Dear Editor,
A recent study by Tré-Hardy et al. published in this journal reported a marked and statistically significant antibody decrease between 3 and 6 months of receiving the second dose of mRNA-1273 vaccine. 1
Initial randomized trials of severe acute respiratory coronavirus 2 (SARS-CoV-2) messenger RNA (mRNA) vaccines BNT162b2 and mRNA-1273 have each shown more than 90% efficacy in preventing corona virus disease 19 (COVID-19).2 , 3 More recent real world studies with longer follow up report waning immunity against infection over time .4 We undertook a study to compare effectiveness of BNT162b2 and mRNA-1273 in Qatar as there is limited data on the longitudinal comparative effectiveness of mRNA vaccines.
A retrospective cohort study design was employed. Polymerase Chain Reaction (PCR) test and vaccination data for individuals (≥12 years) was extracted from Primary Health Care Corporation's (PHCC, Qatar's public sector primary care provider) electronic medical records (EMR). It included all national level data from the start date of the vaccination programme (Point Zero) with a follow up period of 45 weeks (16th December 2020 to 31st October 2021).
Vaccinated individuals received one of the only two approved vaccines in Qatar - BNT162b2 or mRNA-1273. Administration of BNT162b2 was initiated in December 2020 and mRNA-1273 in March 2021. Where individuals received two doses of the vaccine, the time interval between them was approximately 20–31 days. The ‘effective date’ of the vaccine was defined as 14 days after receiving the second dose. A positive PCR test (regardless of symptom status) was used for the diagnosis of a SARS-CoV2 infection.
Hazard ratios (HR) were calculated for each vaccine product and time duration since vaccination after adjusting for age, gender, comorbidities and previous SARS-CoV2 infection (before point zero) using the extended Cox-regression model with time dependent covariates .5 The vaccine product, number of doses and time duration were modeled using the segmented time covariate variable. Vaccine effectiveness was calculated as (1- adjusted HR) x 100. Further details of the methodology and additional analyses are provided in the Supplementary file.
A total of 1,241,501 individuals aged 12 years and above were enroled in the study (Table S2 Supplementary file) - 236,735 (19.1%) were unvaccinated, 11,655 (0.9%) received one vaccine dose and 993,111 (80%) received two vaccine doses. Of those vaccinated, 699,239 (69.6%) received BNT162b2 and 305,527 (30.4%) received mRNA-1273 vaccine products (Table S3 Supplementary file).
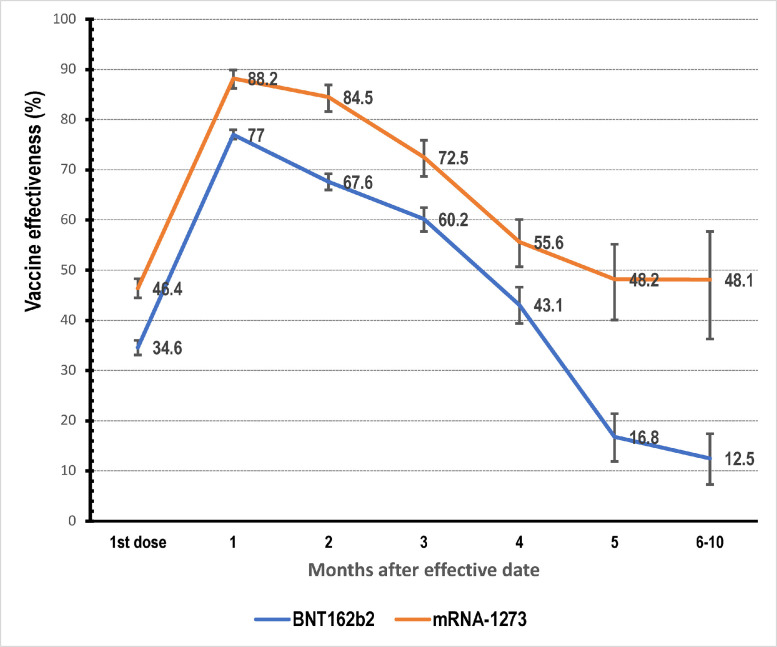
Vaccine effectiveness after the first dose was 34.6% for BNT162b2 and 48.4% for mRNA-1273 (See Fig. 1 ). At month 1, vaccine effectiveness increased to 77% for BNT162b2 and 88.2% for mRNA-1273. From this time point onwards, a steady decline in effectiveness was observed for both vaccine products.
Fig. 1.
Vaccine effectiveness over time.
Between month 2 and month 10 of follow up, effectiveness of BNT162b2 declined from 67.6% to 12.5%. Similarly, effectiveness of mRNA-1273 declined from 84.5% to 48.1% (See Fig. 1). The decline was consistent between the two vaccine products until month 4. From month 4 onwards, the decline in BNT162b2 was significantly higher compared to mRNA-1273. At months 6–10, the difference between mRNA-1273 and BNT162b2 35.6%.
The findings of the study demonstrate vaccine effectiveness of BNT162b2 and mRNA-1273 peaked at month 1. Following this, a significant decline was observed until the end of the follow up period for both vaccine products. Overall, mRNA-1273 was more effective in reducing SARS-CoV2 infections compared to BNT162b2, particularly after month 5. Similar findings have been reported in a sub-population of United State veterans .6
The difference in effectiveness could be attributed to higher humoral immunogenicity of mRNA-1273 compared with the BNT162b2 .7 Other reasons may be related to differences in mRNA content and time interval between the two doses of the vaccine products. mRNA-1273 has higher mRNA content and longer interval between priming and boosting .8
While differences in vaccine effectiveness against risk of SARS-CoV2 between the two vaccine products are observed, the primary objective of mRNA vaccines is to protect against severe disease. Several studies have shown mRNA vaccine products offer high levels of protection against severe disease .9 , 10
Limitations of this study include the exclusion of data for individuals aged < 12 years old and the timing differences between the roll out of the two vaccine products.
In conclusion, there are differences in vaccine effectiveness between the two vaccine products against SARS-CoV2. However, both provide substantial protection against severe disease.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.02.034.
Appendix. Supplementary materials
References
- 1.Tré-Hardy M., Cupaiolo R., Wilmet A., Antoine-Moussiaux T., Della Vecchia A., Horeanga A., et al. Immunogenicity of mRNA-1273 COVID vaccine after 6 months surveillance in health care workers; a third dose is necessary. J Infect. 2021;83(5):559–564. doi: 10.1016/j.jinf.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., Gurtman A., Lockhart S., Perez J.L., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.L., et al. Waning Immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickerman B.A., Gerlovin H., Madenci A.L., Kurgansky K.E., Ferolito B.R., Figueroa Muñiz M.J., et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med. 2021;386(2):105–115. doi: 10.1056/NEJMoa2115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steensels D., Pierlet N., Penders J., Mesotten D., Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326(15):1533–1535. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason T.F.D., Whitston M., Hodgson J., Watkinson R.E., Lau Y.S., Abdulrazeg O., et al. Effects of BNT162b2 mRNA vaccine on COVID-19 infection and hospitalisation amongst older people: matched case control study for England. BMC Med. 2021;19(1):275. doi: 10.1186/s12916-021-02149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Self W.H., Tenforde M.W., Rhoads J.P., Gaglani M., Ginde A.A., Douin D.J., et al. Comparative effectiveness of moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions - United States, March-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(38):1337–1343. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.