Abstract
Mesenchymal stem/stromal cells (MSCs) are known as the issue in biology because of some unpredictable characteristics in the different microenvironments especially in their bone marrow niche. MSCs are used in the regenerative medicine because of their unique potentials for trans-differentiation, immunomodulation, and paracrine capacity. But, their pathogenic and pro-survival effects in tumors/cancers including hematologic malignancies are indisputable. MSCs and/or their derivatives might be involved in tumor growth, metastasis and drug resistance in the leukemias. One of important relationship is MSCs and hematologic malignancy-derived cells which affects markedly the outcome of disease. The communication between these two cells may be contact-dependent and/or contact-independent. In this review, we studied the crosstalk between MSCs and malignant hematologic cells which results the final feedback either the progression or suppression of blood cell malignancy.
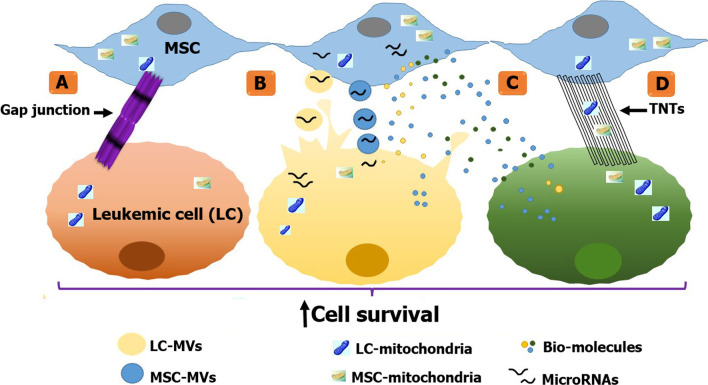
Graphical abstract
Video abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-022-00822-6.
Keywords: Mesenchymal stem cell, Hematologic malignancy, Tumor
Background
Bone marrow-derived mesenchymal stromal cells (BM-MSCs) are a rare population of non-hematopoietic multipotent cells that are recognizable and sortable by being positive for MSC markers such as CD90, CD73 and CD105 and being negative for hematopoietic markers like CD34 and CD45 [1, 2]. It has been revealed that it is not just the physical support provided by MSCs in microenvironment for target cells, but a bidirectional conversation between them is routed [3, 4]. A substantial number of studies declare that BM-MSCs adopt several methods to establish this communication. These mechanisms can be divided into two main categories: contact-dependent including tunneling nanotubes (TNTs) [5, 6], gap junctions [7], and contact-independent including paracrine activity and microvesicle/exosomes delivery through which microRNA (miRNA), immuno-modulatory molecules and mitochondria can be transferred [8–12]. MSCs are mainly being used in the regenerative medicine [13–15]. But in terms of malignancy, it is a subject of controversial that whether MSCs are pro- or anti-tumorigenic [16]. However, to digress from malignancies, MSCs are also used in myocardial infarction (MI) [17]. They play an indirect role in MI by recruitment of macrophages in angiogenesis to promote tissue regeneration [18]. The study of these effects and conversations provides a fertile ground of investigation [19].
Returning to malignancies, although MSCs have shown therapeutic properties mainly by their potentials for trans-differentiation, immunomodulation, and apoptosis induction [2, 20, 21], their pathogenic, leukemogenic and pro-survival effects in hematologic malignancies are indisputable [22, 23]. Cellular mechanisms include RNA processing, ubiquitin–proteasome pathway, cell cycle regulation, cellular stress and non-canonical Wnt signaling are modulated in the leukemia cell lines co-cultured with MSCs [24].
Indeed, MSCs have been suspected of being the culprit of elevated tumor growth, metastasis and drug resistance in the leukemias [25]. Particularly in chronic lymphocytic leukemia (CLL) cells, survival and drug resistance signals as well as CLL-cell trafficking and tissue homing signals are under bone marrow stromal cells (BMSCs) control [26]. Even in a mouse model of the pre-leukemic disorder Schwachman-Diamond syndrome (SDS), genotoxic stress and subsequently DNA damage response (DDR) activation in hematopoietic stem and progenitor cells have been reported as the main consequence of abnormal activity of mesenchymal cells [27]. Therefore, this study covers a lot of ground in this matter and offers a full explanation of mechanisms by which MSCs counter treatment strategies. Herein, the contact-dependent and contact-independent mechanisms that involve in the MSC and leukemic cells conversation are presented.
Contact-dependent
Tunneling nanotubes (TNTs)
TNTs are intercellular transient structures of about 50–200 nm in diameter which are made of polymerization of F-actin; thereby, they are naturally vanishing and henceforth uncontrollable. Likewise, biomolecules and cellular organelles can be transported through them [28].
First of all, chemoresistance can be modulated in other solid tumor cells like SKOV3 ovarian cancer cells and MCF7 breast cancer cells as a consequence of preferential transfer of mitochondria from endothelial to cancer cells through TNTs modulates [29]. Pinto and et al. have reviewed that TNT-like connections are used by cancers to modify their potential chemoresistance, migration, metabolism, metastasis and angiogenesis [30].
However, in the leukemias TNTs serve the reliable infrastructure for trafficking both vesicle and protein to leukemic cells [31]. This type of intercellular relationship is forged, and as it has been proven in B cell acute lymphoblastic leukemia (B-ALL), it is not a monologue delivered by MSCs but dialogues can be held. As an example, using TNTs, BM-MSC secretome profile is pervasively converted to a leukemia pro-survival profile in B-lymphoblastic leukemia [32]. Bidirectional cytoplasmic transport has also been observed between MSCs and activated benign T cells which exerts suppressive effect on the proliferation rate and IFN-γ production of T cells [33].
Furthermore, through a TNT routed communication, primary B-cell precursor ALL (BCP-ALL) cells deliver autophagosomes, mitochondria, intercellular adhesion molecule 1 (ICAM1) and other lipophiles to MSCs leading to cytokine secretion, leukemic cell survival and drug resistance [34]. These pro-survival cytokines include interferon-γ-inducible protein 10 (IP-10), CXCL10, IL-8, monocyte chemotactic protein-1 (MCP-1) and CCL2 [35]. Disruption of TNTs would physically affect this support and significantly re-sensitizes BCP-ALL cells to chemotherapeutics like prednisolone [35]. However, being exposed to chemotherapeutic drugs, mitochondrial transfer from Jurkat cells, a T cell acute lymphoblastic leukemia (T-ALL) cell line, to MSCs is promoted. Although, it is primarily conducted by contact-dependent manners including TNTs and gap junctions (GJs), nevertheless microvesicles (contact-independent) are also to some extent responsible for this trafficking [36]. In fact, Jurkat cells exhibit a trafficking arabinoside (ARA-C) or methotrexate. This leads to a substantial promotion of Jurkat cells' survival, due to a dramatic reduction of reactive oxygen species (ROS) levels inside them [36, 37]. The resulting increased production of mitochondrial adenosine triphosphate (ATP), mitochondrial transfer leads to increased chemoresistance [38].
By the same token, in acute myelogenous leukemia (AML), mitochondrial transfer primarily through TNT has been contingently associated with chemoresistance [39]. Both leukemic blasts and leukemia initiating cells of AML are given the privilege of further survival in battle with some chemotherapies by the TNT-routed mitochondrial transfer from MSC [40]. By up to 14%, mitochondrial mass in AML cells increases in co-cultures with MSCs. Consequently, cytotoxic effects of the nucleoside analog ARA-C would be hedged against AML cell lines [40].
To enhance transferring from MSC to AML blasts through TNT, mitochondrial biogenesis in MSC is stimulated by AML-derived NADPH oxidase 2 (NOX2) superoxide. Correspondingly, this facilitation can be taken place in multiple myeloma (MM) cells by CD38 expressing MM cells [41]. Inhibition of NOX2 is characterized by a downward trend in mitochondrial transfer and an upward trend in AML cells apoptosis [42]. Autophagy, on the other hand is increased in AML cells chiefly because autophagosomes transfer from MSCs to AML cells using TNTs [32].
Tyrosine kinase inhibitors and interferon‐α used in chronic myeloid leukemia (CML) cell lines increase TNT formation and cell adhesion [43]. The so-called stroma-mediated imatinib resistance would be resulted from this TNT formation. To clarify, CML cells receive cellular vesicles from and send them out to MSCs through TNTs. These vesicles finally attenuate imatinib mediated caspase activity and thereby apoptosis [44]. Notably in CML, TNTs facilitate exosomes transportation and exosomes in turn stimulate TNT formation; so, they can synergistically interact and multiply their communication [45].
Turning to lymphoma, we must note that malignant B cells upregulate BCL-2 family proteins following receiving soluble factors from MSCs. Activation of the oncogenic pathways like NOTCH1 signaling is also stimulated by MSCs reported in different B cell malignancies, MM and CLL [46, 47]. Examples of MSCs and malignant hematologic cells communications via TNTs have been summarized in Table 1
Table 1.
Communication between MSCs and hematologic malignancies/cell lines through tunneling nanotubes (TNTs) and its effects
| Type of malignancy/cell | Kind of material transferred | Target | Results | References |
|---|---|---|---|---|
| ALL | Pro-survival cytokines | ALL cells | Conversion to a leukemia pro-survival | [35] |
| ALL | IL-6/TNFα/IL-1β | ETV6‐RUNX1 harboring cells | DNA damage accumulation | [48] |
| BCP-ALL | Autophagosomes, mitochondria, ICAM1 and other lipophiles | MSCs | Cytokine secretion, leukemic cell survival, drug resistance | [34] |
| Jurkat cells | Mitochondria | Mostly MSCs | Leukemic cell survival, ↓ROS, chemoresistance | [36, 37] [38] |
| AML | Mitochondria | AML cells | Chemoresistance, cytotoxic effects of the nucleoside analog ARA-C | [39] |
| AML | Autophagosome | AML cells | Autophagy | [32] |
| AML | NADPH oxidase 2 (NOX2) superoxide | MSCs | Enhances transferring from MSC to AML | [41] |
| CML | Cellular vesicles | Bi-directional | Imatinib resistance, ↓imatinib mediated caspase activity, apoptosis | [44] |
| Multiple myeloma | NADPH oxidase 2 (NOX2) superoxide | MM cell | Enhances transferring from MSC to MM cells | [41] |
| B-cell lymphoma | Mitochondria, soluble factors | MSCs | Anti-apoptosis: upregulate BCL-2 family proteins Pro-oncogenic: Activation of NOTCH1 signaling | [46] |
| MDS | Proangiogenic factors (VEGF-A, IGFs, and EGFs) and mediators of fibrosis (LOXL, TGF-β, and LIF) | Bi-directional | MSCs adopt MDS-desirable features, Mitochondria dysfunction, genotoxic stress in HSCs, ↑ risk of developing to AML, Impaired myeloid and lymphoid differentiation in mice with MDS, Modulated expression of several cytokines in MSCs | [49] |
Gap junctions
Gap junctions (GJs) are comprised of two hemichannels (HCs) or connexons which in turn composed of arrays of connexin proteins. Ions, small metabolites, and organelles can be transferred through GJs. GJ intercellular communication (GJIC) in the HSC niche may lead to improvement of cellular bioenergetics, and rejuvenates the damaged recipient cells [50].
In normal hematopoiesis, mitochondria can transfer from BMSC to HSC using GJ by which GJs modulate granulopoiesis and differentiation to myeloid blood cell precursor. Hematopoietic stem/progenitor cells (HSPCs) quiescence and stemness are also determinable by GJ communication named as connexin-32 (Cx32). HSC quiescence retention and survival in the BM is dependent on CXCL12 secretion which is regulable by the expression of both connexin-43 (Cx43( and connexin-45 (Cx45) in MSC. During stress, Cx43 transfers ROS to BMSC which reduces HSC senescence [50].
Turning to malignancy, in co-culture with MM cells, MSCs exhibit an abrupt increase in Cx43 level representative of improved GJ-mediated intercellular communication [51]. Cx43 has also been postulated to be a putative player of adhesion and migration of MM cells demonstrated in primary MM cells and cell lines RPMI 8226, U266, and XG-7 which can finally increase cell proliferation and chemoresistance [18]. Adhesion and migration of MM cells is blocked by the gap junction blocker 18α-glycyrrhetinic acid (18α-GA) which decrease stromal cell-derived factor-1α (SDF-1α) secretion [51]. Divagating from MM, HL-60 and PBL-985 cells would see a downward trend in differentiation potential when GJ communication has not been abrogated [50, 52].
Carbenoxolone-induced GJ disruption could interfere with MSC and different malignant hematologic cell line communication and alters drug resistance pattern [53]. It indicates the important role of GJ between these cells in the outcome of leukemia treatment.
Cx26, Cx32, Cx37, Cx43, and Cx45 are responsible for exponentially elevated chemoresistance and substantially reduced apoptosis in primary AML cells. Leukemia pathogenesis is interconnected with connexin-based modifications on target cells. For example, mitochondria can transfer in a Cx43-mediated manner which affect adversely not only the pathogenesis but also chemoresistance [50]. Therefore, MSCs induced chemoresistance can be modulated by disruption of gap junctions in AML [53].
A significant amounts of prostaglandin E2 (PGE2), which suppresses DNA damage-induced p53 accumulation, is released from the HCs of stromal cells leading to promoted survival and metastasis of cancer cells [54]. However, having the permeability to small molecules and macromolecules, Cx43 may provide a target for cytoplasmic drug delivery [55]. Table 2 represents details of the GJ-mediated crosstalk between MSCs and malignant hematologic cells that has reported in different studies.
Table 2.
Crosstalk between MSCs and hematologic malignancies/cell lines via gap junction and its effects
| Type of malignancy/cell | Kind of gap junction's component(s) involved | Target | Results | References |
|---|---|---|---|---|
| HL-60 and PBL-985 cells | Cx43 | HL-60 and PBL-985 cells | Downward trend in differentiation potential | [56] |
| U937, KG-1, KG-1a, HL-60, OCI-AML3, MV4-11, MoLM-13 Jurkat, and THP1 cells | Cx25, Cx26, Cx30, Cx31, Cx32, Cx36, Cx37, Cx40, Cx46, and Cx62 | MSCs | Proliferation | [56] |
| Primary AML cells | Cx26, Cx32, Cx37, Cx43, and Cx45 | AML cells | Chemoresistance, ↓ apoptosis | [50] |
| MM cells: RPMI 8226, U266, and XG-7 | Cx43 | MM cells | ↑Cell proliferation, chemoresistance | [57] |
| AML | Cx43 | MSCs | Pathogenesis, chemoresistance | [53] |
Contact-independent
Paracrine activity
MSC produce cytokines such as IL-6, IL-11, SCF, TPO, Flt-3 ligand, CXCL12, G-CSF, GM-CSF and M-CSF [58–61] to support hematopoiesis. On the other hand, HSC quiescence is affected by Wnt released from MSC. Wnt expression in HSC also downregulated kit ligand, angiopoietin-1, CXCL12 and vascular cell adhesion molecule 1 (VCAM-1) [62, 63].
To turn to malignant hematopoiesis, BM-MSCs mainly produce Wnt ligands which leads to the intracellular accumulation of β-catenin. Gene expression of several downstream growth factors are subsequently elevated and proliferation of leukemia stem cells (LSCs) is guaranteed by this way [64, 65]. To elucidate, the effects of Wnt/β-catenin signaling as a pro-growth signal is essentially counteracted by the bone morphogenetic protein (BMP) anti-growth signals. Imbalance toward higher growth rate results in a leukemogenic phenotype [66]. However, dealing with Wnt/β‐catenin signaling, scientists have found this pathway to be complex and regulated by MSCs themselves. Interferon‐β (IFN‐β) released from MSCs exhibits anti‐tumorigenic effects in erythroleukemic cells based on its ability to negatively regulate Wnt/β‐catenin signaling pathways [67]. Dickkopf‐1 (DKK‐1) is also a negative regulator of Wnt signaling pathway and has antiproliferative activity in MM [68]. Considering the stimulatory effect of MSCs in production of DKK-1, IL-6, and IL-10, a potential role has been ascribed to the crosstalk between myeloma and MSCs in the development of disease into a bone lytic phase [57, 69]. Interestingly, in co-culture studies with multiple myeloma-derived mesenchymal stem cells (MM-MSCs), granulocytic-myeloid-derived suppressor cells (G-MDSCs) have been examined. MM-MSC educated G-MDSCs demonstrate supportive effects in MM by upregulation of immune-suppressive and proangiogenic factors including arginase 1 (ARG1), tumor necrosis factor α (TNF-α), and prokineticin 2 (PROK2) [70]. Besides, MSC ensures MM cell survival, disease progression, and drug resistance having upregulated levels of gene expression of angiogenic and growth factors such as CD40/40L, VCAM-1, ICAM-1, lymphocyte function-associated antigen-3 (LFA-3), and immunomodulated level of cytokines: increased IL-6 and reduced IL-10 [71]. Pro-angiogenic profile accompanied by anti-osteogenic pattern in MM cells co-cultivated by MM-MSCs can be a consequence of increased the vascular endothelial growth factor (VEGF) and IL-6 expression. As a matter of interest, this phenomenon is followed from activation of Notch signaling in MM-MSCs [72].
Regarding other cytokines produced by MSCs, they secrete promyelocytic leukemia protein followed by production of pro-inflammatory molecules, including CXCL1 and IL-6 which is considered as the major cause of leukemogenesis in the different types of leukemia [73]. For the most part, MSC secreted factors, especially IL-6, shelter CML cells from imatinib-induced apoptosis basically through NFκB-mediated signaling [74]. Chemoresistance in the diffuse large B-cell lymphoma (DLBCL) can be acquired by MSC secretion of IL-6 and upregulation of IL-17A [75].
In CLL, MSCs also demonstrate protective activities against cytotoxic effects of Forodesine [76]. To illustrate, interaction of MSC with CLL cells increases the production platelet-derived growth factor (PDGF), which binds to its receptor, PDGFR, leading to secretion of VEGF and making an angiogenic switch, associated with drug protection and disease progression [77]. Compared to MDS-derived mesenchymal stromal cells (MDS-MSCs), MSCs from B-CLL patients produce aberrant SDF-1, B-cell activating factor (BAFF), and transforming growth factor β (TGF-β) resulting in exponentially promoted normal B-cell proliferation and IgG production [78]. Elevated VEGF and hypoxia-inducible factor 1 (HIF-1) production is representative for proangiogenic profile and therefore additional CLL cell survival and resistance to rituximab/alemtuzumab [71].
Disease progression resulted from shifting to proangiogenic profile is the fatal outcome of interaction between conditioned medium (CM) obtained from CLL cells (CLL-CM) and MSCs. PDGFR in MSCs is converted to the active form after exposure to CLL-CM. Microenvironment must face devastating consequences of this phenomenon including MSC proliferation and MSC VEGF production [79]. Finally, survival of CLL cells is also insured by the interaction between their hepatocyte growth factor receptor (c-MET) and hepatocyte growth factor secreted by MSCs [80]. Some important MSCs-derived molecules and their related effects on HSCs and leukemic cells were shown in Table 3.
Table 3.
Paracrine effects of MSCs on HSCs and different leukemic cells
| Type of molecule released by MSC | Target | Results | References |
|---|---|---|---|
| Wnt | HSCs | Quiescence | [81] |
| IL-6, IL-11, SCF, TPO, Flt-3 ligand, CXCL12, G-CSF, GM-CSF, and M-CSF | HSCs | Ensures hematopoiesis | [82] |
| Wnt | HSCs | Downregulates kit ligand, angiopoietin-1, CXCL12, and VCAM-1 | [82] |
| Wnt ligands | LSCs | Proliferation | [64, 65] |
| Wnt ligands | LSCs | Counteracted by BMP anti-growth signals | [66] |
| Interferon‐β (IFN‐β) | Erythroleukemic cells | Anti‐tumorigenic, negative regulation of Wnt/β‐catenin | [67] |
| Dickkopf‐1 (DKK‐1) | MM cells | Negative regulation of Wnt, development of disease into a bone lytic phase | [57, 68, 69] |
| ↑CD40/40L, VCAM-1, ICAM-1, LFA-3, HO-1, IL-6, VEGF, and ↓ IL-10 | MM cells/ endothelial cells | MM cell survival, disease progression, drug resistance, pro-angiogenic profile | [71, 72] |
| Promyelocytic leukemia protein (PML) protein | Different types of leukemic cells | CXCL1 and IL-6 production, leukemogenesis | [73] |
| IL-6 | CML cells | Shelters CML cells from imatinib induced apoptosis | [74] |
| IL-6 | Diffuse large B cell lymphoma | Chemoresistance, ↑IL-17A level | [75] |
| PDGF | CLL cells | Making an angiogenic switch, protective activities against cytotoxic effects of Forodesine | [76, 77] |
| SDF-1, BAFF, TGF-β | CLL cells | B-cell proliferation, IgG production | [78] |
| VEGF, HIF-1, HGF | CLL cells | Proangiogenic profile, CLL cell survival, resistance to rituximab/alemtuzumab | [71] |
Chemokines and bio active molecules
There are also some chemokines that are regulated by MSCs. First of all, through CXCL12-CXCR4 interaction between MSC and CML cells (respectively), imatinib-induced cell death is reduced as a consequence of attenuated caspase-3 activity [76]. Niches with CXCL12 devoid of MSCs, cannot support the LSCs from tyrosine kinase inhibitor (TKI) treatment, while CXCL12 + MSC niches offer a full guarantee for LSCs to maintain quiescent and TKI-resistant [83].
Secondly, CXCL8 derived from MSCs supports the survival and proliferation of AML cells through the PI3K/AKT pathway [84]. By the same token, via activation of NF-κB, MSC is involved in the residual disease maintenance in AML and on the other hand in therapy-resistance. This activation of NF-κB may be the consequence of interaction of VCAM-1 on MSC and its ligand, VLA-4, on leukemic cells [85]. Indeed, the underlying molecular mechanisms in BM niche by which the drug resistance and disease relapse are caused in AML include SDF-1/CXCL12, Wnt/β-catenin, VCAM/VLA-4/NF-κB, CD44, and hypoxia [86]. Axl is a member of the Tyro3 and has been approved of prognostic value and therapeutic target in AML that has been claimed as a mediator in the paracrine signaling between the leukemia cells and BM-MSCs. The expression of Axl ligand, growth arrest–specific gene 6 (Gas6), on MSCs can be elicited by AML cells.[87].
Other factors including Periostin (POSTN) is a multifunctional extracellular component. BM-MSC-derived POSTN promotes B-ALL cell-derived CCL2 which increases the leukemia burden [88]. Lumican (LUM) is an extracellular matrix protein secreted by MSCs. LSCs such as Nalm-6 (an ALL cell line) acquires anti-apoptotic properties and resistance to chemotherapy by downregulation of LUM expression in BM-MSCs [89].
Bone destruction in MM is mainly orchestrated by osteoclasts that undergo differentiation induced by the production of CCL3 and CCL4, matrix metalloproteinases (MMP)-13, IL-1, IL-3, IL-6 and IL-17 released by MSCs [72]. Conversely, AML cells shift the niche towards an osteoblastic one by the induction of connective tissue growth factor (CTGF) expression in BM-MSCs [90].
Co-cultured with MSCs, CML cells reduce caspase-3 activation and modulate Bcl-XL (anti-apoptotic protein) expression after treatment with imatinib which signify MSC-mediated protection of CML cells [91, 92]. This has been proven to be interceded with CXCR4/CXCL12; hence, combinational therapy with anti-CXCR4 antagonists and TKIs may represent a powerful approach in the treatment of CML [93].
Apart from intracellular signaling pathways, inhibiting the intercellular trafficking routes provides a promising therapeutic approach in leukemia. Using AMD3100 for example, the SDF-1α/CXCR4 axis is interrupted leading to hindrance of intercellular trafficking of CLL cells, and disturbance of microenvironment-mediated support [94]. AMD3100 is the first generation CXCR4 antagonist; therefore, it can inhibit proliferation of HSC and trafficking of leukocytes. However, BL8040 is the CXCR4 new generation inhibitor exhibiting higher affinity than AMD3100. As it has been presented in Table 4, chemokines and biomolecules interfere with malignant hematologic cells using different mechanisms.
Table 4.
MSCs-secreted chemokines/biomolecules and their impacts on the hematologic malignancies
| Kind of chemokine/biomolecule | Target | Results | References |
|---|---|---|---|
| CXCL12 | AML cells | Dampening effect on MSC-mediated resistance to FLT3 inhibition | [95] |
| CXCL12 | CML cells | ↓Imatinib-induced cell death | [76] |
| CXCL12 | LSCs of CML | Maintain quiescent of LSCs and TKI-resistant | [83] |
| Periostin | B-ALL cells | ↑B-ALL cell-derived CCL2, ↑ leukemia burden | [88] |
| Lumican | LSCs of Nalm-6 cell line | Downregulation of anti-apoptotic, resistance to chemotherapy | [89] |
| CCL3, CCL4, matrix metalloproteinases (MMP)-13, IL-1, IL-3, IL-6, and IL-17 | MM cells | Differentiation to osteoclast, bone destruction in MM | [72] |
| VCAM-1, SDF1, Wnt | AML cells | Residual disease maintenance, drug resistance and disease relapse | [85, 86] |
| Axl | AML cells | Prognostic factor and therapeutic target | [87] |
| SDF-1α/CXCR4 | CLL cells | Intercellular trafficking of CLL cells | [94] |
| CXCR4/CXCL12 | CML cells | ↓Caspase-3 activation, Bcl-XL expression modulation after treatment with imatinib | [93] |
Microvesicles and exosomes
Microvesicles (MVs) and exosomes shed from MSCs membrane [10] and affect on different cell processes. Cell viability, clonogenic capacity and miRNA and gene expression profile of CD34+ cells in patients with MDS were all modified after receiving MVs derived from MSCs [49].
On the other hand, BM-MSCs ability to support CD34+ cells declines, after getting affected by extracellular vesicles (EVs) containing miR-7977 derived from AML/MDS CD34+ cells. miR-150 EVs target the CXCR4/SDF-1 axis which is fundamental for retention and differentiation of HSPC in BM. Instructed by human primary MDS cells, normal donor MSCs (ND-MSCs) adopt MDS-desirable features such as high expression of proangiogenic factors (VEGFA, IGFs, and EGFs) and mediators of fibrosis (LOXL, TGF-β, and LIF) [49].
Exosomes can be defined as the small, extracellular vesicles carrying a variety of biologic molecules, including proteins, DNA, mRNA and non-coding RNA. These proteins include antigen presenting molecules, adhesion molecules, membrane transport and fusion molecules, cytoskeletal proteins, pyruvate kinase, histones and others [10].
For the first consideration, MSC-derived EVs in the kidney, neurological, cardiovascular and liver diseases are of precious value that influence disease trajectory, patient survival and treatment strategy [96]. Secondly, cell-fate determination in stem cells is an EV-mastered process [97]. Modified by EVs, cancer stem cells (CSCs) and normal HSCs can develop and differentiate to various hematologic malignancies. These EVs are secreted by MSCs reprogrammed by CSCs and the neoplastic cells [98]. EVs content also modifies CD34+ cells viability as well as colony forming unit-granulocyte monocyte (CFU-GM) production. Precisely, some microRNAs like miR-10a and miR-15a are overexpressed in EVs from MSCs of MDSs patients and transferred to CD34+ cells. Modifying the expression of MDM2 and P53 genes, these microRNAs augment cell viability and increase clonogenic capacity [99]. On Immune cells, they induce immunosuppression [100]. EVs from MSC promote both proliferation and apoptosis of regulatory T cells [101]. They decrease Th17 cells and increase regulatory T cells on the peripheral blood mononuclear cells [102].
Based on studies on K562 cells-derived exosomes, these EVs may directly stimulate the target cells or transfer receptors between cells. They may deliver functional proteins and transfer the genetic materials like mRNA, miRNA, or transcription factors to target cell [103]. Another CML cell line, LAMA84, generates EVs that have effects on the human vascular endothelial cells leading to ICAM-1, VCAM‐1, and IL‐8 expression upregulation which indeed shift the tumor microenvironment (TME) to a pro-angiogenic pattern and therefore unfavorable prognosis [104].
Enhancement of angiogenesis is mainly mediated by the well-known pro-angiogenic factors such as VEGF, basic fibroblast growth factor (bFGF), and angiopoietin-1 secreted by the MM cells or stromal cells interacting with MM cells. Osteoclast differentiation and osteoclast bone resorption activity in MM is modulated and supported by MM cell-derived exosomes containing osteoclast activating factors which in turn enhance MM cell growth and survival by secretion of IL-6 and B-cell-activating factor [105].
Generally speaking, escaping from spontaneous or drug-induced apoptosis, migrating in higher rate and modifying genes more suitably are the main results of transferring EVs from leukemia patient MSCs compared to EVs from healthy donor MSCs [106]. IL-6 and IL-8 inhibit hematopoiesis by downregulating the CXCL12, angiopoietin 1, and kit ligand. In hematologic malignancies, IL-6 and IL-8 are upregulated in MSCs and are delivered to microenvironment by EVs [71].
LAMA84-derived exosomes promote IL-8 secretion in the MSC cell line, HS5, leading to enhance the survival, proliferation, and migration of LAMA84 cells in vitro. [71]. Based on a study about K562 cell, K562 exosomal miR-711 has been credited for suppressed adhesion abilities of BM-MSCs because of the fact that miR-711 is capable of silencing CD44—an adhesion molecule‒expression in BM-MSCs [107].
Exosomes from BM-MSCs contain miR-222-3p which is responsible for interferon regulatory factor 2/inositol polyphosphate 4-phosphatase type II (IRF2/INPP4B) signaling inhibition and has been greatly observed in co-culture with AML cell line [108]. IRF2/INPP4B signaling is involved in autophagy and apoptosis [109]. Exosomes secreted by AML cells alter the behavior of MSCs [110]. AML cell resistance to TKIs is effectively guaranteed by TGF-β1, miR-155, and miR-375 rich exosomes released by BM-MSC. Similarly, exosomes rich in miR-150 can disrupt the CXCR4/CXCL12 axis; disruption of CXCR4/CXCL12 axis supports the leukemia growth. These EVs are derived from AML cells and destined to be taken up by BM-MSCs [111].
Another in vitro study clarify that exosomes from AML cell lines HEL 92.1.7, HL-60, MOLM-14, and U937 transfer mRNA of insulin-like growth factor 1 receptor (IGF1R), matrix metalloproteinase 9 (MMP-9), nuclear matrix protein 1 (NPM1), CXCR4, and internal tandem duplication mutations in FLT3 (FLT3-ITD) into BM-MSC [71].
By the same token, exosomes derived from MM-MSC aim to ensure disease progression in vivo by delivery of IL-6, CCL2, and fibronectin and by attenuating the expression of the tumor suppressor miR-15a [71].
Tax viral oncoprotein of human T-cell lymphotropic virus type I (HTLV-I) causes adult T-cell leukemia/lymphoma (ATL). BM-MSCs pick exosomes up from ATL cells containing the Tax oncoprotein and leading to reduced MSC stemness and improved angiogenesis due to the multiplied levels of VEGF, CXCR4, and MMP-9 [71]. IL-8 secretion is initiated by CML cell-derived exosomes and finally contributes to CML cell survival [112].
Mechanistically, it has been proposed that the content of tumor-suppressor miR-15a in MSC-EVs is determinative of the MSC communication with MM cells. Decreased miR-15a content in MM-MSCs induces tumor growth and promotes myeloma dissemination [113, 114]. Proliferation, cancer-associated fibroblast (CAF) transformation, and IL-6 secretion of MSCs increases in co-culture with MM cells and these have been partially guided by miR-21 and miR-146a delivered by MM cells [115]. Minimal residual disease (MRD) after treatment can be monitored by measuring the circulating EVs in MM. Remarkably, transforming from monoclonal gammopathy of undetermined significance into symptomatic myeloma can gain the advantage of predictability by identifying and measuring the circulating EVs [116].
Based on both in vitro and in vivo studies, the leukemia-surviving subpopulation of MSCs in CLL cells is created and developed following secretion of protein- and miRNA-containing exosomes by CLL cells [32]. MSCs from CLL patients support in vitro neoplastic B cell survival [117]
EVs would be brought highly on agenda considering the fact that tumor stage, risk of recurrence, drug resistance, and overall clinical outcome of patients correlate to a great extent with number, phenotype and the molecular content of EVs [116]. Table 5 indicates some of studies and their reports about MV-mediated communication of MSCs and malignant cells.
Table 5.
Interaction between MSCs and hematologic neoplasms by microvesicles transferring
| Type of malignancy/cell (source) | Target | Content | Results | References |
|---|---|---|---|---|
| MDS-MSCs | CD34+ cells | miR-10a and miR-15a | Modifying CD34+ cell viability, CFU-GM production, MDM2 and P53 genes expression | [99] |
| CML cell line LAMA84 | Human vascular endothelial cells | Different biomolecules | Upregulation of ICAM‐1, VCAM‐1 and IL‐8, pro-angiogenic pattern | [104] |
| MM | MSCs | Osteoclast activating factors | Osteoclast differentiation, osteoclast bone resorption activity | [105] |
| MM | MSCs | miR-21 and miR-146a | MM cell growth, survival and proliferation, CAF transformation, IL-6 secretion of MSCs | [115] |
| B-CLL | Leukemia B cells | CCL3/4, EGR1/2/3, and MYC | Escaping from spontaneous or drug-induced apoptosis, migrating in higher rate and modifying genes more suitably | [106] |
| MSCs | Microenvironment | IL-6 and IL-8 | Hematopoiesis inhibition by downregulating the CXCL12, angiopoietin 1, and kit ligand | [71] |
| MSC cell line HS5 | CML cell line LAMA84 | IL-8 | Survival, proliferation, migration | [71] |
| K562 | BM-MSCs | miR-711 | Suppressed adhesion abilities of BM-MSCs | [107] |
| MSCs | AML cell line | miR-222-3p | IRF2/INPP4B signaling inhibition | [108] |
| MSCs | AML cell line | TGF-β1, miR-155, and miR-375 | AML cell resistance to tyrosine kinase inhibitors | [111] |
| AML | MSCs | miR-150 | Disruption of the CXCR4/ CXCL12 axis | [111] |
| AML cell lines; HEL 92.1.7, HL-60, MOLM-14, and U937 | MSCs | mRNA of IGF1R, MMP-9, NPM1, CXCR4, FLT3 FLT3-ITD | Leukemia progression | [71] |
| MM-MSC | MM | IL-6, CCL2 Fibronectin | ↓ miR-15a content in MM-MSCs, induces tumor growth and promotes myeloma dissemination | [71, 113, 114] |
| ATL | MSCs | Tax viral oncoprotein of HTLV-I | ↓ MSC stemness and improved angiogenesis | [71] |
| CML | MSCs | IL-8 | IL-8 secretion, CML cell survival | [112] |
| CLL | MSCs | Protein and miRNA | Creation of leukemia-surviving subpopulation of MSCs | [117] |
Discussion
MSCs could communicate with malignant hematologic cells by different contact-dependent and/or contact-independent mechanisms. Notably in CML, TNTs facilitate exosomes transportation and exosomes in turn stimulate TNT formation; so, they can synergistically interact and multiply their communication [45]. However, having permeability to small molecules and macromolecules, Cx43 may provide a target for the cytoplasmic drug delivery [55]. Inhibition of oxidative phosphorylation (Oxphos) pathway in mitochondria also contributes to drug-resistance of AML based on the fact that TNT formation and mitochondrial transfer from BM-MSCs to AML is facilitated and promoted in this way [118]. In Jurkat cells, MSC-induced chemoresistance can be controlled by inhibition of mitochondrial transfer [36]. On the other hand, TNT formation is downregulated by NF-κB inhibitor BAY-117082 in AML [119]. Currently, BM-MSCs are found to be able to significantly enhance the drug resistance to various chemotherapy drugs, such as vincristine and cytarabine in ALL cells [120].
To clarify, in TME, MSCs-derived MVs can block the anti-tumor activity on immune cells and/or converts them into suppressor cells [111]. Another ascribed anti-tumor activity to MSCs is restoration of BM microenvironment via reprogramming the host macrophages [121]. Furthermore, MSC can inhibit the responses to alloreactive T lymphocytes as well as proliferation and cytotoxicity of natural killer (NK) cells [122].
Surprisingly, MSCs play an essential role for leukemia progression and chemoresistance by mitochondrial transfer, though the fate of transferred mitochondria in leukemic cells remains unclear. MSCs from patients with MDS and AML have a wide range of chromosomal aberrations, genetic and transcriptomic alterations. Deficiency of focal adhesion kinase (FAK) in MDS-MSCs correlates with ineffective hematopoiesis as it regulates the adhesion and mobility of cells [123].
It has been by the way proposed that the quiescence of AML blasts is ensured and outlasted in coculture with MSC resulting in increased leukemic survival in the presence of cytarabine [124]. Primary human AML cells remain proliferative for long-term by growth-enhancing effects of normal MSCs which is mediated by increased phosphorylation of the mammalian or mechanistic target of rapamycin (mTOR) and its downstream targets [125]. Diminished apoptosis is representative of tumor promoting effects of MSCs on MM cells and generally results from downregulation in caspase‐3 and poly (ADP‐ribose) polymerase expression which is associated with and mediated by enhanced AKT and ERK activities in MM cells [126].
Conclusions
In summary, bidirectional relationship between MSCs and hematologic malignancy-derived cells has different contact-dependent and contact-independent mechanisms. These cross-talks affect disease progression and outcome. The fate of malignant cells, drug resistance conditions, MRD status and other cellular processes are regulated by the MSC behavior. There are many studies conducted to understand the exact underlying mechanisms of MSCs and malignant hematologic cells communication. Their results could be applicable to design an improved treatment protocol and ameliorated patient’s survival. Hence, focus on this field and conducting additional studies or review with more confirmed information are emphatically suggested in this regard. We finally can infer that MSC does not behave similarly against different malignant hematologic cells and it basically extracted from the diverse responses and signals emitted from MSC in TME. It seems that the nursing role of MSCs in one hematologic neoplasm may be reversed in another by tumor progression and anti-apoptotic benefit.
Acknowledgements
This work was confirmed and founded by Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences, Ethic Code: IR.UMSHA.REC.1400.184.
Abbreviations
- MSCs
Mesenchymal stem/stromal cells
- BM-MSCs
Bone marrow-derived mesenchymal stromal cells
- TNTs
Tunneling nanotubes
- miR
MicroRNA
- MI
Myocardial infarction
- CLL
Chronic lymphocytic leukemia
- BMSCs
Bone marrow stromal cells
- SDS
Schwachman–Diamond syndrome
- DDR
DNA damage response
- B-ALL
B cell acute lymphoblastic leukemia
- BCP-ALL
B-cell precursor ALL
- ICAM1
Intercellular adhesion molecule 1
- IP-10
Interferon-γ-inducible protein 10
- MCP-1
Monocyte chemotactic protein-1
- T-ALL
T cell acute lymphoblastic leukemia
- GJs
Gap junctions
- ARA-C
Cytosine arabinoside
- ROS
Reactive oxygen species
- AML
Acute myelogenous leukemia
- NOX2
NADPH oxidase 2
- MM
Multiple myeloma
- CML
Chronic myeloid leukemia
- HCs
Hemichannels
- GJIC
GJ intercellular communication
- HSPCs
Hematopoietic stem/progenitor cells
- Cx
Connexin
- SDF-1α
Stromal cell-derived factor-1α
- PGE2
Prostaglandin E2
- VCAM-1
Vascular cell adhesion molecule 1
- BMP
Bone morphogenetic protein
- LSCs
Leukemia stem cells
- DKK‐1
Dickkopf‐1
- MM-MSCs
Multiple myeloma-derived mesenchymal stem cells
- G-MDSCs
Granulocytic-myeloid-derived suppressor cells
- ARG1
Arginase 1
- TNF-α
Tumor necrosis factor α
- PROK2
Prokineticin 2
- LFA-3
Lymphocyte function-associated antigen-3
- VEGF
Vascular endothelial growth factor
- DLBCL
Diffuse large B-cell lymphoma
- PDGF
Platelet-derived growth factor
- MDS-MSCs
MDS-derived mesenchymal stromal cells
- BAFF
B-cell activating factor
- TGF-β
Transforming growth factor β
- HIF-1
Hypoxia-inducible factor 1
- CM
Conditioned medium
- TKI
Tyrosine kinase inhibitor
- Gas6
Growth arrest–specific gene 6
- POSTN
Periostin
- LUM
Lumican
- MMP
Matrix metalloproteinase
- EVs
Extracellular vesicles
- ND-MSCs
Normal donor MSCs
- CSCs
Cancer stem cells
- CFU-GM
Colony forming unit-granulocyte monocyte
- TME
Tumor microenvironment
- bFGF
Basic fibroblast growth factor
- IRF2/INPP4B
Interferon regulatory factor 2/inositol polyphosphate 4-phosphatase type II
- IGF1R
Insulin-like growth factor 1 receptor
- FLT3-ITD
Internal tandem duplication mutations in FLT3
- HTLV-I
Human T-cell lymphotropic virus type I
- ATL
Adult T-cell leukemia/lymphoma
- CAF
Cancer-associated fibroblast
- MRD
Minimal residual disease
- FAK
Focal adhesion kinase
- Oxphos
Oxidative phosphorylation
- mTOR
Mammalian (mechanistic) target of rapamycin
Authors' contributions
MV and AS have participated to write the first manuscript. FA and AG have revised and completed some shortcomings to improve it. All authors read and approved the final manuscript.
Funding
This work was founded by the Hamadan University of Medical Sciences (Grant No. 140004012855).
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors contributed in this review clearly state that there is no conflict of interest either in authorship or financial benefits.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alireza Goodarzi, Email: a_goodarzi58@yahoo.com.
Mohsen Valikhani, Email: mohsenvalikhan@gmail.com.
Fatemeh Amiri, Email: amirif2012@gmail.com, Email: f.amiri@umsha.ac.ir.
Armita Safari, Email: armita.sf24@gmail.com.
References
- 1.Crippa S, Santi L, Bosotti R, Porro G, Bernardo ME. Bone marrow-derived mesenchymal stromal cells: a novel target to optimize hematopoietic stem cell transplantation protocols in hematological malignancies and rare genetic disorders. J Clin Med. 2020;9(1):2. doi: 10.3390/jcm9010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amiri F, Jahanian-Najafabadi A, Roudkenar MH. In vitro augmentation of mesenchymal stem cells viability in stressful microenvironments. Cell Stress Chaperone. 2015;20(2):237–251. doi: 10.1007/s12192-014-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahmatizadeh F, Aziz SG-G, Khodadadi K, Ataei ML, Ebrahimie E, Rad JS, et al. Bidirectional and opposite effects of naïve mesenchymal stem cells on tumor growth and progression. Adv Pharm Bull. 2019;9(4):539. doi: 10.15171/apb.2019.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terraza-Aguirre C, Campos-Mora M, Elizondo-Vega R, Contreras-López RA, Luz-Crawford P, Jorgensen C, et al. Mechanisms behind the immunoregulatory dialogue between mesenchymal stem cells and Th17 cells. Cells. 2020;9(7):1660. doi: 10.3390/cells9071660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33(9):994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figeac F, Lesault PF, Le Coz O, Damy T, Souktani R, Trébeau C, et al. Nanotubular crosstalk with distressed cardiomyocytes stimulates the paracrine repair function of mesenchymal stem cells. Stem Cells. 2014;32(1):216–230. doi: 10.1002/stem.1560. [DOI] [PubMed] [Google Scholar]
- 7.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinclair KA, Yerkovich ST, Hopkins PM-A, Chambers DC. Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung. Stem Cell Res Ther. 2016;7(1):91. doi: 10.1186/s13287-016-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125. doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asgarpour K, Shojaei Z, Amiri F, Ai J, Mahjoubin-Tehran M, Ghasemi F, et al. Exosomal microRNAs derived from mesenchymal stem cells: cell-to-cell messages. Cell Commun Signal. 2020;18(1):1–16. doi: 10.1186/s12964-020-00650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar P, Kandoi S, Misra R, Vijayalakshmi S, Rajagopal K, Verma RS. The mesenchymal stem cell secretome: a new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1–9. doi: 10.1016/j.cytogfr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Abbasi-Malati Z, Roushandeh AM, Kuwahara Y, Roudkenar MH. Mesenchymal stem cells on horizon: a new arsenal of therapeutic agents. Stem Cell Rev Rep. 2018;14(4):484–499. doi: 10.1007/s12015-018-9817-x. [DOI] [PubMed] [Google Scholar]
- 13.Amiri F, Molaei S, Bahadori M, Nasiri F, Deyhim MR, Jalili MA, et al. Autophagy-modulated human bone marrow-derived mesenchymal stem cells accelerate liver restoration in mouse models of acute liver failure. Iran Biomed J. 2016;20(3):135. doi: 10.7508/ibj.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amiri F, Halabian R, Salimian M, Shokrgozar MA, Soleimani M, Jahanian-Najafabadi A, et al. Induction of multipotency in umbilical cord-derived mesenchymal stem cells cultivated under suspension conditions. Cell Stress Chaperones. 2014;19(5):657–666. doi: 10.1007/s12192-014-0491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhaleh F, Amiri F, Mohammadzadeh-Vardin M, Bahadori M, Harati MD, Roudkenar MH, et al. Nuclear factor erythroid-2 related factor 2 overexpressed mesenchymal stem cells transplantation, improves renal function, decreases injuries markers and increases repair markers in glycerol-induced Acute kidney injury rats. Iran J Basic Med Sci. 2016;19(3):323. [PMC free article] [PubMed] [Google Scholar]
- 16.Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Mol Cancer. 2017;16(1):1–10. doi: 10.1186/s12943-017-0597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han J, Kim B, Shin J-Y, Ryu S, Noh M, Woo J, et al. Iron oxide nanoparticle-mediated development of cellular gap junction crosstalk to improve mesenchymal stem cells’ therapeutic efficacy for myocardial infarction. ACS Nano. 2015;9(3):2805–2819. doi: 10.1021/nn506732n. [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Zhang G, Wang Y, Liu T, Zhang Y, An Y, et al. Crosstalk of mesenchymal stem cells and macrophages promotes cardiac muscle repair. Int J Biochem Cell Biol. 2015;58:53–61. doi: 10.1016/j.biocel.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Bergfeld SA, DeClerck YA. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev. 2010;29(2):249–261. doi: 10.1007/s10555-010-9222-7. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed ES, Ahmed NH, Medhat AM, Said UZ, Rashed LA, Abdel Ghaffar ARB. Mesenchymal stem cells targeting PI3K/AKT pathway in leukemic model. Tumour Biol. 2019;41(4):1010428319846803. doi: 10.1177/1010428319846803. [DOI] [PubMed] [Google Scholar]
- 21.Harati MD, Amiri F, Jaleh F, Mehdipour A, Harati MD, Molaee S, et al. Targeting delivery of lipocalin 2-engineered mesenchymal stem cells to colon cancer in order to inhibit liver metastasis in nude mice. Tumour Biol. 2015;36(8):6011–6018. doi: 10.1007/s13277-015-3277-6. [DOI] [PubMed] [Google Scholar]
- 22.Behrmann L, Wellbrock J, Fiedler W. The bone marrow stromal niche: a therapeutic target of hematological myeloid malignancies. Expert Opin Ther Targets. 2020;24(5):451–462. doi: 10.1080/14728222.2020.1744850. [DOI] [PubMed] [Google Scholar]
- 23.Ciciarello M, Corradi G, Loscocco F, Visani G, Monaco F, Curti A, et al. The Yin and Yang of the bone marrow microenvironment: pros and cons of mesenchymal stromal cells in acute myeloid leukemia. Front Oncol. 2019;9:1135. doi: 10.3389/fonc.2019.01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Gomez A, De Las RJ, Ocio EM, Díaz-Rodríguez E, Montero JC, Martín M, et al. Transcriptomic profile induced in bone marrow mesenchymal stromal cells after interaction with multiple myeloma cells: implications in myeloma progression and myeloma bone disease. Oncotarget. 2014;5(18):8284. doi: 10.18632/oncotarget.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Low JH, Ramdas P, Radhakrishnan AK. Modulatory effects of mesenchymal stem cells on leucocytes and leukemic cells: a double-edged sword? Blood Cells Mol Dis. 2015;55(4):351–357. doi: 10.1016/j.bcmd.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Ten Hacken E, Burger JA. Microenvironment interactions and B-cell receptor signaling in chronic lymphocytic leukemia: implications for disease pathogenesis and treatment. Biochim Biophys Acta. 2016;1863(3):401–413. doi: 10.1016/j.bbamcr.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zambetti NA, Ping Z, Chen S, Kenswil KJ, Mylona MA, Sanders MA, et al. Mesenchymal inflammation drives genotoxic stress in hematopoietic stem cells and predicts disease evolution in human pre-leukemia. Cell Stem Cell. 2016;19(5):613–627. doi: 10.1016/j.stem.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Gerdes H-H, Rustom A, Wang X. Tunneling nanotubes, an emerging intercellular communication route in development. Mech Dev. 2013;130(6–8):381–387. doi: 10.1016/j.mod.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Pasquier J, Guerrouahen BS, Al Thawadi H, Ghiabi P, Maleki M, Abu-Kaoud N, et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med. 2013;11(1):1–14. doi: 10.1186/1479-5876-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinto G, Brou C, Zurzolo CJ. Tunneling nanotubes: the fuel of tumor progression? Trends Cancer. 2020;6:874–888. doi: 10.1016/j.trecan.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Kolba MD, Dudka W, Zaręba-Kozioł M, Kominek A, Ronchi P, Turos L, et al. Tunneling nanotube-mediated intercellular vesicle and protein transfer in the stroma-provided imatinib resistance in chronic myeloid leukemia cells. Cell Death Dis. 2019;10(11):1–16. doi: 10.1038/s41419-019-2045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griessinger E, Moschoi R, Biondani G, Peyron J-F. Mitochondrial transfer in the leukemia microenvironment. Trends Cancer. 2017;3(12):828–839. doi: 10.1016/j.trecan.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Matula Z, Németh A, Lőrincz P, Szepesi A, Brózik A, Buzás EI, et al. The role of extracellular vesicle and tunneling nanotube-mediated intercellular cross-talk between mesenchymal stem cells and human peripheral T cells. Stem Cell Dev. 2016;25(23):1818–1832. doi: 10.1089/scd.2016.0086. [DOI] [PubMed] [Google Scholar]
- 34.de Rooij B, Polak R, Stalpers F, Pieters R, Den Boer M. Tunneling nanotubes facilitate autophagosome transfer in the leukemic niche. Leukemia. 2017;31(7):1651–1654. doi: 10.1038/leu.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polak R, de Rooij B, Pieters R, den Boer ML. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood. 2015;126(21):2404–2414. doi: 10.1182/blood-2015-03-634238. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Liu X, Qiu Y, Shi Y, Cai J, Wang B, et al. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J Hematol Oncol. 2018;11(1):11. doi: 10.1186/s13045-018-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burt R, Dey A, Aref S, Aguiar M, Akarca A, Bailey K, et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood. 2019;134(17):1415–1429. doi: 10.1182/blood.2019001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar R, Godavarthy PS, Krause DS. The bone marrow microenvironment in health and disease at a glance. J Cell Sci. 2018;131(4):jcs201707. doi: 10.1242/jcs.201707. [DOI] [PubMed] [Google Scholar]
- 39.Forte D, Krause DS, Andreeff M, Bonnet D, Méndez-Ferrer S. Updates on the hematologic tumor microenvironment and its therapeutic targeting. Haematologica. 2019;104(10):1928–1934. doi: 10.3324/haematol.2018.195396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moschoi R, Imbert V, Nebout M, Chiche J, Mary D, Prebet T, et al. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood. 2016;128(2):253–264. doi: 10.1182/blood-2015-07-655860. [DOI] [PubMed] [Google Scholar]
- 41.Marlein C. Intercellular mitochondrial transfer in the bone marrow microenvironment of acute myeloid leukaemia and multiple myeloma. Norwich: University of East Anglia; 2018. [Google Scholar]
- 42.Marlein CR, Zaitseva L, Piddock RE, Robinson SD, Edwards DR, Shafat MS, et al. NADPH oxidase-2 derived superoxide drives mitochondrial transfer from bone marrow stromal cells to leukemic blasts. Blood. 2017;130(14):1649–1660. doi: 10.1182/blood-2017-03-772939. [DOI] [PubMed] [Google Scholar]
- 43.Omsland M, Andresen V, Gullaksen SE, Ayuda-Durán P, Popa M, Hovland R, et al. Tyrosine kinase inhibitors and interferon-α increase tunneling nanotube (TNT) formation and cell adhesion in chronic myeloid leukemia (CML) cell lines. FASEB J. 2020;34:3773–3791. doi: 10.1096/fj.201802061RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolba MD, Dudka W, Zaręba-Kozioł M, Kominek A, Ronchi P, Turos L, et al. Tunneling nanotubes contribute to the stroma-mediated imatinib resistance of leukemic cells. bioRxiv. 2018;10. [DOI] [PMC free article] [PubMed]
- 45.Lou E, Sperduto W, Subramanian S. Exosomes and Tunneling Nanotube Conduits: Synergistic Interaction That Facilitates Intercellular Communication Between Malignant and Stromal Cells in the Tumor Microenvironment. Diagnostic and Therapeutic Applications of Exosomes in Cancer. Amsterdam: Elsevier; 2018. pp. 219–234. [Google Scholar]
- 46.Mangolini M, Ringshausen IJ. Bone marrow stromal cells drive key hallmarks of B cell malignancies. Int J Mol Sci. 2020;21(4):1466. doi: 10.3390/ijms21041466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Rooij B, Polak R, van den Berk LC, Stalpers F, Pieters R, den Boer ML. Acute lymphoblastic leukemia cells create a leukemic niche without affecting the CXCR4/CXCL12 axis. Haemetologica. 2017;102(10):e389. doi: 10.3324/haematol.2016.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beneforti L, Dander E, Bresolin S, Bueno C, Acunzo D, Bertagna M, et al. Pro-inflammatory cytokines favor the emergence of ETV6-RUNX1-positive pre-leukemic cells in a model of mesenchymal niche. Br J Haematol. 2020;190(2):262–273. doi: 10.1111/bjh.16523. [DOI] [PubMed] [Google Scholar]
- 49.Medyouf H, Mossner M, Jann J-C, Nolte F, Raffel S, Herrmann C, et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell. 2014;14(6):824–837. doi: 10.1016/j.stem.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 50.Singh AK, Cancelas JA. Gap junctions in the bone marrow lympho-hematopoietic stem cell niche, leukemia progression, and chemoresistance. Int J Mol Sci. 2020;21(3):796. doi: 10.3390/ijms21030796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Sun Y, Wang Z, Huang Z, Li B, Fu J. Up-regulation of connexin-43 expression in bone marrow mesenchymal stem cells plays a crucial role in adhesion and migration of multiple myeloma cells. Leuk Lymphoma. 2015;56(1):211–218. doi: 10.3109/10428194.2014.913289. [DOI] [PubMed] [Google Scholar]
- 52.Weber MC, Tykocinski ML. Bone marrow stromal cell blockade of human leukemic cell differentiation. Blood. 1994;83(8):2221–2229. [PubMed] [Google Scholar]
- 53.Kouzi F, Zibara K, Bourgeais J, Picou F, Gallay N, Brossaud J, et al. Correction: Disruption of gap junctions attenuates acute myeloid leukemia chemoresistance induced by bone marrow mesenchymal stromal cells. Oncogene. 2020;39(10):2227. doi: 10.1038/s41388-019-1089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valdebenito S, Lou E, Baldoni J, Okafo G, Eugenin E. The novel roles of connexin channels and tunneling nanotubes in cancer pathogenesis. Int J Mol Sci. 2018;19(5):1270. doi: 10.3390/ijms19051270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonacquisti EE, Nguyen J. Connexin 43 (Cx43) in cancer: implications for therapeutic approaches via gap junctions. Cancer Lett. 2019;442:439–444. doi: 10.1016/j.canlet.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 56.Foss B, Tronstad KJ, Bruserud Ø. Connexin-based signaling in acute myelogenous leukemia (AML) Biochim Biophys Acta. 2010;1798(1):1–8. doi: 10.1016/j.bbamem.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 57.Fu J. Cx43 expressed on bone marrow stromal cells plays an essential role in multiple myeloma cell survival and drug resistance. Arch Med Sci. 2017;13(1):236. doi: 10.5114/aoms.2017.64722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aqmasheh S. Effects of mesenchymal stem cell derivatives on hematopoiesis and hematopoietic stem cells. Adv Pharma Bull. 2017;7(2):165. doi: 10.15171/apb.2017.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khodadi E, Shahrabi S, Shahjahani M, Azandeh S, Saki N. Role of stem cell factor in the placental niche. Cell Tissue Res. 2016;366(3):523–531. doi: 10.1007/s00441-016-2429-3. [DOI] [PubMed] [Google Scholar]
- 60.Farahbakhshian E, Verstegen MM, Visser TP, Kheradmandkia S, Geerts D, Arshad S, et al. Angiopoietin-like protein 3 promotes preservation of stemness during ex vivo expansion of murine hematopoietic stem cells. PLoS ONE. 2014;9(8):e105642. doi: 10.1371/journal.pone.0105642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oubari F, Amirizade N, Mohammadpour H, Nakhlestani M, Zarif MN. The important role of FLT3-L in ex vivo expansion of hematopoietic stem cells following co-culture with mesenchymal stem cells. Cell J (Yakhteh) 2015;17(2):201. doi: 10.22074/cellj.2016.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richter J, Traver D, Willert K. The role of Wnt signaling in hematopoietic stem cell development. Crit Rev Biochem Mol Biol. 2017;52(4):414–424. doi: 10.1080/10409238.2017.1325828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang J, Nguyen-McCarty M, Hexner EO, Danet-Desnoyers G, Klein PS. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat Med. 2012;18(12):1778–1785. doi: 10.1038/nm.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duchartre Y, Kim Y-M, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141–149. doi: 10.1016/j.critrevonc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rees WD, Sly LM, Steiner TS. How do immune and mesenchymal cells influence the intestinal epithelial cell compartment in inflammatory bowel disease? Let's crosstalk about it! J Leukoc Biol. 2020;108:309–321. doi: 10.1002/JLB.3MIR0120-567R. [DOI] [PubMed] [Google Scholar]
- 66.Saki N, Abroun S, Hagh MF, Asgharei F. Neoplastic bone marrow niche: hematopoietic and mesenchymal stem cells. Cell J (Yakhteh) 2011;13(3):131. [PMC free article] [PubMed] [Google Scholar]
- 67.Melzer C, von der Ohe J, Hass R. Concise review: crosstalk of mesenchymal stroma/stem-like cells with cancer cells provides therapeutic potential. Stem Cells. 2018;36(7):951–968. doi: 10.1002/stem.2829. [DOI] [PubMed] [Google Scholar]
- 68.Shojaei S, Hashemi SM, Ghanbarian H, Salehi M, Mohammadi-Yeganeh S. Effect of mesenchymal stem cells-derived exosomes on tumor microenvironment: tumor progression versus tumor suppression. J Cell Physiol. 2019;234(4):3394–3409. doi: 10.1002/jcp.27326. [DOI] [PubMed] [Google Scholar]
- 69.Spath C, Schlegel F, Leontyev S, Mohr FW, Dhein S. Inverse relationship between tumor proliferation markers and connexin expression in a malignant cardiac tumor originating from mesenchymal stem cell engineered tissue in a rat in vivo model. Front Pharmacol. 2013;4:42. doi: 10.3389/fphar.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giallongo C, Tibullo D, Parrinello NL, La Cava P, Di Rosa M, Bramanti V, et al. Granulocyte-like myeloid derived suppressor cells (G-MDSC) are increased in multiple myeloma and are driven by dysfunctional mesenchymal stem cells (MSC) Oncotarget. 2016;7(52):85764. doi: 10.18632/oncotarget.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cominal JG, da Costa CM, Pinto-Simões B, Kolb H-J, Malmegrim KCR, de Castro FA. Emerging role of mesenchymal stromal cell-derived extracellular vesicles in pathogenesis of haematological malignancies. Stem Cells Int. 2019;2019:1–12. doi: 10.1155/2019/6854080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu S, De Veirman K, De Becker A, Vanderkerken K, Van Riet I. Mesenchymal stem cells in multiple myeloma: a therapeutical tool or target? Leukemia. 2018;32(7):1500–1514. doi: 10.1038/s41375-018-0061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Alvarenga EC, Silva WN, Vasconcellos R, Paredes-Gamero EJ, Mintz A, Birbrair A. Promyelocytic leukemia protein in mesenchymal stem cells is essential for leukemia progression. Ann Hematol. 2018;97(10):1749–1755. doi: 10.1007/s00277-018-3463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar A, Anand T, Bhattacharyya J, Sharma A, Jaganathan BG. K562 chronic myeloid leukemia cells modify osteogenic differentiation and gene expression of bone marrow stromal cells. J Cell Commun Signal. 2018;12(2):441–450. doi: 10.1007/s12079-017-0412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sircar A, Chowdhury SM, Hart A, Bell WC, Singh S, Sehgal L, et al. Impact and Intricacies of bone marrow microenvironment in B-cell lymphomas: from biology to therapy. Int J Mol Sci. 2020;21(3):904. doi: 10.3390/ijms21030904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Timaner M, Tsai KK, Shaked Y. The multifaceted role of mesenchymal stem cells in cancer. Semin Cancer Biol. 2020;60:225–237. doi: 10.1016/j.semcancer.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Audrito V, Vaisitti T, Serra S, Bologna C, Brusa D, Malavasi F, et al. Targeting the microenvironment in chronic lymphocytic leukemia offers novel therapeutic options. Cancer Lett. 2013;328(1):27–35. doi: 10.1016/j.canlet.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 78.Barcellos-de-Souza P, Gori V, Bambi F, Chiarugi P. Tumor microenvironment: bone marrow-mesenchymal stem cells as key players. Biochim Biophys Acta. 2013;1836(2):321–335. doi: 10.1016/j.bbcan.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 79.Ding W, Knox TR, Tschumper RC, Wu W, Schwager SM, Boysen JC, et al. Platelet-derived growth factor (PDGF)–PDGF receptor interaction activates bone marrow–derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood. 2010;116(16):2984–2993. doi: 10.1182/blood-2010-02-269894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giannoni P, Scaglione S, Quarto R, Narcisi R, Parodi M, Balleari E, et al. An interaction between hepatocyte growth factor and its receptor (c-MET) prolongs the survival of chronic lymphocytic leukemic cells through STAT3 phosphorylation: a potential role of mesenchymal cells in the disease. Haematologica. 2011;96(7):1015–1023. doi: 10.3324/haematol.2010.029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma M, Ross C, Srivastava S. Ally to adversary: mesenchymal stem cells and their transformation in leukaemia. Cancer Cell Int. 2019;19(1):1–9. doi: 10.1186/s12935-019-0855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee G-Y, Jeong S-Y, Lee H-R, Oh I-H. Age-related differences in the bone marrow stem cell niche generate specialized microenvironments for the distinct regulation of normal hematopoietic and leukemia stem cells. Sci Rep. 2019;9(1):1–12. doi: 10.1038/s41598-018-36999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Agarwal P, Isringhausen S, Li H, Paterson AJ, He J, Gomariz Á, et al. Mesenchymal niche-specific expression of Cxcl12 controls quiescence of treatment-resistant leukemia stem cells. Cell Stem Cell. 2019;24(5):769–784.e6. doi: 10.1016/j.stem.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng J, Li Y, Liu S, Jiang Y, Ma J, Wan L, et al. CXCL8 derived from mesenchymal stromal cells supports survival and proliferation of acute myeloid leukemia cells through the PI3K/AKT pathway. FASEB J. 2019;33(4):4755–4764. doi: 10.1096/fj.201801931R. [DOI] [PubMed] [Google Scholar]
- 85.Sharma M, Ross C, Srivastava S. Ally to adversary: mesenchymal stem cells and their transformation in leukaemia. Cancer Cell Int. 2019;19(1):139. doi: 10.1186/s12935-019-0855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou H-S, Carter BZ, Andreeff M. Bone marrow niche-mediated survival of leukemia stem cells in acute myeloid leukemia: Yin and Yang. Cancer Biol Med. 2016;13(2):248. doi: 10.20892/j.issn.2095-3941.2016.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ben-Batalla I, Schultze A, Wroblewski M, Erdmann R, Heuser M, Waizenegger JS, et al. Axl, a prognostic and therapeutic target in acute myeloid leukemia mediates paracrine crosstalk of leukemia cells with bone marrow stroma. Blood. 2013;122(14):2443–2452. doi: 10.1182/blood-2013-03-491431. [DOI] [PubMed] [Google Scholar]
- 88.Ma Z, Zhao X, Deng M, Huang Z, Wang J, Wu Y, et al. Bone marrow mesenchymal stromal cell-derived periostin promotes B-ALL progression by modulating CCL2 in leukemia cells. Cell Rep. 2019;26(6):1533–1543. doi: 10.1016/j.celrep.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 89.Yu Z, Liu L, Shu Q, Li D, Wang R. Leukemia stem cells promote chemoresistance by inducing downregulation of lumican in mesenchymal stem cells. Oncol Lett. 2019;18(4):4317–4327. doi: 10.3892/ol.2019.10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Battula VL, Le PM, Sun JC, Nguyen K, Yuan B, Zhou X, et al. AML-induced osteogenic differentiation in mesenchymal stromal cells supports leukemia growth. JCI Insight. 2017;2(13): e90036. [DOI] [PMC free article] [PubMed]
- 91.Lee MW, Ryu S, Kim DS, Lee JW, Sung KW, Koo HH, et al. Mesenchymal stem cells in suppression or progression of hematologic malignancy: current status and challenges. Leukemia. 2019;33(3):597–611. doi: 10.1038/s41375-018-0373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liang W, Chen X, Zhang S, Fang J, Chen M, Xu Y, et al. Mesenchymal stem cells as a double-edged sword in tumor growth: focusing on MSC-derived cytokines. Cell Mol Biol Lett. 2021;26(1):1–25. doi: 10.1186/s11658-020-00246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vianello F, Villanova F, Tisato V, Lymperi S, Ho K-K, Gomes AR, et al. Bone marrow mesenchymal stromal cells non-selectively protect chronic myeloid leukemia cells from imatinib-induced apoptosis via the CXCR4/CXCL12 axis. Haematologica. 2010;95(7):1081–1089. doi: 10.3324/haematol.2009.017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stamatopoulos B, Meuleman N, De Bruyn C, Pieters K, Mineur P, Le Roy C, et al. AMD3100 disrupts the cross-talk between chronic lymphocytic leukemia cells and a mesenchymal stromal or nurse-like cell-based microenvironment: pre-clinical evidence for its association with chronic lymphocytic leukemia treatments. Haematologica. 2012;97(4):608–615. doi: 10.3324/haematol.2011.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kojima K, McQueen T, Chen Y, Jacamo R, Konopleva M, Shinojima N, et al. p53 activation of mesenchymal stromal cells partially abrogates microenvironment-mediated resistance to FLT3 inhibition in AML through HIF-1α–mediated down-regulation of CXCL12. Blood. 2011;118(16):4431–4439. doi: 10.1182/blood-2011-02-334136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23(5):812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nawaz M, Fatima F, Vallabhaneni KC, Penfornis P, Valadi H, Ekström K, et al. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016;2016:1–17. doi: 10.1155/2016/1073140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laurenzana I, Lamorte D, Trino S, De Luca L, Ambrosino C, Zoppoli P, et al. Extracellular vesicles: a new prospective in crosstalk between microenvironment and stem cells in hematological malignancies. Stem Cells Int. 2018;2018:1–11. doi: 10.1155/2018/9863194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muntión S, Ramos TL, Diez-Campelo M, Rosón B, Sánchez-Abarca LI, Misiewicz-Krzeminska I, et al. Microvesicles from mesenchymal stromal cells are involved in HPC-microenvironment crosstalk in myelodysplastic patients. PLoS ONE. 2016;11(2):e0146722. doi: 10.1371/journal.pone.0146722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kordelas L, Rebmann V, Ludwig A, Radtke S, Ruesing J, Doeppner T, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 101.Del Fattore A, Luciano R, Pascucci L, Goffredo BM, Giorda E, Scapaticci M, et al. Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant. 2015;24(12):2615–2627. doi: 10.3727/096368915X687543. [DOI] [PubMed] [Google Scholar]
- 102.Favaro E, Carpanetto A, Lamorte S, Fusco A, Caorsi C, Deregibus MC, et al. Human mesenchymal stem cell-derived microvesicles modulate T cell response to islet antigen glutamic acid decarboxylase in patients with type 1 diabetes. Diabetologia. 2014;57(8):1664–1673. doi: 10.1007/s00125-014-3262-4. [DOI] [PubMed] [Google Scholar]
- 103.Mineo M, Garfield SH, Taverna S, Flugy A, De Leo G, Alessandro R, et al. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis. 2012;15(1):33–45. doi: 10.1007/s10456-011-9241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taverna S, Flugy A, Saieva L, Kohn EC, Santoro A, Meraviglia S, et al. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int J Cancer. 2012;130(9):2033–2043. doi: 10.1002/ijc.26217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang J, Faict S, Maes K, De Bruyne E, Van Valckenborgh E, Schots R, et al. Extracellular vesicle cross-talk in the bone marrow microenvironment: implications in multiple myeloma. Oncotarget. 2016;7(25):38927. doi: 10.18632/oncotarget.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Crompot E, Van Damme M, Pieters K, Vermeersch M, Perez-Morga D, Mineur P, et al. Extracellular vesicles of bone marrow stromal cells rescue chronic lymphocytic leukemia B cells from apoptosis, enhance their migration and induce gene expression modifications. Haematologica. 2017;102(9):1594–1604. doi: 10.3324/haematol.2016.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang Y-H, Liu J, Lin J, Li S-Q, Xu Y-M, Min Q-H, et al. K562 cell-derived exosomes suppress the adhesive function of bone marrow mesenchymal stem cells via delivery of miR-711. Biochem Biophys Res Commun. 2020;521(3):584–589. doi: 10.1016/j.bbrc.2019.10.096. [DOI] [PubMed] [Google Scholar]
- 108.Zhang F, Lu Y, Wang M, Zhu J, Li J, Zhang P, et al. Exosomes derived from human bone marrow mesenchymal stem cells transfer miR-222-3p to suppress acute myeloid leukemia cell proliferation by targeting IRF2/INPP4B. Mol Cell Probes. 2020;51:101513. doi: 10.1016/j.mcp.2020.101513. [DOI] [PubMed] [Google Scholar]
- 109.Zhang F, Li J, Zhu J, Liu L, Zhu K, Cheng S, et al. IRF2–INPP4B-mediated autophagy suppresses apoptosis in acute myeloid leukemia cells. Biol Res. 2019;52(1):11. doi: 10.1186/s40659-019-0218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gruszka AM, Valli D, Restelli C, Alcalay M. Adhesion deregulation in acute myeloid leukaemia. Cells. 2019;8(1):66. doi: 10.3390/cells8010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Whiteside TL. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin Immunol. 2018;35:69–79. doi: 10.1016/j.smim.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dostert G, Mesure B, Menu P, Velot É. How do mesenchymal stem cells influence or are influenced by microenvironment through extracellular vesicles communication? Front Cell Dev Biol. 2017;5:6. doi: 10.3389/fcell.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lopatina T, Gai C, Deregibus MC, Kholia S, Camussi G. Cross talk between cancer and mesenchymal stem cells through extracellular vesicles carrying nucleic acids. Front Oncol. 2016;6:125. doi: 10.3389/fonc.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roccaro AM, Sacco A, Maiso P, Azab AK, Tai Y-T, Reagan M, et al. BM mesenchymal stromal cell–derived exosomes facilitate multiple myeloma progression. J Clin Investig. 2013;123(4):1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheng Q, Li X, Liu J, Ye Q, Chen Y, Tan S, et al. Multiple myeloma-derived exosomes regulate the functions of mesenchymal stem cells partially via modulating miR-21 and miR-146a. Stem Cells Int. 2017;2017:1–9. doi: 10.1155/2017/9012152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.De Luca L, Laurenzana I, Trino S, Lamorte D, Caivano A, Musto P. An update on extracellular vesicles in multiple myeloma: a focus on their role in cell-to-cell cross-talk and as potential liquid biopsy biomarkers. Expert Rev Mol Diagn. 2019;19(3):249–258. doi: 10.1080/14737159.2019.1583103. [DOI] [PubMed] [Google Scholar]
- 117.Trimarco V, Ave E, Facco M, Chiodin G, Frezzato F, Martini V, et al. Cross-talk between chronic lymphocytic leukemia (CLL) tumor B cells and mesenchymal stromal cells (MSCs): implications for neoplastic cell survival. Oncotarget. 2015;6(39):42130. doi: 10.18632/oncotarget.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang H, Tabe Y, Saito K, Yamatani K, Jacamo R, Ma H, et al. Oxphos inhibition induces formation of tunneling nanotubes in AML cells and facilitates mitochondrial transfer from BM stroma to AML that contributes to microenvironment-mediated drug-resistance of AML. Washington, DC: American Society of Hematology; 2019. [Google Scholar]
- 119.Omsland M, Bruserud Ø, Gjertsen BT, Andresen V. Tunneling nanotube (TNT) formation is downregulated by cytarabine and NF-κB inhibition in acute myeloid leukemia (AML) Oncotarget. 2017;8(5):7946. doi: 10.18632/oncotarget.13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Y, Hu K, Hu Y, Liu L, Wang B, Huang H. Bone marrow mesenchymal stromal cells affect the cell cycle arrest effect of genotoxic agents on acute lymphocytic leukemia cells via p21 down-regulation. Ann Hematol. 2014;93(9):1499–1508. doi: 10.1007/s00277-014-2069-1. [DOI] [PubMed] [Google Scholar]
- 121.Xia C, Wang T, Cheng H, Dong Y, Weng Q, Sun G, et al. Mesenchymal stem cells suppress leukemia via macrophage-mediated functional restoration of bone marrow microenvironment. Leukemia. 2020;34:1–9. doi: 10.1038/s41375-020-0775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jackson MV, Krasnodembskaya AD. Analysis of mitochondrial transfer in direct co-cultures of human monocyte-derived macrophages (MDM) and mesenchymal stem cells (MSC). Bio Protoc. 2017;7(9): e2255. [DOI] [PMC free article] [PubMed]
- 123.Wu Y, Campos L, Daguenet E, He Z, Picot T, Tavernier-Tardy E, et al. FAK deficiency in bone marrow stromal cells alters their homeostasis and drives abnormal proliferation and differentiation of haematopoietic stem cells. Cells. 2020;9(3):646. doi: 10.3390/cells9030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ito S, Barrett AJ, Dutra A, Pak E, Miner S, Keyvanfar K, et al. Long term maintenance of myeloid leukemic stem cells cultured with unrelated human mesenchymal stromal cells. Stem Cell Res. 2015;14(1):95–104. doi: 10.1016/j.scr.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brenner AK, Nepstad I, Bruserud Ø. Mesenchymal stem cells support survival and proliferation of primary human acute myeloid leukemia cells through heterogeneous molecular mechanisms. Front Immunol. 2017;8:106. doi: 10.3389/fimmu.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu S, Menu E, Becker AD, Van Camp B, Vanderkerken K, Van Riet I. Bone marrow-derived mesenchymal stromal cells are attracted by multiple myeloma cell-produced chemokine CCL25 and favor myeloma cell growth in vitro and in vivo. Stem Cells. 2012;30(2):266–279. doi: 10.1002/stem.787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.