Abstract
The coronavirus disease2019 (COVID-19) pandemic has highlighted the need for disposable biosensors that can detect viruses in infected patients quickly due to fast response and also at a low cost.The present review provides an overview of the applications of disposable biosensors based on metal nanoparticles in enzymatic and non-enzymatic sensors with special reference to glucose and H2O2, immunosensors as well as genosensors (DNA biosensors in which the recognized event consists of the hybridization reaction)for point-of-care diagnostics. The disposable biosensors for COVID19 have also been discussed.
Keywords: Disposable biosensor, Metal nanoparticles, Electrochemical sensor, Enzymatic biosensor, Immunosensor, COVID-19
1. Introduction
1.1. Basic principles and applications
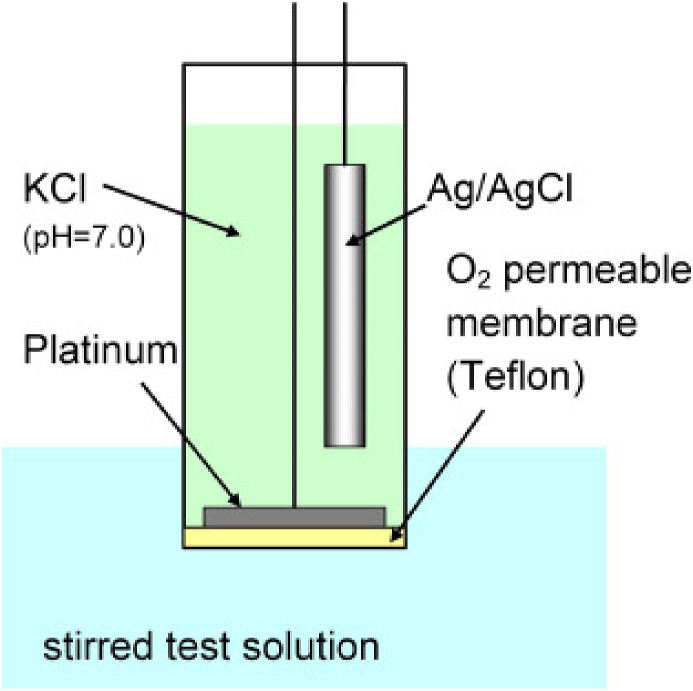
A biosensor is a tool that monitors biological or chemical reactions by producing a signal proportional to an analyte's concentration in a specific reaction. The operational principle of a biosensor is that the biological element interacts with the analyte and produces a physical or chemical change, which can then be sensed by the transducer or the detector producing a signal proportional to the concentration of this analyte (Fig. 1 ). Leland C. Clark Jr., the ‘father of biosensors', invented the first ‘real’ biosensor for the detection of oxygen in 1956 and the oxygen electrode discovery bears the name: the ‘Clark electrode’ [1,2].
Fig. 1.
Schematic representation of a Clark electrode. Reproduced from ref.2 with the permission from Elsevier.
Biosensors have been employed in various fields such as healthcare, food, and environmental protection. Clear, rapid and inexpensive detection tests for diagnostic applications, monitoring of food and water contamination by bacteria and other pathogens, environmental analysis, among others, are required. Such applications fall under the category of ‘single shot’ analytics tools, i.e., where cost-effective, disposable sensing platforms and miniaturization of detection systems are needed for such applications [3,4].
Progress made in the field of nanomedicine and nanobiotechnology has been amply demonstrated by the recent advances reported in drug delivery systems, biosensing and bio detection as well as the development of new drug delivery systems for chronic diseases such as cancer [[5], [6], [7]]. Nanobiosensors essentially comprise biosensors which are integrated with nanomaterials including metal nanoparticles (MNPs). The desirable properties exhibited by MNPs (like large surface area) enhances biorecognition sites and receptor immobilization, better catalytic efficiency, enhanced electron transfer, biocompatibility, etc., making them ideal candidates for biosensing. Advantages of MNP based sensors include significant signal amplification, higher sensitivity, and greater improvements in the detection and quantification of biomolecules and different ions. The immobilization of biomolecules can be achieved using several different strategies and many materials can be used as label. As a demonstration of this growing research area in nanomaterials [[8], [9], [10]], especially in electrochemical sensors [[11], [12], [13], [14], [15], [16], [17]] and catalysis [[18], [19], [20]], one needs to look no further than the number of research papers, review articles and books which has significantly increased over the past decade. Even though there are 146 critical reviews of disposable biosensors published in the present decade, there are none on the application of disposable biosensors based on metal nanoparticles in the health care sector.
1.2. The role of metal nanoparticles in different types of biosensors
Nanostructures from metal, metal oxide, carbon nanotubes, graphene, have been extensively investigated for chemiresistive sensing applications. The small size and high surface to volume ratio of nanomaterials provides several advantages for sensing when compared to bulk films (Fig. 2 ). Metal nanoparticles-based sensors are found to exhibit enhanced sensitivity and selectivity. A variety of metals such as Au, Pt, Pd, Ag, Cu, Co, including rare earth metals have been employed for sensing [21,22]. Metal oxide nanowires have been used extensively because of their easy fabrication techniques and chemical stability [23]. Porous nanostructures are also employed in this field due to their high surface area, accessible surface chemistry, and short pathway for mass and electron transfer.
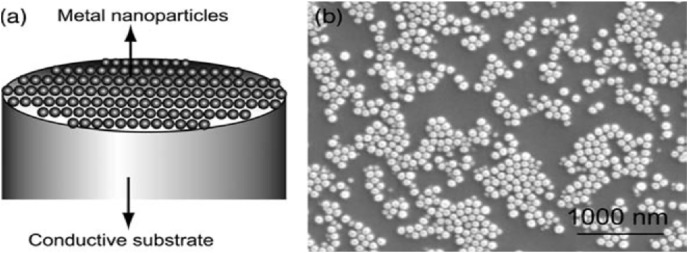
Fig. 2.
(a) Graphical representation of an electrode (b) Scanning electron microscope (SEM) image of a metal nanoparticle-modified electrode surface. Reproduced from ref.30 with the permission from Wiley-VCH.
Several methods which are reported for the preparation of metallic nanoparticles can be divided into two categories: bottom-up approach and top-down approach. The top-down approach involves the use of bulk materials which are reduced to nanoparticles by means of physical, chemical, or mechanical processes. (e.g., mechanical energy, high energy lasers, thermal, and lithography). The examples for these processes are: atomization, arc discharge, laser ablation, electron beam evaporation, focused ion beam lithography, vapor condensation, condensation in inert gas, and electrodeposition [24]. The bottom-up method involves the construction of a structure atom-by-atom, molecule-by-molecule, or cluster-by-cluster and is divided into different categories such as: gaseous phase, liquid phase, solid phase, and biological method. The most commonly employed technique is the wet chemical, liquid-phase reduction [24]. MNPs have a unique combination of biocompatibility, large surface area, good conductivity and also act as a “mass enhancer” or carrier of biorecognition systems in piezoelectric biosensors. The physical or electrochemical changes that occur after binding the biomolecular analyte and the immobilized receptor-target on the surface of the MNPs define the role of MNPs and they can act as immobilizing platforms [25]accelerate electron transfer [26] catalyze the reaction of chemiluminescent materials with their substrates [27] amplify changes in mass [28] and enhance refractive index (RI) changes [29]. MNPs can function as “electron wires” in electrochemical biosensors besides immobilizing the bioreceptors, which allow electrons produced in bioreactions to be transported to sensing electrodes or convert other physicochemical changes to measurable signals that are proportional to the analyte concentration [26].
Fig. 2 shows a schematic of a nanoparticle modified electrode and its SEM image [30]. The surface area available in this type of electrode is much higher when compared to that of a planar macroelectrode and when this electrode is coupled with a nanoparticle possessing high catalytic activity, this results in a sensor which is more selective as well as sensitive (Fig. 3 ).
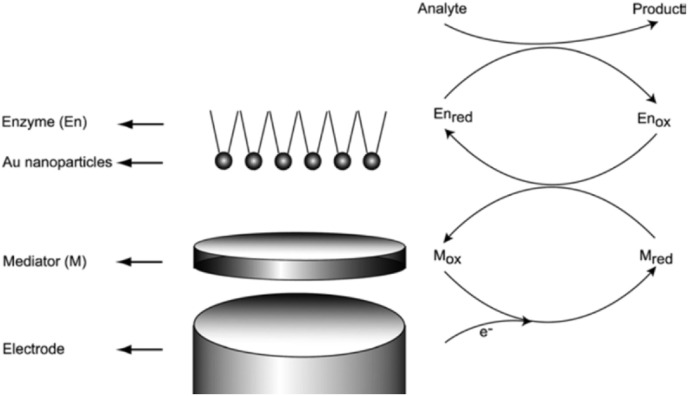
Fig. 3.
Detection mechanism for a nanoparticle based mediated biosensor. Reproduced from ref.30 with the permission from Wiley-VCH.
The above diagram (Fig. 3) shows the initial deposition of a mediator species which has the capacity to catalyze the oxidation or reduction of products from an enzyme reaction onto the surface of macro electrode. It is followed by the adsorption of nanoparticles onto a mediator-electrode surface upon which the enzyme is immobilized [31,32]. The figure also indicates the detection mechanism of the biosensor. The enhanced sensitivity is attributed to the larger surface area available at the nanoparticle immobilized layer and thus increases the concentration of an enzyme on the surface. Metal-based nanoparticles showed good electrical conductivity and can be selected as the suitable mediator for modified electrodes. There is a significant difference in the cost between the metal macroelectrodes derived from noble metals such as gold, platinum and palladium and manufactured nanoparticles, which is one of the major advantages of MNPs while developing commercial sensors. The synthesis of MNPs can be achieved by the reduction of precursor metal salt in the presence of a capping agent such as phosphines, thiols, polymers, and amines [33,34]. The characteristics of MNPs can be altered by tailoring the capping agents, which are capable of stabilizing and dispersing the nanoparticles [11]. When functional groups, which are redox active, are catalytic, bioactive, or act as a selective recognition site, are added to the nanoparticles, then the improved tailored nanoparticles can be employed in a variety of applications such as bioelectronics [[35], [36], [37], [38]], electronic wiring [39,40] opto-electronic [41] and sensor [42] applications.
2. Disposable enzymatic biosensor
A biosensor comprises a bioreceptor (e.g., enzyme) and an effective transducer that can have unique advantages such as sensitivity, portability, quick response user-friendliness and low cost [43]. A broad biosensor community is based on one type of enzyme, viz. Oxidase, e.g. oxidase glucose and oxidase cholesterol.
2.1. Disposable enzymatic glucose biosensor
The determination of glucose is quite important for several fields such as scientific, chemical, biological and the food industry [44]. Several reports are available in the literatures which identify methods for analyzing glucose, such as spectroscopic, chromatographic, and electrochemical techniques. Electrochemical methods are the ones which have been extensively investigated due to their simple manipulation, strong analytical performance and low cost [45]. The amperometric enzyme electrode [2], which is based on glucose oxidase, was first reported in 1962. The primary objective of the researchers, working in this area during that period, was to develop a new glucose biosensor with a lower detection level, wider linear scope, and greater selectivity. Mini-glucose biosensors which exhibit a very good response to glucose (a linear calibration from 0 to 15 mM), offer a favorable anti-interference capability even at a relatively high concentration of interfering species, and have been developed. This suggests the applicability of the mini-sensor based on iridium-modified carbon (Ir-C) to advance a single-use, glucose based disposable electrochemical biosensor [46]. A wide linearity (0–15 nM) with a LOD of 28 μM have been achieved in this technique which is much higher when compared to other glucose sensors. The presence of iridium nanoparticles has improved the efficacy of the enzymatic glucose sensors when compared to other nanomaterials employed in these types of sensors.
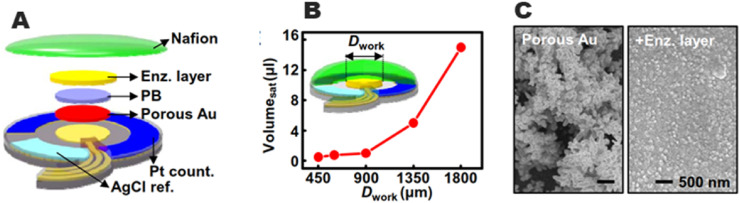
A wearable disposable sweat-based glucose monitoring system which is combined with a transdermal drug delivery module for feedback has been reported by Lee et al. [47].This non-invasive efficiency method eliminates the painful process of blood collection for glucose monitoring. The sweat collection and sensing process can be improved considerably by using a multilayer patch design and miniaturization of sensors. To minimize the quantum of a sweat sample, the reference and counter electrodes should be kept as close as possible (Fig. 4 A). The sample quantity can be reduced to as low as 1 μl (Fig. 4B), which is a 20-fold decrease from that of an earlier report [48]. The working electrode is made up of porous gold and glucose oxidase [Goxdrop-casted on it and then covered by Nafion® and sequentially cross-linked by glutaraldehyde (Fig. 4C). The porous structure of the electrode results in a larger electrochemically active surface area [49,50] and stronger enzyme immobilization [51,52]. The design of the system dictates whether the device is a wearable patch-type or disposable strip type, and it provides a painless and stress-free point-of-care treatment for diabetes mellitus.
Fig. 4.
Graphical representation of the glucose sensor.(B) Minimum volume of the artificial sweat required for sensing with different sizes of the glucose sensor. (C) SEM images before (left) and after (right) immobilization of the enzyme on the porous gold electrode. Reproduced from ref. [47] with the permission from AAAS.
A polyethylene terephthalate based gold electrode (PGE) glucose sensor which is easy to operate at low cost with relatively minimum instrumentation when compared to most wearable thin film gold electrode sensors, was reported and this sensor showed a sensitivity of 22.05 μAmM−1 cm−2 in a linear range of 0.02–1.11 mM with a low detection limit of 2.7 μM (S/N=3) [53]. Nanoparticles of MnO2decorated graphene nanoribbon (MnO2/GNR) composites were prepared by Vukojević et al. by surface modification with drop coating using GOx and Nafion®. Their investigation demonstrated that the synergetic effect of MnO2 decorated graphene nanoribbons is capable of enhancing both the characteristics of the electrode surface and the glucose sensor [54]. Glucose can also be detected easily when the conducting polymer viz., poly (9,9-di-(2-ethylhexyl)-fluorenyl-2,7-diyl)-end capped with 2,5-diphenyl-1,2,4-oxadiazole was used as a matrix [55]. It was also found that there is a strong adherence between AuNPs and a graphene paper surface where the enzyme is uniformly distributed. The combination of a conjugated polymer and AuNPs accelerates electron transfer between an enzyme and the polymer coated transducer due to the wiring effect. The combined effect of these two systems results in high sensitivity and reproducibility. There was another report by Li et al., which describes a matrix nanofilm with iron oxide and AuNPs in chitosan which acts as a matrix for the immobilization of GOx to create a glucose biosensor. When the composite film was employed in the experiment, it significantly increased the effective electrode surface area for GOx loading [56].
2.2. Disposable enzymatic hydrogen peroxide sensors
Hydrogen peroxide (H2O2) is obtained along with the other products for a particular enzyme catalyzed reaction which involves an oxidase as indicated in the following equation:
The detection of H2O2by titration [57], fluorescence [58],chemiluminescence [59], spectrophotometry [60], and electrochemical [61] methods have been reported. The conventional methods are not suitable because of several drawbacks such as low sensitivity, selectivity, long detection time, and complicated instrumentation involved [62]. The electrochemical methods that are based on the electron transfer are preferred over the conventional methods owing to the ease of operation and integration.
Hydrogen peroxide (H2O2) is a reactive oxygen species (ROS) that is present throughout the body, playing various roles in physiological processes, including cellular signaling, where it regulates cell growth, immune activation, and apoptosis [[63], [64], [65], [66]]. However, at high levels, H2O2 can be detrimental to the body, causing cell damage [67], inflammatory disease [68], and cancer [66]. An electrochemical technique based on enzyme biosensors has been widely used for the rapid determination of H2O2 with ease, intrinsic selectivity and efficiency [69,70]. Horseradish peroxidase (HRP) has been the most widely studied in the production of enzyme-based amperometric biosensors due to its easy availability, high purity, and low cost [71]. A highly stable H2O2 biosensor on a screen-printed carbon electrode based on horseradish peroxidase in a chitosan matrix (HRP/AuNP/CHIT) bound with gold nanoparticles exhibited an immediate response to H2O2 [72]. The incorporation of AuNPs provided a larger conductive area which resulted in improved electron transfer kinetics and enhanced the reduction current for H2O2. A novel disposable H2O2 biosensor based on HRP immobilized on the electrode of AuNPs electrodeposited on indium tin oxide (ITO) showed excellent reproducibility, long term selectivity and high stability [73]. A new electrochemical framework for the amperometric sensing of H2O2 and glutamate oxidase (GlutOx) based on platinum nanoparticles (PtNPs) modified three-dimensional (3D) gold nanowire arrays (PtNP/NAEs) can detect 1 μM of H2O2 [74]. PtNPs exhibits a wide linearity of 0.02–20 nM with a LOD of 1 μM has been achieved in this technique which is much higher when compared to other hydrogen peroxide sensors.
Fig. 5A shows the assembly of a GlutOx/PtNP/NAEs electrode. When H2O2 was added repeatedly, a reproducible response was obtained with a sensitivity of 194.6 μAmM−1 cm−2 at 20 °C and a linear range of up to 20 mM (Fig. 5B). The platinum nanoparticles possessed three dimensional surfaces and quite a large surface area to improve the sensitivity and response time. Zhang et al. demonstrated the detection of H2O2 in food samples by employing thionine-catalase functionalized AuNPs on graphene oxide (GO). The AuNPs present in this system resulted in increased conductivity, increase in the amount of catalase as well as the improved sensitivity of biosensor [75].
Fig. 5.
(A) Schematic illustration of the stepwise fabrication of GlutOx/PtNP/NAE electrodes.(B)Amperometric response of a GlutOx modified PtNP/NAE electrode for the successive addition of 0.2 mM glutamate (Eapp= 0.65V vs. Ag/AgCl) and a corresponding concentration plot (inset). Reproduced from ref.74 with the permission from Elsevier.
3. Disposable non-enzymatic sensor
New electrochemical glucose sensors, particularly non-enzymatic sensors, have been developed without any biological catalysts. Functionalized nanomaterials serve as catalyst or immobilization tool or as electro-optical labels to increase sensitivity and detection specificity [[76], [77], [78]]. The selection of the right catalyst for direct electrochemical operation is the main step in manufacturing non-enzymatic glucose sensors. Nanomaterials such as noble metals [79], metal alloys [80,81], metal nanoparticles [82], metal nanoparticles coated with carbon nanotubes (CNT) [83], and graphene [84] are of great interest in the manufacturing of non-enzymatic sensors because of their specific physicochemical characteristics.
These nanostructured materials generally have large surface areas that are suited to the non-enzymatic electrochemical sensing of glucose and hydrogen peroxide. However, they do not provide surface areas large enough for the detection of all the electrochemically active species in a blood sample. Sluggish electrochemical reactions, such as glucose oxidation, benefit from larger electrode surfaces in order to approach diffusion control, while redox-active species that undergo fast electron transfer only use the outermost surface, the electrode that is equivalent to its geometrical planar area. Therefore, a nanostructured electrode produces a faradaic current that is proportional to the concentration of every electrochemically active species regardless of their electrokinetics.
3.1. Disposable non-enzymatic glucose sensors
The major advantage observed for non-enzymatic glucose sensors is that they sense the oxidized product of the analyte obtained by electrocatalytic oxidation unlike the case of bioenzymes. The characteristics of the enzymeless glucose sensor depend on the electrode material. Noble metals such as silver [85], gold [86], palladium [87], and platinum [88] have been employed to construct these types of sensors. The electrooxidation of glucose is a slow process and the modification of electrodes by introducing nanostructures [89] and alloys [90,91] as well as increasing the electrode surface [88] enhances their sensitivity [μ A/(cm2mM)].
In their study on non-enzymatic glucose sensors, Park et al. highlighted the advantages of non-enzymatic sensors such as reliability, usability, reproducibility and non-sensitivity to oxygen [92]. Zengjie Fan et al., developed a non-enzymatic glucose sensor based on copper nanowires (CuNWs), a modified transparent graphene electrode (GTE) as a substitute for an ITO electrode or a glass carbon electrode (GCE) [93]. Copper nanowires were subsequently deposited onto GTE to achieve a CuNWs/GTE hybrid electrode by spin-coating. It was also found that the sensor also displayed a broader linear glucose response over concentrations ranging from 0.005 to 6.0 mM with a sensitivity of 1100 μA/(m · cm2), a low detection limit of 1.6 μM (S/N = 3), and an excellent anti-interference behavior. Dhara et al. reported a one-step synthesis of nanoflowers of CuO and graphene-decorated platinum nanocubes and their use in the amperometric sensing of glucose [94]. In the case of non-enzymatic glucose sensors, a range of metal nanoparticles such as Cu, Pt, Au and Ni have been employed. The efficacy of Pt/CuO LOD is very low (0.01 μM) when it is compared to other non-enzymatic glucose sensors. The sensor demonstrated very high sensitivity (3577 A mM−1cm−2), and glucose oxidation occurred at a low potential (+0.35 V). Tehrani et al. employed direct laser engraved graphene (DLEG) decorated with copper nanocubes coated in pulses as a disposable glucose sensor and it exhibited excellent sensitivity (4532.2 μA/mM.cm2) and selectivity [95]. This sensor also had a low detection limit (LDL) (250 nM) and a linear range of 25 μM–4 mM. The characteristics of this sensor are ideally suited for the detection of glucose in physiological fluids such as tears, saliva, and sweat.
A non-enzymatic electrochemical glucose sensor using reduced graphene oxide decorated with gold – copper oxide nanoparticles (Au – CuO/rGO) has been reported [96]. The Au – CuO/rGO nanocomposite was dispersed in DMF and deposited on an indigenously produced screen-printed electrode (SPE) working field. For direct electrooxidation of glucose with a linear detection range of 1 μM–12 mM, and a lower detection limit of 0.1 μM, the sensor showed strong electrocatalytic behavior in alkaline water. Disposable pencil graphite electrodes (PGE) modified by copper nanoparticles [Cu (NP)-PGE] have been reported by Sima et al. [97]. The modified electrode showed an adsorption-controlled charge transfer process up to 90.0 mVs−1. For a high performance non-enzymatic glucose sensor, the modified PGE is directly utilized as a binder-free and disposable electrode. Three-dimensional copper−cobalt (Cu−Co)/rGO is a strong, interconnected and open porous framework equipped with high conductivity, large exposed space, and unique pore properties that enhance glucose penetration and fast ion and electron transport. The reported sensor in this study exhibits specificity to glucose and can be used for real samples without any modification [98].
Yang and his coworkers adopted a one-pot hydrothermal synthesis of a non-enzymatic nanocomposite disposable electrochemical sensor, containing copper sulfide nanoflake-reduced graphene oxide (rGO/CuSNFs). They observed a simultaneous reduction of graphene oxide and in situ generation of CuSnanoflakes [99]. The catalytic activity of the sensor is quite appreciable with a fast response time of <6 s, a wide linear range from 1 to 2000 μM, a high sensitivity of 53.5 μM(cm2mM−1)and a LDL of 0.19 μM. The rGO/CuSNFs/GCE was quite stable over a long period and exhibited very high reproducibility as well as negligible interference from other species during glucose sensing (Fig. 6 ). Besides, the sensor was employed for the detection of glucose in human urine and blood serum samples and hence it is a potential candidate for non-enzymatic glucose sensing in real samples.
Fig. 6.
Schematic illustration of a rGO/CuSNFs composite formation and their application in non-enzymatic glucose sensing. Reproduced from ref.99 with the permission from RSC.
A novel ultrasensitive and highly flexible laser scribing carbon paper/nickel nanoparticle (LSCP/NN) glucose biosensor was documented by Hou et al. [100]. The LSCP/NN biosensor was enzyme-free and binder-free and had a wide range of linear glucose determinations (0.80 mM–2.50 mM; 4.5 mM - 15.2 mM). This sensor displayed excellent sensitivity (3415 mAmM−1 cm−2), a fast response time (<1s) and an ultra-low limit of detection (20 nM). Similarly, a disposable electrochemical sensor based on 3D porous nickel nanostructures was employed to detect glucose. The performance of this enzymeless sensor was found to be excellent for the selective analysis of glucose and it also exhibited a very low detection limit employable for monitoring blood glucose [101]. Chelaghmia et al. described disposable PGE modified with nickel hydroxide Ni(OH)2, which preserved 93% of its original response towards glucose even after 28 days and exhibited relatively high selectivity in the presence of interfering species [102]. The incorporation of Ni(OH)2 with PGE showed exceptional stability, a relatively low detection limit and high sensitivity towards glucose, which could be attributed to the low background current, large specific surface area and an electrochemically stable structure.
3.2. Disposable non-enzymatic hydrogen peroxide sensors
Hydrogen peroxide is a major messenger molecule in various redox-dependent cellular signaling transductions [103]. It is also known that H2O2 is abnormally produced in the progress of inflammation by causing oxidative damage [104]. Therefore, sensitive detection of a trace level of H2O2 is of great importance in health inspection and environment protection [105]. The sensing of H2O2 by an electrochemical method has become really important in clinical, food, commercial and environmental settings. This has generated a constant need for simpler and more reliable H2O2 sensors and also manufacturing strategies for these sensors [106]. H2O2 is also generated in many enzyme reactions that require sensing of the participating chemical species [[107], [108], [109]]. The use of peroxidases and heme proteins in the construction of highly sensitive and selective electrochemical H2O2 sensors has been reported [[110], [111], [112]]. However, enzyme-modified electrodes usually suffer from high cost, limited lifetime, inherent instability, and complicated immobilization procedure [113]. Consequently, it is imperative to develop non-enzymatic H2O2 sensors with high sensitivity [114].
In a study by Teker et al., a disposable electrochemical sensor based on a poly (2-aminophenylbenzimidazole)/gold nanoparticle (P2AB/AuNPs) coated PGE was designed for the electrocatalytic reduction of H2O2 [115]. A wide linearity of AuNPs (0.06–100 nM) with a LOD of 0.367 μM have been achieved in this technique which is much higher when compared to other non-enzymatic hydrogen peroxide sensors. The presence of AuNPs has improved the efficacy of the non-enzymatic hydrogen peroxide sensor when compared to other nanomaterials like Ag and Pd-Cu employed in these types of sensors.
The sensor exhibited a linear relationship in the range from 0.06 mM to 100 mM with a limit of detection 3.673 × 105 M. This study demonstrated that AuNPs could efficiently catalyze the reduction of H2O2 while increasing the conductivity of the P2AB film. Dhara et al. demonstrated enhanced catalytic activity towards H2O2using gold nanoparticle decorated reduced graphene oxide (Au/rGO) nanocomposite modified SPE. The enhanced electrochemical performance can be attributed to the synergetic signal amplification effect of two different nanomaterials specifically rGO and Au nanoparticles [116]. When the ITO electrode was modified with AuNPs, it was used to detect H2O2. The reduction of H2O2 occurs in the linear concentration range from 0.1 to 15 mM because of the AuNPs which was found to possess very good electrocatalytic activity. The LDL of the disposable paper-based device sensor was found to be 0.08 mM and it showed supremacy over some of the enzymes and other nanomaterial-based sensors such as HRP–HAP/GCE, graphene/pectin–CuNPs/GCE and Pt@Au/EDA/GCE [117].
The combination of silver nanoparticles (AgNPs) and CNT as the base electrode material was effective at catalyzing the electrochemical reduction of H2O2 [118]. The on-site measurement of the H2O2 concentration was made in an alkaline solution at a linear range of 1–700 μM even at a very low concentration of 1 μM by a paper-based enzyme-free sensor in conjunction with a portable readout system. This technique offers a reliable, accurate and cost-effective method for the determination ofH2O2. The high catalytic activity is due to the synergetic effect of AgNPs along with CNT. Fig. 7 a depicts the reduction of H2O2which occurs at the modified electrode and the structure of the sensors is given in Fig. 7b & c. In this set-up, a hydrophobic barrier (such as silicone oil) was used to maintain the electrode surface area and it also helped to prevention liquid entry.
Fig. 7.
Electrochemical sensors: (a)The H2O2 reduction mechanism at the Ag modified MWCNT electrode, (b) electrochemical sensor structures with a single working electrode, and (c) electrochemical sensor structures with two working electrodes. Reproduced from ref.118 with the permission from IOP Science.
Garcia et al. reported palladium-copper/screen printed carbon electrode (PdCu/SPCE) sensors [119]. The alloy formation and the dispersion of Pd on the electrode surface resulted in its enhanced catalytic activity [120]. The sensitivity of these sensors was found to be 396.7 A mM−1cm−2. The LDL was also very low (0.7 M) at the applied potential of −0.3 V. A summary of metal based disposable sensors and their characteristics is provided in Table 1 .
Table 1.
Comparison of metal nanoparticles (MNPs) based disposable sensors.
| Analyte | Electrode Material |
Techniques | Potential (V) | Linearity (mM) | Sensitivity (μAmM−1cm−2) | Limit of Detection (μM) | Ref | ||
|---|---|---|---|---|---|---|---|---|---|
| Glucose | Enzymatic glucose sensors | ||||||||
| Ir-C | Cyclic Voltammetry/Amperometry | 0.2 | 0–15 | – | 28 | 46 | |||
| PGE | Amperometry | 0.5 | 0.02–1.11 | 22.05 | 2.7 | 53 | |||
| GOx/Naf/MnO2−/GNR/SPCE | Amperometry | 0.5 | 0.1–1.4 | 56.32 | 0.050 | 54 | |||
| GOx/graphene/PFLO/AuNPs | Amperometry | −0.7 | 0.1–1.5 | 7.357 | 0.081 | 55 | |||
| CS-GNPs-Fe3O4/GOx | Cyclic Voltammetry/Amperometry | −0.4 | 0.003–0.57 | – | 1.2 | 56 | |||
| Non-enzymatic glucose sensors | |||||||||
| CuNWs/GTE | Cyclic Voltammetry | +0.6 | 0.005–6.0 | 1100 | 1.6 | 93 | |||
| PtCuO/rGO/SPE | Linear Sweep Voltammograms/Amperometry |
+0.6 | 0.005–12 | 3577 | 0.01 | 94 | |||
| CuNCs/DLEG | Chronoampero-metry/Cyclic Voltammetry | +0.55 | 0.025–4.5 | 4532 | 0.25 | 95 | |||
| AuCuO/rGO/SPE | Linear Sweep Voltammograms/Chronoampero-metry |
+0.6 | 0.001–12 | 2356 | 0.1 | 96 | |||
| Cu NPs/PGE | Cyclic Voltammetry | +0.55 | 0.1–6.0 | 1467.5 | 0.44 | 97 | |||
| Cu/Co/rGO/PGE | Cyclic Voltammetry | +0.4 | 0.001–4 | 2400 | 0.15 | 98 | |||
| rGO/CuSNFs/GCE | Cyclic Voltammetry | +0.4 | 0.001–2 | 53.5 μM/(cm2 mM) | 0.19 | 99 | |||
| LSCP/NN | Cyclic Voltammetry/Amperometry | +0.50 | 0.0008–2.50 (Enzyme free) 4.5–15.2 (Binder free) |
3415 | 0.020 | 100 | |||
| 3D porous Ni Networks |
Cyclic Voltammetry/Chronoampero-metry | +0.50 | 0.0005–4 | 2900 | 0.07 | 101 | |||
| Ni(OH)2/PGE | Cyclic Voltammetry/Amperometry | +0.57 | 0.004–3.5 3.5–9 |
948 | 2.0 | 102 | |||
| H2O2 | Enzymatic hydrogen peroxide sensors | ||||||||
| HRP/AuNP/CHIT/SPCE | Cyclic Voltammetry/Amperometry | −0.4 | 0.01–11.3 | 176 | 0.65 | 72 | |||
| AuNP/ITO | Cyclic Voltammetry | 0.167 | 0.008–3.0 | – | 2 | 73 | |||
| PtNP/NAE | Cyclic Voltammetry/Amperometry | 0.65 | 0.02–20 | 194.6 | 1.0 | 74 | |||
| Non-enzymatic hydrogen peroxide sensors | |||||||||
| P2AB/AuNPs/PGE | Chronoampero -metry/Cyclic Voltammetry |
−0.8 | 0.06–100 | 3950 | 0.367 | 115 | |||
| Au-rGO-SPE | Linear Sweep Voltammograms/Amperometry |
−0.4 | 0.02–10 | 1238 | 0.1 | 116 | |||
| AuNPs/ITO | Cyclic Voltammetry/Amperometry | −0.4 | 0.1–15 | – | 0.08 | 117 | |||
| Ag/MWCNT/ | Cyclic Voltammetry | −0.3 | – | – | 119 | ||||
| PdCu/SPCE | Cyclic Voltammetry | −0.3 | 0.5–11 | 396.7 | 0.7 | 100 | |||
Chronoamperometry is a time-dependent technique where a square-wave potential is applied to the working electrode. The current of the electrode, measured as a function of time, fluctuates according to the diffusion of an analyte from the bulk solution toward the sensor surface. Chronoamperometry can therefore be used to measure current–time dependence for the diffusion-controlled process occurring at an electrode.
Amperometry is based on the measurement of the current resulting from the electrochemical oxidation or reduction of an electroactive species. The working electrode of an amperometric biosensor (e.g., noble metal (e.g., gold electrode), screen-printed electrode (SPE), carbon paste electrode (CPE)) is grafted with the biosensing element. When the constant potential is applied, the electroactive species are conversed at the electrode surface and the resulting current is measured.
3.3. Nature of enzymatic and non-enzymatic sensors
Enzymatic sensors are extremely sensitive, possess strong binding capacity, can catalyze or inhibit the reactions involving target analytes, exhibit improved direct visualization ability and excellent stability for years. However, they have certain limitations such as enzyme denaturation due to environmental changes (pH, humidity, and temperature), digestion by proteases, expensive preparation, time-consuming purification, high cost, thermo-chemical deformation, poor reproducibility, lack of stability, and tedious enzyme immobilization techniques [121]. These disadvantages of enzymatic biosensors, as mentioned, can be adequately defined by nanomaterial assisted electrochemical processes through non-enzymatic sensing.
4. Disposable immunosensors
Yalow and Berson (1959) were the first to establish the principle of the modern immunoassay [122]. Antibodies produced in the human body form complexes with corresponding antigens and this principle is used in the immunoassay technique. The most important requirement of immunosensors is a suitable design and preparation of an ideal interface between the biomaterial and the detector. The popularly known radioimmunoassay (RIA) technique is a development from this idea and has been used to investigate the properties of insulin-binding antibodies in human serum, using samples obtained from patients who had been treated with insulin. The basic principle of radioimmunoassay is competitive binding, where a radioactive antigen (tracer-typically 125I) competes with a non-radioactive antigen for a fixed number of antibody or receptor binding sites. The target antigen is labeled radioactively and bound to its specific antibodies (a limited and known amount of the specific antibody must be added). A sample, for example a blood-serum, is then added in order to initiate a competitive reaction of the labeled antigens from the preparation, and the unlabeled antigens from the serum-sample, with the specific antibodies. The competition for the antibodies will release a certain amount of labeled antigen. This amount is proportional to the ratio of labeled to unlabeled antigen. A binding curve can then be generated which allows the amount of antigen in the patient's serum to be derived [123]. In 1962, Clark and Lyons independently pioneered the concept of a biosensor [2]. The original method involved immobilizing enzymes on the surface of electrochemical sensors to exploit the selectivity of the enzymes for analytical investigation (Fig. 8 ) [124]. A fast response and ease of operation are the major advantages of these sensors. A large number of compounds can be detected with very high sensitivity by immunosensors and hence they can be employed in various applications.
Fig. 8.
Schematic representation of electrochemical immunosensor. Reproduced from ref. [124] with the permission from MDPI.
SPEs are nowadays extensively used as immunosensors and they are quite useful in point-of-care/on-site monitoring. The miniaturization of sensors is possible because SPEs are mechanically robust electrochemical transducers which facilitate the integration of both reference and working electrodes in the same chip at an affordable price, and these are disposable devices. The problems, such as electrode surface fouling by products obtained from redox processes and unintentional adsorption that can arise by using solid electrode materials (e.g., metal, amalgam, composite electrodes) can be overcome by the disposable nature of these devices. The SPE immunosensors developed in recent years can analyze a number of biomaterials such as enzymes, microorganisms, antigens, biomarkers and receptors [[125], [126], [127], [128], [129], [130], [131]].
Gold nanoparticles seem to be responsible for the improved performance of bioelectrodes. It is already known that gold enhances protein adsorption when compared to carbon. On the other hand, gold nanoparticles formed in situ are probablythe most adequate for protein adsorption. The nanogold surface is quite different from that of bulk gold. Nanogold particles have very high surface to volume ratio, high surface energy, highly active and hence they can strongly bind with protein molecules. Manfredi et al. developed a competitive disposable amperometric immunosensor based on gliadin-functionalized carbon/nanogold SPEs for the rapid determination of celiotoxicprolamins [132]. The immune competitive assay achieved strong sensitivity for gliadin in ethanol extracts, with a detection limit and a quantitative limit of 8 and 22 ng mL−1, respectively. A novel disposable competitive amperometric determination of p53 protein in urine using CNT/GNP-SPCEs has also been reported [133]. The transcription factor phosphorylated p53 at serine 392 (phospho-p53392) was found to be the target of a nanomaterial enhanced disposable immunosensor. The immune device achieved a low detection limit of 14 pM in synthetic urine samples.
Non-enzymatic labels derived from mesoporous platinum nanoparticles (M-PtNPs) were employed on a disposable immunosensor array. The preparation of these nanoparticles was achieved by ultrasonication and subsequently they were used to label the antibody (Ab2) which could amplify the signal due to the high conductivity and electrocatalytic activity of PtNPs. When a panel of tumor markers (CA125, CA153 and CEA) linked to breast cancer as model analytes were employed, the data obtained from the experiment indicated large linear ranges of more than four orders of magnitude with detection limits of 0.002 U mL−1, 0.001 U mL−1 and 7.0 pg mL−1 for CA125, CA153 and CEA (carcinoembryonic antigen), respectively [134]. Qiuyu Hu et al. documented a novel electrochemiluminescence (ECL) immunosensor obtained by the combination of enzyme-free and label-free strategies, using the catalytic effect of a gold nanoflower (AuNF) co-reactant accelerator [135]. The concentration of α-fetoprotein (AFP), a protein produced in the liver of a developing fetus which is used as a tumor marker to help detect and diagnose cancers of the liver, testicles, and ovaries, can be determined using this sensor from 0.01 to 100 ng/mL, and at a low limit of 3.4 pg mL−1.
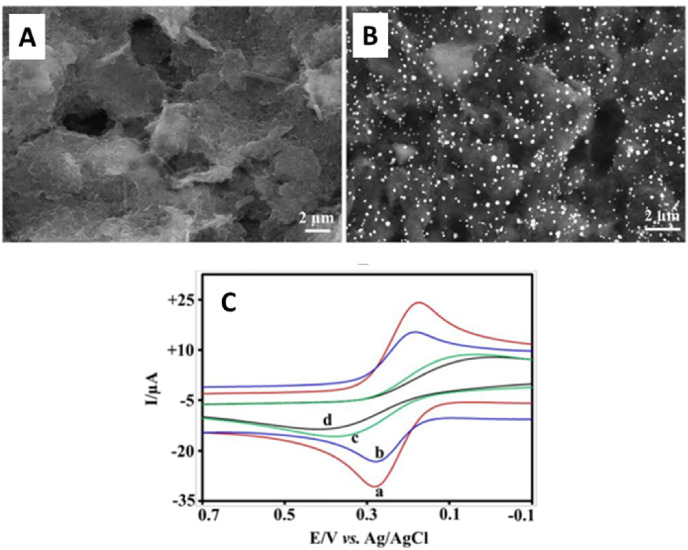
A label free electrochemical immunoassay measurement of C-reactive protein (CRP) using a self-assembled monolayer of AuNPs on a SPCE immunosensor has also been reported [136]. SEM images (Fig. 9 A and B) of bare SPE and AuNPs modified SPE indicate the porous nature of the bare SPE. The entire electrode was covered by metal nanoparticles (20–60 nm) randomly by electrodeposition. The immobilization of anti-CRP without any loss in its biological activity is possible in this arrangement and the fast electron transfer between SPE and the electrochemical probe was also achieved. When the antibodies are not present, the SAM-AuNPs-SPE (Fig. 9 (C) curve a) exhibited a higher redox current than bare SPE (Fig. 9 (C) curve c). This behavior is attributed to the larger surface area and enhanced electron transfer rates provided by AuNPs. However, in the presence of antibodies, both anti-CRP-SAM-AuNPs-SPE (Fig. 9 (C) curve b) and anti-CRP-SPE (Fig. 9 (C) curve d) exhibited reduced currents than that of their corresponding counterparts [137].
Fig. 9.
SEM images of (A) bare screen-printed electrodes (SPE) and (B)AuNPs-SPE. (C) Cyclic voltammetric responses of the AuNPs modified electrode (a) SAM-AuNPs-SPE and (b) anti-CRP-SAM-AuNPs-SPE, and without the AuNPs electrode (c) bare SPE, and (d) anti-CRP-SPE for 1 nM CRP in PBS containing 0.5 mM Fe(CN)64−at a scan rate of 50 mV s−1. Reproduced from ref. [136] with the permission from MDPI.
SPCE modified nanohybrid multi-walled carbon nanotubes (MWCNT) with AuNPs were fabricated by Neves et al. [138]. Cyclic voltammograms obtained for the detection of anti-tTG IgA antibodies using a tTG-modified SPCE as well as a tTG-modified SPCE–MWCNT– AuNPs are shown in Fig. 10 . It was found that when the nanomaterials are present, they improved the faradaic/capacitive current ratio. It was also reported that the conjugation of MWCNTs and AuNPs enhanced the capability of the biosensors to act as point-of-care diagnostic due to the ability of the MNPs to adsorb proteins without compromising their bioactivity, and the electrocatalytic properties of the carbon nanotubes themselves [139,140].
Fig. 10.
Effect of the transducer surface on the analytical signal for the detection of anti-tTG IgA autoantibodies using tTG-modified SPCE (grey line) and tTG-modified SPCE–MWCNT– AuNPs (black line). Experimental conditions: tTG 0.1 g L−1; BSA 2%; control samples diluted 1:2; anti-H-IgA-AP 1:30,000; 3-IP, 1.0 mM; Ag+, 0.4 mM. Cyclic voltammetric scans from −0.002 V to +0.4 V at a scan rate of 50 mV s−1. Reproduced from ref. [138] with the permission from Elsevier.
A new disposable electrochemical immunosensor was developed for the detection of gluten-induced celiac disease-specific anti-tissue transglutaminase IgA and IgG auto-antibodies in real patient samples. An open circuit potential strategy using SPCE/PtNPs as an electrochemical label with hydrazine was reported capable of detecting human chorionic gonadotropin hormone (hCG) at 0.28 ng mL−1 due to the high electrocatalytic activity of PtNPs [141]. Novel and disposable electrochemical immunosensors based on AuNPs/MWCNTs–chitosan (Chits) composite films were developed by Huang and coauthors for the detection of the carcinoembryonic antigen (CEA) [142]. AuNPs were synthesized onto the MWCNTs–Chits composite film in situ for antibody (CEA) immobilization (anti CEA/AuNPs/MWCNTs–Chits). This enhanced electrochemical signals as well as the adsorption capacity of the antibody thereby improving sensitivity (0.01 ngmL−1).
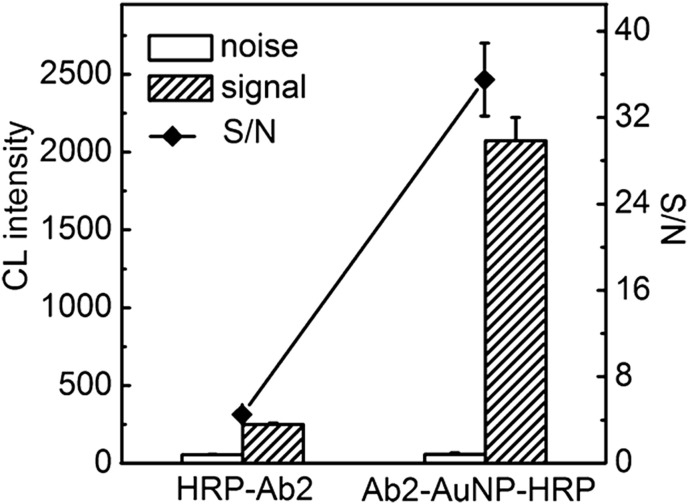
An amperometry technique using a biocompatible composite film composed of AuNPs, porous chitosan and thionine has been reported [143]. The immunosensor was highly sensitive to the carcinoembryonic antibody with a detection limit of 0.08 ng mL−1. There was particularly good adsorption of AuNPs onto the electrode surface due to its opposite charge and also due to the chemisorption of thionine. AuNPs possess a large surface area as well as biocompatibility and fast electron transfer. Afonso et al. employed an electrochemical based magneto-immunosensor using anti-Salmonella magnetic beads (MBs-pSAb) sandwiched with AuNPs modified antibodies (sSAb-AuNPs) for the detection of Salmonella in skim milk [144]. In this approach, the bacteria are captured from the skimmed milk and preconcentrated by immunomagnetic separation, followed by labeling with AuNPs modified with a polyclonal anti-Salmonella antibody. Then, the modified MBs are captured by applying a magnetic field below the SPCE and have been used as transducer for the electro-chemical detection. The developed immunosensor can detect up to 143 Salmonella mL−1over a rather shorter time (up to1:30 h). Zong et al. developed Ab2−AuNP−HRP bioconjugates based on an immunoassay array for the detection of multiple tumor markers such as α-fetoprotein, carcinoma antigen 125, carbohydrate antigen 153, and the carcinoembryonic antigen [145]. The authors prepared four different tags by binding a high loading ratio of HRP to detection antibodies (Ab2) to AuNPs. It has been clearly demonstrated that the use of AuNP-based multienzymatic amplification leads to a wide linear detection range and a much lower detection limit for biomarkers as compared to the assay with single enzyme tags (Fig. 11 ).
Fig. 11.
Performance of aAb2−AuNP−HRP tag compared with a Ab2−HRP label on the sensing array with the same detection conditions. Signal and noise are the CL intensities obtained from the immunoassay in the presence and absence of 0.1 ng mL−1 CEA. Results are expressed as the average of three independent experiments. Reproduced from ref. [145] with the permission from ACS.
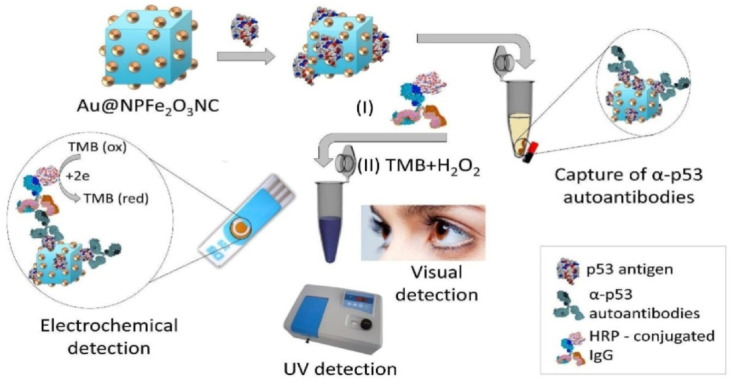
The early diagnosis of cancer is important, and this is facilitated by non-invasive biomarkers. One such example is an autoantibody produced against tumor associated antigens much earlier than any observed symptoms. The currently available methodologies for the detection of autoantibodies are not only invasive but provide diagnosis only at advanced stages of cancer. A group of researchers from Australia and Japan developed a method for the early detection of p53 autoantibodies against colon cancer which employs a strategy that combines the strength of gold-loaded nanoporous iron oxide nanocube (Au@NPFe2O3NC) based capture and purification. The reported method involves two steps: i) magnetic capture and isolation of autoantibodies using p53/Au@NPFe2O3NC as dispersible nanocapture agents in serum samples followed by: ii) detection of autoantibodies through a peroxidase-catalyzed reaction on a commercially available disposable SPE or naked-eye detection in an Eppendorf tube. This method exhibits good sensitivity (LOD = 0.02 U mL−1) and reproducibility (relative standard deviation, %RSD = <5%, for n = 3) in samples obtained from colorectal cancer and is inexpensive, rapid, and specific (Fig. 12 ) [146].
Fig. 12.
Schematic representation of an assay for the detection of p53 autoantibodies. P53-functionalized Au@NPFe2O3NC was used as a ‘dispersible nanocapture agent’ for capturing target autoantibodies in serum. The bionanoconjugates were treated with HRP-IgG antibodies and a TMB-substrate solution after magnetic purification and separation. The level of autoantibody concentration against p53 antigen was detected by the naked-eye, UV–vis and an electrochemical detection technique. Reproduced from ref. [146] with the permission from RSC.
C-reactive protein (CRP) is a protein made by our liver which is sent into the bloodstream in response to inflammation. Boonkaew et al. reported a label-free origami paper-based electrochemical immunoassay for the detection of CRP at 15 ng mL−1. AuNPs were initially electrodeposited onto the graphene/SPCE which was followed by a self-assembled monolayer (SAM) of L-cysteine. The diameter of AuNPs was found to be 50–70 nm. The uniform distribution of AuNPs on the electrode surface considerably increased the surface area and affected the number of biomolecule anchoring sites [147]. A summary of metal based disposable immunosensors and their characteristics is provided in Table 2 .
Table 2.
Disposable electrochemical immunosensors and their characteristics.
| Immunosensor | Linear range | Detection limit | Ref |
|---|---|---|---|
| Glaiden/C/GNP/SPCE | 22 (ng/ml) | 8 (ng/ml) | 132 |
| p53-modified/CNT/GNP-SPCEs | 20 pM-10 nM | 14 pM | 133 |
| M-Pt/Ab2/SPCE/CA125 | – | 0.002U mL−1 | 134 |
| M-Pt/Ab2/SPCE/CA153 | 0.001UmL−1 | ||
| M-Pt/Ab2/SPCE/CEA | 7.0 pg mL−1 | ||
| AuNFs/ITO | 0.01–100 ngmL−1 | 3.4 PgmL−1 | 135 |
| Anti CRP-SAM-AuNPs-SPE | 0.4–200 nM | 0.150 nM | 136 |
| Anti-tTG IgA/SPCE/CNT/AuNPs | – | – | 138 |
| SPCE/PtNPs | 0.05–10 ng mL−1 | 0.28 ng mL−1 | 141 |
| anti-CEA/AuNPs/MWCNTs–Chits/GCE | 0.3–2.5 and 2.5–20.0 ng mL−1 | 0.01 ng mL−1 | 142 |
| BSA/anti-CEA/GNPs/Thi/pChit-modified GCE | 10.0–160.0 ng mL−1 | 0.08 ng mL−1 | 143 |
| SPCE/AuNPs | – | – | 144 |
| Ab2−AuNP−HRP | – | – | 145 |
| Au@NPFe2O3NC | – | 0.7 and 0.02 U mL−1 | 146 |
| AuNPs/G/SPCE | 0.05–100 (μg mL−1) | 0.015 (μg mL−1) | 147 |
5. Disposable genosensors
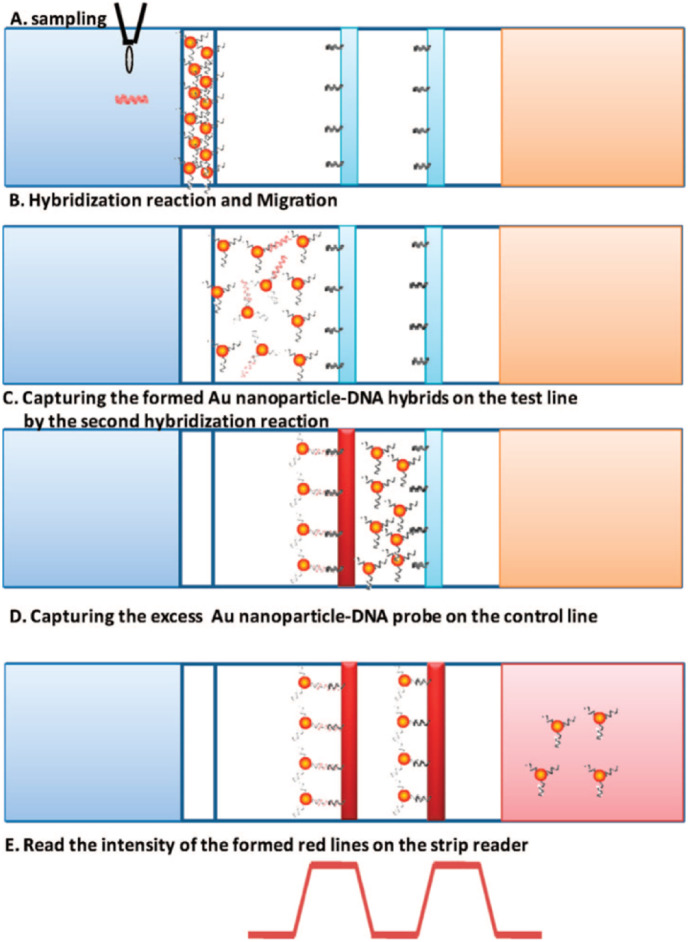
DNA biosensor (genosensor) technologies have been successfully employed for detecting microbial contamination in food and water, early detection of anomalies caused by genetic disorders, screening of drugs, and tissue matching besides crime investigation by forensic analysis [[148], [149], [150], [151], [152]]. The principle behind this technology is the detection of a target DNA sequence which is achieved by the combination of recognition surface with a single stranded DNA (ssDNA) and an optical sensitive, mass sensitive, or electrochemical transducer [[153], [154], [155], [156], [157]]. Ihalainen et al., reported a disposable paper supported inkjet printed AuNPs recognition architecture which essentially consists of multiple layers of biotinylated SAM and subsequent backfill with 11-mercapto-1-undecanol (MUOH) SAM for the impedimetric detection of DNA hybridization [158]. The result obtained from electrochemical impedance spectroscopy suggests that both recognition architectures have good selectivity, whereas surface plasmon resonance of AuNPs indicated very high unspecific binding for the mixed HSDNA (thiol functionalized DNA probe) probe and MUOH SAM system. There is also another report by Mao et al. which describes the use of AuNPs for the detection of nucleic acid samples in a very short time period (15 min). This is an inexpensive and sensitive disposable nucleic acid biosensor (DNAB) that is capable of detecting human genomic DNA (1.25 Fm) (Fig. 13 ) [159]. The number of AuNP-DNA conjugates varied inversely with size of the AuNPs and hence 15 nm diameter AuNPs were employed among four different sizes (15, 50, 100, and 200 nm).
Fig. 13.
Principles of measuring the disposable nucleic acid biosensor (DNAB).Reproduced from ref. [159] with the permission from ACS. Biosensors have two parts(i) a biorecognition interface which enables the selective detection of the analyte, and (ii) the transducer, which converts the recognition event into an electronic signal. The transducer is an electrode onto which DNA as the biorecognition species is immobilized. The two main approaches to the electrochemical transduction of DNA hybridization are: labeled methods and label-free methods. Labeled methods use redox active molecules that bind to DNA either in the minor groove, intercalating a planar aromatic ring between base pairs or by interaction with one of the bases. Label-free methods depend on either changes to the electrical characteristics of the DNA-modified interface upon hybridization or on the natural electroactivity of DNA. In the ideal case, a DNA biosensor would be able to discriminate complementary target DNA from DNA with a single base-pair mismatch without requiring amplification of the sample [160].
Shiddiky et al. developed a sandwich type electrochemical DNA and protein sensor on SPEs based on the catalytic activity of hydrazine [161]. Poly-5,2′,5′,2″-terthiophene-3′-carboxylic acid poly (TTCA)/dendrimer (DEN) was loaded with AuNPs onto which the analyte (target protein)-linked avidin-labeled hydrazine (Av–Hyd) was adsorbed. They reported that DNA and proteins can be detected as low as 30 fM and 25 fg/ml, respectively. There was a negligible non-specific adsorption of proteins on the electrode surface which resulted in the specific and sensitive detection of the target protein. The disposable nature of the electrode coupled with easy detection and high sensitivity contributes to numerous opportunities for the fabrication of one-time use analytical devices which can be employed for solving forensic issues, detection of the nature of infection, and also to identify genetic mutations. Ingrosso et.al.developed genosensors derived from AuNPs in situ and were decorated with rGO nanocomposites with an LOD of 0.7 pM [162]. The nucleic acid biosensing behavior of this genosensor can be attributed to its fast heterogeneous electron transfer kinetics, a concomitant decrease in the electron transfer resistance at the electrode/electrolyte interface, high electroactivity as well as the large surface area of the AuNPs/rGO hybrid material modified SPCEs.
6. Disposable biosensors for COVID-19
Paper-based biosensors have several advantages over chip-based biosensors for point-of-care testing such as being biodegradable, cheap, as well as easy to fabricate and modify. The most commonly used paper strips to detect IgG and IgM for the detection of COVID-19 in blood, serum and plasma samples are based on lateral flow test strips [163,164]. The underlying principle is the formation of a complex obtained by the reaction of antibodies with a gold-COVID-19 antigen when IgG and or IgM are present in the patient sample. The complex thus formed is capable of moving across the nitrocellulose membrane and finally interacts with anti-IgM and/or IgG at their respective test line. The formation of red color at the control line indicates the reaction of gold-rabbit IgG with anti-rabbit IgG. The severity of the infection is ascertained as follows: primary or acute infection is inferred by a positive IgM and a negative IgG or positive at both lines. However, a secondary or later stage of infection is inferred with a positive IgG and with a negative IgM [163,165]. The mechanism is similar to lateral flow strips and the interaction between AuNP-Ab and target increases with a fluidic delay in the thread.
Biosensors which are based on films, carbon and textiles (as a thread, fabric or cloth) have also been employed for detecting infections [[166], [167], [168], [169]]. An oligonucleotide capture monolayer was assembled onto disposable gold nanostructured SPCE for the detection of SARS RNA. The enzymatic amplification signal was observed after hybridization which could be measured with voltammetry at a detection limit of 2.5 pmol/L [170]. A recent report indicated a rapid detection (10min) of the SARS-CoV-2 from the isolated RNA samples by spectrophotometry in which Au NPs capped with thiol-modified DNA antisense oligonucleotides specific for the N-gene (nucleocapsid phosphoprotein) of SARS-CoV-2 was employed [171]. The combination of a plasmonic photothermal effect and localized surface plasmon resonance sensing transduction can be employed in a biosensor for the detection of the virus [172]. The hybridization of the selected sequences from the virus enables the detection of the virus by gold nano-islands functionalized with complementary DNA receptors (detection limit: 0.22 pM).
Metallic nanoparticles such as AgNPs, CuNPs as well as TiO2 NPs have been found to possess antiviral properties, persistence, as well as efficacy at a very low dosage [173]. A facial mask coated with an Ag nanocluster/SiO2 composite was found to inhibit the SARS-CoV-2 virus. It has been accepted that the detection of an IgM antibody in blood during the acute infection period indicates the presence of this virus. An indirect immunochromatography method was developed to achieve diagnosis over a very short time span by a colloidal AuNP based assay which facilitates the on-site detection of an IgM antibody to confirm the presence of the COVID-19 virus (Fig. 14 ). In this method, an analytical membrane coated with a SARS-CoV-2 nucleoprotein was employed for sample capture and subsequently an antihuman IgM antibody conjugated with AuNPs was used for detection of the virus. The efficacy of this method was demonstrated by comparing the results with those of a real-time polymerase chain reaction (RT-PCR). The assay can be completed within 15min and requires only 10–20 μLof serum with a sensitivity and specificity of 100 and 93.3% respectively [174].
Fig. 14.
Operation principle of the AuNP-LF-strip for SARS-CoV-2. Reproduced from ref. [174] with the permission from ACS.
7. Conclusion and future perspectives
We have reviewed a comprehensive range of disposable metal nanoparticle-based biosensors for biomedical applications. As highlighted, a lot of progress has been made in the development of disposable electrochemical devices for medical applications and this area of research is still at its infancy because of the hurdles to be overcome in the stabilization of the biological molecules at the platform and the miniaturization of the disposables without compromising selectivity and specificity. The metal nanoparticles, especially gold nanoparticles provide a route for signal transduction due to their unique optoelectronic and physico-chemical properties. By combining or coating the metal nanoparticles with materials, sensing technologies with improved sensing properties can be achieved. Even though they possess several advantages over conventional diagnostic strategies such as portability, disposability and low cost, these sensors are still at the early stage of development. The recently developed advanced nanodiagnostic techniques have laid the foundation for affording easy, rapid, low-cost, and multiplexed identification of biomarkers. Optimization parameters for the sensors is one of the most important criteria for achieving reliability in diagnosis. Although electrochemical biosensors have been shown to be adequate for the high-performance analysis in various field applications, matrix interference affecting the biomolecular interaction of real samples (blood, food, etc.) remains as the most important issue to be tackled in order to improve analytical performance. However, disposable electrochemical sensors based on metal nanoparticles have the potential to be employed in the detection of microbes which play havoc in human life, such as COVID-19.
The future trends and challenges concerning disposable biosensors include: I) development of new classes of disposable devices using “green” materials for sustainable, biodegradable and low-cost production; ii) miniaturization and use with portable devices like handheld analyzers or smartphones; iii) implementation of fully integrated, standalone “use-and-throw instruments” containing the elements for readout (such as disposable displays/LEDs, microcontrollers, opamps or even potentiostats) and a source of electrical power (batteries, solar panels, etc.); vii) Disposable sensors may also be combined with systems capable of delivery of therapeutics. Theranostics can monitor healing of a wound and release drugs on demand when an infection is detected. Future challenges in MNP-based biosensors generally include expansion of the range of different biomolecules, which can be sensed or detected by enhancing the sensitivity and providing more rapid and versatile detection methods. However, the most demanding aspect of the progress in this field is to provide more opportunities for translational application of these nanobiosensors.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgements
S. Malathi acknowledges the financial grant by Dr. D. S. Kothari Post-Doctoral Fellowship (No. F.4-2/2006 (BSR)/CH/18–19/0122 dated May 27th, 2019) University Grants Commission, New Delhi, India. S.Narayana Kalkura acknowledges the financial grant by BSR faculty fellowship (No.F.4-2(11)/2019(BSR) dated June 4, 2020, UGC, New Delhi, India.
References
- 1.Heineman W.R., Jensen W.B., Leland C., Clark Biosens. Bioelectron. 2006;21(8):1403–1404. 1918–2005. [Google Scholar]
- 2.Kral A., Aplin F., Maier H. In: Prostheses for the Brain. Kral A., Aplin F., Maier H., editors. Academic Press; 2021. Chapter-10 - advanced concepts physical chemistry: electrodes and electrolytes; pp. 167–208. [Google Scholar]
- 3.Figueiredo-Filho L.C.S., Janegitz B.C., Fatibelilo-Filho O., Marcolino-Junior L.H., Banks C.E. Inexpensive and disposable copper mini-sensor modified with bismuth for lead and cadmium determination using square-wave anodic stripping voltammetry. Anal. Methods. 2013;5(1):202–207. [Google Scholar]
- 4.Dincer C., Bruch R., Costa-Rama E., Fernandez-Abedul M.T., Merkoci A., Manz A., Urban G.A., Guder F. Disposable sensors in diagnostics, food, and environmental monitoring. Adv. Mater. 2019;31(30) doi: 10.1002/adma.201806739. [DOI] [PubMed] [Google Scholar]
- 5.Freire F., Ferraresi C., Jorge A.O., Hamblin M.R. Photodynamic therapy of oral Candida infection in a mouse model. J. Photochem. Photobiol., B. 2016;159:161–168. doi: 10.1016/j.jphotobiol.2016.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y., Wu X., Chen J., Amin R., Lu M., Bhayana B., Zhao J., Murray C.K., Hamblin M.R., Hooper D.C., Dai T. Antimicrobial blue light inactivation of gram-negative pathogens in biofilms: in vitro and in vivo studies. J. Infect. Dis. 2016;213(9):1380–1387. doi: 10.1093/infdis/jiw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnan S., Dusane A., Morajkar R., Venkatb A., Vernekar A.A. Deciphering the role of nanostructured materials in the point-of-care diagnostics for COVID-19: a comprehensive review. J. Mater. Chem. B. 2021;9(30):5967–5981. doi: 10.1039/d1tb01182k. [DOI] [PubMed] [Google Scholar]
- 8.Fahrner W. first ed. Springer-Verlag Berlin Heidelberg; 2005. Nanotechnology and Nanoelectronics. [Google Scholar]
- 9.Lockwood D.J. Springer Nature; New York: 2002. Nanostructure Science and Technology. [Google Scholar]
- 10.Cao G., Wang Y. World Scientific; 2011. Nanostructures and Nanomaterials. [Google Scholar]
- 11.Kumar S., Bukkitgar S.D., Singh S., Pratibha, Singh V., Raghava Reddy K., Venkata Reddy Ch, Shetti N.P., Sadhu V., Naveen S. Electrochemical sensors and biosensors based on graphene functionalized with metal oxide nanostructures for healthcare applications. ChemistrySelect. 2019;4(18):5322–5337. [Google Scholar]
- 12.Guo X., Liang T., Yuan C., Wang J. Controllable morphology design of an ecofriendly tamarind seedcase-derived porous carbonaceous biomass as a versatile electrochemical biosensor platform for reactive oxygen species. ACS Sustain. Chem. Eng. 2021;9(2):765–775. [Google Scholar]
- 13.Bukkitgar S.D., Shetti N.P., Malladi R.S., Raghava Reddy K., Kalanur S.S., Aminabhavi T.M. Novel ruthenium doped TiO/reduced graphene oxide hybrid as highly selective sensor for the determination of ambroxol. J. Mol. Liq. 2018;300:112368. [Google Scholar]
- 14.Iniesta J., García-Cruz L., Gomis-Berenguer A., Ania C.O. Carbon materials based on screen-printing electrochemical platforms in biosensing applications. Electrochemistry. 2016;13:133–169. [Google Scholar]
- 15.Saha U., Todi K., Malhotra B.D. Emerging DNA-based multifunctional nano-biomaterials towards electrochemical sensing applications. Nanoscale. 2021;13(23):10305–10319. doi: 10.1039/d1nr02409d. [DOI] [PubMed] [Google Scholar]
- 16.Calam T.T. A novel, efficient and sensitive method for the simultaneous determination of riboflavin (vitamin B2) and pyridoxine hydrochloride (vitamin B6) in food and pharmacological samples using an electrochemical sensor based on 4,4 -diamino benzophenone. Microchem. J. 2021;169:106557. [Google Scholar]
- 17.Welch C.M., Compton R.G. The use of nanoparticles in electroanalysis: a review. Anal. Bioanal. Chem. 2006;384:601–619. doi: 10.1007/s00216-005-0230-3. [DOI] [PubMed] [Google Scholar]
- 18.Schlogl R., Abd Hamid S.B., Nanocatalysis Mature science revisited or something really new? Angew Chem. Int. Ed. Engl. 2004;43(13):1628–1637. doi: 10.1002/anie.200301684. [DOI] [PubMed] [Google Scholar]
- 19.Astruc D., Lu F., Aranzaes J.R. Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis. Angew Chem. Int. Ed. Engl. 2005;44(48):7852–7872. doi: 10.1002/anie.200500766. [DOI] [PubMed] [Google Scholar]
- 20.Roucoux A., Schulz J., Patin H. Reduced transition metal colloids: a novel family of reusable catalysts? Chem. Rev. 2002;102(10):3757–3778. doi: 10.1021/cr010350j. [DOI] [PubMed] [Google Scholar]
- 21.Hua Z., Yu T., Liu D., Xianyu Y. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021;179:113076. doi: 10.1016/j.bios.2021.113076. [DOI] [PubMed] [Google Scholar]
- 22.Si P., Razmi N., Nur O., Solanki S., Pandey C.M., Gupta R.K., Malhotra B.D., Willander M., de la Zerda A. Gold nanomaterials for optical biosensing and bioimaging. Nanoscale Adv. 2021;3(10):2679–2698. doi: 10.1039/d0na00961j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munawar A., Ong Y., Schirhagl R., Tahir M.A., Khan W.S., Bajwa S.Z. Nanosensors for diagnosis with optical, electric and mechanical transducers. RSC Adv. 2019;9(12):6793–6803. doi: 10.1039/c8ra10144b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacioni N.L., Borsarelli C.D., Rey V., Veglia A.V. In: Silver Nanoparticle Applications. Engineering Materials. Alarcon E., Griffith M., Udekwu K., editors. Springer; Cham: 2015. Synthetic routes for the preparation of silver nanoparticles; pp. 13–46. [Google Scholar]
- 25.Turkmen E., Bas S.Z., Gulce H., Yildiz S. Glucose biosensor based on immobilization of glucose oxidase in electropolymerized poly(o-phenylenediamine) film on platinum nanoparticles-polyvinylferrocenium modified electrode. Electrochim. Acta. 2014;123:93–102. [Google Scholar]
- 26.Li Y., Schluesener H.J., Xu S. Gold nanoparticle-based biosensors. Gold Bull. 2010;43:29–41. [Google Scholar]
- 27.Tian D., Duan C., Wang W., Li N., Zhang H., Cui H., Lu Y. Sandwich-type electrochemiluminescence immunosensor based on N-(aminobutyl)-N-ethylisoluminol labeling and gold nanoparticle amplification. Talanta. 2009;78(2):399–404. doi: 10.1016/j.talanta.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 28.Yan Z., Yang M., Wang Z., Zhang F., Xia J., Shi G., Xia L., Li Y., Xia Y., Xia L. A label-free immunosensor for detecting common acute lymphoblastic leukemia antigen (CD10) based on gold nanoparticles by quartz crystal microbalance. Sensor. Actuator. B Chem. 2015;210:248–253. [Google Scholar]
- 29.Sugawa K., Tahara H., Yamashita A., Otsuki J., Sagara T., Harumoto T., Yanagida S. Refractive index susceptibility of the plasmonic palladium nanoparticle: potential as the third plasmonic sensing material. ACS Nano. 2015;9(2):1895–1904. doi: 10.1021/nn506800a. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence N.S., Liang H.-P. In: Nanostructured Materials in Electrochemistry. Eftekhari A., editor. Wiley; 2008. Metal nanoparticles: applications in electroanalysis; pp. 435–457. [Google Scholar]
- 31.Xue M.-H., Xu Q., Zhou M., Zhu J.-J. In situ immobilization of glucose oxidase in chitosan–gold nanoparticle hybrid film on Prussian Blue modified electrode for high-sensitivity glucose detection. Electrochem. Commun. 2006;8(9):1468–1474. [Google Scholar]
- 32.Zhang S., Wang N., Yu H., Niu Y., Sun C. Covalent attachment of glucose oxidase to an Au electrode modified with gold nanoparticles for use as glucose biosensor. Bioelectrochemistry. 2005;67(1):15–22. doi: 10.1016/j.bioelechem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro Caroline A.S., Albuquerque Lindomar J.C., de Castro Carlos E., Pereira Rodrigo M., Albuquerque Brunno L., Pavlova Ewa, Luiza Gabriela Schlüter. Batista Bruno L., Bellettini Ismael C., Giacomelli Fernando C. Ready-to-use room temperature one-pot synthesis of surface-decorated gold nanoparticles with targeting attributes. J. Colloid Interface Sci. 2022;614:489–501. doi: 10.1016/j.jcis.2022.01.145. [DOI] [PubMed] [Google Scholar]
- 34.Alizadeh M., Zarei M., Ebratkhahan M., Amjadi M. Synthesis of different morphologies of metal and metal oxide nanoparticles and investigation of their catalytic properties by optical methods. J. Mol. Struct. 2021;1244:130943. [Google Scholar]
- 35.Marzana M., Alam Khan M.M., Ahmed A., Jalil M.A., Hossain M.M. In: In Micro and Nano Technologies, Nanomaterials for Biocatalysis. Castro G.R., Nadda A.K., Nguyen T.A., Qi X., Yasin G., editors. Elsevier; 2022. Chapter 23 - nanocarbon for bioelectronics and biosensing; pp. 689–714. [Google Scholar]
- 36.Yao Y., Lan L., Liu X., Ying Y., Ping J. Spontaneous growth and regulation of noble metal nanoparticles on flexible biomimetic MXene paper for bioelectronics. Biosens. Bioelectron. 2020;148:111799. doi: 10.1016/j.bios.2019.111799. [DOI] [PubMed] [Google Scholar]
- 37.Ramesh G.V., Chandaluri C.G., Kumar Tadi K., Dandu N.K., Reddy N.M. In: In Woodhead Publishing Series in Electronic and Optical Materials, Functional Materials Processing for Switchable Device Modulation. Pal K., Thomas S., editors. Woodhead Publishing; 2022. 12-Recent advances in functional materials: bioelectronics-integrated biosensor applications; pp. pp.221–239. [Google Scholar]
- 38.Min H., Baik S., Kim J., Lee J., Bok B.-G., Song J.H., Kim M.S., Pang C. Tough carbon nanotube-implanted bioinspired three-dimensional electrical adhesive for isotropically stretchable water-repellent bioelectronics. Adv. Funct. Mater. 2022;32(8):2107285. [Google Scholar]
- 39.Zhao X., Yang L., Guo J., Xiao T., Zhou Y., Zhang Y., Tu B., Li T., Grzybowski B.A., Yan Y. Transistors and logic circuits based on metal nanoparticles and ionic gradients, Nat. Electron.4. 2021:109–115. [Google Scholar]
- 40.Xiao Y., Patolsky F., Katz E., Hainfeld J.F., Willner I. Plugging into Enzymes": nanowiring of redox enzymes by a gold nanoparticle. Science. 2003;299(5614):1877–1881. doi: 10.1126/science.1080664. [DOI] [PubMed] [Google Scholar]
- 41.Amirjani A., Rahbarimehr E. Recent advances in functionalization of plasmonic nanostructures for optical sensing. Microchim. Acta. 2021;188(2):57–74. doi: 10.1007/s00604-021-04714-3. [DOI] [PubMed] [Google Scholar]
- 42.Lu C., Zhou S., Gao F. J. Lin, J. Liu, J. Zheng, DNA-mediated growth of noble metal nanomaterials for biosensing applications. TrAC-Trends Anal. Chem.148. 2022:116533. [Google Scholar]
- 43.Malhotra B.D., Chaubey A. Biosensors for clinical diagnostics industry. Sensor. Actuator. B Chem. 2003;91(1–3):117–127. [Google Scholar]
- 44.Xu J.-J., Feng J.-J., Zhong X., Chen H.-Y. Low-potential detection of glucose with a biosensor based on the immobilization of glucose oxidase on polymer/manganese oxide layered nanocomposite. Electroanalysis. 2008;20(5):507–512. [Google Scholar]
- 45.Chen C., Xie Q., Yang D., Xiao H., Fu Y., Tan Y., Yao S. RSC Adv. 2013;(3):4473–4491. [Google Scholar]
- 46.Shen J., Dudik L., Liu C.-C. An iridium nanoparticles dispersed carbon based thick film electrochemical biosensor and its application for a single use, disposable glucose biosensor. Sensor. Actuator. B Chem. 2007;125(1):106–113. [Google Scholar]
- 47.Lee H., Song C., Hong Y.S., Kim M.S., Cho H.R., Kang T., Shin K., Choi S.H., Hyeon T., Kim D.-H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017;3(3) doi: 10.1126/sciadv.1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H., Choi T.K., Lee Y.B., Cho H.R., Ghaffari R., Wang L., Choi H.J., Chung T.D., Lu N., Hyeon T., Choi S.H., Kim D.H. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016;11(6):566–572. doi: 10.1038/nnano.2016.38. [DOI] [PubMed] [Google Scholar]
- 49.Niu Z., Liu L., Zhang L., Shao Q., Zhou W., Chen X., Xie S. A universal strategy to prepare functional porous graphene hybrid architectures. Adv. Mater. 2014;26(22):3681–3687. doi: 10.1002/adma.201400143. [DOI] [PubMed] [Google Scholar]
- 50.Wang W., You S., Gong X., Qi D., Chandran B.K., Bi L., Cui F., Chen X. Bioinspired nanosucker array for enhancing bioelectricity generation in microbial fuel cells. Adv. Mater. 2016;28(2):270–275. doi: 10.1002/adma.201503609. [DOI] [PubMed] [Google Scholar]
- 51.Pan L., Yu G., Zhai D., Lee H.R., Zhao W., Liu N., Wang H., Tee B.C., Shi Y., Cui Y., Bao Z. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. U. S. A. 2012;109(24):9287–9292. doi: 10.1073/pnas.1202636109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Claussen J.C., Kumar A., Jaroch D.B., Khawaja M.H., Hibbard A.B., Porterfield D.M., Fisher T.S. Nanostructuring platinum nanoparticles on multilayered graphene petal nanosheets for electrochemical biosensing. Adv. Funct. Mater. 2012;22(16):3399–3405. [Google Scholar]
- 53.Wang Y., Wang X., Lu W., Yuan Q., Zheng Y., Yao B. A thin film polyethylene terephthalate (PET) electrochemical sensor for detection of glucose in sweat. Talanta. 2019;198:86–92. doi: 10.1016/j.talanta.2019.01.104. [DOI] [PubMed] [Google Scholar]
- 54.Vukojević V., Djurdjić S., Ognjanović M., Fabián M., Samphao A., Kalcher K., Stanković D.M. Enzymatic glucose biosensor based on manganese dioxide nanoparticles decorated on graphene nanoribbons. J. Electroanal. Chem. 2018;823:610–616. [Google Scholar]
- 55.Gokoglan T.C., Kesik M., Soylemez S., Yuksel R., Unalan H.E., Toppare L. Paper based glucose biosensor using graphene modified with aConducting polymer and gold nanoparticles. J. Electrochem. Soc. 2017;164(6):G59–G64. [Google Scholar]
- 56.Li J., Yuan R., Chai Y. Simple construction of an enzymatic glucose biosensor based on a nanocomposite film prepared in one step from iron oxide, gold nanoparticles, and chitosan. Microchim. Acta. 2011;173(3–4):369–374. [Google Scholar]
- 57.Reichert J.S., McNeight S.A., Rudel H.W. Determination of hydrogen peroxide and some related peroxygen compounds. Ind. Eng. Chem., Anal. Ed. 1939;11(4):194–197. [Google Scholar]
- 58.Gomes A., Fernandes E., Lima J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods. 2005;65(2–3):45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Lee Y.-D., Lim C.-K., Singh A., Koh J., Kim J., Kwon I.C., Kim S. Dye/Peroxalate aggregated nanoparticles with enhanced and tunable chemiluminescence for biomedical imaging of hydrogen peroxide. ACS Nano. 2012;6(8):6759–6766. doi: 10.1021/nn3014905. [DOI] [PubMed] [Google Scholar]
- 60.Wu C., Xia G., Sun J., Song R. Synthesis and anisotropic self-assembly of Ag nanoparticles immobilized by the Pluronic F127 triblock copolymer for colorimetric detection of H2O2. RSC Adv. 2015;5(118):97648–97657. [Google Scholar]
- 61.Maji S.K., Sreejith S., Mandal A.K., Ma X., Zhao Y. Immobilizing gold nanoparticles in mesoporous silica covered reduced graphene oxide: a hybrid material for cancer cell detection through hydrogen peroxide sensing. ACS Appl. Mater. Interfaces. 2014;6(16):13648–13656. doi: 10.1021/am503110s. [DOI] [PubMed] [Google Scholar]
- 62.Thirumalraj B., Zhao D.-H., Chen S.-M., Palanisamy S. Non-enzymatic amperometric detection of hydrogen peroxide in human blood serum samples using a modified silver nanowire electrode. J. Colloid Interface Sci. 2016;470:117–122. doi: 10.1016/j.jcis.2016.02.049. [DOI] [PubMed] [Google Scholar]
- 63.Wang X., Martindale J.L., Liu Y., Holbrook N.J. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem. J. 1998;333(2):291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abe J., Berk B.C. Fyn and JAK2 mediate Ras activation by reactive oxygen species. J. Biol. Chem. 1999;274(30):21003–21010. doi: 10.1074/jbc.274.30.21003. [DOI] [PubMed] [Google Scholar]
- 65.Elias H., Vayssié S. In: Peroxide Chemistry. Adam W., editor. 2000. Reactive peroxo compounds generated in situ from hydrogen peroxide: kinetics and catalytic application in oxidation processes; pp. 128–138. [Google Scholar]
- 66.Wang L., Wang E. A novel hydrogen peroxide sensor based on horseradish peroxidase immobilized on colloidal Au modified ITO electrode. Electrochem. Commun. 2004;6(2):225–229. [Google Scholar]
- 67.Imlay J.A., Linn S. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J. Bacteriol. Res. 1987;169(7):2967–2976. doi: 10.1128/jb.169.7.2967-2976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxidants Redox Signal. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang J., Oyama M. Gold nanoparticle-attached ITO as a biocompatible matrix for myoglobin immobilization: direct electrochemistry and catalysis to hydrogen peroxide. J. Electroanal. Chem. 2005;577(2):273–279. [Google Scholar]
- 70.Ferapontova E., Schmengler K., Börchers T., Ruzgas T., Gorton L. Effect of cysteine mutations on direct electron transfer of horseradish peroxidase on gold. Biosens. Bioelectron. 2002;17(11–12):953–963. doi: 10.1016/s0956-5663(02)00087-8. [DOI] [PubMed] [Google Scholar]
- 71.Maduraiveeran G., Ramaraj R. A facile electrochemical sensor designed from gold nanoparticles embedded in three-dimensional sol–gel network for concurrent detection of toxic chemicals. Electrochem. Commun. 2007;9(8):2051–2055. [Google Scholar]
- 72.Tangkuaram T., Ponchio C., Kangkasomboon T., Katikawong P., Veerasai W. Design and development of a highly stable hydrogen peroxide biosensor on screen printed carbon electrode based on horseradish peroxidase bound with gold nanoparticles in the matrix of chitosan. Biosens. Bioelectron. 2007;22(9–10):2071–2078. doi: 10.1016/j.bios.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Wang J., Wang L., Di J., Tu Y. Electrodeposition of gold nanoparticles on indium/tin oxide electrode for fabrication of a disposable hydrogen peroxide biosensor. Talanta. 2009;77(4):1454–1459. doi: 10.1016/j.talanta.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 74.Jamal M., Xu J., Razeeb K.M. Disposable biosensor based on immobilisation of glutamate oxidase on Pt nanoparticles modified Au nanowire array electrode. Biosens. Bioelectron. 2010;26(4):1420–1424. doi: 10.1016/j.bios.2010.07.071. [DOI] [PubMed] [Google Scholar]
- 75.Zhang B., Cui Y., Chen H., Liu B., Chen G., Tang D. A new electrochemical biosensor for determination of hydrogen peroxide in food based on well-dispersive gold nanoparticles on graphene oxide. Electroanalysis. 2011;23(8):1821–1829. [Google Scholar]
- 76.Bai Y., Sun Y., Sun C. Pt–Pb nanowire array electrode for enzyme-free glucose detection. Biosens. Bioelectron. 2008;24(4):579–585. doi: 10.1016/j.bios.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y., Xu F., Sun Y., Guo C., Cui K., Shi Y., Wen Z., Li Z. Seed-mediated synthesis of Au nanocages and their electrocatalytic activity towards glucose oxidation. Chem. Eur J. 2010;16(30):9248–9256. doi: 10.1002/chem.200903552. [DOI] [PubMed] [Google Scholar]
- 78.Rathod D., Dickinson C., Egan D., Dempsey E. Platinum nanoparticle decoration of carbon materials with applications in non-enzymatic glucose sensing. Sensor. Actuator. B Chem. 2010;143(2):547–554. [Google Scholar]
- 79.Cherevko S., Chung C.-H. Gold nanowire array electrode for non-enzymatic voltammetric and amperometric glucose detection. Sensor. Actuator. B Chem. 2009;142(1):216–223. [Google Scholar]
- 80.Ryu J., Kim K., Kim H.S., Hahn H.T., Lashmore D. Intense pulsed light induced platinum–gold alloy formation on carbon nanotubes for non-enzymatic glucose detection. Biosens. Bioelectron. 2010;26(2):602–607. doi: 10.1016/j.bios.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 81.Cui H.F., Ye J.S., Zhang W.D., Li C.M., Luong J.H., Sheu F.S. Selective and sensitive electrochemical detection of glucose in neutral solution using platinum-lead alloy nanoparticle/carbon nanotube nanocomposites. Anal. Chim. Acta. 2007;594(2):175–183. doi: 10.1016/j.aca.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 82.Stephen Inbaraj B., Chen B.H. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J. Food Drug Anal. 2016;24(1):15–28. doi: 10.1016/j.jfda.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu H., Cao W., Li Y., Liu G., Wen Y., Yang H., Yang S. In situ growth of copper nanoparticles on multiwalled carbon nanotubes and their application as non-enzymatic glucose sensor materials. Electrochim. Acta. 2010;55(11):3734–3740. [Google Scholar]
- 84.Wu J.W., Wang C.H., Wang Y.C., Chang J.K. Ionic-liquid-enhanced glucose sensing ability of non-enzymatic Au/graphene electrodes fabricated using supercritical CO2 fluid. Biosens. Bioelectron. 2013;46:30–36. doi: 10.1016/j.bios.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 85.Baciu A., Pop A., Remes A., Manea F., Burtica G. Non-enzymatic electrochemical determination of glucose on silver-doped zeolite-CNT composite electrode. Adv. Sci. Eng. Med. 2011;3(1–2):13–19. [Google Scholar]
- 86.Xie F., Huang Z., Chen C., Xie Q., Huang Y., Qin C., Liu Y., Su Z., Yao S. Preparation of Au-film electrodes in glucose-containing Au-electroplating aqueous bath for high-performance nonenzymatic glucose sensor and glucose/O2 fuel cell. Electrochem. Commun. 2012;18:108–111. [Google Scholar]
- 87.Zhong X., Yuan R., Chai Y. n situ spontaneous reduction synthesis of spherical Pd@Cys-C60nanoparticles and its application in nonenzymatic glucose biosensors. Chem. Commun. 2012;48(4):597–599. doi: 10.1039/c1cc16081h. [DOI] [PubMed] [Google Scholar]
- 88.Wang J., Thomas D.F., Chen A. Nonenzymatic electrochemical glucose sensor based on nanoporous PtPb networks. Anal. Chem. 2008;80(4):997–1004. doi: 10.1021/ac701790z. [DOI] [PubMed] [Google Scholar]
- 89.Wei G., Xu F., Li Z., Jandt K.D. Protein-promoted synthesis of Pt nanoparticles on carbon nanotubes for electrocatalytic nanohybrids with enhanced glucose sensing. J. Phys. Chem. C. 2011;115(23):11453–11460. [Google Scholar]
- 90.Song Y.Y., Zhang D., Gao W., Xia X.H. Nonenzymatic glucose detection by using a three-dimensionally ordered, macroporous platinum template. Chem. Eur J. 2005;11(7):2177–2182. doi: 10.1002/chem.200400981. [DOI] [PubMed] [Google Scholar]
- 91.Sun Y., Buck H., Mallouk T.E. Combinatorial discovery of alloy electrocatalysts for amperometric glucose sensors. Anal. Chem. 2001;73(7):1599–1604. doi: 10.1021/ac0015117. [DOI] [PubMed] [Google Scholar]
- 92.Park S., Boo H., Chung T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta. 2006;556(1):46–57. doi: 10.1016/j.aca.2005.05.080. [DOI] [PubMed] [Google Scholar]
- 93.Fan Z., Liu B., Liu X., Li Z., Wang H., Yang S., Wang J. A flexible and disposable hybrid electrode based on Cu nanowires modified graphene transparent electrode for non-enzymatic glucose sensor. Electrochim. Acta. 2013;109:602–608. [Google Scholar]
- 94.Dhara K., Stanley J., Ramachandran T., Nair B.G., Satheesh Babu T.G. Pt-CuO nanoparticles decorated reduced graphene oxide for the fabrication of highly sensitive non-enzymatic disposable glucose sensor. Sensor. Actuator. B Chem. 2014;195:197–205. [Google Scholar]
- 95.Tehrani F., Bavarian B. Facile and scalable disposable sensor based on laser engraved graphene for electrochemical detection of glucose. Sci. Rep. 2016;6:27975. doi: 10.1038/srep27975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dhara K., Ramachandran T., Nair B.G., Satheesh Babu T.G. Single step synthesis of Au–CuO nanoparticles decorated reduced graphene oxide for high performance disposable nonenzymatic glucose sensor. J. Electroanal. Chem. 2015;743:1–9. [Google Scholar]
- 97.Pourbeyram S., Mehdizadeh K. J. Food Drug Anal. 2016;24:894–902. doi: 10.1016/j.jfda.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Justice Babu K., Sheet S., Lee Y.S., Gnana kumar G. Three-dimensional dendrite Cu–Co/reduced graphene oxide architectures on a disposable pencil graphite electrode as an electrochemical sensor for nonenzymatic glucose detection. ACS Sustain. Chem. Eng. 2018;6(2):1909–1918. [Google Scholar]
- 99.Yan X., Gu Y., Li C., Zheng B., Li Y., Zhang T., Zhang Z., Yang M. A non-enzymatic glucose sensor based on the CuS nanoflakes–reduced graphene oxide nanocomposite. Anal. Methods. 2018;10(3):381–388. [Google Scholar]
- 100.Hou L., Bi S., Lan B., Zhao H., Zhu L., Xu Y., Lu Y. A novel and ultrasensitive nonenzymatic glucose sensor based on pulsed laser scribed carbon paper decorated with nanoporous nickel network. Anal. Chim. Acta. 2019;1082:165–175. doi: 10.1016/j.aca.2019.07.056. [DOI] [PubMed] [Google Scholar]
- 101.Niu X., Lan M., Zhao H., Chen C. Highly sensitive and selective nonenzymatic detection of glucose using three-dimensional porous nickel nanostructures. Anal. Chem. 2013;85(7):3561–3569. doi: 10.1021/ac3030976. [DOI] [PubMed] [Google Scholar]
- 102.Chelaghmia M.L., Nacef M., Affoune A.M., Pontié M., Derabla T. Facile synthesis of Ni(OH)2 modified disposable pencil graphite electrode and its application for highly sensitive non-enzymatic glucose sensor. Electroanalysis. 2018;30(6):1117–1124. [Google Scholar]
- 103.Giorgio M., Trinei M., Migliaccio E., Pelicci P.G. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007;8(9):722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 104.Zhang K., Kaufman R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee Y.D., Lim C.K., Singh A., Koh J., Kim J., Kwon I.C., Kim S. Dye/peroxalate aggregated nanoparticles with enhanced and tunable chemiluminescence for biomedical imaging of hydrogen peroxide. ACS Nano. 2012;6(8):6759–6766. doi: 10.1021/nn3014905. [DOI] [PubMed] [Google Scholar]
- 106.Oungpipat W., Alexander P.W., Southwell-Keely P. A reagentless amperometric biosensor for hydrogen peroxide determination based on asparagus tissue and ferrocene mediation. Anal. Chim. Acta. 1995;309(1–3):35–45. [Google Scholar]
- 107.Rassaei L., Olthuis W., Tsujimura S., Sudholter E.J., van den Berg A. Lactate biosensors: current status and outlook. Anal. Bioanal. Chem. 2014;406:123–137. doi: 10.1007/s00216-013-7307-1. [DOI] [PubMed] [Google Scholar]
- 108.Zhao Y., Yan X., Kang Z., Fang X., Zheng X., Zhao L., Du H., Zhang Y. Zinc oxide nanowires-based electrochemical biosensor for L-lactic acid amperometric detection. J Nanopart Res. 2014;16:2398. [Google Scholar]
- 109.Anzai J.-i., Takeshita H., Kobayashi Y., Osa T., Hoshi T. Layer-by-Layer construction of enzyme multilayers on an electrode for the preparation of glucose and lactate sensors: elimination of ascorbate interference by means of an ascorbate oxidase multilayer. Anal. Chem. 1998;70(4):811–817. doi: 10.1021/ac970536b. [DOI] [PubMed] [Google Scholar]
- 110.Majidi M.R., Pournaghi-Azar M.H., Saadatirad A., Alipour E. Simultaneous determination of nitrite and hydrogen peroxide using hemoglobin modified pencil lead electrode as a novel biosensor: application to the analysis of mother's milk. J. Chin. Chem. Soc. 2015;62(1):83–89. [Google Scholar]
- 111.Nandini S., Nalini S., Manjunatha R., Shanmugam S., Melo J.S., Suresh G.S. Electrochemical biosensor for the selective determination of hydrogen peroxide based on the co-deposition of palladium, horseradish peroxidase on functionalized-graphene modified graphite electrode as composite. J. Electroanal. Chem. 2013;689:233–242. [Google Scholar]
- 112.Safavi A., Farjami F. Hydrogen peroxide biosensor based on a myoglobin/hydrophilic room temperature ionic liquid film. Anal. Biochem. 2010;402(1):20–25. doi: 10.1016/j.ab.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 113.Zhang X., Li L., Peng X., Chen R., Huo K., Chu P.K. Non-enzymatic hydrogen peroxide photoelectrochemical sensor based on WO3 decorated core-shell TiC/C nanofibers electrode. Electrochim. Acta. 2013;108:491–496. [Google Scholar]
- 114.Zou G., Ju H. Electrogenerated chemiluminescence from a CdSe nanocrystal film and its sensing application in aqueous solution. Anal. Chem. 2004;76(23):6871–6876. doi: 10.1021/ac049012j. [DOI] [PubMed] [Google Scholar]
- 115.Teker M.Ş., Karaca E., Pekmez N.Ö., Tamer U., Pekmez K. An enzyme-free H2O2 sensor based on poly(2-aminophenylbenzimidazole)/gold nanoparticles coated pencil graphite electrode. Electroanalysis. 2019;31(1):75–82. [Google Scholar]
- 116.Dhara K., Ramachandran T., Nair B.G., Satheesh Babu T.G. Au nanoparticles decorated reduced graphene oxide for the fabrication of disposable nonenzymatic hydrogen peroxide sensor. J. Electroanal. Chem. 2016;764:64–70. [Google Scholar]
- 117.Wang Q., Li W., Qian D., Li Y., Bao N., Gu H., Yu C. Paper–based analytical device for detection of extracellular hydrogen peroxide and its application to evaluate drug–induced apoptosis. Electrochim. Acta. 2016;204:128–135. [Google Scholar]
- 118.Shamkhalichenar H., Choi J.-W. An inkjet-printed non-enzymatic hydrogen peroxide sensor on paper. J. Electrochem. Soc. 2017;164:B3101–B3106. [Google Scholar]
- 119.Fernández-García M., Anderson J.A., Haller G.L. alloy formation and stability in Pd−Cu bimetallic catalysts. J. Phys. Chem. 1996;100(40):16247–16254. [Google Scholar]
- 120.Uzunoglu A., Scherbarth A.D., Stanciu L.A. Bimetallic PdCu/SPCE non-enzymatic hydrogen peroxide sensors. Sensor. Actuator. B Chem. 2015;220:968–976. [Google Scholar]
- 121.Jiang D., Chu Z., Peng J., Luo J., Mao Y., Yang P., Jin W. One-step synthesis of three-dimensional Co(OH)2/rGO nano-flowers as enzyme-mimic sensors for glucose detection. Electrochim. Acta. 2018;270:147–155. [Google Scholar]
- 122.Yalow R.S., Berson S.A. Immunoassay of endogenous plasma insulin in manj. Clin. Invest. 1960;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.https://www.antibodies-online.com/resources/17/1215/radioimmunoassay-ria/
- 124.Cho I.-H., Lee J., Kim J., Kang M.-S., Paik J.K., Ku S., Cho H.-M., Irudayaraj J., Kim D.-H. Current technologies of electrochemical immunosensors: perspective on signal amplification. Sensors. 2018;18(1):207. doi: 10.3390/s18010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Silva B.V., Cavalcanti I.T., Silva M.M., Dutra R.F. A carbon nanotube screen-printed electrode for label-free detection of the human cardiac troponin T. Talanta. 2013;117:431–437. doi: 10.1016/j.talanta.2013.08.059. [DOI] [PubMed] [Google Scholar]
- 126.Tallapragada S.D., Layek K., Mukherjee R., Mistry K.K., Ghosh M. Development of screen-printed electrode based immunosensor for the detection of HER2 antigen in human serum samples. Bioelectrochemistry. 2017;118:25–30. doi: 10.1016/j.bioelechem.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 127.Zhan P., Du X.-W., Gan N., Lin S.-C., Li T.-H., Cao Y.-T., Sang W.-G. Amperometric immunosensor for determination of clenbuterol based on enzyme-antibody coimmobilized ZrO2 nano probes as signal tag. Chin. J. Anal. Chem. 2013;41(6):828–834. [Google Scholar]
- 128.Sánchez-Tirado E., González-Cortés A., Yudasaka M., Iijima S., Langa F., Yáñez-Sedeño P., Pingarrón J.M. Electrochemical immunosensor for the determination of 8-isoprostane aging biomarker using carbon nanohorns-modified disposable electrodes. J. Electroanal. Chem. 2017;793:197–202. [Google Scholar]
- 129.Zhang X., Wang H., Yang C., Du D., Lin Y. Preparation, characterization of Fe3O4 at TiO2 magnetic nanoparticles and their application for immunoassay of biomarker of exposure to organophosphorus pesticides. Biosens. Bioelectron. 2013;41:669–674. doi: 10.1016/j.bios.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 130.Yan M., Zang D., Ge S., Ge L., Yu J. A disposable electrochemical immunosensor based on carbon screen-printed electrodes for the detection of prostate specific antigen. Biosens. Bioelectron. 2012;38(1):355–361. doi: 10.1016/j.bios.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 131.Xu Q.-Y., Tan Z., Liao X.-W., Wang C. Recent advances in nanoscale metal-organic frameworks biosensors for detection of biomarkers. Chin. Chem. Lett. 2022;33:22–32. [Google Scholar]
- 132.Manfredi A., Giannetto M., Mattarozzi M., Costantini M., Mucchino C., Careri M. Competitive immunosensor based on gliadin immobilization on disposable carbon-nanogold screen-printed electrodes for rapid determination of celiotoxic prolamins. Anal. Bioanal. Chem. 2016;408:7289–7298. doi: 10.1007/s00216-016-9494-z. [DOI] [PubMed] [Google Scholar]
- 133.Giannetto M., Bianchi M.V., Mattarozzi M., Careri M. Competitive amperometric immunosensor for determination of p53 protein in urine with carbon nanotubes/gold nanoparticles screen-printed electrodes: a potential rapid and noninvasive screening tool for early diagnosis of urinary tract carcinoma. Anal. Chim. Acta. 2017;991:133–141. doi: 10.1016/j.aca.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 134.Cui Z., Wu D., Zhang Y., Ma H., Li H., Du B., Wei Q., Ju H. Ultrasensitive electrochemical immunosensors for multiplexed determination using mesoporous platinum nanoparticles as nonenzymatic labels. Anal. Chim. Acta. 2014;807:44–50. doi: 10.1016/j.aca.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 135.Hu Q., Yang J., Zheng Z., Ding Y., Chen Y., Gao W. In situ H2O2 generation with gold nanoflowers as the coreactant accelerator for enzyme-free electrochemiluminescent immunosensing. Biosens. Bioelectron. 2019;143:111627. doi: 10.1016/j.bios.2019.111627. [DOI] [PubMed] [Google Scholar]
- 136.Thangamuthu M., Santschi C., Martin O.J.F. Label-free electrochemical immunoassay for C-reactive protein. Biosensors. 2018;8(2):34. doi: 10.3390/bios8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Madasamy T., Santschi C., Martin O.J. A miniaturized electrochemical assay for homocysteine using screen-printed electrodes with cytochrome c anchored gold nanoparticles. Analyst. 2015;140(17):6071–6078. doi: 10.1039/c5an00752f. [DOI] [PubMed] [Google Scholar]
- 138.Neves M.M., Gonzalez-Garcia M.B., Nouws H.P., Costa-Garcia A. Celiac disease detection using a transglutaminase electrochemical immunosensor fabricated on nanohybrid screen-printed carbon electrodes. Biosens. Bioelectron. 2012;31(1):95–100. doi: 10.1016/j.bios.2011.09.044. [DOI] [PubMed] [Google Scholar]
- 139.Agui L., Yanez-Sedeno P., Pingarron J.M. Role of carbon nanotubes in electroanalytical chemistry: a review. Anal. Chim. Acta. 2008;622(1–2):11–47. doi: 10.1016/j.aca.2008.05.070. [DOI] [PubMed] [Google Scholar]
- 140.Pereira da Silva Neves M.M., García M.B.G., Delerue-Matos C., García A.C. Nanohybrid materials as transducer surfaces for electrochemical sensing applications. Electroanalysis. 2011;23(1):63–71. [Google Scholar]
- 141.Charoenkitamorn K., Tue P.T., Kawai K., Chailapakul O., Takamura Y. Electrochemical immunoassay using open circuit potential detection labeled by platinum nanoparticles. Sensors. 2018;18(2):444. doi: 10.3390/s18020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang K.J., Niu D.J., Xie W.Z., Wang W. A disposable electrochemical immunosensor for carcinoembryonic antigen based on nano-Au/multi-walled carbon nanotubes–chitosans nanocomposite film modified glassy carbon electrode. Anal. Chim. Acta. 2010;659(1–2):102–108. doi: 10.1016/j.aca.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 143.Liu Y., Yuan R., Chai Y., Hong C., Liu K., Guan S. Ultrasensitive amperometric immunosensor for the determination of carcinoembryonic antigen based on a porous chitosan and gold nanoparticles functionalized interface. Microchim. Acta. 2009;167:217–224. [Google Scholar]
- 144.Afonso A.S., Perez-Lopez B., Faria R.C., Mattoso L.H., Hernandez-Herrero M., Roig-Sagues A.X., Maltez-da Costa M., Merkoci A. Electrochemical detection of Salmonella using gold nanoparticles. Biosens. Bioelectron. 2013;40(1):121–126. doi: 10.1016/j.bios.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 145.Zong C., Wu J., Wang C., Ju H., Yan F. Chemiluminescence imaging immunoassay of multiple tumor markers for cancer screening. Anal. Chem. 2012;84(5):2410–2415. doi: 10.1021/ac203179g. [DOI] [PubMed] [Google Scholar]
- 146.Yadav S., Masud M.K., Islam M.N., Gopalan V., Lam A.K., Tanaka S., Nguyen N.T., Hossain M.S.A., Li C., Yamauchi Y., Shiddiky M.J.A. Gold-loaded nanoporous iron oxide nanocubes: a novel dispersible capture agent for tumor-associated autoantibody analysis in serum. Nanoscale. 2017;9(25):8805–8814. doi: 10.1039/c7nr03006a. [DOI] [PubMed] [Google Scholar]
- 147.Boonkaew S., Chaiyo S., Jampasa S., Rengpipat S., Siangproh W., Chailapakul O. An origami paper-based electrochemical immunoassay for the C-reactive protein using a screen-printed carbon electrode modified with graphene and gold nanoparticles. Mikrochim. Acta. 2019;186:153. doi: 10.1007/s00604-019-3245-8. [DOI] [PubMed] [Google Scholar]
- 148.Rusling J.F., Hvastkovs E.G., Schenkman J.B. Toxicity screening using biosensors that measure DNA damage. Curr. Opin. Drug Discov. Dev. 2007;10(1):67–73. [PubMed] [Google Scholar]
- 149.Takács K., Némedi E., Márta D., Gelencsér É., Kovács E. Use of the enzyme transglutaminase for developing glutenfree noodle products from pea flour. Acta Aliment. 2007;36(2):195–205. [Google Scholar]
- 150.Waggoner P.S., Craighead H.G. Micro- and nanomechanical sensors for environmental, chemical, and biological detection. Lab Chip. 2007;7(10):1238–1255. doi: 10.1039/b707401h. [DOI] [PubMed] [Google Scholar]
- 151.Patolsky F., Zheng G., Lieber C.M. Nanowire sensors for medicine and the life sciences. Nanomedicine. 2006;1(1):51–65. doi: 10.2217/17435889.1.1.51. [DOI] [PubMed] [Google Scholar]
- 152.White D.C., Gouffon J.S., Peacock A.D., Geyer R., Biernacki A., Davis G.A., Pryor M., Tabacco M.B., Sublette K.L. Forensic analysis by comprehensive rapid detection of pathogens and contamination concentrated in biofilms in drinking water systems for water resource protection and management. Environ. Forensics. 2003;4(1):63–74. [Google Scholar]
- 153.Paleček E., Fojta M., Tomschik M., Wang J. Electrochemical biosensors for DNA hybridization and DNA damage. Biosens. Bioelectron. 1998;13(6):621–628. doi: 10.1016/s0956-5663(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 154.Marrazza G., Chianella I., Mascini M. Disposable DNA electrochemical sensor for hybridization detection. Biosens. Bioelectron. 1999;14(1):43–51. doi: 10.1016/s0956-5663(98)00102-x. [DOI] [PubMed] [Google Scholar]
- 155.Piunno P.A.E., Krull U.J., Hudson R.H.E., Damha M.J., Cohen H. Fiber-optic DNA sensor for fluorometric nucleic acid determination. Anal. Chem. 1995;67(15):2635–2643. doi: 10.1021/ac00111a022. [DOI] [PubMed] [Google Scholar]
- 156.Okahata Y., Kawase M., Niikura K., Ohtake F., Furusawa H., Ebara Y. Kinetic measurements of DNA hybridization on an oligonucleotide-immobilized 27-MHz quartz crystal microbalance. Anal. Chem. 1998;70(7):1288–1296. doi: 10.1021/ac970584w. [DOI] [PubMed] [Google Scholar]
- 157.Zhang H., Tan H., Wang R., Wei W., Yao S. Immobilization of DNA on silver surface of bulk acoustic wave sensor and its application to the study of UV-C damage. Anal. Chim. Acta. 1998;374(1):31–38. [Google Scholar]
- 158.Ihalainen P., Pettersson F., Pesonen M., Viitala T., Maattanen A., Osterbacka R., Peltonen J. An impedimetric study of DNA hybridization on paper-supported inkjet-printed gold electrodes. Nanotechnology. 2014;25(9) doi: 10.1088/0957-4484/25/9/094009. [DOI] [PubMed] [Google Scholar]
- 159.Mao X., Ma Y., Zhang A., Zhang L., Zeng L., Liu G. Disposable nucleic acid biosensors based on gold nanoparticle probes and lateral flow strip. Anal. Chem. 2009;81(4):1660–1668. doi: 10.1021/ac8024653. [DOI] [PubMed] [Google Scholar]
- 160.Odenthal K.J., Justin Gooding J. An introduction to electrochemical DNAbiosensors. Analyst. 2007;132:603–610. doi: 10.1039/b701816a. [DOI] [PubMed] [Google Scholar]
- 161.M. J. Shiddiky, M. A. Rahman, C. S. Cheol and Y. B. Shim, Fabrication of disposable sensors for biomolecule detection using hydrazine electrocatalyst,Anal. Biochem. 379(2) 2008170-2008175. [DOI] [PubMed]
- 162.Ingrosso C., Corricelli M., Bettazzi F., Konstantinidou E., Bianco G.V., Depalo N., Striccoli M., Agostiano A., Curri M.L., Palchetti I. Au nanoparticle in situ decorated RGO nanocomposites for highly sensitive electrochemical genosensors. J. Mater. Chem. B. 2019;7:768–777. doi: 10.1039/c8tb02514b. [DOI] [PubMed] [Google Scholar]
- 163.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., Zhang Y., Wang J., Huang B., Lin Y., Yang J., Cai W., Wang X., Cheng J., Chen Z., Sun K., Pan W., Zhan Z., Chen L., Ye F. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020;92(9):1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sheridan C. Coronavirus and the race to distribute reliable diagnostics. Nat. Biotechnol. 2020;38:382–384. doi: 10.1038/d41587-020-00002-2. [DOI] [PubMed] [Google Scholar]
- 165.Du Z., Zhu F., Guo F., Yang B., Wang T. Detection of antibodies against SARS-CoV-2 in patients with COVID-19. J. Med. Virol. 2020;92(10):1735–1738. doi: 10.1002/jmv.25820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shetti N.P., Mishra A., Bukkitgar S.D., Basu S., Narang J., Raghava Reddy K., Aminabhavi T.M. Conventional and nanotechnology-based sensing methods for SARS coronavirus (2019-nCoV) ACS Appl. Bio Mater. 2021;4:1178–1190. doi: 10.1021/acsabm.0c01545. [DOI] [PubMed] [Google Scholar]
- 167.Mogha N.K., Sahu V., Sharma R.K., Masram D.T. Reduced graphene oxide nanoribbon immobilized gold nanoparticle based electrochemical DNA biosensor for the detection of Mycobacterium tuberculosis. J. Mater. Chem. B. 2018;6:5181–5187. doi: 10.1039/c8tb01604f. [DOI] [PubMed] [Google Scholar]
- 168.Bukkitgar S.D., Shetti N.P., Aminabhavi T.M. Electrochemical investigations for COVID-19 detection-A comparison with other viral detection methods. Chem. Eng. J. 2021;420(2):127575. doi: 10.1016/j.cej.2020.127575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Choi J.R., Nilghaz A., Chen L., Chou K.C., Lu X. Modification of thread-based microfluidic device with polysiloxanes for the development of a sensitive and selective immunoassay. Sensor. Actuator. B Chem. 2018;260:1043–1051. [Google Scholar]
- 170.Martínez-Paredes G., González-García M.B., Costa-García A. Genosensor for SARS virus detection based on gold nanostructured screen-printed carbon electrodes. Electroanalysis. 2009;21(3–5):379–385. [Google Scholar]
- 171.Alafeef M., Dighe K., Moitra P., Pan D. Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano. 2020;14(12):17028–17045. doi: 10.1021/acsnano.0c06392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14(5):5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- 173.Sportelli M.C., Izzi M., Kukushkina E.A., Hossain S.I., Picca R.A., Ditaranto N., Cioffi N. Can nanotechnology and materials science help theFight against SARS-CoV-2? Nanomaterials. 2020;10(4):820. doi: 10.3390/nano10040802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Huang C., Wen T., Shi F.J., Zeng X.Y., Jiao Y.J. Rapid detection of IgM antibodies against the SARS-CoV-2 virus via colloidal gold nanoparticle-based lateral-flow assay. ACS Omega. 2020;5(21):12550–12556. doi: 10.1021/acsomega.0c01554. [DOI] [PMC free article] [PubMed] [Google Scholar]