Abstract
Cardiovascular lesions, including coronary artery stenosis, are frequently associated and can cause sudden death in patients with genetic defects of glycosaminoglycan (GAG) metabolism. Early diagnosis of coronary artery lesions is difficult, although potentially lifesaving. Histopathological similarities between atherosclerotic changes in adults and in patients with genetic GAG metabolism defects have been known. Atherosclerosis is the result of a complex process involving metabolism of GAGs and proteoglycans preceded by endothelial dysfunction as a key event. Decreased nitric oxide (NO) bioavailability is considered the hallmark of endothelial dysfunction. Reduced NO synthase (NOS) has been reported in atherosclerotic arteries. Impairment in reactive hyperemia-digital peripheral arterial tonometry (RH-PAT) with EndoPAT has been validated to correlate coronary microvascular function in patients with atherosclerosis. RH-PAT is thought to reflect endothelial NO production. Immunohistological staining of endothelial NOS was performed in the stenotic lesions in the coronary artery of a 3-year-old patient with Mucopoly- saccharidosis-I, showing decreased activities. This prompted a study to measure endothelial function in patients with GAG metabolism defects for early diagnosis of endothelial dysfunction in the coronary arteries as an early sign of coronary artery changes. Evaluation by RH-PAT in 30 patients with variable genetic defects in GAG metabolism revealed significantly decreased Reactive Hyperemia Indexes compared with 12 controls. Evaluation of endothelial function with RH-PAT in patients with GAG metabolism defects may detect coronary artery lesions that can be underdiagnosed by the other measures such as coronary angiography. Use of this method may prove vital in the management of patients with GAG metabolism defects.
Introduction
The pathogenesis of atherosclerosis is unclear, but thought to be a complex multifactorial process involving shear stress induced by blood flow, inflammation, lipoprotein metabolism, extracellular matrix of cells of the vessel wall consisting of proteoglycans, and cells of the vessel wall. Proteoglycans consist of hyaluronic acid, core proteins, and glycosaminoglycans (GAGs). Low density lipoprotein (LDL), trapped in an arterial wall through apolipoprotein B-proteoglycan binding, undergo progressive oxidation. T cells and macrophages infiltrate the vessel wall triggered by endothelial dysfunction. Autoreactive T cells recognize oxidized LDL (oxLDL) and GAGs (heparin, chondroitin, and dermatan sulfate), and locally release proinflammatory cytokines and growth factors, resulting in vascular smooth muscle cell proliferation and secretion of proteoglycans with elongated GAG chains that have increased affinity for LDL (Fogelstand and Boren 2012). Macrophages stimulated by T cell derived cytokines transform into foam cells after uptake of oxLDL. Lipoprotein lipase (LPL) produced by macrophages in the vascular wall is thought to promote lipid accumulation, contributing to the development of atherosclerosis (Sartippour and Renier 2000). Chondroitin and dermatan sulfate-rich proteoglycans are considered proatherogenic because of their ability to retain LDL (Wight and Merrilees 2004). Increase in vascular proteoglycans content precede and contribute to atherosclerosis development (Huang et al 2008). Structural changes in GAG chains in proteoglycans, observed during the development of endothelial dysfunction, may enhance LDL binding to these molecules, which might be the initiating event in atherosclerosis (Oemar et al 1998).
The mucopolysaccharidoses (MPS) are a group of genetic disorders caused by deficiencies of lysosomal enzymes involved in GAG metabolism. Several types of MPS are well characterized, each distinguished by a deficiency in a different enzyme in degradation of GAGs: MPS-I (Hurler syndrome), II (Hunter syndrome), III (Sanfilippo syndrome), IV (Morquio syndrome), VI (Maroteaux-Lamy syndrome), VII (Sly syndrome), and IX (hyaluronidase deficiency). Systemic gradual accumulation of GAGs in the lysosomes typically causes chronic, progressive, multi-system organ dysfunction resulting in early death, often due to cardiopulmonary causes (Mohan et al 2002). All types of MPS, except for IX, have been reported to present with cardiovascular manifestation, although MPS-I, II, and VI seem to have more severe involvement than MPS-III or IV (Fesslova et al 2009; Honjo et al 2005; Natowictz et al 1996; Oudit et al 2007). This may be due to involvement of the defective dermatan sulfate metabolism in MPS-I, II, and VI. Cardiovascular lesions often seen in patients with MPS include valvular diseases (often involving the mitral and aortic valves), hypertrophic cardiomyopathy, endocardial fibroelastosis, and coronary artery stenosis (Krovetz et al 1965; Yano et al 2009). Histopathologic similarity in the coronary artery lesions between the atherosclerotic changes in adults and in MPS-I has been reported (Brosius and Roberts 1981; Renteria et al 1976).
Based on the aforementioned multifactorial process in atherogenesis, patients with genetic defects in GAG metabolism likely develop early atherosclerosis leading to the coronary arteries lesions as early as 3 years of age (Brosius and Roberts 1981; Yano et al 2009), suggesting that GAG metabolism defects have significantly more atherogenic effects compared to the commonly known risk factors for the coronary artery diseases.
Reduced nitric oxide synthase (NOS) has been reported in the endothelial cells in atherosclerotic arteries (Oemar et al 1998). Decreased nitric oxide (NO) bioavailability is considered the hallmark of endothelial dysfunction (Flammer et al 2012). Histological evidence showing reduced endothelial NOS (eNOS) in atherosclerotic arteries has been reported, however this has not been reported in patients with MPS (Oemar et al 1998).
Although it is well recognized that early diagnosis of coronary artery involvement in patients with MPS is of extreme importance for proper clinical management, detection of early coronary artery changes is difficult (Braunlin et al 2011). Even coronary angiography has limited value since it can underestimate the extent of the stenotic vascular lesions (Braunlin et al 1992). Because of these difficulties, the prevalence of coronary artery lesions in patients with MPS is not well known (Braunlin et al 2011).
In the coronary arteries, impairment of endothelial function occurs early in the course of atherosclerosis (Vita et al 1990). Measuring endothelial function with peripheral arterial tonometry (PAT) has recently gained increased attention; the principle of which has been described elsewhere (Kuvin et al 2003; Lavie et al 2000; Tierney et al 2009). The EndoPAT 2000 (Itamar Medical) is a device that can evaluate endothelial function in a non-invasive manner based on reactive hyperemia peripheral arterial tonometry (RH-PAT). The reactive hyperemia index (RHI) is validated to serve as a marker for endothelial function (Flammer et al 2012). RHI reflects changes in flow and digital microvessel dilatation and is partly dependent on NO (Nohria et al 2006). Although the direct link between RHI and coronary vascular lesions has not been established, RHI with EndoPAT is validated to correlate with coronary microvascular function in patients with early atherosclerosis (Bonetti et al 2003). The correlation between RHI and the age of subjects was reported as not statistically significant (Hamburg et al 2008). Endothelial function by RH-PAT with EndoPAT has also been studied in the pediatric population (Sivamurthy et al 2009).
We studied immunohistological staining of eNOS in the coronary arteries of a patient with MPS-I who died at age 3.5 years due to an ischemic heart attack caused by coronary artery stenosis (Yano et al 2009) and evaluated the eNOS activities in the coronary stenotic lesions in the MPS-I patient and in an age-matched control. Subsequently, we conducted the present study to evaluate endothelial function in 30 subjects with variable genetic defects in GAG metabolism to investigate whether a diagnosis of endothelial dysfunction suggesting coronary artery involvement could be made based on non-invasive RH-PAT with the EndoPAT.
Methods
This study was approved by the University of Southern California Institutional Review Board (HS-10–00375). All procedures followed regulatory and institutional guidelines.
1). Immunohistological studies for NOS
Immunohistological studies on coronary artery eNOS in the specimens from the 3.5-year-old patient with MPS-I (Yano et al 2009) were performed and compared with the age-matched control specimen. Formalin-fixed 5-um sections were prepared from paraffin-embedded cardiac specimens and mounted on poly-L-lysine–coated slides. The slides were then deparaffinized in xylene and washed with 100 % ethanol, followed by rehydration in 95 % ethanol. A 3 % hydrogen peroxide solution in absolute methanol was used to quench endogenous peroxidase. Antigen retrieval was done using citrate buffer (pH 6) and microwaving for 30 min, followed by cooling at room temperature for 20 min. The slides were then blocked with normal horse serum for 20 min and incubated for 1 h with primary antibody (eNOS, Epitomics, Burlingame, CA). The slides were washed with PBS (Vector Laboratories, Inc., Burlingame, CA) for 10 min. Bound anti- bodies were detected by biotinylated horse anti-mouse secondary antibody followed by avidin-biotin conjugate of Chromogen of 0.03 % diaminobenzidine. Structure of the tissue was shown by hematoxylin counterstaining.
2). Endothelial function studies on patients with genetic GAG metabolism defects
The EndoPAT 2000 system measures pulsatile arterial volume by finger plethysmography. With peripheral arterial tonometry, beat-to-beat plethysmographic recording of the finger arterial pulse wave amplitudes are captured with pneumatic probes. The study consists of three 5-minutes phases (base-line, occlusion, and dilation). Pneumatic probes are placed on the control arm and the experimental arm, on which a blood pressure cuff is placed. An increase in arterial blood volume in the fingertip in dilation phase causes an increase in pulsatile arterial column changes, thus increasing the measured signal (Flammer et al 2012). Reactive hyperemia peripheral arterial tonometry studies were performed in the fasting state (for 3 or more hours). The RH-PAT data was analyzed with the proprietary software package (Hamburg et al 2008).
The study subjects consisted of 30 individuals with variable genetic GAG metabolism defects: MPS-I (n=13), II: (n=5), IIIA: (n=3), IIIB: (n=3), IVA: (n=4), VI: (n=1), Mucolipidosis type 3: (n=1) and 12 controls (Table 1). The ages of the subjects ranged from 4–50 years, with a mean of 18.1 years, while the controls ranged in age from 15–40 years, with a mean of 25.3 years. Eleven male and 19 female subjects participated, while data was obtained from three male and nine female controls. Patients were recruited at the National MPS Society Meetings, October 2010 in Buena Park, California and July 2011 in St. Louis, Missouri, and at LAC+ Medical Center in Los Angeles. Except for MPS-II, more female study subjects were enrolled since more female patients with MPS attended the meetings. Consent was obtained from participants or a parent or guardian for minors and the intellectually disabled.
Table 1.
Endothelial function (Reactive Hyperemia Index: RHI) in the study subjects
| Subject | M/F | Age | Condition | RHI | N/Abn | Therapy | Duartion |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | F | 7 | MPS-I | 0.78 | Abn | ERT | 3Y |
| 2 | F | 9 | MPS-I | 0.89 | Abn | ERT | 7Y |
| 3 | F | 13 | MPS-I | 1.88 | N | ERT | 7Y |
| 4 | M | 13 | MPS-I | 0.7 | Abn | ERT | 7Y |
| 5 | F | 17 | MPS-I | 1.41 | Abn | ERT | 7Y |
| 6 | M | 17 | MPS-I | 1.13 | Abn | ERT | 8Y |
| 7 | F | 18 | MPS-I | 0.83 | Abn | ERT | 7Y |
| 8 | M | 19 | MPS-I | 1.32 | Abn | ERT | 8Y |
| 9 | F | 19 | MPS-I | 0.94 | Abn | ERT | 7Y |
| 10 | F | 20 | MPS-I | 1.28 | Abn | ERT | 4Y |
| 11 | F | 21 | MPS-I | 0.9 | Abn | ERT | 6Y |
| 12 | F | 23 | MPS-I | 1.04 | Abn | ERT | 6Y |
| 13 | F | 27 | MPS-I | 1.66 | Abn | ERT | 7Y |
| 14 | M | 10 | MPS-II | 0.72 | Abn | BMT at age 5Y | |
| 15 | M | 12 | MPS-II | 1.29 | Abn | ERT | 3Y |
| 16 | M | 16 | MPS-II | 1.78 | N | ERT | 3Y |
| 17 | M | 30 | MPS-II | 0.62 | Abn | ERT | 4Y |
| 18 | M | 37 | MPS-II | 2.05 | N | ERT | 4Y |
| 19 | M | 4 | MPS-IIIA | 1.14 | Abn | ||
| 20 | F | 9 | MPS-IIIA | 1.31 | Abn | ||
| 21 | F | 12 | MPS-IIIA | 2.14 | N | ||
| 22 | F | 15 | MPS-IIIB | 1.46 | Abn | ||
| 23 | M | 20 | MPS-IIIB | 2.49 | N | ||
| 24 | M | 24 | MPS-IIIB | 1.14 | Abn | ||
| 25 | F | 11 | MPS-IVA | 1.37 | Abn | ||
| 26 | F | 11 | MPS-IVA | 0.8 | Abn | ||
| 27 | F | 23 | MPS-IVA | 0.36 | Abn | ||
| 28 | F | 50 | MPS-IVA | 2.99 | N | ||
| 29 | F | 20 | MPS-VI | 1.38 | Abn | BMT at age 3 Y | |
| 30 | F | 18 | MLP-III | 1.84 | N | ||
The RHI in controls (n=12; age 15 y–40 y; female-9, male-3) was 2.40+0.72 (mean+SD). RHI of less than 1.67 is considered abnormal (EndoPAT Itamar Medical)
All subjects with MPS-I had been treated with enzyme replacement therapy (ERT) with laronidase for at least 3 years and no MPS-I subjects had bone marrow transplantation (BMT). Four MPS-II patients have been treated with ERT with idursulfase for at least 3 years. One patient with MPS-II and one with MPS-VI received BMT at age 5 years and 3 years, respectively.
No study subjects reported common risk factors for cardiovascular disease including smoking, diabetes, hyperlipidemia, obesity, or diabetes. The correlation between RHI and the age of subjects was reported as not statistically significant (Hamburg et al 2008).
Statistical analysis
Prior to statistical analyses, RHI values were log-transformed in order to normalize the distributions. Means of log-transformed RHI were compared between controls and all MPS patients with Student’s 2-sample t-test. Analysis of variance was used to compare controls and subgroups of MPS patients, based on diagnosis, excluding MPS-VI (n=1) and MLP-III (n=1), with Tukey adjustment for multiple comparisons. The association between RHI and age was examined with Spearman correlation analysis. Categorical variables were compared between various groups with Chi-square or Fisher’s exact test. RHI values are reported as mean ± s.d. and median of untransformed values. All statistical tests were 2-sided at alpha=0.05, and performed using SAS/STAT® software version 9.2.
Results
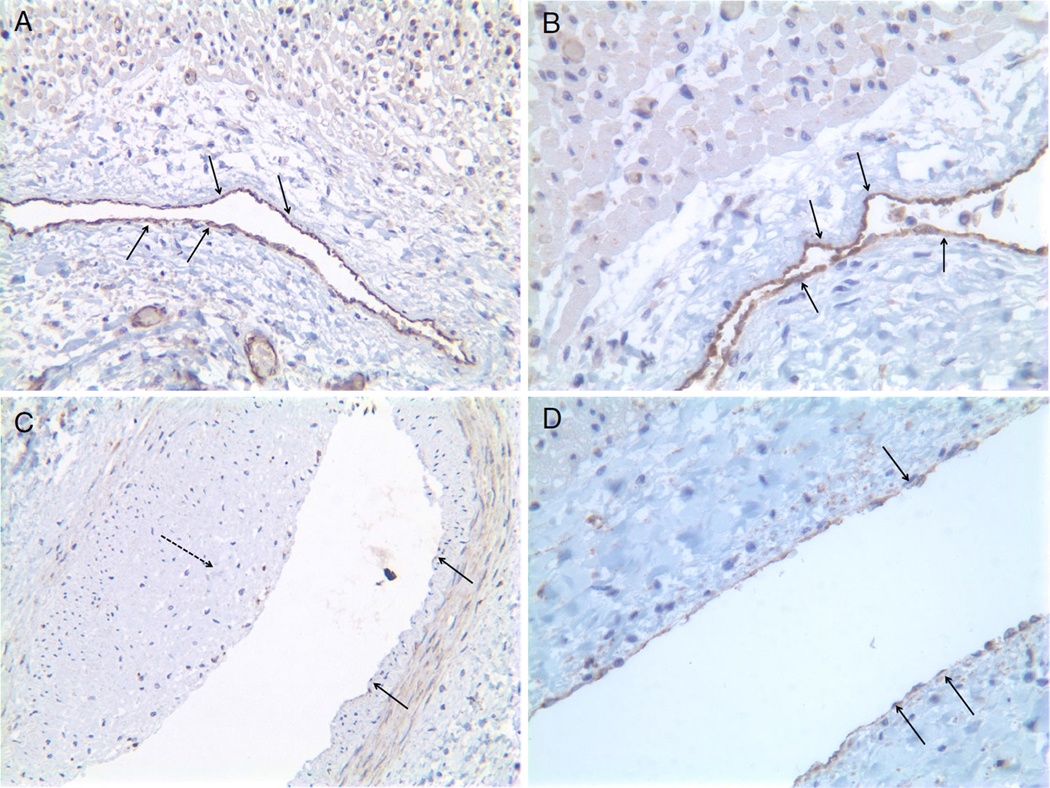
eNOS staining in the coronary artery specimens from the age-matched control (Fig. 1a and b) and the patient with MPS-I (Fig. 1c and d) showed no eNOS staining in the stenotic lesions of the extramural coronary artery (Fig. 1c) and decreased eNOS staining in the endothelium of the intramural coronary artery (Fig. 1d) in the MPS-I patient compared to the control.
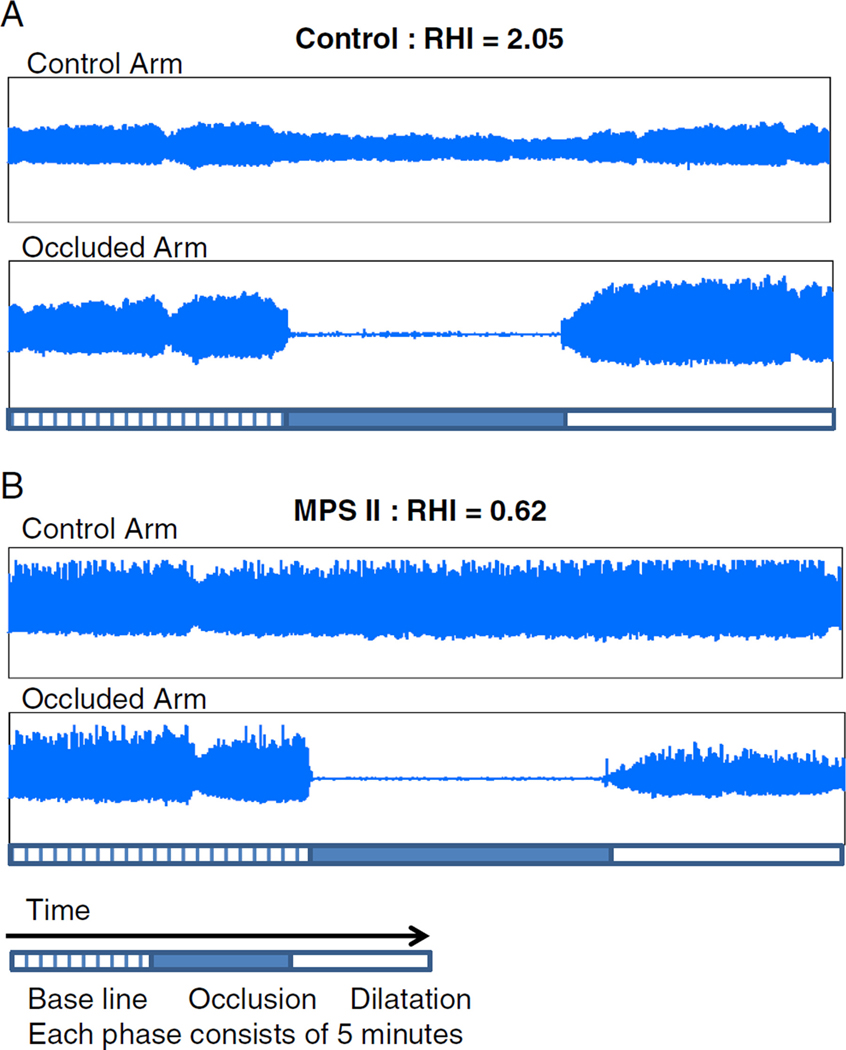
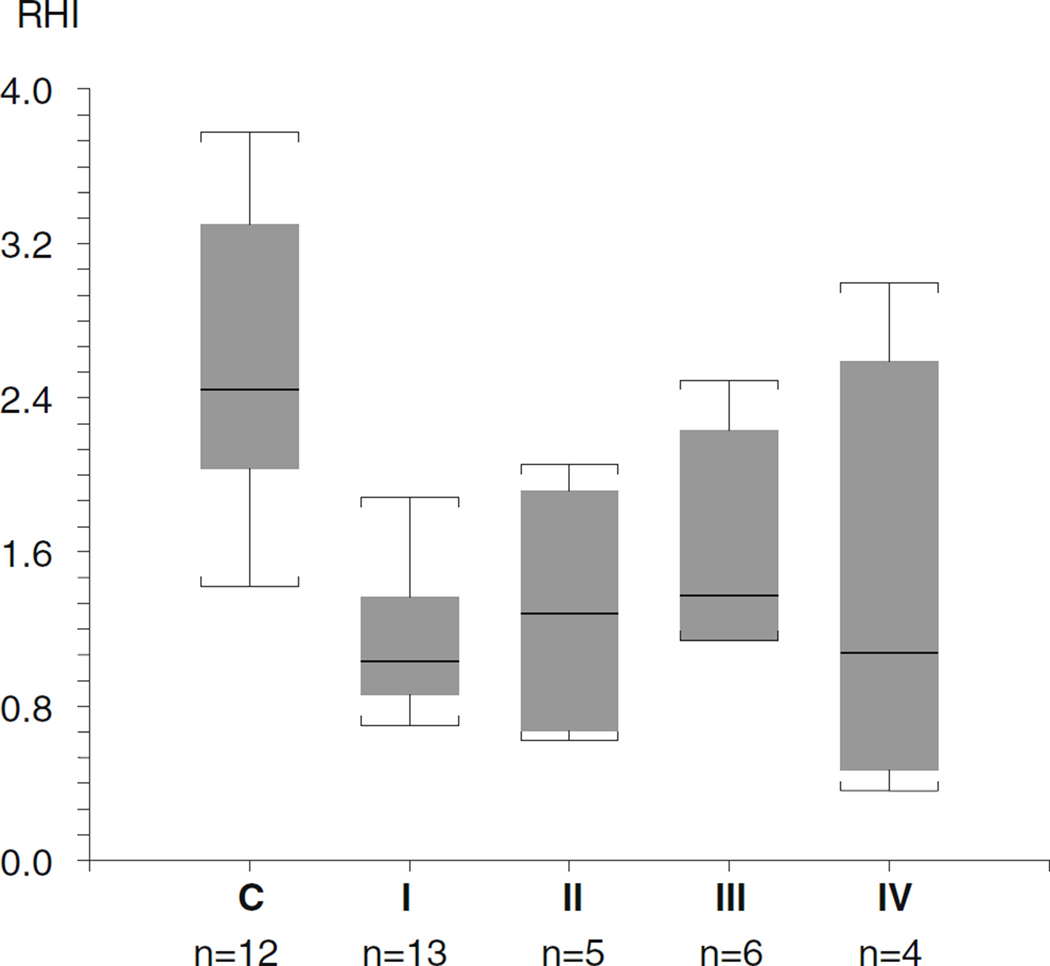
Figure 2 shows representative results of control (a) and abnormal RHI observed in a patient with MPS-II (b). Twenty three out of 30 study subjects showed abnormal results. Twelve of 13 subjects with MPS-I, three out of five subjects with MPS-II, four out of six subjects with MPS-III, three out of four subjects with MPS-IV, and the subject with MPS-VI showed abnormal RHI (Table 1). The cut off value of RHI of 1.67 has been used: lower than 1.67 is considered abnormal (Costa and Virag 2009). The 10-year-old male with MPS-II and the 23-year-old female with MPS-VI who each previously received BMT showed abnormal RHIs. The RHI of 30 patients with MPS as a whole, including one subject with mucolipidosis, was 1.32±0.59 (median 1.29) and the RHI of 12 controls was 2.40±0.72 (median 2.14). The RHIs of MPS patients were significantly lower than the controls’ (p<0.0001). RHI was not correlated with age in 30 patients with MPS (r=0.19, p=0.32). While females were disproportionally represented (63 % vs. 37 %), there was no difference between males and females in age (p=0.96), RHI (p=0.93) or percent with abnormal RHI (p=0.44). The RHIs of the subgroup of MPS are: MPS-I 1.13±0.36 (median 1.04), MPS-II 1.29±0.63 (median 1.29), MPS-III 1.61±0.57 (median 1.39), and MPS-IV 1.38±1.15 (median 1.09). The RHI of the MPS-VI subject was abnormal with 1.38 while the mucolipidosis type III subject’s RHI was normal at 1.84. The RHI of MPS subgroups I, II, and IV were significantly decreased compared to the controls (p<0.05) but no significant differences were found between MPS-III and controls, or among any of the MPS subgroups (Fig. 3).
Fig. 1.
Immunohistology of atherosclerotic coronary artery eNOS stain. a: Control X 200, b: Control X 400, c: Patient X 200, d: Patient X 400. a and b: showing NOS activities in the coronary endothelial cells in the age-matched control specimen. Positive staining (arrows) is indicated by purple∼brown color in the luminal endothelial cells. c: lacking NOS activities in the endothelial cells (arrows) in the extramural coronary artery. Note intimal proliferation (dashed arrow). d: significantly decreased activities of NOS in the endothelial cells (arrows) of intramural coronary artery
Fig. 2.
EndoPAT study results of control (a) and MPS-II (b) Control (a) shows normal endothelial vasodilator function (Reactive Hyperemia Index, RHI: 2.05) characterized by an increase in the signal amplitude after cuff release relative to baseline. MPS II patient (b) shows endothelial vasodilator dysfunction without an increase in signal amplitude after cuff release (RHI: 0.62)
Fig. 3.
The Reactive Hyperemia Indexes of the subgroup of MPS C: controls I: MPS-I II: MPS-II III: MPS-III IV: MPS-IV. The horizontal lines in the columns and the ones outside the boxes indicate median and the highest and the lowest values, respectively. The top and bottom of each box represent with 90th and 10th percentiles, respectively
Discussion
The pathogenesis of atherosclerosis is not fully understood. Accumulating evidence suggests that endothelial dysfunction is an early marker for atherosclerosis (Davignon and Ganz 2004). Major functions of endothelial cells are mediated by nitric oxide produced by eNOS. A defect in NO production has been proposed as a major mechanism of endothelial dysfunction and a contributor to atherosclerosis (Davignon and Ganz 2004). Reduced eNOS expression in the human atherosclerotic arteries has been reported (Oemar et al 1998).
Atherosclerosis is the result of complex interactions involving shear stress, T cell and macrophage mediated inflammation, lipid metabolism, GAGs in the proteoglycans and cells consisting of the vessel walls including endothelial cells, fibroblasts, and smooth muscle cells.
GAGs, core proteins, and hyaluronic acid consist of proteoglycans. At least 30 different types of proteoglycans are known based on its size, the composition of the GAGs, distributions, and functions. Several genetic diseases are known to have defects in the lysosomal enzymes responsible for the metabolism of GAGs. MPS-I and II are due to deficient alpha-L-iduronidase and L-iduronate sulfatase, respectively, resulting in accumulation of dermatan sulfate and heparan sulfate. MPS-IIIs (A, B, C, and D) are due to the defect of heparan sulfate metabolism, resulting in heparan sulfate accumulation. MPS-IVA is due to defective galactose-6-sulfatase, resulting in keratan sulfate and chondroitin 6-sulfate accumulation. MPS-VI is due to defective arylsulfatase B, resulting in accumulation of dermatan sulfate. It is a reasonable consequence that abnormal accumulation of GAGs, which occurs in patients with genetic GAG metabolism defects, leads to the development of atherosclerosis and endothelial dysfunction. Considering the fact that there are common pathologic mechanisms in atherosclerosis in adults and in patients with genetic GAG metabolism defects, eNOS was significantly reduced, as expected, in the coronary arteries in the 3.5 year-old patient with MPS-I (Yano et al 2009). This is the first report of a decreased eNOS in the coronary arteries in a patient with MPS-I. Having had this observation, evaluation of endothelial function was performed in patients with variable GAG metabolism defects, mostly with MPS. Endothelial function evaluation by finger RH-PAT with EndoPAT showed a significantly impaired endothelial function in patients’ population with genetic GAG metabolism defects. Even the majority of subjects who had been treated with enzyme replacement therapy for at least 3 years as well as subjects who received BMT more than 5 years ago showed endothelial dysfunction. Kelly et al (2013) recently reported the similar findings of endothelial dysfunction in 12 patients with MPS with EndoPAT (MPS-I: n=4, II: n=5, and VI: n=3).
Development of cardiovascular complications, including cardiomyopathy and valvular abnormalities, are well known in patients with MPS-I who have been treated with enzyme replacement therapy (Sifuentes et al 2007). There are reports that cardiac function improved after ERT (Harada et al 2011). Since this study was conducted in each subject at only one point without follow up, it is uncertain that the RHIs would follow progression of atherosclerotic changes and the cardiac lesions including hypertrophic cardiomyopathy and valvular diseases. Although evaluation of cardiac ultrasound studies were not performed to correlate the RHI in this study, it may be important to follow up the RHIs correlating with cardiac ultrasound findings in the future.
Because of the frequent association of these cardiovascular lesions in patients with MPS, sudden death due to ischemic heart attack is not uncommon (Krovetz et al 1965; Yano et al 2009). The pathogeneses of these cardiac lesions are still not well understood. It is, however, likely that there are common mechanisms leading to atherosclerosis as well as the cardiac lesions including hypertrophic cardiomyopathy. Accumulation of proteoglycans, particularly chondroitin and dermatan sulfate proteoglycans in the coronary arteries in patients with GAG metabolism defects increases the accumulation of LDL derived lipids in the extracellular matrix leading to atherosclerotic changes. eNOS decreases in the coronary arteries, resulting in endothelial dysfunction. Nitric oxide is also known to regulate transforming growth factor-ß (TGF-ß) signaling in the endothelial cells (Saura et al 2005). NOS deficiency in the endothelial cells results in activation of TGF-ß/Smad2 signaling, leading not only to enhanced collagen type I expression and myointimal proliferation causing stenotic lesions in the coronary arteries, but also to increases in extracellular matrix proteoglycan synthesis (McCaffrey 2000). Increased TGF-ß activity is known to up-regulate ß1,3-glucuronosyltransferase-I in fibroblasts, a rate-limiting enzyme in glycosaminoglycan synthesis, which leads to increased synthesis of GAGs including dermatan sulfate, hyaluronic acid, and chondroitin sulfate A and C (Matalon and Dorfman 1968; Venkatesan et al 2011).
Hyperactive TGF-ß signals in the intimal layer with myointimal proliferation causing stenosis in the coronary arteries as well as in the thickened endocardium and in the myocardial cells have been reported in a patient with MPS-I (Yano et al 2013). Canine models with MPS-I showed consistent findings (Lyons et al 2011). Accumulation of negatively charged GAGs may directly activate TGF-ß in the extracellular matrix in the myocardium and may lead to hypertrophic cardiomyopathy. Another possible mechanism of fibrotic changes in the cardiac tissues is that accumulated dermatan sulfate activates STAT proteins, which increase productions of elastin-degrading proteins, i.e., matrix metalloproteinase-12 and cathepsin S (Ma et al 2008). Reductionof elastin in myocardial cells, endocardium, and the vascular wall likely results in proliferation of connective tissues, leading to increased fibrous tissues in the ventricular walls, endocardium, and vascular walls (Hinek and Wilson 2000; Karnik et al 2003). Heparan sulfate proteoglycans (HSPGs) are necessary for the structural and functional integrity of the endothelium. Endothelial cells synthesize HSPGs, which are degraded in the lysosomal compartment, which is defective in MPS-III.
Keratan sulfate proteoglycans (KSPGs) include Aggrecan, a component of cartilage, Lumican, the major component of proteoglycan of the cornea and also distributed in interstitial collagenous matrices of the heart, aorta, muscle, skin, and intervertebral discs, and others. The small-size proteoglycans from atherosclerotic tissue were found to contain keratan sulfate (Nikitovic et al 2008). HSPGs and KSPGs are less involved in atherogenesis because of their lesser ability to retain LDL compared to chondroitin and dermatan sulfate-rich proteoglycans (Wight and Merrilees 2004).
There were no significant differences in the RHI among any of the MPS subgroups. This might be due to a small number of study participants in each MPS subgroup, particularly in MPS-II, III, and IV.
Patients with MPS often require surgical intervention for complications including inguinal hernia, spinal instability, hydrocephalus, and cardiac valve diseases. Evaluation of cardiac structure and reserve function including coronary artery endothelial function by RH-PAT prior to stressful intervention is critically important in clinical management and potentially lifesaving.
Further studies are needed to understand involvement of GAG metabolism, eNOS, and cytokines including TGF-ß in atherogenesis which may lead to a new therapeutic approach for prevention of endothelial dysfunction and atherosclerosis.
Acknowledgments
This study was approved by the University of Southern California Institutional Review Board (HS10-00375). We appreciate the National MPS Society for their collaboration toward this study. We also appreciate Ms. Carolina Coleman for her dedication to and compassion for this project. This publication was supported by NIH/NCRR SC-CTSI Grant Number UL1 RR031986. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Sources of Funding This investigator sponsored research was supported by the intramural research fund at USC and by the Genzyme Corporation.
Footnotes
Conflict of interest Shoji Yano has received research grants from Genzyme.
Kathryn Moseley, Lawrence Wong, Claudia Castelnovi, Colleen Azen, Zdena Pavlova declare that they have no conflict of interest.
Contributor Information
Shoji Yano, Genetics Division, Department of Pediatrics, LAC+USC Medical Center, Keck School of Medicine, University of Southern California, 1801 Marengo Street General Laboratory Building, Room 1G-24, Los Angeles, CA 90033, USA.
Kathryn Moseley, Genetics Division, Department of Pediatrics, LAC+USC Medical Center, Keck School of Medicine, University of Southern California, 1801 Marengo Street General Laboratory Building, Room 1G-24, Los Angeles, CA 90033, USA.
Lawrence Wong, Genetics Division, Department of Pediatrics, LAC+USC Medical Center, Keck School of Medicine, University of Southern California, 1801 Marengo Street General Laboratory Building, Room 1G-24, Los Angeles, CA 90033, USA.
Claudia Castelnovi, Genetics Division, Department of Pediatrics, LAC+USC Medical Center, Keck School of Medicine, University of Southern California, 1801 Marengo Street General Laboratory Building, Room 1G-24, Los Angeles, CA 90033, USA.
Colleen Azen, Southern California Clinical and Translational Science Institute, Children’s Hospital Los Angeles and Keck School of Medicine, University of Southern California, 4650 Sunset Blvd, Los Angeles, CA 90027, USA.
Zdena Pavlova, Department of Pathology, Children’s Hospital Los Angeles, Keck School of Medicine, University of Southern California, 4650 Sunset Blvd, Los Angeles, CA 90027, USA.
References
- Bonetti PO, Barsness GW, Keelan PC et al. (2003) Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol 41:1761–1768 [DOI] [PubMed] [Google Scholar]
- Braunlin EA, Hunter DW, Krivit W et al. (1992) Evaluation of coronary artery disease in the Hurler syndrome by angiography. Am J Cardiol 69(17):1487–1489 [DOI] [PubMed] [Google Scholar]
- Braunlin EA, Harmatz PR, Scarpa M et al. (2011) Cardiac disease in patients with mucopolysaccharidosis: presentation, diagnosis and management. J Inherit Metab Dis 34:1183–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius FC, Roberts WC (1981) Coronary artery disease in the Hurler syndrome: qualitative and quantitative analysis of the extent of coronary narrowing at necropsy in six children. Am J Cardiol 47(3):649–653 [DOI] [PubMed] [Google Scholar]
- Costa C, Virag R (2009) The endothelial-erectile dysfunction connection: an essential update. J Sex Med 6(9):2390–2404 [DOI] [PubMed] [Google Scholar]
- Davignon J, Ganz P (2004) Atherosclerosis: evolving vascular biology and clinical implications. Role of endothelial dysfunction in athero- sclerosis. Circulation 109:III-27–III-32 [DOI] [PubMed] [Google Scholar]
- Fesslova V, Corti P, Sersale G et al. (2009) The natural course and the impact of therapies of cardiac involvement in the mucopolysaccharidoses. Cardiol Young 2:170–178 [DOI] [PubMed] [Google Scholar]
- Flammer AJ, Anderson T, Celermajer DS et al. (2012) The assessment of endothelial function: from research into clinical practice. Circulation 126(6):753–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelstand P, Boren J (2012) Retention of atherogenic lipoproteins in the artery wall and its role in atherogenesis. Nutr Metab Cardiovasc 22(1):1–7 [DOI] [PubMed] [Google Scholar]
- Hamburg NM, Keyes MJ, Larson MG et al. (2008) Cross-Sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 117(19):2467–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Uchiwa H, Nakamura M et al. (2011) Laronidase replacement therapy improves myocardial function in mucopolysaccharidosis I. Mol Genet Metab 103(3):215–219 [DOI] [PubMed] [Google Scholar]
- Hinek A, Wilson SE (2000) Impaired elastogenesis in Hurler disease: dermatan sulfate accumulation linked to deficiency in elastin-binding protein and elastic fiber assembly. Am J Pathol 156(3):925–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo O, Ishino K, Kawada M, Ohtsuki S, Sano S (2005) Coarctation of thoraco-abdominalaorta associated with mucopolysacchridosis VII in a child. Ann Thorac Surg 80(2):729–731 [DOI] [PubMed] [Google Scholar]
- Huang F, Thompson JC, Wilson PG, Aung HH, Rutledge JC, Tannock LR (2008) AngiotensinII Increases vascular proteoglycan content preceding and contributing to atherosclerosis development. J Lipid Res 49(3):521–530 [DOI] [PubMed] [Google Scholar]
- Karnik SK, Brooke BS, Bayes-Genis A et al. (2003) A critical role of elastin signaling in vascular morphogenesis and disease. Development 130(2):411–423 [DOI] [PubMed] [Google Scholar]
- Kelly AS, Metzig AM, Steinberger J, Braunlin EA (2013) Endothelial function in children and adolescents with mucopolysaccharidosis. J Inherit Metab Dis 36:221–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krovetz LJ, Lorincz AE, Schiebler GL (1965) Cardiovascular manifestations of the Hurler syndrome: hemodynamic and angiocardiographic observations in 15 patients. Circulation 31:132–141 [DOI] [PubMed] [Google Scholar]
- Kuvin JT, Patel AR, Sliney KA et al. (2003) Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J 146:168–174 [DOI] [PubMed] [Google Scholar]
- Lavie P, Shcnall RP, Sheffy J, Shlitner A (2000) Peripheral vasoconstricion during REM sleep detected by a new plethysmographic method. Nat Med 6(6):606. [DOI] [PubMed] [Google Scholar]
- Lyons JA, Dickson P, Wall J et al. (2011) Arterial pathology in canine Mucopolysaccharidosis-I and response to therapy. Lab Invest 91(5):665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Tittiger M, Knutsen RH et al. (2008) Upregulation of elastase proteins results in aortic dilation in mucopolysaccharidosis I mice. Mol Genet Metab 94(3):298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon R, Dorfman A (1968) The structure of acid mucopolysaccharides produced by hurler fibroblasts in tissue culture. Proc Natl Acad Sci USA 60(1):179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey TA (2000) TGFs and TGF receptors in atherosclerosis. Cytokine Growth Factor Rev 11:103–114 [DOI] [PubMed] [Google Scholar]
- Mohan UR, Hay AA, Cleary MA, Wraith JE, Patel RG (2002) Cardiovascular changes in children with mucopolysaccharide disorders. Acta Paediatr 91(7):799–804 [DOI] [PubMed] [Google Scholar]
- Natowictz MR, Short MP, Dickersin GR et al. (1996) Clinical and bio-chemical manifestations of hyaluronidase deficiency. N Engl J Med 335:1029–1033 [DOI] [PubMed] [Google Scholar]
- Nikitovic D, Katonis P, Tsatsakis A, Karamanos NK, Tzanakakis GN (2008) Lumican, a small leucine-rich proteoglycan. IUBMB Life 60(12):818–823 [DOI] [PubMed] [Google Scholar]
- Nohria A, Gerhard-Herman M, Creager MA, Mitra D, Ganz P (2006) Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol 101(2):545–548 [DOI] [PubMed] [Google Scholar]
- Oemar BS, Tschudi MR, Godoy N, Brovkovich, Malinski T, Lüscher TF (1998) Reduced endothelial nitric oxide synthase expression and production in human atherosclerosis. Circulation 97(25):2494–2498 [DOI] [PubMed] [Google Scholar]
- Oudit GY, Butany J, Williams WG, Clarke JTR, Iwanochko RM (2007) Left ventricular aneurysm associated with mucopolysaccharidosis type VI syndrome (Maroteaux-Lamy syndrome). Circulation 115(5):e60–e62 [DOI] [PubMed] [Google Scholar]
- Renteria VG, Ferrans VJ, Roberts WC (1976) The heart in the Hurler syndrome: gross, histologic and ultrastructural observations in five necropsy cases. Am J Cardiol 38(4):487–501 [DOI] [PubMed] [Google Scholar]
- Sartippour MR, Renier G (2000) Upregulation of macrophage lipoprotein lipase in patients with type 2 diabetes: role of peripheral factors. Diabetes 49(4):597–602 [DOI] [PubMed] [Google Scholar]
- Saura M, Zaragoza C, Herranz B et al. (2005) Nitric oxide regulates transforming growth factor-ß signaling in endothelial cells. Circ Res 97(11):1115–1123 [DOI] [PubMed] [Google Scholar]
- Sifuentes M, Doroshow R, Hoft R et al. (2007) A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years. Mol Genet Metab 90(2):171–180 [DOI] [PubMed] [Google Scholar]
- Sivamurthy KM, Dampier C, MacDermott M, Maureen M, Cahill M, Hsu LL (2009) Peripheral arterial tonometry in assessing endothelial dysfunction in pediatric sickle cell disease. Pediatr Hem Onc 26:589–596 [DOI] [PubMed] [Google Scholar]
- Tierney ESS, Newburger JW, Gauvreau K (2009) Endothelial pulse amplitude testing: feasibility and reproducibility in adolescents. J Pediatr 154(6):901–905 [DOI] [PubMed] [Google Scholar]
- Venkatesan N, Ouzzine M, Kolb M, Netter P, Ludwig MS (2011) Increased deposition of chondroitin/dermatan sulfate glycosaminoglycan and upregulation of ß1,3glucuronosyltransferase I in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 300(2):L191–L203 [DOI] [PubMed] [Google Scholar]
- Vita JA, Treasure CB, Nable EG et al. (1990) Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation 81:491–497 [DOI] [PubMed] [Google Scholar]
- Wight TN, Merrilees MJ (2004) Proteoglycans in atherosclerosis and restenosis. Key roles for versican. Circ Res 94:1158–1167 [DOI] [PubMed] [Google Scholar]
- Yano S, Moseley K, Pavlova Z (2009) Postmortem studies on a patient with mucopolysaccharidosis type I: histopathological findings after one year of enzyme replacement therapy. J Inherit Metab Dis 32(Suppl 1):53–57 [DOI] [PubMed] [Google Scholar]
- Yano S, Li C, Pavlova Z (2013) The transforming growth factor-beta signaling pathway involvement in cardiovascular lesions in Mucopolysaccharidosis-I. JIMD Rep 7:55–58 [DOI] [PMC free article] [PubMed] [Google Scholar]