disease Surveillance for 2030 and Beyond: A Policy Statement From the American Heart Association. Circulation. 2020;141:e104–e119. [DOI] [PubMed] [Google Scholar]
- 77.Khoury MJ, Engelgau M, Chambers DA and Mensah GA. Beyond public health genomics: can big data and predictive analytics deliver precision public health? Public health genomics. 2018;21:244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horvitz E and Mulligan D. Data, privacy, and the greater good. Science. 2015;349:253–255. [DOI] [PubMed] [Google Scholar]
- 79.Gostin LO, Halabi SF and Wilson K. Health Data and Privacy in the Digital Era. JAMA. 2018;320:233–234. [DOI] [PubMed] [Google Scholar]
- 80.Hunter RF, Gough A, O’Kane N, McKeown G, Fitzpatrick A, Walker T, McKinley M, Lee M and Kee F. Ethical Issues in Social Media Research for Public Health. American Journal of Public Health. 2018;108:343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mosca L, Grundy SM, Judelson D, King K, Limacher M, Oparil S, Pasternak R, Pearson TA, Redberg RF and Smith SC Jr. Guide to preventive cardiology for women. Circulation. 1999;99:2480–2484. [DOI] [PubMed] [Google Scholar]
- 82.Smith GN, Louis JM and Saade GR. Pregnancy and the Postpartum Period as an Opportunity for Cardiovascular Risk Identification and Management. Obstet Gynecol. 2019;134:851–862. [DOI] [PubMed] [Google Scholar]
- 83.Cusimano MC, Pudwell J, Roddy M, Cho CK and Smith GN. The maternal health clinic: an initiative for cardiovascular risk identification in women with pregnancy-related complications. Am J Obstet Gynecol. 2014;210:438 e1–9. [DOI] [PubMed] [Google Scholar]
- 84.Sattar N and Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325:157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kassebaum NJ, Barber RM, Bhutta ZA, Dandona L, Gething PW, Hay SI, Kinfu Y, Larson HJ, Liang X and Lim SS. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388:1775–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davis MB, Arany Z, McNamara DM, Goland S and Elkayam U. Peripartum cardiomyopathy: JACC state-of-the-art review. Journal of the American College of Cardiology. 2020;75:207–221. [DOI] [PubMed] [Google Scholar]
- 87.Adedinsewo DA, Johnson PW, Douglass EJ, Attia IZ, Phillips SD, Goswami RM, Yamani MH, Connolly HM, Rose CH, Sharpe EE, Blauwet L, Lopez-Jimenez F, Friedman PA, Carter RE and Noseworthy PA. Detecting Cardiomyopathies in Pregnancy and the Postpartum Period with an Electrocardiogram-Based Deep Learning Model. European Heart Journal - Digital Health. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davidson L and Boland MR. Enabling pregnant women and their physicians to make informed medication decisions using artificial intelligence. Journal of Pharmacokinetics and Pharmacodynamics. 2020;47:305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoppe KK, Williams M, Thomas N, Zella JB, Drewry A, Kim K, Havighurst T and Johnson HM. Telehealth with remote blood pressure monitoring for postpartum hypertension: A prospective single-cohort feasibility study. Pregnancy Hypertension. 2019;15:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leon LJ, McCarthy FP, Direk K, Gonzalez-Izquierdo A, Prieto-Merino D, Casas JP and Chappell L. Preeclampsia and Cardiovascular Disease in a Large UK Pregnancy Cohort of Linked Electronic Health Records: A CALIBER Study. Circulation. 2019;140:1050–1060. [DOI] [PubMed] [Google Scholar]
- 91.Rich-Edwards JW, Stuart JJ, Skurnik G, Roche AT, Tsigas E, Fitzmaurice GM, Wilkins-Haug LE, Levkoff SE and Seely EW. Randomized Trial to Reduce Cardiovascular Risk in Women with Recent Preeclampsia. J Womens Health (Larchmt). 2019;28:1493–1504. [DOI] [PubMed] [Google Scholar]
- 92.Davis MB, Arendt K, Bello NA, Brown H, Briller J, Epps K, Hollier L, Langen E, Park K, Walsh MN, Williams D, Wood M, Silversides CK, Lindley KJ, American College of Cardiology Cardiovascular Disease in Women C and the Cardio-Obstetrics Work G. Team-Based Care of Women With Cardiovascular Disease From Pre-Conception Through Pregnancy and Postpartum: JACC Focus Seminar 1/5. J Am Coll Cardiol. 2021;77:1763–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.ACOG. ACOG Practice Bulletin No. 212: Pregnancy and Heart Disease. Obstet Gynecol. 2019;133:e320–e356. [DOI] [PubMed] [Google Scholar]
- 94.Thiel de Bocanegra H, Braughton M, Bradsberry M, Howell M, Logan J and Schwarz EB. Racial and ethnic disparities in postpartum care and contraception in California’s Medicaid program. American Journal of Obstetrics and Gynecology. 2017;217:47.e1–47.e7. [DOI] [PubMed] [Google Scholar]
- 95.Hauspurg A, Lemon LS, Quinn BA, Binstock A, Larkin J, Beigi RH, Watson AR and Simhan HN. A Postpartum Remote Hypertension Monitoring Protocol Implemented at the Hospital Level. Obstet Gynecol. 2019;134:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stuebe AM, Kendig S, Suplee PD and D’Oria R. Consensus Bundle on Postpartum Care Basics: From Birth to the Comprehensive Postpartum Visit. Obstetrics & Gynecology. 2021;137. [DOI] [PubMed] [Google Scholar]
- 97.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Munoz D, Smith SC Jr., Virani SS, Williams KA Sr., Yeboah J and Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carey RM and Whelton PK. The 2017 American College of Cardiology/American Heart Association Hypertension Guideline: A Resource for Practicing Clinicians. Ann Intern Med. 2018;168:359–360. [DOI] [PubMed] [Google Scholar]
- 99.Mosca L, Linfante AH, Benjamin EJ, Berra K, Hayes SN, Walsh BW, Fabunmi RP, Kwan J, Mills T and Simpson SL. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation. 2005;111:499–510. [DOI] [PubMed] [Google Scholar]
- 100.Isakadze N, Mehta PK, Law K, Dolan M and Lundberg GP. Addressing the gap in physician preparedness to assess cardiovascular risk in women: a comprehensive approach to cardiovascular risk assessment in women. Current treatment options in cardiovascular medicine. 2019;21:1–11. [DOI] [PubMed] [Google Scholar]
- 101.Manson JE, Ames JM, Shapiro M, Gass ML, Shifren JL, Stuenkel CA, Pinkerton JV, Kaunitz AM, Pace DT and Kagan R. Algorithm and mobile app for menopausal symptom management and hormonal/non-hormonal therapy decision making: a clinical decision-support tool from The North American Menopause Society. Menopause. 2015;22:247–253. [DOI] [PubMed] [Google Scholar]
- 102.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B and Sutton-Tyrrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? Journal of the American College of Cardiology. 2009;54:2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.My Life Check | Life’s Simple 7. URL: https://www.heart.org/en/healthy-living/healthy-lifestyle/my-life-check--lifes-simple-7 Access Date: September 8, 2021
- 104.Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang W, Wen J, Xing Y, Wu F, Xia Y, Liu T, Wu F, Liang Z, Liu F, Zhao Y, Li R, Li X, Zhang L, Guo J, Burnside G, Chen Y and Lip GYH. Mobile Health Technology to Improve Care for Patients With Atrial Fibrillation. J Am Coll Cardiol. 2020;75:1523–1534. [DOI] [PubMed] [Google Scholar]
- 105.Cho L, Vest AR, O’Donoghue ML, Ogunniyi MO, Sarma AA, Denby KJ, Lau ES, Poole JE, Lindley KJ and Mehran R. Increasing Participation of Women in Cardiovascular Trials: JACC Council Perspectives. J Am Coll Cardiol. 2021;78:737–751. [DOI] [PubMed] [Google Scholar]
- 106.Wang J, Ding H, Bidgoli FA, Zhou B, Iribarren C, Molloi S and Baldi P. Detecting Cardiovascular Disease from Mammograms With Deep Learning. IEEE Transactions on Medical Imaging. 2017;36:1172–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rogers MA and Aikawa E. Cardiovascular calcification: artificial intelligence and big data accelerate mechanistic discovery. Nat Rev Cardiol. 2019;16:261–274. [DOI] [PubMed] [Google Scholar]
- 108.Motwani M, Dey D, Berman DS, Germano G, Achenbach S, Al-Mallah MH, Andreini D, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJ, Cury RC, Delago A, Gomez M, Gransar H, Hadamitzky M, Hausleiter J, Hindoyan N, Feuchtner G, Kaufmann PA, Kim YJ, Leipsic J, Lin FY, Maffei E, Marques H, Pontone G, Raff G, Rubinshtein R, Shaw LJ, Stehli J, Villines TC, Dunning A, Min JK and Slomka PJ. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: a 5-year multicentre prospective registry analysis. Eur Heart J. 2017;38:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dey D, Slomka PJ, Leeson P, Comaniciu D, Shrestha S, Sengupta PP and Marwick TH. Artificial Intelligence in Cardiovascular Imaging: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:1317–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin A, Kolossváry M, Išgum I, Maurovich-Horvat P, Slomka PJ and Dey D. Artificial intelligence: improving the efficiency of cardiovascular imaging. Expert Rev Med Devices. 2020;17:565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alaa AM, Bolton T, Di Angelantonio E, Rudd JHF and van der Schaar M. Cardiovascular disease risk prediction using automated machine learning: A prospective study of 423,604 UK Biobank participants. PLOS ONE. 2019;14:e0213653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yuan N, Pevnick JM, Botting PG, Elad Y, Miller SJ, Cheng S and Ebinger JE. Patient Use and Clinical Practice Patterns of Remote Cardiology Clinic Visits in the Era of COVID-19. JAMA Network Open. 2021;4:e214157–e214157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goldsack JC, Coravos A, Bakker JP, Bent B, Dowling AV, Fitzer-Attas C, Godfrey A, Godino JG, Gujar N, Izmailova E, Manta C, Peterson B, Vandendriessche B, Wood WA, Wang KW and Dunn J. Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs). npj Digital Medicine. 2020;3:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shufelt CL, Kim A, Joung S, Barsky L, Arnold C, Cheng S, Dhawan S, Fuller G, Speier W and Lopez M. Biometric and Psychometric Remote Monitoring and Cardiovascular Risk Biomarkers in Ischemic Heart Disease. Journal of the American Heart Association. 2020;9:e016023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schmittdiel J, Selby JV, Swain B, Daugherty SL, Leong TK, Ho M, Margolis KL, O’Connor P, Magid DJ and Bibbins-Domingo K. Missed opportunities in cardiovascular disease prevention? Low rates of hypertension recognition for women at medicine and obstetrics-gynecology clinics. Hypertension. 2011;57:717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Manta C, Jain SS, Coravos A, Mendelsohn D and Izmailova ES. An Evaluation of Biometric Monitoring Technologies for Vital Signs in the Era of COVID‐19. Clinical and Translational Science. 2020;13:1034–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, Howard VJ, Lichtman JH, Lisabeth LD, Piña IL, Reeves MJ, Rexrode KM, Saposnik G, Singh V, Towfighi A, Vaccarino V, Walters MR, American Heart Association Stroke C, Council on C, Stroke N, Council on Clinical C, Council on E, Prevention and Council for High Blood Pressure R. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1545–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A and Cheung L. Large-scale assessment of a smartwatch to identify atrial fibrillation. New England Journal of Medicine. 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Attia ZI, Dugan J, Maidens J, Rideout A, Lopez-Jimenez F, Noseworthy PA, Asirvatham S, Pellikka PA, Ladewig DJ and Satam G. Prospective analysis of utility of signals from an ECG-enabled stethoscope to automatically detect a low ejection fraction using neural network techniques trained from the standard 12-lead ECG. Circulation. 2019;140:A13447–A13447. [Google Scholar]
- 120.Peters SAE, Colantonio LD, Zhao H, Bittner V, Dai Y, Farkouh ME, Monda KL, Safford MM, Muntner P and Woodward M. Sex Differences in High-Intensity Statin Use Following Myocardial Infarction in the United States. J Am Coll Cardiol. 2018;71:1729–1737. [DOI] [PubMed] [Google Scholar]
- 121.Li S, Fonarow GC, Mukamal K, Xu H, Matsouaka RA, Devore AD and Bhatt DL. Sex and racial disparities in cardiac rehabilitation referral at hospital discharge and gaps in long‐term mortality. Journal of the American Heart Association. 2018;7:e008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun EY, Jadotte YT and Halperin W. Disparities in Cardiac Rehabilitation Participation in the United States: A SYSTEMATIC REVIEW AND META-ANALYSIS. Journal of Cardiopulmonary Rehabilitation and Prevention. 2017;37:2–10. [DOI] [PubMed] [Google Scholar]
- 123.Ritchey MD, Maresh S, McNeely J, Shaffer T, Jackson SL, Keteyian SJ, Brawner CA, Whooley MA, Chang T and Stolp H. Tracking cardiac rehabilitation participation and completion among Medicare beneficiaries to inform the efforts of a national initiative. Circulation: Cardiovascular Quality and Outcomes. 2020;13:e005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vidal-Almela S, Czajkowski B, Prince SA, Chirico D, Way KL, Pipe AL and Reed JL. Lessons learned from community- and home-based physical activity programs: A narrative review of factors influencing women’s participation in cardiac rehabilitation. European Journal of Preventive Cardiology. 2020;28:761–778. [DOI] [PubMed] [Google Scholar]
- 125.Höchsmann C, Müller O, Ambühl M, Klenk C, Königstein K, Infanger D, Walz SP and Schmidt-Trucksäss A. Novel Smartphone Game Improves Physical Activity Behavior in Type 2 Diabetes. American Journal of Preventive Medicine. 2019;57:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bond S, Laddu DR, Ozemek C, Lavie CJ and Arena R. Exergaming and Virtual Reality for Health: Implications for Cardiac Rehabilitation. Current Problems in Cardiology. 2021;46:100472. [DOI] [PubMed] [Google Scholar]
- 127.Vadiveloo M, Lichtenstein AH, Anderson C, Aspry K, Foraker R, Griggs S, Hayman LL, Johnston E, Stone NJ and Thorndike AN. Rapid diet assessment screening tools for cardiovascular disease risk reduction across healthcare settings: a scientific statement from the American Heart Association. Circulation: Cardiovascular Quality and Outcomes. 2020;13:e000094. [DOI] [PubMed] [Google Scholar]
- 128.Garcia M, Miller VM, Gulati M, Hayes SN, Manson JE, Wenger NK, Bairey Merz CN, Mankad R, Pollak AW, Mieres J, Kling J and Mulvagh SL. Focused Cardiovascular Care for Women: The Need and Role in Clinical Practice. Mayo Clin Proc. 2016;91:226–40. [DOI] [PubMed] [Google Scholar]
- 129.Knowles JW, Rader DJ and Khoury MJ. Cascade screening for familial hypercholesterolemia and the use of genetic testing. Jama. 2017;318:381–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kind AJ and Buckingham WR. Making neighborhood-disadvantage metrics accessible—the neighborhood atlas. The New England journal of medicine. 2018;378:2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Angell SY, McConnell MV, Anderson CA, Bibbins-Domingo K, Boyle DS, Capewell S, Ezzati M, De Ferranti S, Gaskin DJ and Goetzel RZ. The American Heart Association 2030 impact goal: a presidential advisory from the American Heart Association. Circulation. 2020;141:e120–e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matheny ME, Whicher D and Israni ST. Artificial intelligence in health care: a report from the National Academy of Medicine. Jama. 2020;323:509–510. [DOI] [PubMed] [Google Scholar]
- 133.Kuan R. Adopting AI in Health Care Will Be Slow and Difficult. 2019;2021. [Google Scholar]
- 134.Ashrafian H and Darzi A. Transforming health policy through machine learning. PLoS Medicine. 2018;15:e1002692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Benjamens S, Dhunnoo P and Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ digital medicine. 2020;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.The Medical Futurist : FDA-approved A.I.-based algorithms. 2021.
- 137.Muehlematter UJ, Daniore P and Vokinger KN. Approval of artificial intelligence and machine learning-based medical devices in the USA and Europe (2015–20): a comparative analysis. The Lancet Digital Health. 2021. [DOI] [PubMed] [Google Scholar]
- 138.Quer G, Arnaout R, Henne M and Arnaout R. Machine Learning and the Future of Cardiovascular Care: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2021;77:300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Krittanawong C, Rogers AJ, Johnson KW, Wang Z, Turakhia MP, Halperin JL and Narayan SM. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nature Reviews Cardiology. 2021;18:75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.CVD Prevention Toolbox - Risk assessment and management tools for health professionals and policy makers. 2021.
- 141.Cirillo D, Catuara-Solarz S, Morey C, Guney E, Subirats L, Mellino S, Gigante A, Valencia A, Rementeria MJ and Chadha AS. Sex and gender differences and biases in artificial intelligence for biomedicine and healthcare. NPJ digital medicine. 2020;3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tseng AS, Thao V, Borah BJ, Attia IZ, Medina Inojosa J, Kapa S, Carter RE, Friedman PA, Lopez-Jimenez F, Yao X and Noseworthy PA. Cost Effectiveness of an Electrocardiographic Deep Learning Algorithm to Detect Asymptomatic Left Ventricular Dysfunction. Mayo Clin Proc. 2021;96:1835–1844. [DOI] [PubMed] [Google Scholar]


