Abstract
The laboratory diagnosis of acute bacterial prostatitis is straightforward and easily accomplished in clinical laboratories. Chronic bacterial prostatitis, and especially chronic idiopathic prostatitis (most often referred to as abacterial prostatitis), presents a real challenge to the clinician and clinical microbiologist. Clinically, the diagnosis of chronic idiopathic prostatitis is differentiated from that of acute prostatitis by a lack of prostatic inflammation and no “significant” (controversial) leukocytes or bacteria in the expressed prostatic secretions. Despite these diagnostic criteria, the etiology of chronic idiopathic prostatitis is unknown. While this review covers the entire spectrum of microbially caused acute prostatitis (including common and uncommon bacteria, viruses, fungi, and parasites) and microbially associated chronic prostatitis, a special focus has been given to chronic idiopathic prostatitis. The idiopathic syndrome is commonly diagnosed in men but is poorly treated. Recent data convincingly suggests a possible bacterial etiology for the condition. Provocative molecular studies have been published reporting the presence of 16S rRNA bacterial sequences in prostate biopsy tissue that is negative for ordinary bacteria by routine culture in men with chronic idiopathic prostatitis. Additionally, special culture methods have indicated that difficult-to-culture coryneforms and coagulase-negative staphylococci are present in expressed prostatic secretions found to be negative by routine culture techniques. Treatment failures are not uncommon in chronic prostatitis. Literature reports suggest that antimicrobial treatment failures in chronic idiopathic prostatitis caused by organisms producing extracellular slime might result from the virulent properties of coagulase-negative staphylococci or other bacteria. While it is difficult to definitively extrapolate from animal models, antibiotic pharmokinetic studies with a murine model have suggested that treatment failures in chronic prostatitis are probably a result of the local microenvironment surrounding the persistent focal and well-protected small bacterial biofilms buried within the prostate gland. These conclusions support the molecular and culture data implicating bacteria as a cause of chronic idiopathic prostatitis.
INTRODUCTION
Definition of a Problem
Prostatitis is a common urologic condition that many clinicians find difficult to treat effectively. It has been estimated that up to half of all men suffer from symptoms of prostatitis at some time in their lives (70). Culture diagnosis of acute bacterial prostatitis is straightforward and easily accomplished in the laboratory. On the other hand, the microbiologic diagnosis of chronic prostatitis and chronic idiopathic (nonbacterial) prostatitis (more commonly referred to as prostatodynia) represents a particular challenge. Chronic idiopathic prostatitis, when diagnosed clinically, has a poor record of treatment success. A large study of 597 prostatitis patients indicated that nearly one-third were diagnosed with prostatodynia, which is a significant fraction of the urological population (10). The recent literature suggests that the condition referred to as chronic idiopathic (nonbacterial) prostatitis may actually have an infectious etiology (18, 32, 38, 53). Some patients relate the onset of their symptoms to sexual activity—sometimes associated with acute urethritis (7)—while others have indicated no relationship with sexual activity. The use of antimicrobial therapy may or may not elicit transient relief of symptoms. A number of organisms have been reported to possibly cause this syndrome: Trichomonas vaginalis (25, 34–36), Chlamydia trachomatis (1, 8, 9, 12, 67), genital mycoplasmas (10, 73), staphylococci (3, 8, 53), coryneforms (18, 60), and genital viruses (4, 16). These data are controversial, since other researchers have either failed in their attempts to demonstrate the presence of these microorganisms in clinical specimens or have found them in only rare circumstances (6, 17, 66). The major difficulty in interpreting these microbiologic findings is the presence of contaminating, indigenous microbiota. Specimens such as voided urine, urethral swabs, and expressed prostatic secretions (EPS), used to evaluate a patient with suggestive symptoms, become contaminated with organisms colonizing the distally contaminated urethra. Although the ideal specimen would be uncontaminated prostatic tissue, there are few reports of this in the literature. The microbiological workup of these specimens is further complicated by the presence of inhibitory substances known to exist in the prostatic secretions (21) and the history of multiple previous courses of antibiotics (34).
The detection of difficult-to-culture (cell wall-deficient/defective bacteria [CWDB] and nutritionally deficient bacteria) or nonculturable bacteria in chronic idiopathic prostatitis must necessarily take into account not only the morphologic and metabolic diversity of organisms (CWDB and nutritionally deficient bacteria) but also the consequences of their interaction with other microorganisms (i.e., exchange of genetic material) and their host (20). When first recognized as causing disease, bacteria were seen to be relatively stable vegetative cells that grew and expressed toxic by-products in vitro. These organisms tended to be quite virulent, and so associating them with particular diseases was straightforward. Since then, technologic developments have revealed a spectrum of microbial agents, including viruses, fungi, and protozoa, with each group being diverse and continually changing. The interaction of these organisms within the host can lead to the enhancement or depression of their individual properties (CWDB, production of extracellular slime, biofilms). Clinical expression, i.e., disease symptoms, in the host depends on the genetic vulnerability of the host, the particular environmental stresses, host susceptibility, and the number and location of such consortia. Clinical microbiologists and treating physicians who face this tangled scenario when confronted with patients suffering from chronic idiopathic prostatitis must objectively quantitate and define the process that has led to the illness. Because cryptic organisms, whether intra- or extracellular, are ubiquitous, proving their role in disease requires more than the mere demonstration of their presence. Quantifying and identifying the cells that are most heavily parasitized are impractical clinical approaches, and it is often impossible to satisfy Koch’s postulates. The concept of normal microbiota is a statistical one that is derived from the immunocompetence of most of the population. It is unwise to dismiss the pathogenic capacities of any “benign” microbe in a patient with a mysterious illness. This precept is particularly relevant for patients with chronic idiopathic prostatitis. Bacteriologic advances, which include the use of specialized culture media and stains, electron microscopy, and PCR for amplifying microbial sequences in tissues and body fluids, have revealed an increasing number of previously unidentifiable organisms in a variety of pathologic conditions (19, 58, 59). The present trend toward using sequence-based identification of difficult-to-culture and nonculturable organisms in chronic idiopathic prostatitis will ultimately achieve this end.
Acute and Chronic Bacterial Prostatitis
Bacterial infection of the prostate gland may occur as a result of ascending urethral infection or by reflux of infected urine into prostatic ducts emptying into the posterior urethra. Other possible routes of infection include invasion of rectal bacteria through direct extension or by lymphogenous or hematogenous spread (43). There is an association between bacterial prostatitis and urinary tract infection (UTI), including host responses that result in excessive numbers of polymorphonuclear leukocytes and macrophages in the prostatic secretions. It has been demonstrated that positive cultures (segmented cultures [see Specimen Collection and Bacteriologic Culture, below]) can localize the etiologic agent(s) to the prostatic secretions. When the patient has acute bacterial prostatitis, there is an abrupt onset of fever and genitourinary and constitutional signs and symptoms. Chronic bacterial prostatitis is a more subtle illness, which is characterized by relapsing, recurrent UTI and persistence of bacteria in the prostatic secretory system despite multiple courses of antibacterial therapy (43). A third syndrome, chronic idiopathic prostatitis (sometimes called abacterial prostatitis or nonbacterial prostatitis and prostatodynia) may or may not be associated with excessive numbers of inflammatory cells in the prostatic secretions and with lack of culturally documented bacteriuria. The prostatic secretions from many patients appear normal.
In a study by Brunner et al. in 1983, it was reported that of 600 men attending a special prostatitis clinic in Germany, 5% had bacterial prostatitis, 64% had nonbacterial prostatitis, and 31% had prostatodynia (10). The recent literature suggests that there is no clear reason to distinguish prostatodynia from nonbacterial prostatitis since subjects with prostatodynia may at times have excessive numbers of leukocytes in their expressed prostatic secretions, demonstrate negative routine bacterial urine cultures, and undergo similar therapy for both conditions. During the past few years, molecular data and cultures performed with special media (discussed below) strongly suggested that chronic idiopathic prostatitis may actually be a cryptic bacterial infection of the prostate gland that is usually missed or undetected by routine conventional cultures in clinical microbiology laboratories. The definitions of abacterial prostatitis or nonbacterial prostatitis and prostatodynia are still controversial. For the purposes of this review, these conditions will be grouped into one and referred to as chronic idiopathic prostatitis. Additionally, our primary focus will be on recent findings (1990s) rather than on the historical literature about prostatitis.
SPECIMEN COLLECTION AND BACTERIOLOGIC CULTURE
Quantitative bacteriologic cultures confirm the diagnosis of bacterial prostatitis when the infectious agent(s) is localized to the prostate gland (i.e., segmented cultures). The technique for obtaining segmented cultures of the male lower urinary tract was first described in 1968 by Meares and Stamey (44). This method (although rarely used today in clinical practice) is still considered by many to be the “gold standard” for localizing infection to the prostate gland. The sampling conditions require a sufficiently full bladder, and the samples must be collected by using rigorous aseptic techniques. The first step of the examination must not be preceded by urethral swabbing. All urine samples must have a well-defined volume. Prostatic secretions are obtained by a systematic massage of each lobe of the prostate gland. Bacterial prostatitis is confirmed by the presence of bacteria in the prostatic secretions and in the VB3 (voided bladder) postprostatic massage urine sample in numbers greatly exceeding the bacterial counts of the VB1 and VB2 urine specimens. The traditional criterion for diagnosing chronic bacterial prostatitis is a 10-fold increase in the concentration of culturable microorganisms when the bacterial count of the postmassage urine sample or expressed prostatic secretion sample is compared with that of the first-void (VB1) urine sample (43, 44). The segmented-culture technique is not widely used in primary care settings, and even most urologists appear to have abandoned the procedure because of its labor intensity and overall costs. In 1997, Nickel (46) proposed a simple and cost-effective screen for prostatitis which involves the culture and microscopic examination of urine before and after prostatic massage. This pre- and postmassage test (PPMT) was applied to a series of 53 patients as well as 59 patients for whom segmented-culture results were available from the literature. In these selected populations, the PPMT alone led to the same diagnosis in 102 patients (91.1%). Within the expected limitations of this retrospective review, the calculated sensitivity and specificity of the PPMT were both 91%. Based on these findings, this method warrants further investigation and may motivate researchers to review their prostatitis data and stimulate discussion. Importantly, physicians might then be convinced to adopt a simpler diagnostic plan for prostatitis because it is far more efficient in terms of diagnosing the disease than is doing no work-up of the patient for localization of infection.
COMMON BACTERIAL ETIOLOGIC AGENTS
Most of the urinary pathogens are also the causative agents of acute and chronic prostatitis. Escherichia coli predominates as a cause of culturable prostatitis. Other members of the Enterobacteriaceae, such as Klebsiella, Enterobacteria, Proteus, and Serratia, can be isolated from patients with acute and chronic prostatitis, as can the pseudomonads and less common gram-negative bacteria. Obligate anaerobes have rarely been implicated as a cause of prostatitis. Gram-positive bacteria, particularly the cocci, remain controversial as possible etiologic agents. Recently, coagulase-negative staphylococcal species and coryneforms have been found in segmented specimens (including prostatic secretions) and are postulated to play a role in chronic idiopathic prostatitis (38, 53). It is generally agreed that Enterococcus faecalis can cause chronic bacterial prostatitis and related recurrent enterococcal bacteriuria. Although many published reports have indicated that gram-positive bacteria other than enterococci rarely cause bacterial prostatitis (43), emerging molecular data and special culture results suggest that these organisms and other less well known bacteria may well be true pathogens in the poorly understood condition referred to as chronic idiopathic prostatitis (18, 32, 38, 53, 60).
CHRONIC IDIOPATHIC PROSTATITIS
Since the infectious etiology of acute and chronic recurrent prostatitis in which a single or multiple bacterial isolates can be clearly identified is well understood and accepted, this review will focus on the microbiology of the more controversial syndrome of chronic idiopathic prostatitis. Clinically, this condition frustrates the patient and physician due to its chronicity and resistance to therapy. The syndrome is usually characterized by persistent perineal pain and by functional and somatic urologic complaints, including abnormal urine flow, frequency, urgency, and dysuria. The diagnosis of chronic idiopathic prostatitis is differentiated from that of acute prostatitis by a lack of prostatic inflammation with no significant leukocytes or bacteria in the expressed secretions, and the patients are not acutely ill. Despite these diagnostic criteria, the etiology of chronic idiopathic prostatitis is unknown. Recent studies suggest that the etiology of chronic idiopathic prostatitis may be of bacterial origin (18, 22). Three types of provocative data have demonstrated the presence of bacteria in prostatic specimens (tissue and secretions) that were negative by traditional clinical microbiologic tests: (i) bacterial gene sequences encoding 16S rRNA and tetracycline resistance (tetM-tetO-tetS) were present in prostatic tissue (32); (ii) culture findings indicated that coagulase-negative staphylococci were the most common isolates in patients with prostatodynia (chronic idiopathic prostatitis) (38, 53); and (iii) culture of difficult-to-grow coryneforms in EPS and direct microscopic observation of these pleomorphic bacteria in EPS thought to be negative by routine culture were reported (18, 60).
Prokaryotic DNA Sequences in Patients
In a provocative study by Krieger et al. (32), 135 men with chronic prostatitis refractory to multiple previous courses of antimicrobial therapy were evaluated. These individuals had no evidence of structural or functional lower genitourinary tract abnormalities, bacterial prostatitis by traditional clinical tests, or urethritis or urethral pathogens by bacteriologic culture. PCR assays that were organism specific detected Mycoplasma genitalium, Chlamydia trachomatis, or Trichomonas vaginalis in 10 patients (8%). Broad-spectrum PCR tests detected tetM-tetO-tetS in 25% of the subjects and sequences encoding 16S rRNA in 77% of the patients. The tetM-tetO-tetS-positive patients constituted a subset of the 16S rRNA-positive patients. Patients with 16S rRNA genes were more likely to have at least 1,000 leukocytes per mm3 in their EPS than were men whose prostate biopsy specimens were negative for 16S rRNA (P < 0.001). Multiple bacterial sources of 16S rRNA were observed in individual patients based on direct sequencing and repetitive cloning. Sequences of 29 cloned PCR products revealed 16S rRNAs distinct from those of members of the common skin and gastrointestinal flora. In a case-control design, it is impossible to conclusively determine the cause and effect; however, these data suggest that the prostate harbors bacteria that are not detectable by conventional microbiologic culture. These molecular studies are particularly significant because tissue specimens from prostate biopsies were obtained from a population of men who could not be diagnosed by optimal clinical and microbiologic methods. Therefore, potential study subjects were excluded, unlike in earlier studies (1, 4, 8–10, 12, 16, 22, 25, 33, 35, 36, 42, 53, 73) if they exhibited bacteriuria, bacterial prostatitis, or urethritis or if they had a urethral culture that was positive for urogenital pathogens. The most convincing finding is the strong correlation between inflammation in the EPS and detection of bacterial gene sequences in prostatic tissue. It is unlikely that the demonstrated molecular evidence represents contamination because of the extreme care reported to have been taken in procuring and handling the clinical samples, including the use of a double-needle biopsy method to limit skin contamination and positive and negative controls incorporated in the molecular experiments as well as an internal housekeeping gene control. It will be important to classify the sequences at the bacterial genus and species level, since this has not yet been accomplished for the amplified bacterial nucleic acids derived from prostate tissue. Identification, cloning, and sequencing of prokaryotic DNA in prostatic tissue may help elucidate the etiologic agents of chronic prostatitis syndromes.
Bacterial Cultures for Commensal and Fastidious Bacteria
In a study from our laboratory, Lowentritt et al. (38) proposed that prostatodynia (chronic idiopathic prostatitis) may be caused by an ascending subclinical infection of the prostate by bacteria, including recognized uropathogens and commensals. This research evaluated segmented cultures of urine and EPS from 22 patients and 16 controls. Nine patients had positive cultures from prostatic secretions; compared to the results for the controls, this finding was statistically significant (P < 0.025). Coagulase-negative staphylococci were the most common isolates (68%). The most commonly isolated organism, Staphylococcus epidermidis, has also been implicated by Nickel and Costerton (53) and Wedren et al. (72) in the etiology of chronic idiopathic bacterial prostatitis. Another coagulase-negative Staphylococcus species isolated in this study, Staphylococcus haemolyticus, was reported by Gunn and Davis (26) and Sanchis-Bayarri et al. (62) to cause UTI in men. It has been postulated that these coagulase-negative staphylococci probably possess virulence factors that may be operative in chronic idiopathic prostatitis (29). Coagulase-negative staphylococci have been shown to adhere to urothelial cells (40) and cellular proteins including laminin, fibronectin, vitronectin, and collagen (55), which may allow ascending infection and subsequent colonization of the prostate. Furthermore, these bacteria produce an extracellular slime substance with antiphagocytic and antichemotactic properties that affect neutrophils as well as antiproliferative characteristics that affect lymphocytes (29). Thus, extracellular slime substance may further impair host defenses. Extracellular slime substance also has cytoprotective properties, which can protect bacteria from exposure to otherwise bactericidal levels of antibiotics, and hence can lead to recrudescent infections resistant to therapy (53). Therefore, antimicrobial treatment failures of organisms producing extracellular slime might result from the virulent properties of coagulase-negative staphylococci or other bacteria.
The patients in the study by Lowentritt et al. described above (38) were diagnosed with chronic idiopathic prostatitis clinically and microscopically. They had noninflammatory and minimally inflammatory intraprostatic disease, as indicated by the concentration of leukocytes (WBC) in EPS. Thus, this syndrome may involve a type of host immune deficiency. Wedren et al. (72) reported on several nonbacterial prostatitis patients in whom leukocytes had diminished phagocytic activity against autologous bacterial isolates, suggesting that there exists a selective process involving prostatic pathogens and specific host defenses. Similarly, Sanchis-Bayarri et al. (62) concluded that coagulase-negative staphylococci, particularly Staphylococcus haemolyticus, can function as opportunistic pathogens in immunodepressed hosts.
In 1997, Berger et al. (5) suggested that bacterial colonization and invasion of the prostate may be associated with inflammatory prostatitis in certain cases. They examined the relationship of genitourinary infection to inflammatory prostatitis in 85 patients without bacteriuria. Cultures of the urethra, urine, and transperineal prostate biopsy specimens were performed. In addition to specifically targeting commensal and fastidious bacteria in cultures, leukocyte counts of expressed prostatic secretions were determined. The 25 subjects with inflamed prostatic secretions were more likely to have any type of positive bacteria culture (P = 0.01), positive cultures for anaerobic bacteria (P = 0.03), higher total bacterial counts (P = 0.02), and more bacterial species isolated (P = 0.02) in prostate biopsy cultures than were the 60 men without expressed prostatic secretion inflammation. This group of investigators also developed a symptom index for patients with chronic prostatitis, which may prove valuable in the clinical evaluation of these patients and interpretation of their laboratory results (31).
Difficult-to-Culture Coryneforms in Expressed Prostatic Secretions
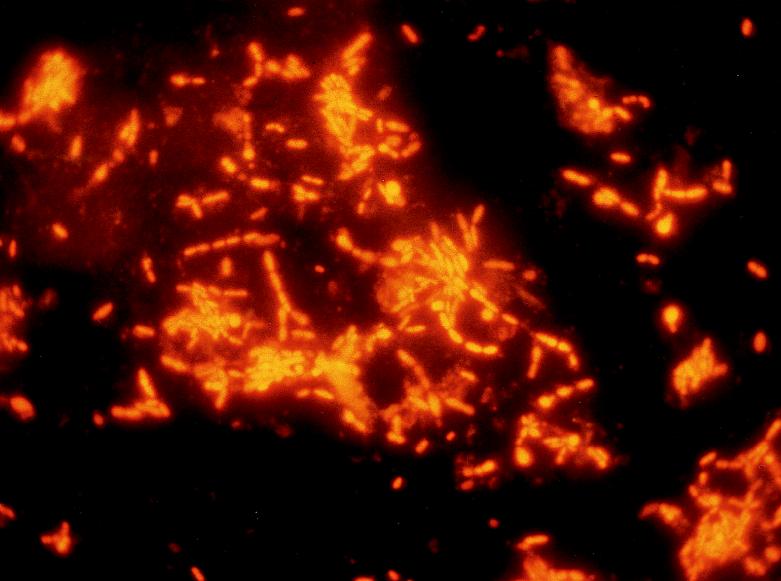
Domingue et al. in 1997 (18) reported that difficult-to-culture coryneforms were missed by routine culture of EPS on blood agar but that careful microscopic observation and culture of EPS on enriched media revealed the presence of these pleomorphic bacteria. Direct Gram staining of EPS showed gram-variable pleomorphic coccobacillary rods that did not grow on routine media within 72 h at 35°C. The presence of these pleomorphic swollen rods were also shown by fluorescent acridine orange staining (Fig. 1). Culture of the EPS demonstrated numerous rod-like forms on culture media enriched with yeast extract and horse serum and incubated for at least 72 h under 5% CO2 at 35°C. EPS inoculated into tryptose broth demonstrated the presence of gram-variable rod forms (microscopically) and, when subcultured to routine and specialized media, produced the slow-growing bacilli on routine and enriched solid media (incubated aerobically, anaerobically, or under 5% CO2 for 72 h). From a representative subject, the routine culture initially grew 200 colonies from the EPS and 100 colonies from the voided bladder urine (VB3) of S. epidermidis. A subculture of the tryptose broth inoculated with EPS grew <100 colonies of other staphylococci (Staphylococcus hominis/warneri) in addition to S. epidermidis. Biochemical identification of the difficult-to-culture rods revealed two different species of Corynebacterium: Corynebacterium group ANF and C. minitussimum. Antibiotic susceptibility studies showed that Corynebacterium group ANF was resistant to ampicillin, erythromycin, penicillin, oxacillin, nalidixic acid, nitrofurantoin, tobramycin, and bactrim while C. minutissimum was resistant only to oxacillin and nalidixic acid. PCR studies to specifically target the persistence of coryneform genes would be of value to determine the frequency of these organisms in prostatic specimens that are negative by conventional culture. Domingue et al. (18) suggested that cryptic coryneforms could be hidden etiologic agents in chronic idiopathic prostatitis and that they are often missed or overlooked in routine clinical microbiology laboratories. A new coryneform species, Corynebacterium seminale sp. nov., was reported by Riegel et al. (60) to be associated with genital infections in male patients. These investigators studied 12 coryneform isolates having similar biochemical profiles which did not permit their assignment to any recognized taxa. Human semen was the source of seven of these strains, whereas the other five strains were isolated from urethra, urine, and blood specimens from adult males. These bacteria were found in significant quantities (104 to 105 CFU/ml) in semen specimens from infertile male patients with a diagnosis of prostatitis. The bacterial strains had characteristics of the genus Corynebacterium (60% G+C in the DNA, and corynemycolic acids, meso-diaminopimelic acid, arabinose, and galactose in the cell wall). Quantitative DNA-DNA hybridizations involving the S1 nuclease procedure and phylogenies based on comparisons of almost-complete small-subunit rDNA sequences confirmed that these strains constitute a single new species within the genus Corynebacterium. All 12 strains showed similar phenotypic features, i.e., good on growth on sheep blood agar, in contrast to poor growth on the same medium supplemented with 1% Tween 80; a positive CAMP test in the presence of Staphylococcus aureus; glucose and sucrose fermentation; and the presence of β-glucuronidase. Some strains were reported to reduce nitrate and hydrolyze urea or esculin. The characteristics described allowed these strains to be distinguished from members of any other coryneform taxon; the authors proposed the name of Corynebacterium seminale with strain IBS B 12915 (CIP 104297) as the type strain. This description and delineation of these strains as a new species should be useful for further studies, including evaluations of their prevalence among the normal flora, particularly of the genitourinary tract, and their clinical implications.
FIG. 1.
Fluorescent acridine orange stain of difficult-to-grow coryneforms from expressed prostatic secretions. Magnification, ×1,000.
Chlamydia trachomatis
Because chlamydiae are fastidious bacteria that require cell systems for growth, they cannot be propagated on artificial culture media used for ordinary bacteria. Researchers in Japan (30) suggested that Chlamydia trachomatis is often a causative organism in chronic idiopathic prostatitis. In this study, immunoglobulin A (IgA) antibodies specific for C. trachomatis were correlated with EPS by Western immunoblotting. IgA was measured in the EPS of 192 subjects, including 92 patients showing symptoms of chronic idiopathic prostatitis. These investigators also looked for the presence of anti-heat shock protein (HSP; 60 kDa) IgA. Anti-C. trachomatis IgA in EPS was found in 44 (26%) of 169 subjects and in 29% of those with more than 10 WBC/high-power field (hpf) (20 of 69 subjects). Of patients with greater than 10 WBC/hpf, 38.5% had anti-HSP IgA; this is in contrast to 0% for the group with 5 to 9 WBC/hpf. Immunologic data of the 69 subjects with >10 WBC/hpf suggested that 20 of these subjects had chronic idiopathic prostatitis caused by C. trachomatis. From the data presented, it was not possible to determine whether some of the 44 subjects positive for anti-chlamydia IgA were control subjects without symptoms. In a prospective study of C. trachomatis antibodies in the serum and ejaculate of male patients without acute urethritis, Ludwig et al. (39) reported the prevalence of chlamydial antibodies by using a genus-specific immunofluorescence test in 101 men. The results were compared to the clinical diagnosis, cell culture of urethral swabs, demonstration of chlamydial DNA by PCR in the ejaculate, and signs of genital inflammation by counting peroxidase-positive leukocytes and elastase level in semen. Serum-specific IgG and IgA antibodies were found in 26 and 15% of men, respectively; seminal IgG and IgA antibodies were present in 6 and 7% of men, respectively. Serum-specific antibodies were not associated with the clinical diagnosis of infection nor with C. trachomatis cell culture, PCR findings, peroxidase-positive leukocytes, or the polymorphonuclear leukocyte-elastase level. These investigators concluded that serum antibodies are not useful in detecting a chlamydial infection. Seminal plasma antibodies were not correlated with the clinical diagnosis of infection, positive cell culture, polymorphonuclear leukocyte-elastase levels, or WBC in semen. However, a significant correlation was found for positive C. trachomatis PCR in the ejaculate (P < 0.001 for IgG, P < 0.05 for IgA, P < 0.001 when combined). This group concluded that although seminal antibodies may be more useful in detecting ascended or occult chlamydial infection, their significance remains unclear and their absence does not exclude chlamydial infection. Nevertheless, the uncertain role of this organism in chronic prostatitis warrants further research, especially into the biological significance of locally derived IgA.
A preliminary report published by Kadar et al. (27) demonstrated detection of C. trachomatis in chronic prostatitis by in situ hybridization (biotin-labeled DNA probe) on formalin-fixed, paraffin-embedded specimens from patients attending a urology clinic in Hungary. Of 79 biopsy specimens, 34 had a diagnosis of chronic abacterial prostatitis, and 3 of 11 specimens were positive for C. trachomatis. It would be of value to perform further studies to determine the incidence of detecting Chlamydia in prostate gland specimens derived from patients with chronic idiopathic prostatitis and to determine whether there is any relationship to infertility in the relevant age groups.
Ureaplasma urealyticum
Although Ureaplasma urealyticum has long been implicated as sometimes causing nongonococcal urethritis, its role as an etiologic agent of prostatitis is controversial. In a study by Teng et al. (71), PCR was compared with culture for the detection of U. urealyticum in 50 specimens including sperm, urine, and prostate secretions from hospitalized patients (n = 50) with urogenital infections. Five positive diagnoses and an additional four doubtful diagnoses were made by culture, whereas PCR detected U. urealyticum in 12 samples. Eight specimens were derived from prostatic secretions; one was positive by culture and two were positive by PCR. Of 14 semen specimens from infertile subjects, 2 were positive by culture, 4 were doubtful, and 6 were positive by PCR. In addition to its greater sensitivity and lesser dependence on careful specimen handling between collection and testing, PCR had a further advantage of yielding faster results. The assay time was reduced from 2 to 3 days for culture to 1 to 2 days for PCR. PCR may show considerable promise for the rapid and specific diagnosis of U. urealyticum from an appropriately obtained specimen localizing the infection to the prostate, provided that there is sufficient demand to justify the cost of a thermal cycler and the requirements for more costly reagents than are needed for culture. It will also be of value to use PCR primers that distinguish between biovars when considering the biological significance of Ureaplasma in clinical specimens, since specific biovars may be associated with different diseases of the genitourinary system (i.e., is there a prostatitis-specific biovar?).
OTHER, LESS COMMON TYPES OF PROSTATITIS
Mycobacterial Infection
Mycobacterial infection of the prostate causes granulomatous prostatitis. This condition may occur as a sequela of miliary tuberculosis. Recovery of the organism from prostatic fluid cultures confirms the diagnosis (43). In a review of the diagnostic and therapeutic aspects of prostatic abscess in patients with AIDS, Galbis et al. (24) and Rivas-Escudero (61) reported on a patient with AIDS and prostatic abscess due to tuberculosis in 1997. The incidence of granulomatous prostatitis and acid-fast bacilli (AFB) after intravesical bacillus Calmette-Guérin (BCG) therapy was recently published by LaFontaine et al. (37). These patients were part of a group of over 100 patients undergoing radical cystoprostatectomy. Granulomatous prostatitis was identified in 9 of 12 patients from this group who had received BCG treatment; AFB were identified in 7 of 9 patients with granulomatous prostatitis. It seems that the pathologic consequence of granuloma prostatitis with AFB is common after intravesical BCG therapy. These incidences are far greater than the reported incidence of symptomatic granulomatous prostatitis. In an assessment of the pathologic findings of granulomatous prostatitis by Oppenheimer et al. (54), 94 cases of granulomatous prostatitis were found in 25,852 (incidence, 0.36%) men who consecutively underwent needle biopsy; clinical correlations were obtained for 75 men. The cases were categorized as nonspecific (77.7%), infectious (18.1%), or indeterminate (4.3%) granulomatous prostatitis based on histologic and clinical criteria. All the patients with infectious granulomatous prostatitis had a history of prior BCG therapy for transitional cell carcinoma. Histologically, 57% of the nonspecific cases mimicked infection and 4% mimicked cancer. Caseating necrosis was identified in 76% of patients with infectious granulomatous prostatitis. While nonspecific granulomatous prostatitis is the most commonly seen on needle biopsy, BCG granulomas are not infrequently found. These investigators indicate that granulomatous prostatitis may be clinically indistinguishable from cancer and that nonspecific granulomatous prostatitis may also histologically mimic carcinoma.
Gonococcal Prostatitis
Gonococcal prostatitis was first demonstrated in 1931 by Sargent and Irwin (63). Forty-two cases of prostatic abscess were studied, and 75% were found to be caused by Neisseria gonorrhoeae. Most studies suggest that prostatitis caused by this organism is rare. Danielsson and Molin in 1971 (15) used a fluorescent-antibody test to demonstrate the persistence of gonococci in the prostatic fluid of 40% of men who were supposedly cured of their gonococcal infection (based on conventional diagnostic techniques and short-term antigonococcal therapy). Colleen and Maardh in 1975 (13) used a fluorescent-antibody test with anti-gonococcal antibodies to show that six men with repeatedly negative cultures had positive findings for the presence of gonococci in the seminal fluid. After treatment with metacycline, five of the six men were negative for the anti-gonococcal antibodies.
Parasitic Prostatitis
Parasitic prostatitis is rare in the United States and elsewhere. An excellent chapter on parasitic diseases of the genitourinary system, documenting the role of various parasites, has been written by Smith and von Lichtenberg (68).
Fungal Prostatitis
Fungal prostatitis is confirmed by means of prostatic histology and culture of prostatic fluid and tissue. Granulomatous prostatitis caused by fungi is associated with systemic mycoses (coccidiodomycosis, cryptococcosis, histoplasmosis, paracoccidiodomycosis, blastomycosis, and candidiasis) (43). Aspergillis prostatitis is rare overall but is more common in patients with immunodeficiencies. A case of Aspergillis prostatitis associated with pulmonary tuberculosis after corticosteroid treatment for retroperitoneal fibrosis as a complication of methysergide therapy was reported by Cherasse et al. in 1997 (11). Cryptococcal prostatitis in a patient with Behcet’s disease was reported by Fuse et al. (23). The 55-year-old man presented with acute urinary retention due to Cryptococcus neoformans infection of the prostate. The disease was localized to the prostate and successfully treated only with fluconazole. The patient remained well without evidence of systemic or local infection at 32 months. Prostatic sequestration of C. neoformans in an immunocompromised patient treated for cryptococcal meningoencephalitis was reported by Ndimbie et al. in 1994 (45). Although the patient was successfully treated for cryptococcal meningoencephalitis with amphotericin B and 5-flucytosine, he died from other sequelae of AIDS 2 years later. Cryptococci were found neither in the central nervous system nor in other anatomic sites. The autopsy files yielded seven other cases of men with a history of cryptococcal meningoencephalitis. These authors emphasized the possibility that the prostate sequesters C. neoformans, thereby contributing to systemic relapse. To prove sequestration in the prostate, cryptococci must be cultured either from the prostate or from a midstream voided specimen after prostatic massage; the prostate must also be the only focus of infection.
Abscesses of the Prostate Gland
Abscesses of the prostate gland have been associated with a variety of microorganisms: Escherichia coli, Pseudomonas species, staphylococci, and, occasionally, obligate anaerobic bacteria. A few cases of prostatic abscess have been reported to be caused by Staphylococcus aureus, suggesting a hematogenous pathogenesis (43). Prostatic abscess is most often seen in patients who are diabetic, on maintenance dialysis for chronic renal failure, immunocompromised, undergoing urethral instrumentation, or requiring indwelling catheters (43). A case of a prostate abscess caused by Brucella in a 44-year-old patient was reported by Sevillano-Guida et al. (64). There is a high incidence of brucellosis in the province of Soria, Spain, from which this report originated. These authors highlighted the crucial role of the endocavitary ultrasound technique for both diagnosis and treatment and the importance of case follow-up and recognition of special culture methods for diagnosis of a Brucella infection. A metastatic prostatic abscess was reported in a 12-year-old boy by Shokeir et al. (65). Rectal examination revealed a tender, fluctuating prostatic mass, causing acute retention. These authors emphasize the value of prostatic imaging by computed tomography and transrectal ultrasound as important in the diagnosis and management of this patient.
The possibility of development of pyogenic vertebral osteomyelits should be kept in mind when treating a serious genitourinary tract infection. Soda et al. (69) described a case of ostemyelitis after acute bacterial prostatitis in a 78-year-old man. The rarity and subtle clinical presentation of this condition, coupled with the delayed appearance of radiologic signs of progression to destructive osteomyelitis, contributed to a significant delay in diagnosis. Positive arterial blood culture for bacterial growth during the episode of acute prostatitis suggested that bacteremia might result from hematogenous spread of the infection to the vertebral column via the venous system.
Viral Prostatitis
There are few literature reports on viral prostatitis. Doble et al. in 1991 (16) described a case of herpes simplex virus isolated from the prostatic fluid of a patient with symptoms of prostatodynia. The demonstration of cytomegalovirus (CMV) in tissue within the male genital tract in an AIDS patient dying of disseminated CMV infection was first reported by Benson and Smith in 1992 (4). Another case report on CMV prostatitis and a review of the literature was published in 1994 by McKay et al. (41). In this case, a patient undergoing chemotherapy for multiple myeloma was found to have CMV prostatitis. The findings of a hypoechoic prostatic lesion on ultrasound and a slightly elevated prostatic specific antigen level (4.6 ng/ml) prompted a prostate biopsy. Immunohistochemical staining demonstrated CMV within the prostate. Although this virus is a common pathogen in immunosuppressed patients, its presence in the male genital tract is relatively rare.
PROSTATITIS IN BENIGN PROSTATIC HYPERPLASIA
Bedalov et al. in 1994 (3) reported the incidence of prostatitis in benign prostatic hyperplasia (BPH). These investigators concluded that patients with gram-negative prostatitis had the largest number of complications and the longest period of postoperative hospitalization. The incidence of prostatitis in their study was 90.3%. Tissue was obtained by transurethral prostatectomy. Gram-positive microorganisms were isolated in 32.8% of the tissue samples, and Staphylococcus epidermidis was found in 26.6% of the tissue specimens from BPH patients. Gram-negative microorganisms were isolated in 30.8% of tissue specimens from BPH patients, and fungi were found in 2.9%. In 27.9% of the tissue samples, a microorganism could not be isolated, although pathohistologic examination demonstrated evidence of prostatitis. Preoperative and postoperative antibiotic therapy reduced the incidence of postoperative complications and shortened the period of hospitalization of patients with significant gram-negative bacteriuria before the operation (BPH patients with gram-negative prostatitis).
UROVIRULENCE DETERMINANTS OF BACTERIA CAUSING PROSTATITIS
Although many studies on the virulence properties of Escherichia coli causing UTI have been published, there are only a few reports devoted to the virulence of strains causing prostatitis. Andreu et al. (2) in 1997 studied E. coli isolated from men with acute (7 strains) or chronic (23 strains) prostatitis and compared these strains with E. coli strains isolated from women with pyelonephritis (30 isolates), acute cystitis (60 isolates), or complicated UTI (30 isolates). Strains from prostatitis patients were significantly more likely to express hemolysin than were strains causing complicated UTI (73 and 43%, respectively; P = 0.02). Cytotoxic necrotizing factor 1 (CF-1) (using a DNA probe) was demonstrated more often in prostatitis strains than in strains obtained from women. P fimbrial expression was highest among strains from patients with pyelonephritis (73%) or prostatitis (53%) and lowest among E. coli strains from women with either complicated UTI (92%) or cystitis (30%) (P < 0.05 for strains from prostatitis patients versus either of the last two groups). These results suggest that E. coli strains causing prostatitis generally possess urovirulence properties similar to those of strains from women with acute, uncomplicated pyelonephritis and that hemolysin and CF-1 are especially prevalent in strains causing prostatitis.
ANTIBIOTIC PHARMACOKINETICS IN PROSTATITIS
Treatment failures are not uncommon in prostatitis. Nickel et al. (47) hypothesized that altered pharmacokinetics in the inflamed prostate gland might account for the treatment failure of clinically diagnosed chronic bacterial prostatitis. These investigators used a rat model of chronic bacterial prostatitis to investigate the presence of any pharmacokinetic differences that may exist between the noninflamed and inflamed prostate glands. The rat prostate glands were infected with a strain of E. coli K-235 (K1:O8:AC:H7). After 7 days of norfloxacin therapy, 60% of the animals with a well-established bacterial prostatitis were cured (36 animals per group), compared with a spontaneous cure rate of 10% in the group with nontreated prostatitis (36 animals per group). The norfloxacin levels did not change significantly between the infected and noninfected prostate glands. These investigators concluded that failure of norfloxacin therapy in chronic bacterial prostatitis may not be due to significantly altered norfloxacin pharmacokinetics in the chronically inflamed prostate gland but, rather, may have been due to the difficulty in eradicating bacteria protected within infection-induced microcolonies and biofilms (40, 49, 50). Nickel et al. (48, 51–53) have previously implicated microcolonies and biofilms in animal models of chronic prostatitis and demonstrated the similarities to chronic prostatitis in humans.
While it is always difficult to definitively extrapolate from animal models, it is the contention of these researchers that failure of antibiotic therapy of chronic prostatitis is probably a result of the local microenvironment surrounding the focal and protected small bacterial biofilms buried within the prostate gland. Their conclusions support the more recent molecular and cultural data implicating bacteria as a cause of chronic idiopathic prostatitis.
IS NONBACTERIAL PROSTATITIS CAUSED BY A CHEMICAL INFLAMMATORY REACTION IN THE PROSTATE?
Persson and Ronquist (56) studied the EPS of 56 patients with nonbacterial prostatitis to find whether urine reflux in the prostatic ducts was responsible for increased concentrations of creatinine, urate, and WBC. They reported a relationship between pain, estimated in accordance with a scoring scale, and WBC in EPS, urate, and creatinine. These results provide further support for the role of reflux into the prostatic ducts, which serves as an underlying mechanism initiating a chemical inflammatory reaction; urate appears to be the chemical agent eliciting this inflammatory response.
Although currently available data strongly suggest a bacterial association with chronic idiopathic prostatitis, the role of endogeneous chemicals should not be overlooked. It is important to design well-controlled studies that would provide information on whether some cases of chronic idiopathic prostatitis are caused by microorganisms that result from the overuse of antibiotics due to selective pressure and development of resistance. Persson and Ronquist (57) also reported that allopurinol treatment resulted in elevated prostate-specific antigen levels in prostatic fluid and serum of patients with nonbacterial prostatitis. These authors speculated that the allopurinol-induced release might be explained by an induction of prostate-specific antigen synthesis via an effect of allopurinol on the cellular genome. However, they could not rule out an increased leakage of the prostatic cells elicited by allopurinol.
IS CHRONIC IDIOPATHIC PROSTATITIS AN AUTOIMMUNE DISEASE?
Keetch et al. (28) have suggested that nonbacterial prostatitis may be an autoimmune process. They developed a mouse model for nonbacterial prostatitis and characterized the immune system parameters of nonbacterial prostatitis. Prostates from SJL, AJ, BALB/c, C57bL/6 1pr mice were harvested, homogenized, and injected into syngeneic mice. Controls were injected with Freund’s complete adjuvant only. Mice from each group were sacrificed 30 days after injection, and the prostates were harvested. Prostatic tissue was examined histologically for the degree of inflammation. None of the BALB/c mice had prostatic inflammation. The SJL and AJ mice showed different degrees of prostatic inflammation. All of the C57bL/6 1pr mice were found to have lymphocytic infiltration of the stroma and periglandular region. These mice did not appear to be more susceptible than the parental strain. Adoptive transfer studies demonstrated that the prostatic inflammation was at least in part immune system mediated. These researchers concluded that injection of syngenic prostate antigen induces prostatic inflammation similar to clinical nonbacterial prostatitis. In 1997, Correa et al. (14) reported on a model of autoimmune prostatitis induced by intraperitoneal administration of saline extract of rat male accessory glands (RAG) associated with liposomes. The intraperitoneal administration of RAG-liposomes elicits both primary and secondary cellular autoimmune responses to RAG as well as organ-specific lesions. To evaluate the participation of dendritic cells (DC) in the induction of the autoimmune response, they purified peritoneal DC (PDC) after a single injection of RAG-liposomes and characterized this population by morphology and phenotype. Based on adherence and morphologic criteria, they determined that PDC comprised approximately 1% of the total peritoneal cells. The ultrastructure of the DC-enriched fraction was assessed by electron microscopy. By fluorescence-activated cell sorter analysis, PDC showed a two- to threefold increase in expression of the IAS molecule compared to that in macrophages. These cells expressed low but positive levels of the CD14 marker and intermediate levels of both CD11b (Mac-1) and CD54 (ICAM-1) adhesion molecules. Additionally, PDC transferred either intravenously or intraperitoneally efficiently caused the autoimmune response to RAG in normal receptors. The investigators concluded that their results support the involvement of PDC in the induction of autoimmune prostatitis, modifying the idea of macrophages as the single antigen-presenting cells in the peritoneal cavity. These provocative data warrant further exploration, in particular the complete characterization of the antigen(s) that may be involved in eliciting an autoimmune response and investigation of whether there is any relationship to human chronic idiopathic prostatitis.
CONCLUSIONS AND REMAINING PROBLEMS
Reports published within the past 2 years strongly suggest an association between bacteria and chronic idiopathic prostatitis. Both molecular and specialized culture findings designed to detect fastidious and difficult-to-culture bacteria in prostatic tissue and fluids point to a possible etiologic role for these microorganisms. The molecular data were particularly significant because prostate biopsy specimens were obtained for a population of men who could not be diagnosed by optimal clinical and microbiologic methods. Therefore, potential study subjects were excluded if they exhibited bacteriuria, bacterial prostatitis, or urethritis or if they had a urethral culture that was positive for urogenital pathogens. The most convincing finding is the strong correlation between inflammation in the expressed prostatic secretions and detection of 16S rRNA genes in prostatic tissue (P < 0.001). It is unlikely that the demonstrated molecular and cultural evidence represents contamination, because of the extreme care taken in procuring and handling the clinical specimens, including the use of a double-needle biopsy method to limit skin contamination and positive and negative controls incorporated in the molecular experiments as well as an internal housekeeping gene control. It will be important to classify the sequences of the isolated organism at the genus and species level, since this has not yet been accomplished for the amplified bacterial nucleic acids derived from prostate tissue.
Future studies should be directed toward more nucleic acid-based experimentation to define the microbiology of the prostate gland and to determine the relationship of these bacteria to chronic idiopathic prostatitis. Once the etiology is known, a logical next step would be to devise methods for delivery of antimicrobial or immune reagents which might help eliminate the foci of infection in prostatic tissue. There is an urgent need to better understand the virulence properties of bacteria that are associated with chronic infection of the prostate. Identifying such a factor(s) would be helpful in devising effective treatment strategies. It is important to determine whether there is persistence of bacterial antigens in prostatic tissue and fluids, since these antigens could trigger immunologic and biochemical events that may result in initiation and maintenance of chronic inflammation in this troublesome condition. For those ascribing to an autoimmune theory, it will be necessary to identify the antigen(s) in human idiopathic prostatitis that initiates immune system pathologic changes and to rule out the possibility that this antigen(s) is not derived from microbes.
REFERENCES
- 1.Abdelatif O M, Chandler F W, McGuire B S J. Chlamydia trachomatis in chronic abacterial prostatitis: demonstration of calorimetric in situ hybridization. Hum Pathol. 1991;22:41–44. doi: 10.1016/0046-8177(91)90059-x. [DOI] [PubMed] [Google Scholar]
- 2.Andreu A, Stapleton A E, Fennell C, Lockman H A, Xercavins M, Fernandez F, Stamm W E. Urovirulence determinants in Escherichia coli strains causing prostatitis. J Infect Dis. 1997;176:464–469. doi: 10.1086/514065. [DOI] [PubMed] [Google Scholar]
- 3.Bedalov G, Vuckovic I, Fridrih S, Bruk M, Puskar D, Bartolin Z. Prostatitis in benign prostatic hyperplasia: a histological, bacteriological and clinical study. Acta Med Croat. 1994;48:105–109. [PubMed] [Google Scholar]
- 4.Benson P J, Smith C S. Cytomegalovirus prostatitis. Urology. 1992;40:165–167. doi: 10.1016/0090-4295(92)90520-7. [DOI] [PubMed] [Google Scholar]
- 5.Berger R E, Krieger J N, Rothman I, Muller C H, Hillier S L. Bacteria in the prostate tissue of men with idiopathic prostatic inflammation. J Urol. 1997;157:863–865. [PubMed] [Google Scholar]
- 6.Berger R E, Kreiger J N, Kessler D, Ireton R C, Close C, Holmes K K, Roberts P L. Case-control study of men with suspected chronic idiopathic prostatitis. J Urol. 1989;141:328–331. doi: 10.1016/s0022-5347(17)40757-9. [DOI] [PubMed] [Google Scholar]
- 7.Bowie W. Urethritis in males. In: Holmes K, Mardh P S, Welsner P P, Cates W, Lemon S, Stamm W, editors. Sexually transmitted disease. 2nd ed. New York, N.Y: McGraw-Hill Book Co.; 1990. pp. 627–640. [Google Scholar]
- 8.Bruce A W, Chadwick O, Willett W S, O’Shaughnessy M. The role of chlamydia in genitourinary disease. J Urol. 1981;126:625–629. doi: 10.1016/s0022-5347(17)54660-1. [DOI] [PubMed] [Google Scholar]
- 9.Bruce A W, Reid G. Prostatitis associated with Chlamydia trachomatis in 6 patients. J Urol. 1989;142:1006–1007. doi: 10.1016/s0022-5347(17)38970-x. [DOI] [PubMed] [Google Scholar]
- 10.Brunner H, Weidner W, Schiefer H G. Studies on the role of Ureaplasma urealyticum and Mycoplasma hominis in prostatitis. J Infect Dis. 1983;147:807–813. doi: 10.1093/infdis/147.5.807. [DOI] [PubMed] [Google Scholar]
- 11.Cherasse A, Herin M, Oana M, Marievoet C. Aspergillus prostatitis and prolonged corticotherapy. Apropos of a case report. Acta Urol Belg. 1997;65:43–48. [PubMed] [Google Scholar]
- 12.Chiarini F, Mansi P, Tamao V, Gentile F, De-Marco F, Brunori S, Wongher L, Di-Silverio F. Chlamydia trachomatis genitourinary infections: laboratory diagnosis and therapeutic aspects. Evaluation of in vitro and in vivo effectiveness of azithromycin. J Chemother. 1994;6:238–242. doi: 10.1080/1120009x.1994.11741158. [DOI] [PubMed] [Google Scholar]
- 13.Colleen S, Mardh P. Effect of metacycline treatment on non-acute prostatitis. Scand J Urol Nephrol. 1975;9:198–204. doi: 10.3109/00365597509134211. [DOI] [PubMed] [Google Scholar]
- 14.Correa S G, Riera D M, Iribarren P. Involvement of peritoneal dendritic cells in the induction of autoimmune prostatitis. J Autoimmun. 1997;10:107–113. doi: 10.1006/jaut.1996.0118. [DOI] [PubMed] [Google Scholar]
- 15.Danielson D, Molin L. Demonstration of N. gonorrhea in prostatic fluid after treatment of uncomplicated gonorrheal urethritis. Acta Dermatol Venereol. 1971;51:73. [PubMed] [Google Scholar]
- 16.Doble A, Harris J R W, Taylor-Robinson D. Prostatodynia and Herpes simplex virus infection. Urology. 1991;38:247–248. doi: 10.1016/s0090-4295(91)80355-b. [DOI] [PubMed] [Google Scholar]
- 17.Doble A, Thomas B J, Walker M M, Harris J R, Witherow R O, Taylor R D. The role of Chlamydia trachomatis in chronic abacterial prostatitis: a study using ultrasound guided biopsy. J Urol. 1989;141:332–333. doi: 10.1016/s0022-5347(17)40758-0. [DOI] [PubMed] [Google Scholar]
- 18.Domingue G J, Human L G, Hellstrom W J G. Hidden microorganisms in “abacterial” prostatitis/prostatodynia. J Urol. 1997;157:243. [Google Scholar]
- 19.Domingue G J, Ghonieum G M, Bost K, Human L. Dormant bacteria in interstitial cystitis. J Urol. 1995;153:1321–1326. [PubMed] [Google Scholar]
- 20.Domingue G J, Sr, Woody H B. Bacterial persistence and expression of disease. Clin Microbiol Rev. 1997;10:320–344. doi: 10.1128/cmr.10.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fair W R, Couch J, Wehner N. Prostatic antibacterial factor. Identity and significance. Urology. 1976;7:169–177. doi: 10.1016/0090-4295(76)90305-8. [DOI] [PubMed] [Google Scholar]
- 22.Fowler J E., Jr . Prostatitis. In: Gillenwater J Y, Grayhack J T, Howards S S, Duckett J W, editors. Adult and pediatric urology. 2nd ed. St. Louis, Mo: Mosby—Year Book; 1991. pp. 1395–1423. [Google Scholar]
- 23.Fuse H, Ohkawa M, Yamaguchi K, Hirata A, Matsubara F. Cryptococcal prostatitis in a patient with Behcet’s disease treated with fuconazole. Mycopathologia. 1995;130:147–150. doi: 10.1007/BF01103097. [DOI] [PubMed] [Google Scholar]
- 24.Galbis S F, Jimenez C M, Rodriguez-Rodriguez R, Perez-Elias M J, Gomez do Santos V, Rivas-Escudero J A. Tuberculous prostatic abscess in acquired immunodeficiency syndrome. Arch Esp Urol. 1997;50:393–395. [PubMed] [Google Scholar]
- 25.Gardner W, Jr, Culberson D, Bennett B. Trichomonas vaginalis in the prostate gland. Arch. 1994, Pathol. Lab Med. 1986;110:430–432. [PubMed] [Google Scholar]
- 26.Gunn B A, Davis C D E., Jr Staphylococcus haemolyticus urinary tract infection in a male patient. J Clin Microbiol. 1988;26:1055–1057. doi: 10.1128/jcm.26.5.1055-1057.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadar A, Bucsek M, Kardos M, Corradi G. Detection of Chlamydia trachomatis in chronic prostatitis by in situ hybridization (preliminary methodical report) Orv Hetil. 1995;136:659–662. [PubMed] [Google Scholar]
- 28.Keetch D W, Humphrey P, Ratliff T L. Development of a mouse model for nonbacterial prostatitis. J Urol. 1994;152:247–250. doi: 10.1016/s0022-5347(17)32871-9. [DOI] [PubMed] [Google Scholar]
- 29.Kloos W E, Bannerman T L. Update on clinical significance of coagulase-negative staphylococci. Clin Microbiol Rev. 1994;7:117–140. doi: 10.1128/cmr.7.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koroku M, Kumamoto Y, Hirose T. A study of the role of Chlamydia trachomatis in chronic prostatitis—analysis of anti-Chlamydia trachomatis specific IgA in expressed prostate secretion by western-blotting method. Kansenshogaku Zasshi. 1995;69:426–437. doi: 10.11150/kansenshogakuzasshi1970.69.426. [DOI] [PubMed] [Google Scholar]
- 31.Krieger J N, Egan K J, Ross S O, Jacobs R, Berger R E. Chronic pelvic pains represent the most prominent urogenital symptoms of “chronic prostatitis.”. Urology. 1996;48:715–721. doi: 10.1016/S0090-4295(96)00421-9. [DOI] [PubMed] [Google Scholar]
- 32.Krieger J N, Riley D E, Roberts M C, Berger R E. Prokaryotic DNA sequences in patients with chronic idiopathic prostatitis. J Clin Microbiol. 1996;34:3120–3128. doi: 10.1128/jcm.34.12.3120-3128.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krieger J N. Prostatitis, epididymitis, and orchitis. In: Mandell G L, Bennett J E, Dolin R, editors. Mandell, Douglas and Bennett’s principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 1098–1102. [Google Scholar]
- 34.Krieger J N, Rein M F. Zinc sensitivity of Trichomonas vaginalis: in vitro studies and clinical implications. J Infect Dis. 1982;146:341–345. doi: 10.1093/infdis/146.3.341. [DOI] [PubMed] [Google Scholar]
- 35.Kuberski T. Trichomonas vaginalis associated with nongonococcal urethritis and prostatitis. Sex Transm Dis. 1980;7:135–136. doi: 10.1097/00007435-198007000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Kurnatowska A, Kurnatowski A, Mazurek L, Wedzikowski P. Rare cases of prostatitis caused by invasion of Trichomonas vaginalis with Candida albicans. Wiad Parazytol. 1990;36:229–236. [PubMed] [Google Scholar]
- 37.LaFontaine P D, Middleman B R, Graham S D, Jr, Sanders W H. Incidence of granulomatous prostatitis and acid-fast bacilli after intravesical BCG therapy. Urology. 1997;49:363–369. doi: 10.1016/s0090-4295(96)00507-9. [DOI] [PubMed] [Google Scholar]
- 38.Lowentritt J E, Kawahara K, Human L G, Hellstrom W J G, Domingue G J. Bacterial infection in prostatodynia. J Urol. 1995;154:1378–1381. [PubMed] [Google Scholar]
- 39.Ludwig M, Hausmann G, Hausmann W, Scriba M, Zimmerman O, Fischer D, Thiele D, Weidner W. Chlamydia trachomatis antibodies in serum and ejaculate of male patients without acute urethritis. Ann Urol. 1996;30:139–146. [PubMed] [Google Scholar]
- 40.Mardh P-A, Colleen S, Hovelius B. Attachment of bacteria to exfoliated cells from the urogenital tract. Investig Urol. 1979;16:322–326. [PubMed] [Google Scholar]
- 41.McKay T C, Albala D M, Sendelbach K, Gattuso P. Cytomegalovirus prostatitis. Case report and review of the literature. Int Urol Nephrol. 1994;26:535–540. doi: 10.1007/BF02767655. [DOI] [PubMed] [Google Scholar]
- 42.Meares E M., Jr Prostatitis syndromes: new perspectives about old woes. J Urol. 1980;123:141–147. doi: 10.1016/s0022-5347(17)55828-0. [DOI] [PubMed] [Google Scholar]
- 43.Meares E M., Jr . Prostatitis and related disorders. In: Walsh P C, Retik A B, Vaughan E D Jr, Wien A J, editors. Campbell’s urology. Philadelphia, Pa: The W. B. Saunders Co.; 1997. pp. 615–630. [Google Scholar]
- 44.Meares E M, Jr, Stamey T A. Bacteriologic localization patterns in bacterial prostatitis and urethritis. Invest Urol. 1968;5:492–518. [PubMed] [Google Scholar]
- 45.Ndimbie O K, Dekker A, Martinez A J, Dixon B. Prostatic sequestration of Cryptococcus neoformans in immunocompromised persons treated for cryptococcal meningoencephalitis. Histol Histopathol. 1994;9:643–648. [PubMed] [Google Scholar]
- 46.Nickel J C. The pre and post massage test (PPMT): a simple screen for prostatitis. Tech Urol. 1997;3:38–43. [PubMed] [Google Scholar]
- 47.Nickel J C, Downey J, Clark J, Ceri H, Olson M. Antibiotic pharmacokinetics in the inflamed prostate. J Urol. 1995;153:527–529. doi: 10.1097/00005392-199502000-00076. [DOI] [PubMed] [Google Scholar]
- 48.Nickel J C, Costerton J W. Bacterial localization in antibiotic-refractory chronic bacterial prostatitis. Prostate. 1993;23:107–114. doi: 10.1002/pros.2990230204. [DOI] [PubMed] [Google Scholar]
- 49.Nickel J C, Olson E, Barabas A, Benediktsson H, Dasgupta M K, Costerton J W. Pathogenesis of chronic bacterial prostatitis in an animal model. Br J Urol. 1990;66:47–54. doi: 10.1111/j.1464-410x.1990.tb14864.x. [DOI] [PubMed] [Google Scholar]
- 50.Nickel, J. C., M. E. Olson, and J. W. Costerton. 1991. Rat model of experimental prostatitis. Infection 19(Suppl. 3):126–130. [DOI] [PubMed]
- 51.Nickel J C. Experimental prostatitis. In: Weidner W, Madsen P O, Schiefer H G, editors. Prostatitis: etiopathology, diagnosis and therapy. Berlin, Germany: Springer-Verlag KG; 1994. pp. 123–132. [Google Scholar]
- 52.Nickel J C, Bruce A W, Reid G. Pathogenesis, diagnosis and treatment of the prostatitis syndrome. In: Krane R J, Siroky M B, Fitzpatrick J M, editors. Clinical urology. J. B. Philadelphia, Pa: Lippincott Co.; 1994. pp. 925–938. [Google Scholar]
- 53.Nickel J C, Costerton J W. Coagulase-negative staphylococcus in chronic prostatitis. J Urol. 1992;147:398–400. doi: 10.1016/s0022-5347(17)37247-6. [DOI] [PubMed] [Google Scholar]
- 54.Oppenheimer J R, Kahane H, Epstein J I. Granulomatous prostatitis on needle biopsy. Arch Pathol Lab Med. 1997;121:724–729. [PubMed] [Google Scholar]
- 55.Paulsson M, Ljungh A, Wadstrom T. Rapid identification of fibronectin, vitronectin, laminin, and collagen cell surface binding proteins on coagulase-negative staphylococci by particle agglutination assays. J Clin Microbiol. 1992;30:2006–2012. doi: 10.1128/jcm.30.8.2006-2012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Persson B E, Ronquist G. Evidence for a mechanistic association between nonbacterial prostatitis and levels of urate and creatinine in expressed prostatic secretion. J Urol. 1996;155:958–960. [PubMed] [Google Scholar]
- 57.Persson B E, Ronquist G. Allopurinol treatment results in elevated prostate-specific antigen levels in prostatic fluid and serum of patients with non-bacterial prostatitis. Eur Urol. 1996;29:111–114. doi: 10.1159/000473728. [DOI] [PubMed] [Google Scholar]
- 58.Relman D A, Loutit J S, Schmidt T M, Falkow S, Tompkins L S. The agent of bacillary angiomatosis. N Engl J Med. 1990;323:1573–1580. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- 59.Relman D A, Schmidt T M, MacDermott R P, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med. 1992;327:293–301. doi: 10.1056/NEJM199207303270501. [DOI] [PubMed] [Google Scholar]
- 60.Riegel P, Ruimy R, De Briel D, Prevost G, Jehl F, Bimet F, Christen R, Monteil H. Corynebacterium seminale sp. nov., a new species associated with genital infections in male patients. J Clin Microbiol. 1995;33:2244–2249. doi: 10.1128/jcm.33.9.2244-2249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rivas-Escudero J A. Tuberculous prostatic abscess in acquired immunodeficiency syndrome. Arch Esp Urol. 1997;50:393–395. [PubMed] [Google Scholar]
- 62.Sanchis-Bayarri V V, Sanchez Sanchez R, Marcaida B G, Sanchis-Bayarri B V. Staphylococcus haemolyticus study in urinary infections. An analysis of 8 cases. Rev Clin Esp. 1992;190:443–446. [PubMed] [Google Scholar]
- 63.Sargent J C, Irwin R. Prostatic abscess: clinical study of 42 cases. Am J Surg. 1931;11:334–337. [Google Scholar]
- 64.Sevillano-Guida C, Arevalo-Velasco J M, Orgaz-Espuela R, Nogeuras-Gimeno M A, Martinez-Perez E, Perez-Arbgez J A, Arnaiz-Esteban F. Abscessed brucellar prostatitis. An infrequent location. Actas Urol Esp. 1995;19:647–650. [PubMed] [Google Scholar]
- 65.Shokeir A A, Dwaba M, Abdel-Gawad M, el-Azab M, Shokeir M A. Prostatic abscess in a child. Scand J Urol Nephrol. 1995;29:525–526. doi: 10.3109/00365599509180040. [DOI] [PubMed] [Google Scholar]
- 66.Shortliffe L K M, Sellers R G, Schachter J. The characterization of nonbacterial prostatitis: search for an etiology. J Urol. 1992;148:1461–1466. doi: 10.1016/s0022-5347(17)36940-9. [DOI] [PubMed] [Google Scholar]
- 67.Shurbaji M S, Gupta P K, Myers J. Immunohistochemical demonstration of chlamydial antigens in association with prostatitis. Mod Pathol. 1988;1:348–351. [PubMed] [Google Scholar]
- 68.Smith J H, von Lichtenberg F. Parasitic diseases of the genitourinary system. In: Walsh P C, Retik A B, Vaughan E D Jr, Wein A J, editors. Campbell’s urology. Philadelphia, Pa: The W. B. Saunders Co.; 1997. pp. 733–778. [Google Scholar]
- 69.Soda T, Ogura K, Ishitoya S, Niibayashi H, Yoshida O. Pyogenic vertebral osteomyelitis after acute bacterial prostatitis: a case report. Int J Urol. 1996;3:402–404. doi: 10.1111/j.1442-2042.1996.tb00564.x. [DOI] [PubMed] [Google Scholar]
- 70.Stamey T. Pathogenesis and treatment of urinary tract infections. Baltimore, Md: The Williams & Wilkins Co.; 1980. pp. 342–429. [Google Scholar]
- 71.Teng K, Li M, Yu W, Li H, Shen D, Liu D. Comparison of PCR with culture for detection of Ureaplasma urealyticum in clinical samples from patients with urogenital infections. J Clin Microbiol. 1994;32:2232–2234. doi: 10.1128/jcm.32.9.2232-2234.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wedren H, Holm S E, Bergman B. Can decreased phagocytosis and killing of autologous gram-positive bacteria explain the finding of gram-positive bacteria in “non-bacterial prostatitis?”. Acta Pathol Microbiol Immunol Scand Sect B. 1987;95:75–78. doi: 10.1111/j.1699-0463.1987.tb03089.x. [DOI] [PubMed] [Google Scholar]
- 73.Weidner W, Brunner H, Krause W. Quantitative culture of Ureaplasma urealyticum in patients with chronic prostatitis or prostatosis. J Urol. 1980;124:622–625. doi: 10.1016/s0022-5347(17)55586-x. [DOI] [PubMed] [Google Scholar]