Abstract

Due to the dramatically increased atmospheric CO2 concentration and consequential climate change, significant effort has been made to develop sorbents to directly capture CO2 from ambient air (direct air capture, DAC) to achieve negative CO2 emissions in the immediate future. However, most developed sorbents have been studied under a limited array of temperature (>20 °C) and humidity conditions. In particular, the dearth of experimental data on DAC at sub-ambient conditions (e.g., −30 to 20 °C) and under humid conditions will severely hinder the large-scale implementation of DAC because the world has annual average temperatures ranging from −30 to 30 °C depending on the location and essentially no place has a zero absolute humidity. To this end, we suggest that understanding CO2 adsorption from ambient air at sub-ambient temperatures, below 20 °C, is crucial because colder temperatures represent important practical operating conditions and because such temperatures may provide conditions where new sorbent materials or enhanced process performance might be achieved. Here we demonstrate that MIL-101(Cr) materials impregnated with amines (TEPA, tetraethylenepentamine, or PEI, poly(ethylenimine)) offer promising adsorption and desorption behavior under DAC conditions in both the presence and absence of humidity under a wide range of temperatures (−20 to 25 °C). Depending on the amine loading and adsorption temperature, the sorbents show different CO2 capture behavior. With 30 and 50 wt % amine loadings, the sorbents show weak and strong chemisorption-dominant CO2 capture behavior, respectively. Interestingly, at −20 °C, the CO2 adsorption capacity of 30 wt % TEPA-impregnated MIL-101(Cr) significantly increased up to 1.12 mmol/g from 0.39 mmol/g at ambient conditions (25 °C) due to the enhanced weak chemisorption. More importantly, the sorbents also show promising working capacities (0.72 mmol/g) over 15 small temperature swing cycles with an ultralow regeneration temperature (−20 °C sorption to 25 °C desorption). The sub-ambient DAC performance of the sorbents is further enhanced under humid conditions, showing promising and stable CO2 working capacities over multiple humid small temperature swing cycles. These results demonstrate that appropriately designed DAC sorbents can operate in a weak chemisorption modality at low temperatures even in the presence of humidity. Significant energy savings may be realized via the utilization of small temperature swings enabled by this weak chemisorption behavior. This work suggests that significant work on DAC materials that operate at low, sub-ambient temperatures is warranted for possible deployment in temperate and polar climates.
Keywords: metal−organic framework, amine, direct air capture, sub-ambient conditions, temperature swing adsorption
Introduction
Due to the vast amount of anthropogenic CO2 emissions caused by fossil fuel utilization, the atmospheric CO2 concentration has climbed from 280 ppm in the preindustrial era to 410 ppm in modern times.1 This rapid rise in the atmospheric CO2 concentration has caused the global temperature to increase by 1.2 °C relative to preindustrial levels.2 If this trend goes on unchecked, it is expected that the global temperature will further increase by 4 °C by the end of the 21st century.3 An uncontrolled increase in the global temperature can cause an array of ecological and climatic consequences,4−6 and the repercussions on human society are likely to be catastrophic.7,8 Therefore, it is essential to curb CO2 emissions into the atmosphere via CO2 mitigation approaches.
One approach to restrict CO2 emissions from point sources such as power plants is to apply technologies such as amine scrubbing,9 oxy-combustion,10 chemical looping,11 or other approaches to capture the CO2 before it is emitted into the atmosphere. However, these “avoided emissions” methods, like the transition to renewable energy, are only capable of slowing the rate of increase of the atmospheric CO2 concentration. To reverse the ascending global temperature, another type of approach that seeks to directly reduce the atmospheric CO2 concentration, producing “negative emissions”, is necessary. Two technologies belonging to this approach are especially promising. One is bioenergy with carbon capture and storage (BECCS), which consists of extracting bioenergy from biomass and capturing the CO2 produced in the process.12,13 The other aims to directly extract CO2 from the air. This technology is known as direct air capture (DAC).14,15
The development of DAC has attracted increased research attention in recent years, and multiple types of technologies have been proposed, as summarized in a review paper by Sanz-Perez and co-workers.16 Among these technologies, two have been the most extensively studied, namely aqueous hydroxide CO2 absorption17,18 and supported amine-based CO2 adsorption. Both approaches show promise for practical scalability, though the supported amine-based CO2 adsorption appears to show the potential for lower costs because it requires lower temperatures for sorbent regeneration and consumes less energy per unit of CO2 captured.19−21
In the past two decades, DAC technology has been increasingly studied, and hundreds of scientific articles have been published on this topic. However, the studies conducted so far have been confined to ambient and above-ambient temperatures (>20 °C). To date, only one paper in the literature reported studies of DAC at sub-ambient (<20 °C) conditions.3 In addition, published studies investigating DAC under humid conditions have all been conducted at ambient or higher temperatures.22−31 This dearth of literature data on DAC at sub-ambient conditions, especially in the presence of humidity, severely hinders both DAC technology development and the potential for widespread implementation at a large scale because more than 80% of the land in the world has an annual average temperature below 25 °C (Figure 1) and essentially no place in the world has an absolute humidity of zero.32
Figure 1.

Map of world average temperature (°C), adapted from a ref (33).
Therefore, for DAC to be implemented around the world, significant research efforts need to be dedicated to assessing the performance of both sorbent materials and processes under sub-ambient temperatures, which are defined here as <20 °C. Similarly, research on DAC under humid conditions across the whole temperature range needs to be conducted to assess the performance of the sorbent materials in the presence of moisture. At sub-ambient temperatures (especially <0 °C), the physical and chemical properties of many CO2 sorbents are expected to undergo significant changes. Consequently, sorbents optimized for ambient DAC need to be reevaluated and in many cases redesigned to achieve desirable performance at sub-ambient conditions.
DAC under sub-ambient temperature conditions may offer some advantages.32 The absolute humidity is much lower at cold temperatures, which may limit water sorption as well as the energy expended desorbing water across temperature or temperature–vacuum swing adsorption cycles. Additionally, cold temperatures may enable the utilization of physisorbents instead of chemisorbents, which are dominantly used in DAC studies today. Physisorbent materials, which have smaller enthalpies of adsorption, could potentially enable smaller temperature swings and reduce the overall energy costs for DAC operation. Among the many supported amine sorbent materials described in the literature for DAC, we identified amine-loaded MIL-101(Cr) as a promising candidate for sub-ambient temperature operation. We chose supported amine materials as the CO2 sorbents for this study because supported amine adsorbents require much lower regeneration temperatures and have a lower energy consumption for CO2 desorption compared to aqueous hydroxide absorbents or solid-state alkali carbonates.19−21 Branched poly(ethylenimine) (PEI) and tetraethylenepentamine (TEPA) were identified in previous studies as two promising amine materials that showed good CO2 adsorption performance when supported on solid substrates and hence were chosen for this work.3,28,34−38 As for the choice of the support, MIL-101(Cr) is moisture-stable (it is stable in boiling water), suggesting it will not catastrophically degrade during sorbent regeneration by steam; it is stable across a range of pH conditions, making it resistant to the basicity of amines and the acidity of CO2; and it has a high surface area and high density of open metal sites, enabling the loading of a large quantity of amines into the pores to attain high CO2 capacities even at concentrations as low as 400 ppm.39,40 The feasibility of amine-loaded MIL-101(Cr) for DAC at ambient temperatures and dry conditions has been validated by previous studies. Darunte et al.36 studied the uptake capacity and kinetics of MIL-101(Cr) loaded with TREN and PEI-800 for DAC applications. The highest CO2 capture capacities were shown to be 1.35 mmol/g on PEI-loaded MIL-101(Cr) and 2.8 mmol/g on TREN-loaded MIL-101(Cr). Sinha et al.41,42 conducted an economic analysis of DAC through temperature–vacuum swing adsorption using PEI-800-loaded MIL-101(Cr) coated on monolithic contactors. The results showed that the energy requirement per mole and the cost per metric ton of CO2 production could be as low as 0.172 MJ and $77–142, respectively.
Here we demonstrate that amine-loaded MIL-101(Cr) sorbents are promising sorbent materials for DAC under sub-ambient conditions. Furthermore, we demonstrate that extrapolating the performance of the materials to −20 °C from 25 °C is not straightforward, necessitating cold temperature experimentation, and that optimal sorbent structures under each condition exist. Finally, we demonstrate that amine-loaded MIL-101(Cr) sorbents are effective DAC sorbents at cold temperatures, operating effectively as weak chemisorbents and allowing for CO2 desorption at very low temperatures of 25 °C without the implementation of vacuum during desorption. The results presented here suggest that an untapped manifold of DAC operation may exist at cold temperatures that may offer promise for the application of a wide array of sorbents.
Results and Discussion
Characterization of MIL-101(Cr)
Three different batches of MIL-101(Cr) were synthesized as described in the Experimental Section to confirm the reproducibility of the synthesis method. After synthesis, the three MIL-101(Cr) powders were characterized by XRD to confirm their crystalline structure. As shown in Figure S1, the XRD patterns revealed that the MIL-101 structure was successfully obtained with high crystallinity for all samples. Figure S2 shows an SEM image of the synthesized MIL-101(Cr) (a mixture of the three smaller batches), which confirmed the formation of typical bipyramidal crystals with a crystal size of about 500 nm, similar to the results of our prior study.36
The BET surface area was calculated from N2 physisorption isotherms and the results are summarized in Figure S3a. The synthesized MIL-101(Cr) powders from the three different batches have similar BET surface areas and showed BET surface areas (3493 ± 198 m2/g) comparable with those from prior literature (3340 m2/g).36Figure S3b shows the CO2 adsorption isotherms of plain unfunctionalized MIL-101(Cr) (mixture of the three batches) obtained at 25 °C and −20 °C. The logarithmic plots give a clearer view of the CO2 uptake at 0.4 mbar (marked by the orange vertical line), which corresponds to 400 ppm of CO2. The CO2 uptakes of MIL-101(Cr) were significantly higher at −20 °C because CO2 adsorption is thermodynamically favorable at lower temperature conditions. However, the CO2 isotherms of MIL-101(Cr) were almost linear, without a significant increase at extremely low pressures. As a result, the uptake of MIL-101(Cr) at 400 ppm of CO2 was very low, no higher than 0.01 mmol/g, even at −20 °C. The synthesized MIL-101(Cr) powders were then impregnated with poly(ethylenimine) (PEI, branched, MW = 800) and tetraethylenepentamine (TEPA) to enhance the CO2 adsorption capacity.
Based on the measured pore volume of amine-impregnated MIL-101(Cr) by N2 physisorption,36 the extent of pore filling of 10, 30, and 50 wt % amine-impregnated MIL-101(Cr) sorbents was estimated (Table S1). The bulk densities of pristine MIL-101(Cr) and 30 and 50 wt % amine-loaded MIL-101(Cr) were also measured, providing an estimation of the volume occupied by the sorbents. The data are shown in Table S2.
CO2 Adsorption Behavior of Amine-Impregnated (PEI and TEPA) MIL-101(Cr) under Dry Ambient Conditions (25 °C)
The measurements of CO2 adsorption at 25 °C were conducted on branched poly(ethylenimine)- (PEI) and tetraethylenepentamine- (TEPA) impregnated MIL-101(Cr) powder sorbents with different amine loadings (0, 10, 30, and 50 wt %) to set the baseline for subsequent tests at sub-ambient temperatures (−20 °C). The CO2 adsorption capacities of the sorbents at 400 ppm CO2 were determined volumetrically by a surface area and porosity (SAP) system and are plotted in Figure 2a. The 400 ppm CO2 uptake of the sorbents at 25 °C was significantly enhanced as the amine loading increased. Interestingly, a dramatic increase in CO2 adsorption was observed between 30 wt % at about 10 mmol N per gram of MOF amine loading and 50 wt % at about 20 mmol N per gram of MOF amine loading, while poor CO2 adsorption performance was observed at <10 mmol N per gram of MOF amine loading, indicating that differences in the CO2 adsorption mechanism(s) may be present between the two loading conditions. This dramatic step-change in the CO2 adsorption was also observed in our previous study.36 Previously, we hypothesized that the impregnated amine groups strongly interact with the MOF framework at lower loadings of amine, and many amine sites may not be available for CO2 capture, resulting in lower CO2 adsorption.
Figure 2.
(a) Effect of amine loading on the 400 ppm of CO2 adsorption capacity of PEI- and TEPA-impregnated MIL-101(Cr) powders at 25 °C (measured by SAP). (b) CO2-TPD profiles of PEI- and TEPA-impregnated MIL-101(Cr) powders (30 and 50 wt % loadings). Adsorption conditions are as follows: gas, 400 ppm of CO2/He; flow rate, 90 mL/min; T = 25 °C; and adsorption time, 12 h.
The CO2 uptakes of 50 wt % PEI- and TEPA-loaded samples were 1.81 and 2.14 mmol/g, respectively, at 400 ppm CO2, indicating that the TEPA sample was more effective than the branched PEI sample for direct air capture under ambient dry conditions (25 °C). This may be because TEPA mainly consists of primary and secondary amines, which have better CO2 adsorption potentials under dry conditions than tertiary amines, or because of the enhanced amine chain mobility in the shorter TEPA chains relative to that of PEI. Thus, TEPA-impregnated MIL-101(Cr) showed a higher amine efficiency than PEI-impregnated sorbents at 25 °C, as shown in Table 1. Also, the ∼50 wt % TEPA sample contains about 7.5% more amine groups than the ∼50 wt % PEI material, as shown in Figure 2a. Thus, the higher amine loadings are an additional factor contributing to the higher CO2 uptake of 50 wt % TEPA. However, mild regeneration temperature conditions will likely be required to utilize TEPA-containing sorbents for DAC due to their limited thermal stability induced by amine volatility at elevated desorption temperatures.43
Table 1. Summary of Pseudoequilibrium CO2 Uptake at –20 and 25 °C and 400 ppm (Measured by the Volumetric System, SAP).
| –20
°C |
25 °C |
|||||||
|---|---|---|---|---|---|---|---|---|
| adsorption
rate (mmol/g/min)a |
adsorption
rate (mmol/g/min)a |
|||||||
| adsorbent (powders) | CO2 uptake (mmol/g) | amine efficiency | initial | after 2 h | CO2 uptake (mmol/g) | amine efficiency | initial | after 2 h |
| MIL-101(Cr) | 0.01 ± 0.008 | 0.0008 ± 1.46 × 10–4 | ||||||
| MIL-101(Cr) _10 wt % PEI | 0.05 ± 0.004 | 0.02 | 0.006 ± 9.29 × 10–4 | 0.002 | ||||
| MIL-101(Cr) _30 wt % PEI | 0.78 ± 0.03 | 0.11 | 0.018 | 2.13 × 10–4 | 0.24 ± 0.02 | 0.03 | 0.021 | 1.05 × 10–4 |
| MIL-101(Cr) _50 wt % PEI | 1.64 ± 0.17 | 0.13 | 0.015 | 6.21 × 10–4 | 1.81 ± 0.17 | 0.14 | 0.016 | 4.75 × 10–4 |
| MIL-101(Cr) _10 wt % TEPA | 0.02 ± 0.005 | 0.01 | 0.002 ± 6.93 × 10–4 | 0.0005 | ||||
| MIL-101(Cr) _30 wt % TEPA | 1.12 ± 0.02 | 0.14 | 0.021 | 2.66 × 10–4 | 0.39 ± 0.12 | 0.05 | 0.022 | 2.54 × 10–5 |
| MIL-101(Cr) _50 wt % TEPA | 1.26 ± 0.28 | 0.10 | 0.012 | 5.71 × 10–4 | 2.14 ± 0.12 | 0.16 | 0.017 | 2.70 × 10–4 |
The adsorption rate was obtained based on the TGA/DSC data shown in Figure S4.
The 400 ppm CO2 adsorption capacities of 30 and 50 wt % PEI- and TEPA-impregnated MIL-101(Cr) sorbents were also measured by a TGA/DSC system at 25 °C, and the results are shown in Figure S4(a). The pseudoequilibrium CO2 capture capacities obtained from the TGA/DSC system show a trend similar to that of the SAP data shown in Figure 2a. Table S3 shows the CO2 uptake as a function of time for different PEI and TEPA loadings (30 and 50 wt %) at 25 °C. Although the 30 wt % PEI- and TEPA-impregnated MIL-101(Cr) samples showed modest DAC performance at 25 °C due to low amine loadings, they quickly reached their pseudoequilibrium capacities (at 720 min), showing rapid CO2 capture kinetics. The 50 wt % amine-impregnated MIL-101(Cr) samples achieved 1 mmol/g uptakes within 75 (PEI) or 50 min (TEPA), showing promising 400 ppm CO2 capture kinetics at 25 °C. However, the relatively fast initial CO2 adsorption kinetics were followed by a slow increase in the CO2 uptake due to the increased diffusion resistance encountered by CO2 at long times. These results are comparable to our previous work on PEI-impregnated MIL-101(Cr), as shown in Table S3.
To probe the CO2 capture mechanism(s) of PEI- and TEPA-impregnated MIL-101(Cr) samples at 25 °C, CO2 temperature-programmed desorption (TPD) experiments were carried out using the TGA/DSC system, as described in the Experimental Section. Figure S5 shows the CO2 concentration of the outlet stream, the sample mass change, and the temperature profiles during the CO2 TPD experiments using 30 and 50 wt % PEI- and TEPA-impregnated MIL-101(Cr). Even during the helium purging step at 25 °C (first 1 h), some amount of CO2 was desorbed from the sorbents, indicating that the CO2 capture mechanism(s) of the amine-impregnated MIL-101(Cr) materials also included some degree of physisorption. After the 1 h helium purge step, more CO2 was desorbed from the sorbents when the temperature increased. While the 30 wt % PEI- and TEPA-impregnated MIL-101(Cr) sorbents were completely regenerated below 60 °C (Figure S5(a)), a higher temperature was required for the complete desorption of CO2 from the 50 wt % PEI- and TEPA-impregnated sorbents, confirming that the 50 wt % amine-loaded MIL-101(Cr) samples interacted more strongly with 400 ppm of CO2 than the 30 wt % samples at 25 °C (Figure S5b).
The outlet CO2 concentration profiles of 30 and 50 wt % PEI- and TEPA-impregnated MIL-101(Cr) sorbents during desorption shown in Figure S5 are plotted as a function of temperature in Figure 2b. The data clearly show that the dominant CO2 capture mechanism of 30 wt % PEI- and TEPA-impregnated MIL-101(Cr) is weak chemisorption or physisorption, and the amount of captured CO2 (amine efficiency, Table 1) is lower than that for the 50 wt % amine-loaded samples, which are strong chemisorption-dominant sorbents. This result suggests that many of the impregnated amine groups at low loading conditions (∼30 wt %) strongly interacted with the open metal sites in the MOFs and were less available for CO2 capture, resulting in a lower CO2 adsorption performance. In our previous work, a decrease in the intensity of XRD reflections between 2° and 4° (2θ) was observed after the MIL-101(Cr) MOFs were impregnated with PEI, probably due to interaction between amine groups and the MOF framework, and low PEI loadings (about 30 wt %) showed 0.2–0.3 mmol/g CO2 adsorption capacities with only 0.01–0.03 amine efficiencies.36 This previous work also hypothesized that this observation was due to the strong interaction between impregnated amines and the MOF framework. On the other hand, the sufficient amount of excess amine groups in the high amine loading conditions (50 wt %) dramatically enhanced the CO2 adsorption capacity via strong chemisorption. A similar trend was also observed for the amine-impregnated MCM-41 sorbent materials.44 In that case, the amine efficiency increased with the amine loading until steric constraints decreased the accessibility of all the amine sites inside the MCM-41.44
To quantify the weak and strong chemisorption, the heats of adsorption of 30 and 50 wt % PEI- and TEPA-impregnated MIL-101(Cr) for 400 ppm of CO2 were measured by TGA/DSC. As shown in Figure S6, the heat of adsorption of 30 wt % amine-impregnated MIL-101(Cr) was between −40 and −65 kJ/mol, supporting the weak chemisorption-dominant CO2 capture mechanism. The 50 wt % amine-loaded sorbents showed strong chemisorption-dominant behavior, with relatively higher (absolute value) heats of adsorption (−95 to −110 kJ/mol).
CO2 Adsorption Behavior of Amine-Impregnated (PEI and TEPA) MIL-101(Cr) under Dry Sub-Ambient Conditions (−20 °C)
The CO2 adsorption performance (i.e., 400 ppm CO2 adsorption capacity) of PEI- and TEPA-impregnated MIL-101(Cr) was next determined under dry sub-ambient temperature conditions (e.g., −20 °C) with the SAP (volumetric) system and compared with the data obtained at 25 °C. Figure 3a shows the 400 ppm CO2 adsorption capacities of amine-loaded (PEI and TEPA) MIL-101(Cr) with different amine loadings (0, 10, 30, and 50 wt %) obtained at 25 °C and −20 °C. The amine-impregnated MIL-101(Cr) samples showed promising 400 ppm CO2 adsorption capacities even at sub-ambient conditions (−20 °C). Interestingly, while the CO2 adsorption capacities of samples with lower amine loadings (<15 mmol N per gram of MOF) were enhanced at −20 °C, the sub-ambient temperature (−20 °C) had a negative effect on the CO2 adsorption performance of the samples with higher amine loadings on MIL-101(Cr) (>20 mmol N per gram of MOF). The CO2 adsorption capacities of the 50 wt % PEI- and TEPA-impregnated samples were lower at −20 °C, possibly due either to decreased amine mobility that results in dramatically lower accessibility of the gas phase CO2 to the impregnated amine groups or to there being insufficient thermal energy available to surpass chemisorption activation barriers. It is noteworthy that the 50 wt % PEI-impregnated sorbents showed better performance than the 50 wt % TEPA-impregnated materials, which is the opposite trend of the other loading (30 wt %) and temperature (25 °C) conditions. The TGA/DSC CO2 adsorption results at 25 °C and −20 °C also showed the same trends in the pseudoequilibrium capacities (Figure S4(b)). While the macroscopic trends were the same, the reported pseudoequilibrium values on each instrument differed slightly for the same sorbent materials (Figures 3a and S4), as each instrument had unique features and only pseudoequilibria were achieved.
Figure 3.
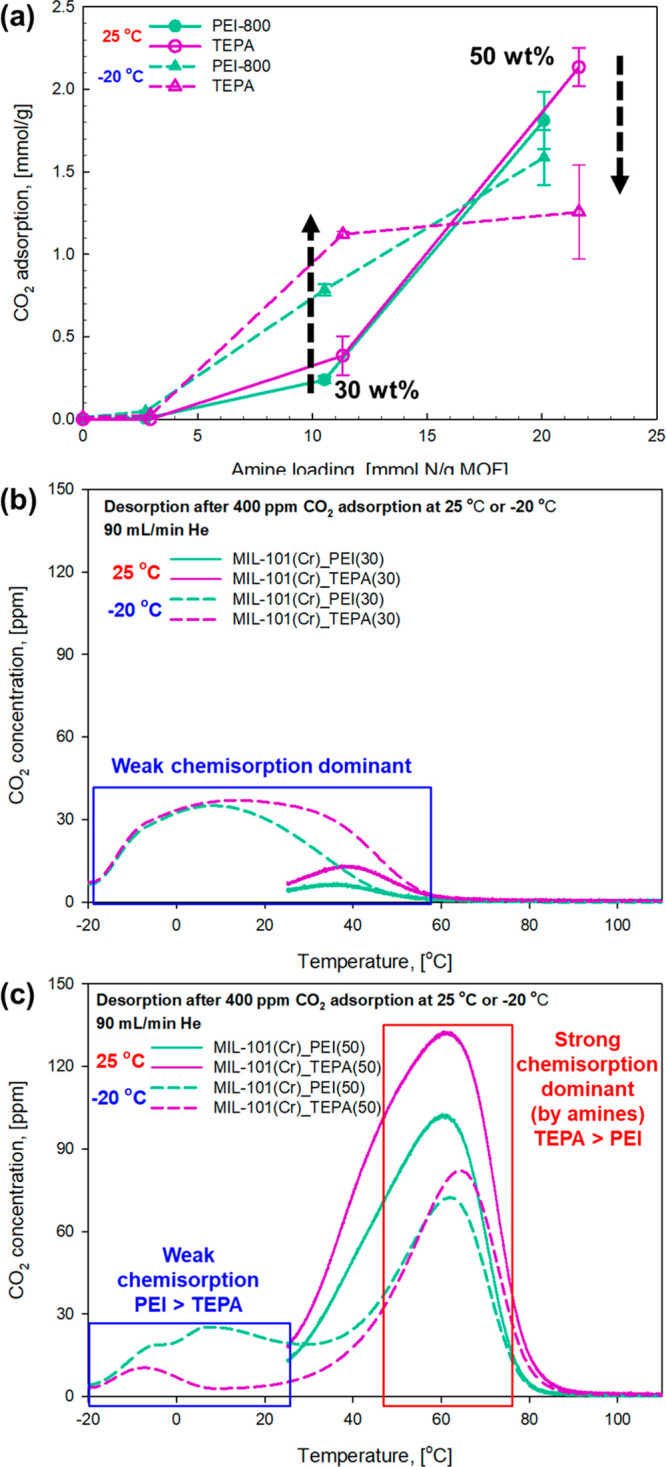
(a) Effect of amine loading on the 400 ppm of CO2 adsorption capacity of PEI- and TEPA-impregnated MIL-101(Cr) powders at 25 °C and −20 °C (measured by SAP). CO2-TPD profiles of PEI- and TEPA-impregnated MIL-101(Cr) powders with (b) 30 and (c) 50 wt % amine loadings. Adsorption conditions are as follows: gas, 400 ppm of CO2/He; flow rate, 90 mL/min; T = 25 and −20 °C; and adsorption time, 12 h.
When increasing the amine loadingsup to 50 wt %, the extent of pore filling increased, resulting in decreased pore volumes (see Table S1). Due to the decreased pore volumes, the overall CO2 capture performance may be impacted by CO2 mass transfer through the impregnated amine phase (pore-blocking).45 Additionally, the effect of pore-blocking should be more significant at cold temperature conditions when polymer chains have slower motions (−20 °C). Thus, the higher amine loading conditions led to reduced DAC performance at the sub-ambient temperature (−20 °C). As shown in Figure S4b, the TGA/DSC data obtained at −20 °C clearly show significantly reduced CO2 capture kinetics for the 50 wt % amine-loaded sorbents after 2 h, after which the amount of CO2 adsorbed only slowly increased up to the end of the 12 h experiment. For example, the initial CO2 capture kinetics of the 50 wt % PEI-impregnated sorbent were dramatically reduced from 0.015 mmol/g/min to 6.21 × 10–4 mmol/g/min after 2 h at −20 °C. Even after 12 h at −20 °C, equilibrium conditions were not achieved. Therefore, the CO2 adsorption capacity even at 12 h is only a pseudoequilibrium value.
As shown in Tables 1 and S3, despite the decrease in the adsorption temperature from 25 to −20 °C, the 30 wt % PEI- and TEPA-impregnated MIL-101(Cr) samples showed promising initial CO2 adsorption kinetics. Table 1 presents the initial adsorption rate and the rate after 2 h, while Table S3 provides key numerical values extracted from Figure S4. The data show that with a lower amine loading, the 30 wt % TEPA-impregnated sorbent showed faster initial uptake kinetics (0.021 mmol/g/min) than the 50 wt % TEPA-impregnated material (0.012 mmol/g/min) at −20 °C. Additionally, we note that the initial CO2 adsorption kinetics of the 50 wt % amine loading samples were not significantly reduced at −20 °C. For example, the initial kinetics of the 50 wt % PEI-impregnated MIL-101(Cr) decreased from 0.016 mmol/g/min at 25 °C to 0.015 mmol/g/min at −20 °C, indicating that promising CO2 working capacities may be obtained with highly loaded amine-impregnated MIL-101(Cr) sorbents even at sub-ambient temperature conditions (−20 °C).
Given the slow approach to the sorption equilibrium for these materials under all conditions, but especially at low temperatures like −20 °C, significant measurement errors may be expected for the 400 ppm of CO2 adsorption capacity of the most sterically hindered materials, specifically the 50 wt % amine-loaded MIL-101(Cr) samples (Figure 3a). Therefore, the CO2 adsorption capacities of the sorbents were measured multiple times on the SAP (volumetric) system, and the pseudoequilibrium capacities are presented with error bars in Figure 3a. While the error bars for the 50 wt % PEI sample are significant, at lower amine loading conditions (30 wt %) the measurement error is much smaller. In this case, the pores are not as highly packed with amine groups, showing relatively larger pore volumes and providing better accessibility to the gas-phase CO2. In this case, the overall CO2 capture capacities appear less impacted by mass transfer with the stronger influence of thermodynamics, with pseudoequilibria being achieved more rapidly.
To understand the interesting CO2 adsorption behavior of the PEI- and TEPA-impregnated MIL-101(Cr) materials at −20 °C, CO2 TPD experiments were also carried out with the TGA/DSC system right after 400 ppm of CO2 adsorption at −20 °C for 12 h. As shown in Figure S7, a small amount of CO2 was desorbed from the sorbents during the helium purge step (first 1 h) at −20 °C due to physisorbed CO2. During the temperature ramp step, a single wide CO2 concentration peak below 60 °C was confirmed from the 30 wt % amine-loaded samples (Figure S7a). Meanwhile, two CO2 concentration peaks at lower and higher temperatures were observed in the case of 50 wt % amine-loaded MIL-101(Cr) (Figure S7b), suggesting that there are two different CO2 capture mechanisms for this material at −20 °C.
Panels b and c of Figure 3 show CO2 TPD profiles of 30 and 50 wt % PEI- and TEPA-impregnated MIL-101(Cr), respectively, that were obtained after 400 ppm of CO2 adsorption at 25 °C and −20 °C. As shown in Figure 3b, a large amount of CO2 was desorbed from the 30 wt % amine-impregnated MIL-101(Cr) sample even at cold temperature conditions (between −20 and 25 °C), and the CO2 desorption was completed around 60 °C, allowing for lower temperature desorption conditions than the commonly used regeneration conditions of amine-based solid sorbents in the absence of a vacuum (80–100 °C).19 This suggests that the dominant CO2 capture mechanism is weak chemisorption (a relatively weak interaction between CO2 and sorbents). Since the reaction between CO2 and the 30 wt % amine-impregnated MIL-101(Cr) is more thermodynamically favorable at lower temperature conditions (exothermic sorption), the 400 ppm CO2 adsorption capacities were enhanced at sub-ambient temperatures (−20 °C). We note that it would be advantageous if the sorbent regeneration step could occur at temperatures lower than 60 °C, as the low regeneration temperature conditions would make the sub-ambient DAC temperature swing process less energy intensive.
On the other hand, as shown in Figure 3c, most adsorbed CO2 was desorbed from the 50 wt % amine-impregnated MIL-101(Cr) between 50 and 90 °C, indicating that the dominant CO2 capture mechanism was strong chemisorption by the excess amine groups. Likely due to significantly reduced mobility of impregnated amines at extreme low-temperature conditions, the CO2 adsorption capacities of 50 wt % PEI- and TEPA-impregnated powders were reduced at −20 °C. Although strong chemisorption is the dominant CO2 capture mechanism of 50 wt % PEI- and TEPA-loaded sorbents, a small amount of CO2 was also weakly adsorbed, as shown by the CO2 desorption peak between −20 and 25 °C. The data also show that PEI-impregnated sorbents have better weak chemisorption (or physisorption) capacities than the TEPA-loaded sorbents at −20 °C. We hypothesize this may be because frozen branched PEI can provide more free volume to physically (or weakly) capture CO2 than linear TEPA. Due to the greater weak chemisorption of CO2 by PEI at −20 °C, the PEI-impregnated MIL-101(Cr) sample showed a better adsorption capacity than the TEPA sample at high amine loadings (50 wt %).
Compared to the 30 wt % amine-loaded MIL-101(Cr), a much higher temperature, about 90 °C, is required for full CO2 desorption from the 50 wt % amine-loaded sorbents due to its strong chemisorption-dominant behavior (relatively strong interaction between CO2 and the sorbent). As shown in Figure 3c, at 60 °C there is a large CO2 desorption peak, making this one of the lower desorption temperatures one might use in the absence of a vacuum for a sub-ambient DAC temperature swing cycle with 50 wt % amine-impregnated MIL-101(Cr). Thus, higher energy consumption for sorbent regeneration would be expected relative to that of the 30 wt % amine-loaded samples shown in Figure 3b.
Air contains 80% N2, and its adsorption on the amine-impregnated MIL-101(Cr) could be significant at sub-ambient conditions (−20 °C). Thus, to confirm selective capture of 400 ppm of CO2 over 80% N2 at −20 °C, the single-gas adsorption isotherms of 30 wt % PEI- and TEPA-impregnated MIL-101(Cr) powders were obtained with CO2 and N2 at −20 °C. As shown in Figure S8, the 400 ppm of CO2 adsorption capacities of 30 wt % PEI- and TEPA-impregnated MIL-101(Cr) powders at −20 °C were 0.78 and 1.12 mmol/g, respectively, which were much larger than the 80% N2 adsorption capacities (0.11 mmol/g) at −20 °C. The calculated CO2/N2 selectivity of 30 wt % amine-impregnated MIL-101(Cr) at −20 °C was about 10–14 mol CO2/kg per mol N2/kg) or 20 000–30 000 (mol CO2/kg × XN2 (0.8) per mol N2/kg × XCO2 (400 ppm)),46 confirming the selective capture of 400 ppm of CO2 over 80% N2 at −20 °C.
The pseudoequilibrium 400 ppm CO2 uptakes at −20 and 25 °C are summarized in Table 1. At −20 °C, the CO2 uptake of MIL-101(Cr) was significantly enhanced by PEI and TEPA impregnation. Notably, 30 wt % TEPA (1.12 mmol/g) and 50 wt % PEI (1.64 mmol/g) materials are potential candidates for sub-ambient DAC at −20 °C. Although 50 wt % PEI shows a better CO2 capture performance at −20 °C, the energy requirement for sorbent regeneration appears higher than that of the 30 wt % TEPA sample because strong chemisorption is the dominant CO2 capture mechanism, as discussed above and as shown in Figure 3b and c. Thus, the 30 wt % TEPA-impregnated MIL-101(Cr) may be a better sorbent for sub-ambient DAC due to its promising adsorption capacity (1.12 mmol/g) and low regeneration temperature below 60 °C (weak chemisorption dominant). To this end, cyclic tests with different regeneration temperatures were conducted, as described in the section below.
To further study the effect of temperature on sub-ambient DAC behavior, the 400 ppm CO2 uptake of the 30 wt % TEPA sample, which is a weak chemisorption-dominant sorbent, was investigated by the TGA/DSC system at −20, −10, 5, and 25 °C (Figure 4a). As discussed in Figure 3, the 30 wt % TEPA sorbent is more highly impacted by thermodynamics (exothermic sorption) than by CO2 mass transfer due to the lower degree of pore-blocking in this sample compared to that for higher amine loadings. Thus, the DAC performance of the 30 wt % TEPA material was enhanced with decreasing temperature within the sub-ambient temperature range, as shown in Figure 4a. The 400 ppm CO2 adsorption trend over the temperature range (−20 °C – 25 °C) clearly shows that lower temperature conditions have a positive effect on sub-ambient DAC with this sorbent. We note that the pseudoequilibrium CO2 uptake was significantly reduced to below 0.7 mmol/g at temperatures above −10 °C, suggesting that this weak chemisorption-dominant sorbent is most effective for DAC at extremely cold temperature conditions (e.g., −20 °C).
Figure 4.
Effect of temperature (−20 °C – 25 °C) on the 400 ppm of CO2 adsorption of MIL-101(Cr) TEPA(30). (a) Uptake profiles for 400 ppm of CO2 (measured by TGA/DSC), (b) CO2-TPD profiles of MIL-101(Cr) TEPA(30). Adsorption conditions are as follows: gas, 400 ppm of CO2/He; flow rate, 90 mL/min; T = −20, −10, 5, and 25 °C; and adsorption time, 12 h.
CO2-TPD experiments with the TGA/DSC system were also conducted right after the CO2 adsorption at each adsorption temperature (−20, −10, 5, and 25 °C) to understand the CO2 desorption trend over the specified temperature range. As shown in Figure 4(b), the CO2 desorption peak was shifted to a lower temperature, and the amount of desorbed CO2 increased with decreasing adsorption temperatures from 25 to −20 °C as the amount of weak chemisorption became more significant.
Small Temperature Swing Adsorption–Desorption Cycles under Dry Sub-Ambient Conditions
Typical desorption temperature conditions for amine-based solid sorbents in the absence of a vacuum are ∼80–100 °C, which accounts for the significant energy demand, on the order of 1750 kWhth/tonne CO2, for operating temperature swing DAC systems at ambient conditions, resulting in elevated CO2 capture costs.19 In the case of sub-ambient DAC, the temperature swing window will be larger than that for ambient DAC if the same regeneration conditions are needed due to the extremely low adsorption temperature (e.g., −20 °C) requiring larger temperature swings. Hence, it is crucial to determine the optimum sorbent regeneration temperature to achieve both a low desorption energy demand and a promising CO2 working capacity for a sub-ambient DAC process. To investigate the effect of the regeneration temperature on the CO2 working capacity and the recyclability of the amine-impregnated MIL-101(Cr) for sub-ambient DAC, the CO2 loadings of 50 wt % PEI- and 30 wt % TEPA-impregnated MIL-101(Cr) sorbents were gravimetrically determined (Figure S9) over 15 consecutive 400 ppm CO2 adsorption–desorption temperature swing cycles (2 h at −20 °C ↔ 2 h at 60 °C for 50 wt % PEI or 25 °C for 20 wt % TEPA), as described in the Experimental Section. Furthermore, Figure S10 shows three replicates of the measured CO2 uptake profiles as a reproducibility test at −20 °C for 30 wt % TEPA and 25 °C for 50 wt % PEI. The error range for the measurements of CO2 uptake with the TGA/DSC system was ±0.03 mmol/g. The calculated CO2 working capacities from the cyclic tests are shown in Figure 5.
Figure 5.
Recyclability of (a) 50 wt % PEI-impregnated MIL-101(Cr) powder sorbents and (b) 30 wt % TEPA-impregnated MIL-101(Cr) powder sorbents over 15 CO2 adsorption and desorption cycles (measured by TGA/DSC). Adsorption conditions are as follows: gas, 400 ppm of CO2/He; flow rate, 90 mL/min; T = −20 °C; and adsorption time, 2 h. Desorption conditions are as follows: gas, He; flow rate, 90 mL/min; T = 60 °C for MIL-101(Cr)_PEI(50); T = 25 °C for MIL-101(Cr)_TEPA(30); and desorption time, 2 h.
As shown in Figure 5(a), the adsorption–desorption cyclic tests were conducted with 50 wt % PEI-impregnated MIL-101(Cr) using a 60 °C regeneration temperature based on the TPD studies described above. This regeneration temperature was chosen since 60 °C showed a significant CO2 desorption peak, and this temperature appeared to be the minimum regeneration temperature needed to achieve a reasonable working capacity with minimized energy consumption, as discussed in Figure 3c. The CO2 adsorption working capacity was about 1.11 mmol/g, which is 68% of the pseudoequilibrium value (1.64 mmol/g, dotted line in Figure 5a) and the performance was stable over all the cycles, indicating that 60 °C is a suitable regeneration temperature for this sorbent during sub-ambient DAC.
The adsorption–desorption cyclic tests were also performed under 25 °C adsorption and 60 °C regeneration conditions with the 50 wt % PEI-impregnated MIL-101(Cr) (Figure S11a). This cyclic test mimics an ambient DAC temperature swing cycle (25 °C ↔ 60 °C) and is a good comparison with the sub-ambient DAC process (−20 °C ↔ 60 °C). As shown in Figure S11b, the working capacity for ambient DAC with a 60 °C regeneration temperature dropped to 1.07 mmol/g across all the cycles, which is only 59% of the pseudoequilibrium adsorption capacity (dotted line in Figure S11b). This value is slightly lower than the working capacity for sub-ambient DAC conditions (1.11 mmol/g). Since strong chemisorption is the dominant CO2 capture mechanism for these sorbents under ambient conditions (25 °C), as discussed in Figure 2b, the sorbent was not as effectively regenerated under the mild temperature conditions (60 °C), showing a relatively lower CO2 working capacity (1.07 mmol/g) compared to the pseudoequilibrium value (1.81 mmol/g) that may be achieved with regeneration at higher temperatures.
On the contrary, under sub-ambient conditions (−20 °C), strong chemisorption is not the sole CO2 capture mechanism for 50 wt % PEI-impregnated MIL-101(Cr). As shown in Figure 3c, a significant amount of CO2 was also captured by the sorbent via weak chemisorption or physisorption at −20 °C. Thus, the 50 wt % PEI-loaded sorbent showed better regeneration performance under mild regeneration temperature conditions (60 °C) than for sorption, at ambient temperature. Similarly, the sorbent had a better working capacity (1.11 mmol/g)under subambient conditions than for that the ambient DAC cycle, even though it had a lower pseudoequilibrium CO2 capture capacity at −20 °C (1.64 mmol/g). A much lower regeneration temperature (≪60 °C) would further improve the sub-ambient DAC process from an energy consumption perspective. However, we expected that the 50 wt % PEI sorbent would not be effectively regenerated below 60 °C and would have a low working capacity under such conditions since strong chemisorption is the dominant CO2 capture mechanism for this material. Figure 3c clearly shows that only the weakly adsorbed CO2 would be fully regenerated below 60 °C, resulting in a low working capacity. Indeed, as shown in Figure S12, the 50 wt % PEI-impregnated MIL-101(Cr) sorbents were not effectively regenerated at 25 °C over five cycles. The average working capacity was 0.66 mmol/g, which is much lower than the working capacity at the 60 °C regeneration conditions (1.11 mmol/g). The recyclability of the 50 wt % TEPA sorbent was also investigated over five CO2 adsorption and desorption cycles with 60 and 25 °C regeneration temperatures (Figure S13). The results showed a similar trend to that of the 50 wt % PEI-impregnated MIL-101(Cr) sorbents.
Next, the adsorption–desorption cyclic tests were conducted with the 30 wt % TEPA material, which is a weak chemisorption-dominant CO2 capture sorbent, as shown in Figure 3b. In this case, a much lower regeneration temperature (25 °C) was used for the temperature swing cyclic tests, allowing an evaluation of the regeneration performance in a very small temperature swing window (−20 °C ↔ 25 °C). As shown in Figure 5b, the sorbents showed promising sub-ambient CO2 adsorption working capacities (0.73 mmol/g) across the entire process, excluding the first two cycles, confirming that the sub-ambient DAC process could be operated with a small temperature swing window with the weak chemisorption dominant sorbents. Since the sorbents were initially activated at 110 °C, the first two cycles showed relatively higher working capacities (0.99 and 0.81 mmol/g), which were the only deviations from the steady cycling. As a comparison, the recyclability of the 30 wt % PEI sorbent was also investigated with a 25 °C regeneration temperature (Figure S14). The average working capacity over five cycles (0.50 mmol/g) was slightly lower than that of the 30 wt % TEPA-impregnated sorbent. This was due to the smaller amount of weakly chemisorbed CO2 captured by the 30 wt % PEI sorbents, as shown in Figure 3b. From the perspective of sorbents and cycles specifically tuned for low-temperature operations, the 30 wt % TEPA-impregnated MIL-101(Cr) may be a preferred sorbent among those reported here due to its promising adsorption working capacity (0.73 mmol/g) and low regeneration temperature (weak chemisorption-dominant). The design of DAC sorbents with dominant but selective weak chemisorption can play an important role in reducing the energy consumption of DAC processes.
CO2 Breakthrough Experiments under Humid Sub-Ambient Conditions (70% RH at −20 °C)
Co-adsorption of CO2 and water vapor by sorbents is inevitable during DAC processes because ambient air always contains moisture. It is known that the CO2 capture performance of amine-based sorbents is usually enhanced by water vapor adsorption due to the increased amine efficiency enabled by bicarbonate formation22,47−49 and the formation of more carbamate ion pairs.50−52 Since sub-ambient air (<0 °C) also contains moisture, the absolute humidity will be an important factor to considered when designing and operating a sub-ambient DAC process with solid sorbents. Although the effects of water vapor on the DAC performance of amine-based solid sorbents under ambient conditions (>20 °C) have been extensively studied,23,27,28,30,31,53 the sub-ambient (<0 °C) DAC behavior of amine-based CO2 capture sorbents under humid conditions has not yet been reported.
Fixed-bed CO2 breakthrough experiments were conducted under dry and humid (70% RH) conditions at −20 °C to investigate the effect of moisture on the 400 ppm CO2 adsorption behavior of 30 wt % TEPA-impregnated MIL-101(Cr) powders, which were the preferred sorbent for sub-ambient DAC among the sorbents presented here. The procedure used for the breakthrough experiments is described in the Experimental Section. In the case of the humid experiments, the sorbents were prehumidified with wet N2 (70% RH, 870 ppm of H2O) at −20 °C before CO2 adsorption. A breakthrough experiment was first conducted with an empty bed to determine the mean residence time of the gas stream through the fixed bed. The background CO2 breakthrough curve is shown in Figure 6a as the black solid line alongside the measured CO2 breakthrough curves for the CO2 adsorption under dry and humid conditions. Breakthrough and pseudoequilibrium capacities were calculated at the times at which 5% of C0 (inlet CO2 concentration) and 95% of C0 were achieved, respectively, based on the measured breakthrough curves in Figure 6a, and the results are shown in Figure 6b for both the dry and humid experiments. We note that breakthrough capacities are less relevant for DAC (an extraction) than for flue gas capture (a purification) and working capacities would likely be obtained at some time and CO2 capture fraction intermediate between these two values. However, reporting such values from breakthrough data are commonplace for adsorption studies in the literature.
Figure 6.
Dry and humid (70% RH) 400 ppm of CO2 adsorption behaviors of 30 wt % TEPA-impregnated MIL-101(Cr) powders in a fixed bed system at −20 °C. (a) CO2 breakthrough curve. (b) Breakthrough (C/C0 = 0.05) and pseudoequilibrium (C/C0 = 0.95) CO2 capture capacities under dry and humid conditions.
As shown in Figure 6a, the CO2 breakthrough time was longer under humid conditions compared to dry conditions. This led to an increase in the breakthrough and pseudoequilibrium capacities of the 30 wt % TEPA-impregnated MIL-101(Cr) (see Figure 6b). The pseudoequilibrium capacity increased to 0.95 mmol/g, which is 34% higher than that of dry conditions (0.71 mmol/g). The breakthrough capacity also improved by 53% from 0.45 to 0.69 mmol/g with humid conditions, demonstrating the positive effect of moisture on sub-ambient DAC with the 30 wt % TEPA sorbent.
CO2 adsorption–desorption cyclic tests under humid (70% RH at −20 °C) conditions with 25 °C regeneration temperature conditions were conducted with the 30 wt % TEPA sorbent to evaluate both material stability and regeneration performance under humid conditions. Since the adsorbed water is also removed during the regeneration step, a prehydrating process was conducted for the sorbents at −20 °C right after every sorbent regeneration step (25 °C) to ensure that the sorbents were fully saturated with water prior to CO2 adsorption over five consecutive cycles. As shown in Figure 7a and b, the CO2 breakthrough times for the five cycles were almost identical, and both the breakthrough (0.64 mmol/g) and pseudoequilibrium (0.82 mmol/g) capacities of each humid cycle were maintained across the full range of cycles, indicating promising sorbent stability under humid conditions. We note that the CO2 capture capacities obtained during the cyclic tests were comparable to the capacities of the fully activated sorbents (110 °C for 3 h), with only 14% and 9% reductions in the pseudoequilibrium and breakthrough capacities, respectively. These results indicate that the 30 wt % TEPA-impregnated MIL-101(Cr) powders, which are weak chemisorption-dominant, can be effectively regenerated with excellent stability at 25 °C even under humid conditions.
Figure 7.
(a) Humid (70% RH) 400 ppm of CO2 breakthrough curves at −20 °C for a 30 wt % TEPA-impregnated MIL-101(Cr) powders. (b) Breakthrough (C/C0 = 0.05) and pseudoequilibrium (C/C0 = 0.95) CO2 capture capacities under humid conditions (70% RH) at −20 °C.
The enhancement in the CO2 capture capacities of the 30 wt % TEPA material under humid conditions at −20 °C is likely due to enhanced amine efficiency under humid conditions. To gain preliminary insight into the CO2 capture mechanisms of the 30 wt % TEPA-impregnated MIL-101(Cr) under dry and humid (70% RH) adsorption conditions at −20 °C, CO2/H2O TPD experiments were conducted with the fixed-bed system after the dry and humid CO2 capture experiments (Figure S15). As shown in Figure 8, while a single wide peak was observed from the CO2 TPD profile of the dry CO2 adsorption run, an additional CO2 peak was confirmed between 20 and 35 °C during desorption after humid CO2 adsorption. The enhanced CO2 adsorption capacity of the 30 wt % TEPA-impregnated MIL-101(Cr) powders under sub-ambient humid conditions appears mostly attributable to the new peak. It is noteworthy that the new CO2 peak starts to appear right after significant water desorption, suggesting that the desorbed CO2 between 20 and 35 °C may be related to HCO3– formation or increased CO2 solubility in adsorbed water at −20 °C. In future work, IR or NMR studies will be conducted to further probe the structure of the adsorbed CO2 under dry and humid conditions at −20 °C.
Figure 8.
CO2/H2O TPD profiles of 30 wt % TEPA-impregnated MIL-101(Cr) powders (ppt, parts per thousand). Adsorption conditions are as follows: gas, dry and humid (70% RH), 400 ppm of CO2/He; flow rate, 50 mL/min; T = −20 °C; adsorption time, 2 h; and activation, 25 °C under 50 mL/min N2 for 2 h.
Conclusions
In this study, the DAC performance of amine-impregnated MIL-101(Cr) powder sorbents under a wide range of humidity (0–70% RH) and temperature (adsorption at −20 to 25 °C) conditions was explored. There is a significant mismatch between the likely DAC deployment conditions (−30 to 50 °C) and nearly all the published studies of DAC material behavior (20 to 35 °C). Furthermore, over 80% of the land on earth has an average temperature below 25 °C. We hypothesized that understanding CO2 adsorption from ambient air at sub-ambient temperatures, below 20 °C, was therefore crucial for two reasons: (i) it represents important practical operating conditions and (ii) it provides for conditions where new sorbent materials or enhanced process performance might be achieved.32 Here, we provide a systematic study that demonstrates the potential for DAC process improvement under such conditions.
Branched PEI (Mw = 800 Da) and TEPA were physically infused into MIL-101(Cr) MOFs with different amine loadings (10, 30, and 50 wt %). First, their CO2 adsorption behaviors were investigated at ambient temperature conditions (e.g., 25 °C) to set up the baseline for subsequent experiments at sub-ambient temperatures (e.g., −20 °C). At relatively low amine loading conditions (30 wt %), the impregnated amine groups are thought to strongly interact with the MOF framework, and some amine sites were not available for CO2 capture, showing weak chemisorption-dominant behavior (−40 to −65 kJ/mol) with low adsorption capacities at ambient adsorption conditions (25 °C), 0.24 mmol/g for PEI and 0.39 mmol/g for TEPA. With increased amine loading conditions (50 wt %), a sufficient number of excess amine groups inside the MOFs were able to interact with CO2, showing dramatically enhanced CO2 adsorption capacities (pseudoequilibrium) via strong chemisorption (−95 to −110 kJ/mol), 1.81 mmol/g for PEI and 2.14 mmol/g for TEPA. However, CO2 adsorption capacities (pseudoequilibrium) of 50 wt % amine-impregnated MOF sorbents were reduced at −20 °C, 1.64 mmol/g for PEI and 1.26 mmol/g for TEPA, probably due to either decreased amine mobility or insufficient thermal energy available to surpass chemisorption activation barriers. A significant pore-blocking effect may be an additional factor causing the reduced pseudoequilibrium CO2 adsorption capacity of the high amine loading materials at −20 °C.
Interestingly, we found that at −20 °C the CO2 adsorption capacity of 30 wt % TEPA-impregnated MIL-101(Cr) significantly increased to 1.12 mmol/g, and a CO2-TPD experiment revealed that the sorbents can be regenerated under mild temperature conditions (25 °C) due to the weak chemisorption-dominant behavior. Thus, the 30 wt % TEPA-loaded sorbent was selected for further investigation as a potential sorbent for sub-ambient DAC, and it showed promising and stable CO2 working capacities over multiple adsorption–desorption cycles with a small temperature swing (−20 °C ↔ 25 °C) under both dry (0.73 mmol/g for 15 cycles) and humid (0.82 mmol/g for 5 cycles) conditions.
There are several limitations of our study. First, while some of the materials presented here are shown to have promising behaviors under sub-ambient conditions, we do not claim that these will be the ultimate optimal materials for deployment under such conditions (one might seek more rapidly scalable materials that do not deploy chromium, for example). Rather, we show that the sub-ambient temperature manifold is important practically and may offer some differing constraints and opportunities for sorbent and process design. Second, we observed significant measurement errors in determining the 400 ppm CO2 pseudoequilibrium adsorption capacity of the 50 wt % amine-loaded MIL-101(Cr) samples using the volumetric system at −20 °C. This may be due to the two-step CO2 capture behavior (with relatively fast initial CO2 adsorption kinetics followed by dramatically reduced kinetics at higher CO2 loadings) caused by significant pore-blocking and hindered CO2 diffusion at higher surface coverage. Thus, the difficulty in determining the equilibrium CO2 adsorption capacity of high amine loading materials under extremely cold temperatures (e.g., −20 °C) is another limitation of this study. A third limitation of this study is a common limitation of all published adsorption DAC research: the instruments used for measuring CO2 uptake kinetics (TGA, fixed beds) do not represent conditions likely to be deployed practically. Indeed, using monolithic54 or fiber31 contactors at very high gas velocities is likely to meaningfully increase the sorption kinetics.
While these MIL-101(Cr) sorbents are not yet fully optimized for sub-ambient operation, the results here clearly demonstrate a new window of potential operation for DAC with solid sorbents that should spur the additional investigation of a wide range of solid sorbents and process condition.
Experimental Section
Materials
Chromium(III) nitrate nonahydrate Cr(NO3)3·9H2O (99%) and terephthalic acid (H2BDC) were purchased from Acros Chemicals. Dimethylformamide (DMF), tetraethylenepentamine (TEPA, technical grade), and poly(ethylenimine) (PEI) (Mw 800) were purchased from Sigma-Aldrich. Methanol (ACS grade) was purchased from BDH Chemicals.
Technical-grade TEPA contains not only linear TEPA (T-LIN) but also branched TEPA (T-BRN), TEPA with an inner piperazine ring (T-IPZ), and TEPA with a piperazine ring at the edge (T-EPZ).55 As shown in Figure S16, the 13C NMR spectra clearly show that the TEPA used in this study is a mixture of T-BRN, T-LIN, T-IPZ, and T-EPZ, indicating that it also contains a small portion of tertiary amines.
Material Synthesis
MIL-101(Cr) was synthesized hydrothermally based on the recipe from the literature.36,56 First, 800 mg of Cr(NO3)3·9H2O and 332 mg of H2BDC (terephthalic acid) were blended in 10 mL of deionized (DI) water for 1 h. To the mixture were also added 1.5 mL of 36% acetic acid and about 5 mg of MIL-101(Cr) crystals as a modulator and a seed, respectively, for the synthesis. The solution was then placed in a Teflon-lined autoclave and kept in an oven at 200 °C for 12 h, followed by slow cooling with a rate of 1 °C/min to room temperature. After the synthesis, the MOF solids were separated using a centrifuge and washed repeatedly with MeOH, DMF, and MeOH two times each. The resulting MOF powders were dried under a high vacuum (about 10 mTorr) at 150 °C overnight for further analysis and amine impregnation.
The synthesized MIL-101(Cr) powders were then impregnated with PEI (Mw 800) and TEPA with 10, 30, and 50 wt % organic loadings. Before impregnation, the starting material (MIL-101(Cr)) was activated at ∼110–120 °C and 20 mTorr for 24 h. The activated material (500 mg, e.g., MIL-101(Cr)) was dispersed in 30 mL of methanol by sonication until a homogeneous suspension formed (∼20 min). Separately, a solution of the amine (PEI or TEPA) dissolved in 10 mL methanol was stirred to ensure complete dissolution (∼15 min). The solution was added to the suspension, and the resulting mixture was stirred at ambient temperature for 24 h, after which the solvent was removed by rotary evaporation. The obtained material was then further dried under about 10 mTorr vacuum at ambient temperature for >24 h to obtain the amine-impregnated MIL-101(Cr) powder sorbents.
Characterization
Powder X-ray Diffraction (PXRD)
To determine the crystal structure of the synthesized MIL-101(Cr), a powder X-ray diffraction pattern (PXRD) was collected in the range of 2–20° 2θ with a step size of 0.017° by a Panalytical XPert Pro Alpha-1 XRD system using Cu Kα radiation.
Scanning Electron Microscopy (SEM)
A scanning electron microscopy (SEM) image of MIL-101(Cr) was obtained by a Hitachi SU 8230 cold field emission microscope to estimate its crystal size. Measurements were conducted with an accelerating voltage of 5 kV and a 10 μA current. Prior to the analysis, the particle samples were coated with gold/palladium.
Nitrogen Physisorption
N2 physisorption experiments at 77 K were conducted with a surface area and porosity (SAP) system (autosorb iQ/Quantachrome). About 50 mg of the powder samples were activated at 110 °C under vacuum for 3 h before the measurement. the BET surface area was estimated using the N2 physisorption data in the P/P0 range of 0.05–0.2.
CO2 Adsorption Experiments
The equilibrium CO2 adsorption performance (i.e., 400 ppm CO2 adsorption capacity) of amine-impregnated MIL-101(Cr) powders was measured under dry ambient temperature (25 °C) and sub-ambient (−20 °C) conditions using the SAP system (autosorb iQ/Quantachrome). About 100 mg of the powder samples were activated at 110 °C under vacuum for 3 h before the CO2 adsorption. An equilibration interval of 1 min was used for 400 ppm CO2. During equilibration, the cell pressure was checked every 1 min and compared until the pressure in the cell was within the P tolerance (manufacturer tolerance setting 0). If the cell pressure droped below the lower limit of the P/P0 tolerance, the data point was then stored. This equilibration interval and P/P0 tolerance resulted in about 6–20 h total equilibration times per point depending on the amine loadings of the samples (10, 30, and 50 wt %) and the adsorption temperatures (−20 and 25 °C).
The CO2 uptake of amine-impregnated MIL-101(Cr) powders was also gravimetrically measured with a thermogravimetric analysis (TGA)/differential scanning calorimetry (DSC) system (STA 449 F3 Jupiter/NETZSCH) under dry conditions at 25 °C and −20 °C. About 20 mg of the sample was first activated at 110 °C under a He flow (90 mL/min) for 3 h, followed by thermal equilibration at adsorption temperature conditions (25 °C or −20 °C). The sample was then exposed to 400 ppm of CO2 balanced in He (90 mL/min) for 12 h.
Temperature swing adsorption–desorption cyclic tests were performed for up to 15 cycles with the TGA/DSC system. The CO2 adsorption step under the 400 ppm of CO2/He gas stream (90 mL/min) at −20 °C and the regeneration step under the He gas stream (90 mL/min) at 25 or 60 °C were performed for 2 h each.
Fixed Bed Breakthrough Experiments under Humid Conditions
A custom-built fixed bed reactor shown in Figure S17 was used to perform dry and humid CO2 breakthrough experiments at −20 °C. After sample activation at 110 °C under a dry N2 flow for 3 husing heating tape, the packed bed column was immersed into a refrigeration liquid bath of a chiller with a set temperature of −20 °C. The inlet gas stream was then switched to dry or humid (70% RH at −20 °C) 400 ppm of CO2 (50 mL/min) gas balanced in He through a three-way valve for the CO2 capture test. In the case of the humid experiment, the humidity of the inlet gas stream was precisely controlled by a wet gas generator (WETSYS/SETARAM), and the sorbents were prehumidified by introducing wet N2 gas (70%RH at −20 °C) prior to the CO2 breakthrough experiment. The prehumidification process was conducted at −20 °C until the water concentration of the outlet gas stream reached that of the inlet gas stream (70% RH). For the small temperature swing experiment (−20 °C ↔ 25 °C) under humid conditions, the bed temperature was precisely controlled by the chiller. During the breakthrough experiments, the CO2 and H2O concentrations of the outlet gas stream were continuously measured every second by an infrared gas analyzer (LI-840/LI-COR).
CO2/H2O Temperature-Programmed Desorption (TPD) Experiments
CO2 temperature-programmed desorption (TPD) experiments were carried out using the TGA/DSC system. Figure S18 shows a schematic diagram of the CO2 TPD experiment. After 400 ppm of CO2 adsorption with the powder sorbents for 12 h at −20, −10, 5, and 25 °C, the inlet gas flow was changed to pure He, and the TGA/DSC chamber was purged for 1 h at the adsorption temperature condition (−20, −10, 5, and 25 °C). The chamber temperature was then slowly increased at a rate of 0.5 °C/min to 110 °C to desorb CO2 from the powder sorbents. During the entire process, the sample mass change and the CO2 concentration of the outlet gas stream were continuously measured, with the gas composition determined by a CO2/H2O sensor from LiCOR to quantify the amount of desorbed CO2 as a function of temperature. In the case of CO2/H2O TPD experiments, the sub-ambient fixed bed system (Figure S17) was used instead of the TGA/DSC system.
Acknowledgments
The authors thank David Elenowitz, Matthew J. Realff, and the Georgia Tech Direct Air Capture Center (DirACC) for fruitful discussions. This research was supported by the National Energy Technology Laboratory of the U.S. Department of Energy under award no. DE-FE-FE0031952 and Zero Carbon Partners, LLC.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.1c00414.
Characterization data (XRD, SEM, and N2 physisorption), CO2 adsorption behavior (TGA data, adsorption isotherms, and CO2/H2O-TPD data) of the amine-impregnated (PEI and TEPA) MIL-101(Cr) powder sorbents, schematic illustration of the sub-ambient fixed bed setup, and schematic illustration of the CO2-TPD setup using the TGA/DSC (PDF)
The authors declare the following competing financial interest(s): C.W.J. has a financial interest in a business that seeks to commercialize a process for CO2 removal from air. This business is not associated with this work.
Supplementary Material
References
- Lindsey R.Climate Change: Atmospheric Carbon Dioxide; NOAA Climate.gov, 2021. https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide#:~:text=The%20global%20average%20atmospheric%20carbon,least%20the%20past%20800%2C000%20years.
- Lindsey R.; Dahlman L.. Climate Change: Global Temperature; NOAA Climate.gov, 2021. https://www.climate.gov/news-features/understanding-climate/climate-change-global-temperature.
- Miao Y.; He Z.; Zhu X.; Izikowitz D.; Li J. Operating temperatures affect direct air capture of CO2 in polyamine-loaded mesoporous silica. Chem. Eng. J. 2021, 426, 131875. 10.1016/j.cej.2021.131875. [DOI] [Google Scholar]
- La Sorte F. A.; Jetz W. Projected range contractions of montane biodiversity under global warming. Proceedings of the Royal Society B: Biological Sciences 2010, 277 (1699), 3401–3410. 10.1098/rspb.2010.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfi J. M.; Connolly S. R.; Marshall D. J.; Cohen A. L. Projecting coral reef futures under global warming and ocean acidification. Science 2011, 333 (6041), 418–422. 10.1126/science.1204794. [DOI] [PubMed] [Google Scholar]
- Trenberth K. E.; Dai A.; Van Der Schrier G.; Jones P. D.; Barichivich J.; Briffa K. R.; Sheffield J. Global warming and changes in drought. Nature Climate Change 2014, 4 (1), 17–22. 10.1038/nclimate2067. [DOI] [Google Scholar]
- Diffenbaugh N. S.; Burke M. Global warming has increased global economic inequality. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (20), 9808–9813. 10.1073/pnas.1816020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegh-Guldberg O.; Jacob D.; Taylor M.; Bindi M.; Brown S.; Camilloni I.; Diedhiou A.; Djalante R.; Ebi K. L.; Engelbrecht F.. et al. Impacts of 1.5 °C global warming on natural and human systems. In Global warming of 1.5 °C; The Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018.
- Dutcher B.; Fan M.; Russell A. G. Amine-based CO2 capture technology development from the beginning of 2013-A Review. ACS Appl. Mater. Interface 2015, 7 (4), 2137–2148. 10.1021/am507465f. [DOI] [PubMed] [Google Scholar]
- Nemitallah M. A.; Habib M. A.; Badr H. M.; Said S. A.; Jamal A.; Ben-Mansour R.; Mokheimer E. M.; Mezghani K. Oxy-fuel combustion technology: current status, applications, and trends. Int. J. Energy Res. 2017, 41 (12), 1670–1708. 10.1002/er.3722. [DOI] [Google Scholar]
- Zhang Y.; Wang D.; Pottimurthy Y.; Kong F.; Hsieh T.-L.; Sakadjian B.; Chung C.; Park C.; Xu D.; Bao J.; et al. Coal direct chemical looping process: 250 kW pilot-scale testing for power generation and carbon capture. Appl. Energy 2021, 282, 116065. 10.1016/j.apenergy.2020.116065. [DOI] [Google Scholar]
- Babin A.; Vaneeckhaute C.; Iliuta M. C. Potential and challenges of bioenergy with carbon capture and storage as a carbon-negative energy source: A review. Biomass Bioenergy 2021, 146, 105968. 10.1016/j.biombioe.2021.105968. [DOI] [Google Scholar]
- Holmes H. E.; Lively R. P.; Realff M. J. Defining Targets for Adsorbent Material Performance to Enable Viable BECCS Processes. JACS Au 2021, 1, 795–806. 10.1021/jacsau.0c00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambhir A.; Tavoni M. Direct air carbon capture and sequestration: how it works and how it could contribute to climate-change mitigation. One Earth 2019, 1 (4), 405–409. 10.1016/j.oneear.2019.11.006. [DOI] [Google Scholar]
- Lackner K.; Ziock H.-J.; Grimes P.. Carbon Dioxide Extraction from Air: Is It an Option?; Los Alamos National Laboratory: Los Alamos, NM, 1999.
- Sanz-Pérez E. S.; Murdock C. R.; Didas S. A.; Jones C. W. Direct capture of CO2 from ambient air. Chem. Rev. 2016, 116 (19), 11840–11876. 10.1021/acs.chemrev.6b00173. [DOI] [PubMed] [Google Scholar]
- de Jonge M. M.; Daemen J.; Loriaux J. M.; Steinmann Z. J.; Huijbregts M. A. Life cycle carbon efficiency of Direct Air Capture systems with strong hydroxide sorbents. Int. J. Greenhouse Gas Control 2019, 80, 25–31. 10.1016/j.ijggc.2018.11.011. [DOI] [Google Scholar]
- Mahmoudkhani M.; Keith D. W. Low-energy sodium hydroxide recovery for CO2 capture from atmospheric air—Thermodynamic analysis. Int. J. Greenhouse Gas Control 2009, 3 (4), 376–384. 10.1016/j.ijggc.2009.02.003. [DOI] [Google Scholar]
- Fasihi M.; Efimova O.; Breyer C. Techno-economic assessment of CO2 direct air capture plants. J. Clean. Prod. 2019, 224, 957–980. 10.1016/j.jclepro.2019.03.086. [DOI] [Google Scholar]
- Sabatino F.; Grimm A.; Gallucci F.; van Sint Annaland M.; Kramer G. J.; Gazzani M. A comparative energy and costs assessment and optimization for direct air capture technologies. Joule 2021, 5 (8), 2047–2076. 10.1016/j.joule.2021.05.023. [DOI] [Google Scholar]
- BNational Academies of Sciences, Engineering, and Medicine . Negative emissions technologies and reliable sequestration: A research agenda; The National Academies Press: Washington, D.C., 2019. [PubMed] [Google Scholar]
- Didas S. A.; Sakwa-Novak M. A.; Foo G. S.; Sievers C.; Jones C. W. Effect of Amine Surface Coverage on the Co-Adsorption of CO2 and Water: Spectral Deconvolution of Adsorbed Species. J. Phys. Chem. Lett. 2014, 5 (23), 4194–4200. 10.1021/jz502032c. [DOI] [PubMed] [Google Scholar]
- Goeppert A.; Czaun M.; May R. B.; Prakash G. K. S.; Olah G. A.; Narayanan S. R. Carbon Dioxide Capture from the Air Using a Polyamine Based Regenerable Solid Adsorbent. J. Am. Chem. Soc. 2011, 133 (50), 20164–20167. 10.1021/ja2100005. [DOI] [PubMed] [Google Scholar]
- Wurzbacher J. A.; Gebald C.; Piatkowski N.; Steinfeld A. Concurrent separation of CO2 and H2O from air by a temperature-vacuum swing adsorption/desorption cycle. Environ. Sci. Technol. 2012, 46 (16), 9191–9198. 10.1021/es301953k. [DOI] [PubMed] [Google Scholar]
- Wang J.; Huang H.; Wang M.; Yao L.; Qiao W.; Long D.; Ling L. Direct capture of low-concentration CO2 on mesoporous carbon-supported solid amine adsorbents at ambient temperature. Ind. Eng. Chem. Res. 2015, 54 (19), 5319–5327. 10.1021/acs.iecr.5b01060. [DOI] [Google Scholar]
- Goeppert A.; Zhang H.; Sen R.; Dang H.; Prakash G. S. Oxidation-Resistant, Cost-Effective Epoxide-Modified Polyamine Adsorbents for CO2 Capture from Various Sources Including Air. ChemSusChem 2019, 12 (8), 1712–1723. 10.1002/cssc.201802978. [DOI] [PubMed] [Google Scholar]
- Gebald C.; Wurzbacher J. A.; Borgschulte A.; Zimmermann T.; Steinfeld A. Single-Component and Binary CO2 and H2O Adsorption of Amine-Functionalized Cellulose. Environ. Sci. Technol. 2014, 48 (4), 2497–2504. 10.1021/es404430g. [DOI] [PubMed] [Google Scholar]
- Rim G.; Feric T. G.; Moore T.; Park A. H. A. Solvent Impregnated Polymers Loaded with Liquid-Like Nanoparticle Organic Hybrid Materials for Enhanced Kinetics of Direct Air Capture and Point Source CO2 Capture. Adv. Funct. Mater. 2021, 31 (21), 2010047. 10.1002/adfm.202010047. [DOI] [Google Scholar]
- Sardo M.; Afonso R.; Juźków J.; Pacheco M.; Bordonhos M.; Pinto M. L.; Gomes J. R.; Mafra L. Unravelling moisture-induced CO2 chemisorption mechanisms in amine-modified sorbents at the molecular scale. J. Mater. Chem. A 2021, 9 (9), 5542–5555. 10.1039/D0TA09808F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayari A.; Liu Q.; Mishra P. Enhanced Adsorption Efficiency through Materials Design for Direct Air Capture over Supported Polyethylenimine. ChemSusChem 2016, 9 (19), 2796–2803. 10.1002/cssc.201600834. [DOI] [PubMed] [Google Scholar]
- Sujan A. R.; Pang S. H.; Zhu G. H.; Jones C. W.; Lively R. P. Direct CO2 Capture from Air using Poly(ethylenimine)-Loaded Polymer/Silica Fiber Sorbents. ACS Sustain. Chem. Eng. 2019, 7 (5), 5264–5273. 10.1021/acssuschemeng.8b06203. [DOI] [Google Scholar]
- Kong F.; Rim G.; Song M. G.; Rosu C.; Priyadarshini P.; Lively R. P.; Realff M. J.; Jones C. W. Research Needs Targeting Direct Air Capture of Carbon Dioxide: Material & Process Performance Characteristics Under Realistic Environmental Conditions. Korean J. Chem. Eng. 2022, 39, 1–19. 10.1007/s11814-021-0976-0. [DOI] [Google Scholar]
- Mourshed M. Climatic parameters for building energy applications: A temporal-geospatial assessment of temperature indicators. Renewable Energy 2016, 94, 55–71. 10.1016/j.renene.2016.03.021. [DOI] [Google Scholar]
- Zhai Y.; Chuang S. S. C. The Nature of Adsorbed Carbon Dioxide on Immobilized Amines during Carbon Dioxide Capture from Air and Simulated Flue Gas. Energy Technol. 2017, 5 (3), 510–519. 10.1002/ente.201600685. [DOI] [Google Scholar]
- Peter S. C.; Cherevotan A.; Raj J. An Overview of Porous Silica Immobilized Amines for Direct Air CO2 Capture. J. Mater. Chem. A 2021, 9, 27271–27303. 10.1039/D1TA05961K. [DOI] [Google Scholar]
- Darunte L. A.; Oetomo A. D.; Walton K. S.; Sholl D. S.; Jones C. W. Direct Air Capture of CO2 Using Amine Functionalized MIL-101(Cr). ACS Sustain. Chem. Eng. 2016, 4 (10), 5761–5768. 10.1021/acssuschemeng.6b01692. [DOI] [Google Scholar]
- Gebald C.; Wurzbacher J. A.; Tingaut P.; Steinfeld A. Stability of amine-functionalized cellulose during temperature-vacuum-swing cycling for CO2 capture from air. Environ. Sci. Technol. 2013, 47 (17), 10063–10070. 10.1021/es401731p. [DOI] [PubMed] [Google Scholar]
- Choi S.; Gray M. L.; Jones C. W. Amine-tethered solid adsorbents coupling high adsorption capacity and regenerability for CO2 capture from ambient air. ChemSusChem 2011, 4 (5), 628–635. 10.1002/cssc.201000355. [DOI] [PubMed] [Google Scholar]
- Hong D. Y.; Hwang Y. K.; Serre C.; Ferey G.; Chang J. S. Porous chromium terephthalate MIL-101 with coordinatively unsaturated sites: surface functionalization, encapsulation, sorption and catalysis. Adv. Funct. Mater. 2009, 19 (10), 1537–1552. 10.1002/adfm.200801130. [DOI] [Google Scholar]
- Liu Q.; Ning L.; Zheng S.; Tao M.; Shi Y.; He Y. Adsorption of carbon dioxide by MIL-101 (Cr): Regeneration conditions and influence of flue gas contaminants. Sci. Rep. 2013, 3 (1), 2916. 10.1038/srep02916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A.; Darunte L. A.; Jones C. W.; Realff M. J.; Kawajiri Y. Systems design and economic analysis of direct air capture of CO2 through temperature vacuum swing adsorption using MIL-101 (Cr)-PEI-800 and mmen-Mg2 (dobpdc) MOF adsorbents. Ind. Eng. Chem. Res. 2017, 56 (3), 750–764. 10.1021/acs.iecr.6b03887. [DOI] [Google Scholar]
- Sinha A.; Darunte L. A.; Jones C. W.; Realff M. J.; Kawajiri Y. Correction to “Systems Design and Economic Analysis of Direct Air Capture of CO2 through Temperature Vacuum Swing Adsorption Using MIL-101 (Cr)-PEI-800 and mmen-Mg2 (dobpdc) MOF Adsorbents. Ind. Eng. Chem. Res. 2020, 59 (1), 503–505. 10.1021/acs.iecr.9b06779. [DOI] [Google Scholar]
- Yang C. R.; Du Z. L.; Jin J. S.; Chen J.; Mi J. G. Epoxide-functionalized tetraethylenepentamine encapsulated into porous copolymer spheres for CO2 capture with superior stability. Appl. Energy 2020, 260, 114265. 10.1016/j.apenergy.2019.114265. [DOI] [Google Scholar]
- Xu X. C.; Song C. S.; Andresen J. M.; Miller B. G.; Scaroni A. W. Preparation and characterization of novel CO2 “molecular basket” adsorbents based on polymer-modified mesoporous molecular sieve MCM-41. Microporous Mesoporous Mater. 2003, 62 (1–2), 29–45. 10.1016/S1387-1811(03)00388-3. [DOI] [Google Scholar]
- Choi S.; Drese J. H.; Eisenberger P. M.; Jones C. W. Application of Amine-Tethered Solid Sorbents for Direct CO2 Capture from the Ambient Air. Environ. Sci. Technol. 2011, 45 (6), 2420–2427. 10.1021/es102797w. [DOI] [PubMed] [Google Scholar]
- Young D. M.; Crowell A. D.. Physical Adsorption of Gases; Butterworths: London, U.K., 1962. [Google Scholar]
- Donaldson T. L.; Nguyen Y. N. Carbon-Dioxide Reaction-Kinetics and Transport in Aqueous Amine Membranes. Ind. Eng. Chem. Fundam. 1980, 19 (3), 260–266. 10.1021/i160075a005. [DOI] [Google Scholar]
- Bollini P.; Didas S. A.; Jones C. W. Amine-oxide hybrid materials for acid gas separations. J. Mater. Chem. 2011, 21 (39), 15100–15120. 10.1039/c1jm12522b. [DOI] [Google Scholar]
- Lee J. J.; Chen C. H.; Shimon D.; Hayes S. E.; Sievers C.; Jones C. W. Effect of Humidity on the CO2 Adsorption of Tertiary Amine Grafted SBAL15. J. Phys. Chem. C 2017, 121 (42), 23480–23487. 10.1021/acs.jpcc.7b07930. [DOI] [Google Scholar]
- Bacsik Z.; Ahlsten N.; Ziadi A.; Zhao G. Y.; Garcia-Bennett A. E.; Martin-Matute B.; Hedin N. Mechanisms and Kinetics for Sorption of CO2 on Bicontinuous Mesoporous Silica Modified with n-Propylamine. Langmuir 2011, 27 (17), 11118–11128. 10.1021/la202033p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. W.; Steib M.; Jentys A.; Lercher J. A. Mechanism and Kinetics of CO2 Adsorption on Surface Bonded Amines. J. Phys. Chem. C 2015, 119 (8), 4126–4135. 10.1021/jp512001t. [DOI] [Google Scholar]
- Li K. J.; Kress J. D.; Mebane D. S. The Mechanism of CO2 Adsorption under Dry and Humid Conditions in Mesoporous Silica-Supported Amine Sorbents. J. Phys.s Chem. C 2016, 120 (41), 23683–23691. 10.1021/acs.jpcc.6b08808. [DOI] [Google Scholar]
- Wijesiri R. P.; Knowles G. P.; Yeasmin H.; Hoadley A. F. A.; Chaffee A. L. CO2 Capture from Air Using Pelletized Polyethylenimine Impregnated MCF Silica. Ind. Eng. Chem. Res. 2019, 58 (8), 3293–3303. 10.1021/acs.iecr.8b04973. [DOI] [Google Scholar]
- Darunte L. A.; Terada Y.; Murdock C. R.; Walton K. S.; Sholl D. S.; Jones C. W. Monolith-Supported Amine-Functionalized Mg2(dobpdc) Adsorbents for CO2 Capture. ACS Appl. Mater. Interface 2017, 9 (20), 17042–17050. 10.1021/acsami.7b02035. [DOI] [PubMed] [Google Scholar]
- Numaguchi R.; Chowdhury F. A.; Yamada H.; Yogo K. Carbon Dioxide Absorption using Solid Sorbents Incorporating Purified Components of Tetraethylenepentamine. Energy Technol. 2017, 5 (8), 1186–1190. 10.1002/ente.201600665. [DOI] [Google Scholar]
- Bromberg L.; Diao Y.; Wu H.; Speakman S. A.; Hatton T. A. Chromium(III) Terephthalate Metal Organic Framework (MIL-101): HF-Free Synthesis, Structure, Polyoxometalate Composites, and Catalytic Properties. Chem. Mater. 2012, 24 (9), 1664–1675. 10.1021/cm2034382. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








