Abstract
Background
Levodopa plus dopamine decarboxylase inhibitor is a common treatment for restless legs syndrome (RLS).
Objectives
To evaluate efficacy and safety of levodopa for RLS compared to placebo and other active agents.
Search methods
We searched CENTRAL (The Cochrane Library 2008, Issue 4), MEDLINE, EMBASE, PsycINFO and CINAHL, from January 1985 to December 2008, reference lists of articles, and contacted pharmaceutical companies.
Selection criteria
We included double‐blind randomised controlled trials (RCT) investigating levodopa treatment versus placebo or other treatment for at least seven days in patients with RLS (age ≥ 18 years). Outcomes included symptom severity, CGI‐I, objective as well as self rated sleep parameters, quality of life, and safety parameters.
Data collection and analysis
Two authors extracted data, assessed risk of bias, and contacted pharmaceutical companies and authors for additional information. We collected dropouts due to adverse events and patients experiencing adverse events.
Main results
Six placebo controlled and three active controlled RCTs were included (521 participants). Symptom severity (11 point rating scale, 0 points indicating no symptoms, 10 points indicating maximally severe symptoms) was more reduced with levodopa than placebo in two studies (mean difference (MD) ‐1.34, 95% confidence interval (CI) ‐2.18 to ‐0.5, P = 0.002). Periodic limb movements in sleep per hour of sleep (PLMS‐Index; PLMSI) improved by ‐26.28/h compared to placebo (95% CI ‐30.53 to ‐22.02, P < 0.00001).The CGI‐I changed more with levodopa than placebo in two studies (MD ‐1.25, 95% CI ‐1.89 to ‐0.62, P = 0.0001). In two studies, sleep quality (sleep questionnaire, visual analogue scale) showed a large effect (standardised mean difference (SMD) 0.92, 95% CI 0.52 to 1.33, P < 0.00001) whereas quality of life (50 mm Visual Analogue Scales) improved by 3.23 compared to placebo (95% CI 1.64 to 4.82, P < 0.0001). Few patients dropped out of treatment (3 of 218 patients) but more levodopa treated patients experienced adverse events than with placebo (odds ratio 2.61, 95% CI 1.35 to 5.04, P = 0.004). Two dopamine agonist controlled studies showed smaller effects with levodopa than cabergoline and pramipexole on the IRLS (MD 5.25, 95% CI 2.10 to 8.40, P =0.001), CGI‐I (MD 0.62, 95% CI 0.37 to 0.87, P < 0.00001), and quality of life (MD 5.54, 95% CI 2.65 to 8.43, P = 0.0002).
Authors' conclusions
Levodopa is efficacious for the short‐term treatment of RLS. Augmentation, the clinically most relevant adverse event, was not investigated sufficiently.
Plain language summary
Levodopa for restless legs syndrome
Restless legs syndrome (RLS) is a common neurological disorder characterised by a nocturnal urge to move the legs that is usually associated with unpleasant sensations in the legs. Symptoms occur predominantly during rest, in the evening, and at night. Sleep disturbances are usually the reason why patients seek medical advice. The disorder is generally considered to be a chronic condition. Levodopa is recommended for the treatment of RLS.
We could include nine trials in the meta‐analysis which compared levodopa treatment to placebo or to other active treatments in RLS and varied from one to eight weeks. Patients suffered from moderate to severe RLS and were treated with doses of 100 mg levodopa/25 mg dopamine decarboxylase up to 400 mg levodopa/100 mg dopamine decarboxylase. The studies were performed in European and Northern American countries.
Levodopa reduced symptom severity to a larger extent than placebo. Also clinicians rated RLS symptoms as more improved with levodopa than placebo. Periodic limb movements in sleep, monitored during polysomnography, were reduced more in levodopa treatment compared to placebo; however, total sleep time was not changed. Self rated quality of sleep and quality of life were markedly improved. Only a very low number of patients discontinued treatment due to adverse events but a larger number of patients on levodopa treatment reported adverse events compared to placebo. Evidence of three active controlled trials comparing levodopa to cabergoline, pergolide, and pramipexole was in favour of dopamine agonists regarding reduction of RLS severity (IRLS questionnaire), symptom improvement (CGI), and quality of life. The results of the other five endpoints do not favour any one treatment over another. However, due to a large range of confidence intervals in these few trials, superiority of one agent cannot be ruled out.
A serious adverse event developing during long‐term dopaminergic medication, the so‐called augmentation, is characterised by an earlier onset of symptoms during the day, faster onset of symptoms when at rest, spreading of symptoms to the upper limbs and trunk, and shorter duration of the treatment effect. Augmentation was not systematically assessed in most of the previous clinical studies. Future trials with longer treatment duration and with comparison to other treatment options are needed to investigate the occurrence of augmentation and the efficacy of levodopa treatment in RLS.
Summary of findings
Background
Description of the condition
Restless legs syndrome (RLS) is a common neurological disorder with a high impact on sleep. RLS was first described in detail by Ekbom 1945. Obligatory diagnosis criteria were established by the International Restless Legs Syndrome Study Group (IRLSSG, Walters 1995). These criteria were revised at a consensus conference held at the National Institute of Health (Allen 2003). The essential criteria, supportive criteria and associated features of the disease are summarised in Table 3.
1. Diagnosis criteria of Restless legs syndrome.
| Essential criteria |
| 1. An urge to move the legs, usually accompanied or caused by uncomfortable and unpleasant sensations in the legs (sometimes the urge to move is present without the uncomfortable sensations and sometimes the arms or other body parts are involved in addition to the legs). 2. The urge to move or unpleasant sensations begin or worsen during periods of rest or inactivity such as lying or sitting. 3. The urge to move or unpleasant sensations are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues. 4. The urge to move or unpleasant sensations are worse in the evening or night than during the day or only occur in the evening or night (when symptoms are very severe, the worsening at night may not be noticeable but must have been present previously). |
| Supportive criteria and associated features of RLS |
|
| Essential criteria |
| 1. An urge to move the legs, usually accompanied or caused by uncomfortable and unpleasant sensations in the legs (sometimes the urge to move is present without the uncomfortable sensations and sometimes the arms or other body parts are involved in addition to the legs). 2. The urge to move or unpleasant sensations begin or worsen during periods of rest or inactivity such as lying or sitting. 3. The urge to move or unpleasant sensations are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues. 4. The urge to move or unpleasant sensations are worse in the evening or night than during the day or only occur in the evening or night (when symptoms are very severe, the worsening at night may not be noticeable but must have been present previously). |
| Supportive criteria and associated features of RLS |
|
Epidemiological surveys in Western Europe and in the USA indicate that up to 10% of the population are afflicted with RLS. The prevalence increases with age and is in females twice as high as in males (Berger 2004; Berger 2007; Högl 2003; Phillips 2000; Rothdach 2000; Ulfberg 2001). Approximately one third of the persons reporting RLS symptoms (i.e. 2% to 3% of the population) may be in need of medical treatment (Happe 2008).
Supporting features of the syndrome include periodic leg movements while awake (PLMW) and during sleep (PLMS), both are recorded in polysomnography (PSG). In PLMS monitoring a bilateral surface electromyogram of the anterior tibial muscles is recorded. Scoring of PLMS is made according to standard criteria (Bonnet 1993; Iber 2007; Zucconi 2006). PLMS occur frequently in several sleep disorders other than RLS and may also be present in subjects who do not complain about sleep disturbance. However, PLMS are seen more frequently in patients with RLS (Allen 2003; Hornyak 2004). Although the presence of PLMS is not specific to RLS, an elevated PLMS index (> 15 PLMS per hour of sleep; PLMSI; American Academy of Sleep Medicine 2005) is supportive of the diagnosis of RLS (Allen 2003). The family history for the disorder is positive in 40% to 60% of cases. RLS is a polygenetic disorder; linkage studies in families with RLS revealed several loci but have not yet identified disease‐causing sequence variants (Stefansson 2007; Winkelmann 2007). Generally, patients with positive family history experience an earlier onset of symptoms (before the age of 45 years) than patients without afflicted relatives. A positive response to levodopa also supports the diagnosis of RLS, with almost 90% patients showing a 50% relief of symptoms when treated with this agent (Stiasny‐Kolster 2006).
Sleep disturbances are a commonly associated feature of the disorder and are usually the reason why patients seek medical advice (Allen 2003). However, patients with milder forms of RLS may not report on disturbed sleep, therefore, sleep disturbance is not considered to be necessary for, or supportive of the diagnosis of RLS (Allen 2003). In patients who seek treatment, typically, the severity and frequency of symptoms increase over time. Thus, the disorder is generally considered to be a chronic condition. Neurological examinations usually result in unremarkable findings for patients with idiopathic (primary) RLS. Secondary forms of the disorder, however, have to be identified, and factors that may exacerbate or trigger symptoms have to be treated. Beside the established causes of secondary RLS (e.g. end‐stage renal disease, pregnancy, and iron deficiency), an increasing number of conditions seems to be associated with the disorder (Allen 2007; Connor 2008; Manconi 2004; Schöls 1998; Walters 2007).
Description of the intervention
Treatment focuses on the relief of limb symptoms and on the sequelae of the disorder such as disturbed sleep and consequent daytime sleepiness and impaired quality of life (Kushida 2007; Talati 2009).
Since the 1980s, therapy has focused on levodopa and dopamine agonists (Stiasny‐Kolster 2009; Trenkwalder 2008). Levodopa was the first dopaminergic substance that was investigated for the treatment of RLS. The very first report on positive effects of levodopa (plus benserazide) on RLS was published by Akpinar 1982. Clinical research on treatment was boosted by the definition of diagnostic criteria (Walters 1995) and by the development of severity rating scales.
Since then, several studies have examined the efficacy of dopaminergic substances in RLS. However, only a few studies have examined treatment effects longer than 12 weeks and no study has examined long‐term effects (i.e. longer than one year). Levodopa trials were conducted mainly in the 1990s using a cross‐over design with few included patients; only one large scale multi‐centre trial is available. Clinical experience revealed the most serious side effect of dopaminergic treatment, the development of augmentation, which was first described by Allen 1996. Augmentation is characterised by an overall increase in severity of RLS symptoms that can be seen in an earlier onset of symptoms during the day, faster onset of symptoms when at rest, spreading of symptoms to the upper limbs and trunk, and shorter duration of the treatment effect (Garcia‐Borreguero 2007a).
Augmentation as a side effect in RLS treatment was prospectively and systematically evaluated only in one actively controlled levodopa trial and a few dopamine agonist trials, currently not yet published. Previous data on frequency and severity of augmentation vary widely, clinical experience shows that augmentation may occur more frequently during levodopa treatment than during treatment with dopamine agonists (Garcia‐Borreguero 2007b). Therefore, levodopa is only recommended in RLS patients with intermittent symptoms or as initial treatment option according to recent treatment guidelines. In case of continuous treatment, the daily dosage of levodopa should not exceed 200 mg to 300 mg. Dopamine agonists are considered first‐line treatment of RLS (Oertel 2007; Trenkwalder 2005).
Alternative treatment options in RLS other than dopaminergic drugs include antiepileptics such as gabapentine, gabapentin enacarbil and valproic acid, as well as pregabalin and opioids (Conti 2008; Eisensehr 2004; Garcia‐Borreguero 2002; Kushida 2009; Trenkwalder 2008; Walters 1993). However, to date, the number of studies investigating substances other than dopaminergic drugs is still limited.
All previous trials investigated the symptomatic therapy of RLS. No cure of RLS has been proposed except when treating causes of secondary RLS such as iron deficiency.
Recently, a few meta‐analyses and reports have been undertaken to summarise evidenced based therapies in RLS (Baker 2008; Conti 2007; Hansen 2009; Quilici 2008; Trenkwalder 2008; Zintzaras 2010).
How the intervention might work
The aetiology of the disorder is not sufficiently understood. It is generally accepted that a dysfunction of the central nervous dopaminergic system may be responsible (Hening 2004; Trenkwalder 2004). The involvement of the dopaminergic system is supported by the effectiveness of dopaminergic drugs for the disorder, at least in those phenotypes which benefit from dopaminergic treatment (Trenkwalder 2010). There is no consistent evidence from neuroimaging studies of how a dopaminergic drug might work in RLS. Newest research indicates a significant decrease of D2 receptor density in the putamen of RLS patients (Connor 2009). Brain iron storage may be involved in many phenotypes of RLS. Currently, it seems that supplying iron is both a symptomatic and in some cases, i.e. in pregnancy and iron deficiency anaemia, a curative way of treating RLS ‐ although the mechanism of low brain iron in RLS is not yet known. Other curative treatments for idiopathic RLS are not known.
Why it is important to do this review
Recently, one review has described efficacy and safety of levodopa treatment for RLS including evaluation of polysomnography. However, clinically relevant outcomes such as severity of the disorder, quality of sleep, and quality of life were not evaluated in placebo or active controlled studies (Conti 2007).
We undertook the present evaluation to systematically assess the therapeutic efficacy and safety of levodopa treatment in RLS. According to a pre‐reviewed protocol, we searched several databases, assessed study quality using a predefined quality assessment, and evaluated a wide range of clinically relevant aspects of treatment effects. We aimed to evaluate the therapeutic efficacy of levodopa treatment for RLS as assessed by self rated symptom severity scales and PSG parameters such as PLMSI and total sleep time. Comprehensive analyses included questionnaires of global improvement, quality of sleep, quality of life, and safety parameters such as patients dropping out of treatment as well as experience of adverse events.
Objectives
To evaluate the efficacy and safety of levodopa in comparison to placebo and other agents for patients with RLS.
Methods
Criteria for considering studies for this review
Types of studies
We included double‐blind and randomised controlled trials (RCTs) investigating the treatment of RLS with levodopa versus placebo or another drug, enclosing parallel group trials as well as cross‐over trials.
Types of participants
Adult patients (18 years or more) had to have a diagnosis of primary or secondary RLS according to diagnosis criteria of the IRLSSG (Allen 2003; Walters 1995). Studies conducted before 1995 (i.e. before standardising diagnostic criteria; Walters 1995) had to present explicitly predefined diagnostic criteria for inclusion in the meta‐analyses.
Types of interventions
The experimental intervention consisted of any dose or regimen of levodopa with carbidopa or benserazide in any method of administration (oral, intravenous or transdermal, regular release, and sustained‐release) for a minimum of seven days. In the control intervention, either placebo or other comparative drugs were used.
Types of outcome measures
Divided into primary and secondary endpoints, we evaluated the following endpoints:
Primary outcomes
Symptoms of RLS experienced subjectively by the patient and assessed with validated instruments (self rated questionnaires assessing severity of symptoms)
PLMS Index (number of PLMS per hour of total sleep time or time in bed)
Total sleep time (min)
Number of dropouts due to adverse events (safety parameter)
Secondary outcomes
Clinical Global Impressions ‐ Improvement (CGI‐I)
Self rated quality of sleep (description of the included questionnaires, see below)
Quality of life (description of the included questionnaires, see below)
Additional outcomes which were expected to be useful for explaining effects:
Number of patients experiencing adverse events (safety parameter)
Number of patients experiencing augmentation (safety parameter)
Search methods for identification of studies
We used the following resources for identification of relevant studies in any language.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2008, Issue 4), MEDLINE (1985 to 2008), EMBASE (1985 to 2008), PsycINFO (1985 to 2008), and CINAHL (1985 to 2008). We did not search the Cochrane Movement Disorders Group's trials register as this database had not been updated by the Cochrane Movement Disorders Group. The respective search strategies are displayed in the Appendices.
Searching other resources
We searched online databases for additional unpublished studies such as the Internet sites www.clinicaltrials.gov and www.clinicalstudyresults.org. Table 4 lists numbers of studies retrieved in these searches. We checked recent reviews and the references of all included studies for further, potentially relevant publications in any language. We contacted the responsible pharmaceutical company for published and unpublished trials.
2. Additional trials/ information retrieved from the Internet.
| Online resource | Retrieved trials | Additional information for published trials |
| Clinical study results | 0 | 1 |
| Boehringer‐Ingelheim | 1 | 0 |
Internet sites:
www.clinicalstudyresults.org
http://trials.boehringer‐ingelheim.com
Data collection and analysis
Selection of studies
Two authors (HS and MH, the latter with support of CL, see acknowledgments) reviewed independently titles and abstracts of all obtained publications to assess their potential relevance. Subsequently, we assessed the potentially relevant publications for inclusion from the full text. Authorship and results were not blinded. In both steps, we resolved disagreements by discussion.
Data extraction and management
Two authors (HS and MH, the latter with support of CL) independently extracted data using a predefined form. We resolved disagreements and errors. Extraction included diagnostic criteria, study type, numbers of patients, doses given and procedures of titration, age, gender, ethnicity, country of trial, treatment duration, occurrence of adverse events and withdrawals due to adverse events.
All questionnaires used in the included studies are presented in Table 5.
3. Summary of questionnaires in trials and their scoring.
| Questionnaire | Description | Measurement |
| IRLS (Walters 2003) |
Symptom severity scale with a total score of 0 to 40 for 10 questions (rating 0 to 4). | Mild RLS: 0 to 10 Moderate RLS: 11 to 20 Severe RLS: 21 to 30 Very severe RLS: 31 to 40 |
| Clinical Global Impressions ‐ Improvement (National Institute of Mental Health 1976) | Evaluation by clinician regarding improvement of condition. | Rating 1 (very much better) to 7 (very much worse) |
| SF‐A (Goertelmeyer 1985) |
Subscore of questions regarding self rated quality of sleep. | Rating 1 to 5 → transformed into SMD |
| RLS‐6 satisfaction with sleep (Kohnen 2004) |
Question regarding satisfaction with sleep in the past 7 days. | Rating 0 (totally satisfied) to 10 (totally unsatisfied) → transformed into SMD |
| QoL‐RLS (Kohnen 2002) |
Total score of 12 questions investigating health related quality of life in RLS patients (6‐point Likert scale). | 0 to 60 (high impairment) → transformed into SMD |
IRLS: International RLS Severity Rating Scale; SF‐A: Schlaffragebogen‐A; QoL‐RLS: Restless Legs Syndrome Quality of Life questionnaire
RLS severity was assessed by a visual analogue scale (VAS, 0‐10), a 11‐point rating scale, and the IRLS scale (Walters 2003). The IRLS is a validated severity rating scale with 10 items rated from 0 to 4 and a total score of 0 to 40. Scores of 1 to 10 represent mild, 11 to 20 moderate, 21 to 30 severe and 31 to 40 points indicate very severe symptoms (Walters 2003). Total sleep time in minutes and the index of periodic limb movements (PLM) per hour of total sleep time (PLMSI) were assessed polysomnographically. One study reported the PLM index, i.e. PLMs per hour of time in bed (PLMI). We extracted observer rated improvement using the Clinical Global Impressions ‐ Improvement (CGI‐I; National Institute of Mental Health 1976). Self rated sleep quality included the scale “quality of sleep” of the questionnaire Schlaffragebogen A (SF‐A; Goertelmeyer 1985), the scale “satisfaction with sleep” of the RLS‐6 scales (Kohnen 2004) and VAS. We extracted data on VAS and the disease‐specific QoL‐RLS by Kohnen 2002 in order to evaluate quality of life. Safety parameters included dropout rates due to adverse events and number of patients experiencing adverse events.
Assessment of risk of bias in included studies
Two authors (HS and MH, the latter with support of CL) independently performed assessment of methodological quality using The Cochrane Collaboration's tool for assessing bias (Reviewer's Handbook, chapter 8). We classified criteria like randomisation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting in each trial indicating the likelihood of risk of bias (Reviewer's Handbook). We resolved any resulting disagreements by discussion. The results of the assessment of risk of bias are displayed in the Characteristics of included studies section for each trial as well as in Figure 1 and Figure 2.
1.
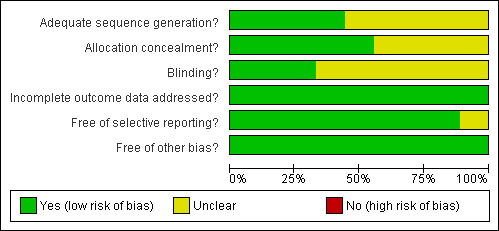
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
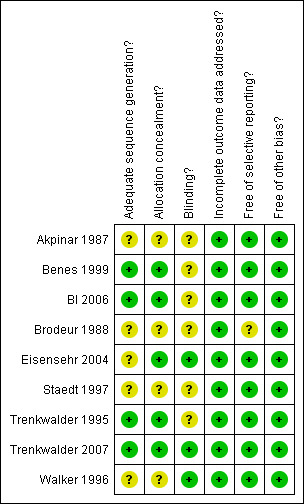
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Measures of treatment effect
Dichotomous data
We analysed safety parameters such as dropout rates due to adverse events and patients experiencing adverse events during treatment using odds ratios with 95% confidence intervals. Odds ratios above 1 indicate more negative events with levodopa compared to negative events with placebo or other dopamine agonists.
Continuous data
We analysed continuous data with the inverse variance method implementing mean differences and standard errors for the outcomes severity of RLS, PLMSI, total sleep time, CGI‐I, and quality of life. All effect estimates include a 95% confidence interval. Quality of sleep was investigated on two different scales. Therefore, we used the inverse variance method implementing standardised mean differences, i.e. Hedges' adjusted g, with standard errors and a confidence interval of 95%. Effects include values such as 0.2 representing a small effect, 0.5 representing a moderate effect, and 0.8 indicating a large effect (Cohen 1988). Negative mean differences indicate better response in the treatment group for severity of RLS, PLMSI and CGI‐I, positive values indicate better response in the treatment group for total sleep time, quality of sleep and quality of life. Active controlled trials were also pooled using the inverse variance method.
Unit of analysis issues
Eight of the nine included trials were cross‐over trials. Four studies provided sufficient information to perform analyses on dependent measures. The other four studies had to be analysed using independent procedures. In three trials, levodopa was compared to another active drug, that is cabergoline, pergolide, and pramipexole.
Dealing with missing data
We extracted end of treatment means or mean changes from baseline and standard deviations or standard errors from the primary analysis population, which consisted of eight of nine trials of patients fulfilling the protocol. We contacted the pharmaceutical companies Hoffmann La‐Roche, Pfizer, and Boehringer Ingelheim for further information on the supported studies. We also contacted authors of the studies Brodeur 1988, Eisensehr 2004, Staedt 1997 and Walker 1996 for missing data, but unfortunately, we could not obtain further information from the contacted authors.
Assessment of heterogeneity
We tested heterogeneity of studies with Chi² tests. Additionally presented I² statistics give an estimate of the degree of heterogeneity between studies. Percentages of 0% to 40% represent low, 30% to 60% moderate, 50% to 90% substantial, and 75% to 100% indicate considerable heterogeneity (Reviewer's Handbook, chapter 9).
Assessment of reporting biases
To identify possible publication bias we visually inspected funnel plots.
Data synthesis
Random‐effects models were used to calculate effect sizes as no common underlying effect could be expected due to the diversity of study populations and medications. As we could consider the cross‐over design in four placebo controlled studies contributing data to all endpoints, we used the generic inverse variance input method for all continuous outcomes. We used the inverse variance method for continuous outcomes of the active controlled studies. We analysed dichotomous outcomes in all meta‐analyses using odds ratios with the Mantel‐Haenszel method.
Subgroup analysis and investigation of heterogeneity
We performed separate meta‐analyses on results of placebo controlled studies and on results of active controlled studies.
Results
Description of studies
Results of the search
Our searches generated 302 publications by searching the electronic databases. Of these, we excluded 279 after screening of titles and abstracts as they were mostly overviews or publications on other aspects of RLS. Of the remaining 23 publications, eight studies could be included after inspection of full texts. A search of online databases yielded one additional trial.
Included studies
Nine randomised, controlled, and double‐blind studies were included in this review comprising a total of 521 patients with five to 32 patients in six placebo controlled trials (Akpinar 1987; Benes 1999; Brodeur 1988; Eisensehr 2004; Trenkwalder 1995; Walker 1996) and 11 to 361 patients in three active controlled trials (BI 2006; Staedt 1997; Trenkwalder 2007). The trials were actively controlled with the dopamine agonists cabergoline (Trenkwalder 2007), pergolide (Staedt 1997), and pramipexole (BI 2006). All trials except Trenkwalder 2007 were cross‐over trials.
Methods, included patients, interventions, and relevant outcomes of all included trials are described in the Characteristics of included studies.
Setting
Six of the nine trials were performed in single centres. Patients were recruited from outpatient clinic settings. Studies were conducted in Germany (Benes 1999; Eisensehr 2004; Staedt 1997; Trenkwalder 1995; Trenkwalder 2007), Canada (Brodeur 1988; Walker 1996), Switzerland (BI 2006), and Turkey (Akpinar 1987).
Treatment durations varied between seven days in one study (Walker 1996), 14 to 16 days in three trials (Akpinar 1987; Brodeur 1988; Staedt 1997) and three to four weeks in four studies (Benes 1999; BI 2006; Eisensehr 2004; Trenkwalder 1995). One actively controlled study investigated treatment effects after eight and 30 weeks (Trenkwalder 2007). Treatment effects after eight weeks were included in the meta‐analyses in order to be able to compare these data to other trial results. Mean duration of the included studies was 23.6 days (SD 14.3).
Patients
All patients had symptoms of RLS which were assessed according to the criteria defined by the IRLSSG (Allen 2003; Walters 1995) in five studies (Benes 1999; BI 2006; Eisensehr 2004; Trenkwalder 1995; Trenkwalder 2007). In four trials, patients were assessed similarly to these diagnostic criteria (Akpinar 1987; Brodeur 1988; Staedt 1997; Walker 1996).
In five studies, patients had a diagnosis of primary RLS. Two studies included patients with primary and secondary RLS (Benes 1999; Trenkwalder 1995). One study only investigated patients with secondary RLS (Walker 1996). In one trial, no information was given (Akpinar 1987).
Age was similarly distributed in all trials with a mean of 57.2 years, ranging from 49.6 years (Akpinar 1987) to 68.2 years (Walker 1996). Mean percentage of included female patients was 57.1%, ranging from 35.7% (Trenkwalder 1995) to 80% (Walker 1996).
Interventions
Study drugs were given orally once daily (Akpinar 1987; Benes 1999; Eisensehr 2004; Staedt 1997; Trenkwalder 1995; Trenkwalder 2007), twice daily (Brodeur 1988) and in controlled or dual‐release form (Walker 1996; BI 2006).
Levodopa was given together with the dopamine decarboxylase inhibitor (DDC) benserazide in seven trials (Akpinar 1987; Benes 1999; BI 2006; Brodeur 1988; Eisensehr 2004; Trenkwalder 1995; Trenkwalder 2007) and with carbidopa in two studies (Staedt 1997; Walker 1996). A fixed dose of 100 mg levodopa/25 mg carbidopa was given in one trial (Walker 1996) and 200 mg levodopa/50 mg benserazide in three trials (Akpinar 1987; Brodeur 1988; Eisensehr 2004). In five studies flexible uptitration from 100 mg levodopa/25 mg benserazide or carbidopa to either 200 mg/50 mg (Benes 1999; Trenkwalder 1995), 300 mg/75 mg (BI 2006; Trenkwalder 2007), or 400 mg/100 mg (Staedt 1997) was used. In four of eight cross‐over studies, medication washout periods between the first and the second treatment were not reported or not used (Akpinar 1987; Benes 1999; Eisensehr 2004; Trenkwalder 1995).
In the active controlled trials cabergoline was uptitrated to a fixed dose of 2.0 mg which could be increased to 3.0 mg (Trenkwalder 2007). Pergolide was flexibly uptitrated from 0.125 mg to 0.25 mg (Staedt 1997) and pramipexole was flexibly uptitrated from 0.25 mg to 0.75 mg (BI 2006).
Outcomes
Self rated assessments included symptoms of severity and symptom improvement as well as self rated quality of sleep and quality of life. Quality of sleep was additionally investigated using polysomnography. Lastly, studies included safety assessment.
Excluded studies
Fifteen publications were excluded while we screened the full texts. In two trials, only a part of the investigated population had a diagnosis of RLS. Three trials had study durations of less than seven days. Open label designs and inadequate control group designs were used in five trials. Duplicate information from conference proceedings published later was found five times. See Characteristics of excluded studies for details.
Risk of bias in included studies
Allocation
Randomisation procedure remained unclear in five studies, the four other studies reported computer‐generated randomisation lists. Allocation of treatment was reported in five studies with allocation of identical, numbered packages assigned to patients.
Blinding
Blinding of participants, investigators and data analysts was sufficiently described in six trials. Blinded polysomnography rating was reported only in one study, six other studies gave insufficient information.
Incomplete outcome data
Three studies with premature withdrawals reported dropouts and reasons for these. All studies except one (Trenkwalder 2007) investigated polysomnography parameters, data analyses of these were based on per protocol population.
Selective reporting
Studies investigated a range of endpoints such as symptom severity, quality of sleep, quality of life and safety parameters. In one study, not all implemented measures were reported fully in the trial publication. In another study, no endpoints had been specified beforehand in the trial publication.
Other potential sources of bias
Two studies were supported by government grants, five studies were sponsored by pharmaceutical companies, two studies did not report on funding. Several studies that used levodopa as the active comparator were sponsored by companies who aimed to licence the comparative substance i.e. a dopamine agonist (BI 2006) and used equivalent dosages of levodopa taken from trials in Parkinson disease. Equivalent dosages of dopaminergic substances are not available for RLS patients.
We could not ascertain any other major source of bias.
Effects of interventions
Summary of findings for the main comparison. Summary of findings: levodopa versus placebo.
| Levodopa compared with placebo for restless legs syndrome | ||||||
|
Patient or population: patients with restless legs syndrome Intervention: levodopa for at least seven days Comparison: levodopa for at least seven days | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Levodopa | |||||
|
1 Severity of symptoms (rating from 0 = not present to 10 = severe) |
The mean symptom severity rating ranged across control groups from 5.2 to 5.5 | The mean symptom severity rating in the intervention groups was ‐1.34 lower (95 CI ‐2.18 to ‐0.50) | 48 (2 studies) |
+++O moderate | Estimated effect based on only two studies. | |
|
2 Periodic limb movements in sleep index (PLMSI) PLMS per hour of sleep (4 trials) or time in bed (1 trial) |
The mean PLMSI ranged across control groups from 36.9 to 101 | The mean PLMSI in the intervention groups was ‐26.28 lower (95 CI ‐30.53 to ‐22.02) | 91 (5 studies) |
+++O moderate | Blinding of polysomnography raters was not reported in most studies. | |
|
3 Total sleep time (TST) |
The mean TST ranged across control groups from 260 to 397.4 | The mean TST in the intervention groups was 5.86 higher (95% CI ‐18.56 to 30.28) | 59 (4 studies) |
++OO low | The confidence interval shows no to a considerable effect. Blinding of polysomnography raters was not reported in most studies. The treatment difference was not significant. |
|
|
4 Dropouts due to adverse events |
0 of 1000 | 0 of 1000 | OR 0.73 (95% CI 0.05 to 11.34) | 109 (6 studies) |
+++O moderate | The confidence interval shows no to a considerable effect. The treatment difference was not significant. |
|
5 Clinical Global Impressions ‐ Improvement of condition (CGI‐I) Rating of 1 = very much improved to 7 = very much worse |
The mean CGI‐I ranged across control groups from 4.4 to 4.8 | The mean CGI‐I in the intervention groups was ‐1.25 lower (95% CI ‐1.89 to ‐0.62) | 60 (2 studies) | +++O moderate | Estimated effect based on only two studies. | |
|
6 Self rated quality of sleep SMD on SF‐A scale quality of sleep and VAS (0 to 10 = very good night) |
The mean SMD in the intervention groups was 0.92 higher (95 % CI 0.52 to 1.33) | 60 (2 studies) |
+++O moderate | Estimated effect based on only two studies. No data for placebo group can be given due to the standardized analysis. |
||
|
7 Quality of life VAS on life satisfaction (0 to 50 = high) |
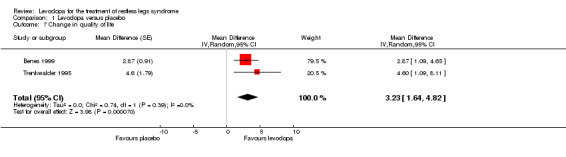
The mean life satisfaction ranged across control groups from 21.6 to 23.8 | The mean life satisfaction in the intervention groups was 3.23 higher (95% CI 1.64 to 4.82) | 60 (2 studies) |
+++O moderate | Estimated effect based on only two studies. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; OR: Odds Ratio; VAS: Visual Analogue Scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2. Summary of findings: levodopa versus other dopamine agonists.
| Dopamine agonists compared with levodopa for restless legs syndrome | ||||||
|
Patient or population: patients with restless legs syndrome Settings: outpatient settings in Europe Intervention: treatment with dopamine agonists cabergoline, pergolide, pramipexole for at least seven days Comparison: treatment with levodopa for at least seven days | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Dopamine agonists | Levodopa | |||||
|
1 IRLS range: 0 to 40 (= severe) |
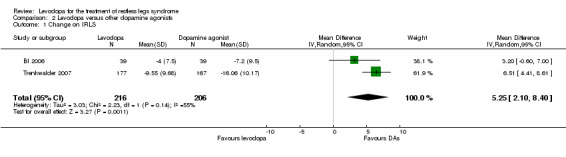
The mean IRLS change ranged across dopamine agonist groups from ‐7.2 to ‐16.06. | The mean IRLS change in the levodopa groups was 5.25 smaller (95% CI 2.1 to 8.4). | 383 (2 studies) | +++O moderate | Estimated effect based on only two studies. | |
|
2 Periodic limb movements per hour of time in bed (PLMI) |
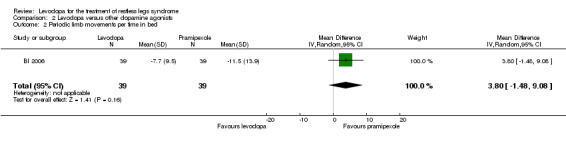
The mean PLMI was ‐11.5 in the pramipexole group. | The mean change on the PLMI in the levodopa group was 3.80 smaller (95% CI ‐1.48 to 9.08, P = 0.16). | 39 (1 study) | ++OO low | Methods of the study were not well reported. Estimated effect based on only one study. The treatment difference was not significant. |
|
|
3 Total sleep time (TST) |
The mean TST was 421 in the pergolide group. | The mean TST in the levodopa group was ‐76.00 smaller (95% CI ‐155.82 to 3.82, P = 0.06). | 11 (1 study) |
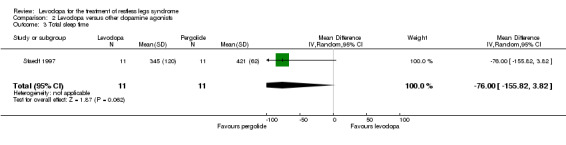
+OOO very low |
Methods of one included study were not well reported, very low doses of pergolide were implemented, the confidence interval of the effect shows no to a considerable effect. The treatment difference was not significant. |
|
|
4 Number of drop outs due to adverse events |
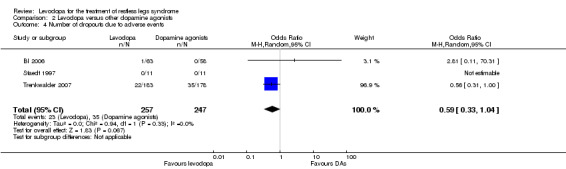
0 per 100 | 0 per 100 | OR 0.59 (95% CI 0.33 to 1.04, P = 0.07) | 504 (3 studies) |
+++O moderate | The confidence interval of the effect shows no to a considerable effect. The treatment difference was not significant. |
|
5 Clinical Global Impressions ‐ Improvement of condition (CGI‐I) Rating of 1 = very much improved to 7 = very much worse |
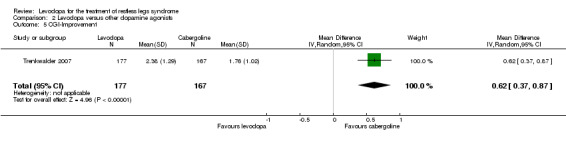
The mean CGI‐I was 1.76 in cabergoline. | The mean change in CGI‐I in the levodopa group was 0.62 (95% CI 0.37 to 0.87), i.e. further apart from a rating of "1 = very much improved". | 344 (1 study) | +++O moderate | Estimated effect based on only one study. | |
|
6 Self rated quality of sleep RLS‐6; scale satisfaction with sleep: 0 to 10 (= low satisfaction) |
The mean change in satisfaction with sleep was ‐3.43 in cabergoline. | The mean change in satisfaction with sleep in the levodopa group was 0.63 smaller (05% CI ‐0.09 to 1.35, P = 0.09). | 344 (1 study) | ++OO low | The confidence interval of the effect shows no to a considerable effect. Estimated effect based on only one study. The treatment difference was not significant. |
|
|
7 Quality of life RLS‐QoL: 0 to 60 (= severe impairment) |
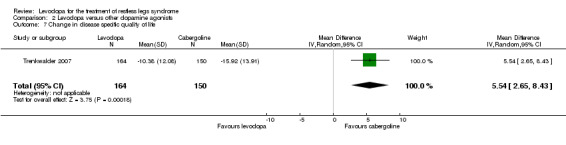
The mean change in quality of life was ‐15.92 in cabergoline. | The mean change in RLS‐QoL in the levodopa group was 5.54 smaller (95% CI 2.65 to 8.43). | 314 (1 study) | +++O moderate | Estimated effect based on only one study. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds Ratio; RR: Risk Ratio; IRLS: International RLS Severity Rating Scale; RLS‐QoL: Restless Legs Syndrome Quality of Life questionnaire | ||||||
GRADE Working Group grades of evidence High quality (++++): Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality (+++O): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality (++OO): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality (+OOO): We are very uncertain about the estimate.
Comparison I: Levodopa versus placebo
1) Change in symptoms on severity scales
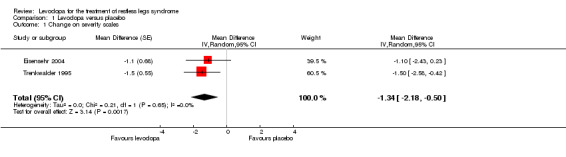
Two studies reported severity ratings of RLS symptoms during the past 24 hours or during the night ranging from 0 to 10 (10 = bad state). These studies were conducted before the IRLS was validated. Treatment doses were 146 mg/36.6 mg (Trenkwalder 1995) and 200 mg/50 mg levodopa/DDC (Eisensehr 2004). The mean treatment difference (MD) of levodopa was ‐1.34 compared to placebo (95% CI ‐2.18 to ‐0.50, P = 0.002; I² = 0%, see Figure 3).
3.

Forest plot of comparison: 1 Levodopa versus placebo, outcome: 1.1 Change on severity scales.
2) Change in periodic limb movements in sleep index (PLMSI)
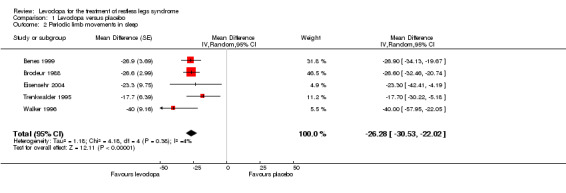
Four trials reported results of PLM per hour of total sleep time, one study which applied an actigraphy method reported data of PLM per time in bed (Benes 1999). Mean doses ranged from 100 mg/25 mg to 200 mg/50 mg levodopa/DDC. Mean difference between levodopa and placebo was ‐26.28/h favouring levodopa (95% CI ‐30.53 to ‐22.02, P < 0.00001; I² = 4%, see Figure 4).
4.
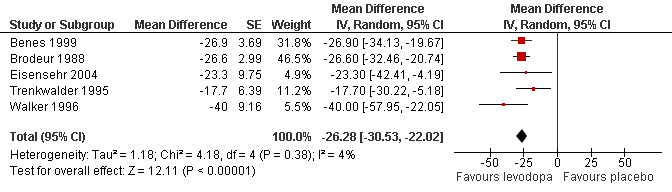
Forest plot of comparison: 1 Levodopa versus placebo, outcome: 1.2 Periodic limb movements in sleep.
3) Change in total sleep time
Patients receiving levodopa did not differ statistically significantly from patients receiving placebo with regard to total sleep time in four trials (see Analysis 1.3).
1.3. Analysis.
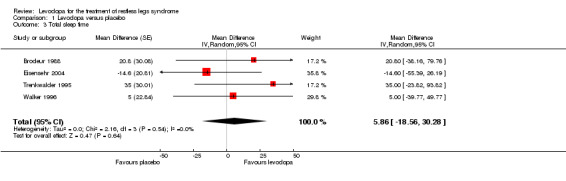
Comparison 1 Levodopa versus placebo, Outcome 3 Total sleep time.
4) Number of dropouts due to adverse events
Patients dropped out of active and placebo treatment only in two of six short term trials. Those two trials with doses of 146 mg/37 mg and 159 mg/40 mg levodopa/DDC showed no difference between levodopa and placebo treatment (see Analysis 1.4).
1.4. Analysis.
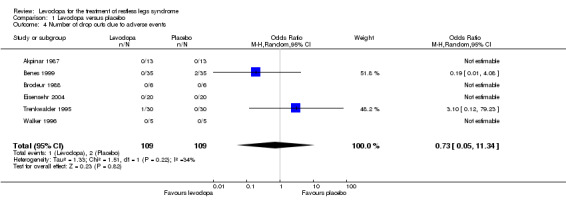
Comparison 1 Levodopa versus placebo, Outcome 4 Number of drop outs due to adverse events.
5) Improvement on CGI‐I
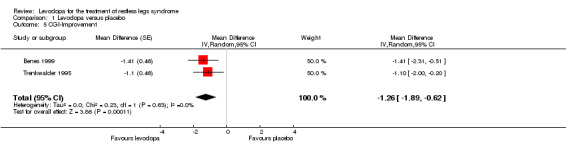
Two trials reported data on CGI improvement. The scale ranges from 1 = very much better to 7 = very much worse. Levodopa doses were 146 mg/37 mg and 159 mg/40 mg levodopa/DDC. Treatment resulted in a ‐1.25 points lower rating compared to placebo treatment (95% CI ‐1.89 to ‐0.62, P = 0.0001, I² = 0%, see Figure 5).
5.

Forest plot of comparison: 1 Levodopa versus placebo, outcome: 1.5 CGI‐Improvement.
6) Change in self rated quality of sleep
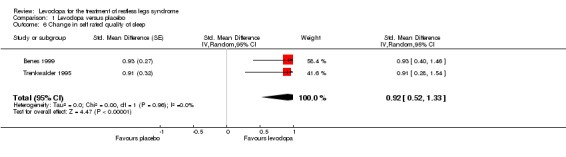
In two trials with mean treatment doses of 146 mg/37 mg and 159 mg/40 mg levodopa/DDC, quality of sleep improved more with levodopa compared to placebo treatment (standardised mean difference (SMD) 0.92, 95% CI 0.52 to 1.33, P < 0.00001, I² = 0%, see Figure 6).
6.
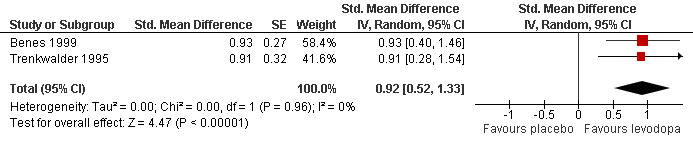
Forest plot of comparison: 1 Levodopa versus placebo, outcome: 1.6 Change in self rated quality of sleep.
7) Change in quality of life
Quality of life improved more with levodopa compared to placebo treatment as shown in two trials with doses of 146 mg/37 mg and 159 mg/40 mg levodopa/DDC (MD 3.23, 95% CI 1.64 to 4.82, P < 0.0001, I² = 0%, see Figure 7).
7.
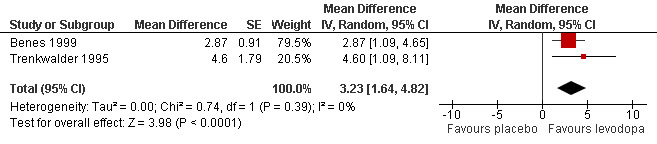
Forest plot of comparison: 1 Levodopa versus placebo, outcome: 1.7 Change in quality of life.
8) Number of patients experiencing adverse events
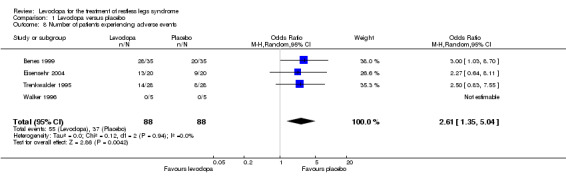
In four of five included trials with doses ranging from 100 mg/25 mg to 200 mg/50 mg levodopa/DDC, patients experienced adverse events. In these trials, a higher number of patients experienced adverse events when treated with levodopa versus those treated with placebo (Odds Ratio (OR) 2.61, 95% CI 1.35 to 5.04, P = 0.004, I² = 0%, see Figure 8).
8.
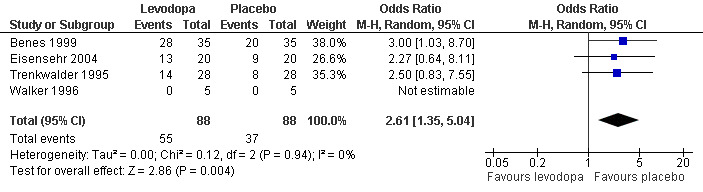
Forest plot of comparison: 1 Levodopa versus placebo, outcome: 1.8 Number of patients experiencing adverse events.
Augmentation was not mentioned in seven trials and not reliably and comparably assessed in the remaining two trials. Therefore we could not perform meta‐analysis on this outcome.
Comparison II: Levodopa versus dopamine agonists
1) Change on severity scale IRLS
Changes on the IRLS were evaluated in two trials comparing cabergoline up to 3.0 mg or pramipexole up to 0.75 mg with levodopa up to 300 mg/75 mg levodopa/DDC. Reductions on the IRLS after treatment were smaller during levodopa treatment compared to dopamine agonist treatment with a mean difference of 5.25 (95% CI 2.1 to 8.4, P = 0.001) and moderate heterogeneity (I² = 55%). Cabergoline showed a quantitatively larger treatment difference from levodopa than pramipexole (see Figure 9).
9.
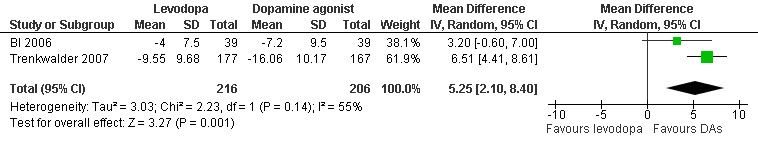
Forest plot of comparison: 2 Levodopa versus other dopamine agonists, outcome: 2.1 Change on IRLS.
2) Change in periodic limb movements per time in bed (PLMI)
One trial investigated PLMI when treated with pramipexole up to 0.75 mg in comparison to levodopa up to 300 mg/75 mg levodopa/DDC. Effects on PLMI did not differ statistically significantly between the substances (MD 3.80/h, 95% CI ‐1.48 to 9.08; P = 0.16).
3) Change in total sleep time
In one study, treatment with levodopa (mean dose of 291 mg levodopa/DDC) tended to show a smaller increase of total sleep time compared to pergolide treatment (up to 0.25 mg; MD ‐76.00 min, 95% CI ‐155.82 to 3.82; P = 0.06).
4) Number of dropouts due to adverse events
Numbers of patients dropping out of treatment due to adverse events were slightly but not significantly lower with levodopa treatment (dose of levodopa up to 300 mg/75 mg levodopa/DDC) compared to patients treated with dopamine agonists (dose of cabergoline up to 3.0 mg or pramipexole up to 0.75 mg; OR 0.59, 95% CI 0.33 to 1.04, P = 0.07; see Figure 10). Whereas the cabergoline trial with the higher weight in the meta‐analysis (weight: 96.9%) favoured levodopa, the pramipexole trial (weight: 3.1%) was in favour of pramipexole. The effect size of one trial investigating pergolide could not be estimated as no patients had dropped out of the study.
10.
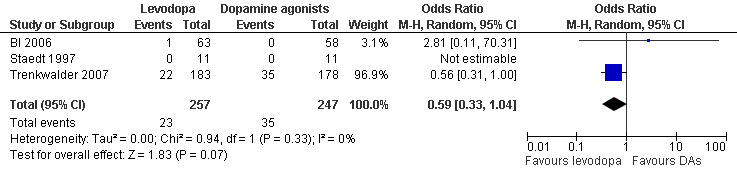
Forest plot of comparison: 2 Levodopa versus other dopamine agonists, outcome: 2.4 Number of dropouts due to adverse events.
5) Improvement on CGI‐I
One trial reported data on CGI improvement ranging from 1 = very much better to 7 = very much worse. Treatment with levodopa up to 300 mg/75 mg levodopa/DDC revealed a smaller improvement compared to cabergoline treatment up to 3.0 mg (MD 0.62; 95% CI 0.37 to 0.87, P < 0.00001).
5) Change in self rated quality of sleep
One trial compared the change in self rated quality of sleep with cabergoline treatment up to 3.0 mg with levodopa treatment up to 300 mg/75 mg levodopa/DDC. Quality of sleep showed a tendency for larger improvement with cabergoline compared to levodopa after treatment but this change was not statistically significant (P = 0.09; see Analysis 2.6).
2.6. Analysis.
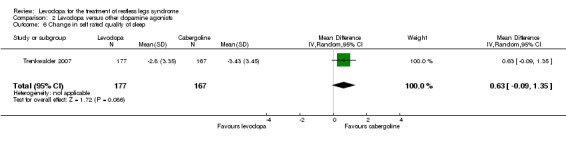
Comparison 2 Levodopa versus other dopamine agonists, Outcome 6 Change in self rated quality of sleep.
6) Change in disease‐specific quality of life
One trial investigated change in disease‐specific quality of life in cabergoline treated patients (up to 3.0 mg) compared to levodopa (up to 300 mg/75 mg levodopa/DDC). A smaller improvement for treatment with levodopa compared to cabergoline was found (MD 5.54, 95% CI 2.65 to 8.43, P = 0.0002).
7) Number of patients experiencing adverse events
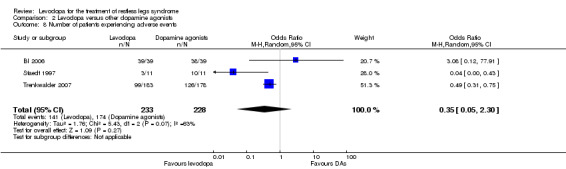
Three trials reported numbers of patients experiencing adverse events. Patients were treated with cabergoline, pergolide or pramipexole and compared to those treated with levodopa. Levodopa treated patients did not differ from patients treated with dopamine agonists regarding the experience of adverse events (OR 0.35, 95% CI 0.05 to 2.30, I² = 63%). The odds ratio was numerically but non‐significantly larger when comparing levodopa treated patients (up to 300 mg/75 mg levodopa/DDC) to those treated with pramipexole (up to 0.75 mg; OR 3.08, 95% CI 0.12 to 77.91), indicating that slightly more levodopa treated patients experienced adverse events than pramipexole treated patients. Cabergoline and pergolide resulted in odds ratios below 1(up to 3.0 mg; OR 0.49, 95% CI 0.31 to 0.75 and up to 0.25 mg; OR 0.04, 95% CI 0.00 to 0.43 respectively) when comparing these to levodopa (300 mg/75 mg and 400 mg/100 mg levodopa/DDC). Hence, fewer levodopa treated patients experienced adverse events in these studies compared to cabergoline and pergolide (see Figure 11).
11.
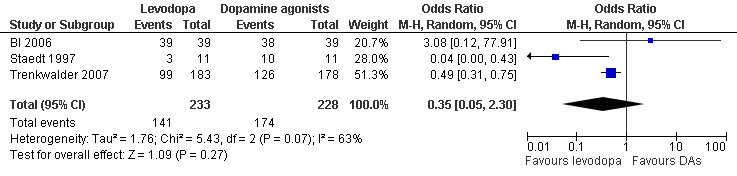
Forest plot of comparison: 2 Levodopa versus other dopamine agonists, outcome: 2.8 Number of patients experiencing adverse events.
Experience of augmentation was not reliably and comparably assessed in the active controlled trials, therefore we could not perform meta‐analysis on this outcome.
Discussion
Summary of main results
Levodopa was superior to placebo treatment regarding improvement of RLS symptoms (presented as severity ratings and CGI‐I), quality of sleep, and quality of life. The severity rating presented a decrease above the minimally important difference (Dworkin 2008). Self rated quality of sleep showed a large effect (Cohen 1988). However, the results are limited owing to the low number of studies included (only two per outcome). The PLMSI showed markedly larger improvement with levodopa treatment whereas treatment effects of total sleep time did not differ from placebo treatment. Patients were not more likely to drop out of levodopa treatment due to adverse events but experienced more adverse events than placebo treated patients (see Table 1).
Trials comparing levodopa to cabergoline, pergolide, and pramipexole showed lower effects with levodopa on the IRLS (cabergoline, pramipexole), the CGI‐I (cabergoline), and quality of life (cabergoline). The mean treatment difference of the IRLS was above the non‐inferiority margin of three points and close to six points which is regarded a difference of clinical relevance. A tendency of levodopa showing lower treatment effects was seen for total sleep time and self rated quality of sleep. There was a further trend of a lower dropout rate due to adverse events with levodopa treatment compared to active comparators. Heterogeneous evidence was present in numbers of levodopa treated patients with adverse events compared to those with other dopaminergic treatments. Also PLMI was not different in pramipexole versus levodopa treated patients. In these comparisons, it has also to be taken into account that all meta‐analyses but those investigating safety parameters were based on one to two studies (see Table 2).
Overall completeness and applicability of evidence
Patients were recruited from outpatient settings with moderate to severe RLS and represent the usual patient population requiring treatment. In five studies, diagnosis was made according to valid diagnostic criteria. The remaining studies were conducted before diagnostic criteria were published, but their description of RLS symptoms closely resembled the IRLS criteria.
Treatment durations varied between studies from one to eight weeks. Only one actively controlled trial also investigated treatment effects after 30 weeks. These are short time periods for the investigation of treatment effects; particularly since RLS is a chronic disease and medication only suppresses symptoms but does not cure the disorder. The lack of long‐term studies is especially relevant for augmentation, which is the most important treatment complication in dopaminergic treatment but was only reported in one placebo controlled and one active controlled study. Augmentation usually develops during long‐term treatment (Allen 2003; Trenkwalder 2008). Therefore, controlled long‐term studies of at least 12 months duration are needed.
In summary, all relevant aspects of symptom severity and well being related improvements were assessed in at least some placebo and active controlled studies. Limiting factors for the validity of the results are the restricted number of studies, low numbers of included patients and short duration of trials.
Quality of the evidence
We searched all relevant databases and public trial registers provided by the pharmaceutical companies and by government. Doing this, we could obtain published as well as unpublished trials. Pharmaceutical companies contributed additional data. Therefore, it seems unlikely that there are additional unpublished trials.
We retrieved six placebo controlled trials and three active controlled studies. Except for one large scale trial, all studies comprised very few patients. Two studies were conducted before the introduction of official diagnosis criteria in 1995. The cross‐over design was implemented in eight trials but could be statistically considered in only four trials (Benes 1999; Brodeur 1988; Trenkwalder 1995; Walker 1996). Duration of medication washout periods between the first and the second treatment were not reported or used in four of the cross‐over trials. However, as treatment periods lasted for at least seven days, carryover effects for assessed post treatment effects were not likely. Two to six placebo controlled trials and one to three active controlled trials contributed data to meta‐analyses. Those meta‐analyses with only few studies have to be interpreted with caution.
Studies were reported with an often incomplete description of study procedures. In five studies, we could not obtain further information and thus had to rate their likelihood for bias conservatively as ”unclear” even if we are aware that an insufficient study report does not necessarily represent inadequate performance of the study.
Marked heterogeneity was not observed in meta‐analyses of placebo controlled studies. Heterogeneous results in active controlled trials were observed on the IRLS with more pronounced effects for cabergoline than pramipexole and regarding patients with adverse events with more patients experiencing adverse events in cabergoline and pergolide than in levodopa and slightly less experience of adverse events in pramipexole than in levodopa. These differences in treatment effects can be traced back to the different medications. A risk of the effective dopamine agonists cabergoline and pergolide is the known side effect valvular fibrosis, which requires medical monitoring and led in recent years to a preference of non‐ergot dopamine agonists. Furthermore, impulse control disorders were observed recently during treatment with dopamine agonists (Cornelius 2010).
Results of the majority of meta‐analyses in the placebo controlled trials showed precise small to large effects. In the placebo controlled trials, only the results on total sleep time and patients dropping out of treatment did not differ between levodopa and placebo and showed wide confidence intervals. In the active controlled trials five of eight evaluated endpoints revealed wide confidence intervals comprising a range from zero to a considerable effect.
Potential biases in the review process
We made an effort to prevent bias in the search for relevant trials and data as well as meta‐analysis of included data. To identify all relevant studies, we searched all relevant databases without language restrictions. Data management was performed by two review authors. We asked pharmaceutical companies and study authors for additional information relevant to bias.
This work is independent of sponsoring of pharmaceutical companies, as it was supported by the German Ministry for Education and Research (Bundesministerium für Bildung und Forschung ‐ BMBF, Project number DLR 01KG0723).
Agreements and disagreements with other studies or reviews
One meta‐analysis investigated levodopa treatment in RLS (Conti 2007). Results showed comparable effects on PLMSI and total sleep time to our meta‐analysis. Other outcome parameters such as relief of symptoms, sleep quality, and quality of life were not analysed due to heterogeneity in outcome measurements between trials. We could analyse self rated data and by this, complete previous results.
Authors' conclusions
Implications for practice.
Levodopa in doses from 100 mg to 200 mg proves to be an effective treatment option compared to placebo for short‐term treatment (up to four weeks) of RLS. Evidence from the active controlled trials was in favour of dopamine agonists regarding three essential outcomes. The results of the other five endpoints do not favour any one treatment over another. However, due to imprecise results in these few trials, superiority of one agent cannot be ruled out. Tolerability proves to be satisfactory for patients treated with levodopa compared to cabergoline (Trenkwalder 2007; N = 361) and pergolide (Staedt 1997; N = 11).
Data concerning long‐term treatment efficacy is limited and the occurrence of augmentation, the most serious adverse event during dopaminergic treatment, is barely investigated.
Implications for research.
Efficacy for short‐term medication was proved in moderate to severely affected patients. However, for patients needing long‐term medication, appropriate long‐term studies including assessment of augmentation are necessary in order to be able to recommend levodopa for these patients. As no mechanism of action is known for levodopa in RLS, further research may concentrate on this aim to better understand dosages and long‐term risks.
Besides comparison to placebo, levodopa needs to be tested against other dopaminergic and non‐dopaminergic treatment options. Agents such as anticonvulsants and opioids present potent comparators. In all those studies, the occurrence and severity of augmentation should be evaluated.
Acknowledgements
This work was supported by a grant from the German Federal Ministry for Education and Research (Bundesministerium für Bildung und Forschung ‐ BMBF, grant number DLR 01KG0723) to MH.
We also thank Carolin Laux for supporting the project during data collection and data management.
Appendices
Appendix 1. MEDLINE (OVID) search strategy
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/ 1‐8
humans.sh.
9 and 10
AF L‐dopa
AF "L dopa"
AF Ldopa
AF levodopa
or/ 12‐15
AF "rls"
AF "restless leg*"
17 or 18
11 and 16 and 19
Appendix 2. CENTRAL (ODVID) search strategy
AF L‐dopa
AF ”L dopa”
AF ”Ldopa”
AF levodopa
or/1‐4
AF “rls”
AF “restless leg*“
6 or 7
5 and 8
Appendix 3. PsycINFO (EBSCO) search strategy
TX L‐dopa
TX Ldopa
TX “L dopa”
TX levodopa
or/ 1‐4
TX “rls”
TX “restless leg*“
6 or 7
5 and 8
Appendix 4. CINAHL (EBSCO) search strategy
TX L‐dopa
TX “L dopa”
TX Ldopa
TX levodopa
or/1‐4
TX “rls”
TX “restless leg*”
6 or 7
5 and 8
Appendix 5. EMBASE search strategy
(random* or factorial* or crossover* or "cross over*" or placebo* or (doubl* adj blind*) or (singl* adj blind*) or assign* or allocat* or volunteer*).mp
Randomized Controlled Trial/
(l‐dopa or levodopa* or ldopa or "l dopa").mp
Levodopa/
(rls or "restless leg*").mp
Restless Legs Syndrome/
(1 or 2) and (3 or 4) and (5 or 6)
Data and analyses
Comparison 1. Levodopa versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change on severity scales | 2 | Mean Difference (Random, 95% CI) | ‐1.34 [‐2.18, ‐0.50] | |
| 2 Periodic limb movements in sleep | 5 | Mean Difference (Random, 95% CI) | ‐26.28 [‐30.53, ‐22.02] | |
| 3 Total sleep time | 4 | Mean Difference (Random, 95% CI) | 5.86 [‐18.56, 30.28] | |
| 4 Number of drop outs due to adverse events | 6 | 218 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 11.34] |
| 5 CGI‐Improvement | 2 | Mean Difference (Random, 95% CI) | ‐1.26 [‐1.89, ‐0.62] | |
| 6 Change in self rated quality of sleep | 2 | Std. Mean Difference (Random, 95% CI) | 0.92 [0.52, 1.33] | |
| 7 Change in quality of life | 2 | Mean Difference (Random, 95% CI) | 3.23 [1.64, 4.82] | |
| 8 Number of patients experiencing adverse events | 4 | 176 | Odds Ratio (M‐H, Random, 95% CI) | 2.61 [1.35, 5.04] |
1.1. Analysis.
Comparison 1 Levodopa versus placebo, Outcome 1 Change on severity scales.
1.2. Analysis.
Comparison 1 Levodopa versus placebo, Outcome 2 Periodic limb movements in sleep.
1.5. Analysis.
Comparison 1 Levodopa versus placebo, Outcome 5 CGI‐Improvement.
1.6. Analysis.
Comparison 1 Levodopa versus placebo, Outcome 6 Change in self rated quality of sleep.
1.7. Analysis.
Comparison 1 Levodopa versus placebo, Outcome 7 Change in quality of life.
1.8. Analysis.
Comparison 1 Levodopa versus placebo, Outcome 8 Number of patients experiencing adverse events.
Comparison 2. Levodopa versus other dopamine agonists.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change on IRLS | 2 | 422 | Mean Difference (IV, Random, 95% CI) | 5.25 [2.10, 8.40] |
| 2 Periodic limb movements per time in bed | 1 | 78 | Mean Difference (IV, Random, 95% CI) | 3.80 [‐1.48, 9.08] |
| 3 Total sleep time | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐76.0 [‐155.82, 3.82] |
| 4 Number of dropouts due to adverse events | 3 | 504 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.04] |
| 5 CGI‐Improvement | 1 | 344 | Mean Difference (IV, Random, 95% CI) | 0.62 [0.37, 0.87] |
| 6 Change in self rated quality of sleep | 1 | 344 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.09, 1.35] |
| 7 Change in disease specific quality of life | 1 | 314 | Mean Difference (IV, Random, 95% CI) | 5.54 [2.65, 8.43] |
| 8 Number of patients experiencing adverse events | 3 | 461 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.05, 2.30] |
2.1. Analysis.
Comparison 2 Levodopa versus other dopamine agonists, Outcome 1 Change on IRLS.
2.2. Analysis.
Comparison 2 Levodopa versus other dopamine agonists, Outcome 2 Periodic limb movements per time in bed.
2.3. Analysis.
Comparison 2 Levodopa versus other dopamine agonists, Outcome 3 Total sleep time.
2.4. Analysis.
Comparison 2 Levodopa versus other dopamine agonists, Outcome 4 Number of dropouts due to adverse events.
2.5. Analysis.
Comparison 2 Levodopa versus other dopamine agonists, Outcome 5 CGI‐Improvement.
2.7. Analysis.
Comparison 2 Levodopa versus other dopamine agonists, Outcome 7 Change in disease specific quality of life.
2.8. Analysis.
Comparison 2 Levodopa versus other dopamine agonists, Outcome 8 Number of patients experiencing adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akpinar 1987.
| Methods | Randomised controlled crossover trial of levodopa versus placebo Dropouts/withdrawals: Total N of 16, alternative medication for 3 patients |
|
| Participants | Included/analysed:16/13 Demographics: 6 male, age 49.6 years Diagnosis: patients with moderate to severe RLS Setting: 1 centre in Turkey Baseline: 6 patients rated as having severe, 10 patients rated as having moderate symptoms |
|
| Interventions | Intervention: fixed single dose of 200 mg/50 mg levodopa/benserazide for 14 days Control: fixed single dose of placebo capsules for 14 days No washout between treatment periods |
|
| Outcomes | Safety: Number of dropouts due to adverse events | |
| funding source | No funding stated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were treated randomly. |
| Allocation concealment? | Unclear risk | Double‐blind crossover basis. |
| Blinding? All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data addressed? All outcomes | Low risk | No incomplete outcome data. |
| Free of selective reporting? | Low risk | All results reported as prespecified. |
| Free of other bias? | Low risk | Low indication of bias. |
Benes 1999.
| Methods | Randomised controlled crossover trial of levodopa versus placebo Dropouts/withdrawals: PP of 32 with premature discontinuations of 3 patients in the treatment group. |
|
| Participants | Included/analysed: 35/32 Demographics: 13 male, age 56 years Diagnosis: RLS according to IRLSSG, PLMS/h > 5, symptoms for at least 2 weeks, sleep onset latency > 30 min and/or sleep efficiency ≤ 85% Setting: 3 centres in Germany Baseline: PLMI (using actigraphy) 49.5 ± 29.2 |
|
| Interventions | Intervention: flexible uptitration of single dose levodopa/benserazide from 100 mg/25 mg to 200 mg/50 mg in 3 weeks, maintenance for 1 week Control: flexible uptitration of single dose placebo capsules in 3 weeks, maintenance for 1 week No washout between treatment periods |
|
| Outcomes | Change of symptoms: CGI‐Improvement Objective quality of sleep: PLM‐Index (using actigraphy) Self rated quality of sleep: SF‐A Quality of life: VAS on life satisfaction Safety: Number of drop outs due to adverse events, number of patients experiencing adverse events |
|
| funding source | The study was supported by a grant from Hoffmann La‐Roche, Inc. | |
| Notes | PLMI: index of number of periodic limb movements per hour time in bed; CGI‐I: Clinical Gobla Impressions ‐ Improvement; SF‐A: Schlaffragebogen‐A; VAS: Visual Analogue Scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation list was produced by the statistical department of the sponsor. |
| Allocation concealment? | Low risk | Patients had to be allocated to a numbered medication in ascending order. Due to the blinding of the medication, investigators and patients were not informed regarding treatment sequence or medication. |
| Blinding? All outcomes | Unclear risk | White capsules of identical size and shape, the capsules were not differentiable. The capsules which had to be taken in one cross‐over period were filled in a glass and labelled with "week 3‐6" and "week 7‐10", respectively. Blinding of polysomnography raters was not mentioned. |
| Incomplete outcome data addressed? All outcomes | Low risk | No incomplete outcome data. |
| Free of selective reporting? | Low risk | All results reported as prespecified with additional sleep data. |
| Free of other bias? | Low risk | Low indication of bias. |
BI 2006.
| Methods | Randomised controlled crossover trial of levodopa dual release versus pramipexole Dropouts/withdrawals: PP of 39 with 28 premature discontinuations |
|
| Participants | Included/analysed: 67/39 Demographics: 41% male, age 56.9 years Diagnosis: RLS according to IRLSSG, symptom presence almost every day, PLMI > 5 Setting: 6 Swiss centres including Basel, Bern, Lugano, Luzern, Zürich, Zurzach Baseline: IRLS score of 21.1 (levodopa) and 20.8 (ppx), PLMI (actigraphy) of 21.1 (levodopa) and 21.5 (ppx) |
|
| Interventions | Intervention 1: flexible uptitration of single dose pramipexole from 0.25 to 0.75 mg in 2 weeks, maintenance for 2 weeks Intervention 2: flexible uptitration of single dose levodopa‐dual‐release from 100/25 mg to 300/75 mg for 2 weeks, maintenance for 2 weeks 2 weeks washout between treatment periods |
|
| Outcomes | Change of symptoms: IRLS, CGI responders Objective quality of sleep: PLMI (using actigraphy) Safety: number of dropouts due to adverse events, number of patients with adverse events |
|
| funding source | The study was supported by Boehringer Ingelheim GmbH. | |
| Notes | IRLS: International RLS Severity Rating Scale; ppx: pramipexole | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation schedule was provided by BI Pharma GmbH & Co KG. |
| Allocation concealment? | Low risk | Medication package with the lowest available number was allocated to the patient. |
| Blinding? All outcomes | Unclear risk | Tablets were packaged in identical hard gelatine capsules, the code was restricted to authorised personnel, such as staff involved in packaging of study medication. Blinding of data analysts not reported. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported by BI on request. |
| Free of selective reporting? | Low risk | Results reported as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
Brodeur 1988.
| Methods | Randomised controlled crossover trial of levodopa versus placebo Dropouts/withdrawals: Total N of 6, no premature discontinuations |
|
| Participants | Included/analysed: 6/6 Demographics: 3 male, age 51.3 years Diagnosis: primary RLS according to clinical evaluation, nocturnal awakenings and daytime sleepiness Setting: 1 centre in Canada Baseline: PLMSI (using PSG) 38.2 ± 12.7 |
|
| Interventions | Intervention: fixed dose of 100 mg/25 mg levodopa/benserazide twice daily (1hour before, 3 hours after going to bed) for 14 days Control: fixed dose of placebo capsules twice daily (1hour before, 3 hours after going to bed) for 14 days Washout for 1 week between treatment periods |
|
| Outcomes | Objective quality of sleep: change in sleep parameters (TST), PLMSI (using PSG) Safety: Number of dropouts due to adverse events |
|
| funding source | The study was supported by the Medical Research Council of Canada, the Fonds de la recherche en santé du Québec and the Fond pour la formation de chercheurs et l'aide à la recherche du Québec. | |
| Notes | TST: total sleep time; PSG: polysomnography | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Insufficient information. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding? All outcomes | Unclear risk | No information concerning blinding of staff, examiners, polysomnography analysis. |
| Incomplete outcome data addressed? All outcomes | Low risk | All patients were included in analyses. |
| Free of selective reporting? | Unclear risk | No outcomes prespecified. |
| Free of other bias? | Low risk | Low indication of bias. |
Eisensehr 2004.
| Methods | Randomised controlled crossover trial of slow‐release levodopa versus slow‐release valproic acid versus placebo Dropouts/withdrawals: Total N of 20, no premature discontinuations |
|
| Participants | Included/analysed: 20/20 Demographics: 8 male, age 58.9 years Diagnosis: primary RLS according to IRLSSG, PLM/h > 10, symptoms daily for at least 6 months Setting: 1 centre in Germany Baseline: PLMSI (using PSG) 42.3 ± 40.7 |
|
| Interventions | Intervention 1: fixed single dose of 200 mg/50 mg slow‐release‐levodopa/benserazide (after 2 days of 100 mg/25 mg) for 3 weeks Intervention 2: fixed single dose of slow‐release‐valproic acid (600 mg after 2 days of 300 mg) for 3 weeks Control: fixed single dose of placebo capsules for 3 weeks No washout between treatment periods |
|
| Outcomes | Change of symptoms: Rating of severity of symptoms in the past 24 hours Objective quality of sleep: TST, PLMSI (using PSG) Safety: Number of dropouts due to adverse events, number of patients experiencing adverse events |
|
| funding source | The study was supported by Sanofi pharmaceutical company. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Insufficient information. |
| Allocation concealment? | Low risk | Capsules were distributed when requested by blinded executor mentioning patient number and treatment session. |
| Blinding? All outcomes | Low risk | Similar looking and tasting capsules, physicians did not know about sequences until end of study when analysis was completed. |
| Incomplete outcome data addressed? All outcomes | Low risk | No incomplete outcome data. |
| Free of selective reporting? | Low risk | All results reported as prespecified with additional sleep data. |
| Free of other bias? | Low risk | Low indication of bias. |
Staedt 1997.
| Methods | Randomised active controlled crossover trial of levodopa versus pergolide Dropouts/withdrawals: Total N of 11, no premature discontinuations |
|
| Participants | Included/analysed: 11 Demographics: 6 male, age 57.6 years Diagnosis: patients with a history of restlessness and paraesthesias at night and/or day Setting: 1 centre in Germany Baseline: PLMS‐disturbed time of sleep 164 ± 80.4 min |
|
| Interventions | Intervention 1: flexible uptitration of single dose levodopa/benserazide from 200 mg/50 mg to 400 mg/100 mg in 16 days Intervention 2: flexible uptitration of single dose pergolide from 0.125 mg to 0.25 mg in 16 days Washout of 24 hours between treatment periods |
|
| Outcomes | Objective quality of sleep: Change in sleep parameters (TST) Safety: Number of dropouts due to adverse events, number of patients experiencing adverse events |
|
| funding source | No funding stated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Insufficient information. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding? All outcomes | Unclear risk | Insufficient information, not mentioned if research staff were blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | No incomplete outcome data. |
| Free of selective reporting? | Low risk | All results reported as prespecified, additional sleep data reported. |
| Free of other bias? | Low risk | Low indication of bias. |
Trenkwalder 1995.
| Methods | Randomised controlled crossover trial of levodopa versus placebo Dropouts/withdrawals: PP of 28, 4 premature discontinuations (2 before drug intake) |
|
| Participants | Included/analysed: 32/28 Demographics: 18 male, age 52.0 years Diagnosis: primary and secondary RLS according to descriptive criteria, PLMS‐AI > 5, sleep onset latency > 25 and/or sleep efficiency < 85% Setting: 1 centre in Germany Baseline: severity on RLS rating scale 7.9 (0 to 10 points = severe) |
|
| Interventions | Intervention: flexible uptitration of single dose levodopa/benserazide from 100 mg/25 mg to 200 mg/50 mg in 2 weeks, maintenance for 2 weeks Control: single dose placebo capsules for 4 weeks No washout between treatment periods |
|
| Outcomes | Change of symptoms: CGI‐I, VAS on severity of RLS symptoms during the night Objective quality of sleep: TST, PLMSI (using PSG) Self rated quality of sleep: VAS on quality of sleep Quality of Life: VAS on life satisfaction Safety: Number of dropouts due to adverse events, number of patients experiencing adverse events |
|
| funding source | No funding stated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was done by the sponsor with a computer based system. |
| Allocation concealment? | Low risk | Patient randomisation numbers were allocated sequentially in the order of recruitment of patients. The allocated number was then the patient code. |
| Blinding? All outcomes | Unclear risk | Unblinding was done after the complete database had been transferred to the sponsor. |
| Incomplete outcome data addressed? All outcomes | Low risk | No incomplete outcome data. |
| Free of selective reporting? | Low risk | All results reported as prespecified with additional questionnaire data. |
| Free of other bias? | Low risk | Low indication of bias. |
Trenkwalder 2007.
| Methods | Randomised active controlled parallel‐group trial of levodopa versus cabergoline Dropouts/withdrawals: ITT (361) and PP (204) with premature discontinuations of 83 (levodopa) and 74 (cabergoline) |
|
| Participants | Included/analysed: long term: 361 patients (178 cabergoline, 183 levodopa), short term: 204 (104 cabergoline, 100 levodopa) Demographics: 46 (levodopa)/58 (cabergoline) male, age 56.9 (levodopa)/58.7 (cabergoline) Diagnosis: RLS according to IRLSSG, symptom severity on IRLS ≥ 10 and RLS‐6 "severity at night" ≥ 4. Setting: 51 centres in 4 European countries Baseline: IRLS score of 25.6 (levodopa) and 25.8 (cabergoline) |
|
| Interventions | Intervention 1: fixed uptitration of single dose cabergoline to 2.0 mg which could be increased to 3.0 mg (in 2 weeks), maintenance up to 6/ 8 weeks and to 24 weeks Intervention 2: fixed uptitration of single dose levodopa/benserazide 200 mg/50 mg (in 8 days) which could be increased to to 300 mg/75 mg, maintenance up to 6/ 8 weeks and to 24 weeks |
|
| Outcomes | Change of symptoms: IRLS, CGI‐I Self rated quality of sleep: RLS‐6 satisfaction with sleep Daytime tiredness: RLS‐6 daytime tiredness Quality of life: RLS‐QoL Safety: number of dropouts due to adverse events, number of patients with adverse events |
|
| funding source | The study was supported by Pfizer GmbH. | |
| Notes | RLS‐QoL: Restless Legs Syndrome Quality of Life questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Sequentially assigned to one of the two treatments by the investigators using medication numbers in ascending order for each block of 4 which was allocated to the study site after central randomisation using the program SAS Proc. Plan Version v8.2. |
| Allocation concealment? | Low risk | Medication numbers in ascending order. See above. |
| Blinding? All outcomes | Low risk | Identical looking tablets. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons described. |
| Free of selective reporting? | Low risk | All results reported as prespecified with further report of short term data for secondary outcomes following request. |
| Free of other bias? | Low risk | Low indication of other bias. |
Walker 1996.
| Methods | Randomised controlled crossover trial of levodopa versus placebo Dropouts/withdrawals: PP (5) with premature discontinuations of 3 patients prior to start of trial |
|
| Participants | Included/analysed: 8/5 Demographics: 1 male, age 68.2 years Diagnosis: RLS according to Diagnostic Classification Steering Committee 1990 Setting: 1 centre in Canada No baseline values given |
|
| Interventions | Intervention: fixed single dose of 100 mg/25 mg levodopa/carbidopa for 1 week Control: fixed single dose of placebo capsules for 1 week Washout for 1 week between treatment periods |
|
| Outcomes | Objective quality of sleep: Change in sleep parameters (TST), PLMSI (using PSG) Safety: number of dropouts due to adverse events, number of patients with adverse events |
|
| funding source | The study was supported by the Kidney Foundation of Canada, Manitoba Branch, The Medical Research Council of Canada and Baxter Healthcare corporation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Insufficient information. |
| Allocation concealment? | Unclear risk | Insufficient information. |
| Blinding? All outcomes | Low risk | Blinded scorers for PSG rating. |
| Incomplete outcome data addressed? All outcomes | Low risk | No incomplete outcome data. |
| Free of selective reporting? | Low risk | All results reported as prespecified with results of additional sleep parameters partly reported. |
| Free of other bias? | Low risk | Low indication of bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Collado‐Seidel 1997 | Results of the congress abstract were published in Collado‐Seidel (1999). |
| Collado‐Seidel 1999 | Double‐blind comparison of sr‐ldopa additionally to rr‐ldopa versus placebo additional to rr‐ldopa. |
| Kaplan 1993 | Diagnosis of PLMS/h > 5 in all patients, RLS‐symptoms were present only in 3 patients. |
| Polo 2007 | Medication period was only two nights. |
| Polo 2008 | Erratum of excluded study Polo 2007. |
| Reuter 1999 | Results of two patients with RLS only analysed descriptively. |
| Saletu 2003 | Medication period was only one night. |
| Staedt 1998 | Open label follow‐up of Staedt 1997. |
| Stiasny 2001 | Preliminary data which were published later in Trenkwalder 2007. |
| Trenkwalder 2005 | Results of the congress abstract were published in Trenkwalder 2007. |
| von Scheele 1986 | Treatment of levodopa and placebo on alternate days. |
| von Scheele 1990 | Two year open label follow‐up. |
| Wetter 1995 | German duplicate of study of Trenkwalder 1995. |
| Wetter 1995a | Results of the congress abstract were published in Trenkwalder 1995. |
| Wu Yh 2008 | Observational study on levodopa versus acupuncture. |
Differences between protocol and review
Two outcomes defined in the protocol were specified in detail, two outcomes were added, and two outcome parameters were dropped after discussion in the light of actual research before the search for publications.
PLMS Index and number of patients dropping out due to adverse events were defined as primary and not as secondary outcome parameters. Total sleep time assessed in polysomnography was appended as a primary outcome.
We added clinician rated general improvement (CGI‐I) and number of patients experiencing augmentation in the secondary outcome section. We excluded the planned outcomes “patient satisfaction with treatment” and “daytime functioning” as investigation of these endpoints in levodopa trials was very unlikely.
We investigated not only the first phase of cross‐over trials, but included both phases of cross‐over trials and corrected statistically for the different design when possible (see Reviewer's Handbook, chapter 16.4).
We set search dates from 1985 (instead of 1990) to 2008 in order to obtain all possibly relevant trials. In order to be able to include all possibly relevant studies, trials did not have to implement explicit diagnostic criteria according to the IRLSSG (Walters 1995) but it was permitted to describe similar inclusion criteria of patients.
Search strategies were modified with respect to newly recommended strategies by the Reviewer's Handbook, chapter 6.
We conducted additional searches in online trial registers provided by pharmaceutical companies, the Pharmaceutical Research and Manufacturers of America (PhRMA) and the U.S. National Institutes for Health.
Instead of implementing fixed‐effect models where data were homogeneous, we performed all meta‐analyses using random‐effects models as recommended by the Reviewer's Handbook. As only data for per protocol populations were reported, we performed analyses based on these data.
We could not perform any subgroup analyses on different types of agents, comparator treatment, duration of treatment or dosage of treatment as these parameters did not vary between the included studies. Also study type could not be investigated separately as all studies but one active controlled trial were cross‐over trials. As only few studies contributed data to the primary outcomes, we did not perform subgroup or sensitivity analysis on studies with differing study quality.
Contributions of authors
MH: Performing previous work that was the foundation of the current review, conceiving the review, designing the review, co‐ordinating the review, providing general advice on the review, securing funding for the review, screening of obtained publications regarding eligibility, appraising quality of papers, data collection for the review, writing to study authors for additional information, interpretation of data, writing the review, providing a clinical perspective, providing a policy perspective, providing a consumer perspective.
HS: Undertaking searches, screening search results, organizing retrieval of papers, screening retrieved publications regarding eligibility, appraising quality of papers, extracting data from papers, writing to study authors and pharmaceutical companies for additional information, obtaining and screening data on unpublished studies, data management for the review, entering data into RevMan, analysis of data, interpretation of data, writing the review, providing a methodological perspective.
CT: Performing previous work that was the foundation of the current review, co‐ordinating the review, securing funding for the review, providing general advice on the review, interpretation of data, writing the review, providing a clinical perspective, providing a policy perspective, providing a consumer perspective.
RK: Performing previous work that was the foundation of the current review, conceiving the review, designing the review, co‐ordinating the review, securing funding for the review, providing general advice on the review, providing additional data on included trials, interpretation of data, providing a methodological perspective.
LK: Conceiving the review, designing the review, providing general advice on the review, writing the protocol, designing search strategies, analysis of data, interpretation of data.
DR: Co‐ordinating the review, securing funding for the review, providing general advice on the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
BMBF, Germany.
Grant from the German Federal Ministry for Education and Research (Bundesministerium für Bildung und Forschung ‐ BMBF, grant number DLR 01KG0723)
Declarations of interest
Hanna Scholz has no conflicts of interest to declare.
Claudia Trenkwalder has been an advisor for Boehringer Ingelheim, Cephalon, Mundipharm, Orion Pharma, Novartis, Solvay, Axxonis and UCB. She has received lecture honoraria from Boehringer Ingelheim, UCB, TEVA and Astra Zeneca.
Ralf Kohnen received honoraria for advisory board membership from Pfizer, USA; Axxonis, Germany; Roche, Germany; UCB, Germany; Jazzpharma, USA.
Dieter Riemann received research support from DFG, BMBF, EU (public funding) and from Takeda, Sanofi‐Aventis, Organon, Actelion and Omron. He was on the speakers bureau of Sanofi‐Aventis, Takeda, Servier, Lundbeck, Boehringer‐Ingelheim, GSK, Cephalon and Merz Pharmaceuticals. He was also a member of advisory boards for Sanofi‐Aventis, Lundbeck, GSK, Takeda and Actelion. Dr. Riemann declares that the above mentioned activities have no influence on the content of this article.
Levente Kriston has no conflicts of interest to declare.
Magdolna Hornyak received honoraria and/or lecture fees from Boehringer‐Ingelheim, Germany; GlaxoSmithKline, Germany; Roche, Germany; Pfizer Inc., Germany.
Edited (no change to conclusions)
References
References to studies included in this review
Akpinar 1987 {published data only}
- Akpinar S. Restless legs syndrome treatment with dopaminergic drugs. Clinical Neuropharmacology 1987;10(1):69‐79. [DOI] [PubMed] [Google Scholar]
Benes 1999 {published data only}
- Benes H, Kurella B, Kummer J, Kazenwadel J, Selzer R, Kohnen R. Rapid onset of action of levodopa in restless legs syndrome: a double‐blind, randomized, multicenter, crossover trial. Sleep 1999;22(8):1073‐81. [DOI] [PubMed] [Google Scholar]
BI 2006 {published and unpublished data}
- 248.518. A double‐blind, randomised, crossover trial investigating the efficacy and safety of the dopamine agonist pramipexole (Sifrol®, 0.25‐0.75 mg per day) versus levodopa / benserazide (Madopar® DR, 125‐375 mg per day) in patients with restless legs syndrome. http://trials.boehringer‐ingelheim.com/res/trial/data/pdf/248.518_U08‐2249_upload.pdf (accessed 16 March 2009).
- Mathis J, Bassetti C. Comparison of pramipexole (PPX) versus levodopa/benserazide (L/B) in the treatment of restless legs syndrome (RLS): A double blind, randomized, Swiss multi‐centre crossover trial. Movement Disorders 2006;21 Suppl 15:S442. [Google Scholar]
Brodeur 1988 {published data only}
- Brodeur C, Montplaisir J, Godbout R, Marinier R. Treatment of restless legs syndrome and periodic movements during sleep with l‐dopa: a double‐blind, controlled study. Neurology 1988;38:1845‐8. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Boucher S, Gosselin A, Poirier G, Lavigne G. Persistence of repetitive EEG arousals (k‐alpha complexes) in RLS patients treated with l‐dopa. Sleep 1996;19(3):196‐9. [DOI] [PubMed] [Google Scholar]
Eisensehr 2004 {published data only}
- Eisensehr I, Ehrenberg B, Rogge Solti S, Noachtar S. Treatment of idiopathic restless legs syndrome (RLS) with slow‐release valproic acid compared with slow‐release levodopa/benserazid. Journal of Neurology 2004;251:579‐83. [DOI] [PubMed] [Google Scholar]
Staedt 1997 {published data only}
- Staedt J, Waßmuth F, Ziemann U, Hajak G, Rüther E, Stoppe G. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double‐blind randomized crossover trial of pergolide versus l‐dopa. Journal of Neural Transmission 1997;104:461‐8. [DOI] [PubMed] [Google Scholar]
Trenkwalder 1995 {published data only}
- Trenkwalder C, Stiasny K, Pollmächster T, Wetter T, Schwarz J, Kohnen R, et al. L‐dopa therapy of uremic and idiopathic restless legs syndrome: A double‐blind, crossover trial. Sleep 1995;18(8):681‐8. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2007 {published data only}
- Trenkwalder C, Benes H, Grote L, Happe S, Högl B, Mathis J, et al. Cabergoline compared to levodopa in the treatment of patients with severe restless legs syndrome: results from a multicenter, randomized, active controlled trial. Movement Disorders 2007;22(5):696‐703. [DOI] [PubMed] [Google Scholar]
Walker 1996 {published data only}
- Walker S, Fine A, Kryger M. L‐DOPA/carbidopa for nocturnal movement disorders in uremia. Sleep 1996;19(3):214‐8. [PubMed] [Google Scholar]
References to studies excluded from this review
Collado‐Seidel 1997 {published data only}
- Collado‐Seidel V, Wetter T, Kohnen R, Kazenwald J, Selzer R, Oertel W, et al. Treatment of the restless legs syndrome with a combination of standard and sustained release levodopa/benserazide (Madopar Depot): A double‐blind controlled study. Pharmacopsychiatry 1997;30:158. [Google Scholar]
Collado‐Seidel 1999 {published data only}
- Collado‐Seidel V, Kazenwadel J, Wetter T, Kohnen R, Winkelmann J, Selzer R, et al. A controlled study of additional sr‐l‐dopa in l‐dopa‐responsive restless legs syndrome with late night symptoms. Neurology 1999;52(2):285‐90. [DOI] [PubMed] [Google Scholar]
Kaplan 1993 {published data only}
- Kaplan P, Allen R, Buchholz D, Walters J. A double‐blind, placebo‐controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep 1993 16;8:717‐23. [DOI] [PubMed] [Google Scholar]
Polo 2007 {published data only}
- Polo O, Ylä‐Sahra R, Hirvonen K, Karvinen J, Vahteristo M, Ellmén J. Entacapone prolongs the reduction of PLM by levodopa/carbidopa in restless legs syndrome. Clinical Neuropharmacology 2007;30(6):335‐44. [DOI] [PubMed] [Google Scholar]
Polo 2008 {published data only}
- Polo O, Ylä‐Sahra R, Hirvonen K, Karvinen J, Vahteristo M, Ellmén J. 'Entacapone prolongs the reduction of PLM by levodopa/carbidopa in restless legs syndrome': Erratum. Clinical Neuropharmacology 2008;31(1):61. [DOI] [PubMed] [Google Scholar]
Reuter 1999 {published data only}
- Reuter I, Ellis C, Ray Chaudhuri K. Nocturnal subcutaneous apomorphine infusion in Parkinson's disease and restless legs syndrome. Acta Neurologica Scandinavica 1999;100(3):163‐7. [DOI] [PubMed] [Google Scholar]
Saletu 2003 {published data only}
- Saletu M, Anderer P, Hogl B, Saletu‐Zyhlarz G, Kunz A, Poewe W, et al. Acute double‐blind, placebo‐controlled sleep laboratory and clinical follow‐up studies with a combination treatment of rr‐L‐dopa and sr‐L‐dopa in restless legs syndrome. Journal of Neural Transmission 2003;110(6):611‐26. [DOI] [PubMed] [Google Scholar]
Staedt 1998 {published data only}
- Staedt J, Hunerjager H, Ruther E, Stoppe G. Pergolide: treatment of choice in Restless Legs Syndrome (RLS) and Nocturnal Myoclonus Syndrome (NMS). Longterm follow up on pergolide. Short communication. Journal of Neural Transmission 1998;105(2‐3):265‐8. [DOI] [PubMed] [Google Scholar]
Stiasny 2001 {published data only}
- Stiasny K. Clinical data on restless legs syndrome: a dose‐finding study with cabergoline. European Neurology 2001;46 Suppl 1:24‐6. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2005 {published data only}
- Trenkwalder C, Kohnen R. Augmentation and lack of efficacy with dopaminergic treatment in patients with RLS double blind comparison between cabergoline and l‐dopa in the treatment of patients with severe restless legs syndrome. Sleep Medicine 2005;6 Suppl 2:S162. [Google Scholar]
von Scheele 1986 {published data only}
- Scheele C. Levodopa in restless legs. Lancet 1986;2(8504):426‐7. [DOI] [PubMed] [Google Scholar]
von Scheele 1990 {published data only}
- Scheele C, Kempi V. Long‐term effect of dopaminergic drugs in restless legs. A 2‐year follow‐up. Archives of Neurology 1990;47(11):1223‐4. [DOI] [PubMed] [Google Scholar]
Wetter 1995 {published data only}
- Wetter T, Trenkwalder C, Stiasny K, Pollmacher T, Kazenwadel J, Kohnen R, et al. [Treatment of idiopathic and uremic restless legs syndrome with L‐dopa‐‐a double‐blind cross‐over study] [Behandlung des idiopathischen und uramischen Restless‐legs‐Syndrom mit L‐Dopa‐‐Eine doppelblinde Cross‐over‐Studie]. Wiener Medizinische Wochenzeitschrift 1995;145(17‐18):525‐7. [PubMed] [Google Scholar]
Wetter 1995a {published data only}
- Wetter T, Trenkwalder C, Pollmacher T, Stiasny K, Kohnen R, Kazenwadel J, et al. Effect of levodopa therapy of idiopathic and uremic restless legs syndrome: a double blind crossover trial. Journal of Neurology 1995;242(6 Suppl 2):S81. [DOI] [PubMed] [Google Scholar]
Wu Yh 2008 {published data only}
- Wu Yh, Sun C, Wu D, Huang Y, Chi C. [Observation on therapeutic effect of acupuncture on restless legs syndrome]. Zhongguo zhen jiu [Chinese acupuncture & moxibustion] 2008;28(1):27‐9. [PubMed] [Google Scholar]
Additional references
Akpinar 1982
- Akpinar S. Treatment of restless legs syndrome with levodopa plus benserazide. Archives of Neurology 1982;39(11):739. [DOI] [PubMed] [Google Scholar]
Allen 1996
- Allen RP, Earley CJ. Augmentation of the restless legs syndrome with carbidopa/levodopa. Sleep 1996;19(3):205‐13. [DOI] [PubMed] [Google Scholar]
Allen 2003
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Medicine 2003;4:101‐19. [DOI] [PubMed] [Google Scholar]
Allen 2007
- Allen RP, Earley CJ. The role of iron in restless legs syndrome. Movement Disorders 2007;22 Suppl 18:S440‐8. [DOI] [PubMed] [Google Scholar]
American Academy of Sleep Medicine 2005
- American Academy of Sleep Medicine. Periodic limb movement disorder. In: American Academy of Sleep Medicine editor(s). The International classification of sleep disorders, 2nd edition: Diagnostic and coding manual. Westchester, Illinois: American Academy of Sleep Medicine, 2005:182‐6. [Google Scholar]
Baker 2008
- Baker WL, White CM, Coleman CI. Effect of nonergot dopamine agonists on symptoms of restless legs syndrome. Annals of Family Medicine 2008;6(3):253‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 2004
- Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Parity and the risk of restless legs syndrome in the general population. Archives of Internal Medicine 2004;164(2):196‐202. [DOI] [PubMed] [Google Scholar]
Berger 2007
- Berger K, Kurth T. RLS epidemiology ‐ Frequencies, risk factors and methods in population studies. Movement Disorders 2007;22:S420‐3. [DOI] [PubMed] [Google Scholar]
Bonnet 1993
- Bonnet M, Carley D, Carskadon M, Easton P, Guilleminault C, Harper R, et al. Recording And Scoring Leg Movements. Sleep 1993;16:748‐59. [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988. [Google Scholar]
Connor 2008
- Connor JR. Pathophysiology of restless legs syndrome: evidence for iron involvement. Current Neurology and Neuroscience Reports 2008;8:162‐6. [DOI] [PubMed] [Google Scholar]
Connor 2009
- Connor JR, Wang XS, Allen RP, Beard JL, Wiesinger JA, Felt BT, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain 2009;132(Pt 9):2403‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conti 2007
- Conti CF, Oliveira MM, Andriolo RB, Saconato H, Atallah AN, Valbuza JS, et al. Levodopa for idiopathic restless legs syndrome: evidence‐based review. Movement Disorders 2007;22(13):1943‐51. [DOI] [PubMed] [Google Scholar]
Conti 2008
- Conti CF, Oliveira MM, Valbuza JS, Prado LB, Carvalho LB, Prado GF. Anticonvulsants to treat idiopathic restless legs syndrome: systematic review. Arquivos de Neuropsiquiatria 2008;66(2b):431‐5. [DOI] [PubMed] [Google Scholar]
Cornelius 2010
- Cornelius JR, Tippmann‐Peikert M, Slocumb NL, Frerichs CF, Silber MH. Impulse control disorders with the use of dopaminergic agents in restless legs syndrome: a case‐control study. Sleep 2010;33(1):81‐7. [PMC free article] [PubMed] [Google Scholar]
Diagnostic Classification Steering Committee 1990
- Diagnostic Classification Steering Committe, Thorpy MJ (chairman). International classification of sleep disorders: diagnostic and coding manual. Rochester, MN: American Sleep Disorders Association, 1990. [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, McDermott MP, Peirce‐Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. The Journal of Pain 2008;9(2):105‐21. [DOI] [PubMed] [Google Scholar]
Eisensehr 2004
- Eisensehr I, Ehrenberg BL, Rogge Solti S, Noachtar S. Treatment of idiopathic restless legs syndrome (RLS) with slow‐release valproic acid compared with slow‐release levodopa/ benserazid. Journal of Neurology 2004;251:579‐83. [DOI] [PubMed] [Google Scholar]
Ekbom 1945
- Ekbom K. Restless legs: a clinical study. Acta Medica Scandinavica 1945;158:1‐123. [Google Scholar]
Garcia‐Borreguero 2002
- García‐Borreguero D, Larrosa O, Llave Y, Verger K, Masramon X, Hernandez G. Treatment of restless legs syndrome with gabapentin: a double‐blind, cross‐over study. Neurology 2002;59(10):1573‐9. [DOI] [PubMed] [Google Scholar]
Garcia‐Borreguero 2007a
- García‐Borreguero D, Allen RP, Kohnen R, Högl B, Trenkwalder C, Oertel W, et al. International Restless Legs Syndrome Study Group. Diagnostic standards for dopaminergic augmentation of restless legs syndrome: report from a World Association of Sleep Medicine‐International Restless Legs Syndrome Study Group consensus conference at the Max Planck Institute. Sleep Medicine 2007;8(5):520‐30. [DOI] [PubMed] [Google Scholar]
Garcia‐Borreguero 2007b
- García‐Borreguero D, Allen RP, Benes H, Earley C, Happe S, Högl B, et al. Augmentation as a treatment complication of restless legs syndrome: concept and management. Movement Disorders 2007;22 Suppl 18:S476‐84. [DOI] [PubMed] [Google Scholar]
Goertelmeyer 1985
- Goertelmeyer R. On the development of a standardized sleep inventory for the assessment of sleep. In: Kubicki S, Herrmann W editor(s). Methods of sleep research. Stuttgart/New York: Fischer, 1985. [Google Scholar]
Hansen 2009
- Hansen RA, Song L, Moore CG, Gilsenan AW, Kim MM, Calloway MO, et al. Effect of ropinirole on sleep outcomes in patients with restless legs syndrome: meta‐analysis of pooled individual patient data from randomized controlled trials. Pharmacotherapy 2009;29(3):255‐62. [DOI] [PubMed] [Google Scholar]
Happe 2008
- Happe S, Vennemann M, Evers S, Berger K. Treatment wish of individuals with known and unknown restless legs syndrome in the community. Journal of Neurology 2008;255(9):1365‐71. [DOI] [PubMed] [Google Scholar]
Hening 2004
- Hening W. The clinical neurophysiology of the restless legs syndrome and periodic limb movements. Part I: diagnosis, assessment, and characterization. Clinical Neurophysiology 2004;115(9):1965‐74. [DOI] [PubMed] [Google Scholar]
Hornyak 2004
- Hornyak M, Trenkwalder C. Restless legs syndrome and periodic limb movement disorder in the elderly. Journal of Psychosomatic Research 2004;56:543‐8. [DOI] [PubMed] [Google Scholar]
Högl 2003
- Högl B, Saletu M, Frauscher B, Brandauer E, Seppi K, Müller J, et al. Prevalence of Restless Legs Syndrome in the central European alpine region of South Tyrol. Sleep 2003;26:A344. [Google Scholar]
Iber 2007
- Iber C, Ancoli‐Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. first. Westchester, Illinois: American Academy of Sleep Medicine, 2007. [Google Scholar]
Kohnen 2002
- Kohnen R, Benes H, Heinrich CR, Kurella B. Development of the disease‐specific Restless Legs Syndrome Quality of Life (RLS‐QoL) questionnaire. Movement Disorders 2002;17 Suppl 5:P743. [Google Scholar]
Kohnen 2004
- Kohnen R, Oertel WH, Stiasny‐Kolster K, Benes H, Trenkwalder C. Severity rating of restless legs syndrome: validation of the RLS‐6 scales. Sleep 2004;27 Suppl 1:A304. Abstract 680. [Google Scholar]
Kushida 2007
- Kushida C, Martin M, Nikam P, Blaisdell B, Wallenstein G, Ferini‐Strambi L, et al. Burden of restless legs syndrome on health‐related quality of life. Quality of Life Research 2007;16(4):617‐24. [DOI] [PubMed] [Google Scholar]
Kushida 2009
- Kushida CA, Becker PM, Ellenbogen AL, Canafax DM, Barrett RW, XP052 Study Group. Randomized, double‐blind, placebo‐controlled study of XP13512/GSK1838262 in patients with RLS. Neurology 2009;72(5):439‐46. [DOI] [PubMed] [Google Scholar]
Manconi 2004
- Manconi M, Govoni V, Vito A, Economou NT, Cesnik E, Mollica G, et al. Pregnancy as a risk factor for restless legs syndrome. Sleep Medicine 2004;5(3):305‐8. [DOI] [PubMed] [Google Scholar]
National Institute of Mental Health 1976
- National Institute of Mental Health (NIMH). Early clinical drug evaluation unit (ECDEU). Clinical Global Impressions. In: Guy W editor(s). ECDEU assessment manual for psychopharmacology. revised. Rockville MD: NIMH, Psychopharmacology Research Branch, 1976. [Google Scholar]
Oertel 2007
- Oertel WH, Trenkwalder C, Zucconi M, Benes H, Borreguero DG, Bassetti C, et al. State of the art in restless legs syndrome therapy: practice recommendations for treating restless legs syndrome. Movement Disorders 2007;22 Suppl 18:S466‐75. [DOI] [PubMed] [Google Scholar]
Phillips 2000
- Phillips B, Young T, Finn L, Asher K, Hening WA, Purvis C. Epidemiology of restless legs symptoms in adults. Archives of Internal medicine 2000;160:2137‐41. [DOI] [PubMed] [Google Scholar]
Quilici 2008
- Quilici S, Abrams KR, Nicolas A, Martin M, Petit C, Lleu PL, et al. Meta‐analysis of the efficacy and tolerability of pramipexole versus ropinirole in the treatment of restless legs syndrome. Sleep Medicine 2008;9(7):715‐26. [DOI] [PubMed] [Google Scholar]
Reviewer's Handbook
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Rothdach 2000
- Rothdach AJ, Trenkwalder C, Haberstock J, Keil U, Berger K. Prevalence and risk factors of RLS in an elderly population. Neurology 2000;54:1064‐8. [DOI] [PubMed] [Google Scholar]
Schöls 1998
- Schöls L, Haan J, Riess O, Amoridis G, Przuntek H. Sleep disturbance in spinocerebellar ataxias: is the SCA3 mutation a cause of restless legs syndrome?. Neurology 1998;51:1603‐7. [DOI] [PubMed] [Google Scholar]
Stefansson 2007
- Stefansson H, Rye DB, Hicks A, Petursson H, Ingason A, Thorgeirsson TE. A genetic risk factor for periodic limb movements in sleep. New England Journal of Medicine 2007;357(7):639‐47. [DOI] [PubMed] [Google Scholar]
Stiasny‐Kolster 2006
- Stiasny‐Kolster K, Kohnen R, Möller JC, Trenkwalder C, Oertel WH. Validation of the "L‐DOPA test" for diagnosis of restless legs syndrome. Sleep Medicine 2006;21(9):1333‐9. [DOI] [PubMed] [Google Scholar]
Stiasny‐Kolster 2009
- Stiasny‐Kolster. Restless legs syndrome [Restless‐Legs‐Syndrom]. Somnology 2009;13(1):115‐27. [Google Scholar]
Talati 2009
- Talati R, Phung OJ, Mather J, Coleman CI. Effect of non‐ergot dopamine agonists on health‐related quality of life of patients with restless legs syndrome. The Annals of Pharmacotherapy 2009;43:813‐21. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2004
- Trenkwalder C, Paulus W. Why do restless legs occur at rest?‐pathophysiology of neuronal structures in RLS. Neurophysiology of RLS (part 2). Clinical Neurophysiology 2004;115(9):1975‐88. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2005
- Trenkwalder C, Benes H, Hornyak M, Riemann D, Stiasny K, Winkelmann J. Restless legs syndrome and periodic limb movement disorder [Restless Legs Syndrom und Periodic Limb Movement Disorder]. In: Diener CH editor(s). Leitlinien für Diagnostik und Therapie in der Neurologie. Stuttgart: Thieme, 2005. [Google Scholar]
Trenkwalder 2008
- Trenkwalder C, Hening WA, Montagna P, Oertel WH, Allen RP, Walters AS, et al. Treatment of restless legs syndrome: an evidence‐based review and implications for clinical practice. Movement Disorders 2008;23(16):2267‐302. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2010
Ulfberg 2001
- Ulfberg J, Nyström B, Carter N, Edling C. Prevalence of restless legs syndrome among men aged 18 to 64 years: An association with somatic disease and neuropsychiatric symptoms. Movement Disorders 2001;16:1159‐63. [DOI] [PubMed] [Google Scholar]
Walters 1993
- Walters AS, Wagner ML, Hening WA, Grasing K, Mills R, Chokroverty S, et al. Successful treatment of the idiopathic restless legs syndrome in a randomized double‐blind trial of oxycodone versus placebo. Sleep 1993;16:327‐32. [DOI] [PubMed] [Google Scholar]
Walters 1995
- Walters AS, The International Restless Legs Syndrome Study Group. Toward a better definition of restless legs syndrome. Movement Disorders 1995;10:634‐42. [DOI] [PubMed] [Google Scholar]
Walters 2003
- Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al. International Restless Legs Syndrome Study Group. Validation of theInternational Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Medicine 2003;4:121‐32. [DOI] [PubMed] [Google Scholar]
Walters 2007
- Walters AS. Restless legs syndrome and periodic limb movements in sleep. Continuum: Lifelong learning in Neurology 2007;13(3):115‐38. [Google Scholar]
Winkelmann 2007
- Winkelmann J, Schormair B, Lichtner P, Ripke S, Xiong L, Jalilzadeh S, et al. Genome‐wide association study of restless legs syndrome identifies common variants in three genomic regions. Nature Genetics 2007;39(8):1000‐6. [DOI] [PubMed] [Google Scholar]
Zintzaras 2010
- Zintzaras E, Kitsios GD, Papathanasiou AA, Konitsiotis S, Miligkos M, Rodopoulou P, et al. Randomized trials of dopamine agonists in restless legs syndrome: a systematic review, quality assessment, and meta‐analysis. Clinical Therapeutics 2010;32(2):221‐37. [DOI] [PubMed] [Google Scholar]
Zucconi 2006
- Zucconi M, Ferri R, Allen R, Baier PC, Bruni O, Chokroverty S, et al. International Restless Legs Syndrome Study Group (IRLSSG). The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Medicine 2006;7(2):175‐83. [DOI] [PubMed] [Google Scholar]


