Abstract
Materials and processes for chemical separations must be used in complex environments to have an impact in many practical settings. Despite these complexities, much research on chemical separations has focused on idealized chemical mixtures. In this paper, we suggest that research communities for specific chemical separations should develop well-defined exemplar mixtures to bridge the gap between fundamental studies and practical applications and we provide a hierarchical framework of chemical mixtures for this purpose. We illustrate this hierarchy with examples, including CO2 capture, capture of uranium from seawater, and separations of mixtures from electrocatalytic CO2 reactions, among others. We conclude with four recommendations for the research community to accelerate the development of innovative separations strategies for pressing real-world challenges.
Keywords: chemical separations, chemical mixtures, CO2 capture, CO2 conversion, uranium
Introduction
Chemical separations are an ubiquitous and vital part of almost every product of the global chemical industry. Traditional separation methods relying on phase changes in the production of commodity chemicals (e.g., distillation) use enormous amounts of energy and water.1 The drive to reduce the costs associated with large-scale separations and the need to develop chemically specific separations that enable new products and applications have created intense interest in development of new separation methods. A recent report from the US National Academies defined a roadmap for fundamental research on chemical separations.2 A key recommendation of that report was that “The research community should study complex systems to understand separations systems under various realistic conditions.”
The recommendation to study “complex systems” is likely to be uncontroversial, but at least two barriers must be confronted by researchers who want to act on this idea. First, studying realistic mixtures can be significantly more challenging than performing work with individual molecules or “simple” binary mixtures. A second barrier, however, is also important: it can be difficult to know what mixture to study or how to judge if a mixture is “realistic”. An additional challenge that limits progress is that if multiple groups perform experiments with a disparate array of mixtures comparing information between studies of different materials or separations processes can be difficult. A recent systematic review of experimental studies of mixture gas adsorption found few examples where experiments from different sources could be directly compared, in part because of variations in the conditions chosen for different experiments.3
The aim of this paper is to reduce the barriers in research on chemical separations to studying “complex systems...under various realistic conditions” by illustrating the concept of exemplar mixtures for a set of separations of wide interest. Our aim in providing these mixtures is not to be definitive, as many choices are possible in each example. Instead, we hope that our examples promote discussion as to what properties of complex mixtures need to be captured in specific applications and motivate research communities to consider “standard” mixtures that would accelerate research progress. Successful examples of defining standard mixtures already exist in other research domains. Automotive emissions catalysts must function when they are exposed to streams containing water, CO2, CO, NOx, and a diverse array of hydrocarbons. Work in this area has been aided by a collaboration of industry, national laboratory, and academic researchers to define a protocol to mimic lean-burn conditions without using real exhaust gases.4
Hierarchy of Separations Complexity
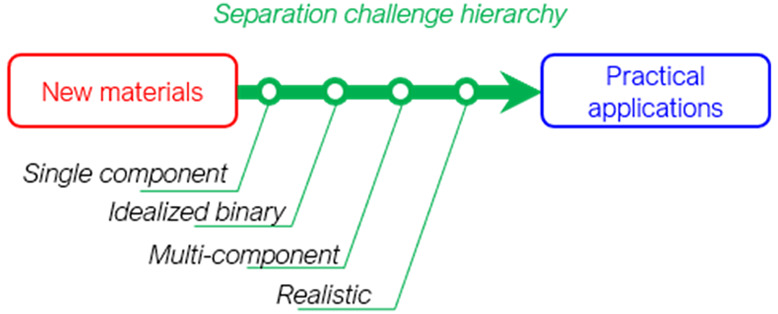
To motivate our discussion, we begin with the hierarchy of complexity shown in Table 1 to give a framework for defining what a “complex” mixture is. This hierarchy divides separations experiments (or computer simulations) into single-component, idealized, multicomponent, realistic, and process stream studies on the basis of the set of chemical species that is used. The single-component category is self-explanatory. Even though no chemical separation is achieved in this category, a large fraction of all separations-based research reports focus on data of this kind. Our terminology deliberately distinguishes between work with binary mixtures, which we denote “idealized”, and “multicomponent” work, a term we reserve for efforts with three or more species. Only a small fraction of the research literature in most areas of chemical separations reports data that are multicomponent by this definition. Our hierarchy includes two levels of complexity above multicomponent experiments. Work with process streams that come directly from real processes obviously satisfies the definition of a “complex” mixture, but this objective is not readily available to many academic research groups. There is great value, however, in using what we describe as a “realistic” stream that includes the key known components, including trace contaminants, that are expected in a real setting. A core aim of this paper is to suggest a set of specific compositions for “realistic” studies in a variety of application areas.
Table 1. Hierarchy of Separations Experiments.
| description | analytes used in experiments or simulations |
|---|---|
| single component | one species studied at a time |
| idealized | binary mixtures of key target species (often 50/50 composition) |
| multicomponent | mixture of three or more components at concentrations relevant to target application |
| realistic | mixtures with representative concentrations of all known species in real-world processes, including trace contaminants |
| process stream | samples taken directly from real-world processes |
To make this hierarchy more concrete, consider the task of capturing CO2 from the flue gas of a coal-fired power plant. This example allows us to illustrate why issues that only arise with the more complex mixtures in the hierarchy can become critical bottlenecks in advancing new approaches toward operating technologies. Societal interest in CO2 capture has led to a vast literature exploring separations based on absorption in liquids, adsorption in porous materials, or membrane permeation.5 Single-component studies on this topic typically examine the response of a material to pure CO2 and to pure N2, the major components of flue gas. Idealized studies extend single-component studies to binary CO2/N2 mixtures, often at a 15/85 composition similar to the composition of actual flue gas. The selectivity observed with binary gas mixtures can differ qualitatively from simple predictions from single-component experiments.6 Real flue gas is typically saturated with water; thus, the most natural multicomponent mixture to consider is a 15/85 CO2/N2 mixture with 100% relative humidity (RH). Testing of new materials in this multicomponent environment is already revealing, since there are many materials that are highly stable in dry environments but which degrade systematically in the presence of humidity.7
Flue gas from coal-fired power plants will contain not only CO2, N2, and water vapor, but also ppb–ppm levels of SO2, NO2, and HgCl2, among other possible trace contaminants. The “realistic” level of our hierarchy would therefore be achieved by testing using a 15/85 CO2/N2 stream saturated with water and containing ppm levels of SO2, NO2, and/or HgCl2. This composition illustrates two important points about doing work with complex streams. First, the details of performing experiments with these streams can be substantially more complicated in comparison to experiments with idealized streams. Using even ppm levels of SO2, NO2, or HgCl2 requires much greater care with laboratory safety and equipment design relative to work with only CO2, N2, and water. Second, work with this realistic stream can indicate critical features of new materials that cannot be deduced from experiments with simpler streams. Multiple examples are known of adsorbents that are stable in pure water and also on exposure to dry acid gases such as SO2 but degrade rapidly in a humid stream containing ppm levels of SO2.8−10 This occurs because of synergistic effects between water and SO2. HgCl2 has been shown to adsorb far more strongly in porous adsorbents in comparison to CO2 or N2, leading to examples in which extremely low levels of HgCl2 can dramatically change the adsorption capacity of adsorbents for CO2.11 In each of these examples, information from “realistic” streams reveals issues that might become critical “showstoppers”12 on the path to real-world implementation that cannot be deduced from the typical single-component or idealized mixture experiments that are common in the research literature.
Our discussion of CO2 from coal-fired power plants also indicates why the exemplar mixtures we suggest in this paper are application-dependent. In many parts of the world, combustion of coal is rapidly being displaced by combustion of natural gas for economic and environmental reasons. It is therefore natural to consider the task of capturing CO2 from natural gas combustion. Pipeline-quality natural gas contains almost no N-containing species aside from low levels of N2 and no appreciable levels of Hg-containing species. It does, however, contain significant levels of sulfur because mercaptans are added to natural gas for safety reasons.13 In addition, trace levels of NOx are common combustion products. An exemplary “realistic” mixture for this application is therefore a 15/85 mixture of CO2/N2 that is saturated with water and contains ppm levels of SO2 and NO2 but no other trace contaminants. The various mixtures we have discussed related to coal-fired power plants and natural gas combustion are summarized in Table 2.
Table 2. Summary of Suggest Exemplar Mixtures for the Applications Discussed in the Text (Compositions in mol %).
| application | typical process conditions (T, P) | single component | idealized | multicomponent | realistic |
|---|---|---|---|---|---|
| CO2 capture (coal-fired combustion) | 25–50 °C, 1 atm | CO2, N2 | 15/85 CO2/NO2 | 15/85 CO2/N2 with 100% RH | 15/85 CO2/N2 with 100% RH and ppm of SO2, NO2, and/or HgCl2 |
| CO2 capture (natural gas fired combustion) | 25–50 °C, 1 atm | CO2, N2 | 15/85 CO2/NO2 | 15/85 CO2/N2 with 100% RH | 15/85 CO2/N2 with 100% RH and ppm of SO2 and NO2 |
| natural gas storage | 25–50 °C, 1–50 atm | CH4 | 95/5 CH4/C2H6 | 95/2.6/0.2/1.5/0.7 CH4/C2H6/C3H8/N2/CO2 | multicomponent mixture + 0.01% C5H12, 0.01% C6H14 and ppm mercaptan |
| capture of U from seawater | ambient T and P | deionized water + sea salt + 5 ppm U | deionized water + sea salt + 5 ppm U + 2 ppm V | “simulated seawater” (ref (28)) | |
| CO2 reduction reaction products | 25–50 °C, 1 atm | C2H4, CO2, | 25/75 C2H4/CH4 or 50/50 C2H4/CO2 | 30/30/20/15/5 CH4/C2H6/H2O/CO2 | 10/20/18/3/2/6/1/40 CH4/C2H4/CO2/H2O/C2H5OH/CO/CH3CHO/H2 |
| crude oil | 100–400 °C (distillation); 25–150 °C (membranes) | toluene, hexane, cyclohexane, 1-methylnaphthalene | 90/10 toluene/triisopropylbenzene or 90/10hexane/isocetane | 90 mol % toluene, <1 mol % of p-xylene, o-xylene, mesitylene, 1-methylnaphthalene, biphenyl, triisopropylenzene, isocetane, dodecane, pristane, dodecylbenzene, n-docosane | “simulated light shale”: toluene, 17 mol %; methylcyclohexane, 28 mol %; n-octane, 22 mol %; iso-octane, 15 mol %; tert-butylbenzene, 2.2 mol %; decalin, 11 mol %; triisopropylbenzene, 1.6 mol %; isocetane, 1.3 mol % |
Exemplar Mixtures for Chemical Separations
To further illustrate the hierarchy of separations complexity defined above, we present below several additional examples of chemical separations that could be relevant in large-scale applications in the future. We view our suggestions of exemplar mixtures for these applications as tentative, since a more robust way to establish standards for these or other applications would be via a consensus process involving a range of researchers and end users.
Adsorptive Storage of Natural Gas
The composition of natural gas that is available for industrial and transportation uses is often dictated by pipeline standards. The simple observation that natural gas delivered by pipelines is not pure CH4 highlights the potential role that other natural gas components could have in downstream processes. In vehicular settings natural gas can be stored in compressed gas tanks, but considerable research has been applied to the alternative concept of adsorbing natural gas in porous materials.14,15 Because of the non-CH4 components in natural gas, cyclic adsorption and desorption of natural gas is best thought of as a separation process, albeit a process in which the separation is an unintentional consequence. Because desorption in these settings is typically designed to release CH4 without incurring large energy inputs, species with larger molecular weights will accumulate in adsorbents.16,17 Examples have been described in which this phenomenon substantially reduces the net energy density of natural gas stored by an adsorbent over the course of many cycles.18,19
This discussion suggests that it is useful to define a hierarchy of natural gas mixtures for consideration in any storage or use process that will rely on natural gas. The mixtures summarized in Table 2 are based on wellhead compositions from a diverse array of sources around the world.20−22 The idealized and multicomponent mixtures include the ethane and propane that make up several percent of typical natural gas supplies. The “realistic” mixture adds low levels of C5 and C6 hydrocarbons as well as ppm levels of mercaptan.
Purification of Products from Electrocatalytic CO2 Conversion
Electrochemical conversion of CO2 to ethylene and other byproducts is a rapidly emerging technological concept driven by the anticipated availability of renewable electricity and the need to obtain near-zero carbon emissions for liquid fuels and chemicals. Significant emphasis has been placed on the design of electrocatalysts that reduce CO2 in the presence of water (or H2), often with the goal of producing ethylene.23,24 Reactions such as these produce a variety of off-target byproducts, including CO, formic acid, acetaldehyde, alcohols, and light alkanes. Moreover, excess H2O is also found in the reaction products. Interestingly, oxidative coupling of methane produces a similar slate of compounds.25
The importance of chemical separations in making electrocatalytic CO2 conversion economically viable has not been widely appreciated. Greenblatt et al. described the significant separations challenges that must be addressed to take raw CO2 reduction reaction conversion streams into products that are compatible with downstream processing (e.g., polyethylene plants).26 Although robust materials capable of selectively removing high-value compounds from these streams are desirable from a process simplicity perspective, it is likely that almost all of the components in electrochemical CO2 conversion product streams have utility either as a fuel to provide process heat or as a feedstock for subsequent conversion. Thus, a key observation emphasized by Greenblatt et al. is that a system of various separations technologies is likely to be needed to sequentially remove target species or target groups of species from these complex streams.
The discussion above highlights the need to consider complex mixtures in considering how to couple separations with electrocatalytic CO2 conversion. An exemplar product mixture from these catalytic processes contains methane in concentration ranges of 4–30 mol %, ethylene in ranges of 4–30 mol %, hydrogen in ranges of 30–60 mol %, carbon monoxide in ranges of 3–17 mol %, acetaldehyde in concentrations of 0.5–1.5 mol %, ethanol in ranges of 0.3–1.6 mol %, inert species at concentrations of up to 2 mol %, and water, higher aldehydes, alcohols, and combustible gases at concentrations of <1 mol %. Moreover, there is often some unreacted CO2 that must be addressed. on application of the separations hierarchy from Table 1, single-component sorption experiments should focus on components such as CO2, ethylene, methane, water, and ethanol; if they are properly managed from a safety perspective, single-component H2 and CO would be beneficial. Idealized separation experiments should include the removal of ethylene from CO2 and light alkanes, while multicomponent separations will incorporate water vapor and CO, and realistic experiments will involve nearly the full slate of reduction reaction products. This suggested hierarchy of mixtures is summarized in Table 2.
Uranium from Seawater and Value-Added Materials from Produced Water
Although the concentration of U in seawater (3.3 μg L–1 = 3.3 ppb) is low, the total quantity of U in the oceans exceeds conventional terrestrial ores by approximately a factor of 1000.27 This capacity has driven decades of work aiming to efficiently extract U from seawater. As emphasized by Abney et al.,27 seawater is inherently complex, so studies that focus on capturing U from simpler aqueous solutions often have little impact on the real challenge of processing seawater. The elements with the highest concentrations in seawater are27 Na (19400 ppm), Cl (10800 ppm), Mg (1290 ppm), Ca (413 ppm), and K (400 ppm). A challenge for capturing U is that materials such as amidoxime-functionalized polymers also typically bind competing species that are present in seawater at dilute concentrations, including V (1.8 ppb) and Fe (3.4 ppb). These effects, plus the impact of pH and (bi)carbonates on ion speciation, led Abney et al. to conclude that “standardization of seawater simulant and normalization of uranium extraction data from environmental seawater is essential for valid head-to-head comparisons of material performance”.27
With the “simulated seawater” described by Gao et al. as motivation, the mixtures given in Table 2 suggest one way to approach the complexity of extracting U from real seawater.28 We followed the suggestion of Das et al.29 that initial experiments be performed with ppm levels of U rather than the ppb levels relevant to true seawater to make the screening of new materials more feasible. A feature of this approach is that it uses real sea salt to create the requisite salinity rather than pristine NaCl, allowing some of the chemical complexity of the most concentrated ions in seawater to be reproduced. The “idealized” and “multicomponent” mixtures in Table 2 could give insight into capturing U in the presence of these ions and, in the multicomponent case, competing ions containing V. An important simplification of these mixtures is that they are not pH controlled. Gao et al. described an approach to control the pH via the addition of a saturated Na2CO3 solution.
This example illustrates how exemplar mixtures need to be defined not only on the basis of the feedstocks to be treated (in this case seawater) but also on the basis of the desired separation. In an exploration of the capture of U, U needs to be included in even the idealized mixture and the key competing ion (V) is included in the multicomponent mixture. If instead one was interested in capturing CO2 or carbonate from seawater,30,31 the inclusion of trace U and V would likely be irrelevant but the addition of carbonate would be required for even the simplest mixtures.
Although seawater is complex, it is more homogeneous than the large volumes of produced water that are a byproduct of oil and gas production. The work of Johnson et al.32 gives an impressive analysis of produced water compositions from more than 4000 samples that focus on natural gas production. They note that produced water has levels of chloride as high as 380,000 mg/L, 20 times higher than that of seawater, a pH as low as 3, and potential “items of concern” including Al, As, Ba, B, Br, Cd, Cu, Fe, Pb, Li, Ni, Zn, H2S, sulfate, sulfide, and bicarbonate. As interest grows in extracting value-added products from produced water,33,34 it seems likely that developing a set of exemplar mixtures that can be adopted by the research community will greatly aid in the comparison of experiments.
Synthetic Crude Oils
Membrane separation processes have recently been explored for the extraction of light compounds from raw crude oil.35 Real crude oil is enormously complex, containing tens of thousands of unique hydrocarbon molecules in addition to a variety of metals, heteroatom-containing molecules, and salts.36 Very few laboratories have the capability to fully analyze these types of mixtures; however, useful approximations have been developed. Light hydrocarbons such as toluene, hexane, cyclohexane, and 1-methylnapthalene are useful single-component representatives of the various classes of hydrocarbons found in crude oil. Idealized separation experiments utilizing binary pairs of a “small” and “large” hydrocarbon are useful for size-based separations (e.g., toluene and triisopropylbenzene37), whereas pairings of different classes of molecules (e.g., toluene and cyclohexane) are useful for exploring sorption- or solubility-based separations. Multicomponent mixtures can be easily explored when a single light hydrocarbon is picked as a “solvent” (e.g., toluene) while a collection of dilute hydrocarbons (<1 mol %) are chosen as solutes. These types of mixtures are useful for exploring size-based separations as well as investigating relative interactions of the solutes with the separation media. Finally, a realistic stream can be formulated in the laboratory using ∼6–10 light hydrocarbons spanning a range of hydrocarbon classes and sizes. Importantly, these solutions should be crafted such that no one compound can be thought of as a “solvent”. One exemplar composition that is intended to mimic a light shale oil is described in Table 2.
Recommendations for the Research Community
The central idea we are advocating in this article is that adoption of a well-defined hierarchy of exemplar mixtures that approximate the full complexity of a practical chemical separation could have far-reaching implications for the productivity and efficiency of the research community. This is not a new idea; it has precedents in applied catalysis,4 in specific separations challenges such as extracting U from seawater,27 and likely in other contexts. We conclude with several recommendations for using this idea to improve research outcomes in the separations community.
Recommendation 1: Use Inclusive Methods to Define Exemplar Mixtures for Target Separations
A challenge associated with defining exemplar mixtures is that different researchers are likely to have a range of views for what will make a separation challenging as it is moved from fundamental research to real-world applications. For this reason we view the examples given above for individual separations as initial suggestions to promote discussion rather than definitive pronouncements. An ideal process to establish a set of exemplar mixtures would involve input from an array of researchers with hands-on experience of the methods of interest and industrial practitioners and end users. We are not, however, suggesting that a complex formal process analogous to IUPAC or ASTM standards be envisioned, since the slow pace of this kind of activity is inconsistent with the goal of accelerating high-quality research. The next recommendation gives a specific suggestion to sidestep this issue.
Recommendation 2: Use Round-Robin Experiments to Establish De Facto Community Standards for Exemplar Mixtures
A useful definition of a community standard for an exemplar mixtures is that an active subset of researchers in a field is motivated to use the standard and that positive peer pressure emerges in the process of peer review to take advantage of the consistency the standard provides. One avenue to create this outcome is for a collection of research groups to perform round-robin experiments that apply the same conditions to a well-defined set of samples. A carefully performed study of this kind can create a de facto standard that might otherwise take years of discussion and debate to establish. This approach has been used to good effect, for example, in the adsorption community for single-component gas adsorption in porous materials.38−40 In addition to addressing the need for well-defined exemplar mixtures, this approach has the advantage of creating data that give systematic insight into uncertainties in replicate experiments.41,42 In efforts of this kind it is important before experiments commence to discuss how data will be anonymized, how outliers will be addressed and issues related to authorship will be handled, etc.
A more formal approach to this issue is the creation of facilities in which separation performance is measured by a respected third-party organization. This approach has become widely accepted in the development of photovoltaic devices.43 Although it may be challenging to establish efforts of this type for chemical separations, a careful discussion of this concept within the research community would likely yield progress toward defining community standards for exemplary mixtures.
Recommendation 3: Use the Hierarchy of Separations to Describe Current and Needed Work
Researchers at all stages on the continuum from fundamental to applied separations research would be well served by explicitly placing their work in the hierarchy summarized in Table 1 and making sincere efforts to understand the complications that could arise in taking their work to higher levels of realism in this hierarchy. As Ph.D. students and other researchers are trained, they should be encouraged to read and ponder examples that span the entire hierarchy. We acknowledge that higher stages in the separations hierarchy are typically associated with more complex equipment and, in some cases, safety concerns. Even if work is constrained by these limitations, explicitly placing reported work in the context of this hierarchy can enhance the value of such reports to the research community.
Recommendation 4: Remember that Real Applications Take Place in Settings That Vary with Time and Location
It is vital to remember that even the “realistic” level of the hierarchy above is an approximation that relies on the idea that the problems that might beset a separations technology in the real world arise from known origins. The composition and properties of any real-world input, particularly if taken from an “open” source such as produced water, will vary with time and location, sometimes in unpredictable ways. No single exemplar mixture can represent the full complexity of these situations; thus, obtaining data that help design processes under diverse operating conditions and performing field tests of promising technologies will remain critical in moving concepts from research to application.
Acknowledgments
This work was supported by the Center for Understanding and Control of Acid Gas-Induced Evolution of Materials for Energy (UNCAGE-ME), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Award No. DE-SC0012577. This manuscript has been authored by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the US Department of Energy (DOE). The publisher acknowledges the US government license to provide public access under the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
The authors declare no competing financial interest.
Originally published ASAP January 19, 2022; Table 2 revised January 24, 2022.
References
- Sholl D. S.; Lively R. P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. 10.1038/532435a. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine A Research Agenda for Transforming Separation Science; The National Academies Press: 2019. 10.17226/25421. [DOI] [Google Scholar]
- Cai X.; Gharagheizi F.; Bingel L. W.; Shade D.; Walton K. S.; Sholl D. S. A Collection of More than 900 Gas Mixture Adsorption Experiments in Porous Materials from Literature Meta-Analysis. Ind. Eng. Chem. Res. 2021, 60, 639–651. 10.1021/acs.iecr.0c05398. [DOI] [Google Scholar]
- Toops T. J.; Binder A. J.; Kunal P.; Kyriakidou E. A.; Choi J.-S. Analysis of Ion-Exchanged ZSM-5, BEA, and SSZ-13 Zeolite Trapping Materials under Realistic Exhaust Conditions. Catalysts 2021, 11, 449. 10.3390/catal11040449. [DOI] [Google Scholar]
- Wilcox J.Carbon Capture; Springer: 2012. [Google Scholar]
- Lang W.-Z.; Ouyang J.-X.; Guo Y.-J.; Chu L.-F. Synthesis of Tubular Faujasite X-Type Membranes with Mullite Supports and their Gas Permeances for N2/CO2 Mixtures. Sep. Sci. Technol. 2011, 46, 1716–1725. 10.1080/01496395.2011.572940. [DOI] [Google Scholar]
- Burtch N. C.; Jasuja H.; Walton K. S. Water Stability and Adsorption in Metal–Organic Frameworks. Chem. Rev. 2014, 114, 10575–10612. 10.1021/cr5002589. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S.; Han R.; Kim W.-G.; Chiang Y.; Jayachandrababu K. C.; Hungerford J. T.; Dutzer M. R.; Ma C.; Walton K. S.; Sholl D. S.; et al. Acid Gas Stability of Zeolitic Imidazolate Frameworks: Generalized Kinetic and Thermodynamic Characteristics. Chem. Mater. 2018, 30, 4089–4101. 10.1021/acs.chemmater.8b01394. [DOI] [Google Scholar]
- Mounfield W. P.; Han C.; Pang S. H.; Tumuluri U.; Jiao Y.; Bhattacharyya S.; Dutzer M. R.; Nair S.; Wu Z.; Lively R. P.; et al. Synergistic Effects of Water and SO2 on Degradation of MIL-125 in the Presence of Acid Gases. J. Phys. Chem. C 2016, 120, 27230–27240. 10.1021/acs.jpcc.6b09264. [DOI] [Google Scholar]
- Han S.; Huang Y.; Watanabe T.; Dai Y.; Walton K. S.; Nair S.; Sholl D. S.; Meredith J. C. High-Throughput Screening of Metal–Organic Frameworks for CO2 Separation. ACS Comb. Sci. 2012, 14, 263–267. 10.1021/co3000192. [DOI] [PubMed] [Google Scholar]
- Tang H.; Fang H.; Duan Y.; Sholl D. S. Predictions of Hg0 and HgCl2 Adsorption Properties in UiO-66 from Flue Gas Using Molecular Simulations. J. Phys. Chem. C 2019, 123, 5972–5979. 10.1021/acs.jpcc.8b11268. [DOI] [Google Scholar]
- Walton K. S.; Sholl D. S. Research Challenges in Avoiding “Showstoppers” in Developing Materials for Large-Scale Energy Applications. Joule 2017, 1, 208–211. 10.1016/j.joule.2017.06.005. [DOI] [Google Scholar]
- Chen G.; Koros W. J.; Jones C. W. Hybrid Polymer/UiO-66(Zr) and Polymer/NaY Fiber Sorbents for Mercaptan Removal from Natural Gas. ACS Appl. Mater. Interfaces 2016, 8, 9700–9709. 10.1021/acsami.6b00897. [DOI] [PubMed] [Google Scholar]
- Sahoo S.; Ramgopal M. Theoretical performance of an adsorbed natural gas storage system subjected to variable charge–discharge conditions. Int. J. Ambient Energy 2016, 37, 372–383. 10.1080/01430750.2014.977495. [DOI] [Google Scholar]
- Bimbo N.; Physick A. J.; Noguera-Díaz A.; Pugsley A.; Holyfield L. T.; Ting V. P.; Mays T. J. High volumetric and energy densities of methane stored in nanoporous materials at ambient temperatures and moderate pressures. Chem. Eng. J. 2015, 272, 38–47. 10.1016/j.cej.2015.02.088. [DOI] [Google Scholar]
- Walton K. S.; LeVan M. D. Natural gas storage cycles: Influence of nonisothermal effects and heavy alkanes. Adsorption 2006, 12, 227–235. 10.1007/s10450-006-0142-3. [DOI] [Google Scholar]
- Pupier O.; Goetz V.; Fiscal R. Effect of cycling operations on an adsorbed natural gas storage. Chem. Eng. Process. 2005, 44, 71–79. 10.1016/j.cep.2004.05.005. [DOI] [Google Scholar]
- Zhang H.; Deria P.; Farha O. K.; Hupp J. T.; Snurr R. Q. A thermodynamic tank model for studying the effect of higher hydrocarbons on natural gas storage in metal–organic frameworks. Energy Environ. Sci. 2015, 8, 1501–1510. 10.1039/C5EE00808E. [DOI] [Google Scholar]
- Wu Y.; Tang D.; Verploegh R. J.; Xi H.; Sholl D. S. Impacts of Gas Impurities from Pipeline Natural Gas on Methane Storage in Metal–Organic Frameworks during Long-Term Cycling. J. Phys. Chem. C 2017, 121, 15735–15745. 10.1021/acs.jpcc.7b03459. [DOI] [Google Scholar]
- Nandanwar S. U.; Corbin D. R.; Shiflett M. B. A Review of Porous Adsorbents for the Separation of Nitrogen from Natural Gas. Ind. Eng. Chem. Res. 2020, 59, 13355–13369. 10.1021/acs.iecr.0c02730. [DOI] [Google Scholar]
- Farzaneh-Gord M.; Niazmand A.; Deymi-Dashtebayaz M.; Rahbari H. R. Effects of natural gas compositions on CNG (compressed natural gas) reciprocating compressors performance. Energy 2015, 90, 1152–1162. 10.1016/j.energy.2015.06.056. [DOI] [Google Scholar]
- Dicks A.; Connor T.; Bradley J.; Lashtabeg A. Impact of Australian natural gas and coal bed methane composition on PEM fuel cell performance. Int. J. Hydrogen Energy 2009, 34, 8892–8904. 10.1016/j.ijhydene.2009.08.019. [DOI] [Google Scholar]
- Hahn C.; Hatsukade T.; Kim Y.-G.; Vailionis A.; Baricuatro J. H.; Higgins D. C.; Nitopi S. A.; Soriaga M. P.; Jaramillo T. F. Engineering Cu surfaces for the electrocatalytic conversion of CO2: Controlling selectivity toward oxygenates and hydrocarbons. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 5918. 10.1073/pnas.1618935114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D.; Deng Y.; Handoko A. D.; Chen C. S.; Malkhandi S.; Yeo B. S. Selective Electrochemical Reduction of Carbon Dioxide to Ethylene and Ethanol on Copper(I) Oxide Catalysts. ACS Catal. 2015, 5, 2814–2821. 10.1021/cs502128q. [DOI] [Google Scholar]
- Farrell B. L.; Igenegbai V. O.; Linic S. A Viewpoint on Direct Methane Conversion to Ethane and Ethylene Using Oxidative Coupling on Solid Catalysts. ACS Catal. 2016, 6, 4340–4346. 10.1021/acscatal.6b01087. [DOI] [Google Scholar]
- Greenblatt J. B.; Miller D. J.; Ager J. W.; Houle F. A.; Sharp I. D. The Technical and Energetic Challenges of Separating (Photo)Electrochemical Carbon Dioxide Reduction Products. Joule 2018, 2, 381–420. 10.1016/j.joule.2018.01.014. [DOI] [Google Scholar]
- Abney C. W.; Mayes R. T.; Saito T.; Dai S. Materials for the Recovery of Uranium from Seawater. Chem. Rev. 2017, 117, 13935–14013. 10.1021/acs.chemrev.7b00355. [DOI] [PubMed] [Google Scholar]
- Gao Q.; Hu J.; Li R.; Xing Z.; Xu L.; Wang M.; Guo X.; Wu G. Radiation synthesis of a new amidoximated UHMWPE fibrous adsorbent with high adsorption selectivity for uranium over vanadium in simulated seawater. Radiat. Phys. Chem. 2016, 122, 1–8. 10.1016/j.radphyschem.2015.12.023. [DOI] [Google Scholar]
- Das S.; Brown S.; Mayes R. T.; Janke C. J.; Tsouris C.; Kuo L. J.; Gill G.; Dai S. Novel poly(imide dioxime) sorbents: Development and testing for enhanced extraction of uranium from natural seawater. Chem. Eng. J. 2016, 298, 125–135. 10.1016/j.cej.2016.04.013. [DOI] [Google Scholar]
- Willauer H. D.; Hardy D. R.; Baldwin J. W.; DiMascio F.; Williams F. W.; Bradley M. J.; Hoheisel R. Economic comparisons of littoral production of low carbon fuel from non-fossil energy sources and seawater. J. Cleaner Prod. 2018, 170, 1473–1483. 10.1016/j.jclepro.2017.09.187. [DOI] [Google Scholar]
- Willauer H. D.; DiMascio F.; Hardy D. R.; Williams F. W. Development of an Electrolytic Cation Exchange Module for the Simultaneous Extraction of Carbon Dioxide and Hydrogen Gas from Natural Seawater. Energy Fuels 2017, 31, 1723–1730. 10.1021/acs.energyfuels.6b02586. [DOI] [Google Scholar]
- Johnson B. M.; Kanagy L. E.; Rodgers J. H.; Castle J. W. Chemical, Physical, and Risk Characterization of Natural Gas Storage Produced Waters. Water, Air, Soil Pollut. 2008, 191, 33–54. 10.1007/s11270-007-9605-8. [DOI] [Google Scholar]
- Almarzooqi K.; Ashrafi M.; Kanthan T.; Elkamel A.; Pope M. A. Graphene Oxide Membranes for High Salinity, Produced Water Separation by Pervaporation. Membranes 2021, 11, 475. 10.3390/membranes11070475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturabul S.; Srirachat W.; Wannachod T.; Ramakul P.; Pancharoen U.; Kheawhom S. Separation of mercury(II) from petroleum produced water via hollow fiber supported liquid membrane and mass transfer modeling. Chem. Eng. J. 2015, 265, 34–46. 10.1016/j.cej.2014.12.034. [DOI] [Google Scholar]
- Thompson K. A.; Mathias R.; Kim D.; Kim J.; Rangnekar N.; Johnson J. R.; Hoy S. J.; Bechis I.; Tarzia A.; Jelfs Kim E.; et al. N-Aryl–linked spirocyclic polymers for membrane separations of complex hydrocarbon mixtures. Science 2020, 369, 310–315. 10.1126/science.aba9806. [DOI] [PubMed] [Google Scholar]
- Marshall A. G.; Rodgers R. P. Petroleomics: The Next Grand Challenge for Chemical Analysis. Acc. Chem. Res. 2004, 37, 53–59. 10.1021/ar020177t. [DOI] [PubMed] [Google Scholar]
- Jang H.-Y.; Johnson J. R.; Ma Y.; Mathias R.; Bhandari D. A.; Lively R. P. Torlon® hollow fiber membranes for organic solvent reverse osmosis separation of complex aromatic hydrocarbon mixtures. AlChE J. 2019, 65, e16757. 10.1002/aic.16757. [DOI] [Google Scholar]
- Fang H.; Findley J.; Muraro G.; Ravikovitch P. I.; Sholl D. S. A Strong Test of Atomically Detailed Models of Molecular Adsorption in Zeolites Using Multilaboratory Experimental Data for CO2 Adsorption in Ammonium ZSM-5. J. Phys. Chem. Lett. 2020, 11, 471–477. 10.1021/acs.jpclett.9b02986. [DOI] [PubMed] [Google Scholar]
- Nguyen H. G. T.; Sims C. M.; Toman B.; Horn J.; van Zee R. D.; Thommes M.; Ahmad R.; Denayer J. F. M.; Baron G. V.; Napolitano E.; et al. A reference high-pressure CH4 adsorption isotherm for zeolite Y: results of an interlaboratory study. Adsorption 2020, 26, 1253–1266. 10.1007/s10450-020-00253-0. [DOI] [Google Scholar]
- Nguyen H. G. T.; Espinal L.; van Zee R. D.; Thommes M.; Toman B.; Hudson M. S. L.; Mangano E.; Brandani S.; Broom D. P.; Benham M. J.; et al. A reference high-pressure CO2 adsorption isotherm for ammonium ZSM-5 zeolite: results of an interlaboratory study. Adsorption 2018, 24, 531–539. 10.1007/s10450-018-9958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.; Howe J. D.; Sholl D. S. How Reproducible Are Isotherm Measurements in Metal–Organic Frameworks?. Chem. Mater. 2017, 29, 10487–10495. 10.1021/acs.chemmater.7b04287. [DOI] [Google Scholar]
- Agrawal M.; Han R.; Herath D.; Sholl D. S. Does repeat synthesis in materials chemistry obey a power law?. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 877. 10.1073/pnas.1918484117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.nrel.gov/pv/device-performance.html, accessed November 1, 2021.


