Abstract
Dominance status in hamsters is driven by interactions between arginine-vasopressin V1a, oxytocin (OT), and serotonin 1A (5-HT1A) receptors. Activation of V1a and OT receptors in the anterior hypothalamus (AH) increases aggression in males, while decreasing aggression in females. In contrast, activation of 5-HT1A receptors in the AH decreases aggression in males and increases aggression in females. The mechanism underlying these differences is not known. The purpose of this study was to determine if dominance status and sex interact to regulate V1a, OT, and 5-HT1A receptor binding. Same-sex hamsters (N = 47) were paired 12 times across six days in five min sessions. Brains from paired and unpaired (non-social control) hamsters were collected immediately after the last interaction and processed for receptor binding using autoradiography. Differences in V1a, OT, and 5-HT1A receptor binding densities were observed in several brain regions as a function of social status and sex. For example, in the AH, there was an interaction between sex and social status, such that V1a binding in subordinate males was lower than in subordinate females and V1a receptor density in dominant males was higher than in dominant females. There was also an interaction in 5-HT1A receptor binding, such that social pairing increased 5-HT1A binding in the AH of males but decreased 5-HT1A binding in females compared with unpaired controls. These results indicate that dominance status and sex play important roles in shaping the binding profiles of key receptor subtypes across the neural circuitry that regulates social behavior.
Keywords: aggression, submission, anterior hypothalamus, medial preoptic area, orbitofrontal cortex, hippocampus, dorsal raphe, dominant, subordinate, social stress
INTRODUCTION
In many species, social interactions among individuals are governed by dominance relationships (Larson et al., 2006; Hattori and Wilczynski, 2009; Loveland et al., 2014). The formation and maintenance of dominance relationships rely on agonistic behaviors, particularly aggression and social communication. Dominance relationships provide a social structure that serves many adaptive functions (e.g., resource distribution) and that ultimately reduce social conflict (Bernstein et al., 1974). Typically, dominance relationships are rapidly established through aggressive interactions but are primarily maintained through social communication (e.g., scent marking, vocalization, non-contact aggression or harassment, etc.) thereby reducing the dangers of continual, intense conflict (Albers et al., 2002; Fernald, 2014). If social communication does not occur or if it is dysfunctional, then social interactions become maladaptive, resulting in continuously high levels of social conflict (Ferris et al., 1987). In extreme cases, continuously high levels of conflict can result in pathological aggression (Covington III et al., 2019; de Boer, 2018), which can predispose those subjected to it to higher incidences of numerous psychopathologies (Huhman, 2006).
Despite the importance of agonistic behaviors in dominance relationships and ultimately pathological aggression, investigation of the neurobiological mechanisms underlying their expression have received only limited attention in males and almost no attention in females (Terranova et al., 2017). Intrasexual selection resulting from male-male competition and intersexual selection resulting from female mate choice were emphasized by Darwin and have played an influential role in our understanding of sexual selection (Darwin, 1871). Because of the emphasis on male agonistic behaviors, offensive aggression and dominance have been studied almost exclusively in males. In contrast, selection as a product of mate choice has been stressed in females with almost no attention paid to the importance of female agonistic behaviors such as offensive aggression. Recently, however, it has been more fully recognized that females compete for resources and for mates to achieve reproductive benefits and that female competition is a widespread and significant evolutionary selective pressure (Been et al., 2019; Huchard and Cowlishaw, 2011; Rosvall, 2011; Stockley and Bro-Jorgensen, 2011).
For many females, aggression serves to define social status and, ultimately, reproductive success (Bernstein, 1976; Stanyon and Bigoni, 2014). As a result, agonistic behaviors are central to defining the nature of social relationships in females as well as males. Given that social behavior is evolutionarily quite old, and the strategies used by males and females evolved as the result of very different selective pressures, it is likely that there are fundamental sex differences in the neural mechanisms regulating the expression of social behaviors. Despite the observed similarities in many of the agonistic behaviors expressed by males and females, there is evidence that the underlying neurochemical and genetic mechanisms regulating these behaviors can differ dramatically between the sexes (McCann et al., 2019; Terranova et al., 2016).
Rodent species such as mice, rats, voles and hamsters have been used extensively for studies of the neural circuitry underlying different types of social behavior (Albers, 2015). Because the types of social organizations observed in these species differ greatly, studies in each species provide important complementary information. Unlike many other laboratory rodents, though, female hamsters as well as males rapidly establish stable hierarchical dominance relationships that persist for extended periods (Drickamer and Vandenbergh, 1973; Drickamer et al., 1973; however, see Williamson et al., 2019). Although comparatively little is known about the social behavior of wild Syrian hamsters (Gattermann et al., 2001; Murphy, 1977), it is clear that male and female hamsters readily display a variety of social behaviors such as social recognition, social reward, social avoidance and social communication, that are critical in determining social status thus providing highly tractable models to the study of their underlying neural mechanisms (Albers, 2015). A great deal is also known about the neural circuitry and the hormones controlling social behavior in hamsters, making them a powerful model system for understanding social status (Albers, 2012; Albers and Bamshad, 1998; Albers et al., 2002).
Social behaviors including agonistic behaviors are mediated by an evolutionarily-ancient social decision-making network (SDMN) (O’Connell and Hofmann, 2011) that is composed of a number of distinct but interconnected nodes. Specifically, the SDMN includes the social behavior neural network (Goodson, 2005; Newman, 1999) and the mesolimbic dopamine system, which are both conserved across numerous taxa. In the present study, we focused on many of the structures that have been identified as key elements within this network to begin to identify how neurochemical signaling varies as a function of social status and sex. Social behaviors, including agonistic behaviors, are regulated by interactions between a number of neurochemical signals including arginine-vasopressin (AVP), oxytocin (OT), and serotonin (5-HT) within the SDMN (Ferris et al., 1989; Harmon et al., 2002a; Terranova et al., 2016). As might be expected, there are significant sex differences in how these neurochemicals regulate aggressive behavior throughout this network. For example, in the anterior hypothalamus AVP increases aggression in males but decreases aggression in females (Albers et al., 2006; Caldwell and Albers, 2004; Ferris et al., 1997; Terranova et al., 2016). The purpose of the present study was to determine how social status influences the receptors of three important neurochemical signals, AVP, OT, and 5-HT, in key regions of the SDMN in both male and female hamsters.
Methods
Animals
Adult male and female Syrian hamsters (120–130 g) purchased from Charles River Laboratories (Wilmington, MA) were housed in polycarbonate cages (23 × 43× 20 cm) with wire grid lids with corncob bedding and cotton nesting material. Hamsters were housed individually for four weeks before behavioral testing began. We have demonstrated that individual housing is not stressful for hamsters (Ross et al., 2017). Chow (LabDiet 5001, Purina Mills, Gray Summit, MO) and water were available continuously. The room was maintained on a 14:10 light:dark cycle as is customary in hamsters to maintain gonadal patency. All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with the standards outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. On each of the eight days prior to the start of behavioral testing, the estrous cycle was determined in all female hamsters by examining vaginal discharge. Male hamsters were handled on each of these days to control for the monitoring of estrous cycles in females.
Behavioral Testing, Scoring and Analysis
Male and female pairs (n = 8/group) were weight-matched, and females were also matched for the stage of the estrous cycle. One hamster of each pair was randomly chosen to be the resident, and the other hamster of the pair was placed into the resident’s home cage for a five min social interaction. Each pair of hamsters interacted within the home cage of the resident for two, five min sessions each day for six days, resulting in a total of 12 sessions (female interactions began on diestrous Day 1 of their cycle). These social encounters were video recorded, and the duration of the following behaviors emitted by each hamster was subsequently scored: Aggression - chasing after, lunging at, rolling over, pinning, or biting the other hamster; Submission – fleeing, flagging, tail lifts, full submissive posture; Nonsocial - exploring the cage, grooming themselves; Social - approaching or sniffing the other conspecific. The number of flank marks were also counted during these sessions. An aggression index was then calculated by subtracting the duration of all submissive behaviors from the duration of all aggressive behaviors for each hamster. Dominance status was determined using the aggression index; the animal with the highest aggression index per pair was categorized as dominant and the other animal in the pair as subordinate. Given the low levels of female aggression and submission on the day of estrus (Solomon et al., 2007), female behavior was not scored on those days. All dominant-subordinate relationships were rapidly established during the first pairing and were stable across sessions. The durations of all behaviors were calculated for each of these sessions and averaged for data analyses. Male (n = 8) and female (n = 7) controls were also yoked to a dominant/subordinate pair and moved from the animal housing room to the testing suite with the dominant/subordinate pair but were placed in a clean cage without another hamster present.
Autoradiography
Immediately after the final trial of behavior testing (diestrous Day 2 for females), hamsters were lightly anesthetized with isoflurane, decapitated, and the brains were collected and frozen in dry ice. Brains were stored at −80°C and cut into 20 μm coronal sections using a cryostat. Sections were thaw-mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and stored at −80°C until processing.
V1a and OT Receptor Binding
V1a receptor binding was determined with the I125-labeled linear V1aR antagonist [125I]-Phenylacetyl-D-Tyr(Me)-Phe-Gln-Asn-Arg-Pro-Arg-Tyr-NH (Perkin-Elmer, Boston, MA). OT receptor binding was determined with the I125-labeled ornithine vasotocin analog Vasotocin, d(CH2)5[Tyr(Me)2,Thr4,Orn8,[125I]Tyr9-NH2] (Perkin-Elmer). The tissue was allowed to thaw and dry. It was then fixed in 0.1% paraformaldehyde for 2 min. Slides were then rinsed twice for 10 min each in buffer (50 mM Tris, pH 7.4) and were then incubated in tracer buffer (0.35 mM bacitracin, Sigma-Aldrich, St. Louis, MO; 0.015 mM bovine serum albumin, Sigma-Aldrich, St. Louis, MO; 50 mM I125 linear V1aR antagonist or 50 mM I125 vasotocin analog) for one hr. Slides were rinsed twice for 5 min each and then again for 35 min with agitation in buffer (50 mM Tris, 21 mM MgCl). All incubations and washes were performed at room temperature. The slides were then dipped in 4°C deionized water and allowed to dry. The slides and a C14 standard calibration strip (American Radiolabeled Chemicals, St. Louis, MO) were loaded into autoradiography cassettes and exposed to film (Kodak, Rochester, NY) for three days (V1a) or seven days (OT) at room temperature.
5-HT1A Receptors
5-HT1A receptor binding was determined with the H3-labeled 5-HT1A receptor full agonist 8-Hydroxy-DPAT,[Propyl-2,3-ring-1,2,3-3H] (Perkin Elmer). After the tissue was allowed to thaw and dry, slides were incubated in buffer (50 mM Tris, 120 mM NaCl, 4 mM CaCl2, pH 7.4) for 15 min, followed by a one hr incubation in tracer buffer (50 mM Tris, 6 μM H3 5-HT1A agonist). The slides were then rinsed twice for 10 min in buffer and dipped in 4°C deionized water and allowed to dry. The slides and an H3 standard calibration strip (American Radiolabeled Chemicals) were loaded into autoradiography cassettes and exposed to film for 18 weeks.
Analysis of Autoradiography
Densitometry analysis was performed using ImageJ software (NIH, Bethesda, MD) and a camera (Panasonic, Newark, NJ) attached to a lightbox (Imaging Research, Inc., Ontario, Canada). Standard curves were created using the C14 microscales on the standard calibration strip for V1a and OT receptors, while standard curves were created using H3 microscales on the standard calibrations strip for 5-HT1A receptors. Using the Morin and Wood Syrian hamster brain atlas (Morin and Wood, 2001), regions of the SDMN, including the nucleus accumbens (NAc; plate 15), lateral septum (LS; plate 20), bed nucleus of the stria terminalis (BNST; plate 20), medial preoptic area (mPOA; plate 20), anterior hypothalamus (AH; plate 23), ventromedial hypothalamus (VMH; plate 28), basolateral amygdala (BLA; plate 28), hippocampus (HC; plate 28), ventral tegmental area (VTA; plate 37), and periaqueductal gray (PAG; plate 40) were analyzed (O’Connell and Hofmann, 2011). Furthermore, regions such as the medial prefrontal cortex (mPFC; plate 13) and the dorsal raphe nucleus (DR; plate 40) were also analyzed given their essential role in regulating agonistic social behavior (Cooper et al., 2009; Dölen et al., 2013; Markham et al., 2012). For each brain area, three tissue sections located 100 μm apart were quantified and collapsed bilaterally for analysis. A 0.35 mm2 box was placed over the center of each brain area, and the optical density was recorded. Background binding measured just outside each region of interest was subtracted from this measurement. Optical densities were calculated as disintegrating units per min per mg tissue (dpm/mg).
Statistical Analysis
All data were analyzed using R software with the lme4 statistical package (Bates et al., 2007). A 3 (social group) by 2 (sex) mixed-effect model nesting subordinates, dominants, and yoked controls together was used. LSD post hoc tests were performed on statistically significant main effects and interactions using R software with the lmerTest package (Kuznetsova et al., 2017) to determine if there were statistically significant effects of sex and dominance status on social behaviors and receptor densitometry. Cohen’s ƒ2 was used as an estimate of effect sizes for the mixed-effects models (Selya et al., 2012). Multiple comparisons were controlled for using a Benjamini-Hochberg correction. Statistical significance was ascribed at p<0.05.
Data availability
All raw data will be made available upon reasonable request.
Results
Behavioral Studies
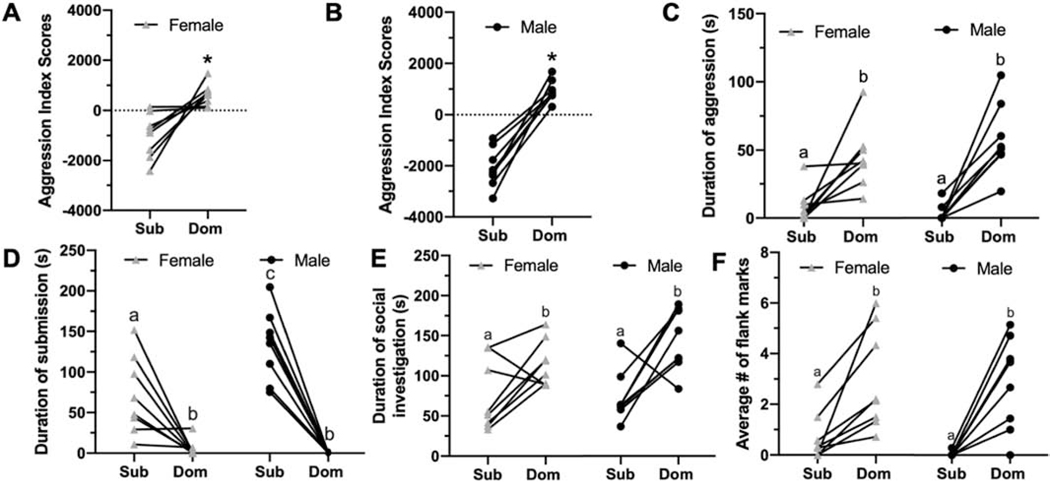
Aggression index allowed us to separate dominant males and females from subordinate males and females [F(1, 32) = 126.53, p < 0.001, ƒ2 = 0.66; Figure 1A, 1B]. Dominant males and females were more aggressive than subordinate males and females [F(1, 32) = 54.02, p < 0.001, ƒ2 = 0.41; Figure 1C], but there was no significant effect of sex on aggressive behavior [F(1, 32) = 0.45, p = 0.51; Figure 1C]. There was an interaction between sex and status on submissive behavior, such that subordinate males were more submissive than subordinate females [F(1, 32) = 9.46, p < 0.01, ƒ2 = 2.18; Figure 1D]. Furthermore, subordinate males and females were more submissive than dominant males and females [F(1, 32) = 83.13, p < 0.001, ƒ2 = 0.41; Figure 1D]. There were also effects of social status on social investigation [F(1, 32) = 21.66, p < 0.001, ƒ2 = 2.96; Figure 1E] and flank marking [F(1, 32) = 27.55, p < 0.001, ƒ2 = 0.95; Figure 1F], with dominant males and females socially investigating and flank marking more than subordinate males and females.
Figure 1.
Aggression index scores between dominant and subordinate, female (A) and male (B) hamsters. Duration of aggression (C), submission (D), social investigation (E) between dominant and subordinate, male and female hamsters. Average number of flank marks during social pairing between dominant and subordinate, male and female hamsters (F). n = 8/group. Lines connect each dominant-subordinate pair. Different letter above bars indicate p < 0.05. * indicates p < 0.05.
V1a receptor binding
The distribution of V1a receptor binding was similar to that seen in previous studies (Dubois-Dauphin et al., 1990; C. F. Ferris et al., 1993; Johnson et al., 1995; Ross et al., 2019; Young et al., 2000). In males and females, V1a binding was detectable throughout key regions in the social decision making network (Figure 2; Table 1). Dense receptor binding was found in the bed nucleus of the stria terminalis (BNST) and lateral septum (LS). Moderate to low binding was observed in the medial preoptic area (mPOA), the anterior hypothalamus (AH), and the CA1 region of the hippocampus (HC). There was an interaction between sex and social status in V1a binding in the AH [F(2, 45) = 6.13, p < 0.01, ƒ2 = 0.27; Figure 3A], such that V1a binding in subordinate females was higher than subordinate males (p < 0.05) and V1a receptor density in dominant females was lower than dominant males (p < 0.05). There was also a main effect of social status on V1a receptor density in the ventromedial hypothalamus (VMH) [F(2, 45) = 9.83, p < 0.01, ƒ2 = 0.26; Figure 3B], such that controls had lower V1a receptor density compared to subordinate (p < 0.05) or dominant (p < 0.05) hamsters regardless of sex. There was also a notable effect of sex on V1a receptor density in the LS, with V1a receptor density significantly higher in females compared to males [F(1, 45) = 7.36, p < 0.01, ƒ2 = 0.17; Figure 3C]. There were no significant effects of dominance status or sex, nor an interaction between those variables, on V1a receptor density in any of the other brain areas tested (Table 1).
Figure 2.
Representative autoradiograms illustrating V1a receptor binding in brain sites analyzed (adapted from (Morin and Wood, 2001). A. AH. B. VMH.
Table 1.
Means ± SEM and P-values for status, sex and interaction effects on V1a receptor densities.
| Brain area | Females | Males | Status effect | Sex effect | Interaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cont. | Sub. | Dom. | Cont. | Sub. | Dom. | ||||
| mPFC | 3449±5 35 | 3704±5 48 | 2967±2 31 | 4113±561 | 3136±343 | 4053±487 | p = 0.70 | p = 0.28 | p = 0.16 |
| NAc | 195±39 | 392±83 | 226±42 | 234±35 | 278±39 | 249±74 | p = 0.08+ | p = 0.70 | p = 0.32 |
| LS | 3106±408 | 2693±328 | 2743±322 | 2652±253 | 1863±235 | 2221±103 | p = 0 10+ | p < 0.01* | p = 0.77 |
| BNST | 3101±327 | 3028±360 | 3159±300 | 3221±344 | 2548±299 | 2842±194 | p = 0.96 | p = 0.35 | p = 0.58 |
| MPOA | 636±98 | 890±246 | 624±83 | 655±76 | 943±150 | 751±108 | p = 0.11 | p = 0.54 | p = 0.91 |
| AH | 452±116 | 557±66 | 287±46 | 430±77 | 321±94 | 543±51 | p = 0.92 | p = 0.99 | p < 0.01* |
| VMH | 1056±160 | 2028±311 | 1343±182 | 1087±145 | 1885±274 | 2337±315 | p < 0.01* | p = 0.02+ | p = 0.03+ |
| BLA | 585±99 | 891±127 | 939±57 | 797±124 | 678±176 | 753±109 | p = 0.35 | p = 0.48 | p = 0.10+ |
| HC | 686±61 | 720±65 | 785±91 | 614±46 | 569±48 | 728±69 | p = 0.13 | p = 0.08+ | p = 0.74 |
| VTA | 1527±106 | 1299±109 | 1535±207 | 1612±164 | 1283±170 | 1316±110 | p = 0.16 | p = 0.66 | p = 0.53 |
| PAG | 2349±341 | 2019±259 | 2366±482 | 2788±230) | 2312±251 | 2340±192 | p = 0.41 | p = 0.33 | p = 0.71 |
| DR | 1099±139 | 910±129 | 918±75 | 927±121 | 756±101 | 889±85 | p = 0.24 | p = 0.17 | p = 0.75 |
Note: Binding density in dpm/mg. Cont.: Controls, Sub.: Subordinate, Dom.: Dominant
indicates significant effect
indicates trend
Figure 3.
V1a receptor binding in the anterior hypothalamus (AH) (A), ventromedial hypothalamus (VMH) (B), and lateral septum (LS) (C) of female and male hamsters who were not socially paired (control), were subordinate in a social pairing, or were dominant in a social pairing. Different letter above bars indicate p < 0.05. * indicates p < 0.05.
Oxytocin receptor binding
The distribution of OTR receptor binding was similar to that reported previously. (Dubois-Dauphin et al., 1992; Ross et al., 2019). In male and female hamsters, high OTR binding was found throughout the nodes of the social decision making network (Table 2). In the VMH, there were effects of both sex [F(1, 45) = 12.73, p < 0.01, ƒ2 = 0.58; Figure 4A] and social status [F(2, 45) = 6.24, p < 0.01, ƒ2 = 0.08; Figure 4A], with males having more OTR density than did females (p < 0.05), and controls having higher levels of OTR densities compared to subordinate (p < 0.05) or dominant (p < 0.05) hamsters regardless of sex. There were also interactions between sex and social status on OTR density in several brain regions. In the HC, there was an interaction such that OTR density was unaffected by social status in females, but subordinate males had more OTR density compared to control males [F(2, 45) = 6.99, p < 0.01, ƒ2 = 0.34; Figure 4B]. In the mPOA, there was an interaction such that OTR density was unaffected by social status in females, but subordinate males had less OTR density compared to control males [F(2, 45) = 4.93, p = 0.01, ƒ2 = 0.30; Figure 4C]. Finally, in the ventral tegmental area (VTA) there was an interaction such that OTR density in control males was higher than control females, but this relationship was inverted in dominant subjects, so that dominant males had less OTR density than dominant females [F(2, 45) = 6.15, p < 0.01, ƒ2 = 0.37; Figure 4D]. There were no significant effects of dominance status or sex, nor an interaction between those variables, on OTR density in any of the other brain areas tested (Table 2).
Table 2.
Means ± SEM and P-values for status, sex and interaction effects on OTR receptor densities.
| Brain area | Females | Males | Status effect | Sex effect | Interaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cont. | Sub. | Dom. | Cont. | Sub. | Dom. | ||||
| mPFC | 2168±289 | 1967±273 | 1744±145 | 1726±246 | 2063±271 | 1697±97 | p = 0.35 | p = 0.45 | p = 0.44 |
| NAc | 653±91 | 521±78 | 504±61 | 436±40 | 576±80 | 501±61 | p = 0.72 | p = 0.30 | p = 0.10 |
| LS | 958±55 | 914±77 | 858±62 | 824±52 | 841±69 | 902±74 | p = 0 97 | p = 0.29 | p = 0.33 |
| BNST | 846±22 | 840±95 | 781±50 | 846±53 | 691±73 | 848±82 | p = 0.46 | p = 0.60 | p = 0.74 |
| MPOA | 294±19 | 303±28 | 266±15 | 366±33 | 232±14 | 294±24 | p = 0.02 | p = 0.61 | p = 0.01* |
| AH | 284±22 | 260±22 | 304±30 | 286±30 | 263±26 | 266±26 | p = 0.37 | p = 0.44 | p = 0.72 |
| VMH | 562±72 | 429±49 | 453±49 | 832±86 | 541±61 | 644±83 | p < 0.01* | p < 0.01* | p = 0.34 |
| BLA | 383±23 | 405±42 | 421±43 | 373±48 | 441±56 | 383±91 | p = 0.53 | p = 0.89 | p = 0.64 |
| HC | 1361±118 | 1096±64 | 1327±93 | 959±65 | 528±195 | 1237±157 | p = 0.36 | p = 0.83 | p < 0.01* |
| VTA | 259±23 | 336±26 | 394±37 | 365±34 | 316±44 | 279±18 | p = 0.89 | p = 0.46 | p < 0.01* |
| PAG | 287±58 | 297±41 | 276±37 | 266±29 | 232±45 | 319±42 | p = 0.70 | p = 0.65 | p = 0.40 |
| DR | 184±31 | 133±25 | 148±45 | 128±22 | 223±46 | 167±18 | p = 0.66 | p = 0.40 | p = 0.03 |
Note: Binding density in dpm/mg. Cont.: Controls, Sub.: Subordinate, Dom.: Dominant
indicates significant effect
Figure 4.
OTR receptor binding in the ventromedial hypothalamus (VMH) (A), dorsal CA1 of the hippocampus (HC) (B), medial preoptic area (mPOA) (C), and ventral tegmental area (VTA) (D) of female and male hamsters who were not socially paired (control), were subordinate in a social pairing, or were dominant in a social pairing. Different letter above bars or next to status group indicate p < 0.05. * indicates p < 0.05.
5-HT1A receptor binding
The distribution of 5-HT1A receptor binding was similar to that reported previously (Duncan et al., 1999; Ross et al., 2019). There were interactions between sex and social status on 5-HT1A receptor density in the AH and VTA. In the AH, 5-HT1A receptor density was higher in control females than control males but was lower in subordinate and dominant females than in subordinate and dominant males [F(2, 45) = 9.25, p < 0.01, ƒ2 = 0.41; Figure 5A]. In the VTA, 5-HT1A receptor density was higher in subordinate males compared to subordinate females [F(2, 45) = 6.50, p < 0.01, ƒ2 = 0.29; Figure 5B]. There were no significant effects of dominance status or sex, nor an interaction between those variables, on 5-HT1A receptor density in any of the other brain areas tested (Table 3).
Figure 5.
5-HT1A receptor binding in the anterior hypothalamus (AH) (A) and ventral tegmental area (VTA) (B) of female and male hamsters who were not socially paired (control), were subordinate in a social pairing, or were dominant in a social pairing. Different letter above bars indicate p < 0.05.
Table 3.
Means ± SEM and P-values for status, sex and interaction effects on 5-HT1A receptor densities.
| Brain area | Females | Males | Status effect | Sex effect | Interaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cont. | Sub. | Dom. | Cont. | Sub. | Dom. | ||||
| mPFC | 20149±2966 | 32241±5016 | 32195±4450 | 28784±2699 | 26419±6793 | 30641±2392 | p = 0.18 | p = 0.90 | p = 0.17 |
| NAc | 53258±6196 | 58352±7491 | 819±106a | 72097±4520 | 55700±9281 | 74986±6398 | p = 0.01+ | p = 0.60 | p = 0.16 |
| LS | 33454±4633 | 35128±4362 | 34393±1651 | 27399±3664 | 41611±9027 | 36646±5307 | p = 0 97 | p = 0.29 | p = 0.33 |
| BNST | 26978±2167 | 26593±3555 | 25187±2477 | 25141±3869 | 21479±3291 | 23897±1940 | p = 0.89 | p = 0.24 | p = 0.77 |
| MPOA | 13372±2230 | 13424±1435 | 13175±2010 | 11687±919 | 12888±2232 | 12953±1550 | p = 0.92 | p = 0.54 | p = 0.89 |
| AH | 13169±1202 | 8661±691 | 9438±1290 | 9758±1049 | 12373±652 | 13111±944 | p = 0.60 | p = 0.10+ | p < 0.01* |
| VMH | 12269±1038 | 11554±989 | 12594±2439 | 13052±1985 | 12254±2620 | 11906±1129 | p = 0.91 | p = 0.85 | p = 0.88 |
| BLA | 16188±4706 | 15475±2467 | 18186±3136 | 14113±1712 | 16822±2413 | 11577±1877 | p = 0.89 | p = 0.27 | p = 0.33 |
| HC | 939±207a | 889±115a | 901±169a | 914±222a | 934±167a | 1014±184a | p = 0.96 | p = 0.75 | p = 0.92 |
| VTA | 368±126a | 198±34a | 396±110a | 320±83a | 662±153a | 269±31a | p = 0.49 | p = 0.19 | p < 0.01* |
| PAG | 4906±1041 | 5602±625 | 4771±637 | 4830±10 54 | 7168±2276 | 6652±1574 | p = 0.43 | p = 0.37 | p = 0.78 |
| DR | 915±177a | 795±170a | 1191±208a | 948±108a | 1395±233a | 1790±348a | p = 0.13 | p = 0.97 | p = 0.08+ |
Note: Binding density in dpm/mg. Cont.: Controls, Sub.: Subordinate, Dom.: Dominant
indicates mean and SEM are X100 value listed
indicates significant effect
Discussion
In the current study, both male and female hamsters established stable dominance relationships, and there were no sex differences in the duration of aggressive behaviors emitted by dominant subjects. However, there was more submissive behavior produced by the subordinate males compared to the subordinate females. This is consistent with previous data demonstrating that female hamsters are more resilient to the effects of losing agonistic encounters than are males (Bath and Johnston, 2007; Huhman et al., 2003). Additionally, dominant males and females flank marked more than did subordinate males and females. Indeed, dominance relationships are primarily maintained through social communication (e.g., scent marking) (Albers et al., 2002; Fernald, 2014), and dominant subjects likely flank mark more to maintain their status. These data represent the first analysis of the effects of sex and social status on V1a, OT, and 5-HT1A receptor densities in key elements of the neural circuitry regulating social behavior. Effects of sex, social status, and an interaction between sex and social status were observed in at least one brain region for all three receptors.
V1aRs
AVP plays a significant role in many social behaviors, including agonistic behaviors, by activating V1aRs. Aggression and other agonistic behaviors are controlled by a neural network composed of reciprocally-connected limbic structures including the AH and lateral VMH (VMHL) (Adams, 2006; Delville et al., 2000; Nelson and Trainor, 2007). In male hamsters, AVP injected into the AH or VMHL stimulates offensive aggression (Caldwell and Albers, 2004; Ferris and Delville, 1994; Ferris et al., 1997) and injection of antagonists to the V1aR significantly inhibits offensive aggression (Ferris and Potegal, 1988; Potegal and Ferris, 1989). Studies in several other mammalian species have confirmed that injection of AVP into the AH facilitates male offensive aggression, and injection of a selective V1aR antagonist inhibits male offensive aggression (Bester-Meredith et al., 2005; Caldwell and Albers, 2004; Gobrogge et al., 2007; Gobrogge et al., 2009; Potegal and Ferris, 1989). Furthermore, in male hamsters, dominance and winning increases the density of V1aRs in the AH and VMHL (Cooper et al., 2005). Consistent with a positive relationship between male aggression and V1aR signaling in the AH, we found that dominant male hamsters had higher V1aR density in the AH compared to subordinate male hamsters. No differences between dominant and subordinate males in V1a receptor density in the VMH were detected in the present study, however. These disparate results might be due to procedural differences between studies. The previous study established the dominant-subordinate relationships across 11 days (Cooper et al., 2005), while in the present study social status was established over six days. Perhaps V1aR density changes within regions such as the VMH are part of the process of transitioning from establishing dominance relationships to maintaining them. Indeed, previous research from our lab has found that social experience influences V1aR binding profiles in male hamsters (Albers et al., 2006; Ross et al., 2019). For example, V1aR binding in the AH is higher in socially-isolated male hamsters compared to male hamsters that have been socially housed (Ross et al., 2019), and social experience in previously-isolated male hamsters reduces V1a receptor binding in the AH (Albers et al., 2006).
In females, AVP acting within the AH has the opposite effect on aggression than it does in males (Gutzler et al., 2010; Terranova et al., 2016). In contrast to males, AVP injected into the AH in females significantly inhibits aggression, while injection of a V1a antagonist into the AH significantly stimulates aggression in females (Gutzler et al., 2010; Terranova et al., 2016). Consistent with a negative relationship between aggression and V1a signaling in the AH in females, we found that dominant female hamsters had lower V1a receptor density in the AH compared to subordinate females. A trend in the same direction was seen in the VMH (p = 0.05). We found that subordinate females had more V1aR density than controls while dominants did not differ from controls. Taken together, these data suggest that there is an opposite relationship between the density of V1aRs in the AH (and possibly the VMH) and dominance status in males and females. In males, the density of V1aRs is greater in dominant males than in subordinate males, while in females the density of V1a receptors is greater in subordinate females than in dominant females.
In addition to the social status by sex interactions in the AH and VMH, we also found sex differences in V1aR within the LS. In the present study, females had significantly more V1a receptor binding in the LS than did males. The difference appeared to be due in part to sex differences in LS V1a binding in the groups that had socially interacted (i.e., the dominant and subordinate groups). In a previous study, no sex differences were detected in V1a binding in the LS in hamsters that had been socially isolated (Ross et al., 2019). It is interesting to note, however, that social status does alter V1aR binding in the LS in male mice (Lee et al., 2019) and male rats (Askew et al., 2006). In other rat studies, no sex differences in V1a receptor binding in the LS were detected (Dumais et al., 2013; Dumais and Veenema, 2016; however, see also Smith et al., 2017).
OTRs
There is also evidence that OTRs are involved in the establishment of dominance hierarchies (Timmer et al., 2011) and that social status can influence the behavioral response to OT (Harmon et al., 2002b; Winslow and Insel, 1991). Consistent with this, we found that social status and sex interacted to affect OTR binding in a number of brain regions. First, while OTR binding in the mPOA was unaffected by social status in females, male subordinates had lower OTR binding compared to controls. OT acting in the mPOA/AH has previously been associated with increased aggression (Harmon et al., 2002a), including in males (Taylor and Albers, unpublished observations). There was also an interaction between sex and social status on OTR binding in the HC, such that OTR density was unaffected by social status in females, but subordinate males had more OTR density compared to control males. OTRs in the HC enhance social recognition in rats (Raam et al., 2017), and OT enhances social recognition in hamsters, as well (Song et al., 2016b). Therefore, OTR binding changes in males might help to enhance the ability of male subordinates to recognize their dominant counterpart. Indeed, many of the changes in OTR binding as a result of social status only occurred in subordinate male hamsters, which may underlie the maintenance of their submission to their dominant counterpart and the increased submissive behavior compared to subordinate females. Furthermore, these interactions are particularly interesting because male hamsters are more sensitive to the effects of losing agonistic encounters than are females (Bath and Johnston, 2007; Huhman et al., 2003).
Finally, there was an interaction between social status and sex on OTR binding in the VTA, such that dominance status increased OTR binding in females but decreased OTR binding in males. Given that OTRs in the VTA are critical for social reward (Borland et al., 2019a; Borland et al., 2018; Hung et al., 2017; Song et al., 2016a), changes in OTR binding as a result of social status are likely important for sex differences in social reward. In fact, females are more sensitive to the rewarding properties of winning agonistic encounters than are males (Borland et al., 2019a; Borland et al., 2018; Borland et al., 2019b). It should also be noted that the increase in OTR binding in dominant females compared to controls might be related to the increased social reward seen in dominant animals compared to controls (Gil et al., 2013). However, OTR binding was decreased in the VTA of dominant males compared to controls. An intriguing possibility is that there are sex differences in OTR-related reward circuitry of the VTA (Borland et al., 2019b).
In addition to interactions between social status and sex, we also found that males had higher OTR binding in the VMH compared to females, which has been reported in numerous studies in rats (Bale et al., 1995; Dumais et al., 2013; Uhl-Bronner et al., 2005). However, unlike rats, we did not find any sex differences in OTR binding in the BNST, mPOA, MeA, NAc, or LS (Dumais et al., 2013). This suggests species-specific effects on regional OTR binding.
5-HT1ARs
The central 5-HT system is a phylogenetically ancient and an anatomically conserved regulator of many forms of social behavior. Indeed, one of the most well-known phenomena in behavioral neuroscience is the ability of 5-HT to inhibit impulsive behavioral traits including aggression in males. These inhibitory effects have been reported in many species including lizards, fish, birds, rodents, and primates (for reviews see (Carrillo et al., 2009; Takahashi et al., 2011). Many of the inhibitory effects of 5-HT on aggression are mediated by 5-HT1A receptors (Delville et al., 1996; Ferris et al., 1999). Despite the large number of studies demonstrating that 5-HT has inhibitory effects on aggression in males, there is little information on the effects of centrally administered 5-HT agonists on offensive aggression in female mammals (Joppa et al., 1997). Recently, it has been shown that activation of 5-HT1ARs in the AH actually stimulates aggression in females (Terranova et al., 2016). Therefore, behavioral changes might also be due to 5-HT1A changes in the AH. Surprisingly, 5-HT1A receptor binding was higher in the AH of males as a result of either subordinate or dominant status. This increased 5-HT1A binding would ultimately decrease male aggression in subordinates and dominants. Conversely, it was surprising that 5-HT1A receptor binding in the AH was lower in both subordinate and dominant females compared to socially naïve controls. This result suggests that availability of 5-HT1A receptors in, the AH would be reduced, which would decrease aggression in both dominant and subordinate female hamsters compared to socially naïve controls. Social isolation does increase aggression in female hamsters but does so without affecting 5-HT1A binding in the AH (Ross et al., 2019). 5-HT1A binding might not have been affected in this previous study because the “socially naïve” hamsters had one five-minute behavioral interaction, whereas in this study, control hamsters had no social interactions. Perhaps behavioral changes occur before changes in 5-HT1A receptor density. This would be in line with the idea that initial establishment of dominance hierarchies is through aggressive interactions, but that these relationships are thereafter maintained through social communication (Albers et al., 2002; Fernald, 2014).
Finally, there was an interaction between sex and social status on 5-HT1A receptor binding in the VTA, such that subordinate males had higher 5-HT1A receptor binding in the VTA compared to all other groups. 5-HT1A agonism in the VTA can reduce dopamine release (Tanahashi et al., 2012), which might decrease the rewarding properties of social interactions in subordinate males. This would be consistent with previous data demonstrating that female hamsters are more resilient to the effects of losing agonistic encounters than are males (Bath and Johnston, 2007; Huhman et al., 2003).
Conclusions
In conclusion, serotonin as well as members of the AVP/OT superfamily of nonapeptides (e.g., vasotocin and isotocin) play critical roles in the regulation of social behavior not only in mammals but across non-mammalian taxa, as well. The demonstration of regional changes in V1a, OT, and 5-HT1A receptor binding densities as a result of social status and sex in the present study suggests that behavioral plasticity as a result of social status might be driven, at least in part, by adaptations in receptor densities across vertebrate species. Although there is evidence in fish and lizards that social status can modulate the vasotocin system, less is known about how social status may influence receptor expression (Greenwood et al., 2008; Hattori and Wilczynski, 2009). It would be interesting for future work to examine the directional relationship between behavioral and receptor changes. In addition, these data are in line with previous work demonstrating that behavioral adaptation occurs in response to social status such that social status is primarily maintained through social communication (Albers et al., 2002; Fernald, 2014) and extends those data to suggest that hierarchies are maintained in part through receptor plasticity. It is important to note all females in this study were sacrificed on the same day of their estrous cycle. Given that ovarian hormones can alter V1a, OTR, and 5-HT1A receptor expression as well as social behavior (Bale and Dorsa, 1997; Funabashi et al., 2000; Kalamatianos et al., 2004; Michopoulos et al., 2014), it will be important for future work to examine how these receptors might interact with female social status across the estrous cycle. Finally, receptor changes as a result of social status occurred more frequently in subordinate male hamsters, which may help elucidate the mechanism whereby male hamsters are more sensitive to the effects of losing agonistic encounters than are females (Bath and Johnston, 2007; Huhman et al., 2003). Overall, social status and sex play important roles in shaping receptor binding profiles, which has critical implications for the treatment of pathological aggression.
Highlights:
Dominant male and female hamsters display similar levels of aggression
Subordinate males display higher levels of submission than subordinate females
Social status can alter V1a, OT, and 5-HT1A receptor binding
Social status can influence receptor binding in a sex-dependent manner
Acknowledgments
Funding: This work was supported by NIH-MH110212.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams DB (2006). Brain mechanisms of aggressive behavior: an updated review. Neurosci Biobehav Rev, 30(3), 304–318. doi: 10.1016/j.neubiorev.2005.09.004 [DOI] [PubMed] [Google Scholar]
- Albers HE (2012). The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm Behav, 61(3), 283–292. doi: 10.1016/j.yhbeh.2011.10.007 [DOI] [PubMed] [Google Scholar]
- Albers HE (2015). Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front Neuroendocrinol, 36, 49–71. doi: 10.1016/j.yfrne.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE, & Bamshad M. (1998). Role of vasopressin and oxytocin in the control of social behavior in Syrian hamsters (Mesocricetus auratus). Prog Brain Res, 119, 395–408. [DOI] [PubMed] [Google Scholar]
- Albers HE, Dean A, Karom MC, Smith D, & Huhman KL (2006). Role of V1a vasopressin receptors in the control of aggression in Syrian hamsters. Brain research, 1073, 425–430. [DOI] [PubMed] [Google Scholar]
- Albers HE, Huhman KL, & Meisel RL (2002). Hormonal Basis of Social Conflict and Communication. In Pfaff D, Arnold AP, Etgen A, Fahrbach SE, & Rubin RT (Eds.), Hormones, Brain and Behavior (Vol. 1, pp. 393–433). Amsterdam: Academic Press. (Reprinted from: NOT IN FILE). [Google Scholar]
- Askew A, Gonzalez FA, Stahl JM, & Karom MC (2006). Food competition and social experience effects on V1a receptor binding in the forebrain of male Long–Evans hooded rats. Hormones and behavior, 49(3), 328–336. [DOI] [PubMed] [Google Scholar]
- Bale TL, & Dorsa DM (1997). Cloning, novel promoter sequence, and estrogen regulation of a rat oxytocin receptor gene. Endocrinology, 138(3), 1151–1158. [DOI] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM, & Johnston CA (1995). Oxytocin receptor mRNA expression in the ventromedial hypothalamus during the estrous cycle. Journal of Neuroscience, 15(7), 5058–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Sarkar D, Bates MD, & Matrix L. (2007). The lme4 package. R package version, 2(1), 74. [Google Scholar]
- Bath KG, & Johnston RE (2007). Dominant–subordinate relationships in hamsters: Sex differences in reactions to familiar opponents. Hormones and behavior, 51(2), 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been LE, Gibbons AB, & Meisel RL (2019). Towards a neurobiology of female aggression. Neuropharmacology, 156, 107451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IS (1976). Dominance, aggression and reproduction in primate societies. J Theor Biol, 60(2), 459–472. [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP, & Rose RM (1974). Aggression and social controls in rhesus monkey (Macaca mulatta) groups revealed in group formation studies. Folia Primatol (Basel), 21(2), 81–107. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Martin PA, & Marler CA (2005). Manipulations of vasopressin alter aggression differently across testing conditions in monogamous and nonmonogamous Peromyscus mice. Aggressive Behavior, 31(2), 189–199. doi: 10.1002/ab.20075 [DOI] [Google Scholar]
- Borland JM, Aiani LM, Norvelle A, Grantham KN, O’Laughlin K, Terranova JI, . . . Albers HE (2019a). Sex-dependent regulation of social reward by oxytocin receptors in the ventral tegmental area. Neuropsychopharmacology, 44(4), 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland JM, Grantham KN, Aiani LM, Frantz KJ, & Albers HE (2018). Role of oxytocin in the ventral tegmental area in social reinforcement. Psychoneuroendocrinology, 95, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland JM, Rilling JK, Frantz KJ, & Albers HE (2019b). Sex-dependent regulation of social reward by oxytocin: an inverted U hypothesis. Neuropsychopharmacology, 44(1), 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, & Albers HE (2004). Effect of photoperiod on vasopressin-induced aggression in Syrian hamsters. Hormones and Behavior, 46(4), 444–449. [DOI] [PubMed] [Google Scholar]
- Carrillo M, Ricci LA, Coppersmith GA, & Melloni RH Jr. (2009). The effect of increased serotonergic neurotransmission on aggression: a critical meta-analytical review of preclinical studies. Psychopharmacology (Berl), 205(3), 349–368. doi: 10.1007/s00213-009-1543-2 [DOI] [PubMed] [Google Scholar]
- Cooper MA, Grober MS, Nicholas CR, & Huhman KL (2009). Aggressive encounters alter the activation of serotonergic neurons and the expression of 5-HT1A mRNA in the hamster dorsal raphe nucleus. Neuroscience, 161(3), 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Karom M, Huhman KL, & Albers HE (2005). Repeated agonistic encounters in hamsters modulate AVP V1a receptor binding. Hormones and Behavior, 48(5), 545–551. [DOI] [PubMed] [Google Scholar]
- Covington III HE, Newman EL, Leonard MZ, & Miczek KA (2019). Translational models of adaptive and excessive fighting: an emerging role for neural circuits in pathological aggression. F1000Research, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. (1871). The Descent of Man and Selection of in Relation to Sex. London: John Murray. [Google Scholar]
- de Boer SF (2018). Animal models of excessive aggression: implications for human aggression and violence. Current opinion in psychology, 19, 81–87. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, & Ferris CF (2000). Neural connections of the anterior hypothalamus and agonistic behavior in golden hamster. Brain Behav Evol, 55(2), 53–76. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, & Ferris CF (1996). Serotonin blocks vasopressin-facilitated offensive aggression: Interactions within the ventrolateral hypothalamus of golden hamsters. Physiology & Behavior, 59(4–5), 813–816. doi:Doi 10.1016/0031-9384(95)02166-3 [DOI] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, & Malenka RCJN (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. 501(7466), 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer LC, & Vandenbergh JG (1973). Predictors of social dominance in the adult female golden hamster (Mesocricetus auratus). Anim Behav, 21(3), 564–570. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Vandenbergh JG, & Colby DR (1973). Predictors of dominance in the male golden hamster (Mesocricetus auratus). Anim Behav, 21(3), 557–563. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Pevet P, Barberis C, Tribollet E, & Dreifuss JJ (1992). Localization of binding sites for oxytocin in the brain of the golden hamster. Neuroreport, 3(9), 797–800. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Pevet P, Tribollet E, & Dreifuss JJ (1990). Vasopressin in the brain of the golden hamster: The distribution of vasopressin binding sites and of immunoreactivity to the vasopressin-related glycopeptide. Journal of Comparative Neurology, 300, 535–548. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, & Veenema AH (2013). Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region-and sex-specific ways. Hormones and behavior, 64(4), 693–701. [DOI] [PubMed] [Google Scholar]
- Dumais KM, & Veenema AH (2016). Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Frontiers in neuroendocrinology, 40, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Short J, & Wheeler DL (1999). Comparison of the effects of aging on 5-HT7 and 5-HT1A receptors in discrete regions of the circadian timing system in hamsters. Brain Res, 829(1–2), 39–45. [DOI] [PubMed] [Google Scholar]
- Fernald RD (2014). Communication about social status. Curr.Opin.Neurobiol, 28, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C, Axelson J, Martin A, & Roberge L. (1989). Vasopressin immunoreactivity in the anterior hypothalamus is altered during the establishment of dominant/subordinate relationships between hamsters. Neuroscience, 29(3), 675–683. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Axelson JF, Shinto LH, & Albers HE (1987). Scent marking and the maintenance of dominant/subordinate status in male golden hamsters. Physiol Behav, 40(5), 661–664. [DOI] [PubMed] [Google Scholar]
- Ferris CF, & Delville Y. (1994). Vasopressin and serotonin interactions in the control of agonistic behavior. Psychoneuroendocrinology, 19(5–7), 593–601. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Delville Y, Grzonka Z, Luber-Narod J, & Insel TR (1993). An iodinated vasopressin (V1) antagonist blocks flank marking and selectively labels neural binding sites in golden hamsters. Physiol Behav, 54(4), 737–747. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH Jr., Koppel G, Perry KW, Fuller RW, & Delville Y. (1997). Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. Journal of Neuroscience, 17(11), 4331–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, & Potegal M. (1988). Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol Behav, 44(2), 235–239. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, & Delville Y. (1999). Serotonin regulation of aggressive behavior in male golden hamster (Mesocricetus auratus). Behav Neurosci, 113(4), 804–815. [DOI] [PubMed] [Google Scholar]
- Funabashi T, Shinohara K, Mitsushima D, & Kimura F. (2000). Estrogen increases arginine-vasopressin V1a receptor mRNA in the preoptic area of young but not of middle-aged female rats. Neuroscience letters, 285(3), 205–208. [DOI] [PubMed] [Google Scholar]
- Gattermann R, Fritzsche P, Neumann K, Al-Hussein I, Kayser A, Abiad M, & Yakti R. (2001). Notes on the current distribution and the ecology of wild golden hamsters (Mesocricetus auratus). Journal of Zoology, 254(3), 359–365. [Google Scholar]
- Gil M, Nguyen NT, McDonald M, & Albers HE (2013). Social reward: interactions with social status, social communication, aggression, and associated neural activation in the ventral tegmental area. European journal of neuroscience, 38(2), 2308–2318. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, & Wang Z. (2007). Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J.Comp Neurol, 502(6), 1109–1122. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, & Wang Z. (2009). Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A, 106(45), 19144–19149. doi: 10.1073/pnas.0908620106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL (2005). The vertebrate social behavior network: evolutionary themes and variations. Hormones and behavior, 48(1), 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood AK, Wark AR, Fernald RD, & Hofmann HA (2008). Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proceedings of the Royal Society B: Biological Sciences, 275(1649), 2393–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzler SJ, Karom M, Erwin WD, & Albers HE (2010). Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus). Eur.J.Neurosci, 31(9), 1655–1663. [DOI] [PubMed] [Google Scholar]
- Harmon A, Huhman KL, Moore T, & Albers H. (2002a). Oxytocin inhibits aggression in female Syrian hamsters. Journal of neuroendocrinology, 14(12), 963–969. [DOI] [PubMed] [Google Scholar]
- Harmon AC, Moore TO, Huhman KL, & Albers HE (2002b). Social experience and social context alter the behavioral response to centrally administered oxytocin in female Syrian hamsters. Neuroscience, 109(4), 767–772. [DOI] [PubMed] [Google Scholar]
- Hattori T, & Wilczynski W. (2009). Comparison of arginine vasotocin immunoreactivity differences in dominant and subordinate green anole lizards. Physiology & behavior, 96(1), 104–107. [DOI] [PubMed] [Google Scholar]
- Huchard E, & Cowlishaw G. (2011). Female-female aggression around mating: an extra cost of sociality in a multimale primate society. Behavioral Ecology, 22(5), 1003–1011. doi: 10.1093/beheco/arr083 [DOI] [Google Scholar]
- Huhman KL (2006). Social conflict models: can they inform us about human psychopathology? Hormones and behavior, 50(4), 640–646. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, & Jasnow AM (2003). Conditioned defeat in male and female Syrian hamsters. Hormones and behavior, 44(3), 293–299. [DOI] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, . . . Dölen G. (2017). Gating of social reward by oxytocin in the ventral tegmental area. Science, 357(6358), 1406–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AE, Barberis C, & Albers HE (1995). Castration reduces vasopressin receptor binding in the hamster hypothalamus. Brain Res, 674(1), 153–158. [DOI] [PubMed] [Google Scholar]
- Joppa MA, Rowe RK, & Meisel RL (1997). Effects of serotonin 1A or 1B receptor agonists on social aggression in male and female Syrian hamsters. Pharmacol Biochem Behav, 58(2), 349–353. [DOI] [PubMed] [Google Scholar]
- Kalamatianos T, Kallo I, Goubillon ML, & Coen CW (2004). Cellular expression of V1a vasopressin receptor mRNA in the female rat preoptic area: effects of oestrogen. Journal of neuroendocrinology, 16(6), 525–533. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RH (2017). lmerTest package: tests in linear mixed effects models. Journal of statistical software, 82(13), 1–26. [Google Scholar]
- Larson ET, O’Malley DM, & Melloni RH Jr (2006). Aggression and vasotocin are associated with dominant–subordinate relationships in zebrafish. Behavioural brain research, 167(1), 94–102. [DOI] [PubMed] [Google Scholar]
- Lee W, Hiura LC, Yang E, Broekman KA, Ophir AG, & Curley JP (2019). Social status in mouse social hierarchies is associated with variation in oxytocin and vasopressin 1a receptor densities. Hormones and behavior, 114, 104551. [DOI] [PubMed] [Google Scholar]
- Loveland JL, Uy N, Maruska KP, Carpenter RE, & Fernald RD (2014). Social status differences regulate the serotonergic system of a cichlid fish, Astatotilapia burtoni. Journal of Experimental Biology, 217(15), 2680–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Luckett CA, & Huhman KL (2012). The medial prefrontal cortex is both necessary and sufficient for the acquisition of conditioned defeat. Neuropharmacology, 62(2), 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann KE, Sinkiewicz DM, Rosenhauer AM, Beach LQ, & Huhman KL (2019). Transcriptomic analysis reveals sex-dependent expression patterns in the basolateral amygdala of dominant and subordinate animals after acute social conflict. Molecular neurobiology, 56(5), 3768–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Perez Diaz M, Embree M, Reding K, Votaw JR, Mun J, Sanchez M. (2014). Oestradiol Alters Central 5 HT 1 A Receptor Binding Potential Differences Related to Psychosocial Stress but not Differences Related to 5 HTTLPR Genotype in Female Rhesus Monkeys. Journal of neuroendocrinology, 26(2), 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, & Wood RI (2001). Stereotaxic atlas of the golden hamster brain: Academic. [Google Scholar]
- Murphy MR (1977). Intraspecific sexual preferences of female hamsters. Journal of Comparative and Physiological Psychology, 91(6), 1337. [Google Scholar]
- Nelson RJ, & Trainor BC (2007). Neural mechanisms of aggression. Nat Rev Neurosci, 8(7), 536–546. doi: 10.1038/nrn2174 [DOI] [PubMed] [Google Scholar]
- Newman SW (1999). The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Annals of the New York Academy of Sciences, 877, 242–257. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, & Hofmann HA (2011). The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. Journal of Comparative Neurology, 519(18), 3599–3639. [DOI] [PubMed] [Google Scholar]
- Potegal M, & Ferris CF (1989). Intraspecific aggression in male hamsters is inhibited by intrahypothalamic vasopressin-receptor antagonist. Aggressive Behav, 15, 311–320. [Google Scholar]
- Raam T, McAvoy KM, Besnard A, Veenema AH, & Sahay A. (2017). Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nature communications, 8(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AP, McCann KE, Larkin TE, Song Z, Grieb ZA, Huhman KL, & Albers HE (2019). Sex-dependent effects of social isolation on the regulation of arginine-vasopressin (AVP) V1a, oxytocin (OT) and serotonin (5HT) 1a receptor binding and aggression. Hormones and behavior, 116, 104578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AP, Norvelle A, Choi DC, Walton JC, Albers HE, & Huhman KL (2017). Social housing and social isolation: Impact on stress indices and energy balance in male and female Syrian hamsters (Mesocricetus auratus). Physiology & behavior, 177, 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall KA (2011). Intrasexual competition in females: evidence for sexual selection? Behavioral Ecology, 22 1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selya AS, Rose JS, Dierker LC, Hedeker D, & Mermelstein RJ (2012). A practical guide to calculating Cohen’s f2, a measure of local effect size, from PROC MIXED. Frontiers in psychology, 3, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Poehlmann ML, Li S, Ratnaseelan AM, Bredewold R, & Veenema AH (2017). Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: focus on the social decision-making network. Brain Structure and Function, 222(2), 981–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Karom MC, & Huhman KL (2007). Sex and estrous cycle differences in the display of conditioned defeat in Syrian hamsters. Hormones and behavior, 52(2), 211–219. [DOI] [PubMed] [Google Scholar]
- Song Z, Borland JM, Larkin TE, O’Malley M, & Albers HE (2016a). Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology, 74, 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Larkin TE, & Albers HE (2016b). Oxytocin (OT) and arginine-vasopressin (AVP) act on OT receptors and not AVP V1a receptors to enhance social recognition in adult Syrian hamsters (Mesocricetus auratus). Hormones and behavior, 81, 20–27. [DOI] [PubMed] [Google Scholar]
- Stanyon R, & Bigoni F. (2014). Sexual selection and the evolution of behavior, morphology, neuroanatomy and genes in humans and other primates. Neurosci Biobehav Rev, 46P4, 579–590. doi: 10.1016/j.neubiorev.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Stockley P, & Bro-Jorgensen J. (2011). Female competition and its evolutionary consequences in mammals. Biol.Rev.Camb.Philos.Soc, 86(2), 341–366. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Quadros IM, de Almeida RM, & Miczek KA (2011). Brain serotonin receptors and transporters: initiation vs. termination of escalated aggression. Psychopharmacology (Berl), 213(2–3), 183–212. doi: 10.1007/s00213-010-2000-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanahashi S, Yamamura S, Nakagawa M, Motomura E, & Okada M. (2012). Dopamine D2 and serotonin 5-HT1A receptors mediate the actions of aripiprazole in mesocortical and mesoaccumbens transmission. Neuropharmacology, 62(2), 765–774. [DOI] [PubMed] [Google Scholar]
- Terranova JI, Ferris CF, & Albers HE (2017). Sex Differences in the Regulation of Offensive Aggression and Dominance by Arginine-Vasopressin. Front Endocrinol (Lausanne), 8, 308. doi: 10.3389/fendo.2017.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova JI, Song Z, Larkin TE 2nd, Hardcastle N, Norvelle A, Riaz A, & Albers HE (2016). Serotonin and arginine-vasopressin mediate sex differences in the regulation of dominance and aggression by the social brain. Proc Natl Acad Sci U S A, 113(46), 13233–13238. doi: 10.1073/pnas.1610446113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer M, Cordero MI, Sevelinges Y, & Sandi C. (2011). Evidence for a role of oxytocin receptors in the long-term establishment of dominance hierarchies. Neuropsychopharmacology, 36(11), 2349–2356. doi: 10.1038/npp.2011.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl-Bronner S, Waltisperger E, Martinez-Lorenzana G, Lara MC, & Freund-Mercier MJ (2005). Sexually dimorphic expression of oxytocin binding sites in forebrain and spinal cord of the rat. Neuroscience, 135(1), 147–154. [DOI] [PubMed] [Google Scholar]
- Williamson CM, Lee W, DeCasien AR, Lanham A, Romeo RD, & Curley JP (2019). Social hierarchy position in female mice is associated with plasma corticosterone levels and hypothalamic gene expression. Scientific reports, 9(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, & Insel TR (1991). Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration. J Neurosci, 11(7), 2032–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Cooper T, & Albers HE (2000). Vasopressin (V1a) receptor binding, mRNA expression and transcriptional regulation by androgen in the Syrian hamster brain. Journal of neuroendocrinology, 12(12), 1179–1185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All raw data will be made available upon reasonable request.