Abstract
Background
Depressive disorders are linked to dysfunction in reward-related behaviors and corticostriatal reward circuitry. Low-grade dysregulation of the immune system, e.g., elevations in plasma interleukin 6 (IL-6) and tumor necrosis factor α, have been thought to affect corticostriatal reward circuitry. Little is presently known about the degree to which these relationships generalize to patients with treatment-resistant depression (TRD) and/or childhood trauma history.
Methods
Resting-state functional connectivity between the ventral striatum (VS) and ventromedial prefrontal cortex (vmPFC) regions and plasma inflammatory marker levels (IL-6, tumor necrosis factor α) were measured in 74 adults with TRD. Regression analyses examined associations of inflammatory markers with VS-vmPFC connectivity and the moderating effects of self-reported childhood trauma on these associations, with exploratory analyses examining trauma subtypes.
Results
IL-6 was negatively associated with VS-vmPFC connectivity (specifically for the left VS). Childhood trauma moderated the relationships between tumor necrosis factor α and VS-vmPFC connectivity (specifically for the right VS) such that greater childhood trauma severity (particularly emotional neglect) was associated with stronger cytokine-connectivity associations.
Conclusions
This study independently extends previously reported associations between IL-6 and reductions in corticostriatal connectivity to a high-priority clinical population of treatment-seeking patients with TRD and further suggests that childhood trauma moderates specific associations between cytokines and corticostriatal connectivity. These findings suggest that associations between elevated plasma cytokine levels and reduced corticostriatal connectivity are a potential pathophysiological mechanism generalizable to patients with TRD and that such associations may be affected by trauma severity.
Keywords: Childhood trauma, Circuitry, Corticostriatal, Cytokine, Moderation, Reward
Depression, characterized by low mood and anhedonia, is a major contributor to lifetime disability in adults (1), highly prevalent (2), and associated with significant morbidity and increased mortality (3), making it a major health concern. In particular, treatment-resistant depression (TRD), commonly characterized by nonresponse to one or more antidepressant trials, is an important subtype of depression affecting at least 30% of patients with depression (4). TRD significantly contributes to the overall disease burden of depression and is associated with poorer clinical and psychosocial outcomes (5). To ultimately help develop more effective individualized treatments for both depression and TRD, researchers in recent decades have attempted to better understand neurobiological correlates associated with depression. One such neurobiological correlate is reward neural circuitry dysfunction, which is consistent with findings of behavioral reward-related impairments in patients with depression (6). Functional neuroimaging studies of patients with depression have demonstrated blunted activity in ventral striatal (VS) regions such as the nucleus accumbens and elevated activity in the ventromedial prefrontal cortex (vmPFC) during reward tasks (7). Given abnormalities in VS and vmPFC activity in depression during reward tasks, preclinical and early clinical studies have built on these findings to further identify that reduced resting-state corticostriatal (CS) connectivity may be a core neural substrate linked to depressive symptomology (10, 11, 8, 9). However, the pathophysiology of dysfunction in VS-vmPFC connectivity in patients with depression is unclear. In parallel with the diverse phenotypic clinical manifestations that characterize depression, notable heterogeneity in reward dysfunction exists within samples of patients with depression.
In recent years, low-grade inflammatory marker elevation has emerged as a potential biological factor that may contribute to abnormalities in corticostriatal (e.g., VS-vmPFC) connectivity in patients with depression (12). Several meta-analyses have demonstrated low-grade elevations in peripheral cytokine levels (specifically interleukin 6 [IL-6] and tumor necrosis factor α [TNF-α]) in patients with depression as compared with healthy control subjects (13). Researchers have theorized that such elevations may contribute to dysfunction in reward circuitry (e.g., CS connectivity) via evolutionarily preserved mechanisms that promote reduced motivation and anhedonic-like behavior in response to acute infection (14). Several preclinical and clinical studies have examined the effects of exogenous immune stimuli on VS-vmPFC circuitry in preclinical studies and healthy control subjects and found that administration of cytokine-inducing stimuli resulted in decreased activation of the VS during reward tasks (15,16). Similarly, other studies have found that inflammatory markers are associated with decreased connectivity of the vmPFC with numerous brain regions (12). Furthermore, such studies identified that immune system activation was linked to depressive-like behaviors or symptoms of depression (e.g., low mood, anhedonia, fatigue) (15,17, 18, 19). Of note, although having some overlap in physiological function, the cytokines TNF-α and IL-6 appear to have distinct biological roles (e.g., TNF-α has been reported to have primarily proinflammatory activity, while IL-6 has been reported to have both pro- and anti-inflammatory activity) and potentially distinct mechanistic effects, with clinical findings suggesting differences between TNF-α and IL-6 in strengths of cytokine level associations with neural connectivity or specific symptoms of depression (20, 21, 22, 23).
To probe associations between inflammatory pathology and effects on VS-vmPFC functional connectivity in the context of depression, a seminal study by Felger et al. found that higher levels of C-reactive protein and IL-6 were associated with reduced VS-vmPFC connectivity in 48 patients with unselected (unipolar and bipolar) depression (23). However, other studies of patients with depression have focused on investigating either cytokine-depression relationships or CS connectivity–depression relationships, separately from one another. Furthermore, the association between cytokines and CS connectivity in the high-priority cohort of TRD has not yet been examined. A deeper understanding of this potential pathophysiological mechanism in TRD would be helpful to inform novel treatment initiatives for TRD, which are a high priority in depression research precisely because front-line treatment options are less efficacious and/or tolerable in these patients (24). In addition, a substantial subset of patients with TRD (e.g., 20% to >50%) report a history of childhood trauma (25,26), which contributes to overall refractory outcomes in depression (27,28). Recent research has highlighted the importance of examining the role of childhood trauma in moderating cytokine-connectivity relationships (29, 30, 31). Childhood trauma may have moderating effects on cytokine-connectivity relationships, with evidence for both positive and negative moderation effects (29, 30, 31), and is independently associated with low-grade cytokine elevation (32).
In light of growing recognition that a large percentage of published research findings later fail to be replicated (33,34), we sought to both replicate and extend previous work regarding the associations of inflammatory-related cytokine markers (IL-6, TNF-α) with VS-vmPFC connectivity. Specifically, we sought to examine these relationships in a cohort of 74 treatment-seeking TRD participants with moderate-to-severe depression—a population of high clinical interest and concern. Additionally, we examined moderating effects of childhood trauma on these relationships. We hypothesized that 1) inflammatory-related markers would be negatively associated with VS-vmPFC connectivity, extending previous findings in a novel sample of patients with TRD and 2) childhood trauma would moderate relationships between inflammatory-related markers and connectivity, with potential for either positive or negative moderation effects. On an exploratory basis, we also assessed subtypes of childhood trauma (e.g., emotional abuse/neglect, physical abuse/neglect, sexual abuse) to identify which particular subtypes of childhood trauma may have driven moderation effects for trauma-related findings.
Methods and Materials
Data were obtained at a pretreatment baseline from participants enrolled in an ongoing parent clinical trial (R01MH113857; ClinicalTrials.gov: NCT03237286). We included all participants with usable data for both peripheral blood and functional magnetic resonance imaging (fMRI) measures.
Participants
Participants included 74 TRD individuals aged 19–60 years, with history of nonresponse to one or more adequate trial(s) of a Food and Drug Administration–approved antidepressant, score ≥ 25 on the Montgomery–Åsberg Depression Rating Scale, and low self-worth based on self-report indices. Exclusion study criteria included lifetime bipolar or psychotic disorder, substance use disorder, current pregnancy, and serious unstable medical illnesses such as severe head injury (see further details and full inclusion/exclusion criteria at ClinicalTrials.gov: NCT03237286). Participants were recruited into a larger, ongoing depression treatment trial from the community and outpatient settings through web advertisements, clinical referral, or a local research registry. Clinical measures, inflammatory marker plasma measures, and blood oxygen level–dependent (BOLD) fMRI data were acquired within a span of approximately 1–2 weeks. All participants denied having recent acute febrile illness on self-reports. Of all participants, 75.7% (56/74) were taking any psychotropic medication, while 40.5% (30/74) were taking a selective serotonin reuptake inhibitors, which was the major class of medication prescribed.
Cytokine Measurement
Venous blood draw was done between 7 and 9 am, with samples then centrifuged and plasma samples immediately stored at −80 °C. IL-6 and TNF-α were measured in plasma samples using a Meso Scale Discovery MESO QuickPlex SQ 120 plate reader (Meso Scale Diagnostics LLC). Samples were loaded in duplicate into Meso Scale Discovery V-Plex Human proinflammatory panel I ELISA plates according to manufacturer instructions and calculated concentrations were generated through Discovery workbench software (version 4.0; Meso Scale Diagnostics LLC). Average coefficient of variation values between plasma duplicates were 8% for IL-6 and 10% for TNF-α. All the samples were above the detection limit for each cytokine. Our cytokine levels (i.e., IL-6 < 10 pg/mL and TNF-α <15 pg/mL) fell uniformly within commonly reported ranges for patients with depression (35).
BOLD fMRI Data Acquisition and Preprocessing
A 3T Siemens PRISMA scanner (Siemens Healthineers) was used to obtain BOLD fMRI data through use of Human Connectome Project sequences (multiband factor = 8, repetition time = 800 ms, echo time = 37, fractional anisotropy = 52°, field of view = 200 × 200, 72 slices, 2 mm isotropic voxels). Standard preprocessing steps were applied in Analysis of Functional NeuroImages (AFNI) consistent with the afni_proc.py pipeline, as described in previous publications (36). Briefly, preprocessing steps included slice timing correction, motion correction, spatial distortion correction, cross-registration to a magnetization prepared rapid acquisition gradient-echo structural scan, warping to the Montreal Neurological Institute-27 template, and smoothing (6 mm full width at half maximum). For single-subject analyses, regression models (using AFNI’s 3dDeconvolve) included motion parameters and their derivatives and were utilized to generate resting-state whole brain maps. The fast ANATICOR tool was used to reduce white matter artifacts. Bandpass filtering (0.01 < f < 0.1 Hz) was done through inclusion of a bandpass regressor in regression models.
BOLD fMRI Data Analysis
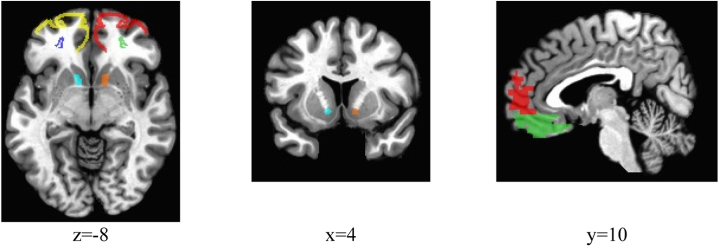
Analysis of resting-state functional connectivity of the VS-vmPFC was done in AFNI, with a priori definitions of seed and target regions of interest (Figure 1). The VS seed region was defined by two bilateral anatomically defined nucleus accumbens seed regions as per AFNI’s Talairach-Tournoux brain structure atlas (see https://afni.nimh.nih.gov/AFNIAtlases). The vmPFC was separately defined by four regions of interest in the AFNI brain structure atlas, specifically the left Brodmann areas 10 and 11 (LBA10/LBA11) and right Brodmann areas 10 and 11 (RBA10/RBA11), which were examined separately, given findings associating inflammatory processes differently with each of these frontal regions (23,37). We analyzed the left and right VS seed regions separately, given that previous studies have noted greater effects of inflammatory processes on neural activity in the left striatum (23). For each subject, voxelwise connectivity cross-correlations were computed separately for each of the bilateral VS seeds, and connectivity correlation coefficients were averaged within each of the four vmPFC target regions and extracted for further analysis in the statistical software R (R Foundation for Statistical Computing). Eight connectivity scores (2 VS seeds × 4 vmPFC targets) were then used in regression analyses as measures of VS-vmPFC connectivity.
Figure 1.
The following seed and target regions and corresponding colors (in parentheses) are demonstrated above: left Brodmann area 10 (yellow), right Brodmann area 10 (red), left Brodmann area 11 (dark blue), right Brodmann area 11 (lime green), right ventral striatum (orange), and left ventral striatum (cyan). Montreal Neurological Institute coordinates are detailed below images.
Clinical Measures
The Childhood Trauma Questionnaire (CTQ) (38), used in moderation analyses, is a 28-item retrospective self-report assessment of lifetime childhood trauma with subscales measuring spectrum of abuse (emotional, physical, and sexual) and neglect (emotional and physical), with greater scores reflecting greater trauma severity. Further information on the CTQ and depression/anxiety scales (respectively, the Montgomery–Åsberg Depression Rating Scale and PROMIS Anxiety item bank) are described in detail in the Supplement.
Statistical Analysis
Statistical analyses were conducted using the statistical software R version 3.5.2 (see https://cran.r-project.org/). Both inflammatory marker and fMRI signal values were winsorized to reduce the effects of outliers, with few values overall being affected by winsorizing (<15%). Inflammatory marker values were log-transformed. Independent regression analyses were used to conduct examination of each of the 8 connectivity measures’ degree of association with inflammatory marker values, while statistically adjusting for immunoassay batch, age, biological sex, race (dichotomized as Caucasian or non-Caucasian), and body mass index in all analyses. For moderation analyses, we similarly conducted regression analyses while examining interaction effects of the CTQ composite and inflammatory marker values on outcome measures of connectivity, while statistically adjusting for covariates as in previous analyses. For all analyses, we present unadjusted p values and adjusted p values using false discovery rate (FDR) correction (corrected for the 8 connectivity indices, applying the FDR correction for each cytokine separately), with α set at 0.05. FDR correction was utilized in this manner, given our conceptualization and prior evidence of IL-6 and TNF-α as having distinct biological roles and potential mechanistic effects (20, 21, 22, 23), each warranting a unique family of hypothesis tests.
Sensitivity analyses were done excluding participants (n = 18) with history of medical illnesses (e.g., HIV, cancer, eczema) or taking current medications (e.g., loratadine, nonsteroidal anti-inflammatory drugs) potentially affecting immune system function, with no appreciable change in significant findings or notable changes in effect sizes of findings (Table S2). Sensitivity analysis examining serotonergic medication use (selective serotonin reuptake inhibitors/serotonin and norepinephrine reuptake inhibitors), neuroleptic use, substance use, depression severity, anxiety severity, and childhood trauma severity (for regression models not including childhood trauma) was done by adding these covariates separately into regression models, without any change in significant findings for our primary analyses (Table S3).
Results
Participant Characteristics
Participants included 74 TRD participants with an average age of 36.2 years (range 19–60), and 65% of participants were female. Plasma IL-6 levels were an average of 1.3 ± 1.2 pg/mL, while plasma TNF-α levels were an average of 3.4 ± 2.1 pg/mL. Consistent with previous studies finding strong associations of body mass index and IL-6, surface validity tests found strong correlations between body mass index and IL-6 (r = 0.44, p < .001, N = 74). See Table 1 for baseline clinical characteristics, with basic intervariable correlations further described in Table S1.
Table 1.
Participant Information Regarding Demographic Characteristics, Clinical Measures, and Cytokine Levels
| Measure | Mean (SD) or % |
|---|---|
| Demographic Characteristics | |
| Age, years | 36.24 (10.83) |
| Biological sex, female | 65% |
| Race, non-Caucasian | 15% |
| BMI | 28.23 (6.32) |
| Clinical Measures | |
| MADRS | 32.93 (5.33) |
| CTQ total | 51.84 (17.8) |
| CTQ emotional abuse | 13.1 (5.95) |
| CTQ emotional neglect | 14.77 (5.35) |
| CTQ physical abuse | 7.9 (3.54) |
| CTQ physical neglect | 8.41 (3.55) |
| CTQ sexual abuse | 7.66 (4.85) |
| Cytokine Levels | |
| IL-6, pg/mL | 1.27 (1.19) |
| TNF-α, pg/mL | 3.41 (2.06) |
N = 74 for all measures except CTQ scores for which n = 73.
BMI, body mass index; CTQ, Childhood Trauma Questionnaire; MADRS, Montgomery–Åsberg Depression Rating Scale.
Associations Between Inflammatory Markers and VS-vmPFC Connectivity in TRD Participants
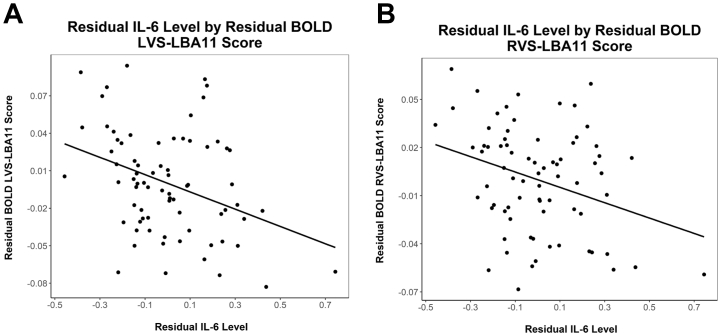
IL-6 was significantly associated with decreased connectivity between the left VS and both the LBA11 (B = −0.07, unadjusted p = .003, adjusted p = .02, N = 74) and RBA11 (B = −0.05, unadjusted p = .009, adjusted p = .034, N = 74) (Figure 2). Nonsignificant associations were noted between IL-6 and left VS (LVS)-LBA10 connectivity (B = −0.07, unadjusted p = .089, adjusted p > .1, N = 74). No significant associations were found for TNF-α (unadjusted ps > .35).
Figure 2.
Partial regression plot of residual IL-6 levels by residual LVS and RVS to LBA11 connectivity scores, respectively (A, B). The partial regression plot permits graphical visualization of these relationships while statistically adjusting for covariates as described in the main text. BOLD, blood oxygen level–dependent; IL-6, interleukin 6; LBA, left Brodmann area; LVS, left VS; RVS, right VS; VS, ventral striatum.
Moderating Effects of Trauma on Cytokine-Connectivity Relationships
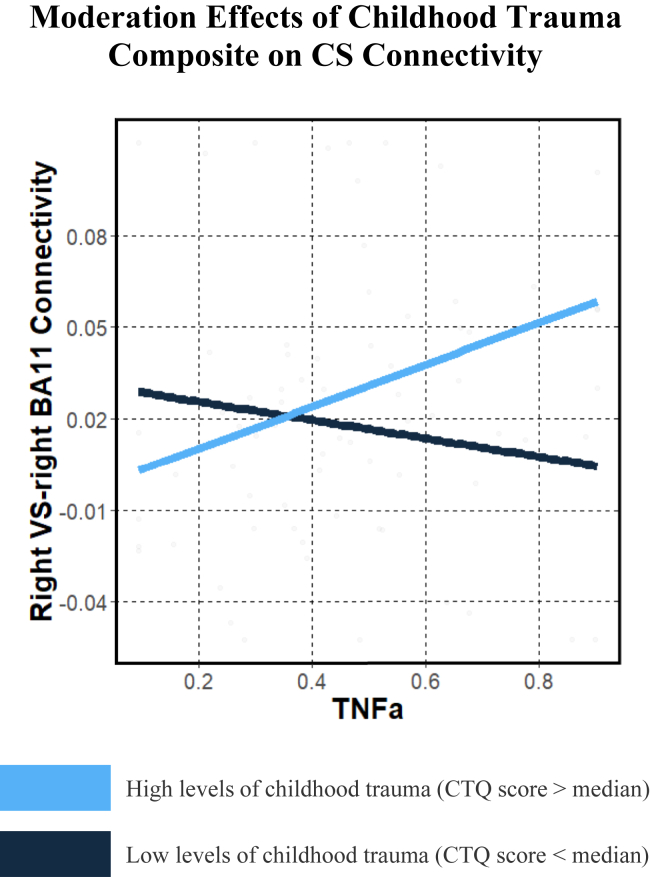
For TNF-α, childhood trauma positively moderated associations between TNF-α and connectivity between the right VS and the RBA11 (B = 0.004, unadjusted p = .005, adjusted p = .037, n = 73), such that participants with higher levels of both trauma and TNF-α had greater connectivity between right VS and RBA11 regions (Figure 3). Post hoc simple slopes analysis of this interaction effect found that for individuals with higher trauma severity (i.e., +1 standard deviation), TNF-α was positively associated with right VS (RVS)-RBA11 connectivity (B = 0.07, p = .03, n = 73), while negative associations of TNF-α with RVS-RBA11 connectivity for individuals with lower trauma severity (i.e., −1 standard deviation) did not reach statistical significance (B = −0.07, p = .089, n = 73). No significant trauma moderation effects were found for IL-6 and connectivity after multiple comparisons adjustment (adjusted ps > .05), although near-significant associations before multiple comparisons adjustment are described in Table S4.
Figure 3.
Graph illustrating that childhood trauma interacted with TNF-α levels (log-transformed) to predict CS connectivity (RVS–right BA11), such that greater childhood trauma and greater TNF-α levels were associated with greater CS connectivity. For visualization purposes, differences between participants with high levels of childhood trauma (blue line; CTQ score > median) and low levels of childhood trauma (black line; CTQ score < median) are presented. BA, Brodmann area; CS, corticostriatal; CTQ, Childhood Trauma Questionnaire; RVS, right VS; TNFa, tumor necrosis factor α; VS, ventral striatum.
Exploratory Analysis Examining CTQ Subtypes
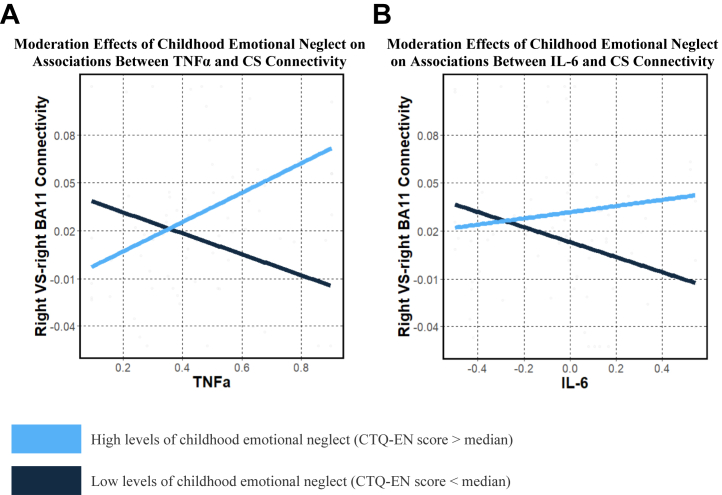
In exploratory analyses, we examined moderation effects of trauma subtypes on inflammatory markers and connectivity relationships. For TNF-α, CTQ emotional neglect positively moderated associations between TNF-α and RVS-RBA11 connectivity (B = 0.015, unadjusted p = .001, adjusted p = .01) (Figure 4). For IL-6, CTQ emotional neglect positively moderated associations between IL-6 and RVS-LBA10 (B = 0.02, unadjusted p = .001, adjusted p = .007) and RVS-RBA10 connectivity (B = 0.018, unadjusted p = .002, adjusted p = .007) (Figure 4). For the findings with TNF-α and IL-6, the nature of the moderation effects of childhood emotional neglect severity on cytokine-connectivity relationships was similar to moderation effects of composite childhood trauma severity as reported in our primary results.
Figure 4.
Graphs illustrating that childhood emotional neglect interacted with log-transformed cytokine levels TNF-α (A) and IL-6 (B) to predict CS connectivity (Right VS–right BA11), such that greater childhood TNF-α and greater cytokine levels were associated with greater CS connectivity. For visualization purposes, differences between participants with high levels of childhood emotional neglect (blue line; CTQ score > median) and low levels of childhood emotional neglect (black line; CTQ score < median) are presented. BA, Brodmann area; CS, corticostriatal; CTQ-EN, Childhood Trauma Questionnaire–emotional neglect; IL-6, interleukin 6; TNFa, tumor necrosis factor α; VS, ventral striatum.
No other CTQ subscale had significant moderation interactions after FDR correction, although certain CTQ subscales had significant findings that were not robust after FDR correction in Table S5.
Discussion
In this study of adults with unipolar treatment-resistant depression, higher IL-6 levels were associated with decreased resting-state functional connectivity in corticostriatal circuitry (LVS-RBA11, LVS-LBA11), shedding light on an association that may help guide future research examining pathophysiological mechanisms relevant for TRD. Furthermore, we identified that childhood trauma and TNF-α levels interacted to predict right VS-vmPFC connectivity (RVS-RBA11) in participants with depression, such that individuals with higher levels of both trauma and TNF-α had greater VS-vmPFC connectivity. In exploratory analyses, these associations appeared to be driven primarily by a specific type of trauma—emotional neglect.
Our study identified robust associations of peripheral IL-6 with reduced left VS to bilateral vmPFC resting-state functional connectivity in patients with unipolar TRD, extending an earlier finding of a negative correlational relationship between cytokines and reward circuitry in patients with depression to the novel, high-priority population of patients with TRD. TRD has been previously associated with both unique (e.g., decreased activity in visual recognition neural circuits) and common (e.g., reduced prefrontal-limbic-thalamic connectivity) pathophysiological mechanisms as compared with treatment-sensitive depression (39, 40, 41, 42, 43). The extension of previous findings reported in unselected unipolar and bipolar depressed patients suggests that inflammation may generally impact reward circuitry irrespective of treatment resistance and that similar inflammatory mechanisms in treatment-sensitive depression are applicable in the context of TRD. Furthermore, some (but not all) studies report higher cytokine levels (e.g., TNF-α, IL-6) in patients with TRD as than in patients with treatment-sensitive depression, suggesting that any such potential impact of cytokines on CS connectivity might be particularly pertinent and commonplace in the context of TRD (44,45). Given the lack of effective and/or safe treatments for this high-priority group of patients with TRD, research to understand the core pathophysiological processes contributing to depressive symptoms is imperative for development of novel, effective treatments. For instance, understanding mechanisms by which certain immunomodulatory treatments (e.g., infliximab) or real-time fMRI neurofeedback may modulate corticostriatal circuitry could uncover more precise treatments for patients with TRD (46).
Moreover, for the first time, we examined the moderating effects of childhood trauma on associations between cytokines and corticostriatal connectivity in TRD—a clinical population where childhood trauma is known to have moderating effects on neurobiology (47, 48, 49). Childhood trauma has significant yet complex effects on brain regions involved in reward neural circuitry (e.g., associated with both reduced VS activation in response to reward and increased VS-vmPFC connectivity—a potential compensatory mechanism) (50), and previous studies suggest that childhood trauma may moderate relationships between stress-related biological markers (e.g., cortisol) and neural circuitry (51, 52, 53). Importantly, the severity of depression, severity of anxiety, and other potential confounders did not change the pattern of these findings in sensitivity analyses (Table S3). Our findings suggest that dissociable mechanistic pathways may alter reward circuitry function for those with, versus those without, a substantial childhood trauma history. Specifically, we found that greater TNF-α activation in the context of greater levels of childhood trauma was predictive of greater corticostriatal connectivity (specifically right VS to RBA11).
Current theories suggest that in individuals with childhood trauma, increased connectivity between PFC and VS regions may exist as a top-down compensatory mechanism for chronic dysfunctional reward processing (29,50,54,55), with a role of the vmPFC in enhancing reward-related VS activity (56). From a pathophysiological perspective, cytokines released from microglia in the central nervous system may contribute to decreased synthesis and reduced presence of dopamine at neuronal synapses in the VS (15). Such effects may occur owing to direct cytokine effects at dopamine transporters or indirectly owing to cytokine activation of the kynurenine pathway or nitro-oxidative stress pathways, which can cause depletion of dopamine precursors such as tyramine. Decreased dopamine synthesis and availability in neuronal synapses likely ultimately lead to reduction in VS activity and signaling in neuronal projections from the VS to the PFC. In individuals with trauma (with chronic deficits in reward signaling), compensatory overactivation of mPFC-VS pathways (and consequently greater VS-vmPFC functional connectivity) may thus occur in the context of decreased VS activity secondary to cytokine activity, while in individuals without significant childhood trauma (with acute deficits in such VS-to-PFC signaling), such compensatory pathways may not be established and thus not activate (29). Such theories could be tested through longitudinal neuroimaging studies and/or studies using treatments that reduce dysregulated low-grade cytokine activity in such dopaminergic pathways.
The described theories help guide a better understanding of the heterogeneity in the pathophysiology of depression related to childhood trauma and allow a clearer understanding of potential differences in treatment targets within reward neurocircuitry between depressed individuals with and without trauma. However, this theoretical conceptualization requires examination in future studies, given the inability to directly measure causal cytokine effects at the molecular level on VS-vmPFC activity in this cross-sectional study. Alternatively, our findings may be due to unmeasured external confounding factors (e.g., stress), which may differently affect cytokine-circuitry associations in individuals with elevated childhood trauma. Of note, one prior study’s exploratory analysis of a community sample of women, not selected for depression, with varying levels of trauma found an opposing pattern to ours—specifically, that an inflammatory composite score was negatively associated with LVS-vmPFC connectivity only in high-trauma groups. These reports emphasize the need for further research to examine potentially contrasting results in TRD and nondepressed cohorts (57).
Our exploratory analysis examining childhood trauma subtypes also suggested that emotional neglect in particular interacted with both IL-6 and TNF-α to predict corticostriatal connectivity, such that higher cytokine levels in participants with greater childhood emotional neglect severity was associated with greater corticostriatal connectivity. Although much of the current literature related to childhood trauma has not focused on emotional neglect (broadly defined as parental disregard for a child’s emotional needs such as love or support) (58), this trauma subtype may play an important role in reward circuitry development in TRD. For instance, one study of 106 adolescents found that greater emotional neglect was associated with blunted reward-related VS activity and that VS activity partially mediated relationships between emotional neglect and prospective depressive symptoms (59). Future studies are needed to clearly dissect the role of emotional neglect specifically in cytokine-connectivity relationships.
Of note, we did not find significant correlations between depression severity and cytokine levels in our cohort of patients with TRD (Table S1). However, given the marked heterogeneity of depression and the vastly heterogeneous symptom presentations that are conflated when looking at overall increased depression severity, different patients may (from a theoretical standpoint) have symptoms of depression that are differentially impacted by low-grade elevations in inflammation (60,61). Thus, these factors may lead to lack of correlations between cytokines and depression severity within a TRD cohort, particularly a cohort with a restricted range of depressive symptoms (given that the participants in our cohort uniformly had moderate-to-severe depression), which is consistent with prior studies examining cohorts of patients with TRD (44,62). However, even in the presence of weak correlations between low-grade cytokine elevation and depression severity, our focus on individual differences within TRD permits identifying significant relationships between low-grade cytokine elevation and neural connectivity, which may nevertheless contribute substantially to depressive symptomology in certain individuals with depression. Similarly, genetic, biological, and psychological factors may contribute to heterogeneity that precludes linear associations between low-grade cytokine elevation and CTQ scores and also between CTQ scores and depression severity (e.g., variable etiologies of depressive symptoms occurring with vs. without frank childhood trauma history) (63,64). Consistently, correlations between CTQ composite scores and both depression severity and peripheral cytokine levels were not significant in our analysis (Table S1).
These study findings should be taken in the context of certain limitations. First, our sample size may not have been sufficient to detect significant differences while adjusting appropriately for multiple comparisons, leading to type II error. Specifically, we found that significant findings related to cytokine type (IL-6 or TNF-α) or VS-vmPFC connectivity indices differed based on the specific VS and vmPFC region, warranting examination in future studies to decipher whether true differences exist in these cytokine-connectivity patterns or whether the pattern of findings is attributable to limited power in this study. However, to our knowledge, this study is the largest one examining cytokine associations with VS-vmPFC connectivity in patients with depression. Second, our study was cross-sectional, and thus we are unable to parse potential causal or longitudinal associations. Third, our lack of a control group limits our ability to directly identify whether low-grade cytokine elevations (as compared with healthy control subjects) were present on average in our sample. We could not accurately compare our values with previously published values from healthy control subjects, given between-laboratory variability and between-assay variability in absolute levels of cytokines; this variability would preclude identification of the low-grade cytokine elevations (e.g., IL-6 or TNF-α elevations of <5 pg/mL) found in patients with depression as compared with healthy control subjects that have commonly been reported in the literature (44,65,66). Moreover, our definition of TRD (one or more failed antidepressant trials) was more liberal than that used in many previous reported studies, but we defined TRD in this manner given that such patients are at a higher risk for worse outcomes and to achieve a balance between generalizability and clinical severity (5,67). Setting too high a bar for treatment resistance would limit generalizability to the large proportion of patients who may attempt and fail only one conventional antidepressant but are nevertheless at a heightened risk of worse outcomes. Finally, our use of a retrospective measure of childhood trauma severity highlights the need for prospective developmental studies examining causal associations of childhood trauma on inflammation and corticostriatal connectivity.
In this study, we examined a cohort of adults with unipolar TRD, identifying that IL-6 was associated with reduced bilateral VS-vmPFC connectivity, which is important given that this was the first replication, to our knowledge, of a seminal finding reported in a smaller sample (N = 48) of patients with mixed (unipolar/bipolar) depression and extends this finding to a high-priority TRD cohort. We also found that childhood trauma severity and TNF-α positively interacted to predict VS-vmPFC connectivity, suggesting that cytokines may interact differently with corticostriatal circuitry in patients depending on severity of childhood trauma. Additionally, in exploratory analyses, childhood emotional neglect positively moderated TNF-α to VS-vmPFC connectivity, indicating that this is a needed area of future research. Larger studies are also needed to parse temporal relationships of these pathways through longitudinal analyses. Overall, this study and future studies could help pinpoint cytokine effects on corticostriatal dysfunction in patients with TRD, helping ultimately develop a better individualized pathophysiological understanding of TRD to drive development of targeted treatments.
Acknowledgments and Disclosures
This research was supported by grants from the National Institutes of Health (Grant No. R01MH113857 [to RP]) and by the Clinical and Translational Sciences Institute at the University of Pittsburgh (Grant No. UL1-TR-001857) and was approved by the Institutional Review Board of the University of Pittsburgh. This research was also supported by funding from the Ruth L. Kirschstein National Research Service Award Institutional Research Training Grants sponsored by the National Institutes of Health (Grant No. NIH T32 MH018951 [principal investigator, Dr. David Brent).
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.06.009.
Supplementary Material
References
- 1.Judd L.L., Akiskal H.S., Zeller P.J., Paulus M., Leon A.C., Maser J.D., et al. Psychosocial disability during the long-term course of unipolar major depressive disorder. Arch Gen Psychiatry. 2000;57:375–380. doi: 10.1001/archpsyc.57.4.375. [DOI] [PubMed] [Google Scholar]
- 2.Brody D.J., Pratt L.A., Hughes J.P. Prevalence of depression among adults aged 20 and over: United States, 2013-2016. NCHS Data Brief. 2018;(303):1–8. [PubMed] [Google Scholar]
- 3.Machado M.O., Veronese N., Sanches M., Stubbs B., Koyanagi A., Thompson T., et al. The association of depression and all-cause and cause-specific mortality: An umbrella review of systematic reviews and meta-analyses. BMC Med. 2018;16:112. doi: 10.1186/s12916-018-1101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemeroff C.B. Prevalence and management of treatment-resistant depression. J Clin Psychiatry. 2007;68(suppl 8):17–25. [PubMed] [Google Scholar]
- 5.Fekadu A., Wooderson S.C., Markopoulo K., Donaldson C., Papadopoulos A., Cleare A.J. What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies. J Affect Disord. 2009;116:4–11. doi: 10.1016/j.jad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Keren H., O’Callaghan G., Vidal-Ribas P., Buzzell G.A., Brotman M.A., Leibenluft E., et al. Reward processing in depression: A conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry. 2018;175:1111–1120. doi: 10.1176/appi.ajp.2018.17101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng T.H., Alloy L.B., Smith D.V. Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Transl Psychiatry. 2019;9:293. doi: 10.1038/s41398-019-0644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers M.A., Bradshaw J.L., Pantelis C., Phillips J.G. Frontostriatal deficits in unipolar major depression. Brain Res Bull. 1998;47:297–310. doi: 10.1016/s0361-9230(98)00126-9. [DOI] [PubMed] [Google Scholar]
- 9.Russo S.J., Nestler E.J. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furman D.J., Hamilton J.P., Gotlib I.H. Frontostriatal functional connectivity in major depressive disorder. Biol Mood Anxiety Disord. 2011;1:11. doi: 10.1186/2045-5380-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rupprechter S., Romaniuk L., Series P., Hirose Y., Hawkins E., Sandu A.L., et al. Blunted medial prefrontal cortico-limbic reward-related effective connectivity and depression. Brain. 2020;143:1946–1956. doi: 10.1093/brain/awaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin L., Xu X., Chen G., Mehta N.D., Haroon E., Miller A.H., et al. Inflammation and decreased functional connectivity in a widely distributed network in depression: Centralized effects in the ventral medial prefrontal cortex. Brain Behav Immun. 2019;80:657–666. doi: 10.1016/j.bbi.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctôt K.L. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 14.Miller A.H., Raison C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felger J.C., Treadway M.T. Inflammation effects on motivation and motor activity: Role of dopamine. Neuropsychopharmacology. 2017;42:216–241. doi: 10.1038/npp.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenberger N.I., Berkman E.T., Inagaki T.K., Rameson L.T., Mashal N.M., Irwin M.R. Inflammation-induced anhedonia: Endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller A.H., Haroon E., Raison C.L., Felger J.C. Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrne M.L., Whittle S., Allen N.B. The role of brain structure and function in the association between inflammation and depressive symptoms: A systematic review. Psychosom Med. 2016;78:389–400. doi: 10.1097/PSY.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 19.Treadway M.T., Cooper J.A., Miller A.H. Can’t or won’t? Immunometabolic constraints on dopaminergic drive. Trends Cogn Sci. 2019;23:435–448. doi: 10.1016/j.tics.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rengasamy M., Marsland A., McClain L., Kovats T., Walko T., Pan L., Price R.B. Longitudinal relationships of cytokines, depression and anhedonia in depressed adolescents. Brain Behav Immun. 2021;91:74–80. doi: 10.1016/j.bbi.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaur S., Bansal Y., Kumar R., Bansal G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg Med Chem. 2020;28:115327. doi: 10.1016/j.bmc.2020.115327. [DOI] [PubMed] [Google Scholar]
- 22.Zelová H., Hošek J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm Res. 2013;62:641–651. doi: 10.1007/s00011-013-0633-0. [DOI] [PubMed] [Google Scholar]
- 23.Felger J.C., Li Z., Haroon E., Woolwine B.J., Jung M.Y., Hu X., Miller A.H. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21:1358–1365. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ionescu D.F., Papakostas G.I. Experimental medication treatment approaches for depression. Transl Psychiatry. 2017;7 doi: 10.1038/tp.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tunnard C., Rane L.J., Wooderson S.C., Markopoulou K., Poon L., Fekadu A., et al. The impact of childhood adversity on suicidality and clinical course in treatment-resistant depression. J Affect Disord. 2014;152–154:122–130. doi: 10.1016/j.jad.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien B., Lijffijt M., Wells A., Swann A.C., Mathew S.J. The impact of childhood maltreatment on intravenous ketamine outcomes for adult patients with treatment-resistant depression. Pharmaceuticals (Basel) 2019;12:133. doi: 10.3390/ph12030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanni V., Uher R., Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am J Psychiatry. 2012;169:141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- 28.Nelson J., Klumparendt A., Doebler P., Ehring T. Childhood maltreatment and characteristics of adult depression: Meta-analysis. Br J Psychiatry. 2017;210:96–104. doi: 10.1192/bjp.bp.115.180752. [DOI] [PubMed] [Google Scholar]
- 29.Herzberg M.P., Gunnar M.R. Early life stress and brain function: Activity and connectivity associated with processing emotion and reward. NeuroImage. 2020;209:116493. doi: 10.1016/j.neuroimage.2019.116493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nusslock R., Miller G.E. Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biol Psychiatry. 2016;80:23–32. doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraynak T.E., Marsland A.L., Hanson J.L., Gianaros P.J. Retrospectively reported childhood physical abuse, systemic inflammation, and resting corticolimbic connectivity in midlife adults. Brain Behav Immun. 2019;82:203–213. doi: 10.1016/j.bbi.2019.08.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumeister D., Akhtar R., Ciufolini S., Pariante C.M., Mondelli V. Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry. 2016;21:642–649. doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shrout P.E., Rodgers J.L. Psychology, science, and knowledge construction: Broadening perspectives from the replication crisis. Annu Rev Psychol. 2018;69:487–510. doi: 10.1146/annurev-psych-122216-011845. [DOI] [PubMed] [Google Scholar]
- 34.Poldrack R.A., Baker C.I., Durnez J., Gorgolewski K.J., Matthews P.M., Munafò M.R., et al. Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nat Rev Neurosci. 2017;18:115–126. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rengasamy M., Price R.B. In: The Neuroscience of Depression: Genetics, Cell Biology, Neurology, Behaviour, and Diet. Martin C., Hunter L.A., Patel V., Preedy V., Rajendram R., editors. Academic Press; Cambridge: 2021. Linking interleukin-6 and depression; pp. 119–125. [Google Scholar]
- 36.Price R.B., Panny B., Degutis M., Griffo A. Repeated measurement of implicit self-associations in clinical depression: Psychometric, neural, and computational properties. J Abnorm Psychol. 2021;130:152–165. doi: 10.1037/abn0000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandey G.N., Rizavi H.S., Ren X., Fareed J., Hoppensteadt D.A., Roberts R.C., et al. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res. 2012;46:57–63. doi: 10.1016/j.jpsychires.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernstein D.P., Ahluvalia T., Pogge D., Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Price R.B., Shungu D.C., Mao X., Nestadt P., Kelly C., Collins K.A., et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: Relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coplan J.D., Gopinath S., Abdallah C.G., Berry B.R. A neurobiological hypothesis of treatment-resistant depression - mechanisms for selective serotonin reuptake inhibitor non-efficacy. Front Behav Neurosci. 2014;8:189. doi: 10.3389/fnbeh.2014.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akil H., Gordon J., Hen R., Javitch J., Mayberg H., McEwen B., et al. Treatment resistant depression: A multi-scale, systems biology approach. Neurosci Biobehav Rev. 2018;84:272–288. doi: 10.1016/j.neubiorev.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lui S., Wu Q., Qiu L., Yang X., Kuang W., Chan R.C., et al. Resting-state functional connectivity in treatment-resistant depression. Am J Psychiatry. 2011;168:642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- 43.Dichter G.S., Gibbs D., Smoski M.J. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord. 2015;172:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rengasamy M., McClain L., Gandhi P., Segreti A.M., Brent D., Peters D., Pan L. Associations of plasma interleukin-6 with plasma and cerebrospinal fluid monoamine biosynthetic pathway metabolites in treatment-resistant depression. Neurol Psychiatry Brain Res. 2018;30:39–46. [Google Scholar]
- 45.Haroon E., Daguanno A.W., Woolwine B.J., Goldsmith D.R., Baer W.M., Wommack E.C., et al. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology. 2018;95:43–49. doi: 10.1016/j.psyneuen.2018.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Z., Zhang C.Y., Huang J., Wang Y., Yan C., Li K., et al. Improving motivation through real-time fMRI-based self-regulation of the nucleus accumbens. Neuropsychology. 2018;32:764–776. doi: 10.1037/neu0000425. [DOI] [PubMed] [Google Scholar]
- 47.Vythilingam M., Heim C., Newport J., Miller A.H., Anderson E., Bronen R., et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paquola C., Bennett M.R., Hatton S.N., Hermens D.F., Lagopoulos J. Utility of the cumulative stress and mismatch hypotheses in understanding the neurobiological impacts of childhood abuse and recent stress in youth with emerging mental disorder. Hum Brain Mapp. 2017;38:2709–2721. doi: 10.1002/hbm.23554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao U., Chen L.A., Bidesi A.S., Shad M.U., Thomas M.A., Hammen C.L. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry. 2010;67:357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cassiers L.L.M., Sabbe B.G.C., Schmaal L., Veltman D.J., Penninx B.W.J.H., Van Den Eede F. Structural and functional brain abnormalities associated with exposure to different childhood trauma subtypes: A systematic review of neuroimaging findings. Front Psychiatry. 2018;9:329. doi: 10.3389/fpsyt.2018.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novick A.M., Levandowski M.L., Laumann L.E., Philip N.S., Price L.H., Tyrka A.R. The effects of early life stress on reward processing. J Psychiatr Res. 2018;101:80–103. doi: 10.1016/j.jpsychires.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riem M.M., van IJzendoorn M.H., Tops M., Boksem M.A., Rombouts S.A., Bakermans-Kranenburg M.J. Oxytocin effects on complex brain networks are moderated by experiences of maternal love withdrawal. Eur Neuropsychopharmacol. 2013;23:1288–1295. doi: 10.1016/j.euroneuro.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 53.Quidé Y., Girshkin L., Watkeys O.J., Carr V.J., Green M.J. The relationship between cortisol reactivity and emotional brain function is differently moderated by childhood trauma, in bipolar disorder, schizophrenia and healthy individuals. Eur Arch Psychiatry Clin Neurosci. 2021;271:1089–1109. doi: 10.1007/s00406-020-01190-3. [DOI] [PubMed] [Google Scholar]
- 54.Hanson J.L., Knodt A.R., Brigidi B.D., Hariri A.R. Heightened connectivity between the ventral striatum and medial prefrontal cortex as a biomarker for stress-related psychopathology: Understanding interactive effects of early and more recent stress. Psychol Med. 2018;48:1835–1843. doi: 10.1017/S0033291717003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCutcheon R.A., Bloomfield M.A.P., Dahoun T., Mehta M., Howes O.D. Chronic psychosocial stressors are associated with alterations in salience processing and corticostriatal connectivity. Schizophr Res. 2019;213:56–64. doi: 10.1016/j.schres.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hiser J., Koenigs M. The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol Psychiatry. 2018;83:638–647. doi: 10.1016/j.biopsych.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehta N.D., Stevens J.S., Li Z., Gillespie C.F., Fani N., Michopoulos V., Felger J.C. Inflammation, reward circuitry and symptoms of anhedonia and PTSD in trauma-exposed women. Soc Cogn Affect Neurosci. 2020;15:1046–1055. doi: 10.1093/scan/nsz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoltenborgh M., Bakermans-Kranenburg M.J., Van Ijzendoorn M.H. The neglect of child neglect: A meta-analytic review of the prevalence of neglect. Soc Psychiatry Psychiatr Epidemiol. 2013;48:345–355. doi: 10.1007/s00127-012-0549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanson J.L., Hariri A.R., Williamson D.E. Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biol Psychiatry. 2015;78:598–605. doi: 10.1016/j.biopsych.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milaneschi Y., Lamers F., Berk M., Penninx B.W.J.H. Depression heterogeneity and its biological underpinnings: Toward immunometabolic depression. Biol Psychiatry. 2020;88:369–380. doi: 10.1016/j.biopsych.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 61.Milaneschi Y., Lamers F., Peyrot W.J., Abdellaoui A., Willemsen G., Hottenga J.J., et al. Polygenic dissection of major depression clinical heterogeneity. Mol Psychiatry. 2016;21:516–522. doi: 10.1038/mp.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park M., Newman L.E., Gold P.W., Luckenbaugh D.A., Yuan P., Machado-Vieira R., Zarate C.A. Change in cytokine levels is not associated with rapid antidepressant response to ketamine in treatment-resistant depression. J Psychiatr Res. 2017;84:113–118. doi: 10.1016/j.jpsychires.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonanno G.A., Mancini A.D. Beyond resilience and PTSD: Mapping the heterogeneity of responses to potential trauma. Psychol Trauma Theor Res Pract Policy. 2012;4:74–83. [Google Scholar]
- 64.Nugent N.R., Koenen K.C., Bradley B. Heterogeneity of posttraumatic stress symptoms in a highly traumatized low income, urban, African American sample. J Psychiatr Res. 2012;46:1576–1583. doi: 10.1016/j.jpsychires.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simon N.M., McNamara K., Chow C.W., Maser R.S., Papakostas G.I., Pollack M.H., et al. A detailed examination of cytokine abnormalities in Major Depressive Disorder. Eur Neuropsychopharmacol. 2008;18:230–233. doi: 10.1016/j.euroneuro.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’donovan A., Rush G., Hoatam G., Hughes B.M., McCrohan A., Kelleher C., et al. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress Anxiety. 2013;30:307–314. doi: 10.1002/da.22087. [DOI] [PubMed] [Google Scholar]
- 67.Rush A.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Stewart J.W., Warden D., et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR∗D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.