Abstract
Bacteria belonging to the genus Klebsiella frequently cause human nosocomial infections. In particular, the medically most important Klebsiella species, Klebsiella pneumoniae, accounts for a significant proportion of hospital-acquired urinary tract infections, pneumonia, septicemias, and soft tissue infections. The principal pathogenic reservoirs for transmission of Klebsiella are the gastrointestinal tract and the hands of hospital personnel. Because of their ability to spread rapidly in the hospital environment, these bacteria tend to cause nosocomial outbreaks. Hospital outbreaks of multidrug-resistant Klebsiella spp., especially those in neonatal wards, are often caused by new types of strains, the so-called extended-spectrum-β-lactamase (ESBL) producers. The incidence of ESBL-producing strains among clinical Klebsiella isolates has been steadily increasing over the past years. The resulting limitations on the therapeutic options demand new measures for the management of Klebsiella hospital infections. While the different typing methods are useful epidemiological tools for infection control, recent findings about Klebsiella virulence factors have provided new insights into the pathogenic strategies of these bacteria. Klebsiella pathogenicity factors such as capsules or lipopolysaccharides are presently considered to be promising candidates for vaccination efforts that may serve as immunological infection control measures.
Klebsiella is well known to most clinicians as a cause of community-acquired bacterial pneumonia, occurring particularly in chronic alcoholics (40) and showing characteristic radiographic abnormalities (81) due to a severe pyogenic infection which has a high fatality rate if untreated.
The vast majority of Klebsiella infections, however, are associated with hospitalization. As opportunistic pathogens, Klebsiella spp. primarily attack immunocompromised individuals who are hospitalized and suffer from severe underlying diseases such as diabetes mellitus or chronic pulmonary obstruction. Nosocomial Klebsiella infections are caused mainly by Klebsiella pneumoniae, the medically most important species of the genus. To a much lesser degree, K. oxytoca has been isolated from human clinical specimens. It is estimated that Klebsiella spp. cause 8% of all nosocomial bacterial infections in the United States and in Europe. No great geographical variations in frequency have been noted. In the United States, Klebsiella accounts for 3 to 7% of all nosocomial bacterial infections, placing them among the eight most important infectious pathogens in hospitals (104, 211), and data collected from the United Kingdom (26) and from Germany (242) are remarkably similar to those reported by the Centers for Disease Control and Prevention.
Table 1 lists the most frequent nosocomial infections caused by Klebsiella. The urinary tract is the most common site of infection. Klebsiella accounts for 6 to 17% of all nosocomial urinary tract infections (UTI) and shows an even higher incidence in specific groups of patients at risk, e.g., patients with neuropathic bladders or with diabetes mellitus (25, 133). As a cause of nosocomial gram-negative bacteremia, Klebsiella is second only to Escherichia coli (35, 70, 181, 258).
TABLE 1.
Hospital-acquired bacterial infections caused by Klebsiella spp.
| Infection | % of infections caused by Klebsiella | Ranka | References |
|---|---|---|---|
| UTI | 6–17 | 5–7 | 26, 46, 104, 242 |
| Pneumonia | 7–14 | 2–4 | 26, 44, 104 |
| Septicemia | 4–15 | 3–8 | 34, 35, 45–47, 64, 70, 135, 181, 242, 258 |
| Wound infections | 2–4 | 6–11 | 104, 142, 242 |
| Nosocomial infections in intensive care unit patients | 4–17 | 4–9 | 26, 104, 226, 242 |
| Neonatal septicemia | 3–20 | 2–8 | 24, 84, 168, 235, 240, 247 |
Ranking of Klebsiella compared to all other bacterial pathogens.
In pediatric wards, nosocomial Klebsiella infections are especially troublesome—particularly in premature infants and intensive care units. Klebsiella species are often the pathogens involved in neonatal sepsis (Table 1), in both early-manifestation and late-manifestation infections (90).
Due to the extensive spread of antibiotic-resistant strains, especially of extended-spectrum β-lactamase (ESBL)-producing strains, there has been renewed interest in Klebsiella infections.
EPIDEMIOLOGY
Klebsiella spp. are ubiquitous in nature. Klebsiellae probably have two common habitats, one being the environment, where they are found in surface water, sewage, and soil and on plants (15, 33, 71, 140, 214), and the other being the mucosal surfaces of mammals such as humans, horses, or swine, which they colonize. In this respect, the genus Klebsiella is like Enterobacter and Citrobacter but unlike Shigella spp. or E. coli, which are common in humans but not in the environment.
In humans, K. pneumoniae is present as a saprophyte in the nasopharynx and in the intestinal tract. Carrier rates differ considerably from study to study. The detection rate in stool samples ranges from 5 to 38%, while rates in the nasopharynx range from 1 to 6% (62, 206, 208, 236). Because gram-negative bacteria do not find good growth conditions on the human skin (207), Klebsiella spp. are rarely found there and are regarded simply as transient members of the flora (126).
These carrier rates change drastically in the hospital environment, where colonization rates increase in direct proportion to the length of stay. Even hospital personnel have elevated rates of Klebsiella carriage (42, 43, 52). Reported carrier rates in hospitalized patients are 77% in the stool, 19% in the pharynx, and 42% on the hands of patients (52, 62, 112, 193, 206, 215, 225). The high rate of nosocomial Klebsiella colonization appears to be associated with the use of antibiotics rather than with factors connected with delivery of care in the hospital (193, 206). Previous antibiotic therapy is significantly associated with acquisition of Klebsiella by the patient. In one study, 2 weeks after admission to the hospital, a two- to fourfold increase in the colonization rates with Klebsiella was observed (193); this increase occurred primarily in patients receiving antibiotics, especially in persons receiving broad-spectrum or multiple antibiotics. In the hospital setting, the local antibiotic policy is a major determinant of the colonization pattern. The significance of increased colonization was illustrated by the observation that the attack rate of Klebsiella nosocomial infection in patients carrying hospital-acquired intestinal Klebsiella was four times as high as for noncarriers (215). Furthermore, widespread use of antimicrobial therapy has often been held responsible for the occurrence of multiply resistant Klebsiella strains in hospitals (215, 239). Because these undesired effects may be reversed by strict control of antibiotic use, demands for strategies to avoid the overuse of antibiotics in prophylaxis and empirical therapy are increasingly being expressed.
Apart from medical equipment (contaminated due to faulty hygienic procedures) and blood products (89, 116, 198), the principal reservoirs for transmission of Klebsiella in the hospital setting are the gastrointestinal tract of patients and the hands of hospital personnel (156). The ability of this organism to spread rapidly (129) often leads to nosocomial outbreaks, especially in neonatal units (98). Of the 145 epidemic nosocomial infections reported in the literature published in English between 1983 and 1991, 13 were caused by Klebsiella (68). According to the statistics of the Centers for Disease Control and Prevention, Klebsiella spp. account for 8% of endemic hospital infections and 3% of epidemic outbreaks (227).
Especially feared are epidemic hospital infections caused by multiresistant strains. In the 1970s, these strains were chiefly aminoglycoside-resistant Klebsiella strains (48, 60, 138, 160). Since 1982, strains that produce ESBLs, which render them resistant to extended-spectrum cephalosporins, have evolved (22, 53, 63, 85, 96, 113, 144, 146, 200). The hallmark of these strains, resistance to ceftazidime, is observed in both K. pneumoniae and K. oxytoca isolates (224). In Europe, the β-lactamases of ceftazidime-resistant Klebsiella strains are commonly of the SHV-5 type, whereas TEM-10 and TEM-12 are more prevalent in the United States (22, 85, 108, 130, 144, 179, 213, 243, 245). The incidence of ESBL-producing Klebsiella isolates in the United States has been reported to be 5% of the K. pneumoniae strains tested in the National Nosocomial Infection Study system (108). In Europe, the frequency of such strains seems to be even higher. A percentage of 14 to 16% ESBL producers among clinical Klebsiella isolates has been reported for France and England (221). In particular regions or hospitals, the incidence can reach 25 to 40% (38). However, the percentage of ceftazidime-resistant strains may be much higher, because the conventional disc diffusion criteria used in the routine laboratory underestimate the incidence of these isolates (109).
ESBLs are usually plasmid mediated. Since these plasmids are easily transmitted among different members of the Enterobacteriaceae, accumulation of resistance genes results in strains that contain multiresistant plasmids. For this reason, ESBL-producing isolates are resistant to a variety of classes of antibiotics. Moreover, the emergence of these multiply resistant Klebsiella strains is unfortunately accompanied by a relatively high stability of the plasmids encoding ESBLs. Even years after discontinuation of ceftazidime and other extended-spectrum cephalosporins, continued colonization of patients by ESBL-producing Klebsiella strains has been observed (101). Risk factors for acquisition of these strains seem to be the length of stay in hospital and the performance of invasive procedures (132).
Since ESBL production frequently is accompanied by multiresistance to antibiotics, therapeutic options become limited. So far, however, ESBL-producing Klebsiella strains have been susceptible to carbapenems such as imipenem or meropenem. Both antibiotics are the drugs of choice in the treatment of infections due to ESBL-producing organisms. In this respect, a recent observation is very disturbing. For the first time, ESBL-producing K. pneumoniae strains which showed an additional resistance to imipenem have been isolated (30). These strains possessed a transmissible plasmid-mediated AmpC-type β-lactamase. This development should be monitored closely, since the emergence of imipenem-resistant ESBL-producing Klebsiella strains will have a serious impact on remaining therapeutic options.
In the last several years, the question has arisen whether it is necessary to determine if each isolated Klebsiella strain is an ESBL producer. The answer depends on the epidemiologic situation of a country or a hospital, but it should definitely be positive if a high percentage of ceftazidime-resistant strains is to be expected. To date, two diagnostic tests have been most commonly used for the detection of such isolates. In the double-disc synergy test, a disc of clavulanic acid and a disc of an extended-spectrum cephalosporin such as ceftazidime are placed close together on an agar surface inoculated with the test organism (111). Enhancement of the zone of inhibition around the cephalosporin disc towards the clavulanate-containing disc indicates the presence of an ESBL-producing strain. A commercially available product is the ESBL screening E test strip (AB Biodisk, Solna, Sweden). This method is based on the evaluation of the difference between the antimicrobial activity of ceftazidime alone compared to that of ceftazidime plus clavulanic acid (119).
It should be kept in mind that a number of measures have been recommended to prevent the nosocomial spread of Klebsiella. Strict adherence to basic epidemiological standards for the management of urinary catheters, intravenous tracheostomies, and wounds, maintenance and care of equipment, and good hand-washing practices all help to prevent the spread of nosocomial Klebsiella infections. Detailed information on this subject is given in an excellent review by Montgomerie (156). Another measure to control Klebsiella infections is the regulation of antibiotic use in the hospital to prevent misuse and overuse of antibiotics. Furthermore, nosocomial infection surveillance is necessary to collect data that are used in the prevention and control of nosocomial Klebsiella infection rates.
TAXONOMY OF THE GENUS KLEBSIELLA
The taxonomy of Klebsiella is characterized by a nomenclature reflecting its colorful taxonomic history. Originally, the medical importance of the genus Klebsiella (family Enterobacteriaceae) led to its being subdivided into three species corresponding to the diseases they caused: K. pneumoniae, K. ozaenae, and K. rhinoscleromatis. As the taxonomy became increasingly refined due to the development of new methods such as numerical taxonomy, the species classification in this genus was continually revised. In time, three main classifications emerged, those of Cowan, Bascomb, and Ørskov (Table 2).
TABLE 2.
Species classification of the genus Klebsiella by different taxonomic systemsa
| Classification by:
| ||
|---|---|---|
| Cowan | Bascomb | Ørskov |
| K. aerogenes K. edwardsii | K. aerogenes/oxytoca/ edwardsii | K. pneumoniae |
| subsp. pneumoniae | ||
| subsp. edwardsii | K. pneumoniae | subsp. ozaenae |
| subsp. atlantae | sensu stricto | subsp. rhinoscleromatis |
| K. pneumoniae | sensu lato | K. oxytoca |
| K. ozaenae | K. ozaenae | K. terrigena |
| K. rhinoscleromatis | K. rhinoscleromatis | K. planticola (syn. K. trevisanii) |
| K. “unnamed group” | ||
| Enterobacter aerogenes | K. ornithinolytica | |
In the early 1980s, Klebsiella isolates from the environment, which had previously been classified as “Klebsiella-like organisms” (groups J, K, L, and M), were increasingly being classified into provisional taxa (87). These groups gave rise to four new species: K. terrigena (107), K. ornithinolytica (210), K. planticola (14), and K. trevisanii (82). In 1986, the last two species were combined into one species, K. planticola, because of their extensive DNA sequence homology (86). While originally considered to be without clinical significance and restricted to aquatic, botanical, and soil environments, K. terrigena and K. planticola have recently been reported as occurring in human clinical specimens (158, 189, 190). According to these findings, particularly K. planticola has been isolated from human infections with a surprisingly high frequency of 3.5 to 18.5% among clinical isolates of Klebsiella species. More than half of these isolates were recovered from respiratory tract secretions; wound and urine isolates were the next most common (189). However, since most of the isolates were obtained from polymicrobial specimens, it is difficult to estimate the significance of these strains as causative agents of disease. Nevertheless, 6 of the 94 isolates were recovered from monomicrobial specimens and could be assigned to corresponding infections. Thus, at present it seems possible that in addition to K. pneumoniae and K. oxytoca, a third Klebsiella species exists that is able to cause human infections.
The adoption of a consistent nomenclature has been further complicated by the fact that Great Britain and the former Commonwealth countries adhere to the classification of Cowan while the USA prefers Ørskov’s classification. Consequently, the same bacterium may be called K. pneumoniae in one country and K. aerogenes in another. Most European countries follow the American example and recognize the worldwide predominant classification of Ørskov.
DIFFERENTIATION OF KLEBSIELLA SPECIES
Klebsiella species are usually identified and differentiated according to their biochemical reactions. The genus is defined as containing gram-negative, nonmotile, usually encapsulated rod-shaped bacteria of the family Enterobacteriaceae, which produce lysine decarboxylase but not ornithine decarboxylase and are generally positive in the Voges-Proskauer test (75). Within the genus Klebsiella, the individual species can be differentiated on the basis of the features listed in Table 3. Whereas most Klebsiella species can be identified by standard microbiological laboratory tests, the species K. terrigena and K. planticola require special, nonconventional reactions (such as utilization of m-hydroxybenzoate or hydroxy-l-proline, pectate degradation, acid from melezitose, or growth at 10°C).
TABLE 3.
Biochemical reactions of Klebsiella speciesa
| Characteristic |
Klebsiella pneumoniae
|
K. oxytoca | K. terrigena | K. planticola | K. ornithinolytica | ||
|---|---|---|---|---|---|---|---|
| subsp. pneumoniae | subsp. ozaenae | subsp. rhinoscleromatis | |||||
| Indole | − | − | − | + | − | vb | + |
| Ornithine decarboxylase | − | − | − | − | − | − | + |
| Lysine decarboxylase | + | v | − | + | + | + | + |
| Pectate degradation | − | − | − | + | − | − | − |
| Gas from lactose at 44.5°C | + | − | − | − | − | − | − |
| Growth at 10°C | − | − | − | + | + | + | + |
| Acid from: | |||||||
| d-Melezitose | − | − | − | v | + | − | − |
| l-Sorbose | v | + | + | + | |||
| Utilization of: | |||||||
| m-Hydroxybenzoate | − | − | − | + | + | − | − |
| Hydroxy-l-proline | v | v | v | + | |||
| Malonate | + | − | + | + | + | + | + |
| Methyl red test | − | + | + | − | + | v | + |
| Voges-Proskauer reaction | + | − | − | + | + | + | + |
TYPING OF KLEBSIELLA ISOLATES
From an epidemiological point of view, it is often necessary to determine the clonality of the strains. This is particularly important in endemic and epidemic nosocomial outbreaks of Klebsiella infections to improve the management of such outbreaks. A variety of methods have been used with various degrees of success in Klebsiella typing and are discussed below.
Biotyping
Biotyping based on an extended panel of biochemical and culture tests is certainly the most practicable method of typing for smaller laboratories that are epidemiologically not optimally equipped. Biotyping can be carried out by using macrotube tests alone (100, 202) or by combining a commercially available miniaturized system such as the API 20E system with additional macrotube tests (185, 217). However, because of the large number of reactions to be tested and the often long cultivation times—up to 90 days for demonstration of gelatinase (228)—biotyping of Klebsiella spp. is not very suitable as an epidemiological tool.
Serotyping
Serotyping is currently the most widely used technique for typing Klebsiella spp. It is based mainly on a division according to the capsule antigens (177). Klebsiellae usually have well-developed polysaccharide capsules, which give their colonies their characteristic mucoid appearance. Of 82 capsule antigens described, 77 types form the basis for an internationally recognized capsule antigen scheme (176). Although 12 different O-antigen types of Klebsiella have also been described, they are difficult to classify because their determination is hampered by the heat-stable capsules (175, 177). Capsule typing, by contrast, shows good reproducibility and is capable of differentiating most clinical isolates (12). The drawback of this method is the large number of serological cross-reactions that occur among the 77 capsule types. Thus, individual sera have to be absorbed with the cross-reacting K-antigens. Moreover, the typing procedure is cumbersome because of the time needed to perform the test and is susceptible to subjective interpretations because of weak reactions that are not always easy to interpret. Since anti-capsule antisera are not commercially available, this technique is practiced mostly in specialized laboratories. However, in contrast to capsule typing, neither biochemical typing, bacteriocin typing, nor phage typing alone is sufficiently discriminative and reproducible for epidemiological purposes except under certain conditions (177). The combined use of biotyping and capsule typing enables the differentiation of a large number of bioserotypes (202).
Phage Typing
Phage typing of Klebsiella was first developed in the 1960s (196, 223). Although the phage reaction is easily read and the reproducibility of the method is acceptable, this technique shows a relatively poor typing rate of 19 to 67% (209, 222). Since it is not an alternative to capsule typing, this procedure has never become widespread and is useful mainly as a secondary method in combination with serologic testing (48, 53, 113). It should be stressed, however, that it is possible to develop capsule- and O-antigen-specific phage typing if appropriate efforts are made, as a number of reports have demonstrated (143, 180, 237).
Bacteriocin Typing
Although capsule typing is the preferred method for Klebsiella, it has been advised to include an additional feature independent of the capsule type to enable more precise epidemiological analysis. Many authors recommend typing Klebsiella via bacteriocins (20, 36, 73, 97, 223). Bacteriocins are bactericidal substances, usually proteins, produced by bacteria to inhibit the growth of other bacteria, usually members of the same species. An isolate can be characterized either by its ability to inhibit specific indicator strains or by its sensitivity to bacteriocins synthesized by a set of producer strains. Since the synthesis of bacteriocins is not frequent enough in Klebsiella, the latter technique has become the method of choice for bacteriocin typing of organisms belonging to this genus. However, the two principal early methods—the growth-in-broth method and the cross-streak method—both show considerable disadvantages. Because of the instability of bacteriocin preparations, the reproducibility of the growth-in-broth method is low. In addition, the conventional cross-streak method results in low typability of strains (36, 73, 97). The limitations of both of these methods have been surmounted by a modification of the “scrape-and-point” procedure (20), which avoids the use of potentially unstable preproduced and stored bacteriocins. Instead, the bacteriocins are synthesized on an agar medium immediately before the strains to be typed are inoculated by a multipoint inoculator. This method has proven superior for bacteriocin typing of clinical and environmental Klebsiella strains (21, 187, 188) as well as of nosocomial outbreaks of Klebsiella (22).
Molecular Typing Methods
Molecular typing methods, as applied to the genus Klebsiella, are still in their infancy. Preliminary descriptions have been presented on plasmid profiles (22, 28, 53, 99, 157, 185, 250), ribotypes (8, 27, 28), multilocus enzyme analyses (51, 161), and applications of pulsed-field gel electrophoresis (8, 91, 192). The procedures vary from laboratory to laboratory and lack standardization, making it difficult to compare them.
PATHOGENICITY FACTORS OF KLEBSIELLA
The terms “pathogenicity factor” and “virulence factor” are used synonymously by some authors (212), while others lay emphasis on a clear-cut distinction between them. In this review, the term “pathogenicity” defines the ability of a bacterium to cause disease while “virulence” is the measurement or degree of pathogenicity of any bacterial species.
Nosocomial Klebsiella infections most commonly involve the urinary and respiratory tracts. Since these two body sites differ considerably with respect to the host defense mechanisms, it should be expected that the pattern of virulence factors found in UTI-causing strains of Klebsiella will differ from that observed in strains isolated from pulmonary sources of patients with pneumonia.
The search for the pathogenic mechanisms of Klebsiella infections has identified a number of bacterial factors that contribute to the pathogenesis of these bacteria. Both in vitro and in vivo models have been established to investigate the interaction of bacterial cells and the host. The use of animal models has been a critical element in the study of Klebsiella pathogenicity by providing vital information that cannot be obtained from in vitro studies. In particular, animal models have been established to study Klebsiella virulence factors in UTIs; mice and rats seem to be appropriate animal types. Lower UTIs have been investigated in the diuresis mouse or rat model of cystitis by intravesicular injection of organisms (77, 134). To study Klebsiella-mediated upper UTI, a rat model of experimental retrograde pyelitis has been established (78). Frequently, both models include scanning electron microscopy of the surface of the bladder or renal pelvis.
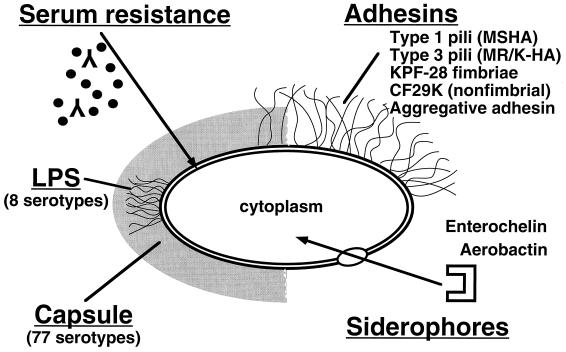
The current research into the pathogenicity of Klebsiella focuses on the group of five factors shown in Fig. 1.
FIG. 1.
Schematic representation of Klebsiella pathogenicity factors.
Capsular Antigens
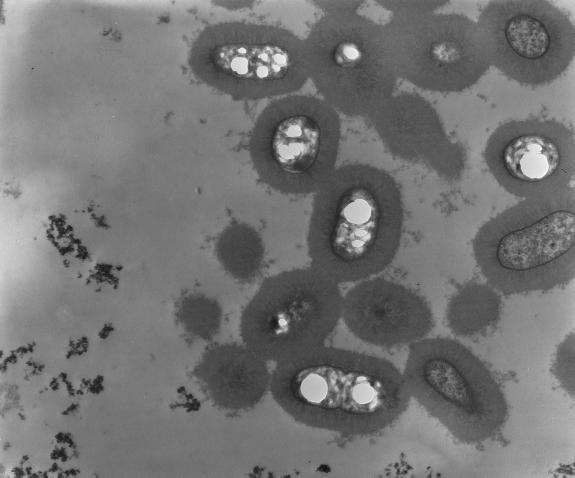
As mentioned above, klebsiellae usually develop prominent capsules composed of complex acidic polysaccharides. The capsular repeating subunits, consisting of four to six sugars and, very often, uronic acids (as negatively charged components), can be classified into 77 serological types (177). Capsules are essential to the virulence of Klebsiella (56, 69, 76, 103). The capsular material forms thick bundles of fibrillous structures covering the bacterial surface in massive layers (Fig. 2) (7). This protects the bacterium from phagocytosis by polymorphonuclear granulocytes, on the one hand (186, 191, 218, 219), and prevents killing of the bacteria by bactericidal serum factors, on the other (252). The molecular mechanism presumably consists of inhibiting the activation or uptake of complement components, especially C3b (254). Apart from their antiphagocytic function, Klebsiella capsule polysaccharides have been reported to inhibit the differentiation and functional capacity of macrophages in vitro (260, 261). Moreover, injection of large doses of Klebsiella capsular polysaccharide (CPS) may even produce immunological paralysis, as has been demonstrated in mice that showed a dose-dependent decrease in the production of antibodies to the specific capsular antigen (19).
FIG. 2.
Transmission electron micrograph of K. pneumoniae cells surrounded by thick layers of fibrillous capsular material. Courtesy of I. Ofek, Tel Aviv University, Israel. Reprinted from reference 163 with permission of the publisher.
While Klebsiella CPS were generally considered to mediate virulence properties, this consideration has recently been abandoned because of the great differences in virulence observed among different capsular types: strains expressing the capsule antigens K1 and K2 were found to be especially virulent in a mouse peritonitis model, whereas isolates of other serotypes showed little or no virulence (120, 151). In experimentally induced skin lesions in mice, Klebsiella strains of serotypes K1, K2, K4, and K5 were more virulent than were those expressing other capsule types (220). At present, strains expressing capsule types K1 and K2 are considered especially likely to be virulent, although only a few of the 77 different K antigens have been systematically studied in this regard.
The degree of virulence conferred by a particular K antigen might be connected to the mannose content of the CPS. Capsular types with low virulence, such as the K7 or K21a antigen (166, 191), contain repetitive sequences of mannose-α-2/3-mannose or l-rhamnose-α-2/3-l-rhamnose. These sequences are recognized by a surface lectin of macrophages, which mediates opsonin-independent (i.e., complement- and antibody-independent) phagocytosis, known as lectinophagocytosis (9). Lectinophagocytosis has been defined as nonopsonic phagocytosis that is based on recognition between surface lectins on one cell and surface carbohydrates on the opposing cell (164). Lectinophagocytosis may be mediated either by bacterial surface lectins such as fimbriae or by phagocyte lectins that act as receptors. Macrophages with the mannose-α-2/3-mannose-specific lectin or mannose receptor recognize, ingest, and subsequently kill Klebsiella serotypes containing the CPS repeating sequences Manα2/3Man or l-Rhaα2/3l-Rha. In contrast, strains that lack these repeating sequences are not recognized by macrophages and hence phagocytosis does not take place. This model is consistent with the marked virulence of K2, which completely lacks mannose-α-2/3-mannose structures (117, 166). Thus, Klebsiella strains bearing capsule types devoid of these mannose or rhamnose sequences should be more closely associated with infectious diseases.
Previous attempts to establish a correlation between individual Klebsiella serotypes and the site of infection or clinical symptoms have produced a profusion of contradictory results. Each study reports different capsular types as predominant (41, 59, 185, 202, 203, 217, 241). Geographical differences in serotypes may have contributed to this confusion. Most reports do agree, however, that the K2 serotype is among the most common capsule types isolated from patients with UTI, pneumonia, or bacteremia. It can be assumed, therefore, that K2 is the predominant serotype of human clinical isolates worldwide whereas K2 strains are very rarely encountered in the environment (33, 74, 140, 182). Thus, the observed predominance of the K2 serotype in Klebsiella infections is quite consistent with the concept of lectinophagocytosis. Since the host innate immune mechanisms interact with structures commonly found on microorganisms, particular Klebsiella serotypes, such as the K2 type, which do not bear such structures, become selected.
The significance of capsular mannose-α-2/3-mannose sequences to the clearance of Klebsiella in the host is further illustrated by recent findings of Ofek’s group (118). They observed that surfactant protein A (SP-A), the main protein component of lung surfactant, enhances the phagocytosis by alveolar macrophages of Klebsiella K21a strains (which bear a mannose-α-2/3-mannose containing capsule) but not of K2 isolates. Since the reaction was inhibited by mannan, the authors suggested that bacterial binding is mediated by the macrophage mannose receptor.
Pili (Fimbriae)
As a critical first step in the infectious process, microorganisms must come as close as possible to host mucosal surfaces and maintain this proximity by attaching to the host cell (adherence). The adhesive properties in the Enterobacteriaceae are generally mediated by different types of pili. Pili (otherwise known as fimbriae) are nonflagellar, filamentous projections on the bacterial surface. These structures are up to 10 μm long and have a diameter of 1 to 11 nm (163); they consist of polymeric globular protein subunits (pilin) with a molecular mass of 15 to 26 kDa (115).
Pili are demonstrated mainly on the basis of their ability to agglutinate erythrocytes of different animal species. Depending on whether the reaction is inhibited by d-mannose, these adhesins are designated as mannose-sensitive or mannose-resistant hemagglutinins (MSHA and MRHA), respectively (178). Of the different types of pili described in enterobacteria, there are two predominant types in Klebsiella spp. (171, 184, 195).
Type 1 (common) pili.
Type 1 pili are the best investigated of the bacterial adhesins. They are MSHA which agglutinate guinea pig erythrocytes. The adhesion protein in this pilus type is located on the fimbrial shaft and is capable of binding to mannose-containing trisaccharides of the host glycoproteins (13, 83). The sugar structures presumably consist of short oligomannose chains bound via N-glycosidic linkages to the glycoproteins (216). The relevance of these pili to bacterial virulence is thought to arise mainly from binding of the bacteria to mucus or to epithelial cells of the urogenital, respiratory, and intestinal tracts (16, 162, 244). Their role in the pathogenesis of UTI was clarified mostly in studies on E. coli but has also been described for K. pneumoniae in animal models (77, 78, 134). Although associated primarily with the pathogenesis of lower UTI (106), type 1 pili may also be involved in the pathogenesis of pyelonephritis (78, 141). In this setting, these structures have been shown to bind effectively to proximal tubulus cells (248). Type 1 fimbriae are also capable of binding to soluble, mannosyl-containing glycoproteins in urine, such as the Tamm-Horsfall protein (199), or in saliva (13). These findings provide an explanation for the fact that type 1 pili mediate bacterial colonization of the urinogenital and respiratory tracts (50). Adherence of bacteria to cells of the respiratory tract (11) leads to impairment of colonization resistance in the upper airways, with a subsequent proliferation of facultative pathogenic bacteria. This impairment may result in the development of pneumonia, especially in patients undergoing long-term mechanical ventilation (254).
In the appraisal of the pathogenic role of type 1 pili, however, the phenomenon of “phase variation” has to be taken into account. As mentioned above, this type of adhesin mediates bacterial colonization of the host mucosal surfaces via a rather nonspecific binding. In pathogenic microorganisms, colonization of the mucous membrane is followed by invasion of the underlying tissue, with all of the subsequent events of infectious pathogenesis. Once in the host tissue, however, the type 1 pili are no longer of use to the bacteria, since they trigger an opsonin-independent leukocyte activity known as lectinophagocytosis (167). The repulsion forces separating bacterium and leukocyte are weakened by the hydrophilic character of these pili (169), thus enabling the adhesins to bind to specific mannose-containing receptors on the leukocyte surface (205). Adhesin-binding triggers stimulation of the leukocyte (137), which ultimately leads to phagocytosis and intracellular killing of the bacterium (131). The bacterium counters this form of host defense by switching off the expression of type 1 pili in tissue (134). Thus, while type 1 pili are important for host colonization, their contribution to subsequent steps of pathogenesis is less clear.
Type 3 pili.
Unlike other fimbriae, type 3 pili agglutinate only erythrocytes that have been treated with tannin. Although its name, mannose-resistant, Klebsiella-like hemagglutination (MR/K-HA), implies that this fimbrial type is synthesized only by Klebsiella, later studies demonstrated that type 3 pili occur in many enteric genera (50). Moreover, type 3 pili apparently are not identical in all genera of enterobacteria, since serological studies showed considerable antigenic diversity (170). Originally described as the adhesion organelles of Klebsiella inhabiting plant roots (128), these pili were later found to be capable of binding to various human cells. Strains of K. pneumoniae expressing type 3 pili adhere to endothelial cells, epithelia of the respiratory tract, and uroepithelial cells (105, 232, 256). In the kidneys, these pili mediate bacterial adhesion to tubular basement membranes, Bowman’s capsules, and renal vessels (231). Binding to tannic acid-treated erythrocytes is inhibited by spermidine, a polyamine that is also secreted in urine (88). Since spermidine is exposed on the cell surface of damaged erythrocytes, it has been suggested that MR/K hemagglutination is mediated by spermidine (88). This might explain why type 3 pili bind to tannic acid- or heat-treated erythrocytes but not to untreated erythrocytes.
The role of this fimbrial type in the pathogenetic process is largely unknown. So far, the only evidence of a correlation between the type 3 MrkD hemagglutinin and disease has been the observation of expression of type 3 pili in Providencia stuartii in catheter-associated bacteriuria (152). This species is not a common cause of UTI in short-term-catheterized or noncatheterized persons but has a much higher prevalence in the urine of patients with long-term indwelling catheters. In the above-mentioned study, it was demonstrated that the higher prevalence of P. stuartii in catheter-associated bacteriuria was due to its ability to adhere and persist to the catheter in the catheterized urinary tract by expression of the MR/K hemagglutinin. Unfortunately, so far, no experimental animal model has been established investigate the role of these pili in infection. The structure of the corresponding host receptors is unknown.
Three new types of Klebsiella adhesins have been recently reported. The R-plasmid-encoded CF29K adhesin of K. pneumoniae has been demonstrated to mediate adherence to the human intestinal cell lines Intestine-407 and CaCo-2 (61). This adhesin type seems to be identical to the CS31-A adhesive protein of human diarrheal E. coli strains (66) and belongs to the K88 adhesin family. The available data suggest that CF29K probably is a product of the transfer of CS31A genetic determinants from E. coli to K. pneumoniae strains in the human intestine. A particular adherence pattern characterized by aggregative adhesion to intestinal cell lines is mediated by another new Klebsiella adhesin that seems to be composed of capsule-like extracellular material (80). While the two adhesins mentioned above are nonfimbrial, a third putative colonization factor of the human gut is a new fimbria that has been termed KPF-28 (67). Interestingly, this fimbrial type has been found in the majority of K. pneumoniae strains producing CAZ-5/SHV-4 type ESBL.
To date, however, little is known about the frequency and distribution of these newly described adhesins, their geographical variations, their expression by different species of Klebsiella, their site of isolation from the host, or their significance in pathogenicity.
Serum Resistance and Lipopolysaccharide
The first line of defense by the host against invading microorganisms includes, in addition to phagocytosis by polymorphonuclear granulocytes, the bactericidal effect of serum. The serum bactericidal activity is mediated primarily by complement proteins. After their cascade-like activation, these proteins accumulate as membrane attack complex on the surface of the microorganism (233). This complex consists of the terminal complement proteins C5b–C9, which produce a transmembranous pore in the outer membrane of gram-negative bacteria (197), leading to an influx of Na+ and subsequent osmotic lysis of the bacteria (234). The complement cascade can be activated by two different mechanisms: the classic complement pathway, which typically requires specific antibodies to be activated, and the alternative complement pathway, which can be activated even in the absence of antibodies. The alternative pathway is also regarded as an early defense system of innate immunity, which enables the host to react to invading microorganisms even before specific antibodies are formed (114). Both complement pathways lead, via the activation of C3, to the formation of the opsonin C3b, which ultimately results in formation of the terminal C5b–C9 complex and thus plays a key role in this defense system.
In response to this host defense, pathogenic microorganisms have developed strategies to counter the serum bactericidal effect. Most commensal gram-negative bacteria are sensitive to the bactericidal effect of human serum, whereas pathogenic strains often exhibit serum resistance properties (172). Thus, clinical isolates of enterobacteria often show resistance to serum (249), and the feature “serum resistance” has been correlated with the onset of infection (172, 204) and severity of symptoms (29, 92). Since the main role of the serum bactericidal system is thought to prevent microorganisms from invading and persisting in the blood, even differences in the degree of bacterial serum susceptibility may determine whether a strain is able to infect as well as the length of time it takes the organisms to establish the infection.
To date, the exact mechanism underlying bacterial serum resistance is unknown. Aside from various proteins of the outer membrane, such as the TraT lipoprotein or porins (3, 155), primarily CPS and O antigens (lipopolysaccharides [LPS]) have been implicated (49, 173, 194, 230, 237, 246, 252). For Klebsiella, two hypotheses have been propounded (145). First, capsule polysaccharides may cover and mask the underlying LPS and exhibit a surface structure that does not activate complement. On the other hand, the O side chains of the LPS may reach through the capsule layer and be exposed to the exterior milieu in certain Klebsiella capsule types (238). Since LPS is generally able to activate complement, C3b is subsequently deposited onto the LPS molecules. However, since it is fixed preferentially to the longest O-polysaccharide side chains, C3b is far away from the bacterial cell membrane. Thus, the formation of the lytic membrane attack complex (C5b–C9) is prevented, and subsequent membrane damage and cell death do not take place.
In addition to this steric hindrance of the lytic complement action by LPS, the quantity of deposited C3b also determines the degree of serum resistance (2). While serum-sensitive strains activate both the classical and the alternative complement pathways, the smooth LPS of serum-resistant strains activates only the alternative pathway. The activation of both complement pathways by serum-sensitive strains leads to higher levels of deposited C3b, resulting in greater damage and bacterial killing.
It should be borne in mind, however, that all previous studies in this field were done with strains expressing the O1 serotype. Even though O1 is the most common O antigen found among clinical Klebsiella isolates, a number of different O serotypes, many of them neutral polysaccharides, are known. Originally there were 12 chemically different O types, but the results of structural investigations later reduced the number of Klebsiella O antigens to 8 (151). To date, it is unclear whether serum resistance is mediated solely by the O1 antigen or whether this feature is generally conferred by Klebsiella LPS.
Nevertheless, even within a given O serotype, serum resistance does not seem to be a stable characteristic; environmental factors affect the composition and effect of LPS. Recently, the influence of different osmolarity conditions on LPS was demonstrated in Aeromonas hydrophila serotype O:34 (1); cells grown at high osmolarity showed smooth LPS, whereas growth at low osmolarity resulted in rough LPS. Correspondingly, cells cultivated at high osmolarity were resistant to normal human serum while bacteria grown at low osmolarity proved to be serum sensitive. Thus, the same bacterial strain may be serum resistant at host body sites with a high-osmolarity milieu, such as the urinary tract, and serum sensitive at low-osmolarity body locations like the respiratory tract.
Siderophores
The growth of bacteria in host tissue is limited not only by the host defense mechanisms but also by its supply of available iron. Iron is an essential factor in bacterial growth, functioning mainly as a redox catalyst in proteins participating in oxygen and electron transport processes (93). The supply of free iron available to bacteria in the host milieu is extremely low, since this element is bound intracellularly to proteins such as hemoglobin, ferritin, hemosiderin, and myoglobin and extracellularly to high-affinity iron-binding proteins such as lactoferrin and transferrin. The level of free, bioavailable iron (10−18 M) is several thousandfold too low for normal bacterial growth (37). The marked effect of the iron supply in the host body on the pathogenesis of infections has been demonstrated for Klebsiella. After parenteral administration of iron in a guinea pig model, the susceptibility to K. pneumoniae infections increased dramatically (121).
Many bacteria attempt to secure their supply of iron in the host by secreting high-affinity, low-molecular-weight iron chelators, called siderophores, that are capable of competitively taking up iron bound to host proteins (94). Under iron-deficient conditions, e.g., in the host milieu, enterobacteria synthesize a variety of siderophores, which belong to two different chemical groups, one consisting of the phenolate-type siderophores and one consisting of the hydroxamate-type siderophores.
The more common group consists of the phenolate-type siderophores. Their best-known representative, enterobactin (also known as enterochelin), is a cyclic trimer of 2,3-dihydroxy-benzoyl-serine. This siderophore appears to comprise the main iron uptake system of enterobacteria and is synthesized by almost all clinical isolates of E. coli and Salmonella spp. (93). Studies on the contribution of enterobactin to virulence have produced conflicting results. For example, Yancey et al. (257) reported that Salmonella typhimurium mutants unable to produce this siderophore were less virulent in mice, while Benjamin et al. (23) found no association between virulence and the ability to synthesize enterobactin. To date, the role of enterobactin in virulence remains uncertain.
Among the hydroxamate-type siderophores, the ferrichromes (which are synthesized only by fungi), the ferrioxamines, and aerobactin are the most important. In contrast to enterobactin, the contribution of aerobactin to bacterial virulence has been clearly demonstrated (65). While the thermodynamic stability constant of ferric enterobactin indicates a much higher affinity for Fe(III) than that of ferric aerobactin (Ks 1052 and 1023, respectively), aerobactin still seems to be far more effective than enterobactin because of a number of physical advantages such as greater stability and better solubility (255). Moreover, while enterobactin becomes hydrolyzed by an esterase after delivery of iron, aerobactin can be recycled after each turn of iron transport.
The observations of Martinez et al. (139) indicate that enterobacterial genera can be divided into two groups according to their incidence of aerobactin synthesis. The group with a low rate of aerobactin-producing strains (<20%) comprises genera such as Serratia, Proteus, and Salmonella. The second group, which includes the genus Escherichia, shows a high incidence of aerobactin synthesis (>40%).
In the genus Klebsiella, the production of both enterobactin and aerobactin has been demonstrated. However, while enterobactin is synthesized by almost all strains (183, 201, 251), aerobactin-positive Klebsiella isolates, irrespective of the species or source of isolation, have been observed rarely (139, 183, 253). Enterobactin-positive Klebsiella isolates in animal models were no more virulent than enterobactin-negative strains (147). In contrast, an association between aerobactin synthesis and the virulence of Klebsiella strains was unequivocally demonstrated by Nassif and Sansonetti (159). In this study, the aerobactin gene was cloned from the plasmids of some K. pneumoniae strains of serotypes K1 and K2 and transferred to a nonvirulent (siderophore-negative) strain. The transformant then exhibited markedly enhanced virulence in a mouse peritonitis model.
Because of the common ability of strains to produce enterobactin, it has been speculated for a long time what additional advantage a bacterium, which already synthesizes enterobactin, might derive from aerobactin. The great advantage of enterobactin over aerobactin is its high iron affinity, the highest ever recorded for a ferric iron chelator. At pH 7.4, the formation constant for ferric enterobactin is 1052, which is magnitudes higher than that of transferrin (93). In contrast to enterobactin, aerobactin is not an effective competitor for transferrin-bound iron because of its much lower affinity for ferric iron. Indeed, investigations have demonstrated that enterobactin sequesters iron predominantly from transferrin, while the iron source of aerobactin is host cells (32). Thus, production of these two siderophores may give access to both sources of iron, resulting in enhanced growth in the host.
Data on the incidence of aerobactin-producing Klebsiella ∼ndicates that this siderophore does not play a central role in the pathogenicity of the genus Klebsiella. It should be pointed out, however, that clinical K. pneumoniae isolates, which do not synthesize aerobactin themselves, are entirely capable of using exogenously introduced aerobactin as their sole source of iron (253). By synthesizing only the intrinsically expressed aerobactin receptor, such strains could derive an advantage over other aerobactin-synthesizing bacteria in mixed infections. The aerobactin-mediated iron uptake system would thus be an indirect contributor to the pathogenicity of the genus Klebsiella.
As in many enterobacteria, and as has been especially well studied in E. coli, other factors have also been demonstrated in Klebsiella spp. Although the production of cytotoxins (102, 127, 149, 150, 229), enterotoxins (95, 122–125, 148) and hemolysin (4–6, 17) has been sporadically described, these features probably play a rather minor role in Klebsiella.
VACCINATION EFFORTS
As stated above, most Klebsiella infections are acquired during hospital stays and account for 5 to 7.5% of all nosocomial infections. The morbidity and mortality of severe systemic infections, such as bacteremia and pneumonia, remain high despite the use of appropriate antibiotic therapy. Fatality rates of 20 to 50% in Klebsiella bacteremia and of more than 50% in Klebsiella pneumonia have been reported (35, 55, 64). Moreover, Klebsiella infections in pediatric wards have become a major concern. In neonatal intensive care units, Klebsiella is one of the three or four most common pathogens (98). This is apparently related to the observation that premature neonates, especially those in neonatal intensive care units are more likely than other neonates to develop an intestinal flora in which Klebsiella spp. are highly prevalent. These findings, taken together with the emergence of ESBL-producing multiresistant strains, indicate the need for a means of immunological control of Klebsiella infections. Such measures may include immunoprophylaxis (active vaccination of patients who are at risk) as well as immunotherapy (passive immunization by hyperimmune sera).
Among the different cell constituents, two surface components are mainly being discussed as candidates for an anti-Klebsiella vaccine: LPS and CPS.
Lipopolysaccharides
Due to their endotoxic properties, LPS are considered important in the pathology of septicemia. Until recently, Klebsiella LPS O antigens were generally considered to be masked by the capsule polysaccharides and thus not to be exposed on surface, leaving them inappropriate as vaccine candidates. Recent studies, however, demonstrated surface exposure of O-antigens in strains expressing particular capsular serotypes (238). The small number of different Klebsiella O-types is a great advantage with respect to their applicability as vaccines. In contrast to the K antigens, only eight O types are known, O1 being the most commonly found O type in clinical isolates. A multivalent LPS vaccine composed of these eight O antigens or at least the inclusion of the common O1 antigen in a broad spectrum capsular polysaccharide vaccine might be a promising approach. Recently, the administration of monoclonal antibodies to the Klebsiella O1 antigen has been reported to be protective in a mouse model of lethal endotoxemia (136). Moreover, the inclusion of O antigens in a multivalent Klebsiella vaccine formulation might be of additional benefit due to strong adjuvant action, as has been demonstrated for the Klebsiella O3 lipopolysaccharide (259). A great drawback of active immunization with LPS-containing vaccines, however, is adverse toxic reactions, which must be expected because of the endotoxin content. Thus, each Klebsiella vaccine composed of O antigens has to be rendered safe by sufficient detoxification of the LPS.
Capsular Polysaccharides
CPS have been the obvious vaccine candidates for several reasons. Capsules are produced by almost all Klebsiella strains; they represent the outermost layer of surface structures in contact with the host milieu, and they have been proven to be highly immunogenic and nontoxic (57). A serious disadvantage of a Klebsiella CPS vaccine is the great number of K antigens (77 different antigens). However, in a study of the incidence of the capsule types among bacteremic Klebsiella isolates, Cryz et al. observed that only 25 serotypes made up 70% of all bacteremic strains (59). Based on their seroepidemiological findings, they formulated a 24-valent Klebsiella CPS vaccine that subsequently was proven to be safe and immunogenic (58). To date, this vaccine seems to be the most promising approach for preventing sepsis caused by Klebsiella and has already passed phase I human trials (72). The most recent study of the 24-valent Klebsiella CPS vaccine demonstrated an excellent antibody response after active immunization in patients with acute blunt or penetrating trauma (39).
CONCLUDING REMARKS
Klebsiellae are opportunistic pathogens and can give rise to severe diseases such as septicemia, pneumonia, UTI, and soft tissue infection. Typically, Klebsiella infections are nosocomial. The hospitalized, immunocompromised patient with underlying diseases is the main target of these bacteria. Thus, Klebsiella infections may serve as a paradigm of hospital-acquired infections. Their incidence of 5 to 7% of all hospital-acquired infections ranks them among the most important nosocomial pathogens.
In this context, some new trends have been observed in the past several years.
(i) An increasing number of endemic and epidemic outbreaks in pediatric wards has been reported. Especially common are Klebsiella infections causing septicemia and meningitis in newborns in neonatal intensive care units. Since more and more of these outbreaks have been caused by multidrug-resistant strains, Klebsiella neonatal infections are becoming a major concern of the pediatrician. Especially peculiar has been the repeated frequent isolation of multidrug-resistant Klebsiella isolates expressing serotype K55. It remains to be seen whether this observation reflects the spread of a particular “neonatal” Klebsiella clone.
(ii) Hospital outbreaks of multidrug-resistant Klebsiella spp. are often caused by a new type of strain, the ESBL producers. The incidence of ESBL-producing strains among clinical Klebsiella isolates has been steadily increasing over the past several years. Frequencies of up to 40% have been reported in certain regions. Currently, the available data suggest a further increase in the incidence of ESBL-producing isolates. As a result, the therapeutic options are becoming limited, so that in the near future there will be an urgent need for hospital infection control measures that counter the spread of ESBL-producing bacteria.
(iii) Until recently, K. pneumoniae and K. oxytoca have been considered to be the only pathogenic Klebsiella species. However, the newer species K. terrigena and K. planticola, formerly regarded as “environmental” Klebsiella species, have been demonstrated to occur in human clinical specimens. K. planticola, in particular, has been isolated with astonishing frequency from human infectious processes. So far, it is unclear what kind of pathogenicity factors K. planticola might possess or whether this species expresses the same factors that have been described for K. pneumoniae. K. planticola can, however, be expected to be of clinical significance, and the question remains whether the identification of this species by standard laboratory procedures should be recommended.
Nosocomial Klebsiella infections continue to be a heavy burden on the economy and on the life expectancy of patients in developed countries. Hospital infection prevention and control programs led in the past to considerable improvements in the management and control of these infections. However, there is a general agreement that further progress in prevention of hospital-acquired infections will require new approaches to infection control. As a future challenge for the advancement of preventive measures against nosocomial Klebsiella infections, the usefulness of new concepts needs to be evaluated. A number of different approaches are conceivable.
One possible measure is the vaccination of persons at risk. Regardless of whether active or passive immunization is performed (the latter preferentially by a cocktail of monoclonal anti-Klebsiella antibodies), the question has to be raised about whom to vaccinate. While little controversy is to be expected for vaccinating immunocompromised hospitalized patients to prevent fatal Klebsiella pneumonia and septicemia, the justification of immunological measures in other patient groups is being debated. A good example in this respect is Klebsiella UTI in elderly individuals. Most cases of bacterial pyelonephritis are not caused by Klebsiella but by E. coli strains. However, although Klebsiella species are not a predominant cause of UTI, they can cause significant renal scarring even after a single episode of infection. Moreover, infections with these uropathogens are more likely to lead to death than are infections with most E. coli strains. The question whether a Klebsiella vaccine should be recommended for persons older than 60 years has to be clarified by cost-benefit analyses.
Another point of interest is the possible eradication of klebsiellae in patients during their hospital stay. One of the new approaches is the use of cranberry juice. This juice shows a pronounced anti-adhesive effect on enterobacteria and therefore might prevent colonization of hospitalized patients or even eradicate these bacteria in colonized persons. Cranberry juice might be of benefit both because of its high content of fructose as an inhibitor of type 1 pili and because of the presence of a high-molecular-weight constituent that blocks mannose-resistant adhesion (165). It has been postulated that the juice acts on the gastrointestinal organisms to eliminate the source of infection rather than on the bladder because recurrent but not acute UTIs were prevented (10). Daily consumption of cranberry juice cocktail has been demonstrated to cause bacteriuric elderly persons to become and remain abacteriuric. The use of cranberry juice cocktail in the hospital setting might be a paradigm for cheap and simple preventive intervention without the use of antibiotics or vaccines.
Fascinating future aspects are also raised by new findings on so far unknown host defense mechanisms. As mentioned above, lung SP-A increases phagocytosis of Klebsiella by acting as an opsonin and by activating alveolar macrophages. Another surfactant protein (SP-D) has been reported to interact with bacterial LPS. It has been suggested that SP-D plays an important role in the defense of the lungs against gram-negative bacteria. Investigations in the near future will show whether surfactant proteins may be useful in new therapeutic approaches and whether they are a meaningful addition to the management of nosocomial Klebsiella infections.
REFERENCES
- 1.Aguilar A, Merino S, Rubires X, Tomas J M. Influence of osmolarity on lipopolysaccharides and virulence of Aeromonas hydrophila serotype O:34 strains grown at 37°C. Infect Immun. 1997;65:1245–1250. doi: 10.1128/iai.65.4.1245-1250.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albertí S, Alvarez D, Merino S, Casado M T, Vivanco F, Tomás J M, Benedí V J. Analysis of complement C3 deposition and degradation on Klebsiella pneumoniae. Infect Immun. 1996;64:4726–4732. doi: 10.1128/iai.64.11.4726-4732.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albertí S, Marqués G, Camprubi S, Merino S, Tomás J M, Vivanco F, Benedí V J. C1q binding and activation of the complement classical pathway by Klebsiella pneumoniae outer membrane proteins. Infect Immun. 1993;61:852–860. doi: 10.1128/iai.61.3.852-860.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albesa I. Klebsiella pneumoniae haemolysin adsorption to red blood cells. J Appl Bacteriol. 1989;67:263–266. doi: 10.1111/j.1365-2672.1989.tb02494.x. [DOI] [PubMed] [Google Scholar]
- 5.Albesa I, Barberis L I, Pájaro M C, Eraso A J. Actividad hemolítica de Klebsiella pneumoniae sobre glóbulos rojos de conejo. Rev Latinoam Microbiol. 1985;27:83–87. [PubMed] [Google Scholar]
- 6.Albesa I, Barberis L I, Pajaro M C, Farnochi M C, Eraso A J. A thiol-activated hemolysin in gram-negative bacteria. Can J Microbiol. 1985;31:297–300. doi: 10.1139/m85-055. [DOI] [PubMed] [Google Scholar]
- 7.Amako K, Meno Y, Takade A. Fine structures of the capsules of Klebsiella pneumoniae and Escherichia coli K1. J Bacteriol. 1988;170:4960–4962. doi: 10.1128/jb.170.10.4960-4962.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arlet G, Rouveau M, Casin I, Bouvet P J M, Lagrange P H, Philippon A. Molecular epidemiology of Klebsiella pneumoniae strains that produce SHV-4 β-lactamase and which were isolated in 14 french hospitals. J Clin Microbiol. 1994;32:2553–2558. doi: 10.1128/jcm.32.10.2553-2558.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Athamna A, Ofek I, Keisari Y, Markowitz S, S. D G G, Sharon N. Lectinophagocytosis of encapsulated Klebsiella pneumoniae mediated by surface lectins of guinea pig alveolar macrophages and human monocyte-derived macrophages. Infect Immun. 1991;59:1673–1682. doi: 10.1128/iai.59.5.1673-1682.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avorn J, Monane M, Gruwitz J H, Glynn J, Choodnovskiy I, Lipsitz L A. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271:751–754. doi: 10.1001/jama.1994.03510340041031. [DOI] [PubMed] [Google Scholar]
- 11.Ayars G H, Altman L C, Fretwell M D. Effect of decreased salivation and pH on the adherence of Klebsiella species to human buccal epithelial cells. Infect Immun. 1982;38:179–182. doi: 10.1128/iai.38.1.179-182.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayling-Smith B, Pitt T L. State of the art in typing: Klebsiella spp. J Hosp Infect. 1990;16:287–295. doi: 10.1016/0195-6701(90)90001-5. [DOI] [PubMed] [Google Scholar]
- 13.Babu J P, Abraham S N, Dabbous M K, Beachey E H. Interaction of a 60-Kilodalton d-mannose-containing salivary glycoprotein with type 1 fimbriae of Escherichia coli. Infect Immun. 1986;54:104–108. doi: 10.1128/iai.54.1.104-108.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagley S, Seidler R J, Brenner D J. Klebsiella planticola sp. nov.: a new species of Enterobacteriaceae found primarily in nonclinical environments. Curr Microbiol. 1981;6:105–109. [Google Scholar]
- 15.Bagley S T, Seidler R J, Talbot H W J, Morrow J E. Isolation of Klebsiellae from within living wood. Appl Environ Microbiol. 1978;36:178–185. doi: 10.1128/aem.36.1.178-185.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balish M J, Jensen J, Uehling D T. Bladder mucin: a scanning electron microscopy study in experimental cystitis. J Urol. 1982;128:1060–1063. doi: 10.1016/s0022-5347(17)53344-3. [DOI] [PubMed] [Google Scholar]
- 17.Barberis L I, Eraso A J, Pájaro M C, Albesa I. Molecular weight determination and partial characterization of Klebsiella pneumoniae hemolysins. Can J Microbiol. 1986;32:884–888. doi: 10.1139/m86-161. [DOI] [PubMed] [Google Scholar]
- 18.Bascomb S, Lapage S P, Willcox W R, Curtis M A. Numerical classification of the tribe Klebsiellae. J Gen Microbiol. 1971;66:279–295. doi: 10.1099/00221287-66-3-279. [DOI] [PubMed] [Google Scholar]
- 19.Batshon B A, Baer H, Shaffer M F. Immunologic paralysis produced in mice by Klebsiella pneumoniae type 2 polysaccharide. J Immunol. 1963;90:121–126. [PubMed] [Google Scholar]
- 20.Bauernfeind A. Epidemiological typing of Klebsiella by bacteriocins. Methods Microbiol. 1984;16:213–224. [Google Scholar]
- 21.Bauernfeind A, Petermüller C, Schneider R. Bacteriocins as tools in analysis of nosocomial Klebsiella pneumoniae infections. J Clin Microbiol. 1981;14:15–19. doi: 10.1128/jcm.14.1.15-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauernfeind A, Rosenthal E, Eberlein E, Holley M, Schweighart S. Spread of Klebsiella pneumoniae producing SHV-5 beta-lactamase among hospitalized patients. Infection. 1993;21:18–22. doi: 10.1007/BF01739303. [DOI] [PubMed] [Google Scholar]
- 23.Benjamin W H, Turnbough C L, Posey B S, Briles D E. The ability of Salmonella typhimurium to produce the siderophore enterobactin is not a virulence factor in mouse typhoid. Infect Immun. 1985;50:392–397. doi: 10.1128/iai.50.2.392-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennet R, Eriksson M, Melen B, Zetterström R. Changes in the incidence and spectrum of neonatal septicemia during a fifteen-year period. Acta Paediatr Scand. 1985;74:687–690. doi: 10.1111/j.1651-2227.1985.tb10014.x. [DOI] [PubMed] [Google Scholar]
- 25.Bennett C J, Young M N, Darrington H. Differences in urinary tract infection in male and female spinal cord injury patients on intermittent catheterization. Paraplegia. 1995;33:69–72. doi: 10.1038/sc.1995.17. [DOI] [PubMed] [Google Scholar]
- 26.Bergogne-Berezin E. Nosocomial pathogens: new pathogens, incidence, prevention. Presse Med. 1995;24:89–97. [PubMed] [Google Scholar]
- 27.Bingen E H, Denamur E, Elion J. Use of ribotyping in epidemiological surveillance of nosocomial outbreaks. Clin Microbiol Rev. 1994;7:311–327. doi: 10.1128/cmr.7.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bingen E H, Desjardins P, Arlet G, Bourgeois F, Marianikurkdjian P, Lambertzechovsky N Y, Denamur E, Philippon A, Elion J. Molecular epidemiology of plasmid spread among extended broad-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates in a pediatric hospital. J Clin Microbiol. 1993;31:179–184. doi: 10.1128/jcm.31.2.179-184.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Björkstén B, Kaijser B. Interaction of human serum and neutrophils with Escherichia coli strains: differences between strains isolated from urine of patients with pyelonephritis or asymptomatic bacteriuria. Infect Immun. 1978;22:308–311. doi: 10.1128/iai.22.2.308-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradford P A, Urban C, Mariano N, Projan S J, Rahal J J, Bush K. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob Agents Chemother. 1997;41:563–569. doi: 10.1128/aac.41.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenner D J, Farmer III J J, Hickman F W, Asbury M A, Steigerwalt A G. Taxonomic and nomenclature changes in Enterobacteriaceae. HEW publication (CDC) 79-8356. Atlanta, Ga: Centers for Disease Control; 1977. [Google Scholar]
- 32.Brock J H, Williams P H, Licéaga J, Wooldridge K G. Relative availability of transferrin-bound iron and cell-derived iron to aerobactin-producing and enterobactin-producing strains of Escherichia coli and to other microorganisms. Infect Immun. 1991;59:3185–3190. doi: 10.1128/iai.59.9.3185-3190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown C, Seidler R J. Potential pathogens in the environment: Klebsiella pneumoniae, a taxonomic and ecological enigma. Appl Microbiol. 1973;25:900–904. doi: 10.1128/am.25.6.900-904.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bryan C S, Hornung C A, Reynolds K L, Brenner E R. Endemic bacteremia in Columbia, South Carolina. Am J Epidemiol. 1986;123:113–127. doi: 10.1093/oxfordjournals.aje.a114205. [DOI] [PubMed] [Google Scholar]
- 35.Bryan C S, Reynolds K L, Brenner E R. Analysis of 1,186 episodes of gram-negative bacteremia in non-university hospitals: the effects of antimicrobial therapy. Rev Infect Dis. 1983;5:629–638. doi: 10.1093/clinids/5.4.629. [DOI] [PubMed] [Google Scholar]
- 36.Buffenmeyer C L, Rychek R R, Yee R B. Bacteriocin (klebocin) sensitivity typing of Klebsiella. J Clin Microbiol. 1976;4:239–244. doi: 10.1128/jcm.4.3.239-244.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bullen J J, Rogers H J, Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- 38.Burwen D R, Banerjee S N, Gaynes R P. Ceftazidime resistance among selected nosocomial gram-negative bacilli in the United States. J Infect Dis. 1994;170:1622–1625. doi: 10.1093/infdis/170.6.1622. [DOI] [PubMed] [Google Scholar]
- 39.Campbell W N, Hendrix E, Cryz S, Cross A S. Immunogenicity of a 24-valent Klebsiella capsular polysaccharide vaccine and an eight-valent Pseudomonas O-polysaccharide conjugate vaccine administered to victims of acute trauma. Clin Infect Dis. 1996;23:179–181. doi: 10.1093/clinids/23.1.179. [DOI] [PubMed] [Google Scholar]
- 40.Carpenter J L. Klebsiella pulmonary infections: occurrence at one medical center and review. Rev Infect Dis. 1990;12:672–682. doi: 10.1093/clinids/12.4.672. [DOI] [PubMed] [Google Scholar]
- 41.Casewell M, Talsania H G. Predominance of certain klebsiella capsular types in hospitals in the United Kingdom. J Infect. 1979;1:77–79. [Google Scholar]
- 42.Casewell M W, Phillips I. Epidemiological patterns of klebsiella colonization and infection in an intensive care ward. J Hyg Camb. 1978;80:295–300. doi: 10.1017/s0022172400053651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casewell M W, Phillips I. Hands as a route of transmission for Klebsiella species. Br Med J. 1977;2:1315–1317. doi: 10.1136/bmj.2.6098.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control. National nosocomial infections study report. Annual Summary 1983. Atlanta, Ga: Centers for Disease Control; 1985. pp. 9SS–21SS. [Google Scholar]
- 45.Centers for Disease Control. National nosocomial infections study report. Annual Summary 1975. Atlanta, Ga: Centers for Disease Control; 1977. [Google Scholar]
- 46.Centers for Disease Control. National nosocomial infections study report. Annual Summary 1979. Atlanta, Ga: Centers for Disease Control; 1982. [Google Scholar]
- 47.Centers for Disease Control. Nosocomial infection surveillance, 1983. CDC Surveillance Summaries. 1984;33:955–22SS. [PubMed] [Google Scholar]
- 48.Christensen S C, Korner B. An endemic caused by multiresistant Klebsiella in an urological unit. Scand J Urol Nephrol. 1972;6:232–238. doi: 10.3109/00365597209132093. [DOI] [PubMed] [Google Scholar]
- 49.Ciurana B, Tomás J M. Role of lipopolysaccharide and complement in susceptibility of Klebsiella pneumoniae to nonimmune serum. Infect Immun. 1987;55:2741–2746. doi: 10.1128/iai.55.11.2741-2746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clegg S, Gerlach G F. Enterobacterial fimbriae. J Bacteriol. 1987;169:934–938. doi: 10.1128/jb.169.3.934-938.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Combe M L, Pons J L, Sesboue R, Martin J P. Electrophoretic transfer from polyacrylamide gel to nitrocellulose sheets: a new method to characterize multilocus enzyme genotypes of Klebsiella strains. Appl Environ Microbiol. 1994;60:26–30. doi: 10.1128/aem.60.1.26-30.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooke E M, Pool R, Brayson J C, Edmondson A S, Munro M E, Shinebaum R. Further studies on the source of Klebsiella aerogenes in hospital patients. J Hyg Camb. 1979;83:391–395. doi: 10.1017/s0022172400026218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coovadia Y M, Johnson A P, Bhana R H, Hutchinson G R, George R C, Hafferjee I E. Multiresistant Klebsiella pneumoniae in a neonatal nursery: the importance of maintenance of infection control policies and procedures in the prevention of outbreaks. J Hosp Infect. 1992;22:197–205. doi: 10.1016/0195-6701(92)90044-m. [DOI] [PubMed] [Google Scholar]
- 54.Cowan S T, Steel K J, Shaw C, Duguid J P. A classification of the Klebsiella group. J Gen Microbiol. 1960;23:601–612. doi: 10.1099/00221287-23-3-601. [DOI] [PubMed] [Google Scholar]
- 55.Cryz S J. Progress in immunization against Klebsiella infections. Eur J Clin Microbiol. 1983;2:523–528. doi: 10.1007/BF02016559. [DOI] [PubMed] [Google Scholar]
- 56.Cryz S J, Fürer E, Germanier R. Experimental Klebsiella pneumoniae burn wound sepsis: role of capsular polysaccharide. Infect Immun. 1984;43:440–441. doi: 10.1128/iai.43.1.440-441.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cryz S J, Jr, Fürer E, Germanier R. Safety and immunogenicity of Klebsiella pneumoniae K1 capsular polysaccharide vaccine in humans. J Infect Dis. 1985;151:665–671. doi: 10.1093/infdis/151.4.665. [DOI] [PubMed] [Google Scholar]
- 58.Cryz S J, Jr, Mortimer P, Cross A S, Fürer E, Germanier R. Safety and immunogenicity of a polyvalent Klebsiella capsular polysaccharide vaccine in humans. Vaccine. 1986;4:15–20. doi: 10.1016/0264-410x(86)90092-7. [DOI] [PubMed] [Google Scholar]
- 59.Cryz S J, Mortimer P M, Mansfield V, Germanier R. Seroepidemiology of Klebsiella bacteremic isolates and implications for vaccine development. J Clin Microbiol. 1986;23:687–690. doi: 10.1128/jcm.23.4.687-690.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curie K, Speller D C E, Simpson R A, Stephens M, Cooke D I. A hospital epidemic caused by a gentamicin-resistant Klebsiella aerogenes. J Hyg Camb. 1978;80:115–123. doi: 10.1017/s0022172400053432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Darfeuille-Michaud A, Jallat C, Aubel D, Sirot D, Rich C, Sirot J, Joly B. R-plasmid-encoded adhesive factor in Klebsiella pneumoniae strains responsible for human nosocomial infections. Infect Immun. 1992;60:44–45. doi: 10.1128/iai.60.1.44-55.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis T J, Matsen J M. Prevalence and characteristics of Klebsiella species: relation to association with a hospital environment. J Infect Dis. 1974;130:402–405. doi: 10.1093/infdis/130.4.402. [DOI] [PubMed] [Google Scholar]
- 63.De Champs C, Rouby D, Guelon D, Sirot J, Sirot D, Beytout D, Gourgand J M. A case-control study of an outbreak of infections caused by Klebsiella pneumoniae strains producing CTX-1 (TEM-3) beta-lactamase. J Hosp Infect. 1991;18:5–13. doi: 10.1016/0195-6701(91)90088-p. [DOI] [PubMed] [Google Scholar]
- 64.de la Torre M G, Romero-Vivas J, Martinez-Beltran J, Guerrero A, Meseguer M, Bouza E. Klebsiella bacteremia: an analysis of 100 episodes. Rev Infect Dis. 1985;7:143–150. doi: 10.1093/clinids/7.2.143. [DOI] [PubMed] [Google Scholar]
- 65.de Lorenzo V, Martinez J L. Aerobactin production as a virulence factor: a reevaluation. Eur J Clin Microbiol Infect Dis. 1988;7:621–629. doi: 10.1007/BF01964239. [DOI] [PubMed] [Google Scholar]
- 66.Di Martino P, Bertin Y, Girardeau P, Livrelli V, Joly B, Darfeuille-Michaud A. Molecular characterization and adhesive properties of CF29K, an adhesin of Klebsiella pneumoniae strains involved in nosocomial infections. Infect Immun. 1995;63:4336–4344. doi: 10.1128/iai.63.11.4336-4344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Di Martino P, Livrelli V, Sirot D, Joly B, Darfeuille-Michaud A. A new fimbrial antigen harbored by CAZ-5/SHV-4-producing Klebsiella pneumoniae strains involved in nosocomial infections. Infect Immun. 1996;64:2266–2273. doi: 10.1128/iai.64.6.2266-2273.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doebbeling B N. Epidemics: identification and management. In: Wenzel R P, editor. Prevention and control of nosocomial infections. 2nd ed. Baltimore, Md: The Williams & Wilkins Co.; 1993. pp. 177–206. [Google Scholar]
- 69.Domenico P, Johanson W G, Straus D C. Lobar pneumonia in rats produced by clinical isolates of Klebsiella pneumoniae. Infect Immun. 1982;37:327–335. doi: 10.1128/iai.37.1.327-335.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duggan J M, Oldfield G S, Ghosh H K. Septicaemia as a hospital hazard. J Hosp Infect. 1985;6:406–412. doi: 10.1016/0195-6701(85)90057-x. [DOI] [PubMed] [Google Scholar]
- 71.Edberg S C, Piscitelli V, Cartter M. Phenotypic characteristics of coliform and noncoliform bacteria from a public water supply compared with regional and national clinical species. Appl Environ Microbiol. 1986;52:474–478. doi: 10.1128/aem.52.3.474-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edelman R, Taylor D N, Wasserman S S, Mcclain J B, Cross A S, Sadoff J C, Que J U, Cryz S J. Phase 1 trial of a 24-valent Klebsiella capsular polysaccharide vaccine and an eight-valent Pseudomonas O-polysaccharide conjugate vaccine administered simultaneously. Vaccine. 1994;12:1288–1294. doi: 10.1016/s0264-410x(94)80054-4. [DOI] [PubMed] [Google Scholar]
- 73.Edmondson A S, Cooke E M. The development and assessment of a bacteriocin typing method for Klebsiella. J Hyg Camb. 1979;82:207–233. doi: 10.1017/s0022172400025626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edmondson A S, Cooke E M, Wilcock A P D, Shinebaum R. A comparison of the properties of Klebsiella strains isolated from different sources. J Med Microbiol. 1980;13:541–550. doi: 10.1099/00222615-13-4-541. [DOI] [PubMed] [Google Scholar]
- 75.Edwards P R, Ewing W H. Identification of Enterobacteriaceae. 4th ed. Minneapolis, Minn: Burgess Publishing Co.; 1986. [Google Scholar]
- 76.Ehrenwort L, Baer H. The pathogenicity of Klebsiella pneumoniae for mice: the relationship to the quantity and rate of production of type-specific capsular polysaccharide. J Bacteriol. 1956;72:713–717. doi: 10.1128/jb.72.5.713-717.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fader R C, Davis C P. Effect of piliation on Klebsiella pneumoniae infection in rat bladders. Infect Immun. 1980;30:554–561. doi: 10.1128/iai.30.2.554-561.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fader R C, Davis C P. Klebsiella pneumoniae-induced experimental pyelitis: the effect of piliation on infectivity. J Urol. 1982;128:197–201. doi: 10.1016/s0022-5347(17)52817-7. [DOI] [PubMed] [Google Scholar]
- 79.Farmer J J, III, Davis B R, Hickman-Brenner F W, McWhorter A, Huntley-Carter G P, Asbury M A, Riddle C, Wathen-Grady H G, Elias C, Fanning G R, Steigerwalt A G, O’Hara C M, Morris G K, Smith P B, Brenner D J. Biochemical identification of new species and biogroups of Enterobacteriaceae isolated from clinical specimens. J Clin Microbiol. 1985;21:46–76. doi: 10.1128/jcm.21.1.46-76.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Favre-Bonte S, Darfeuille-Michaud A, Forestier C. Aggregative adherence of Klebsiella pneumoniae to human Intestine-407 cells. Infect Immun. 1995;63:1318–1328. doi: 10.1128/iai.63.4.1318-1328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Felson B, Rosenberg L S, Hamburger M J. Roentgen findings in acute Friedländer’s pneumonia. Radiology. 1949;53:559–565. doi: 10.1148/53.4.559. [DOI] [PubMed] [Google Scholar]
- 82.Ferragut C, Izard D, Gavini F, Kersters K, De Ley J, Leclerc H. Klebsiella trevisanii: a new species from water and soil. Int J Syst Bacteriol. 1983;33:133–142. [Google Scholar]
- 83.Firon N, Ofek I, Sharon N. Carbohydrate-binding sites of the mannose-specific fimbrial lectins of enterobacteria. Infect Immun. 1984;43:1088–1090. doi: 10.1128/iai.43.3.1088-1090.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Freedman R M, Ingram D L, Gross I, Ehrenkranz R A, Warshaw J B, Baltimore R S. A half century of neonatal sepsis at Yale. Am J Dis Child. 1981;135:140–144. doi: 10.1001/archpedi.1981.02130260032010. [DOI] [PubMed] [Google Scholar]
- 85.French G L, Shannon K P, Simmons N. Hospital outbreak of Klebsiella pneumoniae resistant to broad-spectrum cephalosporins and β-lactam–β-lactamase inhibitor combinations by hyperproduction of SHV-5 β-lactamase. J Clin Microbiol. 1996;34:358–363. doi: 10.1128/jcm.34.2.358-363.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gavini F, Izard D, Grimont P A D, Beji A, Ageron E, Leclerc H. Priority of Klebsiella planticola Bagley, Seidler, and Brenner 1982 over Klebsiella trevisanii Ferragut, Izard, Gavini, Kersters, DeLey, and Leclerc 1983. Int J Syst Bacteriol. 1986;36:486–488. [Google Scholar]
- 87.Gavini F, Leclerc H, Lefèbvre B, Ferragut C, Izard D. Etude taxonomique d’entérobactéries appartenant ou apparentées au genre Klebsiella. Ann Microbiol (Inst Pasteur) 1977;128B:45–49. [PubMed] [Google Scholar]
- 88.Gerlach G F, Allen B L, Clegg S. Type 3 fimbriae among enterobacteria and the ability of spermidine to inhibit MR/K hemagglutination. Infect Immun. 1989;57:219–224. doi: 10.1128/iai.57.1.219-224.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goetz A M, Rihs J D, Chow J W, Singh N, Muder R R. An outbreak of infusion-related Klebsiella pneumoniae bacteremia in a liver transplantation unit. Clin Infect Dis. 1995;21:1501–1503. doi: 10.1093/clinids/21.6.1501. [DOI] [PubMed] [Google Scholar]
- 90.Gotoff S P. Sepsis in the newborn. In: Krugman S, Katz S L, Gershon A A, Wilfert C M, editors. Infectious diseases of children. 9th ed. St. Louis, Mo: Mosby—Year Book; 1992. pp. 402–418. [Google Scholar]
- 91.Gouby A, Neuwirth C, Bourg G, Bouziges N, Carlesnurit M J, Despaux E, Ramuz M. Epidemiological study by pulsed-field gel electrophoresis of an outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a geriatric hospital. J Clin Microbiol. 1994;32:301–305. doi: 10.1128/jcm.32.2.301-305.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gower P E, Taylor P W, Koutsaimanis K G, Roberts A P. Serum bactericidal activity in patients with upper and lower urinary tract infections. Clin Sci. 1972;43:13–22. doi: 10.1042/cs0430013. [DOI] [PubMed] [Google Scholar]
- 93.Griffiths E. The iron-uptake systems of pathogenic bacteria. In: Bullen J J, Griffiths E, editors. Iron and infection. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 69–137. [Google Scholar]
- 94.Griffiths E, Chart H, Stevenson P. High-affinity iron uptake systems and bacterial virulence. In: Roth J A, editor. Virulence mechanisms of bacterial pathogens. Washington, D.C: American Society for Microbiology; 1988. pp. 121–137. [Google Scholar]
- 95.Guarino A, Guandalini S, Alessio M, Gentile F, Tarallo L, Capano G, Migliavacca M, Rubino A. Characteristics and mechanism of action of a heat-stable enterotoxin produced by Klebsiella pneumoniae from infants with secretory diarrhea. Pediatr Res. 1989;25:514–518. doi: 10.1203/00006450-198905000-00018. [DOI] [PubMed] [Google Scholar]
- 96.Gür D, Pitt T L, Hall L M C, Erdal Akalin H, Livermore D M. Diversity of klebsiellae with extended-spectrum β-lactamases at a turkish university hospital. J Hosp Infect. 1992;22:163–178. doi: 10.1016/0195-6701(92)90101-q. [DOI] [PubMed] [Google Scholar]
- 97.Hall F A. Bacteriocine typing of Klebsiella spp. J Clin Pathol. 1971;24:712–716. doi: 10.1136/jcp.24.8.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hart C A. Klebsiellae and neonates. J Hosp Infect. 1993;23:83–86. doi: 10.1016/0195-6701(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 99.Hartstein A I, Morthland V H, Rourke J W, Sykes R, Rashad A L. Plasmid DNA analysis, biotyping, and antimicrobic susceptibility as subtyping tests for Klebsiella pneumoniae and Klebsiella oxytoca. Diagn Microbiol Infect Dis. 1993;16:35–41. doi: 10.1016/0732-8893(93)90128-t. [DOI] [PubMed] [Google Scholar]
- 100.Haverkorn M L, Michel M F. Nosocomial klebsiellas I. Colonization of hospitalized patients. J Hyg Camb. 1979;82:177–193. doi: 10.1017/s0022172400025602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hibbertrogers L C F, Heritage J, Gascoynebinzi D M, Hawkey P M, Todd N, Lewis I J, Bailey C. Molecular epidemiology of ceftazidime resistant Enterobacteriaceae from patients on a paediatric oncology ward. J Antimicrob Chemother. 1995;36:65–82. doi: 10.1093/jac/36.1.65. [DOI] [PubMed] [Google Scholar]
- 102.Higaki M, Chida T, Takano H, Nakaya R. Cytotoxic component(s) of Klebsiella oxytoca on HEp-2 cells. Microbiol Immunol. 1990;34:147–151. doi: 10.1111/j.1348-0421.1990.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 103.Highsmith A K, Jarvis W R. Klebsiella pneumoniae: selected virulence factors that contribute to pathogenicity. Infect Control. 1985;6:75–77. doi: 10.1017/s0195941700062640. [DOI] [PubMed] [Google Scholar]
- 104.Horan T, Culver D, Jarvis W, Emori G, Banerjee S, Martone W, Thornsberry C. Pathogens causing nosocomial infections. Antimicrobic Newsl. 1988;5:65–67. [Google Scholar]
- 105.Hornick D B, Allen B L, Horn M A, Clegg S. Adherence to respiratory epithelia by recombinant Escherichia coli expressing Klebsiella pneumoniae type 3 fimbrial gene products. Infect Immun. 1992;60:1577–1588. doi: 10.1128/iai.60.4.1577-1588.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iwahi T, Abe Y, Nakao M, Imada A, Tsuchiya K. Role of type 1 fimbriae in the pathogenesis of ascending urinary tract infection induced by Escherichia coli. Infect Immun. 1983;39:1307–1315. doi: 10.1128/iai.39.3.1307-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Izard D, Ferragut C, Gavini F, Kersters K, De Ley J, Leclerc H. Klebsiella terrigena, a new species from soil and water. Int J Syst Bacteriol. 1981;31:116–127. [Google Scholar]
- 108.Jacoby G A. Antimicrobial-resistant pathogens in the 1990s. Annu Rev Med. 1996;47:169–179. doi: 10.1146/annurev.med.47.1.169. [DOI] [PubMed] [Google Scholar]
- 109.Jacoby G A, Han P. Detection of extended-spectrum β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J Clin Microbiol. 1996;34:908–911. doi: 10.1128/jcm.34.4.908-911.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jain K, Radsak K, Mannheim W. Differentiation of the oxytocum group from Klebsiella by deoxyribonucleic acid-deoxyribonucleic acid hybridization. Int J Syst Bacteriol. 1974;24:402–407. [Google Scholar]
- 111.Jarlier V, Nicolas M H, Fournier G, Philippon A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 112.Johanson W G, Pierce A K, Sanford J P. Changing pharyngeal bacterial flora of hospitalized patients. Emergence of gram-negative bacilli. N Engl J Med. 1969;281:1137–1140. doi: 10.1056/NEJM196911202812101. [DOI] [PubMed] [Google Scholar]
- 113.Johnson A P, Weinbren M J, Ayling-Smith B, Du Bois S K, Amyes S G B, George R C. Outbreak of infection in two UK hospitals caused by a strain of Klebsiella pneumoniae resistant to cefotaxime and ceftazidime. J Hosp Infect. 1992;20:97–103. doi: 10.1016/0195-6701(92)90111-x. [DOI] [PubMed] [Google Scholar]
- 114.Joiner K A. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- 115.Jones G W, Isaacson R E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10:229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- 116.Jumaa P, Chattopadhyay B. Pseudobacteraemia with multiply-resistant Klebsiella pneumoniae resulting from contamination from the blood gas machine on a neonatal unit. J Hosp Infect. 1992;22:251–255. doi: 10.1016/0195-6701(92)90049-r. [DOI] [PubMed] [Google Scholar]
- 117.Kabha K, Nissimov L, Athamna A, Keisari Y, Parolis H, Parolis L A S, Grue R M, Schlepper-Shafer J, Ezekowitz A R B, Ohman D E, Ofek I. Relationships among capsular structure, phagocytosis, and mouse virulence in Klebsiella pneumoniae. Infect Immun. 1995;63:847–852. doi: 10.1128/iai.63.3.847-852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kabha K, Schmegner J, Keisari Y, Parolis H, Schlepper-Schaefer J, Ofek I. SP-A enhances phagocytosis of Klebsiella by interaction with capsular polysaccharides and alveolar macrophages. Am J Physiol. 1997;272:L344–L352. doi: 10.1152/ajplung.1997.272.2.L344. [DOI] [PubMed] [Google Scholar]
- 119.Katsanis G P, Spargo J, Ferraro M J, Sutton L, Jacoby G A. Detection of Klebsiella pneumoniae and Escherichia coli strains producing extended-spectrum β-lactamases. J Clin Microbiol. 1994;32:691–696. doi: 10.1128/jcm.32.3.691-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kauffmann F. On the serology of the Klebsiella group. Acta Pathol Scand. 1949;26:381–406. [Google Scholar]
- 121.Khimji P L, Miles A A. Microbial iron-chelators and their action on Klebsiella infections in the skin of guinea-pigs. Br J Exp Pathol. 1978;59:137–147. [PMC free article] [PubMed] [Google Scholar]
- 122.Klipstein F A, Engert R F. Enterotoxigenic intestinal bacteria in tropical sprue. III. Preliminary characterization of Klebsiella pneumoniae enterotoxin. J Infect Dis. 1975;132:200–203. doi: 10.1093/infdis/132.2.200. [DOI] [PubMed] [Google Scholar]
- 123.Klipstein F A, Engert R F. Immunological interrelationships between cholera toxin and the heat-labile and heat-stable enterotoxins of coliform bacteria. Infect Immun. 1977;18:110–117. doi: 10.1128/iai.18.1.110-117.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klipstein F A, Engert R F. Purification and properties of Klebsiella pneumoniae heat-stable enterotoxin. Infect Immun. 1976;13:373–381. doi: 10.1128/iai.13.2.373-381.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Klipstein F A, Engert R F, Houghten R A. Immunological properties of purified Klebsiella pneumoniae heat-stable enterotoxin. Infect Immun. 1983;42:838–841. doi: 10.1128/iai.42.2.838-841.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kloos W E, Musselwhite M S. Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl Microbiol. 1975;30:381–395. doi: 10.1128/am.30.3.381-395.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koo F C W, Stein M D. Detection of an extracellular toxin produced by burn isolates of Klebsiella pneumoniae. Curr Microbiol. 1986;13:23–27. [Google Scholar]
- 128.Korhonen T K, Tarkka E, Ranta H, Haahtela K. Type 3 fimbriae of Klebsiella sp.: molecular characterization and role in bacterial adhesion to plant roots. J Bacteriol. 1983;155:860–865. doi: 10.1128/jb.155.2.860-865.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kühn I, Ayling-Smith B, Tullus K, Burman L G. The use of colonization rate and epidemic index as tools to illustrate the epidemiology of faecal Enterobacteriaceae strains in Swedish neonatal wards. J Hosp Infect. 1993;23:287–297. doi: 10.1016/0195-6701(93)90146-q. [DOI] [PubMed] [Google Scholar]
- 130.Legakis N J, Maniatis A, Tzouvelekis L S. Prevalent mechanisms of resistance among common enterobacterial isolates in Greek hospitals. Microb Drug Resist. 1995;1:331–333. doi: 10.1089/mdr.1995.1.331. [DOI] [PubMed] [Google Scholar]
- 131.Lock R, Dahlgren C, Lindén M, Stendahl O, Svensbergh A, Öhman L. Neutrophil killing of two type 1 fimbria-bearing Escherichia coli strains: dependence on respiratory burst activation. Infect Immun. 1990;58:37–42. doi: 10.1128/iai.58.1.37-42.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lucet J C, Chevret S, Decre D, Vanjak D, Macrez A, Bedos J P, Wolff M, Regnier B. Outbreak of multiply resistant enterobacteriaceae in an intensive care unit: Epidemiology and risk factors for acquisition. Clin Infect Dis. 1996;22:430–436. doi: 10.1093/clinids/22.3.430. [DOI] [PubMed] [Google Scholar]
- 133.Lye W C, Chan R K T, Lee E J C, Kumarasinghe G. Urinary tract infections in patients with diabetes mellitus. J Infect. 1992;24:169–174. doi: 10.1016/0163-4453(92)92876-k. [DOI] [PubMed] [Google Scholar]
- 134.Maayan M C, Ofek I, Medalia O, Aronson M. Population shift in mannose-specific fimbriated phase of Klebsiella pneumoniae during experimental urinary tract infection in mice. Infect Immun. 1985;49:785–789. doi: 10.1128/iai.49.3.785-789.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maki D G. Epidemic nosocomial bacteremias. In: Wenzel R P, editor. CRC Handbook of hospital acquired infections. Boca Raton, Fla: CRC Press, Inc.; 1981. pp. 371–512. [Google Scholar]
- 136.Mandine E, Salles M F, Zalisz R, Guenounou M, Smets P. Murine monoclonal antibodies to Klebsiella pneumoniae protect against lethal endotoxemia and experimental infection with capsulated K. pneumoniae. Infect Immun. 1990;58:2828–2833. doi: 10.1128/iai.58.9.2828-2833.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mangan D F, Snyder I S. Mannose-sensitive stimulation of human leucocyte chemiluminescence by Escherichia coli. Infect Immun. 1979;26:1014–1019. doi: 10.1128/iai.26.3.1014-1019.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martin, C. M., N. S. Ikari, J. Zimmerman, and J. A. Waitz. 1971. A virulent nosocomial Klebsiella with a transferable R factor for gentamicin: emergence and suppression. J. Infect. Dis. 124(Suppl.):S24–S29. [DOI] [PubMed]
- 139.Martinez J L, Cercenado E, Baquero F, Pérez-Díaz J C, Delgado-Iribarren A. Incidence of aerobactin production in gram-negative hospital isolates. FEMS Microbiol Lett. 1987;43:351–353. [Google Scholar]
- 140.Matsen J M, Spindler J A, Blosser R O. Characterization of Klebsiella isolates from natural receiving waters and comparison with human isolates. Appl Microbiol. 1974;28:672–678. doi: 10.1128/am.28.4.672-678.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matsumoto T, Mizunoe Y, Sakamoto N, Tanaka M, Kumazawa J. Increased renal scarring by bacteria with mannose-sensitive pili. Urol Res. 1990;18:299–303. doi: 10.1007/BF00300774. [DOI] [PubMed] [Google Scholar]
- 142.Mayhall C G. Surgical infections including burns. In: Wenzel R P, editor. Prevention and control of nosocomial infections. 2nd ed. Baltimore, Md: The Williams & Wilkins Co.; 1987. pp. 614–664. [Google Scholar]
- 143.McCallum K L, Schoenhals G, Laakso D, Clarke B, Whitfield C. A high-molecular-weight fraction of smooth lipopolysaccharide in Klebsiella serotype O1:K20 contains a unique O-antigen epitope and determines resistance to nonspecific serum killing. Infect Immun. 1989;57:3816–3822. doi: 10.1128/iai.57.12.3816-3822.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Medeiros A A. Nosocomial outbreaks of multiresistant bacteria—extended-spectrum beta-lactamases have arrived in North America. Ann Intern Med. 1993;119:428–430. doi: 10.7326/0003-4819-119-5-199309010-00015. [DOI] [PubMed] [Google Scholar]
- 145.Merino S, Camprubí S, Albertí S, Benedí V J, Tomás J M. Mechanisms of Klebsiella pneumoniae resistance to complement-mediated killing. Infect Immun. 1992;60:2529–2535. doi: 10.1128/iai.60.6.2529-2535.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Meyer K S, Urban C, Eagan J A, Berger B J, Rahal J J. Nosocomial outbreak of Klebsiella infection resistant to late-generation cephalosporins. Ann Intern Med. 1993;119:353–358. doi: 10.7326/0003-4819-119-5-199309010-00001. [DOI] [PubMed] [Google Scholar]
- 147.Miles A A, Khimji P L. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J Med Microbiol. 1975;8:477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- 148.Minami J, Katayama S I, Matsushita O, Sakamoto H, Okabe A. Enterotoxic activity of Klebsiella oxytoca cytotoxin in rabbit intestinal loops. Infect Immun. 1994;62:172–177. doi: 10.1128/iai.62.1.172-177.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Minami J, Okabe A, Shiode J, Hayashi H. Production of a unique cytotoxin by Klebsiella oxytoca. Microb Pathog. 1989;7:203–211. doi: 10.1016/0882-4010(89)90056-9. [DOI] [PubMed] [Google Scholar]
- 150.Minami J, Saito S, Yoshida T, Uemura T, Okabe A. Biological activities and chemical composition of a cytotoxin of Klebsiella oxytoca. J Gen Microbiol. 1992;138:1921–1927. doi: 10.1099/00221287-138-9-1921. [DOI] [PubMed] [Google Scholar]
- 151.Mizuta K, Ohta M, Mori M, Hasegawa T, Nakashima I, Kato N. Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsule (K) types. Infect Immun. 1983;40:56–61. doi: 10.1128/iai.40.1.56-61.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mobley H L T, Chippendale G R, Tenney J H, Mayrer A R, Crisp L J, Penner J L, Warren J W. MR/K hemagglutination of Providencia stuartii correlates with adherence to catheters and with persistence in catheter-associated bacteriuria. J Infect Dis. 1988;157:264–271. doi: 10.1093/infdis/157.2.264. [DOI] [PubMed] [Google Scholar]
- 153.Monnet D, Freney J. Method for differentiating Klebsiella planticola and Klebsiella terrigena from other Klebsiella species. J Clin Microbiol. 1994;32:1121–1122. doi: 10.1128/jcm.32.4.1121-1122.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Monnet D, Freney J, Brun Y, Boeufgras J M, Fleurette J. Difficulties in identifying Klebsiella strains of clinical origin. Zentbl Bakteriol. 1991;274:456–464. doi: 10.1016/s0934-8840(11)80081-2. [DOI] [PubMed] [Google Scholar]
- 155.Montenegro M A, Bitter-Suermann D, Timmis J K, Agüero M E, Cabello F C, Sanyal S C, Timmis K N. TraT gene sequences, serum resistance and pathogenicity-related factors in clinical isolates of Escherichia coli and other gram-negative bacteria. J Gen Microbiol. 1985;131:1511–1521. doi: 10.1099/00221287-131-6-1511. [DOI] [PubMed] [Google Scholar]
- 156.Montgomerie J Z. Epidemiology of Klebsiella and hospital-associated infections. Rev Infect Dis. 1979;1:736–753. doi: 10.1093/clinids/1.5.736. [DOI] [PubMed] [Google Scholar]
- 157.Montgomerie J Z, John J F, Atkins L M, Gilmore D S, Ashley M A. Increased frequency of large R-plasmids in Klebsiella pneumoniae colonizing patients with spinal cord injury. Diagn Microbiol Infect Dis. 1993;16:25–29. doi: 10.1016/0732-8893(93)90126-r. [DOI] [PubMed] [Google Scholar]
- 158.Mori M, Ohta M, Agata N, Kido N, Arakawa Y, Ito H, Komatsu T, Kato N. Identification of species and capsular types of Klebsiella clinical isolates, with special reference to Klebsiella planticola. Microbiol Immunol. 1989;33:887–895. doi: 10.1111/j.1348-0421.1989.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 159.Nassif X, Sansonetti P J. Correlation of the virulence of Klebsiella pneumoniae K1 and K2 with the presence of a plasmid encoding aerobactin. Infect Immun. 1986;54:603–608. doi: 10.1128/iai.54.3.603-608.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Noriega, E. R., R. E. Leibowitz, A. S. Richmond, E. Rubinstein, S. Schaefler, M. S. Simberkoff, and J. J. Rahal. 1975. Nosocomial infection caused by gentamicin-resistant, streptomycin-sensitive Klebsiella. J. Infect. Dis. 131(Suppl):S45–S50. [DOI] [PubMed]
- 161.Nouvellon M, Pons J L, Sirot D, Combe M L, Lemeland J F. Clonal outbreaks of extended-spectrum β-lactamase-producing strains of Klebsiella pneumoniae demonstrated by antibiotic susceptibility testing, β-lactamase typing, and multilocus enzyme electrophoresis. J Clin Microbiol. 1994;32:2625–2627. doi: 10.1128/jcm.32.10.2625-2627.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ofek I, Beachey E H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978;22:247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ofek I, Doyle R J. Bacterial adhesion to cells and tissues. London, United Kingdom: Chapman & Hall, Ltd.; 1994. [Google Scholar]
- 164.Ofek I, Goldhar J, Keisari Y. Nonopsonic phagocytosis of microorganisms. Annu Rev Microbiol. 1995;49:239–276. doi: 10.1146/annurev.mi.49.100195.001323. [DOI] [PubMed] [Google Scholar]
- 165.Ofek I, Goldhar J, Zafriri D, Lis H, Adar R, Sharon N. Anti-Escherichia coli adhesin activity of cranberry and blueberry juices. N Engl J Med. 1991;324:1599. doi: 10.1056/NEJM199105303242214. [DOI] [PubMed] [Google Scholar]
- 166.Ofek I, Kabha K, Athamna A, Frankel G, Wozniak D J, Hasty D L, Ohman D E. Genetic exchange of determinants for capsular polysaccharide biosynthesis between Klebsiella pneumoniae strains expressing serotypes K2 and K21a. Infect Immun. 1993;61:4208–4216. doi: 10.1128/iai.61.10.4208-4216.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ofek I, Sharon N. Lectinophagocytosis: a molecular mechanism of recognition between cell surface sugars and lectins in the phagocytosis of bacteria. Infect Immun. 1988;56:539–547. doi: 10.1128/iai.56.3.539-547.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ohlsson A, Bailey T, Takieddine F. Changing etiology and outcome of neonatal septicemia in Riyadh, Saudi Arabia. Acta Paediatr Scand. 1986;75:540–544. doi: 10.1111/j.1651-2227.1986.tb10246.x. [DOI] [PubMed] [Google Scholar]
- 169.Öhman L, Magnusson K-E, Stendahl O. Mannose-specific and hydrophobic interaction between Escherichia coli and polymorphonuclear leucocytes—influence of bacterial culture period. Acta Pathol Microbiol Immunol Scand Sect B. 1985;93:125–131. doi: 10.1111/j.1699-0463.1985.tb02863.x. [DOI] [PubMed] [Google Scholar]
- 170.Old D C, Adegbola R A. Antigenic relationships among type-3 fimbriae of Enterobacteriaceae revealed by immunoelectronmicroscopy. J Med Microbiol. 1985;20:113–121. doi: 10.1099/00222615-20-1-113. [DOI] [PubMed] [Google Scholar]
- 171.Old D C, Tavendale A, Senior B W. A comparative study of the type-3 fimbriae of Klebsiella species. J Med Microbiol. 1985;20:203–214. doi: 10.1099/00222615-20-2-203. [DOI] [PubMed] [Google Scholar]
- 172.Olling, S. 1977. Sensitivity of gram-negative bacilli to the serum bactericidal activity: a marker of the host parasite relationship in acute and persisting infections. Scand. J. Infect. Dis. 10(Suppl.):1–40. [DOI] [PubMed]
- 173.Opal S, Cross A, Gemski P. K antigen and serum sensitivity of rough Escherichia coli. Infect Immun. 1982;37:956–960. doi: 10.1128/iai.37.3.956-960.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ørskov I. Genus Klebsiella. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 461–465. [Google Scholar]
- 175.Ørskov I. O antigens in the Klebsiella group. Acta Pathol Microbiol Scand. 1954;34:145–156. doi: 10.1111/j.1699-0463.1954.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 176.Ørskov I, Fife-Asbury M A. New Klebsiella capsular antigen, K82, and the deletion of five of those previously assigned. Int J Syst Bacteriol. 1977;27:386–387. [Google Scholar]
- 177.Ørskov I, Ørskov F. Serotyping of Klebsiella. Methods Microbiol. 1984;14:143–164. [Google Scholar]
- 178.Ottow J C G. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- 179.Pagani L, Ronza P, Giacobone E, Romero E. Extended spectrum beta-lactamases from Klebsiella pneumoniae strains isolated at an Italian hospital. Eur J Epidemiol. 1994;10:533–540. doi: 10.1007/BF01719569. [DOI] [PubMed] [Google Scholar]
- 180.Pieroni P, Rennie R P, Ziola B, Deneer H G. The use of bacteriophages to differentiate serologically cross-reactive isolates of Klebsiella pneumoniae. J Med Microbiol. 1994;41:423–429. doi: 10.1099/00222615-41-6-423. [DOI] [PubMed] [Google Scholar]
- 181.Pittet D, Li N, Wenzel R P. Association of secondary and polymicrobial nosocomial bloodstream infections with higher mortality. Eur J Clin Microbiol Infect Dis. 1993;12:813–819. doi: 10.1007/BF02000400. [DOI] [PubMed] [Google Scholar]
- 182.Podschun R. Phenotypic properties of Klebsiella pneumoniae and K. oxytoca isolated from different sources. Zentbl Hyg Umweltmed. 1990;189:527–535. [PubMed] [Google Scholar]
- 183.Podschun R, Fischer A, Ullmann U. Siderophore production of Klebsiella species isolated from different sources. Zentbl Bakteriol. 1992;276:481–486. doi: 10.1016/s0934-8840(11)80673-0. [DOI] [PubMed] [Google Scholar]
- 184.Podschun R, Heineken P, Sonntag H G. Hemagglutinins and adherence properties to HeLa and Intestine 407 cells of Klebsiella pneumoniae and Klebsiella oxytoca isolates. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1987;263:585–593. doi: 10.1016/s0176-6724(87)80203-1. [DOI] [PubMed] [Google Scholar]
- 185.Podschun R, Heineken P, Ullmann U, Sonntag H G. Comparative investigations of Klebsiella species of clinical origin: plasmid patterns, biochemical reactions, antibiotic resistances and serotypes. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1986;262:335–345. doi: 10.1016/s0176-6724(86)80006-2. [DOI] [PubMed] [Google Scholar]
- 186.Podschun R, Penner I, Ullmann U. Interaction of Klebsiella capsule type 7 with human polymorphonuclear leucocytes. Microb Pathog. 1992;13:371–379. doi: 10.1016/0882-4010(92)90080-8. [DOI] [PubMed] [Google Scholar]
- 187.Podschun R, Ullmann U. Bacteriocin typing of environmental Klebsiella isolates. Zentbl Hyg Umweltmed. 1993;195:22–26. [PubMed] [Google Scholar]
- 188.Podschun R, Ullmann U. Bacteriocin typing of Klebsiella spp. isolated from different sources. Zentbl Hyg Umweltmed. 1996;198:258–264. [PubMed] [Google Scholar]
- 189.Podschun R, Ullmann U. Incidence of Klebsiella planticola among clinical Klebsiella isolates. Med Microbiol Lett. 1994;3:90–95. [Google Scholar]
- 190.Podschun R, Ullmann U. Isolation of Klebsiella terrigena from clinical specimens. Eur J Clin Microbiol Infect Dis. 1992;11:349–352. doi: 10.1007/BF01962076. [DOI] [PubMed] [Google Scholar]
- 191.Podschun R, Ullmann U. Klebsiella capsular type K7 in relation to toxicity, susceptibility to phagocytosis and resistance to serum. J Med Microbiol. 1992;36:250–254. doi: 10.1099/00222615-36-4-250. [DOI] [PubMed] [Google Scholar]
- 192.Poh C L, Yap S C, Yeo M. Pulsed-field gel electrophoresis for differentiation of hospital isolates of Klebsiella pneumoniae. J Hosp Infect. 1993;24:123–128. doi: 10.1016/0195-6701(93)90074-a. [DOI] [PubMed] [Google Scholar]
- 193.Pollack M, Niemann R E, Reinhardt J A, Charache P, Jett M P, Hardy P H., Jr Factors influencing colonisation and antibiotic-resistance patterns of gram-negative bacteria in hospital patients. Lancet. 1972;ii:668–671. doi: 10.1016/s0140-6736(72)92084-3. [DOI] [PubMed] [Google Scholar]
- 194.Porat R, Johns M A, McCabe W R. Selective pressures and lipopolysaccharide subunits as determinants of resistance of clinical isolates of gram-negative bacilli to human serum. Infect Immun. 1987;55:320–328. doi: 10.1128/iai.55.2.320-328.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Przondo-Hessek A, Pulverer G. Hemagglutinins of Klebsiella pneumoniae and Klebsiella oxytoca. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1983;255:472–478. [PubMed] [Google Scholar]
- 196.Przondo-Hessek A. Bacteriophages of Klebsiella bacilli. Isolation properties and use in typing. Arch Immunol Ther Exp. 1966;14:413–435. [PubMed] [Google Scholar]
- 197.Ramm L E, Whitlow M B, Mayer M M. Size distribution and stability of the trans-membrane channels formed by complement complex C5b-9. Mol Immunol. 1983;20:155–160. doi: 10.1016/0161-5890(83)90126-8. [DOI] [PubMed] [Google Scholar]
- 198.Ransjö U, Good Z, Jalakas K, Kühn I, Siggelkow I, Åberg B, Anjou E. An outbreak of Klebsiella oxytoca septicemias associated with the use of invasive blood pressure monitoring equipment. Acta Anaesthesiol Scand. 1992;36:289–291. doi: 10.1111/j.1399-6576.1992.tb03466.x. [DOI] [PubMed] [Google Scholar]
- 199.Reinhardt H H, Obedeanu N, Sobel J D. Quantitation of Tamm-Horsfall protein binding to uropathogenic Escherichia coli and lectins. J Infect Dis. 1990;162:1335–1340. doi: 10.1093/infdis/162.6.1335. [DOI] [PubMed] [Google Scholar]
- 200.Reish O, Ashkenazi S, Naor N, Samra Z, Merlob P. An outbreak of multiresistant Klebsiella in a neonatal intensive care unit. J Hosp Infect. 1993;25:287–291. doi: 10.1016/0195-6701(93)90115-g. [DOI] [PubMed] [Google Scholar]
- 201.Reissbrodt R, Rabsch W. Further differentiation of Enterobacteriaceae by means of siderophore-pattern analysis. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1988;268:306–317. doi: 10.1016/s0176-6724(88)80015-4. [DOI] [PubMed] [Google Scholar]
- 202.Rennie R P, Duncan I B R. Combined biochemical and serological typing of clinical isolates of Klebsiella. Appl Microbiol. 1974;28:534–539. doi: 10.1128/am.28.4.534-539.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Riser E, Noone P. Klebsiella capsular type versus site of isolation. J Clin Pathol. 1981;34:552–555. doi: 10.1136/jcp.34.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Roantree R J, Rantz L A. A study of the relationship of the normal bactericidal activity of human serum to bacterial infection. J Clin Invest. 1960;39:72–81. doi: 10.1172/JCI104029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rodriguez-Ortega M, Ofek I, Sharon N. Membrane glycoproteins of human polymorphonuclear leucocytes that act as receptors for mannose-specific Escherichia coli. Infect Immun. 1987;55:968–973. doi: 10.1128/iai.55.4.968-973.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rose H D, Schreier J. The effect of hospitalization and antibiotic therapy on the gram-negative fecal flora. Am J Med Sci. 1968;255:228–236. doi: 10.1097/00000441-196804000-00003. [DOI] [PubMed] [Google Scholar]
- 207.Rosebury T. Microorganisms indigenous to man. New York, N.Y: McGraw Hill Book Co.; 1962. [Google Scholar]
- 208.Rosenthal S, Tager I B. Prevalence of gram-negative rods in the normal pharyngeal flora. Ann Intern Med. 1975;83:355–357. doi: 10.7326/0003-4819-83-3-355. [DOI] [PubMed] [Google Scholar]
- 209.Rubin S J. Klebsiella marker systems. Infect Control. 1985;6:59–63. doi: 10.1017/s0195941700062615. [DOI] [PubMed] [Google Scholar]
- 210.Sakazaki R, Tamura K, Kosako Y, Yoshizaki E. Klebsiella ornithinolytica sp. nov., formerly known as ornithine-positive Klebsiella oxytoca. Curr Microbiol. 1989;18:201–206. [Google Scholar]
- 211.Schaberg D R, Culver D H, Gaynes R P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91:72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 212.Schaechter M, Medoff G, Eisenstein B I. Mechanisms of microbial disease. 2nd ed. Baltimore, Md: The Williams & Wilkins Co.; 1993. [Google Scholar]
- 213.Schiappa D A, Hayden M K, Matushek M G, Hashemi F N, Sullivan J, Smith K Y, Miyashiro D, Quinn J P, Weinstein R A, Trenholme G M. Ceftazidime-resistant Klebsiella pneumoniae and Escherichia coli bloodstream infection: a case-control and molecular epidemiologic investigation. J Infect Dis. 1996;174:529–536. doi: 10.1093/infdis/174.3.529. [DOI] [PubMed] [Google Scholar]
- 214.Seidler R J, Knittel M D, Brown C. Potential pathogens in the environment: cultural reactions and nucleic acid studies on Klebsiella pneumoniae from clinical and environmental sources. Appl Microbiol. 1975;29:819–825. doi: 10.1128/am.29.6.819-825.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Selden R, Lee S, Wang W L, Bennett J V, Eickhoff T C. Nosocomial Klebsiella infections: intestinal colonization as a reservoir. Ann Intern Med. 1971;74:657–664. doi: 10.7326/0003-4819-74-5-657. [DOI] [PubMed] [Google Scholar]
- 216.Sharon N, Ofek I. Mannose specific bacterial surface lectins. In: Mirelman D, editor. Microbial lectins and agglutinins. New York, N.Y: John Wiley & Sons, Inc.; 1986. pp. 55–81. [Google Scholar]
- 217.Simoons-Smit A M, Verweij-van Vught A M J J, Kanis I Y R, MacLaren D M. Biochemical and serological investigations on clinical isolates of klebsiella. J Hyg Camb. 1985;95:265–276. doi: 10.1017/s0022172400062690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Simoons-Smit A M, Verweij-van Vught A M J J, Kanis I Y R, MacLaren D M. Chemiluminescence of human leucocytes stimulated by clinical isolates of Klebsiella. J Med Microbiol. 1985;19:333–338. doi: 10.1099/00222615-19-3-333. [DOI] [PubMed] [Google Scholar]
- 219.Simoons-Smit A M, Verweij-van Vught A M J J, MacLaren D M. The role of K antigens as virulence factors in Klebsiella. J Med Microbiol. 1986;21:133–137. doi: 10.1099/00222615-21-2-133. [DOI] [PubMed] [Google Scholar]
- 220.Simoons-Smit A M, Verweij-van Vught A M J J, MacLaren D M. Virulence of Klebsiella strains in experimentally induced skin lesions in the mouse. J Med Microbiol. 1984;17:67–77. doi: 10.1099/00222615-17-1-67. [DOI] [PubMed] [Google Scholar]
- 221.Sirot D. Extended-spectrum plasmid-mediated beta-lactamases. J Antimicrob Chemother. 1995;36:19–34. doi: 10.1093/jac/36.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- 222.Slopek S. Phage typing of Klebsiella. Methods Microbiol. 1978;11:193–222. [Google Scholar]
- 223.Slopek S, Przondo-Hessek A, Milch H, Deak S. A working scheme for bacteriophage typing of Klebsiella bacilli. Arch Immunol Ther Exp. 1967;15:589–599. [PubMed] [Google Scholar]
- 224.Smith J M B, Chambers S T. Klebsiella oxytoca revealing decreased susceptibility to extended spectrum beta-lactams. J Antimicrob Chemother. 1995;36:265–267. doi: 10.1093/jac/36.1.265. [DOI] [PubMed] [Google Scholar]
- 225.Smith R F, Dayton S L, Chipps D D, Blasi D. Intestinal carriage of Klebsiella and Pseudomonas in burned children and their comparative role in nosocomial infection. Health Lab Sci. 1973;10:173–179. [PubMed] [Google Scholar]
- 226.Spencer R C. Predominant pathogens found in the European Prevalence of Infection in Intensive Care Study. Eur J Clin Microbiol Infect Dis. 1996;15:281–285. doi: 10.1007/BF01695658. [DOI] [PubMed] [Google Scholar]
- 227.Stamm W E, Weinstein R A, Dixon R E. Comparison of endemic and epidemic nosocomial infections. In: Dixon R E, editor. Nosocomial infections. Atlanta, Ga: Yorke Medical Books; 1981. pp. 9–13. [DOI] [PubMed] [Google Scholar]
- 228.Stenzel W, Bürger H, Mannheim W. Zur Systematik und Differentialdiagnostik der Klebsiella-Gruppe mit besonderer Berücksichtigung der sogenannten Oxytocum-Typen. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1972;219:193–203. [PubMed] [Google Scholar]
- 229.Straus D C. Production of an extracellular toxic complex by various strains of Klebsiella pneumoniae. Infect Immun. 1987;55:44–48. doi: 10.1128/iai.55.1.44-48.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Suerbaum S, Friedrich S, Leying H, Opferkuch W. Expression of capsular polysaccharide determines serum resistance in Escherichia coli K 92. Zentbl Bakteriol. 1994;281:146–157. doi: 10.1016/s0934-8840(11)80565-7. [DOI] [PubMed] [Google Scholar]
- 231.Tarkkanen A-M, Allen B, Westerlund B, Holthöfer H, Kuusela P, Risteli L, Clegg S, Korhonen T K. Type V collagen as target for type-3 fimbriae, enterobacterial adherence organelles. Mol Microbiol. 1990;4:1353–1361. doi: 10.1111/j.1365-2958.1990.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 232.Tarkkanen A M, Virkola R, Clegg S, Korhonen T K. Binding of the type 3 fimbriae of Klebsiella pneumoniae to human endothelial and urinary bladder cells. Infect Immun. 1997;65:1546–1549. doi: 10.1128/iai.65.4.1546-1549.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Taylor P W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983;47:46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Taylor P W, Kroll H-P. Effect of lethal doses of complement on the functional integrity of target enterobacteria. Curr Top Microbiol Immunol. 1985;121:135–158. doi: 10.1007/978-3-642-45604-6_7. [DOI] [PubMed] [Google Scholar]
- 235.Tessin I, Trollfors B, Thiringer K. Incidence and etiology of neonatal septicaemia and meningitis in Western Sweden 1975–1986. Acta Paediatr Scand. 1990;79:1023–1030. doi: 10.1111/j.1651-2227.1990.tb11378.x. [DOI] [PubMed] [Google Scholar]
- 236.Thom B T. Klebsiella in faeces. Lancet. 1970;ii:1033. doi: 10.1016/s0140-6736(70)92845-x. [DOI] [PubMed] [Google Scholar]
- 237.Tomás J M, Benedí V J, Ciurana B, Jofre J. Role of capsule and O antigen in resistance of Klebsiella pneumoniae to serum bactericidal activity. Infect Immun. 1986;54:85–89. doi: 10.1128/iai.54.1.85-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tomás J M, Camprubi S, Williams P. Surface exposure of the O-antigen in Klebsiella pneumoniae O1:K1 serotype strains. Microb Pathog. 1988;5:141–147. doi: 10.1016/0882-4010(88)90016-2. [DOI] [PubMed] [Google Scholar]
- 239.Tullus K, Berglund B, Fryklund B, Kühn I, Burman L G. Epidemiology of fecal strains of the family Enterobacteriaceae in 22 neonatal wards and influence of antibiotic policy. J Clin Microbiol. 1988;26:1166–1170. doi: 10.1128/jcm.26.6.1166-1170.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tullus K, Olsson-Liljequist B, Lundström G, Burman L G. Antibiotic susceptibility of 629 bacterial blood and CSF isolates from swedish infants and the therapeutic implications. Acta Paediatr Scand. 1991;80:205–212. doi: 10.1111/j.1651-2227.1991.tb11835.x. [DOI] [PubMed] [Google Scholar]
- 241.Ullmann U. The distribution of Klebsiella pneumoniae serotypes from different sources and their sensitivity to cephalosporins. Infection. 1983;11:S28–S31. doi: 10.1007/BF01641102. [DOI] [PubMed] [Google Scholar]
- 242.Ullmann U. Pattern of microbial isolates in hospitalized patients. Zentbl Bakteriol Mikrobiol Hyg Ser B. 1986;183:103–113. [PubMed] [Google Scholar]
- 243.Urban C, Rahal J J. Klebsiella and extended spectrum β-lactamases. Int J Antimicrob Agents. 1997;8:37–43. doi: 10.1016/s0924-8579(96)00355-x. [DOI] [PubMed] [Google Scholar]
- 244.Venegas M F, Navas E L, Gaffney R A, Duncan J L, Anderson B E, Schaeffer A J. Binding of type 1-piliated Escherichia coli to vaginal mucus. Infect Immun. 1995;63:416–422. doi: 10.1128/iai.63.2.416-422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Venezia R A, Scarano F J, Preston K E, Steele L M, Root T P, Limberger R, Archinal W, Kacica M A. Molecular epidemiology of an SHV-5 extended-spectrum beta-lactamase in enterobacteriaceae isolated from infants in a neonatal intensive care unit. Clin Infect Dis. 1995;21:915–923. doi: 10.1093/clinids/21.4.915. [DOI] [PubMed] [Google Scholar]
- 246.Vermeulen C, Cross A, Byrne W R, Zollinger W. Quantitative relationship between capsular content and killing of K1-encapsulated Escherichia coli. Infect Immun. 1988;56:2723–2730. doi: 10.1128/iai.56.10.2723-2730.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Vesikari T, Isolauri E, Tuppurainen N, Renlund M, Koivisto M, Janas M, Ikonen R S, Kero P, Heinonen K, Nyman R, Kunnas M. Neonatal septicaemia in Finland 1981–85. Acta Paediatr Scand. 1989;78:44–50. doi: 10.1111/j.1651-2227.1989.tb10885.x. [DOI] [PubMed] [Google Scholar]
- 248.Virkola R, Westerlund B, Holthöfer H, Parkkinen J, Kekomäki M, Korhonen T K. Binding characteristics of Escherichia coli adhesins in human urinary bladder. Infect Immun. 1988;56:2615–2622. doi: 10.1128/iai.56.10.2615-2622.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Vosti K L, Randall E. Sensitivity of serologically classified strains of Escherichia coli of human origin to the serum bactericidal system. Am J Med Sci. 1970;259:114–119. doi: 10.1097/00000441-197002000-00005. [DOI] [PubMed] [Google Scholar]
- 250.Walia S, Madhavan T, Reuman P, Tewari R, Duckworth D. Plasmid profiles and klebocin types in epidemiologic studies of infections by Klebsiella pneumoniae. Eur J Clin Microbiol Infect Dis. 1988;7:279–284. doi: 10.1007/BF01963102. [DOI] [PubMed] [Google Scholar]
- 251.Williams P, Chart H, Griffiths E, Stevenson P. Expression of high affinity iron uptake systems by clinical isolates of Klebsiella. FEMS Microbiol Lett. 1987;44:407–412. [Google Scholar]
- 252.Williams P, Lambert P A, Brown M R W, Jones R J. The role of the O and K antigens in determining the resistance of Klebsiella aerogenes to serum killing and phagocytosis. J Gen Microbiol. 1983;129:2181–2191. doi: 10.1099/00221287-129-7-2181. [DOI] [PubMed] [Google Scholar]
- 253.Williams P, Smith M A, Stevenson P, Griffiths E, Tomas J M T. Novel aerobactin receptor in Klebsiella pneumoniae. J Gen Microbiol. 1989;135:3173–3181. doi: 10.1099/00221287-135-12-3173. [DOI] [PubMed] [Google Scholar]
- 254.Williams P, Tomas J M. The pathogenicity of Klebsiella pneumoniae. Rev Med Microbiol. 1990;1:196–204. [Google Scholar]
- 255.Wooldridge K G, Williams P H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 256.Würker M, Beuth J, Ko H L, Przondo-Modarska A, Pulverer G. Type of fimbriation determines adherence of Klebsiella bacteria to human epithelial cells. Zentbl Bakteriol. 1990;274:239–245. doi: 10.1016/s0934-8840(11)80106-4. [DOI] [PubMed] [Google Scholar]
- 257.Yancey R J, Breeding S A L, Lankford C E. Enterochelin (enterobactin): virulence factor for Salmonella typhimurium. Infect Immun. 1979;24:174–180. doi: 10.1128/iai.24.1.174-180.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yinnon A M, Butnaru A, Raveh D, Jerassy Z, Rudensky B. Klebsiella bacteremia: community versus nosocomial infection. Monthly J Assoc Physicians. 1996;89:933–941. doi: 10.1093/qjmed/89.12.933. [DOI] [PubMed] [Google Scholar]
- 259.Yokochi T, Inoue Y, Kato Y, Sugiyama T, Jiang G Z, Kawai M, Fukada M, Takahashi K. Strong adjuvant action of Klebsiella O3 lipopolysaccharide and its inhibition of systemic anaphylaxis. FEMS Immunol Med Microbiol. 1995;10:181–184. doi: 10.1111/j.1574-695X.1995.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 260.Yokochi T, Nakashima I, Kato N. Effect of capsular polysaccharide of Klebsiella pneumoniae on the differentiation and functional capacity of macrophages cultured in vitro. Microbiol Immunol. 1977;21:601–610. doi: 10.1111/j.1348-0421.1977.tb00328.x. [DOI] [PubMed] [Google Scholar]
- 261.Yokochi T, Nakashima I, Kato N. Further studies on generation of macrophages in in vitro cultures of mouse spleen cells and its inhibition by the capsular polysaccharide of Klebsiella pneumoniae. Microbiol Immunol. 1979;23:487–499. doi: 10.1111/j.1348-0421.1979.tb00488.x. [DOI] [PubMed] [Google Scholar]