Abstract
Background
Cardenolides are naturally occurring plant toxins which act primarily on the heart. While poisoning with the digitalis cardenolides (digoxin and digitoxin) are reported worldwide, cardiotoxicity from other cardenolides such as the yellow oleander are also a major problem, with tens of thousands of cases of poisoning each year in South Asia. Because cardenolides from these plants are structurally similar, acute poisonings are managed using similar treatments. The benefit of these treatments is of interest, particularly in the context of cost since most poisonings occur in developing countries where resources are very limited.
Objectives
To determine the efficacy of antidotes for the treatment of acute cardenolide poisoning, in particular atropine, isoprenaline (isoproterenol), multiple‐dose activated charcoal (MDAC), fructose‐1,6‐diphosphate, sodium bicarbonate, magnesium, phenytoin and anti‐digoxin Fab antitoxin.
Search methods
We searched MEDLINE, EMBASE, the Controlled Trials Register of the Cochrane Collaboration, Current Awareness in Clinical Toxicology, Info Trac, www.google.com.au, and Science Citation Index of studies identified by the previous searches. We manually searched the bibliographies of identified articles and personally contacted experts in the field.
Selection criteria
Randomised controlled trials where antidotes were administered to patients with acute symptomatic cardenolide poisoning were identified.
Data collection and analysis
We independently extracted data on study design, including the method of randomisation, participant characteristics, type of intervention and outcomes from each study. We independently assessed methodological quality of the included studies. A pooled analysis was not appropriate.
Main results
Two randomised controlled trials were identified, both were conducted in patients with yellow oleander poisoning. One trial investigated the effect of MDAC on mortality, the relative risk (RR) was 0.31 (95% confidence interval (CI) 0.12 to 0.83) indicating a beneficial effect. The second study found a beneficial effect of anti‐digoxin Fab antitoxin on the presence of cardiac dysrhythmias at two hours post‐administration; the RR was 0.60 (95% CI 0.44 to 0.81). Other benefits were also noted in both studies and serious adverse effects were minimal. Studies assessing the effect of antidotes on other cardenolides were not identified. One ongoing study investigating the activated charcoal for acute yellow oleander self‐poisoning was also identified.
Authors' conclusions
There is some evidence to suggest that MDAC and anti‐digoxin Fab antitoxin may be effective treatments for yellow oleander poisoning. However, the efficacy and indications of these interventions for the treatment of acute digitalis poisoning is uncertain due to the lack of good quality controlled clinical trials. Given pharmacokinetic differences between individual cardenolides, the effect of antidotes administered to patients with yellow oleander poisoning cannot be readily translated to those of other cardenolides. Unfortunately cost limits the use of antidotes such as anti‐digoxin Fab antitoxin in developing countries where cardenolide poisonings are frequent. More research is required using relatively cheap antidotes which may also be effective.
Keywords: Humans, Acute Disease, Antidotes, Antidotes/therapeutic use, Cardenolides, Cardenolides/poisoning, Cardenolides/therapeutic use, Cardiac Glycosides, Cardiac Glycosides/poisoning, Charcoal, Charcoal/therapeutic use, Phytotherapy, Poisoning, Poisoning/drug therapy, Randomized Controlled Trials as Topic, Thevetia, Thevetia/poisoning
Plain language summary
Antidotes for acute cardenolide (cardiac glycoside) poisoning
Cardenolides are naturally occurring plant toxins which act primarily on the heart. While poisoning with the digitalis cardenolides (digoxin and digitoxin) are reported worldwide, cardiotoxicity from other cardenolides such as the yellow oleander are also a major problem, with tens of thousands of cases of poisoning each year in South Asia. Because cardenolides from these plants are structurally similar, acute poisonings are managed using similar treatments. The benefit of these treatments is of interest, particularly in the context of cost since most poisonings occur in developing countries where resources are very limited. The objectives of this review are to determine the efficacy of antidotes for the treatment of acute cardenolide poisoning, in particular atropine, isoprenaline (isoproterenol), multiple‐dose activated charcoal (MDAC), fructose‐1,6‐diphosphate, sodium bicarbonate, magnesium, phenytoin and antidigoxin Fab antitoxin.
Two randomised controlled trials were identified; both were conducted in patients with yellow oleander poisoning. One trial investigated the effect of MDAC on mortality, the relative risk (RR) was 0.31 (95% confidence interval (CI) 0.12 to 0.83) indicating a beneficial effect. The second study found a beneficial effect of anti‐digoxin Fab antitoxin on the presence of cardiac dysrhythmias at two hours post‐administration; the RR was 0.60 (95% CI 0.44 to 0.81). Other benefits were also noted in both studies and serious adverse effects were minimal. Studies assessing the effect of antidotes on other cardenolides were not identified. One ongoing study investigating the activated charcoal for acute yellow oleander self‐poisoning was also identified. There is some evidence to suggest that MDAC and anti‐digoxin Fab antitoxin may be effective treatments for yellow oleander poisoning. However, the efficacy and indications of these interventions for the treatment of acute digitalis poisoning is uncertain due to the lack of good quality controlled clinical trials. Given pharmacokinetic differences between individual cardenolides, the effect of antidotes administered to patients with yellow oleander poisoning cannot be readily translated to those of other cardenolides. Unfortunately cost limits the use of antidotes such as anti‐digoxin Fab antitoxin in developing countries where cardenolide poisonings are frequent. More research is required using relatively cheap antidotes which may also be effective.
Background
Cardenolides, sometimes referred to as cardiac glycosides or cardioactive steroids, are naturally occurring plant toxins which act primarily on the heart (Hoffman 2002). The most well known are the digitalis cardenolides (digoxin and digitoxin) which are used therapeutically for the treatment of cardiac failure. Poisoning with digitalis cardenolides are reported worldwide and require admission to a coronary care unit (if available) to monitor for significant cardiotoxicity, and administration of antidotes such as anti‐digoxin Fab antitoxin, as needed. Case fatality ratios up to 20% have been reported, and severe toxicity may not occur until 24 hours post‐admission for digoxin, or up to five days for digitoxin poisonings (Taboulet 1993a).
Cardiotoxicity is reported from other cardenolides also, in particular yellow oleander (Thevetia peruviana) and pink or white oleander (Nerium oleander), as well as the sea mango tree (Cerbera manghas). The oleander plants are found commonly through much of the tropics and subtropics around houses and gardens (Langford 1996). These cardenolides are structurally similar to digitalis, and treatments for digitalis poisoning such as the anti‐digoxin Fab antitoxin have been trialled in the management of acute poisoning with these cardenolides (Eddleston 2000). In parts of India and Sri Lanka, yellow oleander has become a popular means of self harm with tens of thousands of cases in South Asia each year, and probably hundreds of deaths given the case fatality ratio of 5 to 10%. Further, significant dysrhythmias may be delayed for up to 72 hours post ingestion, requiring prolonged hospital admissions (Roberts 2005). Unfortunately, because the anti‐digoxin Fab antitoxin is expensive, it is not readily available worldwide, particularly in developing countries where poisonings with these cardenolides are common (Eddleston 2003). As such, the management of patients with severe cardenolide poisoning is generally difficult and costly in countries with limited resources.
Why it is important to do this review
A number of specific treatments for cardenolide poisoning have been either used or recommended, including atropine, isoprenaline (isoproterenol), multiple‐dose activated charcoal, fructose‐1,6‐diphosphate, sodium bicarbonate, magnesium, phenytoin and anti‐digoxin Fab antitoxins. The purpose of this systematic review is to evaluate the efficacy of all these treatments. The benefit of these treatments in the context of cost is also of high interest as most poisonings occur in developing countries where resources are very limited (Roberts 2005).
Objectives
To determine the efficacy of antidotes for the treatment of acute cardenolide poisoning, in particular atropine, isoprenaline (isoproterenol), multiple‐dose activated charcoal (MDAC), fructose‐1,6‐diphosphate, sodium bicarbonate, magnesium, phenytoin and anti‐digoxin Fab antitoxin.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Patients with acute symptomatic cardenolide poisoning, in particular digitalis or oleander who present within 24 to 48 hours of poisoning.
Types of interventions
Interventions where antidotes are administered, in particular atropine, isoprenaline (isoproterenol), MDAC, frustose‐1,6‐diphosphate, sodium bicarbonate, magnesium, phenytoin and anti‐digoxin Fab anti‐toxin. Randomised controlled trials comparing these results to patients who do not receive the antidote were included. It is likely that all patients will continue to receive standard treatment in addition to the intervention.
Types of outcome measures
Primary outcomes
Mortality
Secondary outcomes
Occurrence of serious cardiac dysrhythmias (in particular second or third degree heart block or cardiac arrest)
Time to reversal of dysrhythmias
Occurrence of hyperkalaemia (serum K+ > 5.5mmol/L)
Time to reversal of hyperkalaemia
Requirement for pacemaker insertion
Adverse effects of the treatment
Where information on cost of the intervention is available, the cost‐benefit would be determined.
Search methods for identification of studies
The searches were not restricted by language or publication status.
Electronic searches
We searched the following electronic databases (details of the strategies used are presented in Appendix 1);
CENTRAL (The Cochrane Library, issue 3, 2006)
MEDLINE (1966 to October 2005)
EMBASE (1980 to October 2005)
Current Awareness in Clinical Toxicology (www.npis.org/cact/cact.htm) (to March 2006)
Info Trac (to March 2006)
http://www.google.com (to March 2006)
Searching other resources
We also searched the reference lists of relevant studies identified by the above search.
We consulted experts, including authors of textbook chapters and review articles on cardenolide poisoning, and other experts in the field of clinical toxicology. We made contact by e‐mail, and encouraged each expert to forward the message to other experts knowledgeable in the area.
Data collection and analysis
Selection of studies
One author (DMR) reviewed the results of all searches and identified any article that may be eligible, given a reference to acute cardenolide poisoning and treatment with a potential antidote. Each study was then discussed between authors to confirm eligibility for inclusion in the systematic review.
Data extraction and management
Data from studies meeting inclusion criteria were entered into a computer spreadsheet. The authors performed this process independently and the results were compared. We extracted data on the following:
number of participants;
method of allocation;
type of study;
participant selection;
treatment regimen of the antidote;
details of concurrent treatments;
outcome measures listed above, including standard deviations if applicable.
Assessment of risk of bias in included studies
Since there is evidence that the quality of allocation concealment particularly affects the results of studies (Schulz 1995), the authors scored quality on the scale used by Schulz as shown below, assigning C to poorest quality and A to best quality:
A = trials deemed to have taken adequate measures to conceal allocation (that is, central randomisation; serially numbered, opaque, sealed envelopes; or other description that contained elements convincing of concealment).
B = trials in which the authors either did not report an allocation concealment approach at all or reported an approach that did not fall into one of the other categories.
C = trials in which concealment was inadequate (such as alternation or reference to case record numbers or to dates of birth).
The overall quality of each trial was independently assessed by both authors according to the method of Jadad using the following criteria, where the maximum possible score for any study is 5/5: (Jadad 1996)
Randomly assigned: A method to generate the sequence of randomisation will be regarded as appropriate if it allowed each study participant to have the same chance of receiving each intervention and the investigators could not predict which treatment was next. This criteria is scored when the method to generate the sequence of randomisation was described (one point) and it was appropriate (table of random numbers, computer generated, etc) (one point).
Double blind: A study must be regarded as double blind if the word "double blind" is used. The method will be regarded as appropriate if it is stated that neither the person doing the assessments nor the study participant could identify the intervention being assessed, or if in the absence of such a statement the use of active placebos, identical placebos, or dummies is mentioned. This criteria is scored when the method of double blinding was described (one point) and it was appropriate (one point).
Withdrawals and dropouts described: Participants who were included in the study but did not complete the observation period or who were not included in the analysis must be described (one point).
Assessment of heterogeneity
If the data were suited to meta‐analysis, we proposed to use a random‐effects model to pool the data given that heterogeneity between studies was considered likely. The presence of heterogeneity of the observed treatment effects was to be assessed using the I2 statistic, which describes the percentage of total variation across studies due to heterogeneity rather than chance. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity. Where heterogeneity appears significant, pooled results were to be interpreted with caution.
Data synthesis
Relative risk (RR) of death plus 95% confidence interval (CI) was calculated such that a RR of more than one indicated a higher risk of death (or serious dysrhythmias, etc) in the first group named. RR was used because it is more readily applied to the clinical situation. For continuous data the weighted mean difference (WMD) plus 95% CI was used.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses. If there were sufficient appropriate data we would have undertaken the following subgroup analyses;
type of intervention
type of cardenolide poisoning (digitalis versus oleander)
time to presentation. Following acute poisoning the sooner that management is initiated the more likely it is to be effective;
severity of toxicity (symptomatic patients versus those with severe toxicity as defined in Roberts 2005). There is a wide range of severity ‐ trivial poisonings where no effect is possible are relatively common. Conversely, many patients present in a moribund state where any intervention is unlikely to have time to be effective. Patients with severe poisoning who are not about to expire are those who are most likely to benefit from a treatment.
However, there were insufficient data to enable such analyses.
Results
Description of studies
We identified a total 24 studies for this systematic review, but 21 were excluded from further consideration because that they were not randomised controlled trials in patients with acute cardenolide poisoning (see 'Characteristics of excluded studies' table for more details).
Of the three studies fulfilling inclusion criteria (de Silva 2003; Eddleston 2000; Eddleston 2005), only two (de Silva 2003; Eddleston 2000) were included in the analyses (see 'Characteristics of included studies' table for more details) as the third (Eddleston 2005) is presently ongoing (see 'Characteristics of ongoing studies' table for more details). Patient recruitment to the ongoing study has been completed and data are being analysed with final results being expected by late 2006.
Both of the included studies were conducted in patients with acute yellow oleander poisoning, one (de Silva 2003) assessed the effect of MDAC and one (Eddleston 2000) assessed the effect of anti‐digoxin Fab antitoxin.
No other studies assessing the effect of antidotes on other cardenolides were identified.
Risk of bias in included studies
de Silva 2003 Allocation concealment was not adequately described (Schulz = B). The overall quality received 2/5 on the Jadad scale as the method of concealing the next allocation in the random sequence was not described (randomisation 1/2) and it was a single blind study (double blinding 0/2). However, it is acknowledged it would be nearly impossible to conduct a double blind study when the intervention is orally administered activated charcoal.
Eddleston 2000 Appropriate randomisation procedures were reported (Schulz = A), suggesting adequate allocation concealment. The overall quality was rated as 5/5 according to the Jadad scale (high quality).
Effects of interventions
As the two included studies assessed the outcomes from different antidotes, the data were not suited to meta‐analysis.
Both of the included studies reported a benefit in the primary outcome from their respective interventions and their effect on outcomes pre‐defined for this review are shown under 'Analyses'.
de Silva 2003 The administration of MDAC (compared to single dose activated charcoal (SDAC)) indicated beneficial effects in terms of mortality (RR 0.31, 95% CI 0.12 to 0.83), occurrence of severe arrhythmias (RR 0.21, 95% CI 0.06 to 0.71) and requirement for temporary pacing (RR 0.09, 95% CI 0.01 to 0.70).
The reported adverse effects were minor in nature (nausea, abdominal discomfort, diarrhoea) and uncommon.
Eddleston 2000 Administration of anti‐digoxin Fab antitoxin reduced the presence of cardiac dysrhythmias two hours post‐administration (RR 0.60, 95% CI 0.44 to 0.81) and increased the mean heart rate at two hours (WMD 16.00, 95% CI 8.18 to 23.82) and at eight hours (WMD 15.00, 95% CI 7.50 to 22.50) post‐administration. Fab antitoxin also reduced the mean serum potassium at two hours post‐administration (WMD ‐0.60, 95% CI ‐1.02 to ‐0.18]) although this effect was not observed at 48 hours post‐administration (WMD 0.00, 95% CI ‐0.19 to 0.19).
Adverse effects were reported to be more frequent from anti‐digoxin Fab antitoxin (13% of patients administered Fab), and while some potentially severe reactions were reported (including bronchospasm in two patients and mild angioedema in one patient), the reactions all responded promptly to standard treatment with epinephrine, antihistamines and corticosteroids.
Discussion
Few high quality studies have been conducted to assess the efficacy of antidotes for the treatment of acute cardenolide poisoning. In particular there are no randomised controlled trials in acute digitalis poisoning, despite the widespread therapeutic use of digitalis cardenolides and the relative frequency of poisonings. Instead, the evidence supporting antidotes for digitalis poisoning (where they have been assessed) is limited to observational and retrospective studies. While these studies have demonstrated an apparent reversal of cardiotoxicity, the role of the antidote in causing this response, independent of the effects of confounding variables such as other treatments and the natural history of the cardiotoxicity, cannot be clearly defined.
The suboptimal study design is of particular importance when considering the effect of the anti‐digoxin Fab antitoxin. Because the Fab antitoxin is expensive, clear guidelines on indications for its use and quantified benefits would be of interest to clinicians and others. However, because of the favourable outcomes from the observational studies and widespread use of this antidote, it would now be considered unethical to conduct a randomised controlled trial. Similarly, given the widespread use of atropine for cardenolide‐induced bradycardia, and clinical experience suggesting its efficacy, it seems unlikely that a randomised controlled trial will be conducted to define its efficacy.
Fortunately, randomised controlled trials have been conducted on antidotes for the treatment of acute yellow oleander poisoning (see 'Characteristics of included studies' table for more details). Yellow oleander poisoning is a particular problem in developing countries where resources are limited and therefore more information on the efficacy of antidotes is required to determine if it should be available. Unfortunately, in the case of antidotes such as anti‐digoxin Fab antitoxin, despite this data the cost of the drug limits its use in developing countries such as Sri Lanka (Eddleston 2003). Given pharmacokinetic differences between individual cardenolides, the effect of antidotes administered to patients with yellow oleander poisoning cannot be readily translated to those of other cardenolides.
The final interim report of the ongoing randomised controlled trial into the efficacy of activated charcoal in acute poisoning did not report a difference in terms of the primary outcome (death) for the subgroup of patients with yellow oleander poisoning (n = 1515 patients) and secondary analysis data is not yet available (Eddleston 2005). This is in contrast to the included study which reported improvements in mortality from MDAC. The data from this ongoing study are undergoing final analysis and the results are eagerly awaited.
There are other potentially useful antidotes for cardenolide poisoning which have not yet been assessed. Of particular interest is fructose‐1,6‐diphosphate which appears to be useful in dogs poisoned with nerium oleander (Markov 1999). A phase II clinical trial is currently underway in Sri Lanka to assess the effect of this antidote in patients with acute yellow oleander poisoning (Dawson 2006).
With the exception of two studies conducted in patients with acute yellow oleander poisoning (Eddleston 2003; Roberts 2006), the excluded studies were all conducted on subjects exposed to digitalis cardenolides (many included patients with both acute and chronic toxicity). A limited range of antidotes were considered in the excluded studies, notably anti‐digoxin Fab antitoxin (15), activated charcoal (4), glucagon (1), phenytoin (1). This highlights the gap in the available data, inviting further research into antidotes for the management of acute cardenolide poisoning.
Authors' conclusions
Implications for practice.
There is some evidence to suggest that MDAC and anti‐digoxin Fab antitoxin may be effective treatments for yellow oleander poisoning. However, the efficacy and indications of these interventions for the treatment of acute digitalis poisoning is uncertain due to the lack of good quality controlled clinical trials. The evidence base supporting the current treatment of acute cardenolide poisoning, in particular digitalis, is limited.
Considering the findings of the two included studies and their limitations, the current treatment recommendations for antidotes may include MDAC and anti‐digoxin Fab antitoxin for patients with yellow oleander poisoning. However due to the absence of high quality controlled trials, the effectiveness of other antidotes for cardenolide poisoning (such as isoprenaline (isoproterenol), frustose‐1,6‐diphosphate, sodium bicarbonate, magnesium, phenytoin) is unknown.
Implications for research.
Further research is required to confirm the efficacy of antidotes which have been suggested to be useful for acute cardenolide poisoning. In particular, research into antidotes which are relatively cheap (for example, magnesium, fructose‐1,6‐diphosphate, sodium bicarbonate and phenytoin) should be encouraged, given the high incidence of yellow oleander poisoning in developing countries.
What's new
| Date | Event | Description |
|---|---|---|
| 26 March 2008 | Amended | Converted to new review format. |
Acknowledgements
DMR is funded by a scholarship from the National Health and Medical Research Council (Australia). The South Asian Clinical Toxicology Research Collaboration is funded by a Wellcome Trust/National Health and Medical Research Council International Collaborative Research Grant GR071669MA.
We thank the Cochrane Injuries Group for their support, in particular Karen Blackhall for assistance with the literature review.
Appendices
Appendix 1. Search strategy
MEDLINE (1966 to October 2005)
#1 explode "Antidotes‐" / all SUBHEADINGS in MIME,MJME #2 explode "Antitoxins‐" / all SUBHEADINGS in MIME,MJME #3 explode "Antibody‐Affinity" / all SUBHEADINGS in MIME,MJME #4 explode "Immunoglobulins‐" / all SUBHEADINGS in MIME,MJME #5 explode "Charcoal‐" / all SUBHEADINGS in MIME,MJME #6 explode "Atropine‐" / all SUBHEADINGS in MIME,MJME #7 explode "Phenytoin‐" / all SUBHEADINGS in MIME,MJME #8 explode "Magnesium‐" / all SUBHEADINGS in MIME,MJME #9 explode "Fructosediphosphates‐" / all SUBHEADINGS in MIME,MJME #10 explode "Isoproterenol‐" / all SUBHEADINGS in MIME,MJME #11 explode "Sodium‐Bicarbonate" / all SUBHEADINGS in MIME,MJME #12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 #13 ( (antidote* or antitoxin* or antibod* or immunoglobulin* or charcoal* or atropine* or phenytoin* or magnesium* or fructosediphosphate* or isoproterenol* or sodium?bicarbonate*) in TI )or( (antidote* or antitoxin* or antibod* or immunoglobulin* or charcoal* or atropine* or phenytoin* or magnesium* or fructosediphosphate* or isoproterenol* or sodium?bicarbonate*) in AB ) #14 #12 or #13 #15 explode "Cardenolides‐" / all SUBHEADINGS in MIME,MJME #16 explode "Apocynaceae‐" / all SUBHEADINGS in MIME,MJME #17 explode "Cardiac‐Glycosides" / all SUBHEADINGS in MIME,MJME #18 explode "Thevetia‐" / all SUBHEADINGS in MIME,MJME #19 explode "Nerium‐" / all SUBHEADINGS in MIME,MJME #20 explode "Digoxin‐" / all SUBHEADINGS in MIME,MJME #21 explode "Digitoxin‐" / all SUBHEADINGS in MIME,MJME #22 #15 or #16 or #17 or #18 or #19 or #20 or #21 #23 ( (cardenolide* or cardiac?glycoside* or apocynacae* or thevetia* or nerium* or digoxin* or digitoxin*) in TI )or( (cardenolide* or cardiac?glycoside* or apocynacae* or thevetia* or nerium* or digoxin* or digitoxin*) in AB ) #24 #22 or #23 #25 #14 and #24 #26 #25 and the 'MEDLINE highly sensitive search strategy' outlined in the Cochrane Handbook for Systematic Review of Interventions (Higgins 2005).
EMBASE (1980 to October 2005)
((explode 'isoprenaline‐' / all subheadings in DEM,DER,DRM,DRR) or (explode 'fructose‐bisphosphatase' / all subheadings in DEM,DER,DRM,DRR) or (explode 'immunoglobulin‐' / all subheadings in DEM,DER,DRM,DRR) or (explode 'drug‐antibody' / all subheadings in DEM,DER,DRM,DRR) or (explode 'bicarbonate‐' / all subheadings in DEM,DER,DRM,DRR) or (explode 'antitoxin‐' / all subheadings in DEM,DER,DRM,DRR) or (explode 'antidote‐' / all subheadings in DEM,DER,DRM,DRR) or (explode 'atropine‐' / all subheadings in DEM,DER,DRM,DRR) or (explode 'activated‐carbon' / all subheadings in DEM,DER,DRM,DRR) or (explode 'charcoal‐' / all subheadings in DEM,DER,DRM,DRR) or (charcoal) or (atropine) or (phenytoin) or (magnesium) or (FDP) or (fructose) or (isoprenaline) or (isoproterenol) or (sodium‐bicarbonate) or (Fab)) AND (explode 'Apocynaceae‐' / all subheadings in DEM,DER,DRM,DRR) or (explode 'digitoxigenin‐' / all subheadings in DEM,DER,DRM,DRR) or (explode 'peruvoside‐' / all subheadings in DEM,DER,DRM,DRR) or (explode 'Nerium‐oleander‐extract' / all subheadings in DEM,DER,DRM,DRR) or (explode 'digitoxin‐' / all subheadings in DEM,DER,DRM,DRR) or (explode 'acetyldigoxin‐' / all subheadings in DEM,DER,DRM,DRR) or (explode 'alpha‐acetyldigoxin' / all subheadings in DEM,DER,DRM,DRR) or (explode 'metildigoxin‐' / all subheadings in DEM,DER,DRM,DRR) or (explode 'digoxin‐' / all subheadings in DEM,DER,DRM,DRR) or (explode 'cardenolide‐derivative' / all subheadings in DEM,DER,DRM,DRR) or (explode 'cardenolide‐' / all subheadings in DEM,DER,DRM,DRR) or (nerium) or (thevetia) or (digoxin) or (cardenolide) or (oleander) or (cardiac‐glycoside) or (apocynacae) or (digitoxin))
Current Awareness in Clinical Toxicology (www.npis.org/cact/cact.htm) (to March 2006)
CACT was searched using the following terms: cardenolides, cardiac glycoside, apocynacae, oleander, thevetia, nerium, digoxin and digitoxin.
Info Trac (to March 2006)
(cardenolide OR cardiac glycoside OR apocynacae OR oleander OR thevetia OR nerium OR digitalis OR digoxin OR digitoxin) AND (poison* OR toxic*)
http://www.google.com (to March 2006)
(cardenolide OR digitalis OR digoxin OR digitoxin OR oleander OR cardiac‐glycoside OR thevetia OR nerium) AND (antidote OR antitoxin OR Fab OR antibody OR immunoglobulin OR charcoal OR atropine OR isoprenaline OR isoproterenol OR phenytoin OR fructose OR bicarbonate OR magnesium). The first 500 entries were reviewed.
Data and analyses
Comparison 1. Activated charcoal for yellow oleander.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 401 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.12, 0.83] |
| 2 Presence of serious cardiac dysrhythmias | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.06, 0.71] |
| 3 Patients requiring temporary cardiac pacing | 1 | 401 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.70] |
1.1. Analysis.
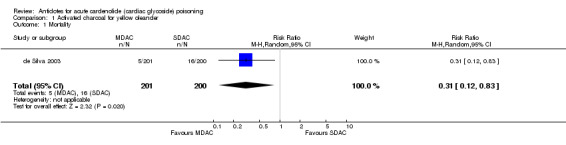
Comparison 1 Activated charcoal for yellow oleander, Outcome 1 Mortality.
1.2. Analysis.
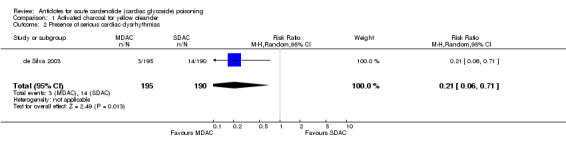
Comparison 1 Activated charcoal for yellow oleander, Outcome 2 Presence of serious cardiac dysrhythmias.
1.3. Analysis.
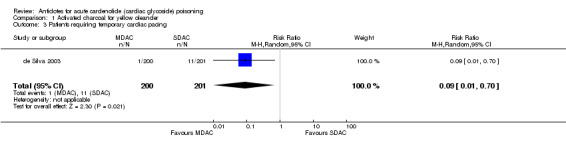
Comparison 1 Activated charcoal for yellow oleander, Outcome 3 Patients requiring temporary cardiac pacing.
Comparison 2. Anti‐digoxin Fab antitoxin for yellow oleander.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistence of presenting dysrhythmia at two hours | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.81] |
| 2 Heart rate at two hours | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 16.0 [8.18, 23.82] |
| 3 Heart rate at eight hours | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 15.0 [7.50, 22.50] |
| 4 Mean serum potassium at two hours | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.02, ‐0.18] |
| 5 Mean serum potassium at 48 hours | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.19, 0.19] |
2.1. Analysis.
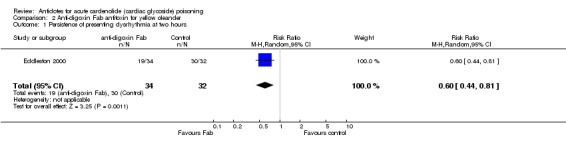
Comparison 2 Anti‐digoxin Fab antitoxin for yellow oleander, Outcome 1 Persistence of presenting dysrhythmia at two hours.
2.2. Analysis.
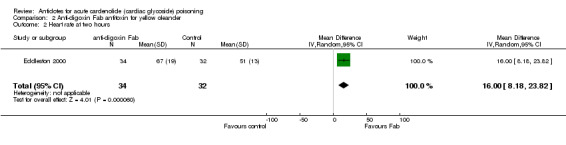
Comparison 2 Anti‐digoxin Fab antitoxin for yellow oleander, Outcome 2 Heart rate at two hours.
2.3. Analysis.
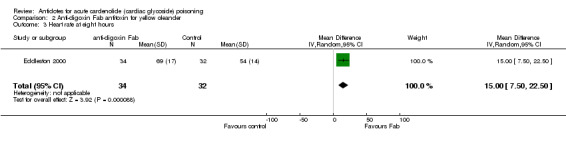
Comparison 2 Anti‐digoxin Fab antitoxin for yellow oleander, Outcome 3 Heart rate at eight hours.
2.4. Analysis.
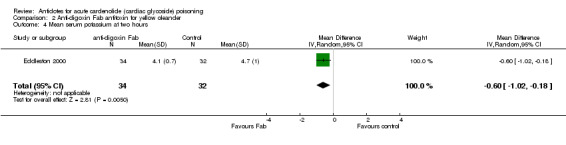
Comparison 2 Anti‐digoxin Fab antitoxin for yellow oleander, Outcome 4 Mean serum potassium at two hours.
2.5. Analysis.
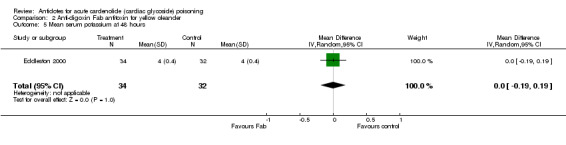
Comparison 2 Anti‐digoxin Fab antitoxin for yellow oleander, Outcome 5 Mean serum potassium at 48 hours.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
de Silva 2003.
| Methods | Randomised controlled trial. | |
| Participants | All patients with a history of acute yellow oleander poisoning. | |
| Interventions | 50g SDAC or MDAC (50g every 6 hours for 12 doses). | |
| Outcomes | Death, admission to intensive care unit, temporary cardiac pacing, administration of anti‐digoxin Fab antitoxin, dose of atropine, duration of hospital stay and frequency of life‐threatening cardiac arrhythmias. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Eddleston 2000.
| Methods | Randomised controlled trial. | |
| Participants | Patients with acute yellow oleander poisoning and clinical evidence of severe toxicity. | |
| Interventions | 1200mg of anti‐digoxin Fab antitoxin or placebo. | |
| Outcomes | Reversal of cardiac arrhythmia within 2 hours, heart rate, potassium, time to first reversal of cardiac arrhythmia. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albrecht 1988 | Uncontrolled case series. |
| Antman 1990 | Uncontrolled case series. |
| Eddleston 2003 | No randomisation ‐ outcomes in patients included in a prospective observational study were compared to patients identified by a retrospective review of hospital records. |
| Hickey 1991 | Uncontrolled surveillance study. |
| Ibanez 1995 | Retrospective study. |
| Kirkpatrick 1991 | Uncontrolled study of adverse reactions. |
| Lavaux 2004 | Uncontrolled retrospective study. |
| Love 1998 | Uncontrolled case series of patients ingesting multiple poisons. |
| Montoya 1995 | Uncontrolled case series of patients ingesting multiple poisons. |
| Oliveri 1971 | Uncontrolled case series. |
| Reissell 1982 | Volunteer study in patients on maintenance digoxin (no acute poisoning). |
| Roberts 2006 | Convenience sample from a randomised controlled trial in patients with acute poisoning. |
| Schaumann 1986 | Uncontrolled case series. |
| Smith 1982 | Uncontrolled case series. |
| Smith 1991 | Uncontrolled case series. |
| Smolarz 1984 | Uncontrolled case series. |
| Taboulet 1993b | Non‐randomised case series. |
| Wenger 1985 | Uncontrolled case series. |
| Wenger 1991 | Patients were a subgroup from an uncontrolled case series. |
| Woolf 1991 | Patients were a subgroup from an uncontrolled case series. |
| Woolf 1992 | Patients were a subgroup from an uncontrolled case series. |
Characteristics of ongoing studies [ordered by study ID]
Eddleston 2005.
| Trial name or title | A randomised controlled trial of single or multiple dose activated charcoal for acute self‐poisoning. (ISRCTN02920054) |
| Methods | |
| Participants | All patients with acute poisoning; 30% present with yellow oleander. |
| Interventions | No activated charcoal or 50g SDAC or MDAC (50g every 4h for 6 doses). |
| Outcomes | Death, proportion of patients receiving anti‐digoxin Fab or requiring transfer for tertiary care (temporary cardiac pacing). |
| Starting date | 31st March 2002 |
| Contact information | Dr Michael Eddleston eddlestonm@eureka.lk |
| Notes | Patients were allocated via a stratified block randomisation procedure using the following strata: (i) ingested toxin; (ii) time between poisoning and recruitment; and (iii) clinical status on admission. |
Contributions of authors
DMR designed the search criteria and drafted the review with the assistance of NAB.
Sources of support
Internal sources
No sources of support supplied
External sources
National Health and Medical Research Council, Australia.
Wellcome Trust, UK.
Declarations of interest
NAB is an investigator in the above‐mentioned ongoing RCT (Eddleston 2005) investigating the effect of single or multiple dose activated charcoal for acute self‐poisoning with yellow oleander. He is also an investigator in the phase II study investigating the effect of fructose‐1,6‐diphosphate for acute self‐poisoning with yellow oleander (Dawson 2006).
Edited (no change to conclusions)
References
References to studies included in this review
de Silva 2003 {published data only}
- Silva HA, Fonseka MM, Pathmeswaran A, Alahakone DG, Ratnatilake GA, Gunatilake SB, Ranasinha CD, Lalloo DG, Aronson JK, Silva HJ. Multiple‐dose activated charcoal for treatment of yellow oleander poisoning: a single‐blind, randomised, placebo‐controlled trial. Lancet 2003;361(9373):1935‐8. [DOI] [PubMed] [Google Scholar]
Eddleston 2000 {published data only}
- Eddleston M, Rajapakse S, Rajakanthan S, Sjöström L, Santharaj W, Thenabadu PN, Sheriff MHR, Warrell DA. Anti‐digoxin Fab fragments in cardiotoxicity induced by ingestion of yellow oleander: a randomised controlled trial. Lancet 2000;355(9208):967‐72. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albrecht 1988 {published data only}
- Albrecht K, Hagen N, Falkowski KP, Ickert K. Treatment of the life‐threatening digitalis‐intoxication with heterologous antibodies [Die behandlung der lebensbedrohlichen digitalis‐intoxikation mit heterologen antikörper‐fragmenten]. Zeitschrift Fur Klinische Medizin 1988;43(8):617‐20. [Google Scholar]
Antman 1990 {published data only}
- Antman EM, Wenger TL, Butler VP Jr, Haber E, Smith TW. Treatment of 150 cases of life‐threatening digitalis intoxication with digoxin‐specific Fab antibody fragments. Final report of a multicenter study. Circulation 1990;81(6):1744‐52. [DOI] [PubMed] [Google Scholar]
Eddleston 2003 {published data only}
- Eddleston M, Senarathna L, Mohamed F, Buckley N, Juszczak E, Sheriff MHR, et al. Deaths due to absence of an affordable antitoxin for plant poisoning. Lancet 2003;362(9389):1041‐4. [DOI] [PubMed] [Google Scholar]
Hickey 1991 {published data only}
- Hickey AR, Wenger TL, Carpenter VP, Tilson HH, Hlatky MA, Furberg CD, et al. Digoxin Immune Fab therapy in the management of digitalis intoxication: safety and efficacy results of an observational surveillance study. Journal of the American College of Cardiology 1991;17(3):590‐8. [DOI] [PubMed] [Google Scholar]
Ibanez 1995 {published data only}
- Ibanez C, Carcas AJ, Frias J, Abad F. Activated charcoal increases digoxin elimination in patients. International Journal of Cardiology 1995;48(1):27‐30. [DOI] [PubMed] [Google Scholar]
Kirkpatrick 1991 {published data only}
- Kirkpatrick CH. Allergic histories and reactions of patients treated with digoxin immune Fab (ovine) antibody. American Journal of Emergency Medicine 1991;9(2 SUPPL. 1):7‐10. [DOI] [PubMed] [Google Scholar]
Lavaux 2004 {published data only}
- Lavaux T, Assemi P, Casset A, Lavigne T, Castelain V, Seydi A, et al. Efficiency of a non‐equimolar neutralisation of digoxin by immune Fab therapy. Journal of Toxicology ‐ Clinical Toxicology. 2004; Vol. 42:464‐5.
Love 1998 {published data only}
- Love JN, Sachdeva DK, Bessman ES, Curtis LA, Howell JM. A potential role for glucagon in the treatment of drug‐induced symptomatic bradycardia. Chest 1998;114(1):323‐6. [DOI] [PubMed] [Google Scholar]
Montoya 1995 {published data only}
- Montoya CMA, Escalante GP, Sauceda GJM, Marquez ELM, Gonzalez CH, Flores Alvarez E. Treatment of acute poisoning caused by carbamazepine, digoxin, and acetylsalicylic acid, with repeated doses of activated charcoal [El tratamiento de las intoxicaciones agudas causadas por carbamazepina, digoxina y acido acetilsalicilico, mediante la administracion de dosis repetidas de carbon activado]. Gaceta Médica de México 1995;131(3):349‐54. [PubMed] [Google Scholar]
Oliveri 1971 {published data only}
- Oliveri R, Balestrini AE. Evaluation of sodium diphenylhydantoin in arrhythmies associated with digitalis poisoning [Valoracion de la difenihidantoina sodica en arritmias asociadas a la intoxicacion digitalica]. Prensa Médica Argentina 1971;58(32):1613‐7. [PubMed] [Google Scholar]
Reissell 1982 {published data only}
- Reissell P, Manninen V. Effect of administration of activated charcoal and fibre on absorption, excretion and steady state blood levels of digoxin and digitoxin. Evidence for intestinal secretion of the glycosides. Acta Medica Scandinavica Supplementum 1982;668:88‐90. [DOI] [PubMed] [Google Scholar]
Roberts 2006 {published and unpublished data}
- Roberts DM, Southcott E, Potter JM, Roberts MS, Eddleston M, Buckley NA. Pharmacokinetics of digoxin cross‐reacting substances in patients with acute yellow oleander (Thevetia peruviana) poisoning, including the effect of activated charcoal. Submitted 2006. [DOI] [PMC free article] [PubMed]
Schaumann 1986 {published data only}
- Schaumann W, Kaufmann B, Neubert P, Smolarz A. Kinetics of the Fab fragments of digoxin antibodies and of bound digoxin in patients with severe digoxin intoxication. European Journal of Clinical Pharmacology 1986;30(5):527‐33. [DOI] [PubMed] [Google Scholar]
Smith 1982 {published data only}
- Smith TW, Butler VP Jr, Haber E, Fozzard H, Marcus FI, Bremner WF, et al. Treatment of life‐threatening digitalis intoxication with digoxin‐specific Fab antibody fragments: experience in 26 cases. New England Journal of Medicine 1982;307(22):1357‐62. [DOI] [PubMed] [Google Scholar]
Smith 1991 {published data only}
- Smith TW. Review of clinical experience with digoxin immune Fab (ovine). American Journal of Emergency Medicine 1991;9(2 Suppl 1):1‐6. [DOI] [PubMed] [Google Scholar]
Smolarz 1984 {published data only}
- Smolarz A, Roesch E, Lenz H. Report on the treatment of 16 cases of severe glycoside poisoning with sheep antidigoxin fragments (Fab) [Erfahrungsbericht uber die behandlung von 16 schweren glykosidvergiftungen mit digitalis antikorper fragmenten (Fab)]. Zeitschrift Fur Kardiologie 1984;73(2):113‐9. [PubMed] [Google Scholar]
Taboulet 1993b {published data only}
- Taboulet P, Baud FJ, Bismuth C, Vicaut E. Acute digitalis intoxication ‐ Is pacing still appropriate?. Journal of Toxicology ‐ Clinical Toxicology 1993;31(2):261‐73. [DOI] [PubMed] [Google Scholar]
Wenger 1985 {published data only}
- Wenger TL, Butler VP Jr, Haber E, Smith TW. Treatment of 63 severely digitalis‐toxic patients with digoxin‐specific antibody fragments. Journal of the American College of Cardiology 1985;5(5 Suppl A):118‐23. [DOI] [PubMed] [Google Scholar]
Wenger 1991 {published data only}
- Wenger TL. Experience with digoxin immune Fab (ovine) in patients with renal impairment. American Journal of Emergency Medicine 1991;9(2 Suppl 1):21‐3. [DOI] [PubMed] [Google Scholar]
Woolf 1991 {published data only}
- Woolf AD, Wenger TL, Smith TW, Lovejoy FH Jr. Results of multicenter studies of digoxin‐specific antibody fragments in managing digitalis intoxication in the pediatric population. American Journal of Emergency Medicine 1991;9(2 Suppl 1):16‐20. [DOI] [PubMed] [Google Scholar]
Woolf 1992 {published data only}
- Woolf AD, Wenger T, Smith TW, Lovejoy FH Jr. The use of digoxin‐specific Fab fragments for severe digitalis intoxication in children. New England Journal of Medicine 1992;326(26):1739‐44. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Eddleston 2005 {published and unpublished data}
- Eddleston M, Juszczak E, Buckley NA, Senarathna L, Mohamed F, Sheriff MHS, Warrell DA. Randomised controlled trial of routine single or multiple dose superactivated charcoal for self‐poisoning in a region with high mortality. Clinical Toxicology 2005;43:442‐3. [Google Scholar]
Additional references
Dawson 2006
- Dawson AH. Fructose‐1,6‐diphosphate (FDP) in yellow oleander poisoning. Correspondence 2006.
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. http://www.cochrane.org/resources/handbook/hbook.htm.
Hoffman 2002
- Hoffman RS. Non‐pharmacological cardioactive steroids. Journal of Toxicology ‐ Clinical Toxicology 2002;40(3):285‐6. [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Langford 1996
- Langford SD, Boor PJ. Oleander toxicity: an examination of the human and animal toxic exposures. Toxicology 1996;109:1‐13. [DOI] [PubMed] [Google Scholar]
Markov 1999
- Markov AK, Payment MF, Hume AS, Rao MR, Markov MA, Skelton TN, et al. Fructose‐1,6‐diphosphate in the treatment of oleander toxicity in dogs. Veterinary and Human Toxicology 1999;41(1):9‐15. [PubMed] [Google Scholar]
Roberts 2005
- Roberts DM, Eddleston M. Yellow oleander poisoning. In: Nayyar V editor(s). Critical Care Update 2004. 1. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd, 2005:189‐200. [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Taboulet 1993a
- Taboulet P, Baud FJ, Bismuth C. Clinical features and management of digitalis poisoning ‐ rationale for immunotherapy. Journal of Toxicology ‐ Clinical Toxicology 1993;31(2):247‐60. [DOI] [PubMed] [Google Scholar]


