In this Journal, Akpolat and Uzun reported data indicating reduced mortality after CoronaVac vaccination (1). Akpolat and Uzun compared the vaccine effectiveness of CoronaVac by retrospectively inspecting the number of confirmed cases and deaths among health workers before and during the vaccination period. Our work, however, compared vaccine effectiveness prospectively from the perspective of immunological responses. We assessed the serological protection conferred by CoronaVac and BNT162b2 in terms of neutralizing antibody titers and IgG level using neutralization tests and an immunosorbent assay.
To compare the vaccine protection efficacy of BNT162b2 and CoronaVac, we used three different methods: live SARS-CoV-2 viruses, vesicular stomatitis virus (VSV) pseudoparticle virus expressing SARS-CoV-2 Spike protein, and ELISA for detecting IgG for Spike (S) and Nucleocapsid (N) proteins. Current published studies that evaluate the seroprotection of common vaccines did not include all of the variants we tested, nor did they include all the methods we employed (2, 3, 4, 5, 6, 7, 8, 9, 10). Most of these studies included only microneutralization or ELISA assays. Furthermore, none provided a comprehensive comparison of the efficacies between mRNA vaccine and inactivated virus vaccine. Therefore, we provide here the most exhaustive and is the only one to include all of Alpha, Beta, Gamma, and Delta variants.
We collected 56 sera from 27 BNT162b2 vaccinees, 26 CoronaVac vaccinees, and 3 unvaccinated individuals on May 18, 2021. All sera from the vaccinated participants were collected at least 21 days after receiving the second dose regardless of which vaccine they had taken. Twenty-eight (50%) of the 56 participants were men (Table 1 ). Twenty people (36%) were aged 18–44, 34 (61%) were aged 45–64, and 2 (4%) were over 65. (Table 1). The mean age was 47.9 (SD 11.4). Forty-five (80%) people had no medical conditions. Sera were collected at mean of 39 days after the second dose (range 21–76 days).
Table 1.
Demographics table of the 56 patients recruited in this study. Percentages of patients n from each category (i.e., each row) within each age group N (i.e., each column) were calculated and reported in the parentheses.
| Demographic variables | Age 18 – 44N=20 | Age 45 – 64N=34 | Age ≥ 65N=2 | Total N = 56 |
|---|---|---|---|---|
| Male | 12 (60%) | 14 (41%) | 2 (100%) | 28 (50%) |
| Medical history | ||||
| No medical history | 17 (85%) | 28 (82%) | 0 (0%) | 45 (80%) |
| Asthma | 1 (5%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Arthritis | 0 (0%) | 2 (6%) | 1 (50%) | 3 (5%) |
| Heart disease | 0 (0%) | 1 (3%) | 0 (0%) | 1 (2%) |
| Hypertension | 1 (5%) | 2 (6%) | 0 (0%) | 3 (5%) |
| Other | 1 (5%) | 1 (3%) | 1 (50%) | 3 (5%) |
| Type of vaccine received | ||||
| BNT162b2 | 10 (50%) | 15 (44%) | 1 (50%) | 26 (46%) |
| CoronaVac | 7 (35%) | 19 (56%) | 1 (50%) | 27 (48%) |
| Unvaccinated | 3 (15%) | 0 (0%) | 0 (0%) | 3 (5%) |
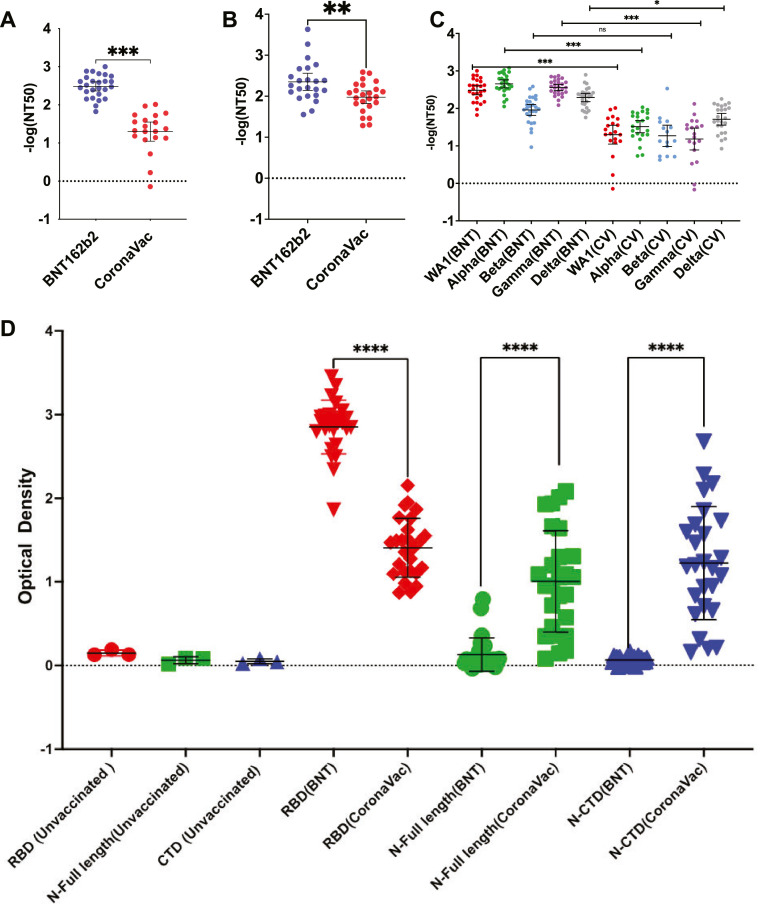
Using natural live viruses, the neutralizing antibody titer at 50% inhibition (NT50) of mRNA vaccine sera was more seroprotective than that of inactivated virus vaccine sera (Fig. 1 A). When tested against the original WA1 strain, BNT162b2 recipients' sera had NT50 values 2.4-fold higher (p<0.001) than CoronaVac. Our unvaccinated controls failed to register a NT50 value as no seroprotection was observed. Additionally, we determined that the number of days between the second dose and blood collection did not correlate with NT50, indicating that variations in the number of days following the second dose at which blood was collected for analysis did not impact our readings. To determine if the neutralization is mainly mediated by Spike (S) protein, we used rVSV pseudoparticles (Fig. 1B) expressing SAR-CoV-2 S protein (see Supplementary methods). We found that BNT162b2 was 13.4-fold more seroprotective (p = 0.0044) than CoronaVac in neutralizing S protein for viral entry inhibition. The unvaccinated controls did not show seroprotection against S protein. Then, we performed PRNT with SARS-CoV-2 live virus to compare their seroprotection against the four variant strains (Alpha, Beta, Gamma, and Delta). BNT162b2 outperformed CoronaVac in the Alpha, Gamma, and Delta variants (Fig. 1C). BNT162b2 sera have a 3.4-fold lower NT50 against Beta than WA1 (p<0.001) (Fig. 1C). We and others have previously shown that changes at E484 in Beta reduce neutralization titers of mRNA vaccines(10). In contrast, the neutralization titers against all four variants were low and comparable to WA1 in sera from CoronaVac-vaccinated individuals (Fig. 1C). The three negative controls (sera from unvaccinated healthy people) did not show seroprotection against all the virus strains tested in our study.
Fig.. 1.
Comparison of the neutralization of BNT162b2 and CoronaVac elicited antibodies against original (WA1) using three separate methods. (A) Comparison of NT50 expressed in -log (NT50) between the 26 human subjects vaccinated with BNT162b2 and 27 human subjects vaccinated with CoronaVac. (B) Same comparison but using rVSV-SARS-CoV-2-S. Non-parametric Mann Whitney tests were performed to calculate the p value. (C) Comparison on NT50 between BNT162b2- and CoronaVac-vaccinated human sera against WA1 and Alpha, Beta, Gamma, and Delta variants. (D) Serum titers of antibodies in BNT162b2- and CoronaVac-vaccinated human sera in optical density from ELISA against purified WA1 Spike RBD, Full length N protein, and CTD domain of N. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; ns, not significant.
Using an ELISA against purified Spike protein receptor-binding domain (RBD), full-length Nucleocapsid (N), and the C-terminal domain (CTD) of N, we tested the vaccinated sera for protein-specific seroneutralization (see Supplementary methods). Seropositivity to the virus targeting antigen (S RBD, full-length or CTD N) was found to be vastly different amongst the two vaccine types (p<0.001) (Fig. 1D). The BNT162b2 sera only tested positive for antibodies against the original WA1 Spike RBD. In contrast, all three antigens were found to be neutralized in eight out of 26 CoronaVac vaccinated human sera (Spike RBD, full-length N, and N CTD).
Finally, we determined if there exists correlation between ELISA neutralizing titer and PRNT NT50 neutralization against WA1 strain. Using Spearman correlation, we found a moderate correlation between ELISA antibody titers against the WA1 Spike RBD and NT50 for the BNT162b2 sera (Spearman R: 0.6144; p = 0.0008). Next, for the BNT162b2, we tested the predictability of the neutralization titer of ELISA assay against WA1 Spike RBD for the four SARS-CoV-2 variants. The WA1 Spike RBD binding profiles of sera antibodies showed a moderate correlation with NT50 against Alpha (Spearman R: 0.5843; p = 0.0017) and Beta (Spearman R: 0.5193; p = 0.0066) variants. Even though BNT162b2 had lower neutralization titers for the Beta variant, the ELISA titer against WA1 Spike RBD is still predictive of NT50 for the Beta variant, albeit being the weakest. ELISA antibody titers against Spike RBD and N (both full-length and CTD) and NT50 for WA1 and Alpha, Beta, Gamma, and Delta variants do not correlate well with CoronaVac sera.
In summary, we showed that CoronaVac exhibited a lower neutralization titer compared to BNT162b2 for prototype virus WA1, and all variants tested in this study (Alpha, Beta, Gamma, and Delta). The inactivated virus vaccine did not exhibit reduced neutralization titer against any of the mutants. Inactivated virus is seropositive to both Spike and Nucleocapsid proteins, whereas BNT162b2 is seropositive to Spike. This leads to a reduced neutralization efficiencies towards Beta, where a single mutation in the Spike protein is responsible for antibody escape. ELISA titers against the RBD of Spike protein can be predictive of neutralization by cVNT against not only the prototype WA1 strain, but also variants of concern. This correlation is lacking in CoronaVac. Even though our present work did not include data on other possible correlates of protection such as T cells or antibody-dependent cellular cytotoxicity antibodies, our findings contribute to a better understanding of how antibodies bind to SARS-CoV-2 antigens.
Declaration of Competing Interest
The authors do not have a commercial or other association that might pose a conflict of interest.
Acknowledgments
Acknowledgment
We are grateful to Dr. Thomas Moran at The Icahn School of Medicine at Mount Sinai for providing the SARS-CoV cross-reactive 1C7C7 N protein monoclonal antibody. We are also thankful to Prof Malik Peiris, Mr. Samuel Cheng, Mr. Chi Hang Tsang, and Mr. Wing Chi Kenny Yam from The School of Public Health, The University of Hong Kong, for assistance on the ELISA assays.
Funding
This study has no funding source to report.
Human Ethics
This study was approved by the Human Research Ethics Committee (HREC) of the Hong Kong University of Science and Technology. The code for the human research ethics approval was HREP-2021-0116. All subjects were presented with our protocol and ethics via a video conference. Electronic informed consent forms were distributed to the recruited participants via email. All biological data were kept confidential and were accessed only among the researchers of this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.02.030.
Appendix. Supplementary materials
References
- 1.Akpolat T., Uzun O. Reduced mortality rate after coronavac vaccine among healthcare workers. J Infect. 2021 Aug 1;83(2):e201. doi: 10.1016/j.jinf.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vacharathit V., Aiewsakun P., Manopwisedjaroen S., Srisaowakarn C., Laopanupong T., Ludowyke N., et al. CoronaVac induces lower neutralising activity against variants of concern than natural infection. Lancet Infect Dis. 2021 Oct 1;21(10):1352–1354. doi: 10.1016/S1473-3099(21)00568-5. [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranzani O.T., Hitchings M.D.T., Dorion M., D'Agostini T.L., de Paula R.C., de Paula O.F.P., et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021 Aug 20;374:n2015. doi: 10.1136/bmj.n2015. http://www.bmj.com/content/374/bmj.n2015.abstract [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunsawong T., Fernandez S., Buathong R., Khadthasrima N., Rungrojchareonkit K., Lohachanakul J., et al. Limited and Short-Lasting Virus Neutralizing Titers Induced by Inactivated SARS-CoV-2 Vaccine. Emerg Infect Dis. 2021;27(12):3178–3180. doi: 10.3201/eid2712.211772. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández J., Bruneau N., Fasce R., Martín H.S., Balanda M., Bustos P., et al. Neutralization of alpha, gamma, and D614G SARS-CoV-2 variants by CoronaVac vaccine-induced antibodies. J Med Virol. 2022;94(1):399–403. doi: 10.1002/jmv.27310. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G.-.L., Wang Z.-.Y., Duan Ll-J, Meng Q.-.C., Jiang M.-.D., Cao J., et al. Susceptibility of Circulating SARS-CoV-2 Variants to Neutralization. N Engl J Med. 2021 Apr 6;384(24):2354–2356. doi: 10.1056/NEJMc2103022. [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melo-González F., Soto J.A., González L.A., Fernández J., Duarte L.F., Schultz B.M., et al. Recognition of Variants of Concern by Antibodies and T Cells Induced by a SARS-CoV-2 Inactivated Vaccine. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.747830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Y., Hao X., Wang X., Wu Q., Song R., Zhao D., et al. Humoral immunogenicity and reactogenicity of CoronaVac or ZF2001 booster after two doses of inactivated vaccine. Cell Res [Internet] 2022;32(1):107–109. doi: 10.1038/s41422-021-00596-5. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J.-.G., Oladunni F.S., Chiem K., Ye C., Pipenbrink M., Moran T., et al. Rapid in vitro assays for screening neutralizing antibodies and antivirals against SARS-CoV-2. J Virol Methods [Internet] 2021;287 doi: 10.1016/j.jviromet.2020.113995. https://www.sciencedirect.com/science/article/pii/S0166093420302470 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jangra S., Ye C., Rathnasinghe R., Stadlbauer D., Alshammary H., Amoako A.A., et al. SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. The Lancet Microbe [Internet]. 2021 Jul 1;2(7):e283–4. Available from: doi:10.1016/S2666-5247(21)00068-9. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.