Abstract
Study Objectives:
Examine the effects of three days of sleep restriction on maximal jump performance, joint coordination variability, and psychomotor response time in elite athletes.
Methods:
Eleven elite athletes obtained a one-week baseline of habitual sleep. Participants were then restricted to four hours of sleep per night for three nights and was measured through both self-report and actigraphy. Pre and post-intervention measures were a box drop maximal vertical jump with 3D motion capture to assess physical performance and biomechanical changes in movement patterns, and the Psychomotor Vigilance Task (PVT) assessed changes in response time. Associations between biomechanical, physical, and cognitive performance measures were assessed.
Results:
Participants restricted reported total sleep time from 7.4 ± 0.5 hours/night at baseline to 4.0 ± 0.2 hours/night and actigraphy indicated sleep was restricted from 6.7 ± 0.7 hours/night to 3.7 ± 0.2 hours/night. Following sleep restriction, maximal jump height decreased (1.39 ± 0.10 vs. 1.37 ± 0.10 m, p = 0.03). Hip sagittal/knee frontal (Δ15.5°, p = 0.04) and hip frontal/knee frontal (Δ11.0°, p < 0.01) plane coordination variability increased after sleep restriction. Hip sagittal/knee frontal plane coordination variability after sleep restriction was associated with increasingly slower PVT response time (P = 0.63, p = 0.03).
Conclusions:
Sleep restriction for three days decreased maximal jump performance. Sleep restriction increased coordination variability and was associated with greater impairment in psychomotor response time in elite athletes.
Keywords: athlete, biomechanics, drop vertical jump, performance, sports, sleep
INTRODUCTION
Physical performance requires the complex interaction between cognitive and psychological outputs and motor control 1. Joint biomechanics describes these patterns of movement and motor control. Chronic sleep loss and circadian factors including jet lag have been shown to impair these cognitive and sensory motor outputs 2–4. Prior studies have demonstrated that sleep restriction impairs cognitive attention, psychomotor response time, and physical performance outcomes 2, 5. No studies, however, have examined the effects of sleep restriction or deprivation on lower extremity joint mechanics. The only prior studies related to biomechanics have been limited to examining individuals with insomnia and effects of hypnotic drugs on balance and postural stability due to risk of falls 6. The impact of chronic sleep loss on common movements in sports such as a vertical jump and the biomechanics during this dynamic movement are unknown.
The sensorimotor system determines the motor patterns required to successfully achieve a dynamic task even under a non-optimal condition or state 7. Lower extremity joint coordination variability is a quantitative method of assessing the sensorimotor system’s ability to accomplish a particular goal by either increasing or decreasing the use of a degree of freedom (i.e. muscle, joint) 8, 9. Vector coding, a method of assessing joint position data, has been used to assess lower extremity joint coordination variability during sidestep cutting 10–12, running 13, 14, walking 13, 15–19, and free-throw shooting 20. Vector coding provides an understanding of the underlying motor strategies required to successfully perform these dynamic tasks. Vector coding has been demonstrated to be a more sensitive measure to assess subtle changes in motion patterns during dynamic activity compared to traditional kinematic analyses 15, 19. To our knowledge, no other studies have examined traditional kinematic analyses or vector coding on dynamic movements following sleep loss. Additionally, the maximal vertical jump is a dynamic task that has been studied extensively to examine the neuromuscular system and associated mechanics 21, 22. Understanding how sleep restriction affects lower extremity biomechanics during a dynamic task may provide insights of the effects on neuromuscular coordination and ultimately how movement patterns are possibly altered in physical performance which may have significant implications for injury risk.
The aim of the present study is to examine the effects of consecutive days of sleep restriction on physical performance and dynamic movement via maximal vertical jump, lower extremity joint coordination variability, and psychomotor response time in elite athletes. Elite athletes are a homogenous population where peak cognitive and physical performance outputs are critical and are more sensitive to sleep loss. It is hypothesized that athletes will demonstrate impaired physical and cognitive performance after consecutive days of sleep restriction. This novel, exploratory study may provide initial understanding of the relationships between chronic sleep loss, changes in performance outcomes, and impact on biomechanical movement patterns.
METHODS
Participants
Participants were recruited from the local community of elite cyclists and competitive cycling teams. Cyclists were included in the study if they were healthy, male, 18–35 years, and classified as Professional/Category 1–3. USA cycling categorizes athletes 1–5 with Category 1 considered the most elite and experienced athletes. Participants were excluded if they reported a prior history of a sleep or psychiatric disorder, significant sleep or circadian issues, or regular use of sleep aids/supplements with sleep-related side effects. Athletes were screened for sleep apnea with STOP-BANG 23. The UCSF Committee on Human Research approved the study and written informed consent was obtained from all participants.
Study Design
This study was a subset of a larger cross-over study examining two independent sleep duration interventions and the present study reports on sleep restriction findings. Participants completed a one-week baseline of habitual sleep in their home environment maintaining their habitual sleep/wake schedule between five to eight hours of sleep/night. Participants were randomized to 1) sleep restriction of four hours time in bed for three days or 2) sleep extension for two weeks. A two-week washout period of habitual sleep was completed between conditions. During sleep restriction, participants were instructed to advance bedtime and maintain habitual wake time. Participants were not allowed daytime naps. Caffeine was standardized for amount and timing prior to testing sessions. Participants refrained from alcohol 24 hours prior to testing sessions.
Sleep/Wake Activity
Participants wore wrist actigraphy (AW-64, Philips Respironics, Andover, MA) to monitor sleep/wake activity on the wrist of the non-dominant hand 24 hours/day with the exception of bathing and training. Sleep data was recorded as 1-minute epoch length and scored by a validated proprietary algorithm within the commercial software (Actiware software, Philips, Respironics, Andover, MA). Participants also recorded daily sleep journals.
Physical and Biomechanical Data Acquisition and Processing
Three-dimensional marker position data were collected at 250 Hz using a 10-camera motion capture system (Vicon, Oxford, UK). Ground reaction force (GRF) data were collected synchronously at 1000 Hz using two in-ground force plates (AMTI, Watertown, MA). A previously-described marker set consisting of 44 retro-reflective markers was used to collect three-dimensional position data. Calibration markers were placed bilaterally at the greater trochanters, medial and lateral femoral epicondyles, medial and lateral malleoli, and first and fifth metatarsal heads. Pelvic tracking was performed using retro-reflective markers placed at the anterior superior iliac spines, iliac crests, and L5/S1 joint (Figure 1). Torso tracking was performed with markers placed bilaterally at the acromioclavicular joint and a marker at the seventh cervical vertebra. Segment tracking was performed using rigid body clusters, each consisting of four markers, and placed at the lateral thighs and shanks, respectively. In addition, tracking of the foot was conducted with clusters consisting of three markers each placed on the heel and a marker at the fifth metatarsal head. A 1-second static calibration trial was obtained and then all calibration markers were removed except at the fifth metatarsal heads.
Figure 1.
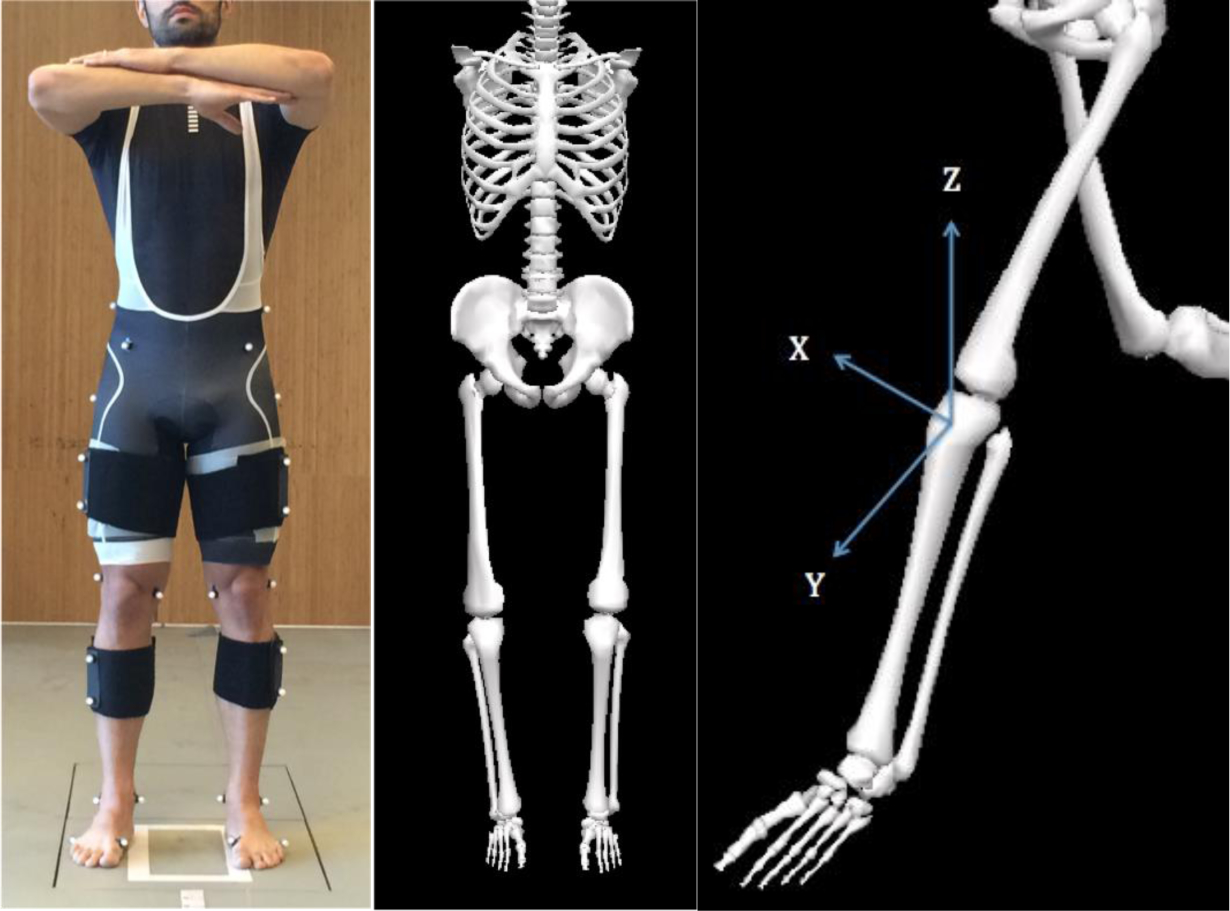
Participant with static calibration markers and segment tracking clusters (left), the corresponding musculoskeletal model created using these marker position data (center), and labeling of X-Y-Z axes on participant’s left knee joint (right).
Each participant was asked to perform four successful drop maximal vertical jumps (DVJ) from a 30-cm box height. Participants were instructed to drop from the box by stepping off, land with one foot on each of the two force platforms simultaneously, and immediately perform a maximal effort jump. Participants were allowed to perform 1–2 warmup trials prior to data collection to acclimate themselves with the drop jump task. Trials were discarded and repeated if the participant 1) landed with an asymmetrical foot strike, 2) landed with part of their foot off of the force platform surface, or 3) jumped from the top of the box rather than stepping off.
All raw marker position data were labeled and gap filled using Nexus (v1.8.5, Vicon, Oxford, UK). The static calibration trial was used to form an 8-segment musculoskeletal model consisting of the trunk, pelvis, bilateral femurs, shanks, and feet using Visual3D (v5.00.16, C-Motion, Germantown, MD). All raw marker position and GRF data were filtered using a fourth-order Butterworth filter at 12 Hz 24. Local joint coordinate systems were created and an unweighted least squares method was used to describe segment position and orientation 25. Joint coordinates were solved using a Cardan sequence of X-Y’-Z”, representing the medial-lateral, anterior-posterior, and superior-inferior directions, respectively. Joint angles were normalized to the standing calibration trial. The stance phase of the DVJ was defined as the first 50 milliseconds (ms) after the feet struck the force plates and the vertical GRF exceeded a 20 Newton threshold. This time frame was chosen as peak knee joint loading occurs during the first 40 – 60 ms after initial contact in a drop landing 26. Hip and knee joint kinematic data during the stance phase were time normalized to 101 points (0 – 100%).
Biomechanical Data Analysis
The biomechanical analyses performed in this study involved the assessment of maximal vertical jump height and lower extremity joint coordination variability during the DVJ. Maximal vertical jump height was calculated as the maximal displacement of the model center of mass in the vertical direction after both feet left the force platforms. A vector coding algorithm 14, 27 was used to analyze hip and knee joint coordination patterns as an effect of sleep loss during the DVJ. The vector coding algorithm provides a quantitative measurement of the coordination patterns between the hip and knee joints. The joint coupling angle (degrees) is described as the angle from the horizontal axis of a vector that connects two successive time points of the stance phase and was repeated for each trial of the DVJ task. The mean joint coupling angle was calculated for each participant. The average standard deviation of each joint coupling angle was determined and used as a measure of the between trial, within participant variability 14. This process was repeated for each participant during the stance phase of the DVJ. The joint couplings analyzed in the current study included the hip sagittal/knee frontal planes and hip frontal/knee frontal planes. These couplings were selected based upon previous work that analyzed lower extremity kinematics during the DVJ 28–31.
Psychomotor Vigilance Task
The Psychomotor Vigilance Task (PVT) was assessed pre and post-intervention. The PVT (Joggle Research) is a 10-minute test that was used to assess changes in response time. Participants were familiarized with a practice session prior to baseline testing. Time of day was standardized within the same two-hour period for each participant.
DATA ANALYSIS
Descriptive statistics are provided as mean ± standard deviation unless otherwise indicated. One-tailed paired t-tests were performed to examine differences in post-intervention sleep/wake activity, physical performance, biomechanics, and PVT. In addition, one-tailed Pearson correlation coefficients (P) were used to assess for associations between cognitive and physical performance as well as hip and knee joint coordination variability. As this is an exploratory study, outcomes greater than two standard deviations of the mean were considered outliers and excluded from analysis. A p-value of < 0.05 was considered statistically significant. Effect sizes were calculated using Hedges’ g 32, 33. All analyses were conducted using SPSS Version 23 (IBM Corporation; Armonk, NY, USA).
RESULTS
Participants
Eleven athletes (mean age 28.8 ± 4.5 years) participated in the study during their off-season and during a period when their training would be consistent (Table 1). Participants were low risk for sleep apnea on STOP-BANG.
Table 1.
Participant demographics for all 11 athletes are presented as mean ± standard deviation; BMI (body mass index).
| Age (years) | 28.8 ± 4.51 |
| Height (m) | 1.81 ± 0.09 |
| Mass (kg) | 74.6 ± 6.65 |
| BMI (kg/m−2) | 22.7 ± 1.64 |
Sleep/Wake Activity
Reported total sleep time decreased from 7.4 ± 0.5 hours/night at baseline to 4.0 ± 0.2 hours/night following sleep restriction. Actigraphy indicated sleep was restricted from 6.7 ± 0.7 hours/night to 3.7 ± 0.2 hours/night (Table 2).
Table 2.
Sleep journal and actigraphy sleep/wake activity. Data are presented as mean ± standard deviation.
| Baseline | Sleep Restriction | p-value | |
|---|---|---|---|
| Sleep Journal | |||
| Total Sleep Time (min) | 442 ± 31 | 240 ± 15* | < 0.001 |
| Actigraphy | |||
| Total Sleep Time (min) | 402 ± 43 | 221 ± 12* | < 0.001 |
| Sleep Onset (min) | 3.8 ± 3.7 | 2.9 ± 3.1 | 0.39 |
| Sleep Efficiency (%) | 87.5 ± 4.2 | 88.4 ± 3.8 | 0.32 |
| Wake After Sleep Onset (min) | 41.7 ± 14.4 | 19.1 ± 7.9* | < 0.001 |
indicates p < 0.001.
Physical, Biomechanical, and Cognitive Performance
Maximal vertical jump height decreased following sleep restriction (Baseline: 1.39 ± 0.09 m, Restriction: 1.37 ± 0.10 m, p = 0.01, g = 0.19) (Figure 2). Athletes demonstrated significantly increased hip sagittal/knee frontal plane (Baseline: 70.1 ± 39.3°, Restriction: 85.7 ± 36.1°, p = 0.04, g = 0.40) and hip frontal/knee frontal plane (Baseline: 41.2 ± 21.9°, Restriction: 52.2 ± 25.6°, p < 0.01, g = 0.44) joint coordination variability during the DVJ (Table 3). One athlete’s PVT measures were removed from the analysis as these PVT measures were greater than two times the standard deviation and considered an outlier. Average change in PVT mean response time (RT) following sleep restriction was 42.2 ± 66.5 ms slower (Baseline: 278.7 ± 28.3 ms, Restriction: 320.8 ± 63.5 ms, p = 0.04, g = 0.82). After sleep restriction, athletes exhibited slower PVT 1/RT (Δ 0.3 ± 0.46 ms−1, Baseline: 3.89 ±0.40 ms−1, Restriction: 3.59 ± 0.39 ms−1, p = 0.03, g = 0.72), and slower PVT Fastest 10% RT (Δ 15.3 ± 23.1 ms, Baseline: 195.2 ± 24.9 ms, Restriction: 210.5 ± 22.2 ms, p = 0.03, g = 0.62).
Figure 2.
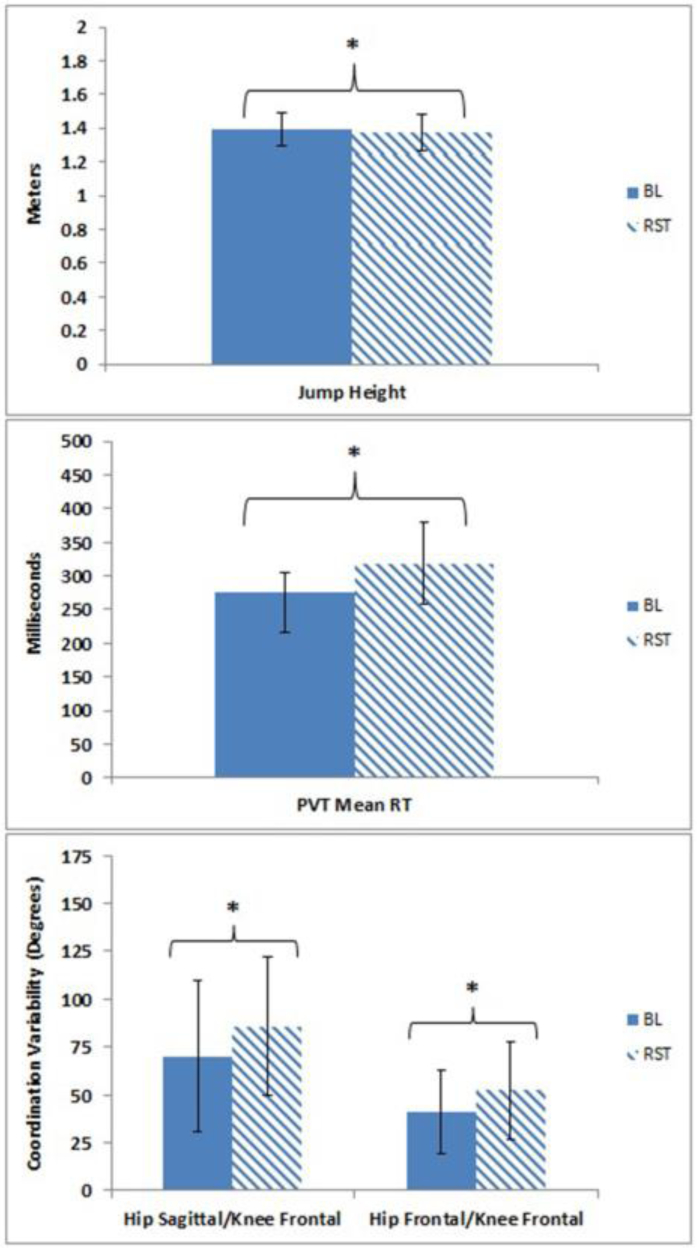
Maximal vertical jump height (top), Psychomotor Vigilance Task (PVT) mean response time (RT) (middle), and hip and knee joint coordination variability (bottom) at baseline (solid) and following sleep restriction (striped). Data provided as mean ± standard deviation. * indicates p < 0.05.
Table 3.
Physical performance, joint coordination variability (CV) and the Psychomotor Vigilance Task (PVT) are reported as the mean ± standard deviation. PVT for 10 of the 11 athletes are presented here.
| Baseline | Sleep Restriction | p-value | |
|---|---|---|---|
| Physical Performance & Joint CV | |||
| Vertical Jump (m) | 1.39 ± 0.10 | 1.37 ± 0.10* | 0.01 |
| Hip Sagittal/Knee Frontal Plane CV (degrees) | 70.1 ± 39.3 | 88.7 ± 36.1* | 0.04 |
| Hip Frontal/Knee Frontal Plane CV (degrees) | 41.2 ± 21.9 | 52.2 ± 25.6* | < 0.01 |
| Psychomotor Vigilance Task | |||
| Mean Response Time (ms) | 278.7 ± 28.3 | 320.8 ± 63.5* | 0.04 |
| 1/Response Time (ms−1) | 3.89 ± 0.40 | 3.59 ± 0.39* | 0.04 |
| Fastest 10% Response Time (ms) | 195.2 ± 24.9 | 210.5 ± 22.2* | 0.03 |
indicates p < 0.05.
Hip and knee joint coordination variability during the DVJ were associated with changes in cognitive measures. The baseline hip frontal/knee frontal plane joint coordination variability (P = 0.62, p = 0.03) and the sleep restriction hip sagittal/knee frontal plane joint coordination variability (P = 0.63, p = 0.03) demonstrated significant, positive associations with changes in PVT mean response time (Figure 3). Specifically, increases in joint coordination variability within these joint couplings were related to an increased slowing of the PVT mean response time. There were no significant associations (p > 0.05) of cognitive or biomechanical measures with maximal jump height.
Figure 3.
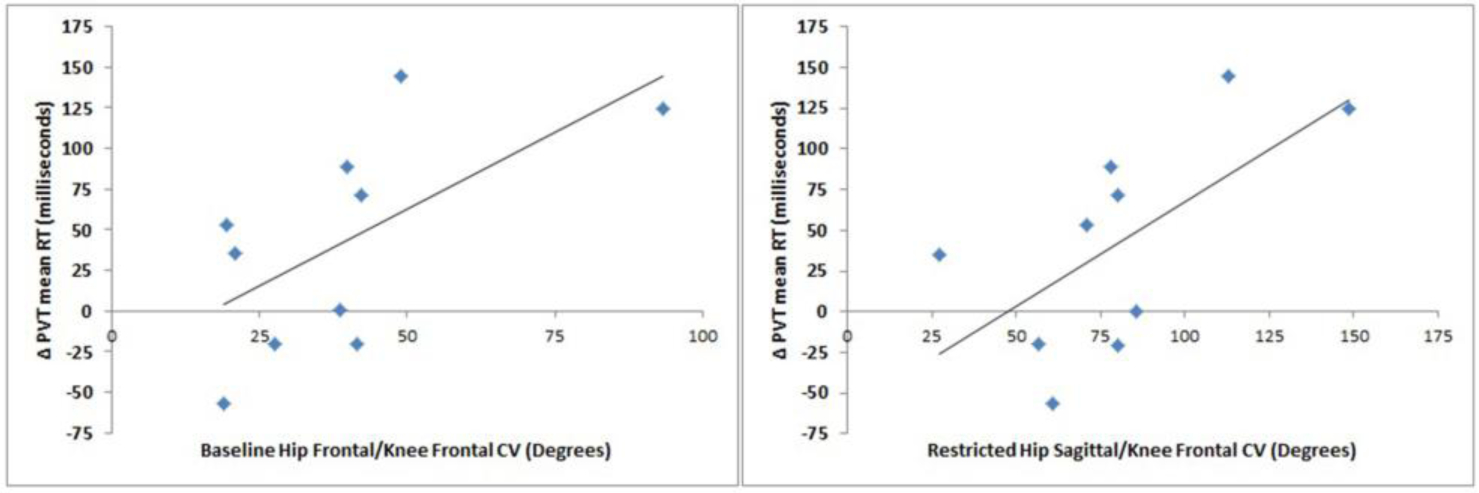
Scatter plots of the baseline hip frontal/knee frontal (left) and sleep restricted hip sagittal/knee frontal (right) joint coordination variability (CV) with the difference in the Psychomotor Vigilance Task (PVT) mean response time (RT) (Δ PVT mean RT) between baseline and sleep restriction.
DISCUSSION
The results of the present study demonstrated that consecutive days of sleep restriction decreased maximal vertical jump, increased coordination variability, and impaired psychomotor response time in elite athletes. In addition, increased coordination variability was found to be associated with increased slowing of psychomotor response time. To our knowledge, this is the first study to demonstrate the impact of sleep restriction on maximal jump performance, the associated biomechanics during dynamic movement, and the relationship with cognitive performance. These exploratory findings suggest chronic sleep loss impaired athletic performance and altered biomechanical function.
Consecutive days of sleep restriction impaired maximal vertical jump decreasing jump height by 1.95 cm. The decrement in jump height is consistent with prior studies that have shown sleep loss impairs vertical jump height 34, 35. One past study demonstrated a significant 2.8 cm decrease in vertical jump height following 64 hours of sleep deprivation 35. Many sports rely on regularly utilizing jumping movements (i.e. basketball, football, volleyball, etc.) and decrements in lower extremity power may have on-field performance and career implications. In the context of football, a 4.0 cm difference in vertical jump height has been demonstrated to distinguish between drafted versus non-drafted skill players in the National Football League 36. This suggests that even several centimeters difference in jump height can be critical for top-level athletes. This finding extends our understanding that physical performance impairments in maximal jump efforts are not limited to complete absence of sleep via sleep deprivation. Notably, consecutive days of inadequate sleep can also result in impairments to lower extremity power and/or coordination.
Assessments of joint coordination variability, using vector coding, may be a useful method in assessing joint biomechanics in athletes under both a normal or perturbed cognitive or physical state. Physiological perturbations such as anterior cruciate ligament reconstruction and neuromuscular fatigue have been shown to alter lower extremity joint coordination variability during dynamic activity 11–13. In addition, altered joint coordination variability has been suggested to be linked with overuse injury 14, 37. In the current study, sleep loss significantly increased hip and knee joint coordination variability during the DVJ task. Prior research has demonstrated that athletes have a preferred coordination strategy for maximal vertical jump 38. The results of the current study suggest that sleep loss impacts the mechanics of preferred coordinated movements. This may be evidence of dynamic instability during the DVJ task and help to explain the reduction in maximal jump height in a sleep restricted state. Furthermore, the present study found that greater impairments in cognitive response time were associated with increased coordination variability. This finding suggests that decrements in cognitive alertness may impact sensorimotor control during dynamic tasks. This is consistent with a recent study of cognitive and sensorimotor capabilities and impaired gait adaptability. This prior study demonstrated slower reaction time was associated with poorer stepping accuracy and increased postural sway in older adults 39.
These findings provide initial insights that sleep loss results in impaired jump height performance with associated changes in biomechanics. These alterations in movement patterns may be a contributing factor for how the resultant performance decrement in jump height is achieved. Future research should elucidate whether changes in neuromuscular coordination, motor control, or other cognitive factors are the main drivers for the resulting performance decrement. It is also unclear whether or not increased variability is detrimental to athletes. Future studies should also examine sleep restriction effects on other dynamic movement patterns and in particular those movements (i.e. sidestep cuts) that are known to contribute to greater injury risk. Previous work has demonstrated that chronic sleep restriction < 8 hours/night is associated with 1.7 times greater injury risk in athletes 40. Another study has suggested that sleep deprivation may be related to increased risk of anterior cruciate ligament injury 41. Ultimately, understanding how lower extremity movement patterns are affected by different sleep durations may help researchers and clinicians elucidate how sensorimotor control and physical performance outcomes are impacted.
LIMITATIONS
The present study had several limitations. The small sample size was limited by the availability of elite athletes, however is consistent with studies of elite athletes that often include small sample sizes due to the difficulty of studying actively competing athletes. Secondly, cyclists were included in the study as a convenient sample. Future studies can include other sports that more regularly rely on jumping movements for sport execution. Third, actigraphy is limited in confirming participants remained awake without microsleeps during sleep restriction. Athletes were instructed to refrain from sedentary activities in the hours prior to bedtime thus likely limiting opportunities for microsleeps. Lastly, this study was unable to determine if changes in coordination variability were due to changes in the underlying muscle activation patterns. Electromyography (EMG) should be included in future studies assessing coordination variability and the effects of sleep restriction.
CONCLUSIONS
Sleep restriction for three days decreased maximal vertical jump height. Sleep loss increased lower extremity coordination variability and was associated with increased slowing of psychomotor response time in elite athletes. Sleep restriction impacts biomechanical movement patterns and further research is warranted to elucidate the impact of sleep on human biomechanics.
Statement of significance:
Sleep loss has been shown to impair cognitive and physical performance; however, it is unknown how the associated biomechanical movement patterns are affected. This exploratory study is the first to provide initial insights that sleep restriction results in biomechanical alterations of the lower extremity during a dynamic jumping task. These changes in movement patterns may help explain the resulting performance decrement and how motor movements are impacted by sleep loss. Cognitive impairments in psychomotor response may also be related. The study findings are support for further study of the impact of sleep and associated biomechanics.
ACKNOWLEDGEMENTS
We would like to thank the athletes that participated in the study.
Funding:
This study was made possible in part by the Clinical and Translational Research Fellowship Program (CTRFP), a program of UCSF’s Clinical and Translational Science Institute (CTSI) that is sponsored in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number TL1 TR000144. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or UCSF.
ABBREVIATIONS
- GRF
Ground reaction force
- DVJ
Drop vertical jump
- COM
Center of mass
- PVT
Psychomotor Vigilance Task
- EMG
Electromyography
References
- 1.Reilly T, Waterhouse J. Sports performance: Is there evidence that the body clock plays a role? Eur J Appl Physiol 2009;106(3):321–32. [DOI] [PubMed] [Google Scholar]
- 2.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J Sleep Res 2003;12(1):1–12. [DOI] [PubMed] [Google Scholar]
- 3.Reilly T, Atkinson G, Budgett R. Effect of low-dose temazepam on physiological variables and performance tests following a westerly flight across five time zones. Int J Sports Med 2001;22(3):166–74. [DOI] [PubMed] [Google Scholar]
- 4.Winget CM, DeRoshia CW, Holley DC. Circadian rhythms and athletic performance. Med Sci Sports Exerc 1985;17(5):498–516. [PubMed] [Google Scholar]
- 5.Fullagar HH, Skorski S, Duffield R, Hammes D, Coutts AJ, Meyer T. Sleep and athletic performance: The effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med 2015;45(2):161–86. [DOI] [PubMed] [Google Scholar]
- 6.Mets MA, Volkerts ER, Olivier B, Verster JC. Effect of hypnotic drugs on body balance and standing steadiness. Sleep Med Rev 2010;14(4):259–67. [DOI] [PubMed] [Google Scholar]
- 7.Latash ML, Scholz JP, Schoner G. Motor control strategies revealed in the structure of motor variability. Exerc Sport Sci Rev 2002;30(1):26–31. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein N The coordination and regulation of movement. London: Pergamon Press, 1967. [Google Scholar]
- 9.Hamill J, van Emmerik RE, Heiderscheit BC, Li L. A dynamical systems approach to lower extremity running injuries. Clin Biomech 1999;14(5):297–308. [DOI] [PubMed] [Google Scholar]
- 10.Pollard CD, Heiderscheit BC, van Emmerik RE, Hamill J. Gender differences in lower extremity coupling variability during an unanticipated cutting maneuver. J Appl Biomech 2005;21(2):143–52. [DOI] [PubMed] [Google Scholar]
- 11.Pollard CD, Stearns KM, Hayes AT, Heiderscheit BC. Altered lower extremity movement variability in female soccer players during side-step cutting after anterior cruciate ligament reconstruction. Am J Sports Med 2015;43(2):460–5. [DOI] [PubMed] [Google Scholar]
- 12.Samaan MA, Hoch MC, Ringleb SI, Bawab S, Weinhandl JT. Isolated hamstrings fatigue alters hip and knee joint coordination during a cutting maneuver. J Appl Biomech 2015;31(2):102–10. [DOI] [PubMed] [Google Scholar]
- 13.Gribbin TC, Slater LV, Herb CC, et al. Differences in hip–knee joint coupling during gait after anterior cruciate ligament reconstruction. Clin Biomech 2016;3264–71. [DOI] [PubMed] [Google Scholar]
- 14.Heiderscheit BC, Hamill J, Van Emmerik EA. Variability of stride characterisitics and joint coordination among individuals with unilateral patellofemoral pain. J Appl Biomech 2002;18110–21. [Google Scholar]
- 15.Armour Smith J, Popovich JM Jr., Kulig K. The influence of hip strength on lower-limb, pelvis, and trunk kinematics and coordination patterns during walking and hopping healthy women. J Orthop Sports Phys Ther 2014;44(7):525–31. [DOI] [PubMed] [Google Scholar]
- 16.Barrett R, Vonk Noordegraaf M, Morrison S. Gender differences in the variability of lower extremity kinematics during treadmill locomotion. J Motor Behav 2008;40(1):62–70. [DOI] [PubMed] [Google Scholar]
- 17.Chang R, Van Emmerik R, Hamill J. Quantifying rearfoot–forefoot coordination in human walking. J Biomech 2008;41(14):3101–5. [DOI] [PubMed] [Google Scholar]
- 18.Miller RH, Chang R, Baird JL, Van Emmerik REA, Hamill J. Variability in kinematic coupling assessed by vector coding and continuous relative phase. J Biomech 2010;43(13):2554–60. [DOI] [PubMed] [Google Scholar]
- 19.Samaan MA, Teng HL, Kumar D, et al. Acetabular cartilage defects cause altered hip and knee joint coordination variability during gait. Clin Biomech 2015;30(10):1202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullineaux DR, Uhl TL. Coordination-variability and kinematics of misses versus swishes of basketball free throws. J Sport Sci 2010;28(9):1017–24. [DOI] [PubMed] [Google Scholar]
- 21.Bobbert MF. Effect of unloading and loading on power in simulated countermovement and squat jumps. Med Sci Sports Exerc 2014;46(6):1176–84. [DOI] [PubMed] [Google Scholar]
- 22.Jaric S, Markovic G. Leg muscles design: The maximum dynamic output hypothesis. Med Sci Sports Exerc 2009;41(4):780–7. [DOI] [PubMed] [Google Scholar]
- 23.Chung F, Yegneswaran B, Liao P, et al. Stop questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108(5):812–21. [DOI] [PubMed] [Google Scholar]
- 24.Ford KR, Shapiro R, Myer GD, Van Den Bogert AJ, Hewett TE. Longitudinal sex differences during landing in knee abduction in young athletes. Med Sci Sports Exerc 2010;42(10):1923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spoor CW, Veldpaus FE. Rigid body motion calculated from spatial co-ordinates of markers. J Biomech 1980;13(4):391–3. [DOI] [PubMed] [Google Scholar]
- 26.Kernozek TW, Ragan RJ. Estimation of anterior cruciate ligament tension from inverse dynamics data and electromyography in females during drop landing. Clin Biomech (Bristol, Avon) 2008;23(10):1279–86. [DOI] [PubMed] [Google Scholar]
- 27.Sparrow WA, Donovan E, van Emmerik R, Barry EB. Using relative motion plots to measure changes in intra-limb and inter-limb coordination. J Motor Behav 1987;19(1):115–29. [DOI] [PubMed] [Google Scholar]
- 28.Bates NA, Ford KR, Myer GD, Hewett TE. Kinetic and kinematic differences between first and second landings of a drop vertical jump task: Implications for injury risk assessments. Clin Biomech (Bristol, Avon) 2013;28(4):459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke SB, Kenny IC, Harrison AJ. Dynamic knee joint mechanics after anterior cruciate ligament reconstruction. Med Sci Sports Exerc 2015;47(1):120–7. [DOI] [PubMed] [Google Scholar]
- 30.Delahunt E, Sweeney L, Chawke M, et al. Lower limb kinematic alterations during drop vertical jumps in female athletes who have undergone anterior cruciate ligament reconstruction. J Orthop Res 2012;30(1):72–8. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz RJ, Cone JC, Tritsch AJ, et al. Changes in drop-jump landing biomechanics during prolonged intermittent exercise. Sports Health 2014;6(2):128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen J Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum, 1998. [Google Scholar]
- 33.Hedges L, Olkin I. Statistical methods for meta-analysis. Orlando: Academic Press, 1985. [Google Scholar]
- 34.Daanen HA, van Ling S, Tan TK. Subjective ratings and performance in the heat and after sleep deprivation. Aviat Space Environ Med 2013;84(7):701–7. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi L, Davis GM, Plyley M, Goode R, Shephard RJ. Sleep deprivation, chronic exercise and muscular performance. Ergonomics 1985;28(3):591–601. [DOI] [PubMed] [Google Scholar]
- 36.Sierer SP, Battaglini CL, Mihalik JP, Shields EW, Tomasini NT. The national football league combine: Performance differences between drafted and nondrafted players entering the 2004 and 2005 drafts. J Strength Cond Res 2008;22(1):6–12. [DOI] [PubMed] [Google Scholar]
- 37.Hamill J, Palmer C, Van Emmerik RE. Coordinative variability and overuse injury. Sports Med Arthrosc Rehabil Ther Technol 2012;4(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandic R, Knezevic OM, Mirkov DM, Jaric S. Control strategy of maximum vertical jumps: The preferred countermovement depth may not be fully optimized for jump height. J Hum Kinet 2016;5285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caetano MJ, Menant JC, Schoene D, Pelicioni PH, Sturnieks DL, Lord SR. Sensorimotor and cognitive predictors of impaired gait adaptability in older people. J Gerontol A Biol Sci Med Sci 2016. [DOI] [PubMed] [Google Scholar]
- 40.Milewski MD, Skaggs DL, Bishop GA, et al. Chronic lack of sleep is associated with increased sports injuries in adolescent athletes. J Pediatr Orthop 2014;34(2):129–33. [DOI] [PubMed] [Google Scholar]
- 41.Elliot DL, Goldberg L, Kuehl KS. Young women’s anterior cruciate ligament injuries. Sports Medicine 2010;40(5):367–76. [DOI] [PubMed] [Google Scholar]


